Abstract
Objective:
To determine risk factors for clinically significant progression during 12 months in patients with mild-to-moderate Alzheimer disease.
Method:
Community-dwelling patients with mild-to-moderate Alzheimer disease were enrolled in a 3-year prospective study, the Canadian Outcomes Study in Dementia (commonly referred to as COSID), at 32 Canadian sites. Assessments included the Global Deterioration Scale (GDS) for disease severity, the Mini-Mental State Examination (MMSE) for cognition, the Functional Autonomy Measurement System (SMAF) for daily functioning, and the NeuroPsychiatric Inventory (NPI) for behaviour, measured at baseline and at 12 months. Logistic regression identified factors associated with GDS decline, and subsequent stepwise regression identified key independent predictors. Area under the curve (AUC) was then calculated for the model.
Results:
Among 488 patients (mean age 76.5 years [SD 6.4], MMSE 22.1 [SD4.6], 44.1% male), 225 (46%) showed GDS decline. After adjusting for age, baseline risk factors for deterioration included the following: poorer cognition (lower MMSE score, OR 0.55; 95% CI 0.4 to 0.72 per 5 points, P ≤ 0.001), greater dependence (lower SMAF, OR 0.72; 95% CI 0.63 to 0.83 per 5 points, P ≤ 0.001), and more neuropsychiatric symptoms (higher NPI, OR 1.11; 95% CI 1.02 to 1.2 per 5 points, P = 0.02), with a protective effect of male sex (OR 0.59; 95% CI 0.39 to 0.9, P = 0.02), and higher (worse) GDS score (very mild, compared with mild OR 0.25; 95% CI 0.09 to 0.70, P ≤ 0.01; compared with moderate, OR 0.08; 95% CI 0.03 to 0.23, P < 0.001; compared with moderately severe, OR 0.03; 95% CI 0.01 to 0.11, P < 0.001). The AUC was 73% (P < 0.001) (sensitivity 90% and specificity 33%).
Conclusion:
The progression of Alzheimer disease in Canada can be predicted using readily available clinical information.
Keywords: Alzheimer disease, dementia, prognosis
Abstract
Objectif :
Déterminer les facteurs de risque de la progression cliniquement significative durant 12 mois chez des patients souffrant de la maladie d’Alzheimer bénigne à modérée.
Méthode :
Des patients résidant dans la communauté et souffrant de la maladie d’Alzheimer bénigne à modérée ont été inscrits dans une étude prospective de 3 ans, l’Étude canadienne de résultat sur la démence (Canadian Outcomes Study in Dementia ou COSID), dans 32 sites au Canada. Les évaluations comprenaient l’Échelle de détérioration globale (GDS) pour la sévérité de la maladie, le Mini-examen de l’état mental (MMSE) pour la cognition, le Système de mesure de l’autonomie fonctionnelle (SMAF) pour le fonctionnement quotidien, et l’Inventaire neuro-psychiatrique (NPI) pour le comportement, mesurées au départ et à 12 mois. La régression logistique a identifié des facteurs associés à la baisse de la GDS, et une régression subséquente progressive a identifié des prédicteurs clés indépendants. La surface sous la courbe (AUC) a ensuite été calculée pour le modèle.
Résultats :
Parmi les 488 patients (âge moyen 76,5 ans [ET 6,4], MMSE 22,1 [ET 4,6], 44,1 % d’hommes), 225 (46 %) présentaient une baisse de la GDS. Après correction pour l’âge, les facteurs de risque de détérioration au départ incluaient ce qui suit: une moins bonne cognition (score plus faible au MMSE, RC 0,55; IC à 95 % 0,4 à 0,72 par 5 points, P ≤ 0,001), une plus grande dépendance (score plus faible au SMAF, RC 0,72; IC à 95 % 0,63 à 0,83 par 5 points, P ≤ 0,001), et plus de symptômes neuropsychiatriques (score plus élevé au NPI, RC 1,11; IC à 95 % 1,02 à 1,2 par 5 points, P = 0,02), avec un effet protecteur du sexe masculin (RC 0,59; IC à 95 % 0,39 à 0,9, P = 0,02), et un score plus élevé (pire) à la GDS (très bénin, comparé à bénin RC 0,25; IC à 95 % 0,09 à 0,70, P ≤ 0,01; comparé à modéré, RC 0,08; IC à 95 % 0,03 à 0,23, P < 0,001; comparé à modérément grave, RC 0,03; IC à 95 % 0,01 à 0,11, P < 0,001). L’AUC était de 73 % (P < 0,001) (sensibilité 90 % et spécificité 33 %).
Conclusion :
La progression de la maladie d’Alzheimer au Canada peut être prédite à l’aide de l’information clinique facilement disponible.
Alzheimer disease is a progressive neurodegenerative disorder associated with cognitive, functional, and behavioural disturbances.1,2 Owing to loss of ability to perform ADLs as the disease progresses, increased cost and caregiver burden are associated with increasing illness severity.3,4 The Alzheimer Society of Canada reported that 63% of dementia patients were affected by Alzheimer disease, making it the most common form of dementia in the nation. The prevalence of Alzheimer disease is expected to more than double from 303 878 in 2008 to 770 811 in 2038.5 Globally, the number of patients with Alzheimer disease account for 92% of the entire dementia population,6 and Brookmeyer et al7 estimated an increase in number of patients with Alzheimer disease from 26.6 million in 2011 to 106.2 million by 2050.
Past studies have noted a wide range of variation in the course of disease progression across patients with Alzheimer disease.8,9 Numerous risk factors were found to be related to the differential rate of Alzheimer disease progression, including age of onset,10,11 sex,8,12 epsilon 4 allele of the apolipoprotein E gene,13 level of education,14 baseline and change in MMSE score,10 aphasia,15,16 visuospatial processing,17,18 neuropsychiatric symptoms,19 hallucinations,20 use of APs,21 and ChEI use.8,22 Being able to estimate disease progression is important, clinically, so that patients at high risk of progression can be identified, as well as for economic modelling, so that impact of treatments can be estimated.
Clinical Implications
Readily accessible clinical information (age, sex, neuropsychiatric symptoms, cognition, and dependence) predict clinically meaningful deterioration in a treated Canadian population.
Four major domains of clinical assessment—cognitive, behavioural, functional, and global—could be used as a tool by clinicians to identify high-risk people who should be closely monitored.
This information could be clinically useful as well as useful for pharmacoeconomic and service delivery planning.
Limitations
The outcome is based on a single time point only, allowing for possible bias due to rare events.
The patients were solely community-dwelling, with mild-to-moderate Alzheimer disease.
Owing to the nature of the recruitment process, more actively treated patients may have enrolled in the study.
Although all of these factors may correlate with Alzheimer disease progression, results have varied, and most studies examined patient populations from the United States and other countries. Further, many of those populations were unmedicated, having been conducted prior to the widespread availability of cognitive-enhancing drugs, such as ChEIs. As such, the magnitude and specific risk factors for disease progression in a Canadian population, with differences in health care funding and availability of services and cognitive-enhancing drug reimbursement, may differ. In our study, a large cohort of patients with Alzheimer disease across Canada was investigated prospectively, with detailed demographic data and commonly administered clinical measurements. The objectives of our analysis were to prospectively quantify the expected decline in a Canadian population with Alzheimer disease, and to determine factors that predict high risk of clinically significant progression over the next 12 months.
Methods
Study and Subjects
COSID was a 3-year prospective observational study that was conducted at 32 sites in major regions across Canada (British Columbia, the Prairies, Ontario, Quebec, and Atlantic Provinces) by investigators specializing in geriatric medicine, geriatric psychiatry, neurology, and family medicine, with special interest in geriatrics. Our study was approved by local review ethics board, and signed informed consents from both patients and caregivers were obtained. Detailed procedures were reported previously.23
In this specific analysis, only patients diagnosed with Alzheimer disease and with 12-month, follow-up data were included. The diagnosis of Alzheimer disease was based on the criteria of the National Institute of Neurological and Communicative Diseases and Alzheimer Disease and Related Disorders Association criteria.24 Other inclusion criteria were age 60 years or older, not residing in a senior’s residence or long-term care facility, and a GDS of 5 or less at the baseline.25
Predictors
Assessments were completed at baseline and at 12 months using the MMSE, SMAF, NPI, and GDS. The MMSE is a screening tool for assessing cognitive function. The resulting score ranges from 0 to 30, with lower values indicating more severe cognitive impairment.26 The SMAF, which measures the functional ability of people, was developed according to the World Health Organization classification of disabilities. The scale measures functional ability in 5 areas: ADLs, instrumental ADLs, mobility, communication, and mental function. Each of the 29 items is scored on a 4-point scale, from 0 (independent) to −3 (dependent), such that lower (more negative) scores indicate greater dependence.27 The NPI is a caregiver-based interview that assesses 10 behavioural areas28 (delusions, hallucinations, agitation, dysphoria, anxiety, euphoria, apathy, disinhibition, irritability, and aberrant motor behaviour), with higher scores indicating more severe and (or) frequent neuropsychiatric symptoms. The GDS, which we used to measure the severity of the disease, is validated and reliable, and scores range from 1 to 7 in order of increasing severity, from no obvious deficits to profound or end-stage disease.25
We collected background demographic information at baseline, including: age, sex, disease duration, cardiovascular risk score, level of education, and the use of any psychotropic (APs, ADs, sedative or hypnotics, and ChEIs). ChEIs included donepezil, galantamine, or rivastigmine. Memantine was not readily available in Canada at the time of our study. A cardiovascular burden score was determined, representing the sum of the number of cardiovascular issues (range 0 to 7) reported from among the following: angiopathy, aortic aneurysm repair, aortic bypass, aortic valve replacement, cardiac pacemaker insertion, coronary artery bypass, and mitral valve replacement.
Outcome Measures
We used the GDS as the primary outcome measure of our study. The GDS is a 7-point, ordinal scale that considers cognitive, functional, and behavioural factors, which makes it useful for assessing clinically significant deterioration of patients with Alzheimer disease.25 This was considered a more comprehensive and clinically relevant marker of change than for example, the MMSE by itself. Progression was defined as a decline by 1 or more points on the GDS during the year of observation.
Statistical Analysis
Baseline characteristics were summarized and compared between the subjects who progressed, from baseline to month 12, using the Wilcoxon test for numeric characteristics and the chi-square test for categorical characteristics. Logistic regression was used to examine the multivariate association between probability of GDS progression and all predictor variables, regardless of the level of significance in the one-at-a-time analyses. As many of the predictor variables in the full model were strongly correlated, stepwise regression analysis was then used to produce a more parsimonious reduced model (with significance levels of 10% to enter and then to leave the model). Age was included in all models so that the influence of other predictors would be adjusted for age.
The adjusted odds ratios, from both the full and reduced models, are presented, along with their 95% confidence intervals. The strength of the multivariate association shown between GDS progression and the predictors of the reduced model is quantified by the AUC from the receiver operating characteristic curve and by the specificity achieved a minimum sensitivity of 90%. In general, these analyses should be interpreted descriptively as they do not account for issues of multiple testing during the modelling process. The analyses were conducted using SAS-STAT software, version 9.3 (SAS Institute Inc, Cary, NC). Figures were produced in R29 using the rms30 and ggplot231 packages.
Results
Baseline Characteristics and Overall Change in Scores
From the original cohort of patients (n = 904),23 731 had a diagnosis of Alzheimer disease. Among diagnosed patients, 243 were excluded because they did not have complete information, leaving 488 patients in this analysis (Table 1). Most of the patients had mild-to-moderate Alzheimer disease (4.7% very mild, 33.0% mild, 48.0% moderate, and 14.3% moderately severe), and 480 (98.4%) of them were Caucasian. Most study subjects were receiving psychotropics at baseline (92.2%), including ChEIs (86.7%), ADs (24.2%), sedatives or hypnotics (13.1%), and APs (9.6%). Common comorbidities included hypertension (44.9%), cardiac disorders (28.5%), osteoarthritis (22.8%), and gastrointestinal disorders (19.0%). On average, patients showed overall declines in performance in all of the assessments after 12 months: MMSE (OR −2.26; 95% CI −2.64 to −1.87, P < 0.001), SMAF (OR −6.37; 95% CI −7.07 to −5.66, P < 0.001), NPI (OR 1.73; 95% CI 0.49 to 2.98, P = 0.007), and GDS (OR 0.49; 95% CI 0.03 to 0.56, P < 0.001). Table 2 shows the breakdown of patients who experienced deterioration during 12 months; in total, 46% of patients declined during 12 months, with 192 (39.2%) declining by 1 point on the GDS, 31 (6.4%) declining by 2 points, and 2 (0.4%) declining by 3 or more points.
Table 1.
Baseline characteristics of patients with Alzheimer disease
| Variable | Overall n = 488 | Progressors n = 225 | Nonprogressors n = 263 | P |
|---|---|---|---|---|
| Age, years, mean (SD) | 76.47 (6.38) | 77.13 (6.61) | 75.90 (6.14) | 0.045 |
| Disease duration, months, mean (SD) | 44.82 (25.89) | 46.42 (27.16) | 43.44 (24.72) | 0.33 |
| Cardiovascular burden score, mean (SD) | 1.11 (1.14) | 1.12 (1.12) | 1.10 (1.16) | 0.65 |
| GDS score, mean (SD) | 3.72 (0.72) | 3.66 (0.81) | 3.77 (0.76) | 0.16 |
| MMSE score, mean (SD) | 22.05 (4.60) | 21.19 (5.27) | 22.78 (3.79) | 0.001 |
| SMAF score, mean (SD) | −17.13 (9.86) | −19.41 (10.5) | −15.18 (8.84) | <0.001 |
| NPI score, mean (SD) | 10.14 (12.55) | 11.77 (12.86) | 8.75 (12.13) | 0.001 |
| Sex, male, n (%) | 215 (44.1) | 95 (42.2) | 120 (45.6) | 0.45 |
| Education, n (%) | ||||
| Grade school | 164 (33.6) | 76 (33.8) | 88 (33.5) | 0.23 |
| High school | 190 (38.9) | 95 (42.2) | 95 (36.1) | |
| >High school | 134 (27.5) | 54 (24.0) | 80 (30.4) | |
| Medication use, n (%) | ||||
| Any psychotropic | 450 (92.2) | 203 (90.2) | 247 (93.9) | 0.13 |
| AP | 47 (9.6) | 27 (12.0) | 20 (7.6) | 0.10 |
| AD | 118 (24.2) | 50 (22.2) | 68 (25.9) | 0.35 |
| Sedative or hypnotic | 64 (13.1) | 30 (13.3) | 32 (12.9) | 0.90 |
| ChEI | 423 (86.7) | 189 (84.0) | 234 (89.0) | 0.11 |
| Marital status, n (%) | 0.45 | |||
| Married or common-law | 325 (66.6) | 145 (64.5) | 180 (68.4) | |
| Single | 6 (1.2) | 4 (1.8) | 2 (0.8) | |
| Widowed, separated, or divorced | 157 (32.1) | 76 (33.8) | 81 (30.8) | |
| Living status, n (%) | 0.21 | |||
| At patient’s home | 408 (83.6) | 181 (80.4) | 227 (86.3) | |
| At caregiver’s home | 26 (5.3) | 15 (6.7) | 11 (4.2) | |
| Other | 54 (11.1) | 29 (12.9) | 25 (9.5) |
AD = antidepressant; AP = antipsychotic; ChEI = cholinesterase inhibitor; GDS = Global Deterioration Scale; MMSE = Mini-Mental State Examination; NPI = NeuroPsychiatric Inventory; SMAF = Functional Autonomy Measurement System
Table 2.
Breakdown of progression in patients with Alzheimer disease 12 months after baseline
| 12-month GDS stage | Baseline GDS stage | |||
|---|---|---|---|---|
|
| ||||
| Very mild n = 23 n (%) | Mild n = 161 n (%) | Moderate n = 234 n (%) | Moderately severe n = 70 n (%) | |
| Very mild | 7 (30.4) | 4 (2.5) | n/a | n/a |
| Mild | 10 (43.5) | 80 (49.7) | 9 (3.8) | n/a |
| Moderate | 5 (21.7) | 63 (39.1) | 126 (53.8) | 6 (8.6) |
| Moderately severe | 1 (4.3) | 13 (8.1) | 86 (36.8) | 31 (44.3) |
| Severe | n/a | 1 (0.6) | 13 (5.6) | 33 (47.1) |
GDS = Global Deterioration Scale; n/a = not applicable
Univariate Analysis
The one-at-a-time analysis compared patients who progressed with patients who did not progress for each of the selected variables. Age, baseline MMSE score, baseline SMAF total scores, and baseline NPI total scores were found to be significantly different between the groups (Table 1).
Multivariate Analysis
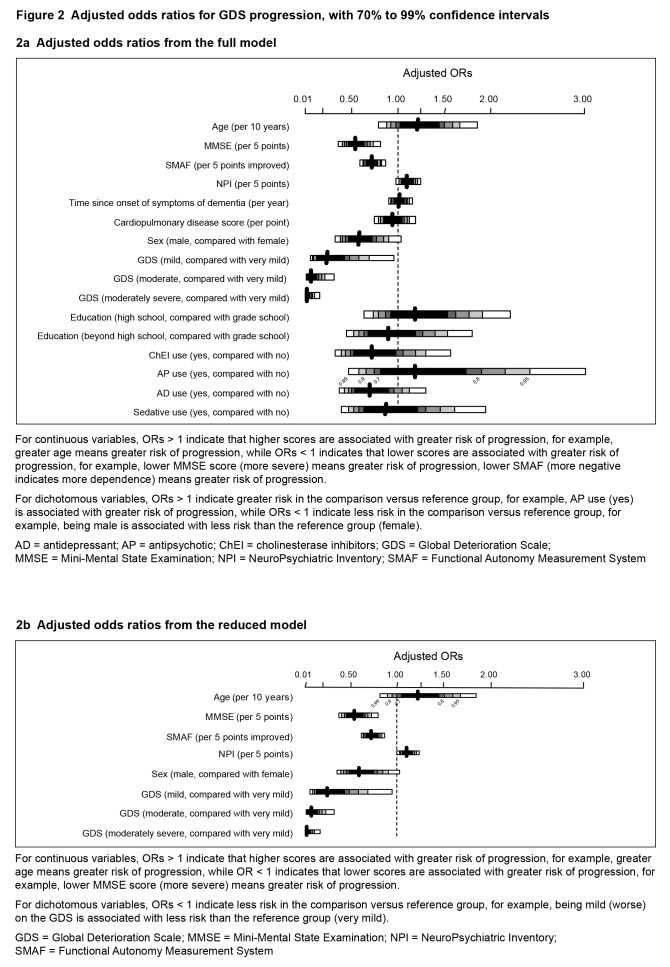
The effect of GDS on MMSE, SMAF, NPI, and age were plotted in Figure 1. Table 3a and Figure 2a show the model results including all the predictors based on the multivariate analysis. The reduced model from stepwise logistic regression, shown in Table 3b and Figure 2b, demonstrated statistically significant baseline risk factors which, after adjusting for age, included: poorer cognition (lower MMSE score 0.55 per 5 points; 95% CI 0.4 to 0.72, P < 0.001), greater dependence (lower SMAF, 0.72 per 5 points; 95% CI 0.63 to 0.83, P < 0.001), and more neuropsychiatric symptoms (higher NPI, 1.11 per 5 points; 95% CI 1.02 to 1.2, P = 0.02), with a protective effect of male sex (OR 0.59; 95% CI 0.39 to 0.9, P = 0.02), and higher baseline GDS score (mild, compared with very mild, 0.25; 95% CI 0.09 to 0.69, P = 0.007; moderate, OR 0.08; 95% CI 0.03 to 0.23, P < 0.001; or moderately severe, OR 0.03; 95% CI 0.01 to 0.11, P < 0.001).The AUC was calculated to be 73% (P < 0.001), yielding a sensitivity of 90% with a specificity of 33%. The cardiovascular burden score was not included in the final model by the stepwise selection algorithm.
Figure 1.
Predicted probability of GDS progression, compared with MMSE, SMAF, and NPI stratified by baseline GDS (reduced model)
Each panel shows the effects of GDS and 1 other predictor; other variables in the model have been fixed at typical values: MMSE = 20, SMAF = −15, NPI = 10, and Age = 7.5 years.
GDS = Global Deterioration Scale; MMSE = Mini-Mental State Examination; NPI = NeuroPsychiatric Inventory; Prob = probability (of progress); SMAF = Functional Autonomy Measurement System
Figure 2.
Adjusted odds ratios for GDS progression, with 70% to 99% confidence intervals
2a Adjusted odds ratios from the full model
For continuous variables, ORs > 1 indicate that higher scores are associated with greater risk of progression, for example, greater age means greater risk of progression, while ORs < 1 indicates that lower scores are associated with greater risk of progression, for example, lower MMSE score (more severe) means greater risk of progression, lower SMAF (more negative indicates more dependence) means greater risk of progression.
For dichotomous variables, ORs > 1 indicate greater risk in the comparison versus reference group, for example, AP use (yes) is associated with greater risk of progression, while ORs < 1 indicate less risk in the comparison versus reference group, for example, being male is associated with less risk than the reference group (female).
AD = antidepressant; AP = antipsychotic; ChEI = cholinesterase inhibitors; GDS = Global Deterioration Scale; MMSE = Mini-Mental State Examination; NPI = NeuroPsychiatric Inventory; SMAF = Functional Autonomy Measurement System
2b Adjusted odds ratios from the reduced model
For continuous variables, ORs > 1 indicate that higher scores are associated with greater risk of progression, for example, greater age means greater risk of progression, while OR < 1 indicates that lower scores are associated with greater risk of progression, for example, lower MMSE score (more severe) means greater risk of progression.
For dichotomous variables, ORs < 1 indicate less risk in the comparison versus reference group, for example, being mild (worse) on the GDS is associated with less risk than the reference group (very mild).
GDS = Global Deterioration Scale; MMSE = Mini-Mental State Examination; NPI = NeuroPsychiatric Inventory; SMAF = Functional Autonomy Measurement System
Discussion
Almost one-half of the patients with Alzheimer disease in COSID had a clinically meaningful deterioration, as measured by the GDS, during 12 months. On average, this group of treated patients worsened by 2.26 points on the MMSE and by 1.73 points on the NPI. The risk factors for GDS deterioration included female sex, older age, lower baseline cognition (lower MMSE score), greater baseline dependence (lower SMAF score), higher baseline neuropsychiatric symptoms (higher NPI score), and a less severe baseline disease severity (lower baseline GDS score). As acceptable AUC values observed in literature range between 0.70 to 0.80,32,33 the AUC reported (73%, P < 0.001) within the range of values observed.
In our study, we found that higher age was significantly associated with increased risk for decline. Previous studies9,34–36 have also reported this finding. This association may be due to other confounding factors associated with older age, such as comorbid chronic medical illnesses. Sona et al35 have particularly suggested that there is a correlation between rapid cognitive decline and the baseline age of the patients. Conversely, Stern et al37 reported an opposite association, with younger age associated with worse disease outcomes. This discrepancy may be due to the relatively younger age of that sample. As such, the impact of age on decline may depend on sample selection. Importantly, the COSID cohort was representative of the average patient with Alzheimer disease in Canada, based on comparisons to the CSHA.38
Several factors emerged as being significant predictors of decline in our study. Being female was associated with a 1.68-fold increase in the risk of any GDS score decline. Numerous previous studies10,34–36,39 have reported a similar impact of being female on risk of deterioration, although some research indicated that there was no significant relation between sex and disease progression.40,41 This difference in disease progression between males and females may be due to hormonal differences,42 as well as psychosocial issues associated with differing roles.
During 12 months, the total population showed a loss of 2.26 points on the MMSE. This finding is similar to previous findings in treated patients with Alzheimer disease. In a prospective observational study,22 the loss on the MMSE was 2.48 points per year in a ChEI-treated sample. More recent long-term, prospective studies with mild-to-moderate Alzheimer disease (90% treated with medications) have found change in MMSE to average 2.4 points decline per year.43 Thus these cognitive findings are consistent with other treated populations. In addition, past studies have shown that untreated patients have a significantly higher risk of rapid cognitive deterioration. Gillette-Guyonnet et al22 reported the risk of rapid cognitive deterioration was increased by 46% for untreated patient groups, compared with ChEI-treated groups, with 47.5% of the untreated patients having a decline of more than 3 points on the MMSE within 12 months. As expected, our population showed less decline than untreated populations, consistent with 86.7% receiving ChEIs. Several studies of Alzheimer disease progression have been conducted using MMSE as the outcome measure. Most of those studies8,20,36,37,40,44–46 found a strong relation between lower baseline MMSE and worse disease outcomes. Among those studies, the baseline MMSE ranged from 17.6 to 25.2. In our study, the baseline MMSE was 22.05, which is consistent with the range found in other studies.
Similarly, the population showed a worsening of behaviour, with a mean increase of 1.73 points on the NPI, and this is consistent with other studies. For example, Gillette-Guyonnet et al43 reported in their prospective longitudinal study that the NPI score of patients with Alzheimer disease worsened by 1.5 points annually. Previous studies have also shown correlations between higher baseline NPI scores and worse Alzheimer disease outcomes.34,39 In particular, neuropsychiatric symptoms, such as psychotic symptoms,47 apathy,48 and depression,49 have previously been associated with steeper declines. The presence of these symptoms has been linked with differing underlying neurobiology50,51 and may indicate more widespread pathology.
Greater dependence at baseline, as measured by the SMAF, was also associated with worse Alzheimer disease outcome. Previously, the PSMS, which examines 6 different functional abilities, was shown to be correlated with disease progression.36 PSMS shares similarities with the SMAF in terms of their areas of measurement. In addition, previous studies52,53 have shown that worsening of the PSMS is correlated with cognitive decline. This was consistent with the result from the COSID population as the patients with greater baseline dependency (such as not easily eating and dressing shown on the SMAF) were more likely to progress in the next 12 months.
In contrast, the GDS indicated that patients with very mild baseline disease severity were 1.38 times more likely to decline, from very mild impairment to moderate or severe impairment, by the end of the year. Given the inclusion criteria of the study (mild Alzheimer disease with GDS ≤ 5), we did not expect a large number of people to progress to the more severe range during the course of 1 year. The higher risk of decline in patients with very mild disease at baseline may seem surprising. A possible explanation for this finding could be the nonlinear progression of Alzheimer disease, or that the GDS was not as sensitive to disease progression at an advanced stage of deterioration. Owing to this possible ceiling effect, there may be some sensitivity limitations when using GDS as a staging instrument for more advanced Alzheimer disease. Because of this, we did not require GDS to have a linear progression in statistical analyses and attempted to reduce the impact of bias by adjusting for baseline differences and other prognostic factors. Additional regressions using baseline GDS level as a categorical predictor to account for the nonlinearity of the GDS progression had little impact on the final model. Specifically, the baseline MMSE, SMAF, and NPI were still strongly predictive of decline in GDS. In addition, lack of sensitivity on the part of the GDS can be overcome by considering other scales measuring function, neuropsychiatric symptoms, and cognition,54 as was done here. Taken with the finding on SMAF, the high-risk population are characterized as those who are still very mild on the GDS, but showing initial losses of instrumental ADLs.
Some predictors correlated with disease progression in previous research were not significant in our study, such as the level of education, disease duration, and the use of concomitant medications. Education data indicated that most participants had a grade school or high school education. With only 27% of the sample educated beyond high school, the 6% difference in the proportion of highly educated patients who progressed was not statistically significant. Disease duration had a fairly small range, as patients were recruited specifically to be in the mild-to-moderate range. ChEIs, which are modestly effective for Alzheimer disease symptoms,55 were also dropped from the models. This finding is likely related to almost every patient receiving therapy with a ChEI (87%), and consistent with their role as symptomatic rather than disease-modifying therapy. In addition, as the study was not randomized, selection bias in administering medications cannot be ruled out. It is possible that when our study was conducted, shortly after the initial introduction of these drugs to Canada, the drugs were given preferentially to more rapidly progressing or high-risk patients, whereas it was not given to the patients who were considered to be relatively stable.
There are several limitations to our study. The generalizability of the data in COSID needs to be considered. Owing to the nature of the patient recruitment process, all the data were acquired from dementia specialists who may have enrolled more actively treated patients or patients who are different clinically from patients with Alzheimer disease in primary care. There may have been differences between patients who were willing to participate in our study and ones who were not. COSID did not collect data on the number of eligible patients at each site who did not provide consent. Specific data on the level of patient support and social engagements were also not collected, although only outpatients in the community or living at home were recruited into the study as part of the inclusion or exclusion criteria. The data used for analyses included 53% of the original 3-year COSID group: 20% were excluded on the basis of diagnosis (non-Alzheimer disease), and the remaining 33% of patients did not have complete information at baseline and (or) at 1-year follow-up. A 1-year follow-up time point was chosen owing to patient attrition as incomplete data could not be used for regression analyses. Despite these factors, demographic features between excluded patients and the 488 included patients were similar (data not shown). Further, as the COSID sample has similar baseline characteristics to the CSHA,38 a population-based Canadian cohort, the study population may still be broadly representative of a population with Alzheimer disease, which strengthens the generalizability of our study. Finally, as the GDS may not be very sensitive to Alzheimer disease progression at advanced stages of deterioration, it should be used in conjunction with other measures of Alzheimer disease progression. The relatively low specificity of our model (33%) may give greater number of false positives; however, high sensitivity (90%) allows for clinicians to err on the side of caution so that patients will be followed more closely and, it is hoped, with more aggressive intervention.
The strengths of our study are that COSID employed one of the largest prospective dementia databases in Canada, and the analyses included clinically accessible predictors and clinically meaningful outcome measurements that can be applied to real-world settings. While previous research has outlined expected progression and predictors of progression for patients with Alzheimer disease, the circumstances for Canadian patients have changed since that time; ChEIs were introduced and evidence-based treatment guidelines for Alzheimer disease have recommended their use as standard of practice for mild, moderate, and severe Alzheimer disease.56 The COSID study was designed to include large numbers of dementia patients across Canada with longitudinal data. This cohort was assembled when all 3 ChEIs, as well as memantine, were available, and the standard of care for patients with Alzheimer disease has not changed significantly since that time. Various domains of clinical measurements predictors were considered in the analysis, including cognitive, functional, and behavioural characteristics, that are thought to play important roles in the progression of Alzheimer disease. No previous models have assessed the impacts of all 4 domains of measurements on the progression of Alzheimer disease at the same time.
Conclusion
Using readily available clinical information, our model characterized Alzheimer disease progression in a Canadian population with acceptable accuracy. While on average, almost one-half of mild patients will have a meaningful decline during 1 year, variability is large and can be predicted based on risk factors. As the prevalence of Alzheimer disease is high and expected to increase dramatically in the future in Canada, the characterization and identification of the risk factors in the Canadian population is urgently needed to establish appropriate health care planning.
Table 3.
Risk factors for clinically significant progression during 12 months in patients with Alzheimer disease
3a.
Prediction of progression: full modela
| Effect | OR | 95% CI |
|---|---|---|
| Age (per 10 years) | 1.216 | 0.883 to 1.674 |
| MMSE (per 5 points) | 0.549 | 0.407 to 0.740 |
| SMAF (per 5 points)a | 0.721 | 0.627 to 0.829 |
| NPI (per 5 points) | 1.109 | 1.016 to 1.209 |
| Time since onset of dementia symptoms (per year) | 1.022 | 0.927 to 1.128 |
| Cardiopulmonary disease score (per point) | 0.947 | 0.794 to 1.130 |
| Sex (male, compared with female) | 0.585 | 0.381 to 0.900 |
| GDS (mild, compared with very mild) | 0.250 | 0.090 to 0.695 |
| GDS (moderate, compared with very mild) | 0.077 | 0.026 to 0.226 |
| GDS (moderately severe, compared with very mild) | 0.030 | 0.008 to 0.109 |
| Education (high school, compared with grade school) | 1.189 | 0.741 to 1.907 |
| Education (beyond high school, compared with grade school) | 0.904 | 0.535 to 1.526 |
| ChEI use (yes, compared with no) | 0.721 | 0.400 to 1.301 |
| AP use (yes, compared with no) | 1.191 | 0.587 to 2.414 |
| AD use (yes, compared with no) | 0.697 | 0.432 to 1.125 |
| Sedative use (yes, compared with no) | 0.879 | 0.481 to 1.606 |
Adjusted odds ratios and 95% confidence intervals from the full logistic regression models for prediction of GDS progression
SMAF is scored to produce negative scores with scores closer to zero indicating less dependence.
AD = antidepressant; AP = antipsychotic; ChEI = cholinesterase inhibitors; GDS = Global Deterioration Scale; MMSE = Mini-Mental State Examination; NPI = NeuroPsychiatric Inventory; SMAF = Functional Autonomy Measurement System
3b.
Prediction of progression: reduced modela
| Effect | OR | 95% CI |
|---|---|---|
| Age (per 10 years) | 1.225 | 0.896 to 1.673 |
| MMSE (per 5 points) | 0.540 | 0.404 to 0.721 |
| SMAF (per 5 points)a | 0.723 | 0.630 to 0.828 |
| NPI (per 5 points) | 1.105 | 1.017 to 1.200 |
| Sex (male, compared with female) | 0.594 | 0.391 to 0.902 |
| GDS (mild, compared with very mild) | 0.249 | 0.090 to 0.685 |
| GDS (moderate, compared with very mild) | 0.079 | 0.027 to 0.229 |
| GDS (moderately severe, compared with very mild) | 0.030 | 0.008 to 0.109 |
Adjusted odds ratios and 95% confidence intervals from the reduced logistic regression models for prediction of GDS progression model after stepwise model selection (that is, stepwise regression with significance levels to stay and exit set to 0.10)
SMAF is scored to produce negative scores with scores closer to zero indicating less dependence.
GDS = Global Deterioration Scale; MMSE = Mini-Mental State Examination; NPI = NeuroPsychiatric Inventory; SMAF = Functional Autonomy Measurement System
Acknowledgments
Funding for COSID was provided by an unrestricted research grant from Janssen-Ortho Inc, Canada. The sponsor had no role in data collection, interpretation of data, or writing of the report. Dr Herrmann has received research support and (or) speaker’s honoraria from Lundbeck Canada Inc, Pfizer Canada Inc, Janssen-Ortho Inc, Novartis, and Sonexa Therapeutics Inc. Mr Harimoto has no potential conflicts of interest to declare. At the start of the analyses, Dr Balshaw was an employee of Syreon Corporation. Syreon Corporation is a contract research organization and was retained by Janssen-Ortho Inc to conduct the COSID Study (study operations, data management, and analyses). Dr Lanctôt has received research support and (or) speaker’s honoraria from Abbott Laboratories, Lundbeck Canada Inc, Pfizer Canada Inc, Janssen-Ortho Inc, Wyeth Pharmaceuticals Inc, and Sonexa Therapeutics Inc.
Abbreviations
- AD
antidepressant
- ADL
activity of daily living
- AP
antipsychotic
- AUC
area under curve
- ChEI
cholinesterase inhibitor
- COSID
Canadian Outcomes Study in Dementia
- CSHA
Canadian Study of Health and Aging
- GDS
Global Deterioration Scale
- MMSE
Mini-Mental State Examination
- NPI
NeuroPsychiatric Inventory
- PSMS
Physical Self-Maintenance Scale
- SMAF
Functional Autonomy Measurement System
References
- 1.Solomon PR, Murphy CA. Early diagnosis and treatment of Alzheimer’s disease. Expert Rev Neurother. 2008;8(5):769–780. doi: 10.1586/14737175.8.5.769. [DOI] [PubMed] [Google Scholar]
- 2.Cohen-Mansfield J, Mintzer JE. Time for change: the role of nonpharmacological interventions in treating behavior problems in nursing home residents with dementia. Alzheimer Dis Assoc Disord. 2005;19(1):37–40. doi: 10.1097/01.wad.0000155066.39184.61. [DOI] [PubMed] [Google Scholar]
- 3.Wimo A, Jönsson L, Bond J, et al. The worldwide economic impact of dementia 2010. Alzheimers Dement. 2013;9(1):1–11.e3. doi: 10.1016/j.jalz.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Herrmann N, Tam DY, Balshaw R, et al. The relation between disease severity and cost of caring for patients with Alzheimer disease in Canada. Can J Psychiatry. 2010;55(12):768–775. doi: 10.1177/070674371005501204. [DOI] [PubMed] [Google Scholar]
- 5.Alzheimer Society of Canada . Rising tide: the impact of dementia on Canadian society [Internet] Toronto (ON): Alzheimer Society of Canada; 2010. [cited 2013 Sep 8]. Available from: http://www.alzheimer.ca/∼/media/Files/national/Advocacy/ASC_Rising%20Tide_Full%20Report_Eng.ashx. [Google Scholar]
- 6.Alzheimer’s Disease International . World Alzheimer report 2010: the global economic impact of dementia [Internet] Stockholm (SE): Alzheimer’s Disease International; 2010. [cited 2013 Sep 8]. Available from: http://www.alz.co.uk/research/files/WorldAlzheimerReport2010.pdf. [Google Scholar]
- 7.Brookmeyer R, Evans DA, Hebert L, et al. National estimates of the prevalence of Alzheimer’s disease in the United States. Alzheimers Dement. 2011;7(1):61–73. doi: 10.1016/j.jalz.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holmes C, Lovestone S. Long-term cognitive and functional decline in late onset Alzheimer’s disease: therapeutic implications. Age Ageing. 2003;32(2):200–204. doi: 10.1093/ageing/32.2.200. [DOI] [PubMed] [Google Scholar]
- 9.Doody RS, Pavlik V, Massman P, et al. Predicting progression of Alzheimer’s disease. Alzheimers Res Ther. 2010;2(1):2. doi: 10.1186/alzrt25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hatoum HT, Thomas SK, Lin SJ, et al. Predicting time to nursing home placement based on activities of daily living scores—a modelling analysis using data on Alzheimer’s disease patients receiving rivastigmine or donepezil. J Med Econ. 2009;12(2):98–103. doi: 10.3111/13696990903004039. [DOI] [PubMed] [Google Scholar]
- 11.Heyman A, Peterson B, Fillenbaum G, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part XIV: demographic and clinical predictors of survival in patients with Alzheimer’s disease. Neurology. 1996;46(3):656–660. doi: 10.1212/wnl.46.3.656. [DOI] [PubMed] [Google Scholar]
- 12.Brodaty H, Woodward M, Boundy K, et al. Patients in Australian memory clinics: baseline characteristics and predictors of decline at six months. Int Psychogeriatr. 2011;23(7):1086–1096. doi: 10.1017/S1041610211000688. [DOI] [PubMed] [Google Scholar]
- 13.Williams JW, Plassman BL, Burke J, et al. Preventing Alzheimer’s disease and cognitive decline. Evid Rep Technol Assess (Full Rep) 2010;(193):1–727. [PMC free article] [PubMed] [Google Scholar]
- 14.Rasmusson DX, Carson KA, Brookmeyer R, et al. Predicting rate of cognitive decline in probable Alzheimer’s disease. Brain Cogn. 1996;31(2):133–147. doi: 10.1006/brcg.1996.0038. [DOI] [PubMed] [Google Scholar]
- 15.Faber-Langendoen K, Morris JC, Knesevich JW, et al. Aphasia in senile dementia of the Alzheimer type. Ann Neurol. 1988;23(4):365–370. doi: 10.1002/ana.410230409. [DOI] [PubMed] [Google Scholar]
- 16.Boller F, Becker JT, Holland AL, et al. Predictors of decline in Alzheimer’s disease. Cortex. 1991;27(1):9–17. doi: 10.1016/s0010-9452(13)80264-x. [DOI] [PubMed] [Google Scholar]
- 17.Burns A, Jacoby R, Levy R. Progression of cognitive impairment in Alzheimer’s disease. J Am Geriatr Soc. 1991;39(1):39–45. doi: 10.1111/j.1532-5415.1991.tb05904.x. [DOI] [PubMed] [Google Scholar]
- 18.Andel R, Gatz M, Pedersen NL, et al. Deficits in controlled processing may predict dementia: a twin study. J Gerontol B Psychol Sci Soc Sci. 2001;56(6):P347–P355. doi: 10.1093/geronb/56.6.p347. [DOI] [PubMed] [Google Scholar]
- 19.Eustace A, Coen R, Walsh C, et al. A longitudinal evaluation of behavioural and psychological symptoms of probable Alzheimer’s disease. Int J Geriatr Psychiatry. 2002;17(10):968–973. doi: 10.1002/gps.736. [DOI] [PubMed] [Google Scholar]
- 20.Ward A, Caro JJ, Kelley H, et al. Describing cognitive decline of patients at the mild or moderate stages of Alzheimer’s disease using the standardized MMSE. Int Psychogeriatr. 2002;14(3):249–258. doi: 10.1017/s1041610202008451. [DOI] [PubMed] [Google Scholar]
- 21.Lopez OL, Wisniewski SR, Becker JT, et al. Psychiatric medication and abnormal behavior as predictors of progression in probable Alzheimer disease. Arch Neurol. 1999;56(10):1266–1272. doi: 10.1001/archneur.56.10.1266. [DOI] [PubMed] [Google Scholar]
- 22.Gillette-Guyonnet S, Andrieu S, Cortes F, et al. Outcome of Alzheimer’s disease: potential impact of cholinesterase inhibitors. J Gerontol A Biol Sci Med Sci. 2006;61(5):516–520. doi: 10.1093/gerona/61.5.516. [DOI] [PubMed] [Google Scholar]
- 23.Sambrook R, Herrmann N, Hébert R, et al. Canadian Outcomes Study in Dementia: study methods and patient characteristics. Can J Psychiatry. 2004;49(7):417–427. doi: 10.1177/070674370404900702. [DOI] [PubMed] [Google Scholar]
- 24.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 25.Reisberg B, Ferris SH, de Leon MJ, et al. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982;139(9):1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- 26.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 27.Hebert R, Carrier R, Bilodeau A. The Functional Autonomy Measurement System (SMAF): description and validation of an instrument for the measurement of handicaps. Age Ageing. 1988;17(5):293–302. doi: 10.1093/ageing/17.5.293. [DOI] [PubMed] [Google Scholar]
- 28.Cummings JL, Mega M, Gray K, et al. The NeuroPsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 29.R Core Team . R: a language and environment for statistical computing [Internet] Vienna (AU): R Foundation for Statistical Computing; 2012. [cited 2013 Nov 14]. Available from: http://www.R-project.org. [Google Scholar]
- 30.Harrell FE., Jr . RMS: regression modeling strategies. R package version 3.6–3 [Internet] Nashville (TN): Vanderbilt University School of Medicine; 2013. [cited 2013 Nov 14]. Available from: http://CRAN.R-project.org/package=rms. [Google Scholar]
- 31.Wickham H. GGPLOT2: elegant graphics for data analysis. New York (NY): Springer; 2009. [Google Scholar]
- 32.Wilson PW, D’Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 33.DeSalvo KB, Fan VS, McDonell MB, et al. Predicting mortality and healthcare utilization with a single question. Health Serv Res. 2005;40(4):1234–1246. doi: 10.1111/j.1475-6773.2005.00404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tschanz JT, Corcoran CD, Schwartz S, et al. Progression of cognitive, functional, and neuropsychiatric symptom domains in a population cohort with Alzheimer dementia: the Cache County dementia progression study. Am J Geriatr Psychiatry. 2011;19(6):532–542. doi: 10.1097/JGP.0b013e3181faec23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sona A, Zhang P, Ames D, et al. Predictors of rapid cognitive decline in Alzheimer’s disease: results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of ageing. Int Psychogeriatr. 2012;24(2):197–204. doi: 10.1017/S1041610211001335. [DOI] [PubMed] [Google Scholar]
- 36.Wattmo C, Wallin ÅK, Londos E, et al. Long-term outcome and prediction models of activities of daily living in Alzheimer disease with cholinesterase inhibitor treatment. Alzheimer Dis Assoc Disord. 2011;25(1):63–72. doi: 10.1097/WAD.0b013e3181f5dd97. [DOI] [PubMed] [Google Scholar]
- 37.Stern Y, Tang MX, Albert MS, et al. Predicting time to nursing home care and death in individuals with Alzheimer disease. JAMA. 1997;277(10):806–812. [PubMed] [Google Scholar]
- 38.Canadian Study of Health and Aging Working Group Canadian study of health and aging: study methods and prevalence of dementia. CMAJ. 1994;150(6):899–913. [PMC free article] [PubMed] [Google Scholar]
- 39.Rabins PV, Schwartz S, Black BS, et al. Predictors of progression to severe Alzheimer’s disease in an incidence sample. Alzheimers Dement. 2013;9(2):204–207. doi: 10.1016/j.jalz.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stern RG, Mohs RC, Davidson M, et al. A longitudinal study of Alzheimer’s disease: measurement, rate, and predictors of cognitive deterioration. Am J Psychiatry. 1994;151(3):390–396. doi: 10.1176/ajp.151.3.390. [DOI] [PubMed] [Google Scholar]
- 41.Lindsay J, Laurin D, Verreault R, et al. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol. 2002;156(5):445–453. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- 42.Vest RS, Pike CJ. Gender, sex steroid hormones, and Alzheimer’s disease. Horm Behav. 2013;63(2):301–307. doi: 10.1016/j.yhbeh.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gillette-Guyonnet S, Andrieu S, Nourhashemi F, et al. Long-term progression of Alzheimer’s disease in patients under antidementia drugs. Alzheimers Dement. 2011;7(6):579–592. doi: 10.1016/j.jalz.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 44.Santillan CE, Fritsch T, Geldmacher DS. Development of a scale to predict decline in patients with mild Alzheimer’s disease. J Am Geriatr Soc. 2003;51(1):91–95. doi: 10.1034/j.1601-5215.2002.51016.x. [DOI] [PubMed] [Google Scholar]
- 45.O’Hara R, Thompson JM, Kraemer HC, et al. Which Alzheimer patients are at risk for rapid cognitive decline? J Geriatr Psychiatry Neurol. 2002;15(4):233–238. doi: 10.1177/089198870201500409. [DOI] [PubMed] [Google Scholar]
- 46.Soto ME, Andrieu S, Cantet C, et al. Predictive value of rapid decline in Mini Mental State Examination in clinical practice for prognosis in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2008;26(2):109–116. doi: 10.1159/000144073. [DOI] [PubMed] [Google Scholar]
- 47.Vilalta-Franch J, López-Pousa S, Calvó-Perxas L, et al. Psychosis of Alzheimer disease: prevalence, incidence, persistence, risk factors, and mortality. Am J Geriatr Psychiatry. 2013;21(11):1135–1143. doi: 10.1016/j.jagp.2013.01.051. [DOI] [PubMed] [Google Scholar]
- 48.Pollero A, Giménez M, Allegri RF, et al. Neuropsychiatric symptoms in patients with Alzheimer disease. Vertex. 2004;15(55):5–9. [PubMed] [Google Scholar]
- 49.Teng E, Lu PH, Cummings JL. Neuropsychiatric symptoms are associated with progression from mild cognitive impairment to Alzheimer’s disease. Dement Geriatr Cogn Disord. 2007;24(4):253–259. doi: 10.1159/000107100. [DOI] [PubMed] [Google Scholar]
- 50.Lanctôt KL, Rajaram RD, Herrmann N. Therapy for Alzheimer’s disease: how effective are current treatments? Ther Adv Neurol Disord. 2009;2(3):163–180. doi: 10.1177/1756285609102724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rountree SD, Chan W, Pavlik VN, et al. Persistent treatment with cholinesterase inhibitors and/or memantine slows clinical progression of Alzheimer disease. Alzheimers Res Ther. 2009;1(2):7. doi: 10.1186/alzrt7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rountree SD, Chan W, Pavlik VN, et al. Factors that influence survival in a probable Alzheimer disease cohort. Alzheimers Res Ther. 2012;4(3):16. doi: 10.1186/alzrt119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schmeidler J, Mohs RC, Aryan M. Relationship of disease severity to decline on specific cognitive and functional measures in Alzheimer disease. Alzheimer Dis Assoc Disord. 1998;12(3):146–151. doi: 10.1097/00002093-199809000-00005. [DOI] [PubMed] [Google Scholar]
- 54.Eisdorfer C, Cohen D, Paveza GJ, et al. An empirical evaluation of the Global Deterioration Scale for staging Alzheimer’s disease. Am J Psychiatry. 1992;149(2):190–194. doi: 10.1176/ajp.149.2.190. [DOI] [PubMed] [Google Scholar]
- 55.Lanctôt KL, Herrmann N, Yau KK, et al. Efficacy and safety of cholinesterase inhibitors in Alzheimer’s disease: a meta-analysis. CMAJ. 2003;169(6):557–564. [PMC free article] [PubMed] [Google Scholar]
- 56.Gauthier S, Patterson C, Chertkow H, et al. Fourth Canadian Consensus Conference on the Diagnosis and Treatment of Dementia. Can J Neurol Sci. 2012;39(6 Suppl 5):S1–S8. doi: 10.1017/s0317167100015183. [DOI] [PubMed] [Google Scholar]