Abstract
Attention deficit hyperactivity disorder (ADHD) and autism spectrum disorder (ASD) share brain function abnormalities during cognitive flexibility. Serotonin is involved in both disorders, and selective serotonin reuptake inhibitors (SSRIs) can modulate cognitive flexibility and improve behavior in both disorders. Thus, this study investigates shared and disorder-specific brain dysfunctions in these 2 disorders during reward reversal, and the acute effects of an SSRI on these. Age-matched boys with ADHD (15), ASD (18), and controls (21) were compared with functional magnetic resonance imaging (fMRI) during a reversal task. Patients were scanned twice, under either an acute dose of Fluoxetine or placebo in a double-blind, placebo-controlled randomized design. Repeated-measures analyses within patients assessed drug effects. Patients under each drug condition were compared with controls to assess normalization effects. fMRI data showed that, under placebo, ASD boys underactivated medial prefrontal cortex (mPFC), compared with control and ADHD boys. Both patient groups shared decreased precuneus activation. Under Fluoxetine, mPFC activation was up-regulated and normalized in ASD boys relative to controls, but down-regulated in ADHD boys relative to placebo, which was concomitant with worse task performance in ADHD. Fluoxetine therefore has inverse effects on mPFC activation in ASD and ADHD during reversal learning, suggesting dissociated underlying serotonin abnormalities.
Keywords: ADHD, ASD, cognitive flexibility, fMRI, serotonin
Introduction
Attention deficit hyperactivity disorder (ADHD) is a neurodevelopmental disorder characterized by age-inappropriate levels of impulsiveness, inattention, and hyperactivity (American Psychiatric Association 1994). Autism spectrum disorder (ASD) is defined by impairments in communication, social interaction, and also by restricted and repetitive behaviors (American Psychiatric Association 1994). ADHD and ASD are highly comorbid and both disorders share executive function deficits (Willcutt et al. 2005; Corbett et al. 2009; Rommelse et al. 2011), including poor cognitive flexibility (Hill 2004; Willcutt et al. 2005; Sanders et al. 2008), which has been linked to repetitive behaviors in ASD (Yerys et al. 2009). The clinical importance of this behavioral and cognitive overlap has been highlighted by changes to the upcoming DSM-V, which allows co-diagnosis of ADHD and ASD (http://www.dsm5.org).
Cognitive flexibility can be measured in switching and reversal tasks, where stimulus-response associations need to be either changed to new, or reversed to previous stimulus-response associations, respectively. It is known that the prefrontal cortex is involved in many aspects of cognition, and that the same region can play a role in a number of functions (Goldman-Rakic et al. 1996; Ashby and Isen 1999). However, the cognitive processes of switching and reward reversal learning involve quite different neuronal circuitry. During switching tasks, healthy children and adults activate inferior frontal cortex (IFC), dorsolateral prefrontal cortex (DLPFC), anterior cingulate cortex (ACC), and parietal lobe (Derrfuss et al. 2005; Loose et al. 2006; Rubia et al. 2006; Ravizza and Carter 2008; Christakou, Halari, et al. 2009). During reward reversal learning tasks, in healthy adults, due to the emotional valence of the reward and punishment present in reward reversal learning tasks, more medial brain regions, including medial prefrontal cortex (mPFC), medial orbitofrontal cortex (OFC), and ACC, are typically recruited (O'Doherty et al. 2001, 2003; Cools et al. 2002; Remijnse et al. 2005; Cohen et al. 2008; Kehagia et al. 2010). Striatal activation is also observed during reward reversal learning due to the role of the striatum in reward-related habitual learning and stimulus-response associations, with more ventral striatal regions involved in reversal learning (Cools et al. 2002; Packard and Knowlton 2002; Remijnse et al. 2005; Yin and Knowlton 2006) and more anterior striatal regions being implicated in switching (Rubia et al. 2006; Christakou, Halari, et al. 2009).
fMRI studies of switch tasks have found decreased activation in ADHD children compared with controls in the IFC, temporo-parietal junction, and striatum (Smith et al. 2006; Rubia, Cubillo, et al. 2010; Rubia, Halari, et al. 2010). ADHD patients have been shown to have abnormal medial frontal and precuneus activation during reward reversal tasks (Finger et al. 2008). In ASD children, no fMRI study has investigated cognitive flexibility. In adult ASD, however, 2 studies have reported conflicting evidence of decreased activation in the DLPFC, ACC, and basal ganglia (Shafritz et al. 2008), and increased activation in the IFC and parietal lobe relative to controls (Schmitz et al. 2006).
5-HT and dopamine interact in the prefrontal cortex, resulting in the fine tuning of neuronal responses and better cognition, particularly in tasks that require the maintenance of stimulus-response representations (Goldman-Rakic 1999). There is evidence that 5-HT is involved in reward reversal learning (Murphy et al. 2002; Evers et al. 2005; Roberts 2011). Furthermore, there is evidence that 5-HT is involved in the pathology of both ADHD and ASD.
Thus, polymorphisms of serotonergic genes have been associated with both ADHD and ASD (Sinzig and Lehmkuhl 2007; Rommelse et al. 2010), and there is evidence that a polymorphism of the 5-HT transporter gene may play a role in the ADHD symptoms observed in ASD (Gadow et al. 2013). Moreover, biochemical serotonergic dysfunction has been implicated in both ADHD (Oades 2007) and ASD (Zafeiriou et al. 2009). In children with ADHD, there is evidence for decreased platelet 5-HT levels (Spivak et al. 1999) and increased ADHD behavior after a reduction in 5-HT (Zepf et al. 2008, 2010). Conversely, one-third of the individuals with ASD have abnormally elevated 5-HT platelet levels (Piven et al. 1991; Mulder et al. 2004; Hranilovic et al. 2007). In addition, in ASD, abnormal 5-HT synthesis (Chugani et al. 1997, 1999), 5-HT transporter (Makkonen et al. 2008; Nakamura et al. 2010), and 5-HT2A receptor binding (Murphy et al. 2006) have also been reported. Clinical trials of Fluoxetine in children with ASD have shown improvement in communication, social interaction, and stereotyped behaviors (DeLong et al. 1998, 2002; Hollander et al. 2005; Carrasco et al. 2012), although effects are small (Williams et al. 2010). In addition, SSRIs have shown to increase metabolic and neurofunctional activity in adults with ASD in areas mediating reward reversal learning such as OFC/mPFC, ACC, and striatum (Mitchell et al. 2008; Freyer et al. 2009), which was associated with reduced obsessive behavior (Buchsbaum et al. 2001; Dichter et al. 2010).
In ADHD children, Fluoxetine monotherapy has been shown to significantly improve inattentiveness and hyperactivity in noncomorbid groups (Barrickman et al. 1991), as well as in groups with co-morbid bipolar disorder (Quintana et al. 2007). Fluoxetine also appears to moderate the efficacy of stimulant medication, as evidenced by the finding that combined Fluoxetine–Methylphenidate treatment reduces ADHD symptoms in co-morbid ADHD children (Gammon and Brown 1993; Findling 1996). There is evidence that 5-HT and dopamine interact, in particular, with respect to impulsiveness (Dalley and Roiser 2012) and the serotonergic system has been shown to play a key regulatory role in dopamine release (Sibley et al. 2007; Di Matteo et al. 2008), which is typically low in ADHD (Volkow et al. 1998; del Campo et al. 2011). The importance of 5-HT-dopamine interactions in ADHD is further reinforced by evidence for abnormal ratios and correlations between 5-HT and dopamine levels in children with ADHD (Castellanos et al. 1994; Oades et al. 1998). There is also evidence that response to stimulants is mediated by 5-HT in animal (Gainetdinov et al. 1999) and human studies as an association between serotonergic genes and Methylphenidate response has been observed (McGough et al. 2009; Banerjee et al. 2012). In addition, the co-administration of 5-HT and dopamine amino acids precursors in children with ADHD has been shown to lead to a significant improvement in symptoms (Hinz et al. 2011). Therefore, when used in combination, Fluoxetine may lead to better regulation of the increased dopamine induced by Methylphenidate, leading to clinical improvement (Barrickman et al. 1991; Gammon and Brown 1993; Findling 1996; Quintana et al. 2007). This is in line with the seminal animal study of Gainetdinov et al. (1999) which showed that, in mice, the effect of Methylphenidate was dependent on 5-HT.
In conclusion, there is evidence for impaired cognitive flexibility and underlying neurofunctional brain mechanisms in ADHD (Willcutt et al. 2005; Smith et al. 2006; Finger et al. 2008; Rubia, Cubillo, et al. 2010; Rubia, Halari, et al. 2010) and in ASD (Hill 2004; Schmitz et al. 2006; Sanders et al. 2008; Shafritz et al. 2008). Furthermore, both disorders have shown 5-HT abnormalities (Piven et al. 1991; Spivak et al. 1999; Mulder et al. 2004; Hranilovic et al. 2007; Oades 2007; Zafeiriou et al. 2009), which may possibly underlie these cognitive flexibility deficits. In addition, Fluoxetine has shown to improve behavior in these 2 disorders (Barrickman et al. 1991; Gammon and Brown 1993; DeLong et al. 1998, 2002; Hollander et al. 2005; Quintana et al. 2007; Carrasco et al. 2012), and to modify cognitive flexibility and underlying neural networks in healthy subjects (Evers et al. 2005; Roberts 2011).
The aim of this study was therefore to investigate (1) shared and disorder-specific brain abnormalities in adolescents with ADHD and those with ASD during reward reversal learning and (2) shared and disorder-specific neurofunctional effects of an acute dose of Fluoxetine on this function in both disorders.
Based on prior evidence (Smith et al. 2006; Finger et al. 2008; Shafritz et al. 2008; Rubia, Cubillo, et al. 2010; Rubia, Halari, et al. 2010), we hypothesized that, under placebo, both disorders would show abnormal switching related activation in compared with controls, with more prominent IFC-striatal deficits in ADHD, and more prominent DLPFC and mPFC abnormalities in ASD. We also hypothesized that Fluoxetine would normalize these neurofunctional abnormalities in both disorders.
Materials and Methods
Participants
Fifty-four right-handed males (assessed with the Edinburgh Handedness Inventory; Oldfield 1971; 21 controls, 15 with ADHD, and 18 with ASD), aged 10–17 years, IQ >70 (assessed with the Wechsler Abbreviated Scale of Intelligence-Revised; Wechsler 1999), participated.
Thirty-two ADHD boys were recruited in total; however, 10 boys dropped out of the study due to their dislike of the MRI scanner, 3 were excluded due to co-morbidities (despite the fact we explicitly aimed to recruit only noncomorbid cases), 1 did not reach the diagnostic criteria for the combined subtype of ADHD, 1 was excluded due to poor task performance, and 2 were excluded due to high levels of motion.
ADHD boys had a DSM-IV diagnosis of noncomorbid ADHD, inattentive/hyperactive-impulsive combined subtype, as assessed by an experienced child psychiatrist using the standardized Maudsley diagnostic interview (Goldberg and Murray 2002). They scored above clinical threshold for ADHD on both the Strengths and Difficulties Questionnaire (SDQ) (Goodman and Scott 1999) and the Conners' Parent Rating Scale-Revised (CPRS-R; Conners et al. 1998) (1 boy was below cut-off on SDQ, but had diagnostic confirmation from a child psychiatrist). Three of the ADHD boys were medication-naïve, 1 had ceased taking Methylphenidate 3 months prior to the study, and 11 were on chronic stimulants, but had a 48-h medication washout prior to scanning. ADHD boys were excluded if they scored >15 on the Social Communication Questionnaire (SCQ; Rutter et al. 2003).
Forty-four ASD boys were recruited in total. Of these, 7 boys dropped out of the study due to their dislike of the MRI scanner, 14 were excluded due to co-morbidities, 1 was excluded due to neurological abnormalities, 2 were excluded due to SSRI use, and 2 were excluded due to high levels of motion. ASD diagnosis was made using ICD-10 diagnostic criteria (World Health Organisation 1994) and confirmed by the Autism Diagnostic Interview-Revised (ADI; Lord et al. 1994) and the Autism Diagnostic Observation Schedule (ADOS; Lord et al. 2000). All ASD subjects were medication-naïve apart from 1 patient, who took melatonin (but underwent 2-week medication washout). ASD exclusion criteria included a score of >7 on the hyperactivity/inattention subscale of the SDQ.
DSM-IV as opposed to DSM-V criteria was used to confirm both ADHD and ASD diagnoses, as DSM-V was not available for clinical use at the time of testing. As previously mentioned, DSM-V allows co-diagnosis of ADHD and ASD and therefore would have enabled easier identification and exclusion of comorbid cases. However, rigorous screening ensured that clinical levels of ADHD and ASD traits were not present in ASD or ADHD participants, respectively.
Patients were recruited from local clinics and support groups. They were scanned twice in a double-blind, randomized, placebo-controlled design, using a Latin square randomization design for counter-balanced effects. Due to the half-life of Fluoxetine (1–3 days) and its metabolite Norfluoxetine (5–16 days) (Wong et al. 1995), each scan was 3–4 weeks apart. To ensure that Fluoxetine had reached its peak plasma levels, shown to be after 5–8 h (Wong et al. 1995), patients were scanned 5 h after administration. Liquid Fluoxetine was titrated to age and weight as follows: boys between 10–13 years and <30 kg received 8 mg, those >30 kg received 10 mg. Boys between 14–17 years and <30 kg received 10 mg, and those >30 kg received 15 mg. Placebo was peppermint water, which was similar in taste to Fluoxetine and measured to the equivalent volume.
Twenty-one handedness and age-matched controls were recruited by advertisement. They all scored below clinical thresholds on the SDQ, SCQ, and CPRS. Controls were scanned only once. Drug/alcohol dependency was an exclusion criterium for all participants.
Written informed consent/assent was given for all participants. The study was approved by the local ethics committee. Participants were paid £50 for each scan.
fMRI Paradigm—Reward Reversal Learning
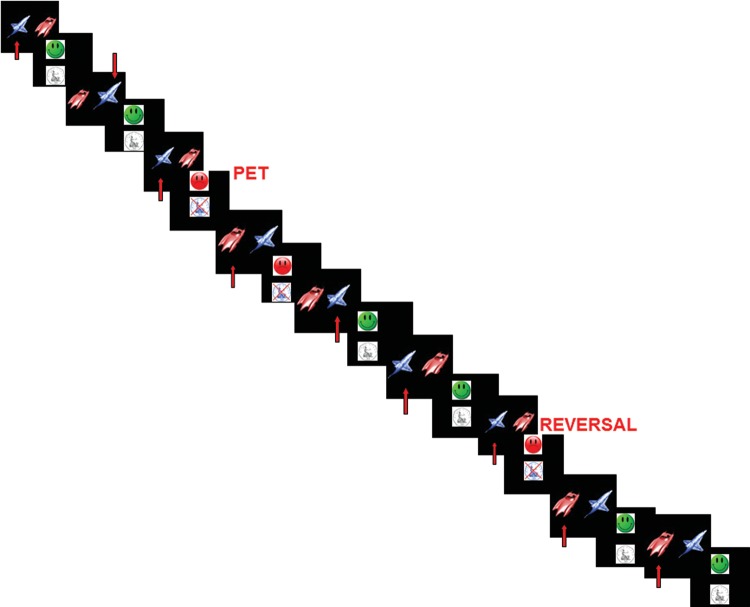
Subjects practiced the task once prior to each scan. Our fMRI adaptation is similar to the probabilistic reward reversal learning task employed by Cools et al. (2002). The semi-self-paced reward reversal learning task requires subjects to learn a stimulus-response association by reward and punishment and to reverse their response when the stimulus-reward contingency changes unexpectedly. Images of a car and a spaceship are displayed simultaneously on the left and right side (randomized) of a black screen for 1950 ms. The subject has to choose with a left or right button press the correct choice, indicated by feedback via an image of a 50 pence piece and a green happy smiley, whereas the incorrect choice is indicated by an image of a crossed-out 50 pence piece and a red unhappy smiley, both displayed after the choice for 950 ms. There is a 100-ms gap between each trial leading to an intertrial interval of 3 s. Reversal of the stimulus-reward contingency occurs after 4–6 consecutive correct responses (i.e., if the car is rewarded and associated with a positive feedback, when a reversal occurs the car is suddenly no longer rewarded, but the spaceship is) (Fig. 1). The task ends after 20 reversal trials or after 20 min, whichever condition is met first. Zero to 2 probabilistic error trials (PETs), where a negative feedback is given for a correct response, are randomly interspersed between reversal trials to prevent subjects from predicting an upcoming reversal trial. PETs are at least 3 trials apart from other PETs and reversal trials. Brain activation to PETs are subtracted from brain activation to the final reversal error before a correct response. This stringent contrast captures the point at which subjects learn to reverse their response, and controls for the brain response to the punishment given in the negative feedback in both trials. This contrast has been used in previous fMRI studies of reward reversal learning (Cools et al. 2002; Remijnse et al. 2005). The main dependent variable is the number of perservative errors made after a reversal trial.
Figure 1.
Reward reversal learning task: subjects select an image (right/left) by pressing the corresponding button (right/left). If is the choice is correct/incorrect, positive/negative feedback is given. Once the subject has made 4–6 correct responses, the stimulus-reward contingency is reversed. PETs, where incorrect feedback is given for a correct response, are included to prevent subjects from predicting an upcoming reversal trial. The task contains on average 20 reversal trials with 20 interspersed PET trials.
Analysis of Performance Data
Two analyses of variance (ANOVAs) compared perseverative errors between controls and patients under either placebo or Fluoxetine. A repeated-measures ANOVA was conducted within the patient groups with group as an independent factor and drug as a repeated measure to test for group by medication interaction effects on performance. Bonferroni correction was used to correct for multiple comparisons.
fMRI Image Acquisition
Gradient-echo echo-planar MR imaging (EPI) data were acquired on a General Electric Signa 3-T Horizon HDx system at the Centre For Neuroimaging Sciences, Institute of Psychiatry, UK. A semi-automated quality control procedure ensured consistent image quality (Simmons et al. 1999). A quadrature birdcage headcoil was used for radio frequency transmission and reception. In each of 23 noncontiguous planes parallel to the anterior–posterior commissure, 800 T2*-weighted MR images depicting blood oxygen level-dependent (BOLD) contrast covering the whole brain were acquired with time echo (TE) = 30 ms, time repetition (TR) = 1.5 s, flip angle = 70°, in-plane voxel size = 3 mm, slice thickness = 5.5 mm (including slice skip = 0.5 mm), and total acquisition time = 20 min. This EPI dataset provided almost complete brain coverage. A whole-brain high-resolution structural scan, (inversion recovery gradient-echo-planar image) on which to superimpose the individual activation maps, was also acquired in the intercommissural plane with TE = 30 ms, TR = 3 s, flip angle = 90°, 43 slices, slice thickness = 3.0 mm, slice skip = 0.3 mm, and in-plane voxel-size = 1.875 mm. The majority of the subjects conducted 3 more fMRI tasks in the same scanning session, which are not analyzed here. Total scanning time was 1 h.
fMRI Image Analysis
The XBAM software package was used (http://www.brainmap.co.uk; Brammer et al. 1997), which makes no normality assumptions (often violated in fMRI data), but instead uses median statistics to control outlier effects and permutation rather than normal theory-based inference. This method of fMRI analysis has been shown to be give excellent Type II error control, and there is evidence that permutation testing results in better sensitivity when compared with the more commonly used theory-based methods of analysis (Thirion et al. 2007).
Individual Analysis
fMRI data were first processed to minimize motion-related artifacts (Bullmore, Brammer, et al. 1999). A 3-dimensional (3D) volume consisting of the average intensity at each voxel over the whole experiment was calculated and used as a template. The 3D image volume at each time point was then realigned to this template by computing the combination of rotations (around the x, y, and z axes) and translations (in x, y, and z) that maximized the correlation between the image intensities of the volume in question and the template (rigid-body registration). Following realignment, data were then smoothed using a Gaussian filter (full-width at half-maximum, 7.2 mm) to improve the signal-to-noise characteristics of the images. After motion correction, global detrending, and spin-excitation history correction, time series analysis for each subject was based on a wavelet-based data resampling method for fMRI data (Bullmore, Suckling, et al. 1999; Bullmore et al. 2001). At the individual-subject level, a standard general linear modeling approach was used to obtain estimates of the response size (beta) to each the reward reversal learning task conditions (final reversal error and probabilistic error) against an implicit baseline (repeat trials) and again for the higher level contrast of final reversal error trials minus PETs. Briefly, we first convolved the main experimental conditions (final reversal and PETs; each separately contrasted with repeat trials) and the higher level contrast (final reversal error trials minus PETs) with 2 Poisson model functions (peaking at 4 and 8 s). We then calculated the weighted sum of these 2 convolutions that gave the best fit (least squares) to the time series at each voxel. A goodness-of-fit statistic (the SSQ ratio) was then computed at each voxel consisting of the ratio of the sum of squares of deviations from the mean intensity value due to the model (fitted time series) divided by the that of squares due to the residuals (original time series minus model time series). The appropriate null distribution for assessing significance of any given SSQ ratio was established using a wavelet-based data re-sampling method (Bullmore et al. 2001) and applying the model-fitting process to the re-sampled data. This process was repeated 20 times at each voxel and the data combined over all voxels, resulting in 20 null parametric maps of SSQ ratio for each subject, which were combined to give the overall null distribution of SSQ ratio. The same permutation strategy was applied at each voxel to preserve spatial correlation structure in the data. Activated voxels, at a <1 level of Type I error, were identified through the appropriate critical value of the SSQ ratio from the null distribution (Brammer et al. 1997; Bullmore, Suckling, et al. 1999). Individual SSQ ratio maps were then transformed into standard space, first by rigid-body transformation of the fMRI data into a high-resolution inversion recovery image of the same subject, and then by affine transformation onto a Talairach template (Talairach and Tournoux 1988).
Group Analysis
A group activation map was produced for the experimental condition (final reversal error—probabilistic error) by calculating the median observed SSQ ratio over all subjects at each voxel in standard space and testing them against the null distribution of median SSQ ratios computed from the identically transformed wavelet re-sampled data (Brammer et al. 1997; Bullmore et al. 2001). The voxel-level threshold was first set to 0.05 to give maximum sensitivity and to avoid Type II errors. Next, a cluster-level threshold was computed for the resulting 3D voxel clusters. The necessary combination of voxel and cluster-level thresholds was not assumed from theory, but rather was determined by direct permutation for each data set, giving excellent Type II error control (Bullmore, Suckling, et al. 1999). Cluster mass rather than a cluster extent threshold was used, to minimize discrimination against possible small, strongly responding foci of activation (Bullmore, Suckling, et al. 1999). In all group activation analyses, less than one false-positive activation locus was expected for P < 0.05 at the voxel level and P < 0.01 at the cluster level.
ANCOVA Between-Group Difference Analyses
For the between-group comparisons between controls and patients under either placebo or Fluoxetine, 1-way ANCOVAs with group as factor and rotational and translation movement in Euclidian 3D space as a covariate were conducted using randomization-based tests for voxel or cluster-wise differences as described in detail elsewhere (Bullmore, Suckling, et al. 1999; Bullmore et al. 2001). For these between-group comparisons, a P-value of P < 0.05 was used for voxel and P < 0.02 for cluster comparisons to achieve an optimal balance between Type II and Type I error. Then, the standardized BOLD response values (SSQ ratios) for each participant were extracted for each of the significant clusters of the 3-group ANCOVAs, and post hoc t-tests (correcting for multiple comparisons using least significant difference, LSD) were conducted to identify the direction of the between-group differences.
ANCOVA Within-Patient Interaction Effects
To investigate the group by drug interaction effects between placebo and Fluoxetine within the patient groups, a 2 × 2 ANCOVA (2 medication conditions and 2 groups) with rotational and translation movement in Euclidian 3D space as a covariate was conducted using randomization-based testing for voxel or cluster-wise differences as described elsewhere (Bullmore et al. 2001). Less than one false-positive 3D cluster was expected at P < 0.05 at the voxel level and P < 0.01 at the cluster level. Statistical measures of BOLD response for each participant were then extracted in each of the significant clusters, and post hoc t-tests (correcting for multiple comparisons with LSD) were conducted to identify the direction of the interaction effects.
Normalization Effects
To test for the statistical significance of any apparent normalization effects of Fluoxetine on case-control activation differences observed under placebo, we used repeated-measures t-tests on the extracted BOLD responses during each medication condition for each of the clusters shown to be significantly different in the comparison between controls and patients during placebo. We conducted this test only within patients, given that controls were only tested once, and hence constant across comparisons.
Results
Participant Characteristics
ANOVAs showed no significant group differences in age [median age: controls: 13.7 (SD = 2.6); ADHD: 15.2 (SD = 1.8); ASD: 15.1 (SD = 1.9)], but did in IQ (Fdf=2,53 = 7, P < 0.002), which was significantly lower in ADHD relative to control and ASD boys (P < 0.005), who did not differ from each other. ADHD children typically have lower IQ than their healthy peers (Bridgett and Walker 2006). Therefore, IQ was not covaried, as when the covariate is intrinsic to the condition, and differs between groups who were not randomly selected, it violates ANCOVA assumptions (Dennis et al. 2009). Nonetheless, to assess the potential impact of IQ on group differences and group by medication interaction effects, the analyses were repeated with IQ as a covariate.
Performance Data
ANOVA between controls and patients under placebo showed no significant group effect (Fdf=2,53 = 2, P = 0.170), although both patient groups made numerically more errors than controls with a relatively large effect size of 0.67 for ADHD and a medium effect size of 0.48 for ASD. When patients were under Fluoxetine, there was a significant group effect for perseverative errors (Fdf=2,53 = 4, P < 0.05) that were significantly higher in ADHD under Fluoxetine relative to controls (P < 0.005), which survived Bonferroni correction for multiple comparisons (P < 0.05) [mean perseverative errors: controls: 1.4 (SD = 0.3); ADHD placebo: 1.7 (SD = 0.5); ADHD Fluoxetine: 1.8 (SD = 0.4); ASD placebo: 1.7 (SD = 0.6); ASD Fluoxetine: 1.6 (0.4)]. However, for the within-patient analyses, no interaction effects were observed between groups (ADHD; ASD) and medication status (placebo; fluoxetine), suggesting that fluoxetine had no differential effect on performance in either group.
fMRI Data
Movement
Repeated-measures ANOVAs using group as an independent factor and maximum x, y, and z rotation or maximum x, y, and z translation as repeated measures showed that there were no significant group by movement interaction effects in rotation (Fdf=4,102 = 2, P = n.s.) or translation (Fdf=4,102 = 2, P = n.s). Nevertheless, to eliminate any potential effects of nonsignificant variance in motion, 3D Euclidean motion parameters were used as covariates in fMRI analysis.
Group Brain Activation Maps
Final Reversal Error—Probabilistic Error
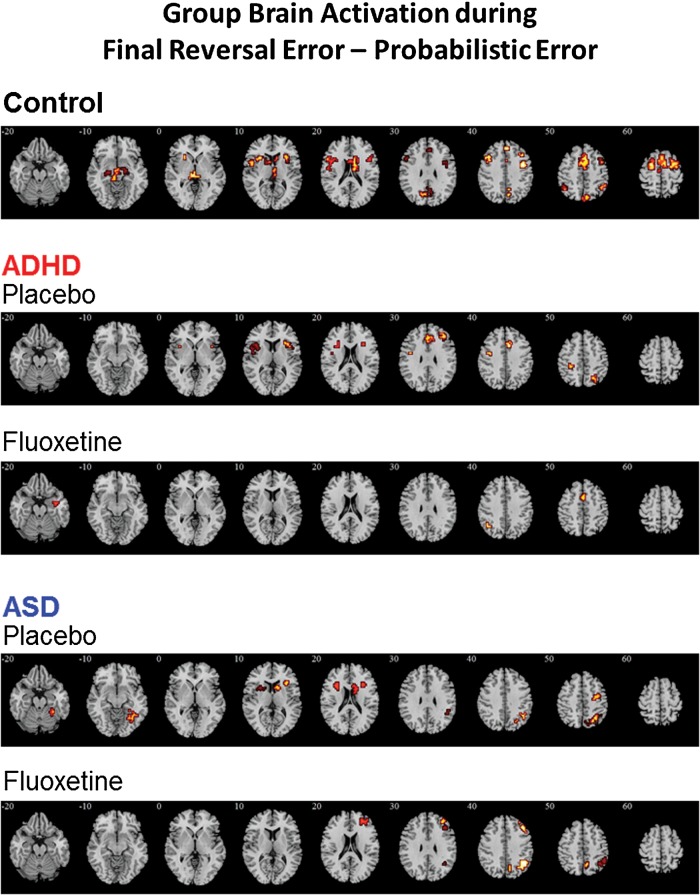
Controls
Controls activated a bilateral network consisting of mPFC, supplementary motor area (SMA), ACC, precentral/postcentral gyri, inferior/middle/superior frontal cortices, basal ganglia, thalamus, midbrain, and posterior cingulate cortex (PCC)/precuneus (Fig. 2A)
Figure 2.
Within-group activation for (A) Healthy controls, (B) adolescents with ADHD under either placebo or Fluoxetine, and (C) adolescents with ASD under either placebo or Fluoxetine for the contrast of final reversal error–probabilistic error. Axial sections showing within-group brain activation for healthy control boys, boys with ADHD under either placebo or Fluoxetine, and boys with ASD under either placebo or Fluoxetine for the contrast of final reversal error – probabilistic error. Talairach z co-ordinates are indicated for slice distance (in mm) from the intercommissural line. The right side corresponds to the right side of the image.
Attention Deficit Hyperactivity Disorder
Under placebo, ADHD subjects activated mPFC/ACC, left precentral/postcentral gyri, right middle frontal cortex, bilateral IFC/insula, putamen, and left inferior- and right superior-parietal lobes. Under Fluoxetine, ADHD subjects activated SMA, left superior parietal lobe, and right hippocampal gyrus (Fig. 2B).
Autism Spectrum Disorder
Under placebo, ASD subjects activated bilateral IFC/caudate/putamen and a right hemispheric network consisting of precentral/postcentral gyrus, inferior/superior parietal lobe, precuneus, and fusiform gyrus/cerebellum. Under Fluoxetine, ASD subjects activated a right hemispheric network consisting of middle/superior frontal cortex, superior parietal lobe, and precuneus (Fig. 2C).
Between-Group Differences Between Controls and Patients Under Placebo
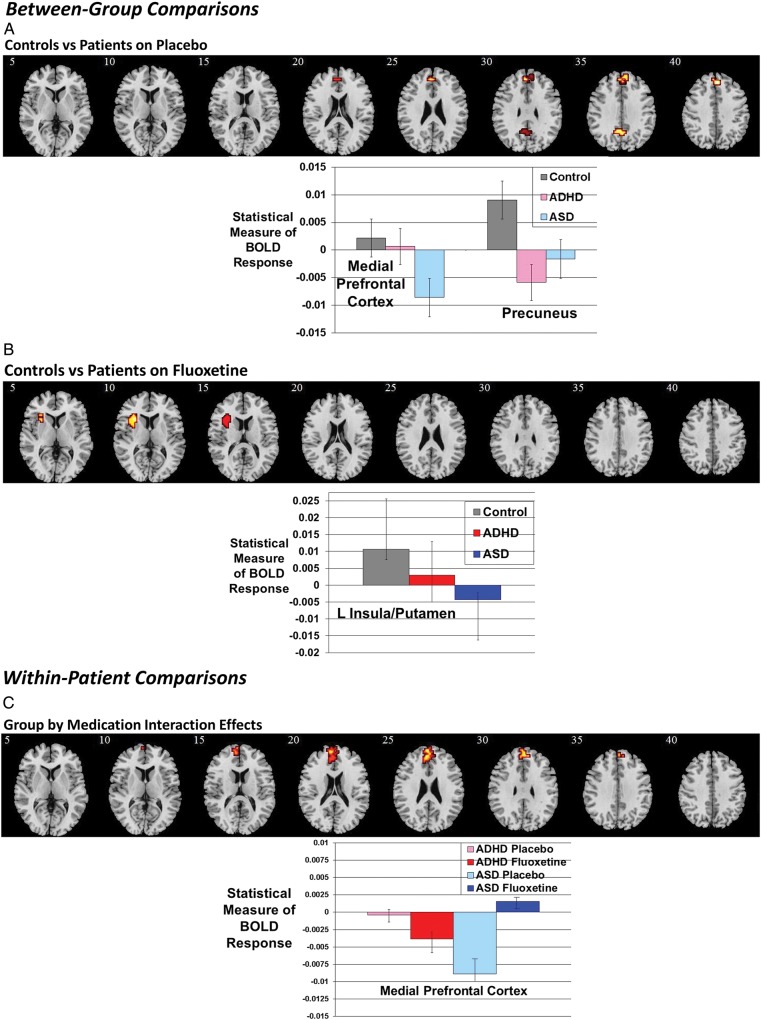
ANCOVA between controls and patients under placebo showed significant group effects in mPFC [23 voxels, peak Talairach co-ordinates (x, y, z): −4, 52, 20; BA 10/9] and precuneus reaching into PCC [11 voxels, peak Talairach co-ordinates (x, y, z): 0, −52, 26; BA 31/7) (Fig. 3A). Post hoc analyses showed that the group effect in mPFC was due to significantly decreased activation in ASD compared with controls (P < 0.0001) and ADHD (P < 0.0001), who did not differ from each other. In precuneus, both the ADHD (P < 0.005) and ASD (P < 0.05) groups, who did not differ from each other, had significantly decreased activation compared with controls.
Figure 3.
(A) Between-group and within-patient comparisons: axial sections showing the between-group ANCOVA findings between controls and patients under placebo. Shown underneath are the statistical measures of BOLD response for each of the 3 groups for each of the brain regions that showed a significant group effect. mPFC, medial prefrontal cortex. Error bars indicate standard error. (B) Axial sections for the between-group ANCOVA comparison between controls and patients under Fluoxetine. (C) Axial sections showing within-patient group by medication interaction effects. Talairach z co-ordinates are indicated for slice distance (in mm) from the intercommissural line. The right side of the image corresponds to the right side of the brain.
To test whether group effects were related to performance or behavior, we correlated the statistical BOLD response in the group difference clusters with perseverative errors and behavioral scores within each group. The activation in precuneus in ASD was positively correlated with perservative errors (r = 0.5, P < 0.05). No other correlations were significant.
Between-Group Differences Between Controls and Patients Under Fluoxetine
ANCOVA between controls and patients under Fluoxetine showed a significant group effect in left insula reaching into putamen [23 voxels, peak Talairach co-ordinates (x, y, z): −33, 19, 4; BA 13) (Fig. 3B). Post hoc analyses showed that this difference was due to significantly reduced activation in the ASD group compared with controls (P < 0.005), who did not differ from ADHD.
Repeated-measures t-tests showed a significant effect of drug condition in mPFC (P = 0.003), which was due to significantly increased activation in mPFC in the ASD group under Fluoxetine relative to placebo. A significant drug effect was also observed in precuneus (P < 0.05), which was due to significantly increased activation in this region in the ADHD group under Fluoxetine relative to placebo. No other significant normalization effects were observed.
Correlation analyses showed that the (reduced) activation in left insula in ASD was negatively correlated with scores on the social domain of the ADI (r = −0.5, P < 0.05). No other correlations were significant.
To assess the potential impact of IQ on case-control group differences, all analyses were repeated with IQ as a covariate. All clusters remained at P < 0.05 for placebo and at P < 0.02 for Fluoxetine.
Within-Patient Group by Medication Interaction Effects
Repeated-measures ANCOVA showed a significant group by medication interaction effect in mPFC [41 voxels, peak Talairach co-ordinates (x, y, z): 0, 63, 15; BA 10/9), due to Fluoxetine increasing activation in this area in the ASD group and decreasing it in the ADHD group (Fig. 3C). This remained significant when IQ was covaried for.
Discussion
This study shows that, during the final stage of reward reversal, contrasted with probabilistic errors, ASD boys have disorder-specific underactivation in mPFC, a key region of reward-related decision-making, relative to ADHD and control boys, and also shared underactivation with ADHD boys, relative to controls, in precuneus, a key region of error processing. Fluoxetine had an inverse, disorder-specific effect in mPFC as it increased activation in ASD boys, leading to normalization of their dysfunction relative to controls, but decreased activation in ADHD boys, concomitant with deteriorated task performance relative to controls. The findings suggest that Fluoxetine has disorder-dissociative, inverse modulation effects on a key region of reward reversal, potentially reflecting differential baseline 5-HT levels in both disorders.
ASD boys compared with the other 2 groups showed disorder-specific underactivation in a key region of reward reversal (Remijnse et al. 2005; Finger et al. 2008; Mitchell et al. 2009) and reward-related decision-making (Euston et al. 2012) that is particularly sensitive to negative feedback, mediating shifting away from disadvantageous responses after negative feedback (Christakou, Brammer, et al. 2009; Ghahremani et al. 2010). Dysfunction in mPFC in ASD may be related to evidence for abnormally increased gray matter in the mPFC in adolescent boys with ASD compared with controls (Bonilha et al. 2008), indicative of poor synaptic pruning and of poor 5-HT binding in mPFC in ASD adolescents (Makkonen et al. 2008). Although ASD patients were not significantly impaired in the task, they had numerically more perseverative errors with a medium effect size (0.48), which may have reached significance in a larger cohort. The disorder specificity of the brain dysfunction to ASD was unexpected. However, the only previous fMRI study that used a similar reward reversal task and contrast found enhanced mPFC activation in ADHD relative to controls (Finger et al. 2008). Taken together, the findings of these 2 studies suggest that medial underactivation may not be a neurofunctional feature of ADHD in the context of reward reversal, while lateral prefrontal underactivation in ADHD patients relative to controls is well documented during other cognitive control tasks that are mediated by lateral prefrontal regions (Rubia et al. 2005, 2009; Smith et al. 2006; Rubia, Cubillo, et al. 2010; Rubia, Halari, et al. 2010; Rubia 2011; Hart et al. 2013).
It is interesting that, during placebo, only individuals with ASD had reduced mPFC activation while ADHD patients did not differ from controls. There is consistent evidence that ADHD patients have abnormal activation in more lateral prefrontal-striato-parietal circuitries, most prominently in inferior prefrontal cortex and caudate during tasks of cognitive control, attention, switching, and timing (Rubia et al. 1999, 2005, 2011; Smith et al. 2006, 2008; Rubia, Cubillo, et al. 2010; Hart et al. 2012, 2013). Therefore, the evidence for functional deficits in ADHD points toward abnormalities in lateral cognitive fronto-striato-parietal networks with relatively little evidence for abnormalities in mPFC-limbic regions during cognitive flexibility or reward-related tasks. In fact, the only prior study of reward reversal learning found increased mPFC activation in ADHD patients during reversal errors (Finger et al. 2008). The deficits in lateral cognitive fronto-striato-parietal circuitries may be related to evidence for delayed maturation of cortical thickness in these fronto-parieto-temporal regions (Shaw et al. 2007, 2011). Adolescents and adults with ASD, on the other hand, have more commonly been shown to have reduced activation in mPFC and their associated limbic-temporal regions during tasks of reward, emotion processing, and cognitive control (Schmitz et al. 2008; Di Martino et al. 2009; Uddin and Menon 2009; Philip et al. 2012), which may be related to abnormal maturation patterns in ASD patients in these regions (Cauda et al. 2011; Radua et al. 2011; Stigler et al. 2011). Children with ASD, unlike ADHD children, who show delayed maturation of fronto-cortical regions (Shaw et al. 2007, 2011), undergo a period of abnormal brain overgrowth in young childhood followed by a period of decreased growth, compared with controls (Courchesne et al. 2001, 2011; Amaral et al. 2008; Stigler et al. 2011). Hence, rather than a delay of brain maturation like in ADHD, there is evidence for a deviance from normal brain maturation in ASD with early overgrowth followed by abnormal growth patterns later on in adolescence and adulthood. Furthermore, in ASD individuals, there is increasing evidence for abnormal white matter integrity between fronto-limbic and fronto-striatal brain regions, compared with controls (Radua et al. 2011; Langen et al. 2012; Pardini et al. 2012; Poustka et al. 2012). This is of particular interest as it has been shown that the ventromedial fronto-basal ganglia-thalamo-cortical loops are involved in habit formation, reward processing, and stimulus-response associations (Packard and Knowlton 2002; Yin and Knowlton 2006; Haber and Calzavara 2009). Although poor fronto-striatal and fronto-parietal white matter tract connectivities have also consistently been reported in children with ADHD, fronto-limbic structural connectivity abnormalities have not been frequently associated with ADHD (Konrad and Eickhoff 2010; van Ewijk et al. 2012). Given that the mPFC is part of a fronto-striato-limbic reward processing network, this may account for the disorder-specific reduced mPFC activation in the ASD group.
The shared dysfunction in precuneus is interesting as this region is closely interconnected with the mPFC (Small et al. 2003) and plays a key role in reversal learning (Dodds et al. 2008; Ghahremani et al. 2010), reward evaluation (McCoy and Platt 2005; Liu et al. 2011), and visual–spatial attention to saliency, in particular error processing (Rubia et al. 2003, 2007; Small et al. 2003; Ridderinkhof et al. 2004; Kravitz et al. 2011). Findings of precuneus dysfunction in ADHD during reward reversal learning extend prior evidence for precuneus dysfunction in response to salient events such as errors and rewarded trials in other tasks (Rubia et al. 2005, 2009; Rubia 2011), presumably reflecting poor saliency and error processing. In ASD, the precuneus has been found to be underactivated during interference (Solomon et al. 2009) and motor inhibition (Kana et al. 2007). The behavioral significance of the abnormal precuneus activation in the ASD group is shown by the positive correlation of this activation with perservative errors. It has been shown that errors elicit the activation of an error detection network that comprises anterior and posterior cingulate as well as precuneus (Small et al. 2003; Ridderinkhof et al. 2004; Rubia et al. 2007; Kravitz et al. 2011). ASD patients committed more peseverative errors with a medium effect size than controls, and the enhanced number of errors may be caused by the diminished precuneus activation, given that higher precuneus activation reflects better error monitoring in ASD patients.
The most intriguing finding is the inverse effect of Fluoxetine on mPFC activation in the 2 disorders, upregulating and normalizing it in ASD, but decreasing it in ADHD. This inverse effect could potentially reflect disorder differences in baseline levels of 5-HT. Approximately 30% of individuals with ASD have enhanced platelet 5-HT levels (i.e., hyperserotonemia) (Piven et al. 1991; Mulder et al. 2004; Hranilovic et al. 2007). There is evidence for reduced binding to 5-HT transporters in the mPFC of individuals with ASD (Makkonen et al. 2008; Nakamura et al. 2010) as well as reduced 5-HT2A receptor binding (Murphy et al. 2006) and altered 5-HT synthesis (Chugani et al. 1997, 1999). This suggests that hyperserotonemia may be an adaptation to counteract poor 5-HT receptor binding and abnormal 5-HT synthesis. An increase in 5-HT with Fluoxetine may have increased ligand-receptor binding sufficiently to enhance activation in areas, where 5-HT receptor density is typically low. In addition, Fluoxetine may have amended an abnormal “balance” of 5-HT, therefore improving the homeostatic role of this key neurotransmitter and potentially leading to an increase in mPFC activation in ASD (Di Pietro and Seamans 2011; Murano et al. 2011). Furthermore, each brain region has a distinct serotonergic profile, with limbic and more medial structures receiving dense serotonergic innervation (Jacobs and Azmitia 1992; Varnäs et al. 2004). This therefore makes regions such as the mPFC highly susceptible to serotonergic manipulation, particularly in a patient group which have shown structural (Bonilha et al. 2008) and biochemical (Murphy et al. 2006; Makkonen et al. 2008; Nakamura et al. 2010) abnormalities in this region. It is also plausible that an increase in 5-HT may be modulating primary 5-HT abnormalities in transporter function (Makkonen et al. 2008; Nakamura et al. 2010) or 5-HT2A receptor binding (Murphy et al. 2006), which have been reported to be impaired in the mPFC of ASD individuals (Murphy et al. 2006; Makkonen et al. 2008; Nakamura et al. 2010), and may have led to the increased activation in mPFC observed in the ASD group. Our finding of an upregulation and normalization of Fluoxetine in the mPFC of adolescents with ASD extends prior evidence that SSRIs increase metabolic and neurofunctional activities in prefrontal areas in adults with ASD (Buchsbaum et al. 2001; Dichter et al. 2010).
Fluoxetine also decreased insula activation in ASD relative to controls. There is consistent evidence for underactivation and underconnectivity of the insula in individuals with ASD (Uddin and Menon 2009; Ebisch et al. 2011). However, this was mainly observed during tasks of emotion processing and there is evidence that this underactivation is associated with alexithymia in ASD, as opposed to social interaction deficits (Bird et al. 2010). The insula forms part of a mPFC-striato-limbic network for reward-related decision-making and, like mPFC, is particularly sensitive to negative feedback and mediates shifting away from disadvantageous choices in both gambling (Christakou, Brammer, et al. 2009; Christakou et al. 2013) and reward reversal learning tasks (O'Doherty et al. 2003; Remijnse et al. 2005; Cohen et al. 2008). The normalization of mPFC activation with Fluoxetine may have resulted in the impairment of a limbic part of the reversal network, suggesting that brain function was not entirely normalized. Alternatively, insula activation has been observed in uncertain conditions during probabilistic tasks (Huettel et al. 2005) and is associated with anxiety to the anticipation of aversive stimuli (Paulus and Stein 2006; Simmons et al. 2006), both of which are key aspects of reward reversal learning. Therefore, the decreased activation in this area in ASD boys may have been a reflection of a reduction in their anxiety to the negative feedback they received when they reversed their response. This is in line with the behavioral correlations which found that the higher the social impairment on the ADI subscale, and therefore the more anxious the individual is likely to be, the more Fluoxetine decreased insula activation.
The reduction of activation in mPFC in ADHD with Fluoxetine was unexpected. However, ADHD boys, unlike ASD boys, showed no underactivation in this region, and hence, the 5-HT modulation may have interfered with normal prefrontal activation. This is further supported by the finding of performance impairment with 5-HT in ADHD. While ADHD patients have shown lateral prefronto-striatal underactivation during switching tasks (Smith et al. 2006; Rubia, Cubillo, et al. 2010; Rubia, Halari, et al. 2010), the only previous fMRI study on a similar contrast in a reward reversal task found increased mPFC activation in ADHD relative to controls (Finger et al. 2008). Hence, reward reversal tasks may not elicit underactivation in key areas of reversal processes and therefore not tap into the dysfunctional brain mechanisms of ADHD. Furthermore, although there is evidence of serotonergic dysfunction in ADHD at both a genetic (Gizer et al. 2009) and biochemical (Spivak et al. 1999) level, potentially leading to low 5-HT, the positive clinical effect of Fluoxetine monotherapy (Barrickman et al. 1991; Quintana et al. 2007) and the modulating effect of Fluoxetine on Methylphenidate therapy (Gammon and Brown 1993; Findling 1996) may be due to the interaction between the increase in 5-HT, a key regulatory neurotransmitter, and dopamine, a neurotransmitter which is known to be low in individuals with ADHD (Volkow et al. 1998; del Campo et al. 2011). Studies in rats have found that increased impulsivity is associated with 5-HT levels in the mPFC, highlighting the importance of 5-HT in this area in disorders of inhibition and impulsivity, and it is known that both 5-HT and dopamine are involved in inhibition (Dalley et al. 2002, 2008). Antagonism of the 5-HT2C receptor, leading to less 5-HT-mediated inhibition of dopamine release, leads to better reversal learning in rats and shows that the interplay between 5-HT and dopamine, as well as absolute 5-HT levels, is involved in reversal learning (Boulougouris et al. 2007). Interestingly, a decrease in 5-HT with acute tryptophan depletion has been shown to lead to increased activation in the mPFC of healthy individuals during a task of reward reversal learning (Evers et al. 2005). Therefore, it appears as if 5-HT agonists may perturb the delicate serotonin-dopamine balance in mPFC, an area which is normal in ADHD in this task context, leading to decreased activation. Given that 11 ADHD patients were withdrawn from medication for 48 h, it cannot be excluded that the withdrawal effect or the interaction of the dopamine withdrawal effect combined with the acute SSRI effect may have affected brain activation or performance (Schweren et al. 2012).It is also possible that the long-term effect of stimulant medication on brain structure and function (Nakao et al. 2011; Hart et al. 2012, 2013; Rubia et al. 2013) in the 11 medicated ADHD participants may have influenced the findings. However, both withdrawal and long-term stimulant effects would have been contrasted out by the placebo control condition.
In ASD, however, 5-HT is thought to be the main abnormal neurotransmitter in the disorder, and there is a wealth of research supporting the presence of genetic and biochemical serotonergic abnormalities, leading to high levels of 5-HT (Piven et al. 1991; Chugani et al. 1997, 1999; Mulder et al. 2004; Murphy et al. 2006; Hranilovic et al. 2007; Makkonen et al. 2008; Zafeiriou et al. 2009; Nakamura et al. 2010), in addition to the positive effect of Fluoxetine on stereotyped behaviors (Fatemi et al. 1998; Hollander et al. 2005, 2012; Carrasco et al. 2012) and brain activation in areas related to reward reversal (Buchsbaum et al. 2001; Dichter et al. 2010) in children and adults with ASD. These differing biochemical abnormalities may have accounted for the positive upregulation effect of Fluoxetine on ASD mPFC activation and its negative downregulating effect on mPFC activation in ADHD.
Despite significant effects on brain activation in both ADHD and ASD boys, Fluoxetine only had a behavioral effect in the ADHD group when compared with controls, leading patients to perform worse than controls under Fluoxetine, but not placebo. It has previously been shown in similar reward reversal learning tasks that brain function is more sensitive to pharmacological manipulations than performance (Evers et al. 2005). In addition, although a sample size of 15–18 is adequate for fMRI analysis (Thirion et al. 2007), it is likely to be insufficient to detect more subtle neuropsychological differences, such as those that may be present between the ASD and control group.
The strengths of this study are the carefully selected, noncomorbid patient groups and the medication-naivety of the ASD group. Although there is a significant clinical overlap between ADHD and ASD (van der Meer et al. 2012; Rao and Landa 2013), rigorous screening ensured that no overlap was present between the 2 patient groups in this study. Although at the time of study DSM-V was not available, and might have enabled easier identification and exclusion of comorbid cases, we are confident that our rigorous screening ensured that none of the patients had comorbidity with the other disorder. This is of great importance as there is an increasing need to find objective, biological biomarkers with the potential to aid in the differential diagnosis of these 2 neurodevelopmental disorders. While testing clearly noncomorbid patient groups is an advantage for studies such as our own which aim to elucidate the differences and commonalities in neural substrates and drug manipulations between the 2 disorders, a limitation is that the data cannot be generalized to the commonly occurring overlapping disorder type. Future studies should compare pure disorder groups as well as comorbid conditions in order to disentangle the underlying neural substrates of these and the effect of serotonin manipulations.
A limitation is the lower IQ in the ADHD group. However, covariance analysis showed that this did not affect the main findings. The use of an acute dose of Fluoxetine may be considered another limitation. However, studies on acute dose effects have the advantage to allow to investigate brain activation effects of medication without the confound of side effects and long-term chronic effects on behavior and cognition, and are often a necessary first step for proof of concept. However, they are limited in that they cannot investigate the association between brain activation effects and clinical improvement over a longer period of time such as several weeks, which is when Fluoxetine typically starts to show clinical efficacy. This is an area which should be focused on in future research, particularly as protracted courses of Fluoxetine are used in the clinical trials that report an improvement in behavior in ADHD (Barrickman et al. 1991; Gammon and Brown 1993; Quintana et al. 2007) and ASD children (DeLong et al. 1998, 2002; Hollander et al. 2005). In addition, the mechanisms of action of these long-term effects need also be understood. Another limitation is that both patient groups were scanned twice, while controls were only scanned once. While training effects are counter-balanced between medication conditions, it cannot be ruled out that the fact that patients conducted the task twice might have affected the performance or brain activation findings.
To summarize, we found disorder-specific underactivation in ASD boys in mPFC, a key region of reversal learning, as well as disorder-dissociated inverse effects of Fluoxetine on this region, which upregulated and normalized dysfunction in ASD but down-regulated activation in ADHD, concomitant with worsening their task performance. The findings may indicate dissociated underlying 5-HT abnormalities in the 2 disorders.
Funding
This work was supported by the UK Department of Health via the National Institute for Health Research (NIHR) Biomedical Research Centre (BRC) for Mental Health at South London and Maudsley NHS Foundation Trust and Institute of Psychiatry, King's College London. Funding to pay the Open Access publication charges for this article was provided by the Biomedical Research Centre.
Notes
Conflict of Interest: K.R. has received funding from Lilly for another project and speaker's honoraria from Lilly, Shire, Novartis, and Medice. M.B. is consultant for P1 Vital, Oxford, UK. The other authors have no conflict of interests to declare.
References
- Amaral DG, Schumann CM, Nordahl CW. 2008. Neuroanatomy of autism. Trends Neurosci. 31:137–145. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. 1994. Diagnostic and Statistical Manual of Mental Disorders. Washington: (DC: ): American Psychiatric Press. [Google Scholar]
- Ashby FG, Isen AM. 1999. A neuropsychological theory of positive affect and its influence on cognition. Psychol Rev. 106:529. [DOI] [PubMed] [Google Scholar]
- Banerjee E, Banerjee D, Chatterjee A, Sinha S, Nandagopal K. 2012. Selective maternal inheritance of risk alleles and genetic interaction between serotonin receptor-1B (5-HTR1B) and serotonin transporter (SLC6A4) in ADHD. Psychiatry Res. 200:1083–1085. [DOI] [PubMed] [Google Scholar]
- Barrickman L, Noyes R, Kuperman S, Schumacher E, Verda M. 1991. Treatment of ADHD with Fluoxetine—a preliminary trial. J Am Acad Child Adolesc Psychiatry. 30:762–767. [PubMed] [Google Scholar]
- Bird G, Silani G, Brindley R, White S, Frith U, Singer T. 2010. Empathic brain responses in insula are modulated by levels of alexithymia but not autism. Brain. 133:1515–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha L, Cendes F, Rorden C, Eckert M, Dalgalarrondo P, Li LM, Steiner CE. 2008. Gray and white matter imbalance—typical structural abnormality underlying classic autism? Brain Dev. 30:396–401. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Glennon JC, Robbins TW. 2007. Dissociable effects of selective 5-HT2A and 5-HT2C receptor antagonists on serial spatial reversal learning in rats. Neuropsychopharmacology. 33:2007–2019. [DOI] [PubMed] [Google Scholar]
- Brammer MJ, Bullmore ET, Simmons A, Williams SCR, Grasby PM, Howard RJ, Woodruff PWR, RabeHesketh S. 1997. Generic brain activation mapping in functional magnetic resonance imaging: a nonparametric approach. Magn Reson Imaging. 15:763–770. [DOI] [PubMed] [Google Scholar]
- Bridgett DJ, Walker ME. 2006. Intellectual functioning in adults with ADHD: a meta-analytic examination of full scale IQ differences between adults with and without ADHD. Psychol Assess. 18:1. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Hollander E, Haznedar MM, Tang C, Spiegel-Cohen J, Wei TC, Solimando A, Buchsbaum BR, Robins D, Bienstock C, et al. 2001. Effect of fluoxetine on regional cerebral metabolism in autistic spectrum disorders: a pilot study. Int J Neuropsychopharmacol. 4:119–125. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Brammer M, Rabe-Hesketh S, Curtis V, Morris R, Williams S, Sharma T, McGuire P. 1999. Methods for diagnosis and treatment of stimulus-correlated motion in generic brain activation studies using fMRI. Hum Brain Mapp. 7:38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E, Long C, Suckling J, Fadili J, Calvert G, Zelaya F, Carpenter TA, Brammer M. 2001. Colored noise and computational inference in neurophysiological (fMRI) time series analysis: resampling methods in time and wavelet domains. Hum Brain Mapp. 12:61–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore ET, Suckling J, Overmeyer S, Rabe-Hesketh S, Taylor E, Brammer MJ. 1999. Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Trans Med Imaging. 18:32–42. [DOI] [PubMed] [Google Scholar]
- Carrasco M, Volkmar FR, Bloch MH. 2012. Pharmacologic treatment of repetitive behaviors in autism spectrum disorders: evidence of publication bias. Pediatrics. 129:1301–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Elia J, Kruesi MJP, Gulotta CS, Mefford IN, Potte WZ, Ritchie GF, Rapoport JL. 1994. Cerebrospinal fluid monoamine metabolites in boys with attention-deficit hyperactivity disorder. Psychiatry Res. 52:305–316. [DOI] [PubMed] [Google Scholar]
- Cauda F, Geda E, Sacco K, D'Agata F, Duca S, Geminiani G, Keller R. 2011. Grey matter abnormality in autism spectrum disorder: an activation likelihood estimation meta-analysis study. J Neurol Neurosurg Psychiatry. 82:1304–1313. [DOI] [PubMed] [Google Scholar]
- Christakou A, Brammer M, Giampietro V, Rubia K. 2009. Right ventromedial and dorsolateral prefrontal cortices mediate adaptive decisions under ambiguity by integrating choice utility and outcome evaluation. J Neurosci. 29:11020–11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakou A, Gershman A, Brammer M, Rubia K. 2013. Neural and psychological maturation of decision-making in adolescence and young adulthood. J Cogn Neurosci. 25(11):807–1823. [DOI] [PubMed] [Google Scholar]
- Christakou A, Halari R, Smith AB, Ifkovits E, Brammer M, Rubia K. 2009. Sex-dependent age modulation of frontostriatal and temporo-parietal activation during cognitive control. Neuroimage. 48:223–236. [DOI] [PubMed] [Google Scholar]
- Chugani DC, Muzik O, Behen M, Rothermel R, Janisse JJ, Lee J, Chugani HT. 1999. Developmental changes in brain serotonin synthesis capacity in autistic and nonautistic children. Ann Neurol. 45:287–295. [DOI] [PubMed] [Google Scholar]
- Chugani DC, Muzik O, Rothermel R, Behen M, Chakraborty P, Mangner T, daSilva EA, Chugani HT. 1997. Altered serotonin synthesis in the dentatothalamocortical pathway in autistic boys. Ann Neurol. 42:666–669. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Elger CE, Weber B. 2008. Amygdala tractography predicts functional connectivity and learning during feedback-guided decision-making. Neuroimage. 39:1396–1407. [DOI] [PubMed] [Google Scholar]
- Conners CK, Sitarenios G, Parker JDA, Epstein JN. 1998. The revised Conners' Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol. 26:257–268. [DOI] [PubMed] [Google Scholar]
- Cools R, Clark L, Owen AM, Robbins TW. 2002. Defining the neural mechanisms of probabilistic reversal learning using event-related functional magnetic resonance imaging. J Neurosci. 22:4563–4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, Constantine LJ, Hendren R, Rocke D, Ozonoff S. 2009. Examining executive functioning in children with autism spectrum disorder, attention deficit hyperactivity disorder and typical development. Psychiatry Res. 166:210–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Campbell K, Solso S. 2011. Brain growth across the life span in autism: age-specific changes in anatomical pathology. Brain Res. 1380:138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, Chisum HJ, Moses P, Pierce K, Lord C, et al. 2001. Unusual brain growth patterns in early life in patients with autistic disorder—an MRI study. Neurology. 57:245–254. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Mar AC, Economidou D, Robbins TW. 2008. Neurobehavioral mechanisms of impulsivity: fronto-striatal systems and functional neurochemistry. Pharmacol Biochem Behav. 90:250–260. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Roiser JP. 2012. Dopamine, serotonin and impulsivity. Neuroscience. 215:42–58. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Theobald DE, Eagle DM, Passetti F, Robbins TW. 2002. Deficits in impulse control associated with tonically-elevated function in rat serotonergic prefrontal cortex. Neuropsychopharmacology. 26:716–728. [DOI] [PubMed] [Google Scholar]
- del Campo N, Chamberlain SR, Sahakian BJ, Robbins TW. 2011. The roles of dopamine and noradrenaline in the pathophysiology and treatment of attention-deficit/hyperactivity disorder. Biol Psychiatry. 69:145–157. [DOI] [PubMed] [Google Scholar]
- DeLong GR, Ritch CR, Burch S. 2002. Fluoxetine response in children with autistic spectrum disorders: correlation with familial major affective disorder and intellectual achievement. Dev Med Child Neurol. 44:652–659. [DOI] [PubMed] [Google Scholar]
- DeLong GR, Teague LA, Kamran MM. 1998. Effects of fluoxetine treatment in young children with idiopathic autism. Dev Med Child Neurol. 40:551–562. [DOI] [PubMed] [Google Scholar]
- Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, Fletcher JM. 2009. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. J Int Neuropsychol Soc. 15:331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrfuss J, Brass M, Neumann J, von Cramon DY. 2005. Involvement of the inferior frontal junction in cognitive control: meta-analyses of switching and Stroop studies. Hum Brain Mapp. 25:22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Sikich L, Mahorney S, Felder JN, Lam KS, Turner-Brown L, Bodfish J. 2010. fMRI tracks reductions in repetitive behaviors in autism: two case studies. Neurocase. 16:307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Ross K, Uddin LQ, Sklar AB, Castellanos FX, Milham MP. 2009. Functional brain correlates of social and nonsocial processes in autism spectrum disorders: an activation likelihood estimation meta-analysis. Biol Psychiatry. 65:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Matteo V, Di Giovanni G, Pierucci M, Esposito E. 2008. Serotonin control of central dopaminergic function: focus on in vivo microdialysis studies. Prog Brain Res. 172:7–44. [DOI] [PubMed] [Google Scholar]
- Di Pietro NC, Seamans JK. 2011. Dopamine and serotonin interactively modulate prefrontal cortex neurons in vitro. Biol Psychiatry. 69:1204–1211. [DOI] [PubMed] [Google Scholar]
- Dodds CM, Mueller U, Clark L, van Loon A, Cools R, Robbins TW. 2008. Methylphenidate has differential effects on blood oxygenation level-dependent signal related to cognitive subprocesses of reversal learning. J Neurosci. 28:5976–5982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisch SJH, Gallese V, Willems RM, Mantini D, Groen WB, Romani GL, Buitelaar JK, Bekkering H. 2011. Altered intrinsic functional connectivity of anterior and posterior insula regions in high-functioning participants with autism spectrum disorder. Hum Brain Mapp. 32:1013–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euston David R, Gruber Aaron J, McNaughton Bruce L. 2012. The role of medial prefrontal cortex in memory and decision making. Neuron. 76:1057–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers EAT, Cools R, Clark L, van der Veen FM, Jolles J, Sahakian BJ, Robbins TW. 2005. Serotonergic modulation of prefrontal cortex during negative feedback in probabilistic reversal learning. Neuropsychopharmacology. 30:1138–1147. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Realmuto GM, Khan L, Thuras P. 1998. Fluoxetine in treatment of adolescent patients with autism: a longitudinal open trial. J Autism Dev Disord. 28:303–307. [DOI] [PubMed] [Google Scholar]
- Findling RL. 1996. Open-label treatment of comorbid depression and attentional disorders with co-administration of serotonin reuptake inhibitors and psychostimulants in children, adolescents, and adults: a case series. J Child Adolesc Psychopharmacol. 6:165–175. [DOI] [PubMed] [Google Scholar]
- Finger EC, Marsh AA, Mitchell DG, Reid ME, Sims C, Budhani S, Kosson DS, Chen G, Towbin KE, Leibenluft E, et al. 2008. Abnormal ventromedial prefrontal cortex function in children with psychopathic traits during reversal learning. Arch Gen Psychiatry. 65:586–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyer T, Valerius G, Kuelz AK, Speck O, Glauche V, Hull M, Voderholzer U. 2009. Test-retest reliability of event-related functional MRI in a probabilistic reversal learning task. Psychiatry Res. 174:40–46. [DOI] [PubMed] [Google Scholar]
- Gadow KD, DeVincent CJ, Siegal VI, Olvet DM, Kibria S, Kirsch SF, Hatchwell E. 2013. Allele-specific associations of 5-HTTLPR/rs25531 with ADHD and autism spectrum disorder. Prog Neuropsychopharmacol Biol Psychiatry. 40:292–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainetdinov RR, Wetsel WC, Jones SR, Levin ED, Jaber M, Caron MG. 1999. Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science. 283:397–401. [DOI] [PubMed] [Google Scholar]
- Gammon GD, Brown TE. 1993. Fluoxetine and methylphenidate in combination for treatment of attention deficit disorder and comorbid depressive disorder. J Child Adolesc Psychopharmacol. 3:1–10. [DOI] [PubMed] [Google Scholar]
- Ghahremani DG, Monterosso J, Jentsch JD, Bilder RM, Poldrack RA. 2010. Neural components underlying behavioral flexibility in human reversal learning. Cereb Cortex. 20:1843–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gizer IR, Ficks C, Waldman ID. 2009. Candidate gene studies of ADHD: a meta-analytic review. Hum Genet. 126:51–90. [DOI] [PubMed] [Google Scholar]
- Goldberg D, Murray R. 2002. Maudsley Handbook of Practical Psychiatry. New York: Oxford. [Google Scholar]
- Goldman-Rakic PS. 1999. The “Psychic” neuron of the cerebral cortex. Ann N Y Acad Sci. 868:13–26. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Cools AR, Srivastava K. 1996. The prefrontal landscape: implications of functional architecture for understanding human mentation and the central executive. Philos Trans R Soc Lond Ser B Biol Sci. 351:1445–1453. [DOI] [PubMed] [Google Scholar]
- Goodman R, Scott S. 1999. Comparing the strengths and difficulties questionnaire and the child behavior checklist: is small beautiful? J Abnorm Child Psychol. 27:17–24. [DOI] [PubMed] [Google Scholar]
- Haber SN, Calzavara R. 2009. The cortico-basal ganglia integrative network: the role of the thalamus. Brain Res Bull. 78:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart H, Radua J, Mataix-Cols D, Rubia K. 2012. Meta-analysis of fMRI studies of timing in attention-deficit hyperactivity disorder (ADHD). Neurosci Biobehav Rev. 36:2248–2256. [DOI] [PubMed] [Google Scholar]
- Hart H, Radua J, Nakao T, Mataix-Cols D, Rubia K. 2013. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatr. 70:185–198. [DOI] [PubMed] [Google Scholar]
- Hill EL. 2004. Executive dysfunction in autism. Trends Cogn Sci. 8:26–32. [DOI] [PubMed] [Google Scholar]
- Hinz M, Stein A, Neff R, Weinberg R, Uncini T. 2011. Treatment of attention deficit hyperactivity disorder with monoamine amino acid precursors and organic cation transporter assay interpretation. Neuropsychiatr Dis Treat. 7:31–38. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hollander E, Phillips A, Chaplin W, Zagursky K, Novotny S, Wasserman S, Iyengar R. 2005. A placebo controlled crossover trial of liquid fluoxetine on repetitive behaviors in childhood and adolescent autism. Neuropsychopharmacology. 30:582–589. [DOI] [PubMed] [Google Scholar]
- Hollander E, Soorya L, Chaplin W, Anagnostou E, Taylor BP, Ferretti CJ, Wasserman S, Swanson E, Settipani C. 2012. A double-blind placebo-controlled trial of fluoxetine for repetitive behaviors and global severity in adult autism spectrum disorders. Am J Psychiatry. 169:292–299. [DOI] [PubMed] [Google Scholar]
- Hranilovic D, Bujas-Petkovic Z, Vragovic R, Vuk T, Hock K, Jernej B. 2007. Hyperserotonemia in adults with autistic disorder. J Autism Dev Disord. 37:1934–1940. [DOI] [PubMed] [Google Scholar]
- Huettel SA, Song AW, McCarthy G. 2005. Decisions under uncertainty: probabilistic context influences activation of prefrontal and parietal cortices. J Neurosci. 25:3304–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. 1992. Structure and function of the brain serotonergic system. Physiol Rev. 72:165–229. [DOI] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Minshew NJ, Just MA. 2007. Inhibitory control in high-functioning autism: decreased activation and underconnectivity in inhibition networks. Biol Psychiatry. 62:198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehagia AA, Murray GK, Robbins TW. 2010. Learning and cognitive flexibility: frontostriatal function and monoaminergic modulation. Curr Opin Neurobiol. 20:199–204. [DOI] [PubMed] [Google Scholar]
- Konrad K, Eickhoff SB. 2010. Is the ADHD brain wired differently? A review on structural and functional connectivity in attention deficit hyperactivity disorder. Hum Brain Mapp. 31:904–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz DJ, Saleem KS, Baker CI, Mishkin M. 2011. A new neural framework for visuospatial processing. Nat Rev Neurosci. 12:217–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langen M, Leemans A, Johnston P, Ecker C, Daly E, Murphy CM, dell'Acqua F, Durston S, Murphy DGM. 2012. Fronto-striatal circuitry and inhibitory control in autism: findings from diffusion tensor imaging tractography. Cortex. 48:183–193. [DOI] [PubMed] [Google Scholar]
- Liu X, Hairston J, Schrier M, Fan J. 2011. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 35:1219–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loose R, Kaufmann C, Tucha O, Auer DP, Lange KW. 2006. Neural networks of response shifting: influence of task speed and stimulus material. Brain Res. 1090:146–155. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, Pickles A, Rutter M. 2000. The autism diagnostic observation schedule generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 30:205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Couteur A. 1994. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 24:659–685. [DOI] [PubMed] [Google Scholar]
- Makkonen I, Riikonen R, Kokki H, Airaksinen MM, Kuikka JT. 2008. Serotonin and dopamine transporter binding in children with autism determined by SPECT. Dev Med Child Neurol. 50:593–597. [DOI] [PubMed] [Google Scholar]
- McCoy A, Platt M. 2005. Expectations and outcomes: decision-making in the primate brain. J Compar Physiol A. 191:201–211. [DOI] [PubMed] [Google Scholar]
- McGough JJ, McCracken JT, Loo SK, Manganiello M, Leung MC, Tietjens JR, Trinh T, Baweja S, Suddath R, Smalley SL, et al. 2009. A candidate gene analysis of methylphenidate response in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 48:1155–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DGV, Luo Q, Avny SB, Kasprzycki T, Gupta K, Chen G, Finger EC, Blair RJR. 2009. Adapting to dynamic stimulus-response values: differential contributions of inferior frontal, dorsomedial, and dorsolateral regions of prefrontal cortex to decision making. J Neurosci. 29:10827–10834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DGV, Rhodes RA, Pine DS, Blair RJR. 2008. The contribution of ventrolateral and dorsolateral prefrontal cortex to response reversal. Behav Brain Res. 187:80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder EJ, Anderson GM, Kema IP, de Bildt A, van Lang NDJ, den Boer JA, Minderaa RB. 2004. Platelet serotonin levels in pervasive developmental disorders and mental retardation: diagnostic group differences, within-group distribution, and behavioral correlates. J Am Acad Child Adolesc Psychiatry. 43:491–499. [DOI] [PubMed] [Google Scholar]
- Murano M, Saitow F, Suzuki H. 2011. Modulatory effects of serotonin on glutamatergic synaptic transmission and long-term depression in the deep cerebellar nuclei. Neuroscience. 172:118–128. [DOI] [PubMed] [Google Scholar]
- Murphy DGM, Daly E, Schmitz N, Toal F, Murphy K, Curran S, Erlandsson K, Eersels J, Kerwin R, Ell P, et al. 2006. Cortical serotonin 5-HT2A receptor binding and social communication in adults with Asperger's syndrome: an in vivo SPECT study. Am J Psychiatry. 163:934–936. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Smith KA, Cowen PJ, Robbins TW, Sahakian BJ. 2002. The effects of tryptophan depletion on cognitive and affective processing in healthy volunteers. Psychopharmacology (Berl). 163:42–53. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Sekine Y, Ouchi Y, Tsujii M, Yoshikawa E, Futatsubashi M, Tsuchiya KJ, Sugihara G, Iwata Y, Suzuki K, et al. 2010. Brain serotonin and dopamine transporter bindings in adults with high-functioning autism. Arch Gen Psychiatry. 67:59–68. [DOI] [PubMed] [Google Scholar]
- Nakao T, Radua Q, Rubia K, Mataix D. 2011. Gray matter volume abnormalities in ADHD and the effects of stimulant medication: voxel-based meta-analysis. Am J Psychiatry. 168(11):1154–1163. [DOI] [PubMed] [Google Scholar]
- Oades RD. 2007. Role of the serotonin system in ADHD: treatment implications. Expert Rev Neurother. 7:1357–1374. [DOI] [PubMed] [Google Scholar]
- Oades RD, Daniels R, Rascher W. 1998. Plasma neuropeptide-Y levels, monoamine metabolism, electrolyte excretion and drinking behavior in children with attention-deficit hyperactivity disorder. Psychiatry Res. 80:177–186. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Critchley H, Deichmann R, Dolan RJ. 2003. Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. J Neurosci. 23:7931–7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. 2001. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 4:95–102. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. 1971. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 9:97–113. [DOI] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ. 2002. Learning and memory functions of the basal ganglia. Annu Rev Neurosci. 25:563–593. [DOI] [PubMed] [Google Scholar]
- Pardini M, Elia M, Garaci F, Guida S, Coniglione F, Krueger F, Benassi F, Emberti Gialloreti L. 2012. Long-term cognitive and behavioral therapies, combined with augmentative communication, are related to uncinate fasciculus integrity in autism. J Autism Dev Disord. 42:585–592. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. 2006. An insular view of anxiety. Biol Psychiatry. 60:383–387. [DOI] [PubMed] [Google Scholar]
- Philip RCM, Dauvermann MR, Whalley HC, Baynham K, Lawrie SM, Stanfield AC. 2012. A systematic review and meta-analysis of the fMRI investigation of autism spectrum disorders. Neurosci Biobehav Rev. 36:901–942. [DOI] [PubMed] [Google Scholar]
- Piven J, Tsai G, Nehme E, Coyle JT, Chase GA, Folstein SE. 1991. Platelet serotonin: a possible marker for familial autism. J Autism Dev Disord. 21:51–59. [DOI] [PubMed] [Google Scholar]
- Poustka L, Jennen-Steinmetz C, Henze R, Vomstein K, Haffner J, Sieltjes B. 2012. Fronto-temporal disconnectivity and symptom severity in children with autism spectrum disorder. World J Biol Psychiatry. 13:269–280. [DOI] [PubMed] [Google Scholar]
- Quintana H, Butterbaugh GJ, Purnell W, Layman AK. 2007. Fluoxetine monotherapy in attention-deficit/hyperactivity disorder and comorbid non-bipolar mood disorders in children and adolescents. Child Psychiatry Hum Dev. 37:241–253. [DOI] [PubMed] [Google Scholar]
- Radua J, Via E, Catani M, Mataix-Cols D. 2011. Voxel-based meta-analysis of regional white-matter volume differences in autism spectrum disorder versus healthy controls. Psychol Med. 41:1539–1550. [DOI] [PubMed] [Google Scholar]
- Rao PA, Landa RJ. 2013. Association between severity of behavioral phenotype and comorbid attention deficit hyperactivity disorder symptoms in children with autism spectrum disorders. Autism. 0:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravizza SM, Carter CS. 2008. Shifting set about task switching: behavioral and neural evidence for distinct forms of cognitive flexibility. Neuropsychologia. 46:2924–2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remijnse PL, Nielen MMA, Uylings HBM, Veltman DJ. 2005. Neural correlates of a reversal learning task with an affectively neutral baseline: an event-related fMRI study. Neuroimage. 26:609–618. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuiss S. 2004. The role of the medial frontal cortex in cognitive control. Science. 306:443–447. [DOI] [PubMed] [Google Scholar]
- Roberts AC. 2011. The importance of serotonin for orbitofrontal function. Biol Psychiatry. 69:1185–1191. [DOI] [PubMed] [Google Scholar]
- Rommelse NNJ, Franke B, Geurts HM, Hartman CA, Buitelaar JK. 2010. Shared heritability of attention-deficit/hyperactivity disorder and autism spectrum disorder. Eur Child Adolesc Psychiatry. 19:281–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelse NNJ, Geurts HM, Franke B, Buitelaar JK, Hartman CA. 2011. A review on cognitive and brain endophenotypes that may be common in autism spectrum disorder and attention-deficit/hyperactivity disorder and facilitate the search for pleiotropic genes. Neurosci Biobehav Rev. 35:1363–1396. [DOI] [PubMed] [Google Scholar]
- Rubia K. 2011. “Cool” inferior frontostriatal dysfunction in attention-deficit/hyperactivity disorder versus “hot” ventromedial orbitofrontal-limbic dysfunction in conduct disorder: a review. Biol Psychiatry. 69:69–87. [DOI] [PubMed] [Google Scholar]
- Rubia K, Alzamora A, Smith ABS, Cubillo A, Brammer M, Radua Q. Forthcoming 2013. Effects of stimulants on brain function in ADHD: a systematic review and meta-analysis. Biol Psych.; http://dx.doi.org/10.1016/j.biopsych.2013.10.016 [DOI] [PMC free article] [PubMed]
- Rubia K, Cubillo A, Smith AB, Woolley J, Heyman I, Brammer MJ. 2010. Disorder-specific dysfunction in right inferior prefrontal cortex during two inhibition tasks in boys with attention-deficit hyperactivity disorder compared to boys with obsessive-compulsive disorder. Hum Brain Mapp. 31:287–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Halari R, Cubillo A, Mohammad AM, Scott S, Brammer M. 2010. Disorder-specific inferior prefrontal hypofunction in boys with pure attention-deficit/hyperactivity disorder compared to boys with pure conduct disorder during cognitive flexibility. Hum Brain Mapp. 31:1823–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Halari R, Mohammad A-M, Taylor E, Brammer M. 2011. Methylphenidate normalizes frontocingulate underactivation during error processing in attention-deficit/hyperactivity disorder. Biol Psychiatry. 70:255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SCR, Simmons A, Bullmore ET. 1999. Hypofrontality in attention deficit hyperactivity disorder during higher-order motor control: a study with functional MRI. Am J Psychiatry. 156:891–896. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Taylor E. 2003. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. Neuroimage. 20:351–358. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Toone B, Taylor E. 2005. Abnormal brain activation during inhibition and error detection in medication-naive adolescents with ADHD. Am J Psychiatry. 162:1067–1075. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Halari R, Matsukura F, Mohammad M, Taylor E, Brammer MJ. 2009. Disorder-specific dissociation of orbitofrontal dysfunction in boys with pure conduct disorder during reward and ventrolateral prefrontal dysfunction in boys with pure ADHD during sustained attention. Am J Psychiatry. 166:83–94. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Taylor E, Brammer M. 2007. Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Hum Brain Mapp. 28:1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, Brammer M. 2006. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Hum Brain Mapp. 27:973–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. 2003. The Social Communication Questionnaire: Manual. Los Angeles: Western Psychological Services. [Google Scholar]
- Sanders J, Johnson KA, Garavan H, Gill M, Gallagher L. 2008. A review of neuropsychological and neuroimaging research in autistic spectrum disorders: attention, inhibition and cognitive flexibility. Res Autism Spectr Disord. 2:1–16. [Google Scholar]
- Schmitz N, Rubia K, Daly E, Smith A, Williams S, Murphy DGM. 2006. Neural correlates of executive function in autistic spectrum disorders. Biol Psychiatry. 59:7–16. [DOI] [PubMed] [Google Scholar]
- Schmitz N, Rubia K, van Amelsvoort T, Daly E, Smith A, Murphy DGM. 2008. Neural correlates of reward in autism. Br J Psychiatry. 192:19–24. [DOI] [PubMed] [Google Scholar]
- Schweren LJS, de Zeeuw P, Durston S. 2012. MR imaging of the effects of methylphenidate on brain structure and function in attention-deficit/hyperactivity disorder. Euro Neuropsychopharm. 23:1151–1164. [DOI] [PubMed] [Google Scholar]
- Shafritz KM, Dichter GS, Baranek GT, Belger A. 2008. The neural circuitry mediating shifts in behavioral response and cognitive set in autism. Biol Psychiatry. 63:974–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, Clasen L, Evans A, Giedd J, Rapoport JL. 2007. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci USA. 104:19649–19654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Gilliam M, Liverpool M, Weddle C, Malek M, Sharp W, Greenstein D, Evans A, Rapoport J, Giedd J. 2011. Cortical development in typically developing children with symptoms of hyperactivity and impulsivity: support for a dimensional view of attention deficit hyperactivity disorder. Am J Psychiatry. 168:143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley D, Hanin I, Kuhar M, Skolnick P. 2007. Handbook of contemporary neuropharmacology. New York: Wiley Blackwell. [Google Scholar]
- Simmons A, Moore E, Williams SCR. 1999. Quality control for functional magnetic resonance imaging using automated data analysis and Shewhart charting. Magn Reson Med. 41:1274–1278. [DOI] [PubMed] [Google Scholar]
- Simmons A, Strigo I, Matthews SC, Paulus MP, Stein MB. 2006. Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety-prone subjects. Biol Psychiatry. 60:402–409. [DOI] [PubMed] [Google Scholar]
- Sinzig J, Lehmkuhl G. 2007. What do we know about the serotonergic genetic heterogeneity in attention-deficit/hyperactivity and autistic disorders? Psychopathology. 40:329–337. [DOI] [PubMed] [Google Scholar]
- Small DM, Gitelman DR, Gregory MD, Nobre AC, Parrish TB, Mesulam MM. 2003. The posterior cingulate and medial prefrontal cortex mediate the anticipatory allocation of spatial attention. Neuroimage. 18:633–641. [DOI] [PubMed] [Google Scholar]
- Smith AB, Taylor E, Brammer M, Halari R, Rubia K. 2008. Reduced activation in right lateral prefrontal cortex and anterior cingulate gyrus in medication-naive adolescents with attention deficit hyperactivity disorder during time discrimination. J Child Psychol Psychiatry. 49:977–985. [DOI] [PubMed] [Google Scholar]
- Smith AB, Taylor E, Brammer M, Toone B, Rubia K. 2006. Task-specific hypoactivation in prefrontal and temporoparietal brain regions during motor inhibition and task switching in medication-naive children and adolescents with attention deficit hyperactivity disorder. Am J Psychiatry. 163:1044–1051. [DOI] [PubMed] [Google Scholar]
- Solomon M, Ozonoff SJ, Ursu S, Ravizza S, Cummings N, Ly S, Carter CS. 2009. The neural substrates of cognitive control deficits in autism spectrum disorders. Neuropsychologia. 47:2515–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivak B, Vered Y, Yoran-Hegesh R, Averbuch E, Mester R, Graf E, Weizman A. 1999. Circulatory levels of catecholamines, serotonin and lipids in attention deficit hyperactivity disorder. Acta Psychiatr Scand. 99:300–304. [DOI] [PubMed] [Google Scholar]
- Stigler KA, McDonald BC, Anand A, Saykin AJ, McDougle CJ. 2011. Structural and functional magnetic resonance imaging of autism spectrum disorders. Brain Res. 1380:146–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. 1988. Co-planar stereotaxic atlas of the human brain. New York: Thieme New York. [Google Scholar]
- Thirion B, Pinel P, Meriaux S, Roche A, Dehaene S, Poline JB. 2007. Analysis of a large fMRI cohort: statistical and methodological issues for group analyses. Neuroimage. 35:105–120. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Menon V. 2009. The anterior insula in autism: under-connected and under-examined. Neurosci Biobehav Rev. 33:1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer JMJ, Oerlemans AM, van Steijn DJ, Lappenschaar MGA, de Sonneville LMJ, Buitelaar JK, Rommelse NNJ. 2012. Are autism spectrum disorder and attention-deficit/hyperactivity disorder different manifestations of one overarching disorder? Cognitive and symptom evidence from a clinical and population-based sample. J Am Acad Child Adolesc Psychiatry. 51:1160–1172. [DOI] [PubMed] [Google Scholar]
- van Ewijk H, Heslenfeld DJ, Zwiers MP, Buitelaar JK, Oosterlaan J. 2012. Diffusion tensor imaging in attention deficit/hyperactivity disorder: a systematic review and meta-analysis. Neurosci Biobehav Rev. 36:1093–1106. [DOI] [PubMed] [Google Scholar]
- Varnäs K, Halldin C, Hall H. 2004. Autoradiographic distribution of serotonin transporters and receptor subtypes in human brain. Hum Brain Mapp. 22:246–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Fowler JS, Gatley SJ, Logan J, Ding Y-S, Hitzemann R, Pappas N. 1998. Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. Am J Psychiatry. 155:1325–1331. [DOI] [PubMed] [Google Scholar]
- Wechsler D. 1999. Wechsler Abbreviated Scale of Intelligence. San Antonio: (TX: ): The Psychological Corporation. [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. 2005. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry. 57:1336–1346. [DOI] [PubMed] [Google Scholar]
- Williams K, Wheeler DM, Silove N, Hazell P. 2010. Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD). Cochrane Database Syst Rev. 8:1–26. [DOI] [PubMed] [Google Scholar]
- Wong DT, Bymaster FP, Engleman EA. 1995. Prozac (fluoxetine, lilly 110140), the first selective serotonin uptake inhibitor and an antidepressant drug: twenty years since its first publication. Life Sci. 57:411–441. [DOI] [PubMed] [Google Scholar]
- World Health Organisation. 1994. ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidlines. Geneva: World Health Organisation. [Google Scholar]
- Yerys BE, Wallace GL, Harrison B, Celano MJ, Giedd JN, Kenworthy LE. 2009. Set-shifting in children with autism spectrum disorders: reversal shifting deficits on the intradimensional/extradimensional shift test correlate with repetitive behaviors. Autism. 13:523–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. 2006. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 7:464–476. [DOI] [PubMed] [Google Scholar]
- Zafeiriou DI, Ververi A, Vargiami E. 2009. The serotonergic system: its role in pathogenesis and early developmental treatment of autism. Curr Neuropharmacol. 7:150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zepf FD, Gaber TJ, Baurmann D, Bubenzer S, Konrad K, Herpertz-Dahlmann B, Stadler C, Poustka F, Wockel L. 2010. Serotonergic neurotransmission and lapses of attention in children and adolescents with attention deficit hyperactivity disorder: availability of tryptophan influences attentional performance. Int J Neuropsychopharmacol. 13:933–941. [DOI] [PubMed] [Google Scholar]
- Zepf FD, Holtmann M, Stadler C, Demisch L, Schmitt M, Wockel L, Poustka F. 2008. Diminished serotonergic functioning in hostile children with ADHD: Tryptophan depletion increases behavioural inhibition. Pharmacopsychiatry. 41:60–65. [DOI] [PubMed] [Google Scholar]