Abstract
Synaptic plasticity comprises a cellular mechanism through which the hippocampus most likely enables memory formation. Neuromodulation, related to arousal, is a key aspect in information storage. The activation of locus coeruleus (LC) neurons by novel experience leads to noradrenaline release in the hippocampus at the level of the dentate gyrus (DG). We explored whether synaptic plasticity in the DG is influenced by activation of the LC via electrical stimulation. Coupling of test-pulses that evoked stable basal synaptic transmission in the DG with stimulation of the LC induced β-adrenoreceptor-dependent long-term depression (LTD) at perforant path–DG synapses in adult rats. Furthermore, persistent LTD (>24 h) induced by perforant path stimulation also required activation of β-adrenergic receptors: Whereas a β-adrenergic receptor antagonist (propranolol) prevented, an agonist (isoproterenol) strengthened the persistence of LTD for over 24 h. These findings support the hypothesis that persistent LTD in the DG is modulated by β-adrenergic receptors. Furthermore, LC activation potently facilitates DG LTD. This suggests in turn that synaptic plasticity in the DG is tightly regulated by activity in the noradrenergic system. This may reflect the role of the LC in selecting salient information for subsequent synaptic processing in the hippocampus.
Keywords: dentate gyrus, hippocampus, locus coeruleus, long-term depression, synaptic plasticity, β-adrenergic receptors
Introduction
The locus coeruleus (LC) is the main source of noradrenaline (NA) in the rodent brain, providing diverse projections to areas involved in memory processing, including the hippocampus (Loughlin et al. 1986; Berridge and Waterhouse 2003). The dentate gyrus (DG) contains the highest NA content and the highest fiber density of noradrenergic innervation relative to the other hippocampal subregions (Loy et al.1980; Fallon and Loughlin 1987). Recent evidence suggests that β-adrenergic receptors are neither evenly distributed neither in the DG (Booze et al. 1993; Milner et al. 2000), nor in other hippocampal regions (Booze et al. 1993; Milner et al. 2000). However, β-adrenoreceptor binding sites are dense in the DG granule cell layer (Booze et al. 1993), on granule cell somata and postsynaptic dendrites from granule cell somata (Milner et al. 2000). This suggests that the DG is subjected to very tight neuromodulory control by the LC.
The LC is activated after novel salient stimuli of various modalities (Aston-Jones et al. 1994; Sara et al. 1994; Vankov et al. 1995) resulting in NA release from this structure (Gibbs et al. 2010), and subsequently in the DG (Yavich et al. 2005). NA release sites are localized near β-adrenoreceptors in the DG (Harley et al. 1996) and NA is an important modulator of hippocampal synaptic plasticity, in the form of long-term potentiation (LTP) (Stanton and Sarvey 1985; Bramham et al. 1997). Effects may be mediated by NA-mediated changes in cellular excitability: Novel environmental stimuli result in increased granule cell excitability in the DG of the rat in vivo that depends on activation of β-adrenoreceptors (Kitchigina et al. 1997). However, the consequences of NA release in the DG are not entirely clear. Whereas inhibition of granule cell excitability has been reported in anesthetized rats after NA application (Rose and Pang 1989), in vivo studies demonstrated both inhibitory (Segal and Bloom 1976) and excitatory effects (Dahl and Winson 1985) on granule cells following high-frequency stimulation (HFS) of the LC.
The LC increases its firing in response to exposure to novel experience (Vankov et al 1995). Strikingly, patterned stimulation of the LC (to trigger NA release) coupled with test-pulse stimulation of the Schaffer collaterals (SCs) causes persistent synaptic plasticity in the form of long-term depression (LTD), in the CA1 region of freely behaving rats (Lemon et al. 2009; Lemon and Manahan-Vaughan 2012). LTD is tightly associated with the learning of information about the content and features of novel space (Dudek and Bear 1992; Manahan-Vaughan 1997; Manahan-Vaughan and Braunewell 1999; Braunewell and Manahan-Vaughan 2001). LTD that is facilitated by LC activation is also closely associated with the encoding of spatial memory and depends on activation of β-adrenoreceptors (Lemon et al. 2009). This finding suggests that activation of the LC in response to novel experience is a key factor in the effective encoding of spatial information by synaptic plasticity in the hippocampus.
LTD that is enabled by spatial experience is different in the CA1 region compared with the DG. Whereas in the CA1 region, LTD is associated with novel learning about the finer, or more subtle, spatial features of an environment, in the DG it is associated with learning about navigational or large orientational elements of an environment (Kemp and Manahan-Vaughan 2007; 2008a). The DG is the “gateway” to the hippocampus and is believed to engage, on the one hand as a barrier for extraneous or irrelevant information (Lemon et al 2009) and on the other hand to subserve pattern separation (Hunsaker et al. 2008). In addition, DG synapses may be crucial for learning about novel information: Granule cells reveal the highest activity of immediate-early genes (IEGs) such arg 3.1 during acquisition of novel information in the mouse brain (Montag-Sallaz et al. 1999). Due to the higher NA content and density of noradrenergic fiber projections from the LC to the DG in comparison to other hippocampal subregions (Loy et al.1980; Fallon and Loughlin 1987), we postulated that the perforant path (PP) synapses of the DG are more susceptible to β-adrenoreceptor modulation than Schaffer collateral–CA1 synapses. We explored whether LTD that is induced by stimulation of the medial PP–DG synapse depends on β-adrenoreceptors. In addition, we explored whether activation of the LC results in synaptic plasticity in this hippocampal subregion. We observed that synaptic plasticity in the DG is modulated by β-adrenoreceptors. Strikingly, activation of these receptors is crucial for the expression of LTD that lasts for very long periods (e.g., >24 h). Furthermore, stimulation of the LC results in a robust form of LTD. This influence of the noradrenergic system on DG function may reflect a mechanism through which the saliency of new information is discriminated for subsequent long-term hippocampal synaptic storage.
Materials and Methods
All experiments were conducted according to the European Communities Council Directive of 22 September 2010 (2010/63/EU) for the care of laboratory animals with prior approval from the local ethics committee (Bezirksamt Arnsberg). All measures were taken to minimize animal suffering and the number of animals used.
Surgery
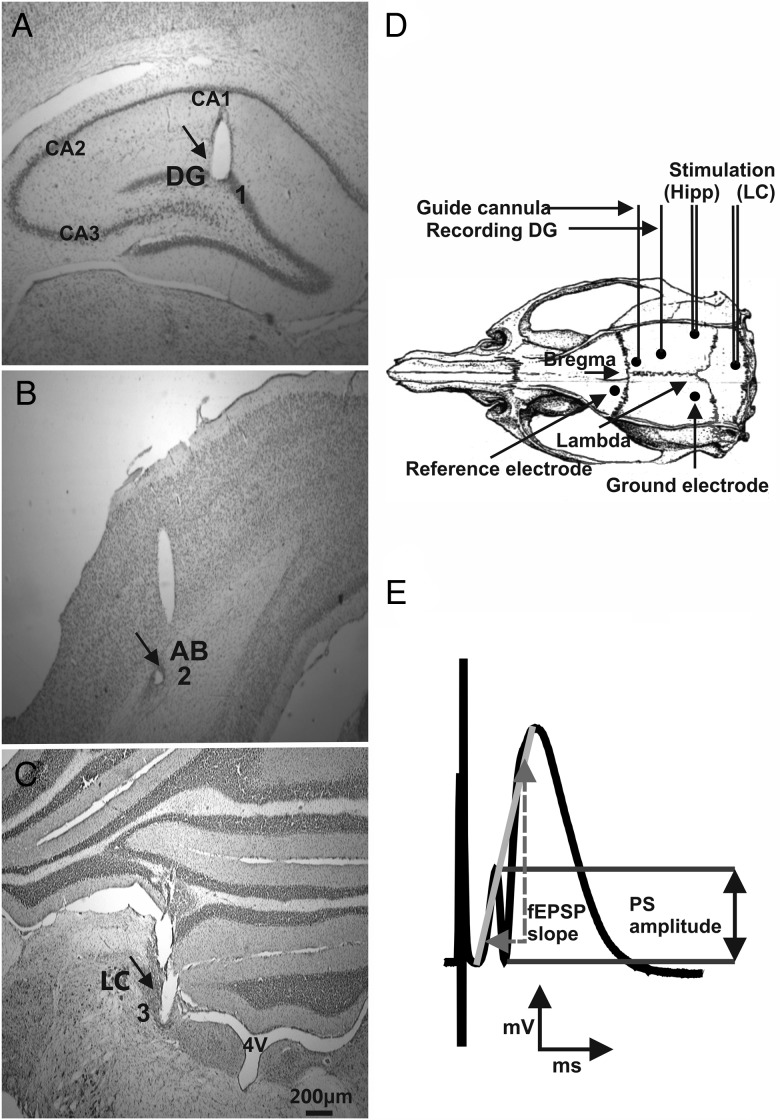
Male Wistar rats (Charles River, Sulzfeld, Germany, 7–8 weeks old), underwent implantation of hippocampal electrodes and a guide cannula under anesthesia as described previously (Manahan-Vaughan 1997; Lemon et al. 2009). Briefly, under sodium pentobarbitone (Synopharm, Germany) anesthesia (“Nembutal,” 52 mg/kg, intraperitoneally), animals underwent implantation of a monopolar recording and bipolar stimulating electrode (made of 0.1-mm-diameter Teflon-coated stainless-steel wire (Biomedical Instruments, Zöllnitz, Germany) attached outside the skull to cardboard to fix and stabilize the wire above the skull. A drill hole (1 mm diameter) was made for the recording electrode, and a second drill hole (1 mm diameter) made for the stimulation electrodes. On the contralateral side, 2 holes were drilled (1.2 mm in diameter) into which anchor screws were inserted. The anchor screws also served as reference or ground electrodes. The coordinates used for DG recordings comprised 3.1 mm posterior to bregma and 1.9 mm lateral to the midline for the recording electrode, and 6.9 mm posterior to bregma and 4.1 mm lateral to the midline for the stimulation electrode. The dura was pierced through both holes, and the recording and stimulating electrodes were lowered into the granule cell layer of the DG and medial PP, respectively, for hippocampus recordings (Fig. 1A,B,D).
Figure 1.
Placement of electrodes and measurement of the population spike amplitude and fEPSP slope. (A) Microphotographs of Nissl-stained hippocampal slices illustrate recording and stimulating electrode placements in the DG. Microphotograph of bipolar stimulation electrode placement in (B) the angular bundle (A,B) of the PP and (C) locus coeruleus. Black arrows indicate electrode tracts in a representative rat slice. Scale bar indicates 200 µm. (D) Schematic of electrode placement in the hippocampus (Hipp) and locus coeruleus (LC) (redrawn from Paxinos and Watson's Atlas 1998). The dots on the skull represent the drilled holes for the electrodes. (E) This illustration indicates how measurement of the population spike (PS) amplitude and the fEPSP slope was conducted. The PS amplitude was measured from the peak of the first positive deflection of the evoked potential to the peak of the subsequent negative potential (black arrows). The fEPSP slope is represented by the maximum slope through the five steepest points of the gray line connecting the starting point of the first positive deflection with the peak of the second positive deflection and (dashed gray arrows).
To enable intracerebroventricular (i.c.v.) injections, a cannula was inserted in the lateral cerebral ventricle with the following specifications (coordinates: 0.5 mm posterior to bregma, 1.6 mm lateral to the midline; size: 5.6 mm length, 0.8 mm diameter, 4.5 mm depth; Fig. 1D).
The electrodes' correct location was verified by means of the electrophysiological characteristics of the field potentials evoked. The following criteria were utilized to discriminate potentials elicited by medial PP stimulation from lateral path stimulation (Abraham and McNaughton 1984): A field excitatory postsynaptic potential (fEPSP) peak latency of ∼3 ms and half-width of ∼5 ms and the appearance of the population spike (PS) within the first positive deflection of the fEPSP (Fig. 1E). A third drill hole (1 mm diameter) was made ipsilateral to the hippocampal electrodes for another stimulation electrode at 3 mm posterior and 1.2 mm lateral to lambda. This bipolar stimulation electrode was implanted in the LC (6.4 mm ventral to dura matter, entering at a 15° angle to the plane of the skull; Fig. 1C,D).
The entire assembly was sealed and fixed to the skull with dental acrylic (Paladur, Heraeus Kulzer GmbH, Hanau, Germany). Seven-to-ten days after surgery recordings were obtained in the DG granule cell layer by stimulating the medial PP. Throughout the experiments, the animals moved freely within the recording chamber (40 × 40 × 40 cm), as the implanted electrodes were connected via a flexible cable and swivel connector to the stimulation unit and amplifier. Aside from the insertion of the connector cable at the start of the experiment, disturbance of the animals was kept to an absolute minimum. Throughout the experiments, the electroencephalogram of each animal was continuously monitored.
Measurement of Evoked Potentials
To measure synaptic activity in the DG, we analyzed both the PS amplitude and fEPSP slope (Fig. 1E). To obtain these measurements, an evoked response was generated by stimulating at low frequency (0.025 Hz) with single biphasic square wave pulses of 0.2-ms duration per half-wave, generated by a constant current isolation unit. For each time-point measured during the experiments, we averaged five recordings of evoked responses. The first 6 time-points recorded at 5-min intervals were used as a “baseline” reference, and subsequently obtained data points were calculated as a percentage of the mean of these 6 points. fEPSP was measured as the maximum slope through the five steepest points obtained on the first positive deflection of the potential and the maximum of the second positive deflection. The PS amplitude was measured from the peak of the first positive deflection (PS onset) to the peak of the first negative deflection of the potential (PS peak). The PS amplitude indicates the summated action potentials of granule cells in the somatic layer of the DG, whereas the fEPSP slope depicts alterations in the dendritic excitability of the DG. By means of input/output curve determination (evaluation of nine different stimulation intensities from 100 to 900 µA in 100 µA steps), we identified the maximum PS amplitude, and during experiments all potentials employed as baseline criteria were evoked at a stimulus intensity which produced 40% of this maximum. Five such evoked responses were recorded every 40 s, and averaged and repeated every 5 min to provide a representative average recording for each 5-min interval. After 90 min of recording, the period between samples of evoked potentials was extended to 15 min. Low-frequency stimulation (LFS; 1 Hz, 900 pulses) or sLFS (1 Hz, 600 pulses) was used to induce LTD of >24 h or <24 h duration, respectively. LTD was defined as a persistent depression of synaptic strength that endured for over 4 h (Manahan-Vaughan 1997). This is in contrast to in vitro studies where a 40-min depression is usually referred to as LTD.
To analyze differences between groups, we conducted a two-way mixed analysis of variance (ANOVA) with the between group factor pharmacological treatment and within group factor time after stimulation. Only significant interaction effects between the factors treatment and time after stimulation are reported. All data periods were expressed as a mean percentage ± SEM of the average baseline value. The level of significance was set to P < 0.05.
Locus Coeruleus Stimulation
The current intensity for LC stimulation was chosen separately for individual animals through a preliminary input /output assessment (referring current injection to behavioral response) done 1 week prior to the experimental recording. The current used was immediately subthreshold for triggering behavioral responses such as: Freezing behavior, production of fecal boli, or slight head twitches. We also verified that the selected current for each rat did not alter the amount of time the animal spent exploring an open field after LC stimulation. LC stimulation consisted of 2 trains of 100 pulses at 100 Hz with each train lasting 1 s with a 20-s intertrain interval. Stimulus strength was 20–115 µA with single biphasic square wave pulses of 0.1-ms duration per half-wave. We used this electrical stimulation protocol because it induces LTD at SC-CA1 synapses in vivo and elicits an increase in NA in the hippocampal CA1 region (Lemon et al. 2009). Furthermore, another study indicated that electrical HFS with 50 Hz causes an increase in NA in the DG in mice (Yavich et al. 2005).
Histology
At the end of the study, brains were removed for histological verification of electrode and cannula localization. Upon removal, the brain tissue was immediately fixed in 4% paraformaldehyde (PFA; IUPAC name polyoxymethylene) solution in phosphate-buffered saline (PBS) at a pH of 7.4. The tissue was then cryoprotected by immersion in 30% sucrose for several days to prevent tissue damage.Frozen sections (30-µm thick) were cut on a freezing microtome. The sections were stored in 0.1 mL PBS and mounted on glass slides coated with 45% sodium chloride solution onto 4% potassium chrome alum-gelatine. The mounted sections were left to air-dry for 7 days. When dried, the glass slides were placed in xylene for 3 min, isopropanol, 96% ethanol, and 70% ethanol (each alcohol for 3 min) and finally washed in distilled water. The slides were then stained in 0.1% cresyl violet for 3 min. After staining the slides, they were washed in distilled water and further differentiated in 70% ethanol, 96% ethanol, and isopropanol (3 min each alcohol) and then cleared 3 min in xylene. Mounting was carried out with DePex mounting medium for histology (Serva Electrophoresis GmbH, Germany). Photomicrographs were taken with a digital video camera system (Visitron Systems, Puchheim, Germany) on a Leica DM LB Microscope (Leica Mikrosysteme Vertrieb GmbH, Wetzlar, Germany).
Brains in which the electrodes had been incorrectly implanted were discarded from the study.
Compounds and Drug Treatment
The β-adrenoreceptor antagonist propranolol (2 µg) and β-adrenoreceptor agonist isoproterenol (20 µg) (Tocris Bioscience, UK), or vehicle (0.9% NaCl) were injected via the ipsilateral i.c.v. via the implanted cannula in a 5-µL volume over 5 min, 25 min before LC or PP stimulation. We used these concentrations as there is evidence that they do not alter basal synaptic transmission (Kemp and Manahan-Vaughan 2008a). The half-life of isoprotenerol is only some minutes, whereas the half-life of propranolol is about 2–3 h (Smits and Struyker-Boudier 1979; Hadwiger et al. 1997).
Results
Locus Coeruleus Stimulation Induces LTD in the Dentate Gyrus That Depends on β-Adrenergic Receptors
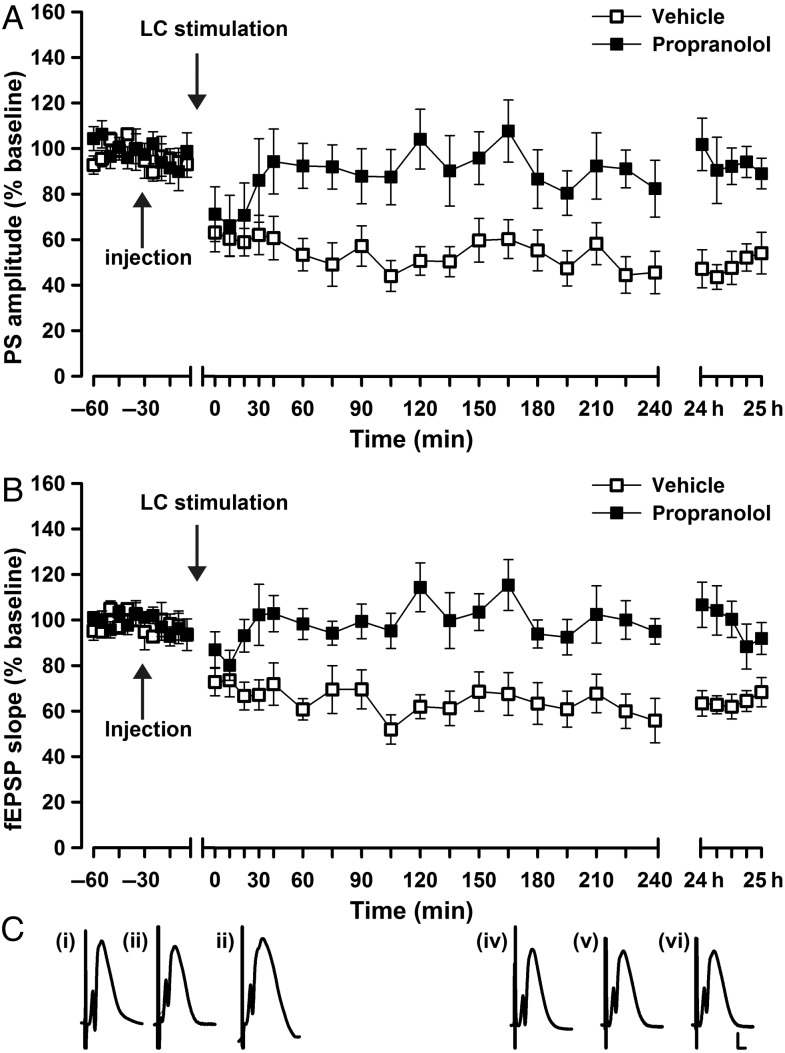
We first assessed whether LC activation modulates synaptic transmission in PP–DG synapses. Bipolar stimulating electrodes were chronically implanted in the LC and PP, and a recording electrode was implanted in the granule cell layer of the DG (Fig. 1A–D). To enable i.c.v. application of pharmacological agents, we also implanted a guide cannula in the lateral cerebral ventricle (Fig. 1D). Synaptic transmission was evoked in the DG by test-pulse stimulation (5 pulses at 0.025 Hz every 5 min) of the PP. LC stimulation (1 s, 100 Hz) resulted in a persistent synaptic depression in the DG lasting >24 h (Fig. 2A–C; LC stimulation/vehicle compared with test-pulse stimulated animals; PS: F1,18 = 70.6, P < 0.0001; fEPSP: F1,18 = 38.2, P < 0.0001; LC stimulation/vehicle n = 10; test-pulse n = 10). Application of the β-adrenergic receptor antagonist, propranolol (2 µg, i.c.v.) prior to LC stimulation significantly impaired the LTD that appeared following LC stimulation (Fig. 2A–C; PS: ANOVA: F1,15 = 16.9, P < 0.001; fEPSP: ANOVA: F1,15 = 23.7, P < 0.001; LC stimulation/vehicle n = 10, LC stimulation/propranolol n = 7).
Figure 2.
LC stimulation induces LTD in the dentate gyrus. (A, B) Locus coeruleus (LC) stimulation (100 Hz) coupled with test-pulse stimulation (0.025 Hz) of perforant path (PP)–dentate gyrus (DG) synapses induces long-term depression (LTD) in the DG lasting over 24 h. Prior treatment with the β-adrenoreceptor antagonist propranolol (2 µg) significantly prevents this form of LTD. The mean population spike (PS) amplitude (A) and the mean fEPSP slope (B) is shown along with the corresponding SEM. (C) Analog traces represent PP–DG field potentials (i) 5 min before, (ii) 5 min after, and (iii) 24 h after vehicle in the presence of LC stimulation, or (iv) 5 min before, (v) 5 min after and (vi) 24 h after propranolol in the presence of LC stimulation. Calibration: vertical bar, 3 mV; horizontal bar, 2.5 ms.
The Late Phase of LTD Elicited by Perforant Path Stimulation is Prevented by a β-Adrenergic Receptor Antagonist
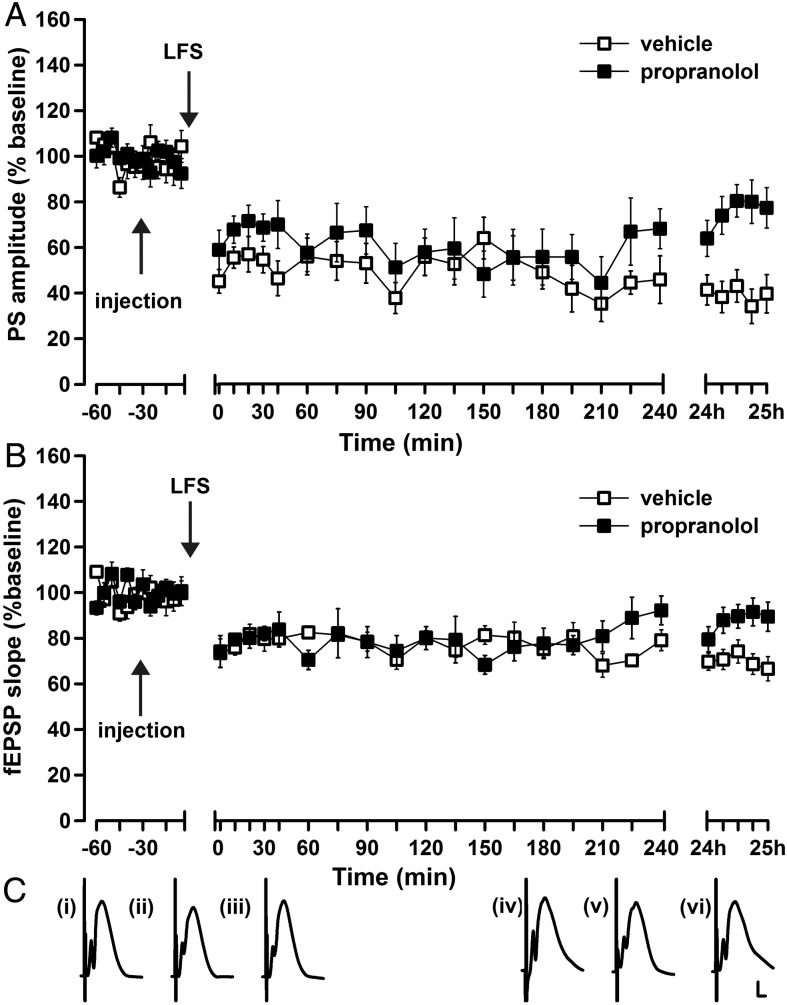
We then explored whether LTD induced by patterned stimulation of the PP is affected by antagonizing β-adrenergic receptors. In vehicle-treated animals, LFS (1 Hz, 900 pulses) elicited LTD that endured for over 24 h (PS: ANOVA, F1, 16 = 47.6; P < 0.0001; fEPSP F1,16 = 49.5, P < 0.0001; LFS/vehicle n = 9; test-pulse n = 9; Fig. 3A–C). LFS in the presence of propranolol (2 μg, i.c.v.), resulted in LTD that persisted for over 4 h, but for <24 h (24 h PS: ANOVA, F1, 16 = 16.9; P < 0.001; 24 h fEPSP: ANOVA, F1,16 = 11.7; P < 0.01; LFS/ vehicle n = 9, LFS/propranolol n = 9; Fig. 3A–C). LTD in the period encompassing 5 min post-LFS through 4-h post-LFS did not differ significantly when comparing vehicle treatment with propranolol treatment (PS: ANOVA, F1,16 = 1.2; P = 0.29; fEPSP: ANOVA, F1, 16 = 0.15; P < 0.71; n = 9 in both groups, Fig. 3A–C).
Figure 3.
Pharmacological antagonism of β-adrenoreceptors prevents persistent LTD in the dentate gyrus. (A, B) Low-frequency stimulation (LFS) (1 Hz, 900 pulses) elicits LTD that lasts for over 24 h in vehicle-treated animals. Prior treatment with the β-adrenoreceptor antagonist, propranolol, (2 µg) significantly impairs the late phase LTD (A: PS, B: fEPSP). (C) Analog traces represent perforant path (PP)–dentate gyrus (DG) field potentials (i) 5 min before, (ii) 5 min after, and (iii) 24 h after vehicle in the presence of LFS, or (iv) 5 min before, (v) 5 min after, and (vi) and 24 h after propranolol in the presence of LFS. Calibration: vertical bar, 3 mV; horizontal bar, 2.5 ms.
The Duration of LTD Elicited by Perforant Path Stimulation is Lengthened by Agonist Activation of β-Adrenergic Receptors
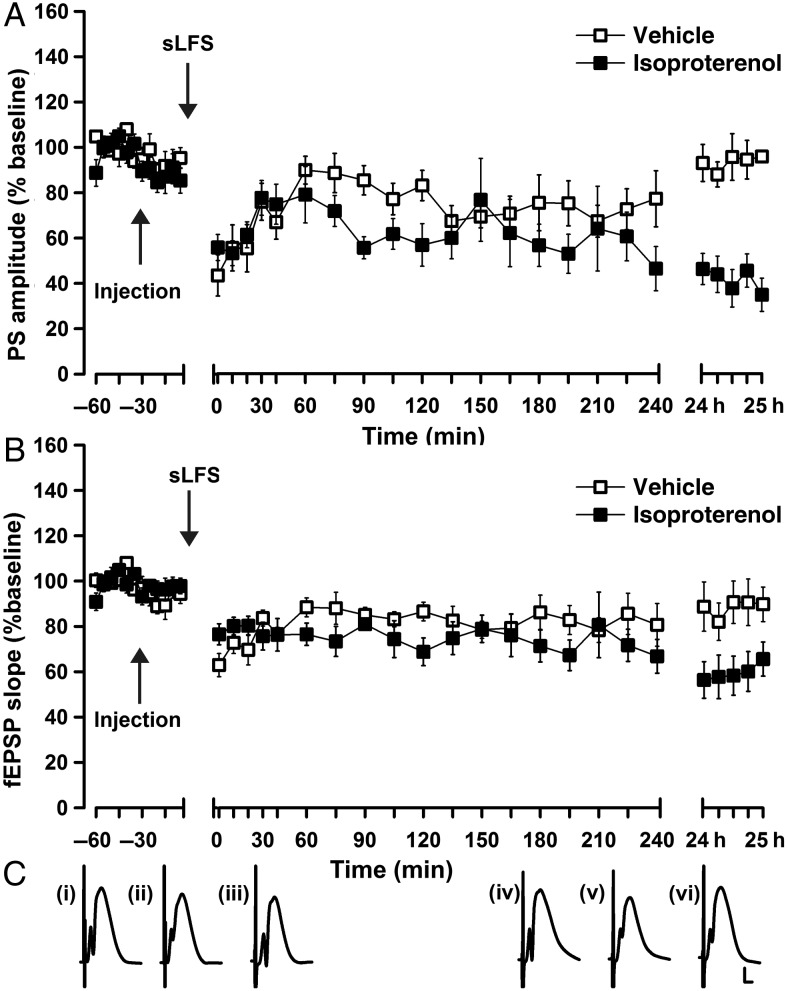
In other hippocampal subregions, weak synaptic plasticity is facilitated into long-term plasticity via agonist activation of β-adrenergic receptors (Kemp and Manahan-Vaughan 2008a; Hagena and Manahan-Vaughan 2012). Here, we investigated if agonist activation of β-adrenergic receptors changes the persistency of synaptic depression in the DG. The infusion of 20 µg of isoproterenol did not affect baseline recordings (isoproterenol vs. vehicle-injected rats: PS ANOVA: F1,16 = 0.1; P = 0.1; fEPSP ANOVA: F1,16 = 0.01, P = 0.96; n = 9 in both groups, Fig. 4A–C). In controls, LFS comprising 1 Hz and 600 pulses resulted in LTD that lasted for over 4 h (4 h PS: ANOVA, F1,16 = 19.9, P < 0.001; 4 h fEPSP: ANOVA, F1,16 = 12.9, P < 0.005; n = 9 in both groups, Fig. 4A–C), but less than 24 h (24 h PS: ANOVA: F1,16 = 0.23, P = 0.64; 24 h fEPSP: ANOVA: F1,16 = 0.46, P = 0.51; n = 9 in both groups, Fig. 4A–C). When applied in the presence of isoproterenol (20 μg, i.c.v.), this LFS protocol resulted in an LTD lasting over 24 h (24 h PS: ANOVA, F1,16 = 37.4, P < 0.0001; 24 h fEPSP: ANOVA, F1,16 = 9.1; P < 0.01; n = 9 in both groups, Fig. 4A–C). LTD in the period encompassing 5-min post-LFS through 4-h post-LFS did not significantly differ when comparing vehicle treatment to isoproterenol treatment (ANOVA PS: F1,16 = 1.32, P = 0.26; ANOVA fEPSP: F1,16 = 0.97, P = 0.34; n = 9 in both groups, Fig. 4A–C).
Figure 4.
Agonist activation of β-adrenoreceptors prolongs LTD in the DG. (A, B) Stimulation at 1 Hz, 600 pulses (sLFS) elicits synaptic depression that lasts for over 4 h but for under 24 h in vehicle-treated animals. Prior treatment with the β-adrenoreceptor agonist isoprotenerol (20 µg) significantly prolongs the synaptic depression, resulting in an LTD that persists for over 24 h (A: PS, B: fEPSP). (C) Analog traces represent perforant path (PP)–dentate gyrus (DG) field potentials (i) 5 min before, (ii) 5 min after, and (iii) 24 h after vehicle in the presence of sLFS, or (iv) 5 min before, (v) 5 min after, and (vi) 24 h after isoprotenerol in the presence of sLFS. Calibration: vertical bar, 3 mV; horizontal bar, 2.5 ms.
Antagonism of β-Adrenergic Receptors Does Not Alter Basal Synaptic Transmission
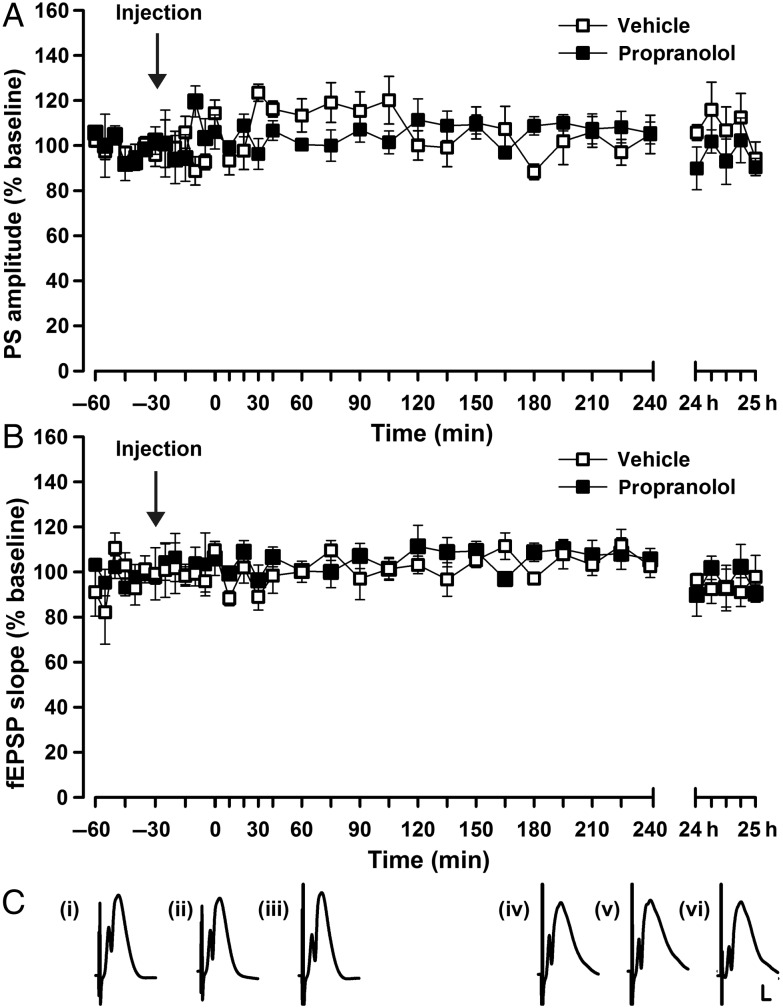
Before performing these experiments, we evaluated whether pharmacological antagonism of β-adrenergic receptors, in the concentration used for plasticity experiments affects basal synaptic transmission. A dose of 2 µg of propranolol did not alter basal synaptic transmission in PP–DG synapses (Fig. 5A,C; ANOVA: PS, F1,12 = 0.82, P = 0.38; fEPSP, ANOVA: F1,12 = 2.3, P = 0.15; n = 8 vehicle and n = 6 propranolol).
Figure 5.
Antagonism of β-adrenoreceptors does not alter basal synaptic transmission. Stable synaptic transmission is elicited in the dentate gyrus (DG) for the duration of the recording period (25 h) by means of test-pulse stimulation (0.025 Hz) of vehicle-treated animals. The β-adrenoreceptor antagonist, propranolol (2 µg) has no influence on basal synaptic transmission of (A) the population spike (PS) amplitude or (B) the field excitatory postsynaptic potential (fEPSP). (C) Analog traces represent DG field potentials (i) 25 min after vehicle application, (ii) 35 min after vehicle, or (iii) 24 h after vehicle, as well as, (iv) 25 min after propranolol treatment, (v) 35 min afterward, and (vi) 24 h after propranolol application. Calibration: vertical bar, 3 mV; horizontal bar, 2.5 ms.
Discussion
In this study, we demonstrate that stimulation of the LC facilitates the expression of LTD in DG synapses of freely behaving rats. Furthermore, we show that LC-mediated LTD in the DG and persistent LTD, elicited by activation of the PP–granule cell synapse, critically depend on β-adrenoreceptor activation. Thus suggests that the LC, by means of NA release onto β-adrenoreceptors in the DG, plays a decisive role in the enablement of long-term information storage in DG synapses.
Locus Coeruleus-Induced LTD at Perforant-Path Synapses
In this study, we observed that activation of the LC led to LTD in the DG, if LC stimulation coincided with test-pulse stimulation of DG synapses. A decisive influence of the LC on synaptic plasticity in other hippocampal subregions has already been reported: Hippocampal LTD elicited by LC stimulation has been described in CA1 synapses in vivo (Lemon et al. 2009; Lemon and Manahan-Vaughan 2012). However, LTD elicited in the DG by LC stimulation is more persistent than that seen in the CA1: In the DG LTD persists for over 24 h, in the CA1 region LTD elicited via LC stimulation lasts for over 4 h but is no longer evident 24 h later (Lemon et al. 2009). LC-mediated LTD in the DG also has a faster onset (compared with CA1) and shows a more pronounced magnitude. These differences may be related to the fact that the NA content and noradrenergic innervation from the LC are higher in DG compared with CA1 (Loy et al. 1980; Oleskevich et al. 1989; Harley 2007). Thus, it is reasonable to assume that LC activation will elicit a stronger effect in DG synapses. The consequences of this potent regulation are intriguing. Our data suggest that LC activation and the subsequent NA release onto β-adrenoreceptors in the DG may comprise a key step in the enablement of persistent information storage in DG synapses.
This is the first time that LC activation has been shown to induce LTD in the DG. Other studies focused on different aspects of the influence of the LC on the hippocampus. For example, HFS of the LC at, for example, 10, 50, or 333 Hz results in a potentiation of the PS amplitude in the DG, when paired with LC stimulation that is given 40–50 ms prior to PP stimulation (Dahl and Winson 1985; Harley et al. 1989). In addition, repeated pairings (50×) of LC and PP test-pulse stimulation cause a long-lasting potentiation of the PS amplitude (Harley et al. 1989). In contrast, stimulation of the LC with 50 Hz causes a transient decrease in the slope and amplitude of evoked potentials at DG dendrites (Dahl and Winson 1985). Taken together, these data indicate that that pairing HFS of the LC with DG stimulation may elicit different forms of synaptic plasticity, depending on precise timing and repetition of the stimulation.
All of these effects are likely to be mediated by NA: The action potential discharge of DG granule cells is increased by NA both in vivo (Chaulk and Harley 1998; Walling and Harley 2004) and in vitro (Lacaille and Harley 1985; Stanton et al. 1989). Effects are mediated by β-adrenoreceptors (Lacaille and Harley 1985; Chaulk and Harley 1998). In line with this, β-adrenoceptor activation facilitates synaptic transmission in PP synapses in vivo after HFS of the vagus nerve (Ura et al. 2013) presumably due to NA release from the LC (Shen et al. 2012). Under other circumstances, electrical LC stimulation in vivo leads to inhibition of granule cell activity, and excitation of interneurons (Rose and Pang 1989). Accordingly, vagus nerve stimulation at 30 Hz leads to reduced PS amplitude for a given EPSP amplitude and increases in the granule cell PS threshold in anesthetized rats (Ura et al. 2013). This latter finding aligns with our observations that LC stimulation results in LTD in the DG that is reflected by changes in both the PS and the fEPSP.
After LC activation via glutamate application, a long-lasting potentiation of PS amplitude in rat DG synapses has been reported (Harley and Sara 1992; Klukowski and Harley 1994; Reid and Harley 2010). Release of NA in the DG after glutamatergic LC activation could enhance the conditions for synaptic plasticity in the DG through neuronal disinhibition. By this means, the activity of feed-forward inhibitory interneurons might be reduced and result in transiently increased granule cell firing (Harley 2007; Brown et al. 2005). Increased presynaptic release of glutamate at DG synapses after NA application via presynaptically located β-adrenoreceptors (Lynch and Bliss 1986) might comprise another potential mechanism for promoting synaptic plasticity in the DG. Combined glutamatergic and β-adrenoreceptor activation may thus lower the threshold to induce LTD for very low-frequency test-pulse stimulation (0.025 Hz) at PP–DG synapses. The noradrenergic system, and in particular the LC (Bouret and Sara 2005) may thereby influence the crossover point for LTD/LTP induction in the hippocampus as a mechanism for network resetting and optimization of synaptic information storage (Lemon et al. 2009).
One cannot exclude, however, that other neurotransmitter receptors contributed to the effects seen. LC-induced changes in synaptic plasticity do not only depend on noradrenergic mediation (Harley et al. 1989). For instance, gamma-aminobutyric acid and dopamine are also released from the LC, albeit to a minor extent, after arousal stimuli (Singewald and Philippu 1998). In line with this, it was reported that dopamine D1/5 receptors play a role in locus-coeruleus-induced hippocampal LTD in SC-CA1 synapses (Lemon and Manahan-Vaughan 2012). However, several studies suggest that memory facilitation after electrical LC stimulation occurs through β-adrenoreceptor activation (Sara and Devauges 1988; Devauges and Sara 1991; Lemon et al. 2009).
Locus Coeruleus Facilitates Memory Storage Through LTD at Dentate Gyrus-Perforant Path Synapses
Spatial learning is tightly associated with the expression of LTD in rodents (Manahan-Vaughan and Braunewell 1999; Goh and Manahan-Vaughan 2012) and hippocampal LTD in CA1 is capable of converting short-term memory into long-term memory (Dong et al. 2012). In rats, DG LTD is enhanced by large directional and self-motion landmark cues that polarize the environment (Kemp and Manahan-Vaughan 2007; Kemp and Manahan-Vaughan 2008b). This form of LTD may serve encoding of spatial orientation. In the CA1 region, LTD is associated with the encoding of finer spatial details (Manahan-Vaughan and Braunewell 1999; Kemp and Manahan-Vaughan 2004), and may enable the encoding of spatial content. In the DG LTD is associated with learning about novel navigational or orientational elements of an environment (Kemp and Manahan-Vaughan 2007; 2008b). The facilitation by LC activation of LTD in the DG may support encoding of spatial information of this kind by raising the saliency of incoming information (Lemon et al. 2009). This may be relevant for the putative role of the DG in pattern separation (Hunsaker et al. 2008; Aimone et al. 2011). The high sensitivity of DG granule cells to noradrenergic neuromodulation may also support the postulated function of the DG in generating novel representations of memory (Leutgeb et al. 2007).
Late LTD at Perforant-Path Dentate Gyrus Synapses is Modulated via β-Adrenoreceptors In Vivo
In line with observations with regard to LTD induced by low-frequency afferent stimulation of CA1 and CA3 synapses of rats or mice in vivo (Straube and Frey 2003; Kemp and Manahan-Vaughan 2008a; Hagena and Manahan-Vaughan 2012; Goh and Manahan-Vaughan 2013) late LTD in medial PP–DG synapses is facilitated by β-adrenoreceptor activation. This effect is surprising: In vitro studies have described that agonist activation of β-adrenoreceptors prevents CA1-LTD (Katsuki et al. 1997) and that modifications of both PS amplitude and dendritic EPSP occur after NA perfusion onto DG slices that last 60 min (Stanton and Sarvey 1987).
The involvement of β-adrenoreceptors in persistent LTP but not LTD at lateral and medial PP–DG synapses was first described in vitro (Bramham et al. 1997). Here, however, plasticity effects were also followed for a matter of minutes (i.e., <60 min) as opposed to for over 24 h as in our study. We observed that it is “late” LTD that is prevented by a β-adrenoreceptor antagonism in the DG. This late effect would have been missed by the short duration of monitoring in in vitro assessments. Our observation of a requirement for β-adrenoreceptor for late LTD in the DG is in contrast to LTD elicited by afferent stimulation in the CA1 and CA3 regions, however (Kemp and Manahan-Vaughan 2008a; Hagena and Manahan-Vaughan 2012). As β-adrenoceptors are known to modulate both forms of long-term plasticity in vivo (Kemp and Manahan-Vaughan 2008a), it is perhaps not illogical however, that the DG, with its higher susceptibility to β-adrenergic neuromodulation compared with the CA1 or CA3 region, expresses a β-adrenoreceptor-dependent form of late LTD in vivo.
Other in vitro studies of the PP input to the DG have suggested a pathway specificity of noradrenergic plasticity in the DG, as shown after test-pulse stimulation of the PP in the presence of the β-adrenoreceptor agonist, isoproterenol. “Norepinephrine long-lasting depression” was reported after lateral PP stimulation (Dahl and Sarvey 1989; Pelletier et al. 1994) and “Norepinephrine long-lasting potentiation” was observed after medial PP stimulation in the presence of isoproterenol (Dahl and Sarvey 1989; Pelletier et al. 1994). However, it is important to note that these β-adrenoreceptor-mediated changes were monitored for maximally 1 h in vitro. It is not known whether these effects are persistent or are dose dependent. In contrast to the abovementioned studies (Dahl and Sarvey 1989; Pelletier et al. 1994), we did not observe any change in basal synaptic transmission when we combined test-pulse stimulation of the medial PP with β-adrenoreceptor agonism. However, we deliberately chose a dose of isoproterenol that did not affect basal synaptic transmission as our goal was to study the role of the β-adrenoreceptors in DG synaptic plasticity as opposed to direct effects on DG cellular excitability. The abovementioned studies raise the interesting possibility that β-adrenoreceptor activation serves to temporarily change signal-to-noise ratios under specific circumstances, thereby acting permissively to subsequent inductions of specific forms of synaptic plasticity: Our finding that β-adrenoreceptor activation facilitated DG LTD is thus not in conflict with the observation that medial PP stimulation combined with β-adrenoreceptor agonism enhances cellular excitability in the DG. NA-mediated increases in cellular excitability would mean that a subsequent attempt to induce LTD (on the background of potentiated synapses) would be likely to be more successful (compared with, e.g., an attempt to induce LTD in synapses that are already depressed). It is also very likely that in the intact animal, fluctuations in basal NA tonus and in phasic NA release onto the DG and hippocampus occur in tight relationship to the current arousal state of the animal. This, in turn, could be expected to exert a potent influence on plasticity thresholds and the direction of change in synaptic strength that results from novel experience.
Conclusions
Our results demonstrate that activation of the LC facilitates expression of robust hippocampal LTD in PP–DG synapses. This process involves β-adrenoreceptors. In contrast to the CA1 (Kemp and Manahan-Vaughan 2008a) and CA3 regions (Hagena and Manahan-Vaughan 2012), LTD that is induced by patterned stimulation of afferent fibers to the DG also depends on β-adrenoreceptors. These findings suggest that LTD in the DG is distinct from LTD in the CA1 region. This may reflect the different functional role attributed to LTD in these subregions (Kemp and Manahan-Vaughan 2007). In the DG activation of β-adrenoreceptors and stimulation of the LC significantly prolongs the duration of synaptic depression resulting in LTD that lasts for over 24 h. LTD is tightly associated with the acquisition of long-term spatial memory (Kemp and Manahan-Vaughan 2004, 2007; 2008b; Dong et al. 2012). Taken together, we postulate that the LC plays an important role in the promotion of the encoding of salient information within the hippocampus. The activation of the LC may thus facilitate long-term memory storage through LTD particularly when salient information should be retained.
Funding
This work was supported by a German Research foundation (DFG) grant to D.M.V. (MA1843/ 6-1). Funding to pay the Open Access publication charges for this article was provided by a departmental budget.
Notes
We gratefully acknowledge the technical assistance of Jens Klausnitzer, Beate Krenzek, Ute Neubacher, and Doris Strehler. We also thank Nadine Kollosch for support in animal care. Conflict of Interest: None declared.
References
- Abraham WC, McNaughton N. 1984. Differences in synaptic transmission between medial and lateral components of the perforant path. Brain Res. 303:251–260. [DOI] [PubMed] [Google Scholar]
- Aimone JB, Deng W, Gage FH. 2011. Resolving new memories: a critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron. 70:589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Kubiak P, Alexinsky T. 1994. Locus coeruleus neurons in monkey are selectively activated by attended cues in a vigilance task. J Neurosci. 14:4467–4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. 2003. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Rev. 42:33–84. [DOI] [PubMed] [Google Scholar]
- Booze RM, Crisostomo EA, Davis JN. 1993. Beta-adrenergic receptors in the hippocampal and retrohippocampal regions of rats and guinea pigs: autoradiographic and immunohistochemical studies. Synapse (New York, N.Y.). 13:206–214. [DOI] [PubMed] [Google Scholar]
- Bouret S, Sara SJ. 2005. Network reset: a simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci. 28:574–582. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Bacher-Svendsen K, Sarvey JM. 1997. LTP in the lateral perforant path is beta-adrenergic receptor-dependent. Neuroreport. 8:719–724. [DOI] [PubMed] [Google Scholar]
- Braunewell KH, Manahan-Vaughan D. 2001. Long-term depression: a cellular basis for learning? Rev Neurosci. 12:121–140. [DOI] [PubMed] [Google Scholar]
- Brown RA, Walling SG, Milway JS, Harley CW. 2005. Locus ceruleus activation suppresses feedforward interneurons and reduces beta-gamma electroencephalogram frequencies while it enhances theta frequencies in rat dentate gyrus. J Neurosci. 25:1985–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaulk PC, Harley CW. 1998. Intracerebroventricular norepinephrine potentiation of the perforant path-evoked potential in dentate gyrus of anesthetized and awake rats: a role for both alpha- and beta-adrenoceptor activation. Brain Res. 787:59–70. [DOI] [PubMed] [Google Scholar]
- Dahl D, Sarvey JM. 1989. Norepinephrine induces pathway-specific long-lasting potentiation and depression in the hippocampal dentate gyrus. Proc Natl Acad Sci USA. 86:4776–4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl D, Winson J. 1985. Action of norepinephrine in the dentate gyrus. I. Stimulation of locus coeruleus. Exp Brain Res. 59:491–496. [DOI] [PubMed] [Google Scholar]
- Devauges V, Sara SJ. 1991. Memory retrieval enhancement by locus coeruleus stimulation: evidence for mediation by beta-receptors. Behav Brain Res. 43:93–97. [DOI] [PubMed] [Google Scholar]
- Dong Z, Gong B, Li H, Bai Y, Wu X, Huang Y, He W, Li T, Wang YT. 2012. Mechanisms of hippocampal long-term depression are required for memory enhancement by novelty exploration. J Neurosci. 32:11980–11990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek SM, Bear MF. 1992. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc Natl Acad Sci USA. 89:4363–4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgersma Y, Fedorov NB, Ikonen S, Choi ES, Elgersma M, Carvalho OM, Giese KP, Silva AJ. 2002. Inhibitory autophosphorylation of CaMKII controls PSD association, plasticity, and learning. Neuron. 36:493–505. [DOI] [PubMed] [Google Scholar]
- Fallon JH, Loughlin SE. 1987. Monoamine innervation of cerebral cortex and a theory of the role of monoamines in cerebral cortex and basal ganglia. In: Jones EG, Peters A, editors. Cerebral cortex, vol. 6 New York: Plenum; p. 41–127. [Google Scholar]
- Gibbs ME, Hutchinson DS, Summers RJ. 2010. Noradrenaline release in the locus coeruleus modulates memory formation and consolidation; roles for α- and β-adrenergic receptors. Neuroscience. 170:1209–1222. [DOI] [PubMed] [Google Scholar]
- Goh JJ, Manahan-Vaughan D. 2013. Hippocampal long-term depression in freely behaving mice requires the activation of beta-adrenergic receptors. Hippocampus. 23:1299–1308. [DOI] [PubMed] [Google Scholar]
- Goh JJ, Manahan-Vaughan D. 2012. Spatial object recognition enables endogenous LTD that curtails LTP in the mouse hippocampus. Cereb Cortex. 23:1118–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadwiger ME, Park S, Torchia SR, Lunte CE. 1997. Simulateneous determination of the elimination profiles of the individual enantiomers of racemic isoprotenerol using capillary electrophoresis and microdialysis sampling. J Pharm Biomed Anal. 15:621–629. [DOI] [PubMed] [Google Scholar]
- Hagena H, Manahan-Vaughan D. 2012. Learning-facilitated long-term depression and long-term potentiation at mossy fiber-CA3 synapses requires activation of β-adrenergic receptors. Front Integr Neurosci. 6:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley C, Milway JS, Lacaille JC. 1989. Locus coeruleus potentiation of dentate gyrus responses: evidence for two systems. Brain Res Bull. 22:643–650. [DOI] [PubMed] [Google Scholar]
- Harley CW. 2007. Norepinephrine and the dentate gyrus. Prog Brain Res. 163:299–318. [DOI] [PubMed] [Google Scholar]
- Harley CW, Lalies MD, Nutt DJ. 1996. Estimating the synaptic concentration of norepinephrine in dentate gyrus which produces beta-receptor mediated long-lasting potentiation in vivo using microdialysis and intracerebroventricular norepinephrine. Brain Res. 710:293–298. [DOI] [PubMed] [Google Scholar]
- Harley CW, Sara SJ. 1992. Locus coeruleus bursts induced by glutamate trigger delayed perforant path spike amplitude potentiation in the dentate gyrus. Exp Brain Res. 89:581–587. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Rosenberg JS, Kesner RP. 2008. The role of the dentate gyrus, CA3a,b, and CA3c for detecting spatial and environmental novelty. Hippocampus. 18:1064–1073. [DOI] [PubMed] [Google Scholar]
- Katsuki H, Izumi Y, Zorumski CF. 1997. Noradrenergic regulation of synaptic plasticity in the hippocampal CA1 region. J Neurophysiol. 77:3013–3020. [DOI] [PubMed] [Google Scholar]
- Kauderer BS, Kandel ER. 2000. Capture of a protein synthesis-dependent component of long-term depression. Proc Natl Acad Sci USA. 97:13342–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. 2008a. Beta-adrenoreceptors comprise a critical element in learning-facilitated long-term plasticity. Cereb Cortex. 18:1326–1334. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. 2008b. The hippocampal CA1 region and dentate gyrus differentiate between environmental and spatial feature encoding through long-term depression. Cereb Cortex. 18:968–977. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. 2007. Hippocampal long-term depression: master or minion in declarative memory processes? Trends Neurosci. 30:111–118. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. 2004. Hippocampal long-term depression and long-term potentiation encode different aspects of novelty acquisition. Proc Natl Acad Sci USA. 101:8193–8197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchigina V, Vankov A, Harley C, Sara SJ. 1997. Novelty-elicited, noradrenaline-dependent enhancement of excitability in the dentate gyrus. Eur J Neurosci. 9:41–47. [DOI] [PubMed] [Google Scholar]
- Klukowski G, Harley CW. 1994. Locus coeruleus activation induces perforant path-evoked population spike potentiation in the dentate gyrus of awake rat. Exp Brain Res. 102:165–170. [DOI] [PubMed] [Google Scholar]
- Lacaille JC, Harley CW. 1985. The action of norepinephrine in the dentate gyrus: beta-mediated facilitation of evoked potentials in vitro. Brain Res. 358:210–220. [DOI] [PubMed] [Google Scholar]
- Lemon N, Aydin-Abidin S, Funke K, Manahan-Vaughan D. 2009. Locus coeruleus activation facilitates memory encoding and induces hippocampal LTD that depends on beta-adrenergic receptor activation. Cereb Cortex. 19:2827–2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon N, Manahan-Vaughan D. 2012. Dopamine D1/D5 receptors contribute to de novo hippocampal LTD mediated by novel spatial exploration or locus coeruleus activity. Cereb Cortex. 22:2131–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser M, Moser EI. 2007. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 315:961–966. [DOI] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. 2002. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 3:175–190. [DOI] [PubMed] [Google Scholar]
- Loughlin SE, Foote SL, Grzanna R. 1986. Efferent projections of nucleus locus coeruleus: morphologic subpopulations have different efferent targets. Neuroscience. 18:307–319. [DOI] [PubMed] [Google Scholar]
- Loy R, Koziell DA, Lindsey JD, Moore RY. 1980. Noradrenergic innervation of the adult rat hippocampal formation. J Comp Neurol. 189:699–710. [DOI] [PubMed] [Google Scholar]
- Lynch MA, Bliss TV. 1986. Noradrenaline modulates the release of [14C]glutamate from dentate but not from CA1/CA3 slices of rat hippocampus. Neuropharmacology. 25:493–498. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D. 1997. Group 1 and 2 metabotropic glutamate receptors play differential roles in hippocampal long-term depression and long-term potentiation in freely moving rats. J Neurosci. 17:3303–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Braunewell KH. 1999. Novelty acquisition is associated with induction of hippocampal long-term depression. Proc Natl Acad Sci USA. 96:8739–8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Kulla A, Frey JU. 2000. Requirement of translation but not transcription for the maintenance of long-term depression in the CA1 region of freely moving rats. J Neurosci. 20:8572–8576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, Shah P, Pierce JP. 2000. Beta-adrenergic receptors primarily are located on the dendrites of granule cells and interneurons but also are found on astrocytes and a few presynaptic profiles in the rat dentate gyrus. Synapse. 36:178–193. [DOI] [PubMed] [Google Scholar]
- Montag-Sallaz M, Welzl H, Kuhl D, Montag D, Schachner M. 1999. Novelty-induced increased expression of immediate-early genes c-fos and arg 3.1 in the mouse brain. J Neurobiol. 38:234–246. [PubMed] [Google Scholar]
- Nichols RA, Sihra TS, Czernik AJ, Nairn AC, Greengard P. 1990. Calcium/calmodulin-dependent protein kinase II increases glutamate and noradrenaline release from synaptosomes. Nature. 343:647–651. [DOI] [PubMed] [Google Scholar]
- Oleskevich S, Descarries L, Lacaille JC. 1989. Quantified distribution of the noradrenaline innervation in the hippocampus of adult rat. J Neurosci. 9:3803–3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfitt KD, Hoffer BJ, Browning MD. 1991. Norepinephrine and isoproterenol increase the phosphorylation of synapsin I and synapsin II in dentate slices of young but not aged Fisher 344 rats. Proc Natl Acad Sci USA. 88:2361–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. 1998. The rat brain in stereotaxic coordinates. 4th ed New York: Academic Press. [Google Scholar]
- Pelletier MR, Kirkby D, Jones SJ, Corcoran ME. 1994. Pathway specificity of noradrenergic plasticity in the dentate gyrus. Hippocampus. 4:181–188. [DOI] [PubMed] [Google Scholar]
- Pöschel B, Manahan-Vaughan D. 2005. Group II mGluR-induced long term depression in the dentate gyrus in vivo is NMDA receptor-independent and does not require protein synthesis. Neuropharmacology. 49(Suppl 1):1–12. [DOI] [PubMed] [Google Scholar]
- Pöschel B, Manahan-Vaughan D. 2007. Persistent (>24 h) long-term depression in the dentate gyrus of freely moving rats is not dependent on activation of NMDA receptors, L-type voltage-gated calcium channels or protein synthesis. Neuropharmacology. 52:46–54. [DOI] [PubMed] [Google Scholar]
- Reid AT, Harley CW. 2010. An associativity requirement for locus coeruleus-induced long-term potentiation in the dentate gyrus of the urethane-anesthetized rat. Exp Brain Res. 200:151–159. [DOI] [PubMed] [Google Scholar]
- Rose GM, Pang KC. 1989. Differential effect of norepinephrine upon granule cells and interneurons in the dentate gyrus. Brain Res. 488:353–356. [DOI] [PubMed] [Google Scholar]
- Sara SJ, Devauges V. 1988. Priming stimulation of locus coeruleus facilitates memory retrieval in the rat. Brain Res. 438:299–303. [DOI] [PubMed] [Google Scholar]
- Sara SJ, Vankov A, Hervé A. 1994. Locus coeruleus-evoked responses in behaving rats: a clue to the role of noradrenaline in memory. Brain Res Bull. 35:457–465. [DOI] [PubMed] [Google Scholar]
- Segal M, Bloom FE. 1976. The action of norepinephrine in the rat hippocampus. IV. The effects of locus coeruleus stimulation on evoked hippocampal unit activity. Brain Res. 107:513–525. [DOI] [PubMed] [Google Scholar]
- Shen H, Fuchino Y, Miyamoto D, Nomura H, Matsuki N. 2012. Vagus nerve stimulation enhances perforant path-CA3 synaptic transmission via the activation of β-adrenergic receptors and the locus coeruleus. J Neuropsychopharmacology. 15:523–530. [DOI] [PubMed] [Google Scholar]
- Singewald N, Philippu A. 1998. Release of neurotransmitters in the locus coeruleus. Prog Neurobiol. 56:237–267. [DOI] [PubMed] [Google Scholar]
- Smits JM, Struyker-Boudier HA. 1979. Propranolol in conscious spontaneously hypertensive rats. II. Disposition after subcutaneous and intracerebroventricular administration. Naunyn Schmiedebergs Arch Pharmacol. 309:18–24. [DOI] [PubMed] [Google Scholar]
- Stanton PK, Mody I, Heinemann U. 1989. A role for N-methyl-D-aspartate receptors in norepinephrine-induced long-lasting potentiation in the dentate gyrus. Exp Brain Res. 77:517–530. [DOI] [PubMed] [Google Scholar]
- Stanton PK, Sarvey JM. 1985. Depletion of norepinephrine, but not serotonin, reduces long-term potentiation in the dentate gyrus of rat hippocampal slices. J Neurosci. 5:2169–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton PK, Sarvey JM. 1987. Norepinephrine regulates long-term potentiation of both population spike and dendritic EPSP in hippocampal dentate gyrus. Brain Res Bull. 18:115–119. [DOI] [PubMed] [Google Scholar]
- Straube T, Frey JU. 2003. Involvement of beta-adrenergic receptors in protein synthesis-dependent late long-term potentiation (LTP) in the dentate gyrus of freely moving rats: the critical role of the LTP induction strength. Neuroscience. 119:473–479. [DOI] [PubMed] [Google Scholar]
- Ura H, Sugaya Y, Ohata H, Takumi I, Sadamoto K, Shibasaki T, Maru E. 2013. Vagus nerve stimulation induced long-lasting enhancement of synaptic transmission and decreased granule cell discharge in the hippocampal dentate gyrus of urethane-anesthetized rats. Brain Res. 1492:63–71. [DOI] [PubMed] [Google Scholar]
- Vankov A, Hervé-Minvielle A, Sara SJ. 1995. Response to novelty and its rapid habituation in locus coeruleus neurons of the freely exploring rat. Eur J Neurosci. 7:1180–1187. [DOI] [PubMed] [Google Scholar]
- Walling SG, Brown RA, Miyasaka N, Yoshihara Y, Harley CW. 2012. Selective wheat germ agglutinin (WGA) uptake in the hippocampus from the locus coeruleus of dopamine-β-hydroxylase-WGA transgenic mice. Front Behav Neurosci. 6:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walling SG, Harley CW. 2004. Locus ceruleus activation initiates delayed synaptic potentiation of perforant path input to the dentate gyrus in awake rats: a novel beta-adrenergic- and protein synthesis-dependent mammalian plasticity mechanism. J Neurosci. 24:598–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavich L, Jäkälä P, Tanila H. 2005. Noradrenaline overflow in mouse dentate gyrus following locus coeruleus and natural stimulation: real-time monitoring by in vivo voltammetry. J Neurochem. 95:641–650. [DOI] [PubMed] [Google Scholar]