Abstract
Individuals born prematurely are at risk for developmental delay, and converging data suggest alterations in neural networks in the developing preterm brain. Nevertheless, those critical period processes such as cerebral lateralization that underlie these findings remain largely unexplored. To test the hypothesis that preterm birth alters the fundamental program of corticogenesis in the developing brain, we interrogated cerebral lateralization at rest in very prematurely born participants and term controls at young adulthood. Employing a novel, voxel-based measure of functional connectivity, these data demonstrate for the first time that cerebral lateralization of functional connectivity in right hemisphere language homologs is altered for very preterm participants. Very preterm participants with no evidence for severe brain injury exhibited a significant decrease in right hemisphere lateralization in the right parietal and temporal lobes in this data driven analysis. Further, for the very preterm participants, but not the term participants, these fundamental alterations in the cerebral lateralization for language significantly correlate with language scores. These findings provide evidence that cerebral asymmetry is both plastic and experiential, and suggest the need for further study of underlying environmental factors responsible for these changes.
Keywords: connectivity lateralization, cross-hemisphere connectivity, functional MRI, preterm, resting state
Introduction
Converging data suggest alterations in neural networks in the developing preterm brain (Ment et al. 2009; Narberhaus et al. 2009; Smyser et al. 2010; Brummelte et al. 2012), yet those critical period processes such as cerebral lateralization that underlie these findings remain largely unexplored (Power et al. 2010; Renteria 2012). Lateralization implies localization of cognitive tasks such as language in a specific cerebral region and lateralization for language has been linked to structural cerebral asymmetry. Asymmetry of the cerebral hemispheres emerges during the late second and third trimesters in typically developing fetuses and healthy preterm neonates and is characterized by enlargement of both the anterior and posterior regions surrounding the sylvian fissure in the left hemisphere compared with the right (Dubois et al. 2009, 2010; Liu et al. 2010; Habas et al. 2012). In contrast, the superior temporal sulcus is deeper in the right. Consistent with these findings, both functional imaging and event-related potential studies demonstrate that, in response to auditory stimuli, newborn infants demonstrate asymmetric responses favoring the left hemisphere (Dehaene-Lambertz 2000; Pena et al. 2003; Dehaene-Lambertz, Hertz-Pannier, Dubois 2006; Dehaene-Lambertz, Hertz-Pannier, Dubois, Meriaux et al. 2006). Taken together, these data suggest that the processing of information relevant for language development is genetically programmed during the late second and third trimesters, a time of rapid neural growth, in the developing infant brain.
Prematurely born children are at high risk for cognitive impairment (Saigal and Doyle 2008; Hack 2009), and sophisticated MRI strategies offer important insights into the impact of preterm birth on developing brain. When compared with term control subjects during infancy, childhood and young adulthood, preterm participants exhibit significant volumetric and microstructural changes, but these data provide only modest predictions of neurodevelopmental outcome (Nosarti et al. 2008; Mathur et al. 2010; Seghier and Huppi 2010). In contrast, functional connectivity data have been highly correlated with measures of intelligence and task performance in adults and older children (Seeley et al. 2007; Nosarti et al. 2009; van den Heuvel et al. 2009; Myers et al. 2010) and have been postulated to be a better predictor of developmental outcome in the prematurely born (Seghier and Huppi 2010).
Notably, task-related functional connectivity studies demonstrate the reliance of preterm participants with unremarkable clinical MRI scans and normal testing scores on right hemisphere language regions compared with term controls (Gozzo et al. 2009; Myers et al. 2010), suggesting impairment of the typically developing pattern of cerebral lateralization. To test the hypothesis that preterm birth alters the fundamental program of corticogenesis in the developing brain, we interrogated cerebral lateralization at rest in very prematurely born subjects and term controls at young adulthood using a novel voxel-based connectivity approach. The advantage of this voxel-based connectivity approach is that cerebral lateralization of connectivity using resting-state functional magnetic resonance imaging (rs-fMRI) can be probed without the requirement to predefine regions of interest (ROI).
Material and Methods
This study was performed at Yale University School of Medicine, New Haven, CT, and Warren Alpert Brown Medical School, Providence RI. The protocols were reviewed and approved by institutional review boards at each location. Informed consent was obtained. All scans were obtained and analyzed at Yale University.
Participants
The participants consisted of a cohort of 20 very prematurely born adolescents with no evidence for intraventricular hemorrhage (IVH), periventricular leukomalacia and/or low pressure ventriculomegaly. Participants had no contraindications to MRI and normal findings on conventional MRI. The very preterm participants enrolled in the follow-up imaging component of the “Multicenter Randomized Indomethacin IVH Prevention Trial” (Ment et al. 1994) were sequentially recruited for the MRI study when they reached 16 years of age. These participants are representative of the cohort of participants with no evidence of neonatal brain injury with respect to gender, handedness, FSIQ scores, minority status, and maternal education. Neonatal characteristics of the very preterm population are shown in Table 1. The very preterm participants weighed between 685 and 1235 g at birth, with a mean birth weight of 970 ± 180 g and had a gestational age between 24 and 32 weeks, with a mean gestational age of 28.2 ± 2.1 weeks. Twenty-three healthy term young adults, aged 16 years, were recruited from the local community and were group-matched with the very preterm participants for age, sex, and minority status for cognitive assessment. The term participants weighed between 2154 and 4252 g at birth, with a mean birth weight of 3290 ± 476 g and had a gestational age between 37.5 and 42 weeks, with a mean gestational age of 39.6 ± 1.3 weeks.
Table 1.
Demographic data for the study participants
| Preterm (N = 20) | Term (N = 23) | P value | |
|---|---|---|---|
| Birth weight, grams ± SD (range) | 970 ± 180 (685–1245) | 3290 ± 476 (2154–4252) | <0.001 |
| Gestational age, weeks ± SD (range) | 28.2 ± 2.1 (24–32) | 39.6 ± 1.3 (37.5–42) | <0.001 |
| Bronchopulmonary dysplasia, N (%) | 8 (40) | N/A | N/A |
| Necrotizing enterocolitis, N (%) | 2 (10) | N/A | N/A |
| Retinopathy of prematurity, N (%) | 8 (40) | N/A | N/A |
| 5 min Apgar score, median (range) | 7 (5–9) | N/A | N/A |
| Male, N (%) | 11 (55) | 8 (34.8) | 0.21 |
| Age at assessment, years ± SD | 16.0 ± 0.03 | 16.2 ± 0.2 | 0.069 |
| Age at scan, years ± SD | 17.8 ± 1.3 | 16.6 ± 1.2 | 0.003 |
| Right-handed, N (%) | 16 (80) | 22 (95.7) | 0.17 |
| Non-white, N (%) | 14 (70) | 17 (73.9) | 0.77 |
| Maternal education less than high school, N (%) | 3 (15) | 1 (4.3) | 0.32 |
The assessments of neonatal health status and neurologic outcome of the very preterm participants have been previously reported (Peterson et al. 2000). Blinded assessment of intelligence was performed using the Wechsler Intelligence Scale for Children-III (WISC). Participants also received the Peabody Picture Vocabulary Test-Revised (PPVT).
Imaging Parameters
Participants were scanned in a Siemens 1.5T Sonata scanner and instructed to keep their eyes open, not to think of anything in particular, and not to fall asleep. No fixation cross was used. Imaging was performed at a later date from the cognitive assessments and as such for the imaging data the subjects were not explicitly matched for age. After a first localizing scan, a high-resolution 3D volume was collected using a magnetization prepared rapid gradient echo (MPRAGE) sequence (155 contiguous sagittal slices, slice thickness 1.2 mm, matrix size = 256 × 256, field of view = 300 mm, TR = 2400 ms, TE = 2.26 ms, flip angle = 45°). Next, a T1-weighted anatomical scan (TR = 420 ms, TE = 11 ms, matrix size = 256 × 256, field of view = 200 mm, thickness = 6 mm thick, gap = 1 mm) was collected with 20 AC–PC aligned axial-oblique slices. After these structural images, acquisition of the resting-state functional connectivity data began in the same slice locations as the axial-oblique T1-weighted data. Functional images were acquired using a T2* sensitive gradient-recalled single shot echo-planar pulse sequence (TR = 2000 ms, TE = 50 ms, flip angle = 80°, bandwidth = 1735 Hz/pixel, matrix size = 64 × 64, field of view = 200 mm, interleaved acquisition). Functional runs consisted of 144 volumes (∼5 min scan length) with the first 4 volumes discarded to allow the magnetization to reach the steady-state and 3 resting runs for each participant were collected.
Preprocessing
Images were slice-time corrected using sinc interpolation and motion corrected using SPM5 (http://www.fil.ion.ucl.ac.uk/spm/software/spm5/). All further analysis was performed using BioImage Suite (Joshi et al. 2011) unless otherwise specified. Several covariates of no interest were regressed from the data including linear and quadratic drift, 6 rigid-body motion parameters, mean cerebral spinal fluid (CSF) signal, mean white matter signal, and overall global signal. The white matter and CSF areas were defined on a template brain (Holmes et al. 1998), eroded to ensure only white matter or CSF signal would be included, and warped to individual-participant space using a series of transformations described below. Finally, the data were temporally smoothed with a zero mean unit variance Gaussian filter (approximate cutoff frequency = 0.12 Hz).
For each participant, all preprocessed resting-state runs were concatenated and the connectivity for each voxel was then calculated in each participant's individual space. First, a gray matter mask was applied to the data so that only voxels in the gray matter were used in the calculation. The gray matter mask was defined on a template brain (Holmes et al. 1998), dilated to ensure full coverage of the gray matter, and warped to individual participant space using a series of transformations described below. Because the removal of the global mean makes the signs of the correlation ambiguous (Buckner et al. 2009), only the positive correlations were used in functional connectivity estimate.
Functional Connectivity Analysis
Connectivity Lateralization
To explore connectivity lateralization (Zuo et al. 2010; Tomasi and Volkow 2012), we developed a new measure labeled cross-hemisphere Intrinsic Connectivity Distribution (ch-ICD). Similar to most voxel-based functional connectivity measures, ch-ICD involves correlating the time course for a voxel x with every other time course in the grey matter and then summarizing these correlations using network theory. However, ch-ICD separates the contribution of each hemisphere to this summary statistic allowing for the interrogation of laterality of a voxel's connectivity (Fig. 1). For each voxel, ch-ICD independently measures connectivity to within the same hemisphere (ipsilateral) and to the opposite hemisphere (contralateral) and these 2 measures of hemisphere connectivity are then compared: ipsilateral connectivity minus contralateral connectivity. Therefore, ch-ICD probes whether a voxel exhibits greater connectivity to other voxels in the ipsilateral or contralateral hemisphere and measures the lateralization of a voxel's connectivity.
Figure 1.
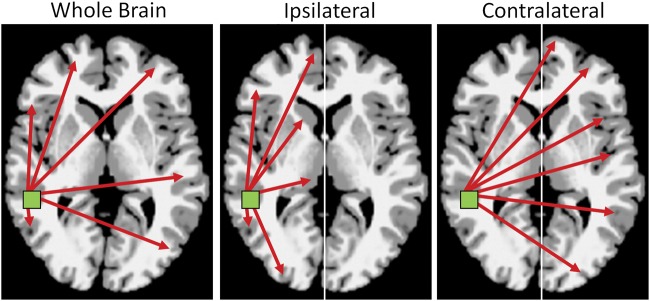
Connectivity lateralization. For any voxel in the brain, ch-ICD describes how connectivity for that voxel is lateralized to a particular hemisphere. Typical metrics of connectivity measure connectivity across the whole brain (bilateral; left panel). ch-ICD independently measures connectivity to within the same hemisphere (ipsilateral; middle panel) and to the opposite hemisphere (contralateral; right panel). These 2 measures of hemisphere connectivity are then compared allowing ch-ICD to measure whether a voxel exhibits greater connectivity to other voxels in the ipsilateral or contralateral hemisphere and, thus, lateralization of connectivity for any voxel.
To calculate ch-ICD, the time course for a voxel x was correlated with the time course for every other voxel in the ipsilateral hemisphere. As previously described (Scheinost et al. 2012), ICD was used to model the distribution of these correlations as a Wiebull distribution. The ICD parameterization efficiently summarizes >10 000 connections to a voxel to a single summary parameter of interest. This procedure was performed for every voxel resulting in a map representing each voxel's connectivity to the same hemisphere, labeled ICD(ipsi). Similarly, a map representing a voxel's connectivity to the contralateral hemisphere was calculated, labeled ICD(contra). Both the ICD(ipsi) and ICD(contra) maps were standardized by converting to Z-scores such that maps across participants could be averaged and compared. This standardization does not change the connectivity patterns but ensures all participants are equally scaled (Buckner et al. 2009). Finally, to detect patterns of between hemisphere connectivity, the ICD(ipsi) and ICD(contra) maps were subtracted creating our ch-ICD metric: ch-ICD = ICD(ipsi) − ICD(contra). For single group ch-ICD maps, a positive ch-ICD value indicates that the connectivity is more lateralized to the ipsilateral hemisphere. A negative ch-ICD value indicates that the connectivity is more lateralized to the contralateral hemisphere. For example, for regions in the left hemisphere, a positive ch-ICD value would indicate connectivity is lateralized to the left hemisphere and, for regions in the right hemisphere, a negative ch-ICD value would indicate connectivity is lateralized to the left hemisphere.
The ch-ICD measure can then be contrasted across groups. Any regions showing significant differences in connectivity lateralization can then be isolated for further investigation in a data driven manner without the need for any a priori ROI information.
Correlation of Connectivity Lateralization and Language
To assess the relationship between language skills and connectivity lateralization (ch-ICD) patterns, we performed a voxel-by-voxel Pearson correlation analysis between ch-ICD and the cognitive outcome measures.
ROI Connectivity
The ch-ICD group analysis described above isolated a specific region in the lateral parietal lobe (the inferior portion of BA 39) showing group differences in connectivity lateralization. Follow-up ROI analysis (similar to Buckner et al. 2009; Constable et al. 2012; Hampson et al. 2012) was performed to explore (post hoc) the nodes which might have altered connectivity for this region. The ROI was defined on the Montreal Neurological Institute (MNI) reference brain as a 9-mm cube centered at the peak voxel and transformed back (via the inverse of the transforms described below) into individual participant space. The time course of the reference region in a given participant was then computed as the average time course across all voxels in the reference region. This time course was correlated with the time course for every other voxel in the gray matter to create a map of r-values, reflecting ROI-to-whole-brain connectivity. These r-values were transformed to z-values using Fisher’'s transform yielding one map for each participant representing the strength of correlation to the seed region.
Motion Analysis
As group differences in motion have been shown to confound functional connectivity results, average frame to frame displacement was calculated for each group (Van Dijk et al. 2012). There were no significant group differences (P > 0.8) in motion.
Imaging Statistics
To facilitate comparisons of imaging data, all single participant ch-ICD results were first spatially smoothed with a 6 mm Gaussian filter and warped to a common template space through the concatenation of a series of linear and non-linear registrations. The functional series were linearly registered to the T1 axial-oblique (2D anatomical) image. The 2D anatomical image was linearly registered to the MPRAGE (3D anatomical) image. The 3D anatomical image was nonlinearly registered to the template brain. All transformation pairs were calculated independently and combined into a single transform warping the single participant results into common space. This single transformation allows the single participant images to be transformed to common space with only one transformation, reducing interpolation error. All transformations were estimated using the intensity-only component of the method implemented by BioImage Suite as previously reported (Papademetris et al. 2004). Significance was assessed at a P < 0.05 level after correcting for multiple comparisons across the grey matter via AFNI's AlphaSim program. Monte Carlo simulation was performed using an estimate of smoothness from the rs-fMRI data via 3dFWHMx, a 10 000 iterations, a nearest neighbor connection radius, and the mask including only grey matter. This produced a cluster threshold of 3750 voxels in 1 mm3 space. All results were also localized in terms of the Brodmann areas (BA) identified using BioImage Suite's digital Brodmann atlas.
Statistics
Demographic and cognitive data were analyzed either using standard χ2 statistics or using Fisher's exact test for categorical data. Continuous-valued data were analyzed either using t-tests or Mann–Whitney U-test when a normal distribution could not be assumed to compare groups. For the purposes of this report, P values < 0.05 were considered significant. All analyses were performed using MATLAB 2011b (MathWorks, Natick, MA).
Results
Participants and Neurodevelopmental Assessment
As shown in Table 1, there were no significant differences between the very preterm and term groups in the number of males, minority status, maternal education, or handedness. While the groups were matched for age for the cognitive assessments, there was a difference between the groups in participant age at the time of scan (P = 0.003). There were no significant differences in the FSIQ or PPVT-R for the groups (Table 2).
Table 2.
Cognitive data
| Preterm | Term | P value | |
|---|---|---|---|
| WISC-III | |||
| Full scale IQ | 97.2 ± 10.0 | 104.3 ± 16.3 | 0.10 |
| Verbal IQ | 100 ± 11.1 | 104 ± 15.1 | 0.32 |
| Performance IQ | 95.1 ± 14.0 | 104.1 ± 17.4 | 0.07 |
| Verbal comprehension | 101.1 ± 11.5 | 104.8 ± 14.7 | 0.37 |
| Vocabulary subtest | 9.9 ± 2.3 | 10.1 ± 3.2 | 0.83 |
| PPVT-R | |||
| PPVT | 101.5 ± 15.5 | 105.3 ± 19.7 | 0.48 |
Connectivity Lateralization Analysis
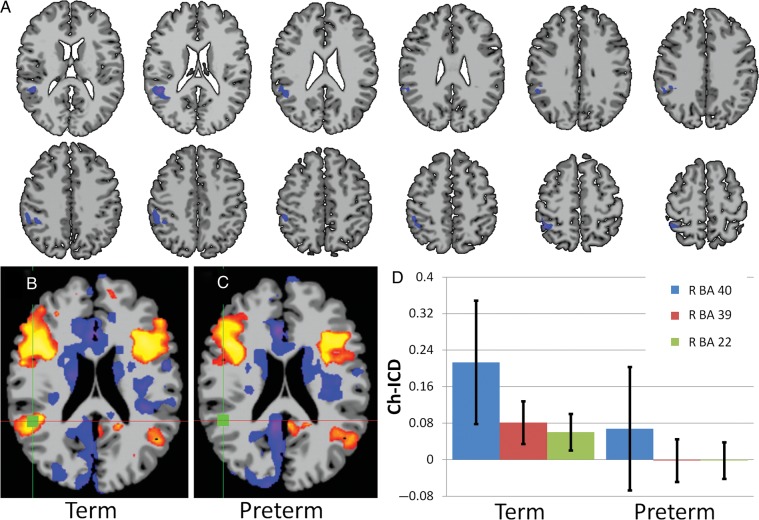
The ch-ICD analysis for this group of participants revealed a single cluster of altered connectivity lateralization in the right temporal–parietal junction for the very preterm participants when compared with term controls (2 sample t-test; P < 0.05 corrected). This region included right BA 22, BA 39, and BA 40, as shown in Figure 2A. Analysis of single group maps (one sample t-test; P < 0.05 corrected) revealed that for term participants this region showed a significantly positive ch-ICD value indicating that connectivity to this region is lateralized to the ipsilateral (right) hemisphere (Fig. 2B,D). However, for the very preterm participants, there was no difference between the connectivity to the ipsilateral and contralateral hemisphere (shown in Fig. 2C,D) suggesting a lack of connectivity lateralization in this region for the very preterm participants. In addition, since there was a significant difference in age at scan for the study groups, we covaried the results by this measure and found no change in the results.
Figure 2.
Group comparison of connectivity lateralization. (A) ch-ICD analysis revealed altered connectivity lateralization in the right temporal–parietal junction for the very preterm participants compared with term controls (P < 0.05 corrected). Single group analysis shows: (B) for term participants, connectivity for this region is significantly lateralized to the ipsilateral hemisphere and (C) for very preterm participants, connectivity for this region is not significantly lateralized to a hemisphere. The green square indicates the region of the maximum t-value from the group difference. (D) Bar graph (mean ± SE) showing the mean ch-ICD values from the 3 regions detected in the group comparison.
Correlation of Connectivity Lateralization and Language
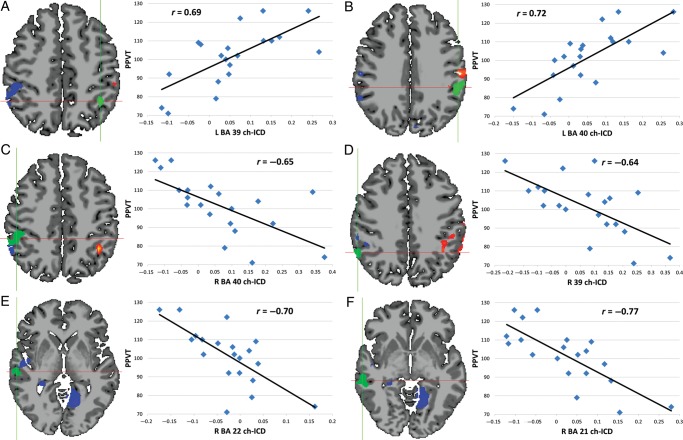
For the very preterm group, several clusters of significant correlation between the PPVT-R scores and ch-ICD were detected in well-known language processing regions and right hemisphere homologs as shown in Figure 3. A cluster of positive correlation between PPVT scores and ch-ICD was observed in the left temporal–parietal junction (left BA 39 and 40; Fig. 3A,B) indicating that as connectivity in these regions is more lateralized to the left hemisphere, a better language score is observed. Clusters of negative correlation between PPVT scores and ch-ICD were observed in the right homologs of several language process areas including right BA 40 (Fig. 3C), BA 39 (Fig. 3D), BA 22 (Fig. 3E), and BA 21 (Fig. 3F). The negative correlation suggests that as connectivity in these regions is less lateralized to the right hemisphere, a better language score is observed. These 6 regions (right and left BA 39 and 40, and right BA 21 and 22) were later used for exploratory ROI analysis with each ROI being defined as the conjunction of the Yale Brodmann Atlas and the significant voxels (P < 0.05 corrected) of the correlation map.
Figure 3.
Correlation of PPVT and ch-ICD for very preterm participants. Clusters of significant correlation (P < 0.05 corrected) between PPVT scores and ch-ICD were detected in several well-known language processing regions and right hemisphere homologs in this data driven voxel-based analysis. (A, B) In the left hemisphere, a positive relationship between connectivity lateralization and language was observed. (C–F) In the right hemisphere, a negative relationship between connectivity lateralization and language was observed. These results suggest an increased importance of left hemisphere connectivity relative to right hemisphere connectivity as a possible response to protect language in preterm participants. Scatterplots show the average ch-ICD value for each region for visualization purposes; significance was calculated in a voxel-wise manner and corrected for multiple comparisons.
Using voxel-wise analysis, no significant correlations were observed in these regions for VIQ, VCI, and vocabulary testing. When significance was reduced (P < 0.1 uncorrected), a similar trend of positive correlation between these measures and ch-ICD was observed in the left hemisphere and negative correlations between these measures and ch-ICD was observed in the right hemisphere.
In contrast, for the term group, no significant correlation between the PPVT scores and ch-ICD was observed using voxel-wise analysis. No significant correlations were observed in the right and left BA 39 and 40, and right BA 21 and 22 regions between VIQ, VCI, and vocabulary testing and ch-ICD for the term group.
Correlation of Laterality
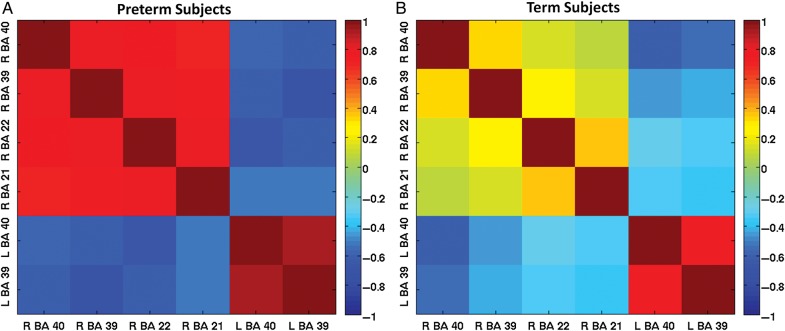
To better understand the different patterns of correlation between ch-ICD values and PPVT scores for the very preterm and term participants at young adulthood, we explored how the average ch-ICD values—for the 6 ROIs based on the correlation between ch-ICD and PPVT-R scores in the very preterm participants; see previous section—were correlated for each group. As shown in Figure 4A (and inferred from Fig. 3), for the very preterm participants, the average ch-ICD value of each ROI showed significant correlation with the other ROIs' average ch-ICD value (P < 0.05 corrected for multiple comparison using false discovery rate correction). For term participants, these correlations are much weaker as shown in Figure 4B. While the ch-ICD values between left BA 39 and BA 40 and right and left BA 40 were significantly correlated (P < 0.05 corrected for multiple comparisons), the remaining pair-wise correlations were not significant for term participants. In contrast to the very preterm participants for whom ROI laterality showed significant network correlation, the laterality of each ROI for the term participants appears largely independent of the others.
Figure 4.
Correlation matrices of connectivity laterality. (A) For the very preterm participants, the average ch-ICD values in the 6 ROIs identified in Figure 2 were highly correlated with each other. (B) For the term participants, the average ch-ICD values in these 6 ROIs were less correlated. For the very preterm participants, these ROIs act together when modulating the laterality of their connectivity; whereas, for the term subjects, these ROIs act more independent from each other.
Exploratory Risk Factor Analyses for the Very Preterm Group
To better understand those environmental factors contributing to cerebral lateralization in the very preterm group, study subjects were categorized into “R lateralized” and “L lateralized” for the right BA 22, BA 39, and BA 40 ROI shown in Figure 2A. Subjects with a positive average ch-ICD value in this region were considered right lateralized while subjects with a negative average ch-ICD value in this region were considered left lateralized. Nine of 20 (45%) very preterm participants were “R lateralized,” compared with 19/23 (83%) term subjects who were “R lateralized” (P = 0.02). In this exploratory analysis there were no significant differences in birth weight, gestational age, handedness, bronchopulmonary dysplasia, necrotizing enterocolitis, retinopathy of prematurity, and testing scores between the very preterm groups; there was, however, a significant difference between 5 min Apgar scores for the 2 groups (P = 0.04) and a trend for more of the “R lateralized” very preterm participants to be males (“R lateralized” vs. “L lateralized”: 7/9 (78%) vs. 4/11 (36.4%), P = 0.09).
ROI Connectivity
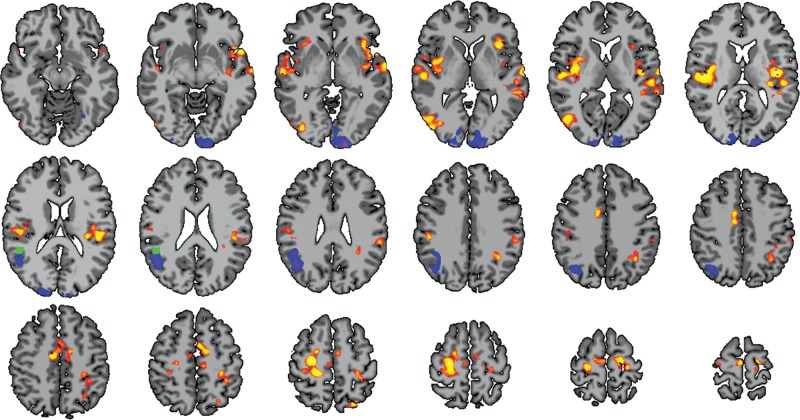
To further investigate the group differences in connectivity lateralization, a conventional ROI connectivity analysis based on this region was performed. When the study groups were compared, ROI connectivity analyses demonstrated several regions of altered connectivity as shown in Figure 5. Strikingly, group comparison revealed a strong reduction in connectivity for very preterm participants to a region immediately next to the ROI. Additionally, bilateral increases to the frontal/temporal lobe (including bilateral BA 21, BA 22, BA 40, primary auditory, and insular cortex), increases to motor circuitry (including primary and supplementary motor areas), and decreases to mainly left visual processing areas were observed.
Figure 5.
Follow-up ROI connectivity analysis. Using the region of group difference detected by ch-ICD (green cube; see also Fig. 2A), very preterm participants showed several regions of significant connectivity when compared with term subjects (P < 0.05 corrected). Striking decreases in connectivity are observed in immediately surrounding structures to the ROI. Additionally, bilateral increases were seen in the frontal and temporal lobes.
Discussion
Employing a novel, voxel-based measure of functional brain organization, these data demonstrate for the first time that cerebral lateralization of functional connectivity in right hemisphere language homologs is altered for very preterm participants. Very preterm participants with no evidence for severe brain injury exhibited a significant decrease in right hemisphere lateralization in the right parietal and temporal lobes. For the very preterm participants but not the term controls, as connectivity was increasingly lateralized to the left hemisphere in the left angular gyrus, and inferior parietal lobule, language scores increased and the brains were more like those of term controls; in contrast, greater lateralization to the right hemisphere homologs resulted in decreasing PPVT scores. Similarly, lateralization values for these 6 temporal–parietal regions were highly correlated with each other for the very preterm participants but not the term controls, suggesting the engagement of a previously unreported bi-hemispheric network for language in very preterm participants at young adulthood. The right angular gyrus appears to be the functional hub of this network, and a follow-up ROI analysis demonstrates not only significant decreases in local connectivity but also marked increases to the left frontal and temporal language region when the very preterm participants were compared with term controls, consistent with the alterations in lateralization we report.
Studies of fetuses during the third trimester of gestation and healthy preterm infants from 29 to 43 weeks postmenstrual age (i.e., the ex utero third trimester of gestation) demonstrate both ROI-based functional connectivity and resting-state auditory networks mimicking those found in typically developing older children and adults (Fransson et al. 2009; Damaraju et al. 2010; Doria et al. 2010; Smyser et al. 2010; Fransson et al. 2011; Perani et al. 2011). Preterm birth impairs neurogenesis and microstructural connectivity in both preclinical models and human neonates (Vaccarino et al. 2007; Scafidi et al. 2009; Salmaso et al. 2012; Malik et al. 2013), and the relationship between putative changes in neuronal populations, oligogenesis and the onset of functional connectivity in the preterm brain has yet to be explored. Notably, high neonatal stressor scores and procedural pain have been shown to impede the development of both microstructural and interhemispheric functional connectivity in healthy preterm neonates (Smith et al. 2011; Brummelte et al. 2012), suggesting a significant impact of the environment on the genome. To the best of our knowledge, however, none of these studies examined cerebral lateralization of connectivity in the preterm brain.
Similarly, ROI-based functional connectivity studies of prematurely born older children and young adults demonstrate the persistence of right hemisphere language systems (Gozzo et al. 2009; Myers and Ment 2009; Myers et al. 2010; Friederici et al. 2011). Since the preterm participants commonly have verbal testing scores similar to the term control subjects with whom they are compared, it is uncertain whether the engagement of these right hemisphere language homologs represents a delay in cerebral maturation or the presence of auxiliary circuits in those preterms with the greatest cognitive need. In children at the age of 6 years, left hemisphere language areas in the inferior frontal and temporal lobes showed stronger connectivity to right hemisphere homologs when compared with adults suggesting that the disengagement of these connections is part of the maturation from childhood to adulthood (Friederici et al. 2011).
The specification of the left hemisphere for language is believed to be present before the neonate is fully exposed to language (Thomason et al. 2013), suggesting that the processing of information relevant for language is genetically determined. The left–right asymmetry of the developing human brain is reportedly mediated by the significant early transcriptional asymmetry of gene expression including FGFR3, ID2, NUMB, and NEUROD6 (Sun et al. 2005; Johnson et al. 2009; Pinel et al. 2012). Of those described, at least one candidate, Lim Domain Only 4 (LMO4), is more highly expressed in the right perisylvian region than the left during the second trimester of gestation, suggesting a role in language lateralization (Sun et al. 2005). In addition, atypical patterns of both structural and functional cortical asymmetries have been reported in children with both ADHD and dyslexia, although these require further replication (Shaw et al. 2009; Renteria 2012). Finally, although speculative, environment perturbations such as hypoxia and infection, common events in preterm neonates, may alter neurogenesis, oligogenesis and ultimately neural connectivity (Vaccarino and Ment 2004).
While we hypothesize that alterations in the lateralization of connectivity is driven by disruption of early development, it is not yet known how speech and language therapies may modulate lateralization and if critical time points for exposure to interventions exist. Very preterm participants with the least lateralization in the right hemisphere and with stronger lateralization in both the right and left hemispheres to the left hemisphere performed the best on language assessments. These results suggest that therapies that strengthen this altered network, rather than normalizing it, could result in the greatest gain. Early intervention has been shown to improve testing scores and fractional anisotropy in both preclinical models for neonatal brain injury (He et al. 2010; Salmaso et al. 2012) and preterm neonates (Als et al. 2004; Milgrom et al. 2010), but the influence of early intervention on connectivity data remain largely unknown. We hypothesize that early intervention services would be advantageous to the prematurely born, and future studies could monitor how interventions change connectivity over time.
The limitations of this study include the small sample size, the lack of voxel-based morphometric data for the cohort, and the failure to collect serial functional connectivity lateralization studies over time. The subjects we report are part of a well-studied group of young adults with neuroimaging data available from the 6th postnatal hour. Although MRI was not readily available in the newborn period for these subjects, sequential MRI studies and neurodevelopmental assessments performed between ages 8 and 16 years demonstrate unremarkable structural MRI studies and normal cognitive development over time for the preterm group as shown in previous reports (Gozzo et al. 2009; Schafer et al. 2009; Myers et al. 2010). The strengths of this study include an innovative voxel-based method, ch-ICD, for assessing cerebral lateralization using resting-state fMRI. The advantage of ch-ICD is that cerebral lateralization of connectivity can be probed without the requirement to predefine ROI.
Almost 2 decades after they were born, prematurely born children exhibit fundamental alterations in the cerebral lateralization for language that significantly correlate with language scores. Cerebral asymmetry is both plastic and experiential, and the underlying environmental factors responsible for these changes remain largely unexplored. If the mandate of neonatologists, neurologists and neuroscientists alike is to improve the neurodevelopmental outcome of premature neonates (Bauer and Msall 2010), then future studies must interrogate those factors contributing to corticogenesis in the prematurely born.
Funding
This work was supported by the National Institutes of Health (R01 NS 027116, R01 NS052344, R01 EB00966, R03 EB012969).
Notes
We thank Drs Deborah Hirtz and Walter Allan for their scientific expertise; Marjorene Ainley for follow-up coordination; Jill Maller-Kesselman and Victoria Watson for neurodevelopmental testing; and Hedy Sarofin and Terry Hickey for technical assistance. Conflict of Interest: None declared.
References
- Als H, Duffy FH, McAnulty GB, Rivkin MJ, Vajapevam S, Mulkern RV, Warfield SK, Huppi PS, Butler SC, Conneman N, et al. 2004. Early experience alters brain function and structure. Pediatrics. 114:1738–1739. [DOI] [PubMed] [Google Scholar]
- Bauer SC, Msall ME. 2010. Optimizing neurodevelopmental outcomes after prematurity: lessons in neuroprotection and early intervention. Minerva Pediatr. 62:485–497. [PubMed] [Google Scholar]
- Brummelte S, Grunau RE, Chau V, Poskitt KJ, Brant R, Vinall J, Gover A, Synnes AR, Miller SP. 2012. Procedural pain and brain development in premature newborns. Ann Neurol. 71:385–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews-Hanna JR, Sperling RA, Johnson KA. 2009. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci. 29:1860–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constable RT, Vohr BR, Scheinost D, Benjamin JR, Fulbright RK, Lacadie C, Schneider KC, Katz KH, Zhang H, Papademetris X, et al. 2012. A left cerebellar pathway mediates language in prematurely-born young adults. NeuroImage. 64C:371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaraju E, Phillips JR, Lowe JR, Ohls R, Calhoun VD, Caprihan A. 2010. Resting-state functional connectivity differences in premature children. Front Syst Neurosci. 4:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene-Lambertz G. 2000. Cerebral specialization for speech and non-speech stimuli in infants. J Cogn Neurosci. 12:449–460. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Hertz-Pannier L, Dubois J. 2006. Nature and nurture in language acquisition: anatomical and functional brain-imaging studies in infants. Trends Neurosci. 29:367–373. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Hertz-Pannier L, Dubois J, Meriaux S, Roche A, Sigman M, Dehaene S. 2006. Functional organization of perisylvian activation during presentation of sentences in preverbal infants. Proc Natl Acad Sci USA. 103:14240–14245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doria V, Beckmann CF, Arichi T, Merchant N, Groppo M, Turkheimer FE, Counsell SJ, Murgasova M, Aljabar P, Nunes RG, et al. 2010. Emergence of resting state networks in the preterm human brain. Proc Natl Acad Sci USA. 107:20015–20020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J, Benders M, Lazeyras F, Borradori-Tolsa C, Leuchter RH, Mangin JF, Huppi PS. 2010. Structural asymmetries of perisylvian regions in the preterm newborn. NeuroImage. 52:32–42. [DOI] [PubMed] [Google Scholar]
- Dubois J, Hertz-Pannier L, Cachia A, Mangin JF, Le Bihan D, Dehaene-Lambertz G. 2009. Structural asymmetries in the infant language and sensori-motor networks. Cereb Cortex. 19:414–423. [DOI] [PubMed] [Google Scholar]
- Fransson P, Aden U, Blennow M, Lagercrantz H. 2011. The functional architecture of the infant brain as revealed by resting-state fMRI. Cereb Cortex. 21:145–154. [DOI] [PubMed] [Google Scholar]
- Fransson P, Skiold B, Engstrom M, Hallberg B, Mosskin M, Aden U, Lagercrantz H, Blennow M. 2009. Spontaneous brain activity in the newborn brain during natural sleep—an fMRI study in infants born at full term. Pediatr Res. 66:301–305. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Brauer J, Lohmann G. 2011. Maturation of the language network: from inter- to intrahemispheric connectivities. PLoS One. 6:e20726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozzo Y, Vohr B, Lacadie C, Hampson M, Katz KH, Maller-Kesselman J, Schneider KC, Peterson BS, Rajeevan N, Makuch RW, et al. 2009. Alterations in neural connectivity in preterm children at school age. NeuroImage. 48:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas PA, Scott JA, Roosta A, Rajagopalan V, Kim K, Rousseau F, Barkovich AJ, Glenn OA, Studholme C. 2012. Early folding patterns and asymmetries of the normal human brain detected from in utero MRI. Cereb Cortex. 22:13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack M. 2009. Adult outcomes of preterm children. J Dev Behav Pediatr. 30:460–470. [DOI] [PubMed] [Google Scholar]
- Hampson M, Tokoglu F, Shen X, Scheinost D, Papademetris X, Constable RT. 2012. Intrinsic brain connectivity related to age in young and middle aged adults. PLoS One. 7:e44067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Ma J, Liu N, Yu X. 2010. Early enriched environment promotes neonatal GABAergic neurotransmission and accelerates synapse maturation. J Neurosci. 30:7910–7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. 1998. Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr. 22:324–333. [DOI] [PubMed] [Google Scholar]
- Johnson MB, Kawasawa YI, Mason CE, Krsnik Z, Coppola G, Bogdanovic D, Geschwind DH, Mane SM, State MW, Sestan N. 2009. Functional and evolutionary insights into human brain development through global transcriptome analysis. Neuron. 62:494–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi A, Scheinost D, Okuda H, Belhachemi D, Murphy I, Staib LH, Papademetris X. 2011. Unified framework for development, deployment and robust testing of neuroimaging algorithms. Neuroinformatics. 9:69–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Baleriaux D, Kavec M, Metens T, Absil J, Denolin V, Pardou A, Avni F, Van Bogaert P, Aeby A. 2010. Structural asymmetries in motor and language networks in a population of healthy preterm neonates at term equivalent age: a diffusion tensor imaging and probabilistic tractography study. NeuroImage. 51:783–788. [DOI] [PubMed] [Google Scholar]
- Malik S, Vinukonda G, Vose LR, Diamond D, Bhimavarapu BB, Hu F, Zia MT, Hevner R, Zecevic N, Ballabh P. 2013. Neurogenesis continues in the third trimester of pregnancy and is suppressed by premature birth. J Neurosci. 33:411–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur AM, Neil JJ, Inder TE. 2010. Understanding brain injury and neurodevelopmental disabilities in the preterm infant: the evolving role of advanced magnetic resonance imaging. Semin Perinatol. 34:57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ment LR, Ehrenkranz RA, Duncan CC, Scott DT, Taylor KJW, Katz KH, Schneider KC, Makuch RW, Oh W, Vohr B, et al. 1994. Low-dose indomethacin and prevention of intraventricular hemorrhage: a multicenter randomized trial. Pediatrics. 93:543–550. [PubMed] [Google Scholar]
- Ment LR, Hirtz D, Huppi PS. 2009. Microstructural markers of outcome in developing brain. Lancet Neurol. 8:1042–1055. [DOI] [PubMed] [Google Scholar]
- Milgrom J, Newnham C, Anderson PJ, Doyle LW, Gemmill AW, Lee K, Hunt RW, Bear M, Inder T. 2010. Early sensitivity training for parents of preterm infants: impact on the developing brain. Pediatr Res. 67:330–335. [DOI] [PubMed] [Google Scholar]
- Myers E, Ment LR. 2009. Long-term outcome of preterm infants and the role of neuroimaging. Clin Perinatol. 36:773–789, vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers EH, Hampson M, Vohr B, Lacadie C, Frost SJ, Pugh KR, Katz KH, Schneider KC, Makuch RW, Constable RT, et al. 2010. Functional connectivity to a right hemisphere language center in prematurely born adolescents. NeuroImage. 51:1445–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narberhaus A, Lawrence E, Allin MP, Walshe M, McGuire P, Rifkin L, Murray R, Nosarti C. 2009. Neural substrates of visual paired associates in young adults with a history of very preterm birth: alterations in fronto-parieto-occipital networks and caudate nucleus. NeuroImage. 47:1884–1893. [DOI] [PubMed] [Google Scholar]
- Nosarti C, Giouroukou E, Healy E, Rifkin L, Walshe M, Reichenberg A, Chitnis X, Williams SCR, Murray RM. 2008. Grey and white matter distribution in very preterm adolescents mediates neurodevelopmental outcome. Brain. 181:205–217. [DOI] [PubMed] [Google Scholar]
- Nosarti C, Sukhwinder S, Shergill S, Allin MP, Walshe M, Rifkin L, Murray RM, McGuire PK. 2009. Neural substrates of letter fluency processing in young adults who were born very preterm: alterations in frontal and striatal regions. NeuroImage. 47:1904–1913. [DOI] [PubMed] [Google Scholar]
- Papademetris X, Jackowski A, Schultz R, Staib L, Duncan J, Barillot C, Haynor D, Hellier P. 2004. Integrated Intensity and Point-Feature Nonrigid Registration. In. Medical Image Computing and Computer-Assisted Intervention—MICCAI 2004. Springer, Berlin/Heidelberg, p 763–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena M, Maki A, Kovacic D, Dehaene-Lambertz G, Koizumi H, Bouquet F, Mehler J. 2003. Sounds and silence: an optical topography study of language recognition at birth. Proc Natl Acad Sci USA. 100:11702–11705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perani D, Saccuman MC, Scifo P, Anwander A, Spada D, Baldoli C, Poloniato A, Lohmann G, Friederici AD. 2011. Neural language networks at birth. Proc Natl Acad Sci USA. 108:16056–16061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BS, Vohr B, Staib LH, Cannistraci CJ, Dolberg A, Schneider KC, Katz KH, Westerveld M, Sparrow S, Anderson AW, et al. 2000. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA. 284:1939–1947. [DOI] [PubMed] [Google Scholar]
- Pinel P, Fauchereau F, Moreno A, Barbot A, Lathrop M, Zelenika D, Le Bihan D, Poline JB, Bourgeron T, Dehaene S. 2012. Genetic variants of FOXP2 and KIAA0319/TTRAP/THEM2 locus are associated with altered brain activation in distinct language-related regions. J Neurosci. 32:817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Fair DA, Schlaggar BL, Petersen SE. 2010. The development of human functional brain networks. Neuron. 67:735–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renteria ME. 2012. Cerebral asymmetry: a quantitative, multifactorial, and plastic brain phenotype. Twin Res Hum Genet. 15:401–413. [DOI] [PubMed] [Google Scholar]
- Saigal S, Doyle LW. 2008. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 371:261–269. [DOI] [PubMed] [Google Scholar]
- Salmaso N, Silbereis J, Komitova M, Mitchell P, Chapman K, Ment LR, Schwartz ML, Vaccarino FM. 2012. Environmental enrichment increases the GFAP+ stem cell pool and reverses hypoxia-induced cognitive deficits in juvenile mice. J Neurosci. 32:8930–8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scafidi J, Fagel DM, Ment LR, Vaccarino FM. 2009. Modeling premature brain injury and recovery. Int J Dev Neurosci. 27:863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer RJ, Lacadie C, Vohr B, Kesler SR, Katz KH, Schneider KC, Pugh KR, Makuch RW, Reiss AL, Constable RT, et al. 2009. Alterations in functional connectivity for language in prematurely-born adolescents. Brain. 132:661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinost D, Benjamin J, Lacadie CM, Vohr B, Schneider KC, Ment LR, Papademetris X, Constable RT. 2012. The intrinsic connectivity distribution: a novel contrast measure reflecting voxel level functional connectivity. NeuroImage. 62:1510–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. 2007. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML, Huppi PS. 2010. The role of functional magnetic resonance imaging in the study of brain development, injury, and recovery in the newborn. Semin Perinatol. 34:79–86. [DOI] [PubMed] [Google Scholar]
- Shaw P, Lalonde F, Lepage C, Rabin C, Eckstrand K, Sharp W, Greenstein D, Evans A, Giedd JN, Rapoport J. 2009. Development of cortical asymmetry in typically developing children and its disruption in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 66:888–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GC, Gutovich J, Smyser C, Pineda R, Newnham C, Tjoeng TH, Vavasseur C, Wallendorf M, Neil J, Inder T. 2011. Neonatal intensive care unit stress is associated with brain development in preterm infants. Ann Neurol. 70:541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser CD, Inder TE, Shimony JS, Hill JE, Degnan AJ, Snyder AZ, Neil JJ. 2010. Longitudinal analysis of neural network development in preterm infants. Cereb Cortex. 20:2852–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, Patoine C, Abu-Khalil A, Visvader J, Sum E, Cherry TJ, Orkin SH, Geschwind DH, Walsh CA. 2005. Early asymmetry of gene transcription in embryonic human left and right cerebral cortex. Science. 308:1794–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason ME, Dassanayake MT, Shen S, Katkuri Y, Alexis M, Anderson AL, Yeo L, Mody S, Hernandez-Andrade E, Hassan SS, et al. 2013. Cross-hemispheric functional connectivity in the human fetal brain. Sci Transl Med. 5:173ra124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. 2012. Laterality patterns of brain functional connectivity: gender effects. Cereb Cortex. 22:1455–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino FM, Fagel DM, Ganat YM, Maragnoli ME, Ment LR, Ohkubo Y, Schwartz ML, Silbereis J, Smith KM. 2007. Astroglial cells in development, regeneration and repair. Neuroscientist. 13:173–185. [DOI] [PubMed] [Google Scholar]
- Vaccarino FM, Ment LR. 2004. Injury and repair in developing brain. Arch Dis Child Fetal Neonatal Ed. 89:F190–F192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Stam CJ, Kahn RS, Hulshoff Pol HE. 2009. Efficiency of functional brain networks and intellectual performance. J Neurosci. 29:7619–7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL. 2012. The influence of head motion on intrinsic functional connectivity MRI. NeuroImage. 59:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo XN, Kelly C, Di Martino A, Mennes M, Margulies DS, Bangaru S, Grzadzinski R, Evans AC, Zang YF, Castellanos FX, et al. 2010. Growing together and growing apart: regional and sex differences in the lifespan developmental trajectories of functional homotopy. J Neurosci. 30:15034–15043. [DOI] [PMC free article] [PubMed] [Google Scholar]