Abstract
The crystal structure of the title compound, [YbCl2(H2O)6]Cl, was determined at 110 K. Samples were obtained from evaporated acetonitrile solutions containing the title compound, which consists of a [YbCl2(H2O)6]+ cation and a Cl− anion. The cations in the title compound sit on a twofold axis and form O—H⋯Cl hydrogen bonds with the nearby Cl− anion. The coordination geometry around the metal centre forms a distorted square antiprism. The ytterbium complex is isotypic with the europium complex [Tambrornino et al. (2014 ▸). Acta Cryst. E70, i27].
Keywords: crystal structure, ytterbium(III), chloride, hydrogen bonding
Related literature
The ytterbium complex is isotypic with the europium complex, the redetermined structure of which was published recently (Tambrornino et al. 2014 ▸) which was in turn similar to studies of other lanthanoid chloride hydrates (Marezio et al., 1961 ▸).
Experimental
Crystal data
[YbCl2(H2O)6]Cl
M r = 387.49
Monoclinic,

a = 7.8158 (11) Å
b = 6.4651 (3) Å
c = 12.7250 (18) Å
β = 131.45 (2)°
V = 481.92 (16) Å3
Z = 2
Mo Kα radiation
μ = 10.52 mm−1
T = 110 K
0.24 × 0.18 × 0.17 mm
Data collection
Oxford Diffraction Xcalibur Sapphire3 diffractometer
Absorption correction: multi-scan (CrysAlis PRO; Oxford Diffraction, 2009 ▸) T min = 0.187, T max = 0.268
12358 measured reflections
1806 independent reflections
1762 reflections with I > 2σ(I)
R int = 0.042
Refinement
R[F 2 > 2σ(F 2)] = 0.018
wR(F 2) = 0.041
S = 1.12
1806 reflections
51 parameters
H-atom parameters constrained
Δρmax = 0.91 e Å−3
Δρmin = −0.96 e Å−3
Data collection: CrysAlis CCD (Oxford Diffraction, 2009 ▸); cell refinement: CrysAlis RED (Oxford Diffraction, 2009 ▸); data reduction: CrysAlis RED; program(s) used to solve structure: SHELXS2014 (Sheldrick, 2008 ▸); program(s) used to refine structure: SHELXL2014 (Sheldrick, 2015 ▸); molecular graphics: PLATON (Spek, 2009 ▸) and ORTEP-3 for Windows (Farrugia, 2012 ▸); software used to prepare material for publication: SHELXL2014.
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989015008488/br2249sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989015008488/br2249Isup2.hkl
. DOI: 10.1107/S2056989015008488/br2249fig1.tif
A view of the title compound (Farrugia, 2012). Displacement ellipsoids are drawn at the 50% probability level. H atoms have been omitted for clarity.
CCDC reference: 1062504
Additional supporting information: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (, ).
| DHA | DH | HA | D A | DHA |
|---|---|---|---|---|
| O1H1ACl2i | 0.91 | 2.37 | 3.2499(19) | 163 |
| O1H1BCl1ii | 0.91 | 2.50 | 3.171(2) | 131 |
| O2H2ACl1iii | 0.87 | 2.36 | 3.1460(18) | 150 |
| O2H2BCl2iv | 0.87 | 2.38 | 3.1806(18) | 154 |
| O3H3ACl2v | 0.88 | 2.33 | 3.179(2) | 163 |
| O3H3BCl1vi | 0.88 | 2.48 | 3.1758(19) | 136 |
Symmetry codes: (i)  ; (ii)
; (ii) 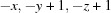 ; (iii)
; (iii)  ; (iv)
; (iv)  ; (v)
; (v)  ; (vi)
; (vi) 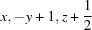 .
.
Acknowledgments
This research was also funded in part by a CCSU–AAUP research grant and CCSU Faculty–Student Research Grants.
supplementary crystallographic information
S1. Comment
Samples gathered from the mother liquor were coated with mineral oil prior to mounting to reduce sample decay. The ytterbium complex is isomorphous with a recently published redetermination of a europium complex (Tambrornino, et al. 2014) which was in turn similar to studies of other lanthanoid chloride hydrates (Marezio, et al. 1961).
Crystals of ytterbium(III) chloride hexahydrate consist of a [YbCl2(H2O)6]1+ cation and a chlorine anion. The covalent nature of the lanthanoid +3 cations are not surprising given their high charge density. An ORTEP of the title compound is shown in Fig. 1.
S2. Experimental
In 40.0 ml of acetonitrile, 0.1945 grams (0.5019 mmol) of ytterbium(III) chloride hexahydrate was added to 0.1000 grams (0.5020 mmol) of di-2-pyridyl ketone oxime (dpko) with the hopes of synthesizing a Yb-dpko complex. The mixture was heated to dissolve the solids. Upon cooling and subsequent evaporation, small colorless crystals of the title compound were isolated. The metal chloride was purchased from Strem chemicals (99.9% purity) whereas the dpko was purchased from Sigma-Aldrich (99.9%). Both were used without additional purification.
S3. Refinement
H atoms were included and were allowed to refine to ideal O—H distances based upon geometric considerations. Thermal parameters for all H atoms were included in the refinement in riding motion approximation with Uiso = 1.5Ueq of the carrier atom.
Figures
Fig. 1.

A view of the title compound (Farrugia, 2012). Displacement ellipsoids are drawn at the 50% probability level. H atoms have been omitted for clarity.
Crystal data
| [YbCl2(H2O)6]Cl | Dx = 2.671 Mg m−3 |
| Mr = 387.49 | Melting point: 350 K |
| Monoclinic, P2/c | Mo Kα radiation, λ = 0.71073 Å |
| a = 7.8158 (11) Å | Cell parameters from 7486 reflections |
| b = 6.4651 (3) Å | θ = 4.9–33.8° |
| c = 12.7250 (18) Å | µ = 10.52 mm−1 |
| β = 131.45 (2)° | T = 110 K |
| V = 481.92 (16) Å3 | Block, light pink |
| Z = 2 | 0.24 × 0.18 × 0.17 mm |
| F(000) = 362 |
Data collection
| Oxford Diffraction Xcalibur Sapphire3 diffractometer | 1806 independent reflections |
| Radiation source: fine-focus sealed tube | 1762 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.042 |
| Detector resolution: 16.1790 pixels mm-1 | θmax = 33.7°, θmin = 4.3° |
| ω scans | h = −11→12 |
| Absorption correction: multi-scan (CrysAlis PRO; Oxford Diffraction, 2009) | k = −9→9 |
| Tmin = 0.187, Tmax = 0.268 | l = −19→19 |
| 12358 measured reflections |
Refinement
| Refinement on F2 | Hydrogen site location: difference Fourier map |
| Least-squares matrix: full | H-atom parameters constrained |
| R[F2 > 2σ(F2)] = 0.018 | w = 1/[σ2(Fo2) + (0.0207P)2] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.041 | (Δ/σ)max = 0.001 |
| S = 1.12 | Δρmax = 0.91 e Å−3 |
| 1806 reflections | Δρmin = −0.96 e Å−3 |
| 51 parameters | Extinction correction: SHELXL2014 (Sheldrick, 2015), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| 0 restraints | Extinction coefficient: 0.0602 (13) |
Special details
| Experimental. Sample was covered in mineral oil prior to mounting in cryo stream.Hydrogen atoms were included and were allowed to refine to ideal O—H distances based upon geometric considerations. Thermal parameters for all H atoms were included in the refinement in riding motion approximation with Uiso = 1.5Ueq of the carrier atom.CrysAlisPro, Oxford Diffraction Ltd., Version 1.171.33.52 (release 06-11-2009 CrysAlis171 .NET) Empirical absorption correction using spherical harmonics, implemented in SCALE3 ABSPACK scaling algorithm (Oxford Diffraction (2009). |
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Yb1 | 0.5000 | 0.65776 (2) | 0.7500 | 0.01599 (6) | |
| Cl1 | 0.32165 (12) | 0.34336 (8) | 0.56141 (7) | 0.02594 (12) | |
| Cl2 | 0.0000 | −0.12652 (14) | 0.2500 | 0.02883 (16) | |
| O1 | 0.1817 (3) | 0.5542 (3) | 0.71889 (19) | 0.0266 (3) | |
| H1A | 0.1626 | 0.4279 | 0.7416 | 0.040* | |
| H1B | 0.0569 | 0.6336 | 0.6823 | 0.040* | |
| O2 | 0.7626 (3) | 0.9242 (3) | 0.85341 (19) | 0.0276 (3) | |
| H2A | 0.7791 | 1.0181 | 0.9087 | 0.041* | |
| H2B | 0.8611 | 0.9467 | 0.8434 | 0.041* | |
| O3 | 0.5420 (3) | 0.8002 (3) | 0.93497 (19) | 0.0273 (3) | |
| H3A | 0.6709 | 0.8455 | 1.0145 | 0.041* | |
| H3B | 0.4315 | 0.8170 | 0.9361 | 0.041* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Yb1 | 0.01594 (8) | 0.01718 (8) | 0.01703 (8) | 0.000 | 0.01184 (6) | 0.000 |
| Cl1 | 0.0283 (3) | 0.0258 (3) | 0.0251 (3) | −0.00427 (18) | 0.0183 (2) | −0.00488 (18) |
| Cl2 | 0.0271 (4) | 0.0336 (4) | 0.0299 (4) | 0.000 | 0.0206 (4) | 0.000 |
| O1 | 0.0229 (8) | 0.0278 (8) | 0.0343 (9) | −0.0001 (6) | 0.0212 (8) | 0.0043 (7) |
| O2 | 0.0293 (9) | 0.0262 (8) | 0.0359 (9) | −0.0096 (7) | 0.0253 (8) | −0.0098 (7) |
| O3 | 0.0310 (9) | 0.0331 (8) | 0.0246 (8) | −0.0050 (7) | 0.0213 (8) | −0.0061 (7) |
Geometric parameters (Å, º)
| Yb1—O2i | 2.3101 (17) | Yb1—O1i | 2.3433 (16) |
| Yb1—O2 | 2.3101 (17) | Yb1—O1 | 2.3434 (17) |
| Yb1—O3i | 2.3392 (17) | Yb1—Cl1i | 2.7211 (7) |
| Yb1—O3 | 2.3392 (17) | Yb1—Cl1 | 2.7212 (7) |
| O2i—Yb1—O2 | 83.56 (10) | O1i—Yb1—Cl1i | 76.75 (5) |
| O2i—Yb1—O3i | 69.72 (6) | O1—Yb1—Cl1i | 78.60 (5) |
| O2—Yb1—O3i | 76.09 (7) | O2i—Yb1—Cl1 | 108.14 (6) |
| O2i—Yb1—O3 | 76.09 (7) | O2—Yb1—Cl1 | 143.38 (5) |
| O2—Yb1—O3 | 69.72 (6) | O3i—Yb1—Cl1 | 75.99 (5) |
| O3i—Yb1—O3 | 133.64 (9) | O3—Yb1—Cl1 | 146.11 (5) |
| O2i—Yb1—O1i | 138.85 (6) | O1i—Yb1—Cl1 | 78.60 (5) |
| O2—Yb1—O1i | 70.92 (6) | O1—Yb1—Cl1 | 76.75 (5) |
| O3i—Yb1—O1i | 73.06 (7) | Cl1i—Yb1—Cl1 | 83.34 (3) |
| O3—Yb1—O1i | 121.09 (7) | Yb1—O1—H1A | 125.5 |
| O2i—Yb1—O1 | 70.92 (6) | Yb1—O1—H1B | 125.7 |
| O2—Yb1—O1 | 138.85 (6) | H1A—O1—H1B | 108.8 |
| O3i—Yb1—O1 | 121.09 (7) | Yb1—O2—H2A | 125.4 |
| O3—Yb1—O1 | 73.06 (7) | Yb1—O2—H2B | 125.4 |
| O1i—Yb1—O1 | 146.79 (9) | H2A—O2—H2B | 109.2 |
| O2i—Yb1—Cl1i | 143.38 (5) | Yb1—O3—H3A | 125.4 |
| O2—Yb1—Cl1i | 108.15 (6) | Yb1—O3—H3B | 125.4 |
| O3i—Yb1—Cl1i | 146.11 (5) | H3A—O3—H3B | 109.2 |
| O3—Yb1—Cl1i | 75.99 (5) |
Symmetry code: (i) −x+1, y, −z+3/2.
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O1—H1A···Cl2ii | 0.91 | 2.37 | 3.2499 (19) | 163 |
| O1—H1B···Cl1iii | 0.91 | 2.50 | 3.171 (2) | 131 |
| O2—H2A···Cl1iv | 0.87 | 2.36 | 3.1460 (18) | 150 |
| O2—H2B···Cl2v | 0.87 | 2.38 | 3.1806 (18) | 154 |
| O3—H3A···Cl2vi | 0.88 | 2.33 | 3.179 (2) | 163 |
| O3—H3B···Cl1vii | 0.88 | 2.48 | 3.1758 (19) | 136 |
Symmetry codes: (ii) −x, −y, −z+1; (iii) −x, −y+1, −z+1; (iv) −x+1, y+1, −z+3/2; (v) −x+1, −y+1, −z+1; (vi) x+1, y+1, z+1; (vii) x, −y+1, z+1/2.
Footnotes
Supporting information for this paper is available from the IUCr electronic archives (Reference: BR2249).
References
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
- Marezio, M., Plettinger, H. A. & Zachariasen, W. H. (1961). Acta Cryst. 14, 234–236.
- Oxford Diffraction (2009). CrysAlis CCD,CrysAlis PRO and CrysAlis RED. Oxford Diffraction Ltd, Abington, England.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sheldrick, G. M. (2015). Acta Cryst. C71, 3–8.
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Tambornino, F., Bielec, P. & Hoch, C. (2014). Acta Cryst. E70, i27. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989015008488/br2249sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989015008488/br2249Isup2.hkl
. DOI: 10.1107/S2056989015008488/br2249fig1.tif
A view of the title compound (Farrugia, 2012). Displacement ellipsoids are drawn at the 50% probability level. H atoms have been omitted for clarity.
CCDC reference: 1062504
Additional supporting information: crystallographic information; 3D view; checkCIF report


