Abstract
Irradiation of blood components with ionizing radiation generated by a specific device is recommended to prevent transfusion-associated graft-versus-host disease. However, a linear accelerator can also be used in the absence of such a device, which is the case of the blood bank facility studied herein. In order to evaluate the quality of the irradiated packed red blood cells, this study aimed to determine whether the procedure currently employed in the facility is effective in inhibiting the proliferation of T lymphocytes without damaging blood components.
The proliferation of T lymphocytes, plasma potassium levels, and the degree of hemolysis were evaluated and compared to blood bags that received no irradiation. Packed red blood cell bags were irradiated at a dose of 25 Gy in a linear accelerator. For this purpose, a container was designed to hold the bags and to ensure even distribution of irradiation as evaluated by computed tomography and dose-volume histogram.
Irradiation was observed to inhibit the proliferation of lymphocytes. The percentage of hemolysis in irradiated bags was slightly higher than in non-irradiated bags (p-value >0.05), but it was always less than 0.4% of the red cell mass. Although potassium increased in both groups, it was more pronounced in irradiated red blood cells, especially after seven days of storage, with a linear increase over storage time.
The findings showed that, at an appropriate dosage and under validated conditions, the irradiation of packed red blood cells in a linear accelerator is effective, inhibiting lymphocyte proliferation but without compromising the viability of the red cells.
Keywords: Blood safety, Hemotherapy service, T-lymphocytes
Introduction
Transfusion-associated graft-versus-host disease (TA-GvHD) is a rare and acute delayed transfusion reaction which occurs after the transfusion of blood components; this complication is correlated to a high mortality rate. The main mechanism for the occurrence of TA-GVHD is the transfer of T lymphocytes from the blood donor that damage and promote a response in the tissues of the recipient. After recognizing host tissues as foreign, the cytokines released by transfused T lymphocytes, such as interleukin-1 (IL-1) and tumor necrosis factor (TNF), drive an inflammatory response. These cytokines activate inflammatory cells, including natural killer (NK) cells, macrophages, and other T lymphocytes, resulting in the destruction of host tissues thereby causing TA-GVHD.1,2 Another mechanism for the occurrence of TA-GVHD occurs through donor-recipient HLA incompatibility.3 The development of this transfusion reaction appears to relate to the number and viability of T lymphocytes transfused in blood components (although this number is not yet known), to the level of immunosuppression of the patient, and to the extent that antigens of the HLA system are common to both the donor and recipient.4,5 Therefore, the greater the number of blood components received, the greater the chance of TA-GVHD occurring in at-risk patients.
In susceptible patients, TA-GVHD occurs when the number of viable transfused lymphocytes is more than 1 × 104 cells/kg of body weight.3,5 It is known that non-leukodepleted packed red blood cell (PRBC) bags have approximately 2–3 × 109 leukocytes, whereas leukodepleted PRBC bags have from 2–3 × 106 leukocytes. Therefore, even leukodepletion is not able to protect these patients from TA-GVHD.3,6 In situations in which the patient has a healthy immune system, lymphocytes are destroyed. However, in immunosuppressed patients, these cells are not destroyed by the recipient's immune system and so after proliferation and producing cytokines, the lymphocytes may cause a TA-GvHD-related inflammatory response. Thus, the only effective method to prevent this disease completely is to inactivate donor lymphocytes by irradiating blood components.
Determination No. 34 of the Ministry of Health of Brazil/National Health Surveillance Agency (ANVISA) states that the irradiation of blood and blood components should be performed in a specific cell irradiator, and that when this equipment is not available, irradiation can be carried out in a linear accelerator used for radiation therapy.7 Using linear accelerators instead of cell irradiators has been the subject of discussion among authors. Although Vetter and Dodd consider the performance of both methods to be similar,8 dose uniformity may fail to meet the quality standards in linear accelerators if the method is not standardized. This was also reported by Janatpour et al. on comparing the irradiation of blood components with X-rays and gamma-rays.9 Bashir et al. demonstrated similar effects in the dosage of potassium and in the degree of hemolysis in red blood cells subjected to X-rays and gamma radiation, and suggested employing linear accelerators in the place of radioactive equipment due to the lower maintenance cost and personnel training, and the dangers of the inappropriate use of cesium.10
Radiation protocols for linear accelerators describe a wide range of types and sizes of containers to be used during radiation. Protocols also differ in respect to the distance between the container and the radiation source, the quantity of bags to be irradiated by each procedure, and the range of results.11,12 Proper irradiation should provide homogeneous distribution of radiation inside the container used, resulting in inhibition of lymphocyte proliferation and lower levels of hemolysis than established by regulatory bodies.13 The PRBC units produced in the Regional Blood Center of Uberaba (HRU) are irradiated in a clinical linear accelerator in the Radiotherapy Department of the Hospital de Clínicas of the Universidade Federal do Triângulo Mineiro (UFTM). This study aimed to evaluate the efficiency of irradiation of PRBCs and possible damage to red blood cells using this method.
Methods
Standardization of the method and irradiation of packed red blood cells
A 30 cm × 30 cm × 15 cm polycarbonate container was designed with a 0.5 cm wall thickness big enough to hold up to 18 PRBC bags (Figure 1). When fewer than 18 bags are being processed, a special lid was created to reduce the size inside the container in order to better control irradiation. A 0.6-mL TN30013 waterproof Farmer ionization chamber coupled to a T10010 Unidos E electrometer (PTW Freiburg) jointly calibrated by the Brazilian Institute of Energy and Nuclear Research (IPEN) were used to measure the dose in the middle of the container. A computed tomography (CT) of the container was performed to provide images for the Eclipse three-dimensional treatment planning system. The analytical anisotropic algorithm (AAA) was used to calculate dose distribution in the irradiated material including identifying regions of uneven irradiation. Radiation indicators (RadTag® RTG 15, RadTag Technologies, Alberta, Canada) were placed on each bag to detect irradiation doses of from 15 to 50 Gy.
Figure 1.

Polycarbonate container designed to irradiate packed red blood cell bags.
Four irradiation procedures involving ten or 12 PRBC bags each were performed at a total dose of 25 Gy in the parallel opposed fields of a Clinac 600™ linear accelerator (Varian) with a nominal power of 6 MV.
Upon confirmation of the irradiation of the bags by the radiation indicators, ten bags from the total of 42 irradiated PRBC bags were randomly taken for testing. Ten non-irradiated bags were used as control. Samples of 20 mL were collected from each irradiated and non-irradiated bag to measure potassium levels and to evaluate the degree of hemolysis on Days 1, 7, 14, 21 and 28. Day 1 (D1) was defined as the day previous to bag irradiation. Another 10 mL sample was collected to compare the lymphocyte proliferation and apoptosis before and 1 h after the irradiation process.
Culture of mononuclear cells of the packed red blood cell sample to evaluate cell proliferation
The proliferation rate of peripheral blood mononuclear cells (PBMCs) was assessed by labeling with carboxyfluorescein diacetate succinimidyl ester (CFSE) and subsequent culture stimulated by phytohemagglutinin. PBMCs were isolated from PRBC by Ficoll-Hypaque separation. The cells were incubated with 5 μM CFSE at room temperature in the dark for five minutes and after 5% fetal bovine serum (FBS) was added. Subsequently, they were washed in complete Roswell Park Memorial Institute (RPMI) medium with 10% FBS, and then 4 × 105 cells were transferred to a 96-well plate to which 5 μg/mL phytohemagglutinin (SIGMA Chemical Co., St. Louis, USA) was added. Some cells were maintained without the addition of the stimulus in order to determine basal fluorescence. The samples were incubated for 72 h at 37 °C in 5% carbon dioxide, and then the cells were collected and resuspended in Dulbecco's Phosphate-Buffered Saline solution (DPBS) for flow cytometry analysis (25,000 events/tube) using a FACSCalibur flow cytometer (BD, USA). The percentage of cell proliferation was determined by subtracting the stimulated CFSE-labeled cell count from control basal fluorescence (cells labeled with CFSE, but without stimulation). All procedures were performed under sterile conditions.
Statistical analysis
At first, the variables were subjected to descriptive analysis using measures of centrality and dispersion. The Shapiro–Wilk test was used to test all variables for normality, whereas Bartlett's test was used to test homogeneity of variance of independent groups (irradiated and non-irradiated). Due to non-normality and non-homogeneity of variances for some variables in the groups, nonparametric analysis of variance was performed using the Mann–Whitney test to compare groups, whereas the Friedman test, Kendall's coefficient of concordance and simple linear regression were used to analyze potassium levels and hemolysis over time. Lymphocyte proliferation was evaluated by comparing the proliferation rates obtained before and after irradiation using the paired Wilcoxon test. An alpha error of 5% was considered acceptable (p-value <0.05) for all tests.
Results
Ten irradiated bags and ten non-irradiated bags were analyzed by comparing the proliferation rates, potassium levels, and the degree of serum hemolysis between the two groups. Distribution of ionizing radiation was also evaluated for the irradiated bags.
Evaluation of the irradiation procedure
Dosimetry showed that the calibration factor of the device met the required factor, that is, 1 cGy/MU. Dose distribution was homogenous in the central section of the CT (Dose = 25 Gy) (Figure 2). A region where the dose reached 26.25 Gy was also observed; this is a variation of 5% above the recommended dose. Analysis of the histogram (Figure 3) shows that the average dose was 28.12 Gy. The figure clearly shows that the minimum dose was 24.37 Gy and the maximum dose was 29.32 Gy. Irradiation of all the ten irradiated PRBC bags was confirmed by RadTag® RTG 15 labels.
Figure 2.
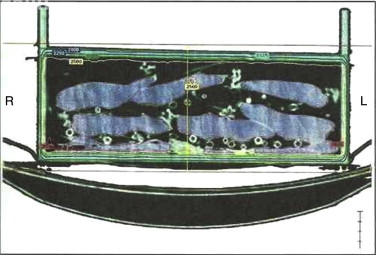
Tomographic image of the container after the irradiation process. Dose distribution was homogenous in the central section of the tomography (yellow line).
Figure 3.
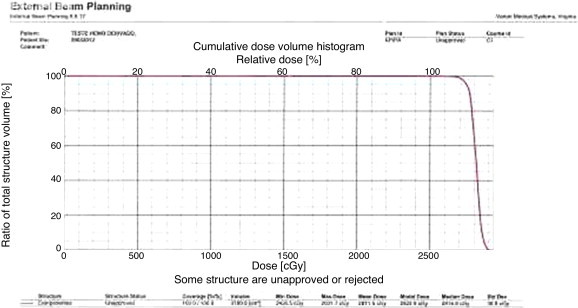
Cumulative dose histogram.
Efficiency of the irradiation process
Regarding the proliferation of lymphocytes, the median proliferation index was 29.8% before irradiation, and 0.5% after irradiation (p-value = 0.005; Figure 4).
Figure 4.

Pre- and post-irradiation pattern of lymphocyte proliferation. Cell proliferation was determined by subtracting carboxyfluorescein diacetate succinimidyl ester labeling from control basal fluorescence – cells labeled with CFSE, but without stimulation.
Storage lesions
The potassium levels (mmol/L) for irradiated and non-irradiated bags during the storage time are shown in Table 1. Over storage days, a significant increase in potassium levels was observed (p-value <0.0001; Friedman test) both in the non-irradiated and irradiated groups, with medians of 61.5 mmol/L and 89.2 mmol/L after 28 days of storage, respectively. A strong linear correlation with the storage time (r = 1 in both cases) was also observed. After adjusting the linear regression model, there was an average increase of 12.96 mmol/L in potassium levels in the non-irradiated group for every week of storage. For the irradiated group, the increase was even higher: 18.69 mmol/L for each week of storage. On Day 7, potassium levels of the non-irradiated group were significantly lower than in and irradiated group (p-value = 0.0002).
Table 1.
Potassium levels (mmol/L) in respect to storage day and bag irradiation.
| Bag irradiation | Storage day | n | Median | Mean (min–max) | Standard deviation |
|---|---|---|---|---|---|
| Non-irradiated | D1 | 10 | 5.05 | 6.85 (4.80–15.10) | 3.66 |
| D7 | 10 | 26.85 | 28.28 (24.30–34.30) | 3.65 | |
| D14 | 10 | 39.05 | 40.89 (35.40–49.80) | 5.21 | |
| D21 | 10 | 51.70 | 50.97 (44.40–58.60) | 4.68 | |
| D28 | 10 | 61.50 | 60.31 (52.90–67.90) | 5.50 | |
| Irradiated | D1 | 10 | 5.85 | 11.05 (4.60–29.90) | 8.59 |
| D7 | 10 | 44.80 | 47.59 (38.00–59.70) | 7.97 | |
| D14 | 10 | 71.90 | 71.94 (54.70–81.10) | 7.82 | |
| D21 | 10 | 82.60 | 80.79 (63.50–92.50) | 8.49 | |
| D28 | 10 | 89.20 | 87.89 (70.60–99.00) | 7.53 |
The percentages of hemolysis, vis-à-vis PRBC storage days and bag irradiation, are shown in Table 2. There was a significant increase in hemolysis (p-value <0.0001; Friedman test) over time both for the non-irradiated and irradiated groups with a strong linear correlation with storage time (r = 0.74 and r = 1, respectively). After adjusting the linear regression model, an average increase of 0.0246% in hemolysis per week of storage was observed in the non-irradiated group; this was higher in the irradiated group (0.044%). However the differences between the non-irradiated and irradiated groups were not significant (p-value > 0.05) at any storage time, and at no time hemolysis was higher than 0.4%.
Table 2.
Hemolysis (%) in relation to the storage day and bag irradiation.
| Bag irradiation | Storage day | n | Median | Average (min–max) | Standard deviation |
|---|---|---|---|---|---|
| Non-irradiated | D1 | 10 | 0.04 | 0.06 (0.02–0.19) | 0.05 |
| D7 | 10 | 0.07 | 0.07 (0.05–0.11) | 0.02 | |
| D14 | 10 | 0.08 | 0.08 (0.04–0.11) | 0.02 | |
| D21 | 10 | 0.11 | 0.11 (0.06–0.18) | 0.04 | |
| D28 | 10 | 0.13 | 0.16 (0.10–0.27) | 0.06 | |
| Irradiated | D1 | 10 | 0.04 | 0.04 (0.02–0.05) | 0.01 |
| D7 | 10 | 0.07 | 0.07 (0.04–0.10) | 0.02 | |
| D14 | 10 | 0.09 | 0.10 (0.05–0.16) | 0.04 | |
| D21 | 10 | 0.15 | 0.16 (0.07–0.24) | 0.06 | |
| D28 | 10 | 0.20 | 0.22 (0.11–0.39) | 0.10 |
Discussion
Due to underreporting of transfusion reactions in Brazil, the incidence and prevalence of TA-GVHD are not yet fully known. In the UK, according to the Annual SHOT Report of 2011, no cases of TA-GVHD were reported from 2001 to 2011, unlike what occurred between 1996 and 2001, when 13 cases were recorded.14 In Japan, a country considered to have a high prevalence of this transfusion reaction, there have been no cases since 2000 according to data published by the Safety Vigilance Division, Blood Service Headquarters of the Japanese Red Cross Society.15 However, 57 cases were confirmed out of 232 suspected cases from 1993 to 1999. The decrease in the frequency of this transfusion reaction is attributed to the implantation of blood component irradiation between 1998 and 1999, thereby demonstrating the importance of this procedure.15 The main goal of the Ministry of Health through ANVISA has been to understand the mechanisms of these transfusion reactions in order to propose measures to prevent their occurrence, as well as to ensure the effectiveness of hemotherapy and the safety of both blood donor and recipient.7
The reason to prevent this transfusion reaction using irradiated PRBCs in susceptible patients is the ineffectiveness of treating this complication. Medications such as corticosteroids, immunoglobulins, anti-thymocyte globulin, and granulocyte-macrophage colony-stimulating factor are not effective and so the only option is prevention by the irradiation of blood components.16,17 Ionizing radiation destroys the DNA of lymphocytes, preventing their proliferation and, thus, reducing the production of cytokines produced in this process.2 The equipment recommended for the irradiation of cellular blood components is a compact gamma-ray irradiator specifically designed for this purpose. Nonetheless, its high cost and the use of radioactive material hinder its use in Brazil, so it has been successfully replaced by the linear accelerator, a procedure which is accepted by Brazilian regulations.7 Regardless of the method, what is really crucial is that the procedure is validated. The recommended radiation dose is 25 Gy, a dose which is considered adequate to inactivate lymphocytes.18 This dose should not be lower than 15 Gy, because this would not inhibit lymphocyte proliferation, nor should it be higher than 50 Gy, as there is a risk of severe red blood cell damage.7 Both gamma rays and X-rays have been studied over the years, and there seems to be no difference between the results.9,10
One of the key requirements for effective irradiation is the homogeneous distribution of radiation inside the container that contains the bags being irradiated. Thus, the first stage of this study aimed to standardize the procedure. Firstly, a container, which would eliminate as far as possible air around the PRBC bags, was developed, as air can cause an uneven distribution of photons leading to a heterogeneous, anisotropic distribution of the radiation.
The container used in this study minimized the presence of air, which may, in part, explain the homogeneous distribution of the dose. Other studies found different kinds of containers were effective.11,12 Another factor which was important for the even dose distribution in this study, as observed by Patton and Skowronski,11 was that irradiation used parallel opposing fields. Two studies employing the single-field technique reported different results; one had homogeneous distribution of radiation12 and the other a heterogeneous distribution.19
Regarding procedure validation, several studies used different methodologies thereby impeding comparisons of the effectiveness of protocols. Pinnarò et al. validated their radiation procedure based only on dosimetry measurements of irradiated blood components.12 On the other hand, Patton and Skowronski and Bashir et al. assessed only the potassium levels and the degree of hemolysis to demonstrate the effectiveness of radiation in a linear accelerator.10,11 Goes et al. studied the proliferation of lymphocytes irradiated with Cobalt-60, and did not find proliferation of these cells at a dose of 25 Gy using a limiting dilution assay.20 Furthermore, Pelszynski et al. did a series of experiments using red cell units irradiated within 24 h after collection. They found that 15 Gy inactivated >4 log10 of T cells, but viable T cells were detected in all experiments. However, when they used 25 or 30 Gy, no T-cell growth (>5 log10 depletion) was detected.21
In this study, we used CT converted into a dose volume histogram to evaluate dose homogeneity. The difference between these methods is that CT produces cross-sectional images of tissue for analysis, whereas the histogram evaluates the total irradiated volume. The dose distribution in the irradiated vessel was found to be homogeneous in the central tomographic section of the container, with a region where the dose reached 26.25 Gy – more than the recommended minimum of 25 Gy – and a variation of 5% of the prescribed dose; this is in accordance with the planning recommended by the International Commission on Radiation Units and Measurements,22 which recommends doses ranging from −5% to +7% of the prescribed dose as satisfactory planning.
The dose volume histogram showed that the dose of 25 Gy was sufficient to uniformly irradiate the entire area occupied by the PRBC bags. These CT and histogram results showed that the dose in none of the regions of the container was lower than 15 Gy or higher than 50 Gy, as determined by Brazilian regulations.7 Another important aspect of blood component irradiation is the confirmation that the irradiation occurred.23 In this study, the use of RadTag® RTG 15 labels ensured that the irradiation procedure had taken place. Even though this label is not a dosimeter, it detects whether irradiation has occurred, thus ensuring a high quality process, as previously demonstrated.11
The efficacy of radiation was demonstrated by a significant reduction in lymphocyte proliferation in irradiated PRBCs (29.8% pre-irradiation versus 0.5% post-irradiation). The fluorescence emission observed in the cytometry of lymphocytes in the irradiated bags (0.5%) may have been caused by the presence of doublets or aggregates or even DNA fragments from these cells, but not by transformed lymphocytes.24 Therefore, this and the proof that an effective radiation dose was delivered7,18 shows that this method prevents the onset of TA-GVHD in patients transfused with these blood components.
In this study, two storage lesions, potassium levels and hemolysis were analyzed in irradiated and non-irradiated PRBCs in order to determine the influence of irradiation. The increase in potassium levels observed in both groups, but significantly more pronounced in irradiated PRBCs from Day 7 of storage, was gradual and linear over time as has been reported in the literature.25 Given the low degree of hemolysis (lower than 0.4%), we believe that increased potassium levels may not be associated with hemolysis, but with the increase in the permeability of the plasma membrane to ions, an occurrence that was reported by Moreira et al.26
The increase in potassium after irradiation was also observed by Janatpour et al.,9 who found a value of 108.7 mequiv./L in PRBC units irradiated by a linear accelerator at a dose of 25 Gy on Day 28. Similar results were found by Bashir et al.10 Transiently increased potassium levels in transfused patients occur due to their redistribution in the body. The number of transfusions, hypovolemia, PRBC storage time, PRBC irradiation, and the infusion time are critical risk factors for hyperkalemia.25 As there is no specific reference value for potassium levels to compare the quality in irradiated PRBC bags7,13 and given that the values found in this study did not differ from those observed by other authors,7,10,25 we concluded that irradiated PRBCs are suitable for transfusion up to the 28th day after irradiation, which is in accordance with current legislation in Brazil.
There was a gradual and linear increase in hemolysis during storage, however, with no significant difference between irradiated and non-irradiated PRBCs. We also found that, even in irradiated PRBCs, the degree of hemolysis was significantly lower than the recommended limit of 0.8%.7,13
Conclusions
In summary, the process of radiation in a linear accelerator, at an appropriate dosage and under validated conditions, is effective to irradiate PRBCs, thereby inhibiting lymphocyte proliferation without compromising the viability of the red blood cells to be transfused.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Dwyre D.M., Holland P.V. Transfusion associated graft versus host disease. Vox Sanguinis. 2008;95(2):85–93. doi: 10.1111/j.1423-0410.2008.01073.x. [DOI] [PubMed] [Google Scholar]
- 2.Ruhl H., Bein G., Sachs U.J. Transfusion associated graft versus host disease. Transfus Med Rev. 2009;23(1):62–71. doi: 10.1016/j.tmrv.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Schroeder M.L. Transfusion associated graft versus host disease. Br J Haematol. 2002;117(2):275–287. doi: 10.1046/j.1365-2141.2002.03450.x. [DOI] [PubMed] [Google Scholar]
- 4.Landi E.P., Oliveira J.S. Doença do enxerto contra hospedeiro pós-transfusional guia para irradiação gama de hemocomponentes. Rev Assoc Med Bras. 1999;45(3):261–272. doi: 10.1590/s0104-42301999000300012. [DOI] [PubMed] [Google Scholar]
- 5.Mathai J. Irradiated blood components. Indian J Med Res. 2005;122(5):371–373. [PubMed] [Google Scholar]
- 6.Asai T., Inaba S., Ohto H., Osada K., Suzuki G., Takahashi K. Guidelines for irradiation of blood and blood components to prevent post-transfusion graft-vs.-host disease in Japan. Transfus Med. 2000;10(4):315–320. doi: 10.1046/j.1365-3148.2000.00264.x. [DOI] [PubMed] [Google Scholar]
- 7.Brasil Ministério da Saúde, Agência Nacional de Vigilância Sanitária. Resolução da Diretoria Colegiada – RDC n° 34, de 11 de junho de 2014. Dispõe sobre as Boas Práticas no Ciclo do Sangue. Available at: http://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2014/rdc0034_11_06_2014.pdf.
- 8.Dodd B., Vetter R.J. Replacement of 137Cs irradiators with x-ray irradiators. Health Phys. 2009;96(2 Suppl.):S27–S30. doi: 10.1097/01.HP.0000334555.78657.bc. [DOI] [PubMed] [Google Scholar]
- 9.Janatpour K., Denning L., Nelson K., Betlach B., Mackenzie M., Holland P. Comparison of X-ray vs. gamma irradiation of CPDA1 red cells. Vox Sanguinis. 2005;89(4):215–219. doi: 10.1111/j.1423-0410.2005.00699.x. [DOI] [PubMed] [Google Scholar]
- 10.Bashir S., Naik F., Cardigan R., Thomas S. Effect of X-irradiation on the quality of red cell concentrates. Vox Sanguinis. 2011;101(3):200–207. doi: 10.1111/j.1423-0410.2011.01479.x. [DOI] [PubMed] [Google Scholar]
- 11.Patton G.A., Skowronski M.G. Implementation of a blood irradiation program at a community cancer center. Transfusion. 2001;41(12):1610–1616. doi: 10.1046/j.1537-2995.2001.41121610.x. [DOI] [PubMed] [Google Scholar]
- 12.Pinnarò P., Soriani A., D’Alessio D., Giordano C., Foddai M.L., Pinzi V. Implementation of a new cost efficacy for blood irradiation using a non dedicated device. J Exp Clin Cancer Res. 2011;30:7. doi: 10.1186/1756-9966-30-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.16th ed. EDQM; Strasbourg: 2011. Guide to the preparation use and quality assurance of blood components: Recommendation No R. (95) 15. [Google Scholar]
- 14.Bolton-Maggs P.H.B., Cohen H., on behalf of the SHOT Steering Group . 2012. The annual SHOT report 2011; p. 22. Available from: http://www.shotuk.org/wp-content/uploads/2012/07/SHOT-ANNUAL-REPORT_FinalWebVersionBookmarked_2012_06_22.pdf. [Google Scholar]
- 15.Safety Vigilance Division, Blood Service Headquarters, Japanese Red Cross Society . 2011. Haemovigilance by JRCS 2008. Tokyo. SVD/BSH/JRCS. Available from: http://www.jrc.or.jp/vcms_lf/anzen_HVreport2008_en.pdf. [Google Scholar]
- 16.Oto O.A., Paydas S., Baslamisli F., Tuncer I., Ergin M., Kalakoc E. Transfusion-associated graft-versus-host disease. Eur J Intern Med. 2006;17(3):151–156. doi: 10.1016/j.ejim.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 17.Agbaht K., Altintas N.D., Topeli A., Gokoz O., Ozcebe O. Transfusion-associated graft-versus-host disease in immunocompetent patients: case series and review of the literature. Transfusion. 2007;47(8):1405–1411. doi: 10.1111/j.1537-2995.2007.01282.x. [DOI] [PubMed] [Google Scholar]
- 18.Rosen N.R., Weidner J.G., Boldt H.D., Rosen D.S. Prevention of transfusion associated graft versus host disease: selection of an adequate dose of gamma radiation. Transfusion. 1993;33(2):125–127. doi: 10.1046/j.1537-2995.1993.33293158043.x. [DOI] [PubMed] [Google Scholar]
- 19.Mergen C., Kunzel R., Góes E.G., Cas E.V., Alves N.M., Botelho M.Z. Dosimetria do sangue irradiado com equipamento de cobaltoterapia. Disc Scientia Série: Ciências Nat Tecnol, S Maria. 2005;6(1):67–77. [Google Scholar]
- 20.Góes E.G., Borges J.C., Covas D.T., Orellana M.D., Palma P.V., Morais F.R. Quality control of blood irradiation: determination T cells radio sensitivity to cobalt 60 gamma rays. Transfusion. 2006;46(1):34–40. doi: 10.1111/j.1537-2995.2005.00669.x. [DOI] [PubMed] [Google Scholar]
- 21.Pelszynski M.M., Moroff G., Luban N.L., Taylor B.J., Quinones R.R. Effect of gamma irradiation of red blood cell units on T-cell inactivation as assessed by limiting dilution analysis: implications for preventing transfusion-associated graft-versus-host disease. Blood. 1994;83(6):1683–1689. [PubMed] [Google Scholar]
- 22.Morgan-Fletcher S.L. Prescribing, recording and reporting photon beam therapy (Supplement to ICRU Report 50). ICRU 62 (1999) Br J Radiol. 2001;74(879):294. [Google Scholar]
- 23.Moroff G., Luban N.L.C. The irradiation of blood and blood components to prevent graft versus host disease: technical issues and guidelines. Transfus Med Rev. 1997;11(1):15–26. doi: 10.1016/s0887-7963(97)80006-5. [DOI] [PubMed] [Google Scholar]
- 24.University of Dundee; Dundee: 2012. Flow cytometry. Core facility. Cell death and apoptosis. Available from: http://www.lifesci.dundee.ac.uk/services/flow_Cytometry/ca/celldeath. [Google Scholar]
- 25.Vraets A., Lin Y., Callum J.L. Transfusion associated hyperkalemia. Transfus Med Rev. 2011;25(3):184–196. doi: 10.1016/j.tmrv.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Moreira O.C., Oliveira V.H., Benedicto L.B., Nogueira C.M., Mignaco J.A., Fontes C.F. Effect of gamma irradiation on the membrane ATPases of human erythrocytes from transfusional blood concentrates. Ann Hematol. 2008;87(2):113–119. doi: 10.1007/s00277-007-0378-3. [DOI] [PubMed] [Google Scholar]


