Abstract
Introduction
Hodgkin's lymphoma is a highly curable disease. Autologous and reduced intensity allogeneic hematopoietic cell transplantations are alternatives to treat relapsed patients. Here, we report on the results of one service using these procedures.
Methods
All patients who underwent transplantations in our institution between 1996 and 2014 were retrospectively studied and demographics, toxicities and survival rate were analyzed.
Results
This study evaluated 24 autologous and five reduced intensity allogeneic transplantations: the median ages of the patients were 29 and 32 years, respectively. At the time of autologous transplantation, ten patients were in complete remission, nine had chemosensitive disease but were not in complete remission, three had refractory disease and the status of two is unknown. In the allogeneic group, two were in complete remission and three had chemosensitive disease. The 5-year overall survival after autologous transplantation was 42% (66% patients were in complete remission, 37% had chemosensitive disease with incomplete remission and 0% had refractory disease) and 1-year overall survival after allogeneic transplantation was 80%. Transplant-related mortality was 0% in patients conditioned with the ifosfamide/carboplatin/etoposide (ICE), carmustine/etoposide/cyclophosphamide (BEC) and carmustine/etoposide/cytarabine/melphalan (BEAM) regimens, 37% in patients conditioned with busulfan-based regimens and 20% in allogeneic transplantations.
Conclusions
Hematopoietic cell transplantation for relapsed Hodgkin's lymphoma is a potentially curative procedure especially in patients in complete remission at the time of autologous transplantations, and possibly after allogeneic transplantations. Further studies are necessary to clarify the role of allogeneic transplantations in the treatment of relapsed Hodgkin's lymphoma.
Keywords: Hodgkin disease, Relapse, Hematopoietic stem cell transplantation
Introduction
Hodgkin's Lymphoma (HL) is a highly curable disease with chemotherapy and radiotherapy.1 However, depending on several risk factors and stage at diagnosis,2,3 a group of patients will relapse during the follow-up. Several studies have shown that in patients with localized disease, depending on the level of risk, the probability of relapse varies between 10 and 30% after treatment with the adriamycin, bleomycin, vinblastine and dacarbazine regimen (ABVD) and radiotherapy.2 However, the relapse rate can be as high as 40–50% for patients with advanced disease.3 Several drugs are under study for the treatment of relapsed/refractory patients but until now, none of them have been able to induce long-term remission.4 In these cases, only autologous hematopoietic cell transplantation (auto HCT) has shown to induce long-term remission.
Two randomized studies have shown a significant benefit of freedom from second failure but not on overall survival (OS) when comparing chemotherapy alone with auto HCT.5,6 On the other hand, the role of reduced intensity allogeneic HCT (allo RIC HCT) has been controversial and several small studies and case series have suggested a potent graft-versus-lymphoma (GVL) effect and a lower relapse rate compared to auto HCT,7 although no direct comparisons between the two procedures exist. Initial studies with myeloablative conditioning regimens showed high transplant-related mortality (TRM) and graft-versus-host disease (GVHD) rates.8,9 More recently, the use of RIC regimens has been associated with lower TRM, however, their role in the treatment of relapsed HL is not very clear yet, with suggestions for their use in cases of relapse after auto HCT, failure to harvest autologous cells or early relapses after chemotherapy.7 Also, the use of haploidentical donors has preliminary shown interesting results with extremely low toxicity and low risk of GVHD, but maintaining the GVL effect.10
Herein the results of a cohort of patients with relapsed HL submitted to auto HCT and allo RIC HCT are reported.
Methods
Patients
A retrospective analysis of the HCT database at the Pontificia Universidad Catolica de Chile, Santiago was performed, which included patients transplanted between 1996 and 2014. Demographic data, as well as date of diagnosis, date of transplant, type of graft and conditioning regimen, age, gender, remission status at transplant, number of CD34+ cells infused, time to neutrophil and platelet engraftment, complications, cause of death, time to event (death or relapse) and OS (time to last follow-up or death) were obtained. For patients submitted to allo RIC HCT, the type of GVHD prophylaxis, and grade and time to the diagnosis of GVHD were also obtained. This study was approved by the Ethics Committee and by the Medical Research Center Committee of the Hospital.
Mobilization and leukapheresis
Two methods were used for autologous cell collection: chemomobilization and chemotherapy followed by filgrastim (10 μg/kg/day) starting on Day +5 after chemotherapy until the day of the leukapheresis (usually 10–14 days after chemotherapy) and filgrastim (10 μg/kg/day) alone for five days. The number of CD34+ cells in the peripheral blood was counted by flow cytometry the day before the programmed collection. If the quantity was >20 × 106/L, collection was performed to obtain a minimum of 2 × 106 CD34+ cells/kg. If the CD34+ count was <20 × 106/L, a dose of plerixafor (0.24 mg/kg) was administered subcutaneously 9–11 h before another collection to target a minimum of 2 × 106 CD34+ cells/kg, with up to five consecutive leukapheresis. For allogeneic collections, filgrastim (10 μg/kg/day) was administered for five days before collection without measuring the CD34+ cells the day before collection.
Definitions
The type of response before the transplant was based on the criteria of Cheson et al. 11,12 depending on the availability of positron emission tomography-computed tomography (PET/CT) and the time of the transplant. The responses were categorized as complete response (CR), incomplete response with chemosensitive disease (non-CR CS) and incomplete response refractory to chemotherapy (non-CR R) according to the stratification proposed by the Center for International Blood and Marrow Transplant Research (CIBMTR)13 and the National Marrow Donor Program (NMDP).14
Donor selection, conditioning regimens, prophylaxis of graft-versus-host disease and infectious diseases, and treatment
After the decision to transplant was made, the majority of patients in second CR (CR2) or non-CR CS underwent auto HCTs. Patients from whom it was impossible to collect an adequate number of CD34+ cells, non-CR R patients, and those in third or more remission with a suitable donor (fully matched or haploidentical sibling or matched unrelated donor) were recommended for allo RIC HCTs.
Conditioning regimens for auto HCT between 1996 and 2003 included busulfan/melphalan/thiotepa (BMT) and busulfan/etoposide/cyclophosphamide (BuEC); in 2004 the service started using the ifosfamide/carboplatin/etoposide (ICE), carmustine/etoposide/cyclophosphamide (BEC) and carmustine/etoposide/cytarabine/melphalan (BEAM) regimens. Allo RIC regimens included fludarabine/melphalan (FluMel), and fludarabine/cyclophosphamide/total body irradiation with or without post-transplant cyclophosphamide (as in the case of haploidentical transplants).
GVHD prophylaxis was made with cyclosporine/methotrexate, or tacrolimus/methotrexate/post-transplant cyclophosphamide (for haploidentical transplants).
Prophylaxis against infectious diseases included levofloxacin (500 mg QD) starting on Day -1 until neutrophil recovery or febrile neutropenia, acyclovir (400 mg TID) starting on Day -1 until Day +365, fluconazole (200 mg QD) starting on Day -1 until Day +100 and sulfametoxazol trimetoprim (QD) three times per week starting on neutrophil recovery until Day +365.
All the patients were kept in isolation rooms with high-efficiency particulate air filters and positive pressure during the neutropenic phase of the transplant. They were given a neutropenic diet and received filgrastim (300 μg IV) starting on Day +5 until neutrophil engraftment.
Statistical analysis
The statistical analysis was performed using GraphPad version 6.0f (GraphPad Software, Inc. La Jolla, CA, USA). Variables are reported as numbers and percentages. OS was measured from transplantation until death by any cause. Patients alive at the time of analysis were censored at the last follow-up date. TRM was defined as death with no evidence of progression or relapse and was measured from the day of transplantation. Death after disease progression was treated as a competing event in the calculation of TRM. Survival curves (OS and TRM) were obtained using the Kaplan–Meier method and were compared with the Log-Rank Test.
Results
Population characteristics
Between 1996 and 2014 29 HCT were performed for HL; 24 auto HCT and five allo RIC HCT. The median age for the 24 auto HCT patients, including 13 men (54%), was 29 years (range: 20–60 years). At the time of the auto HCT, ten patients were in CR, nine were in non-CR CS, three in non-CR R and the status of two was unknown. The median age for the allo RIC HCT patients was 32 years (range: 22–46 years), and all were men; two of the patients were in CR and three were in non-CR CS (Table 1).
Table 1.
Patient characteristics.
| Autologous | Allogeneic | |
|---|---|---|
| Patients – n | 24 | 5 |
| Age – years (range) | 29 (20–60) | 32 (22–46) |
| Gender – male/female | 13/11 | 5/0 |
| Remission status | ||
| CR | 10 | 2 |
| Non-CR, sensitive | 9 | 3 |
| Non-CR, refractory | 3 | 0 |
| Unknown | 2 | 0 |
| Median follow up – days (range) | 493 (9–4822) | 364 (40–778) |
| Cause of death | ||
| respiratory failure | 8 | |
| lymphoma | 4 | |
| MOD | 1 | |
| unknown | 3 | |
CR: complete response; MOD: multiple organ dysfunction.
Transplant procedure
Transplant characteristics are shown in Table 2. Of all the patients submitted to auto HCT, 12 (50%) received conditioning regimens based on busulfan. The rest of the patients (n = 12) were conditioned with the ICE, BEC or BEAM regimens. Patients submitted to allo RIC HCT were all conditioned with RIC regimens as mentioned in the Methods section.
Table 2.
Transplant characteristics.
| Autologous transplantation | |
| Conditioning regimen | |
| Bu/Mel/Tio | 9 |
| Bu/Eto/Cy | 3 |
| ICE | 7 |
| BEC | 4 |
| BEAM | 1 |
| CD34 cell dose – ×106/kg (range) | 2.87 (1.51–16.9) |
| Number of collections – n (range) | 2 (1–4) |
| Neutrophil engraftment – days (range) | 11 (6–28) |
| Platelet engraftment – days (range) | 12 (8–29) |
| Allogeneic transplantation | |
| Donor | |
| Sibling 6/6 | 2 |
| Sibling 3/6 | 1 |
| Unrelated 8/8 | 2 |
| Conditioning regimen | |
| Flu/Mel | 2 |
| Flu/Cy/TBI | 2 |
| Flu/Cy/TBI/Cy | 1 |
| CD34 cell dose – ×106/kg (range) | 6.6 (3.5–12) |
| Number of collections – n (range) | 1 (1) |
| Neutrophil engraftment – days (range) | 13 (10–17) |
| Platelet engraftment – days (range) | 13 (10–22) |
| GVHD prevention – n | |
| CS/MTX | 4 |
| TAC/MTX | 1 |
| GVHD – n (%) | |
| Acute | 2 (40%) |
| chronic | 1 (33%) |
GVHD: graft vs. host disease; Bu/Mel/Tio: busulfan, melphalan, thiotepa; Bu/Eto/Cy: busulfan, etoposide, cyclophosphamide; ICE: ifosfamide, carboplatin, etoposide; BEC: carmustin, etoposide, cyclophosphamide; BEAM: carmustin, etoposide, cytarabine, melphalan; Flu/Mel: fludarabine, melphalan; Flu/Cy/TBI: fludarabine, cyclophosphamide, total body irradiation; CS/MTX: cyclosporine, methotrexate; TAC/MTX: tacrolimus, methotrexate, post-transplant cyclophosphamide.
The CD34+ cell doses in auto HCT and allo RIC HCT were 2.87 × 106 cells/kg (range: 1.51–16.9 × 106 cells/kg) and 6.6 × 106 cells/kg (range: 3.5–12 × 106 cells/kg), respectively. Median number of apheresis required for the minimum CD34+ cell dose was two (range: 1–4) in auto HCT and one in allo RIC HCT.
The median time to neutrophil engraftment was 11 days (range: 6–28 days) with 12 days (range: 8–29 days) for platelet engraftment in patients who underwent auto HCT. Patients submitted to allo RIC HCT had a median time to neutrophil engraftment of 13 days (range: 10–17 days) with 13 days (range, 10–22 days) to platelet engraftment.
Graft-versus-host disease
In the allo RIC HCT group, two of five patients (40%) had Grade 2 acute GVHD of the skin. From three evaluable patients, one had localized chronic GVHD (33%) of the liver, which was adequately controlled by standard immunosuppression.
Survival
Median follow-up time in patients submitted to auto HCT was 429 days (range; 9–4837 days). There were 11 deaths due mainly to respiratory failure and HL relapse (Table 1). The 5-year OS and progression free survival were 42% and 33%, respectively (Figure 1). OS, according to remission status, was 66% in CR patients, 37% in non-CR CS and 0% in non-CR R disease (p-value = 0.03; Figure 2).
Figure 1.
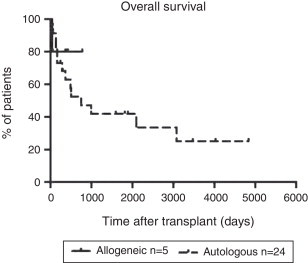
Overall survival after autologous and allogeneic transplantation.
Figure 2.
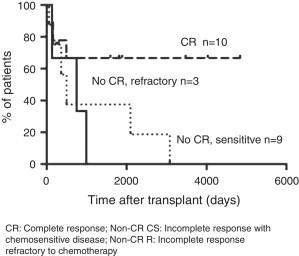
Overall survival according to remission status in autologous transplantation.
CR: Complete response; Non-CR CS: Incomplete response with chemosensitive disease; Non-CR R: Incomplete response refractory to chemotherapy.
Median follow-up time in patients submitted to allo RIC HCT was 364 days (range: 40–778 days). There was one death shortly after transplant (Table 1). The 1-year OS was 80% (Figure 1) and of four evaluable patients, one relapsed (progression free survival: 75%).
Transplant-related mortality
Of the patients conditioned with busulfan and submitted to auto HCT, four (37%) died due to transplant-related complications (Figure 3). Of the patients who underwent allo RIC HCT, one out of five patients (20%) died due to transplant-related complications (Figure 3)
Figure 3.
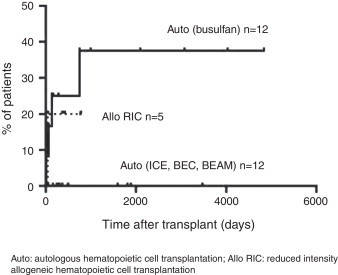
Transplant-related mortality.
Auto: autologous hematopoietic cell transplantation; Allo RIC: reduced intensity allogeneic hematopoietic cell transplantation.
Discussion
HL is a highly curable disease with chemotherapy and radiotherapy, but patients who relapse or have refractory disease are a therapeutic challenge. After relapse, the standard treatment is auto HCT5,6,15,16 with about half of the patients being cured with this procedure. This benefit has been shown only in freedom from treatment failure but not in OS. It has been suggested that this is due to the patients in the chemotherapy arm, who subsequently received an auto HCT when they relapsed after chemotherapy. In our cohort, more than 60% of the auto HCT patients were living five years after transplant. However, the remission status at the time of the transplant is important since patients not in CR had significantly worse OS than CR patients. Similar data were reported by Lazarus et al. where patients in CR had an OS close to 80% compared to 60% in patients with partial response and 40% in those with refractory disease.17 In a recent publication by Jostling et al., the authors also showed that the OS was close to 80% in patients without risk factors compared to 10% in patients with three risk factors at the time of transplant.18
Another finding of this study was the significantly high TRM with older conditioning regimens based on busulfan (37%) compared to more recent conditioning regimens including ICE, BEC and BEAM which, in this series, were not associated with TRM. Considering previous reports of secondary cancers with total body irradiation, this procedure is not included in the preparation for transplant in our service.19 In our group there was no standard criterion to select a specific conditioning regimen, however recently, the BEAM regimen is being used more often due to the results of Jostling et al. 18
Allo RIC HCT was performed in the few patients considered to be at high-risk, and who had failed the CD34+ collection or for whom bad disease control was anticipated using an auto HCT.18 Considering the dismal results with myeloablative regimens mainly related to high TRM,20 only allo RIC HCT was performed. Although no randomized studies compare these two types of transplants, several case series and registry analyses suggest that in advanced disease patients the OS could be over 60% and the TRM lower than 10%.16 Moreover, the evidence suggests that the GVL effect could be significant, especially in patients transplanted early after relapse.21,22 In the current series, although the number of allo RIC HCT patients was low (n = 5), TRM was low and 1-year OS was 80%, which is similar to other studies.23,24 Similarly, the incidence of acute and chronic GVHD in this population, even though the number of patients is low, is in agreement with previous reports. Despite the fact that the results with allo RIC HCT are encouraging, its specific role in the management of relapsed HL is not clear and it is recommended to include these patients in clinical trials, whenever possible.25 Recently, a Phase II study suggested that haploidentical donors could offer better results than matched sibling donors, with very low TRM and GVHD risk, and without evidence of any loss of the GVL effect10 thus opening a new alternative for these patients.
The main problems of the current study are its retrospective nature and the low number of patients. However, this is the first transplant series for HL from Chile and the data is in line with international series. A previous study by Puga et al.26 reported results on engraftment and mucositis in the first ten auto HCT performed in the public health system, where they included seven patients with HL with different response rates before transplant. They did not report on OS or TRM so no comparisons can be made.
Conclusions
The results with auto HCT in this study are similar to previous reports, especially emphasizing the high curability in patients in CR before transplantation. Also, the results with allo RIC HCT are encouraging regarding OS and TRM and are in line with international series remembering that the follow-up and number of patients is still low. Other studies will be necessary to better establish the role of allo RIC HCT in the treatment of relapsed HL.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Sjöberg J., Halthur C., Kristinsson S.Y., Landgren O., Nygell U.A., Dickman P.W. Progress in Hodgkin lymphoma: a population-based study on patients diagnosed in Sweden from 1973–2009. Blood. 2012;119(4):990–996. doi: 10.1182/blood-2010-08-302604. [DOI] [PubMed] [Google Scholar]
- 2.Armitage J.O. Early-stage Hodgkin's lymphoma. N Engl J Med. 2010;363(7):653–662. doi: 10.1056/NEJMra1003733. [DOI] [PubMed] [Google Scholar]
- 3.Hasenclever D., Diehl V. A prognostic score for advanced Hodgkin's disease. International Prognostic Factors Project on Advanced Hodgkin's Disease. N Engl J Med. 1998;339(21):1506–1514. doi: 10.1056/NEJM199811193392104. [DOI] [PubMed] [Google Scholar]
- 4.Younes A. Novel treatment strategies for patients with relapsed classical Hodgkin lymphoma. Hematology Am Soc Hematol Educ Program. 2009:507–519. doi: 10.1182/asheducation-2009.1.507. [DOI] [PubMed] [Google Scholar]
- 5.Linch D.C., Winfield D., Goldstone A.H., Moir D., Hancock B., McMillan A. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin's disease: results of a BNLI randomised trial. Lancet. 1993;341(8852):1051–1054. doi: 10.1016/0140-6736(93)92411-l. [DOI] [PubMed] [Google Scholar]
- 6.Schmitz N., Pfistner B., Sextro M., Sieber M., Carella A.M., Haenel M. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin's disease: a randomised trial. Lancet. 2002;359(9323):2065–2071. doi: 10.1016/S0140-6736(02)08938-9. [DOI] [PubMed] [Google Scholar]
- 7.Brice P. Managing relapsed and refractory Hodgkin lymphoma. Br J Haematol. 2008;141(1):3–13. doi: 10.1111/j.1365-2141.2008.06998.x. [DOI] [PubMed] [Google Scholar]
- 8.Gajewski J.L1, Phillips G.L., Sobocinski K.A., Armitage J.O., Gale R.P., Champlin R.E. Bone marrow transplants from HLA-identical siblings in advanced Hodgkin's disease. J Clin Oncol. 1996;14(2):572–578. doi: 10.1200/JCO.1996.14.2.572. [DOI] [PubMed] [Google Scholar]
- 9.Milpied N., Fielding A.K., Pearce R.M., Ernst P., Goldstone A.H. Allogeneic bone marrow transplant is not better than autologous transplant for patients with relapsed Hodgkin's disease. European Group for Blood and Bone Marrow Transplantation. J Clin Oncol. 1996;14(4):1291–1296. doi: 10.1200/JCO.1996.14.4.1291. [DOI] [PubMed] [Google Scholar]
- 10.Raiola A., Dominietto A., Varaldo R., Ghiso A., Galaverna F., Bramanti S. Unmanipulated haploidentical BMT following non-myeloablative conditioning and post-transplantation CY for advanced Hodgkin's lymphoma. Bone Marrow Transplant. 2014;49(2):190–194. doi: 10.1038/bmt.2013.166. [DOI] [PubMed] [Google Scholar]
- 11.Cheson B.D., Pfistner B., Juweid M.E., Gascoyne R.D., Specht L., Horning S.J. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 12.Cheson B.D., Horning S.J., Coiffier B., Shipp M.A., Fisher R.I., Connors J.M. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17(4):1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 13.Center for International Blood and Marrow Transplant Research (CIBMTR). Available from: www.cibmtr.org [cited 02.12.14].
- 14.National Marrow Donor Program® (NMDP). Available from: www.bethematch.org [cited 02.12.14].
- 15.Eichenauer D.A., Engert A., André M., Federico M., Illidge T., Hutchings M. Hodgkin's lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl. 3):iii70–iii75. doi: 10.1093/annonc/mdu181. [DOI] [PubMed] [Google Scholar]
- 16.Bartlett N.L. Therapies for relapsed Hodgkin lymphoma: transplant and non-transplant approaches including immunotherapy. Hematol Am Soc Hematol Educ Program. 2005;24:5–51. doi: 10.1182/asheducation-2005.1.245. [DOI] [PubMed] [Google Scholar]
- 17.Lazarus H.M., Loberiza F.R., Jr., Zhang M.J., Armitage J.O., Ballen K.K., Bashey A. Autotransplants for Hodgkin's disease in first relapse or second remission: a report from the autologous blood and marrow transplant registry (ABMTR) Bone Marrow Transplant. 2001;27(4):387–396. doi: 10.1038/sj.bmt.1702796. [DOI] [PubMed] [Google Scholar]
- 18.Josting A., Müller H., Borchmann P., Baars J.W., Metzner B., Döhner H. Dose intensity of chemotherapy in patients with relapsed Hodgkin's lymphoma. J Clin Oncol. 2010;28(34):5074–5080. doi: 10.1200/JCO.2010.30.5771. [DOI] [PubMed] [Google Scholar]
- 19.Sureda A., Arranz R., Iriondo A., Carreras E., Lahuerta J.J., García-Conde J. Autologous stem-cell transplantation for Hodgkin's disease: results and prognostic factors in 494 patients from the Grupo Espanol de Linfomas/Transplante Autologo de Medula Osea Spanish Cooperative Group. J Clin Oncol. 2001;19(5):1395–1404. doi: 10.1200/JCO.2001.19.5.1395. [DOI] [PubMed] [Google Scholar]
- 20.Peniket A.J., Ruiz de Elvira M.C., Taghipour G., Cordonnier C., Gluckman E., de Witte T. An EBMT registry matched study of allogeneic stem cell transplants for lymphoma: allogeneic transplantation is associated with a lower relapse rate but a higher procedure-related mortality rate than autologous transplantation. Bone Marrow Transplant. 2003;31(8):667–678. doi: 10.1038/sj.bmt.1703891. [DOI] [PubMed] [Google Scholar]
- 21.Greaves P.J., Gribben J.G. Demonstration of durable graft versus lymphoma effects in Hodgkin's lymphoma. J Clin Oncol. 2011;29(8):952–953. doi: 10.1200/JCO.2010.33.2437. [DOI] [PubMed] [Google Scholar]
- 22.Peggs K.S., Kayani I., Edwards N., Kottaridis P., Goldstone A.H., Linch D.C. Donor lymphocyte infusions modulate relapse risk in mixed chimeras and induce durable salvage in relapsed patients after T-cell-depleted allogeneic transplantation for Hodgkin's lymphoma. J Clin Oncol. 2011;29(8):971–978. doi: 10.1200/JCO.2010.32.1711. [DOI] [PubMed] [Google Scholar]
- 23.Sureda A., Canals C., Arranz R., Caballero D., Ribera J.M., Brune M. Allogeneic stem cell transplantation after reduced intensity conditioning in patients with relapsed or refractory Hodgkin's lymphoma. Results of the HDR-ALLO study – a prospective clinical trial by the Grupo Espanol de Linfomas/Trasplante de Medula Osea (GEL/TAMO) and the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2012;97(2):310–317. doi: 10.3324/haematol.2011.045757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarina B., Castagna L., Farina L., Patriarca F., Benedetti F., Carella A.M. Allogeneic transplantation improves the overall and progression-free survival of Hodgkin lymphoma patients relapsing after autologous transplantation: a retrospective study based on the time of HLA typing and donor availability. Blood. 2010;115(18):3671–3677. doi: 10.1182/blood-2009-12-253856. [DOI] [PubMed] [Google Scholar]
- 25.Kuruvilla J., Keating A., Crump M. How I treat relapsed and refractory Hodgkin lymphoma. Blood. 2011;117(16):4208–4217. doi: 10.1182/blood-2010-09-288373. [DOI] [PubMed] [Google Scholar]
- 26.Puga B., Molina J., Andrade A., Guerra C., Ardila A., Alvarez G. First ten hematopoietic stem cell transplants performed in the adult public health service in Chile. Rev Med Chile. 2012;140(9):1207–1212. doi: 10.4067/S0034-98872012000900016. [DOI] [PubMed] [Google Scholar]


