Abstract
Earthworms (Oligochaeta: Lumbricidae) have substantial effects on the structure and fertility of soils with consequences for the diversity of plant communities and associated ecosystem functions. However, we still lack a clear understanding of the functional role earthworms play in terrestrial ecosystems, partly because easy-to-use methods to quantify their activities are missing. In this study, we tested whether earthworms and their casts can be dual-labelled with 15N and 13C stable isotopes by cultivating them in soil substrate amended with 15N ammonium nitrate and 13C-glucose. Additionally, we also wanted to know whether (i) earthworms from different functional groups (soil-feeders vs. litter-feeders) and their casts would differ in their incorporation of stable isotopes, (ii) if enrichment levels are higher if the same amount of isotopes is applied in one dose or in staggered doses, and (iii) if isotopic enrichment in casts changes when they are stored in a conditioning cabinet or in a pot filled with soil placed in a greenhouse. Our findings show the feasibility of dual-labelling tissues and casts of both litter-feeding (Lumbricus terrestris) and soil-feeding (Aporrectodea caliginosa) earthworms using the same method. The advantage of this method is that earthworms and their casts can be labelled under realistic conditions by cultivating them for only four days in soil that received a one-time addition of commercially available stable isotopes instead of offering labelled plant material. In earthworms, the isotopic enrichment remained at a stable level for at least 21 days; labelled casts could be stored for at least 105 days without significantly decreasing their isotopic signals. This simple and efficient method opens new avenues for studying the role of these important ecosystem engineers in nutrient cycling and their functional relationships with other organisms.
Keywords: Carbon isotope, Ecosystem engineers, Nitrogen isotope, Soil invertebrates, Tracer study, Labelling method
Introduction
In most temperate terrestrial ecosystems, earthworms (Oligochaeta: Lumbricidae) represent the dominant fraction of the soil faunal biomass, often acting as ecosystem engineers (Jones et al. 1994) with substantial effects on the structure and fertility of soils. Most earthworm communities consist of different functional groups comprising litter-dwellers (epigeics), soil-dwellers (endogeics) and vertical-burrowers (anecics; Bouché 1977). In temperate grasslands, up to 1000 earthworms m−2 have been reported (Edwards et al. 1995). By producing huge amounts of nutrient rich casts – from 1.4–7.5 ton ha−1 a−1 (James 1991) up to 40 or even 80 ton ha−1 a−1 (reviewed in Edwards and Bohlen 1996) – such populations are a key component of nutrient cycling in soil (Lavelle, 1988, Scheu, 1993). These earthworm casts contain more plant nutrients than bulk soil (McKenzie and Dexter, 1987, Schrader and Zhang, 1997, Zaller and Arnone, 1997, Chaoui et al., 2002) and are also hot spots of microbial (Scheu, 1987, Brown et al., 2000) and other invertebrate activity (Decaens et al. 1999). Moreover, earthworms are suggested to affect the diversity of grassland communities (Willems and Huijsmans, 1994, Zaller and Saxler, 2007, Eisenhauer and Scheu, 2008) and are themselves affected by plant diversity (Zaller and Arnone, 1999a, Eisenhauer et al., 2008).
Despite the earthworms’ paramount importance, we still lack a mechanistic understanding of many aspects of interactions, mainly trophic links, between earthworms and other ecosystem components such as microorganisms, plant roots, or mycorrhizae (Curry and Schmidt 2007). Several researchers have successfully studied the feeding habits of earthworms by means of analysing stable isotope natural abundances (Spain et al., 1990, Martin et al., 1992a, Martin et al., 1992b, Schmidt et al., 1997, Spain and Feuvre, 1997, Scheu and Falca, 2000, Schmidt et al., 2004, Elfstrand et al., 2008, Seeber et al., 2009). Natural abundances of stable isotopes can reveal patterns in food-webs, mainly by identifying the trophic level of organisms, but they provide only limited information on functional relationships. These functional relationships have been studied successfully using isotopic tracers by feeding earthworms with isotopically labelled plant material (Barois et al., 1987, Scheu, 1991, Binet and Trehen, 1992, Hameed et al., 1994, Curry et al., 1995, Whalen et al., 2000, Whalen and Janzen, 2002). This method seemed to work very well although its wider use is restricted because incorporating stable isotopes into plants requires special growth chambers, which are often not available in ecological laboratories. This was also the motivation for Dyckmans et al. (2005) to test a method whereby the endogeic Aporrectodea caliginosa (Savigny) was kept in soil enriched with 13C and 15N and the label enrichment in tissue and mucus was examined.
In the current study, we tested extensions of the method of Dyckmans et al. (2005) in three major aspects: (i) in addition to the endogeic A. caliginosa we also tested the anecic Lumbricus terrestris L.; (ii) in addition to earthworm tissue, we also tested earthworm casts for tracer signals; and (iii) we tested if the 15N and 13C signal in potentially labelled L. terrestris casts remains stable over a longer period of time so that casts could be used in later experiments. Additionally, we varied the labelling procedure at several stages where we expected to achieve higher 15N and 13C enrichments in earthworm tissue and casts: (i) like Dyckmans et al. (2005) we incubated the labelled soil to improve the availability of nitrogen for earthworms through microbial metabolism of ammonium nitrate, but we also tested a variant without soil incubation. (ii) Since microbial activity could also decrease the amount of N and especially of C available through microbial respiration, we included a variant with a staggered application of glucose (13C-source) and of ammonium nitrate (15N-source). (iii) We set up a variant providing additional food which could improve the earthworms’ condition and thus, the incorporation of stable isotopes. The latter variant was also thought to be more suitable for the litter feeding L. terrestris than the geophagous A. caliginosa (Doube et al. 1997).
Materials and methods
Preparation of 13C and 15N enriched cultivation substrate for earthworms
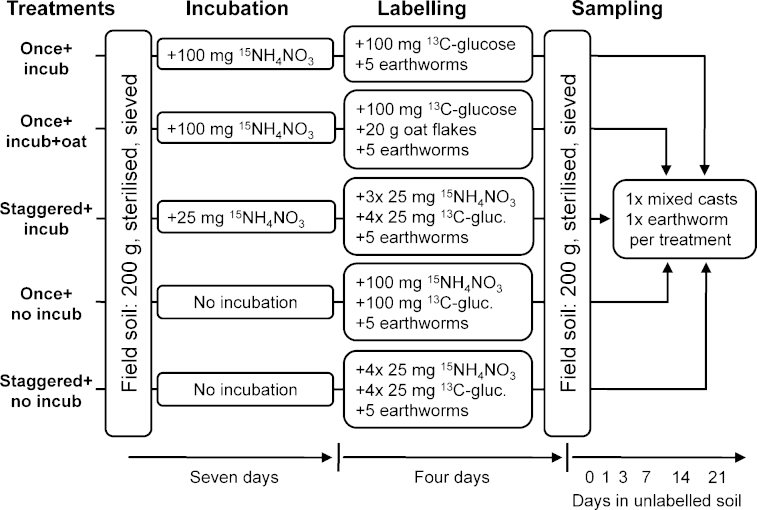
Soil (Haplic Chernozem, silty loam, pH = 7.6, Corg = 2.2 g kg−1, Ntot = 0.117 g kg−1) was collected from an arable field at the research farm of the University of Natural Resources and Applied Life Sciences Vienna near Groß-Enzersdorf, Austria, sieved (2 mm) and steam-sterilized (12 h at 120 °C). Following Dyckmans et al. (2005) we used 13C6H12O6 (99 at.% 13C6-glucose; Sigma–Aldrich, Vienna, Austria) and 15NH4NO3 (95 at.% 15N-ammonium nitrate; Chemotrade, Leipzig, Germany) in order to dual-label earthworm species, with several modifications as follows (Fig. 1): first, we looked at soil containing 15NH4NO3 that was incubated for seven days and soil that was not incubated. Secondly, we either applied 100 mg of 13C6H12O6 and 100 mg of 15NH4NO3 once or split it into four applications of 25 mg 13C6H12O6 and 25 mg 15NH4NO3 over four days. Thirdly, we established treatments with ground oat flakes addition (as an additional food source) and those with no addition. These treatments were combined resulting in five experiments as shown in Fig. 1; one unlabelled control was set up for each experiment.
Fig. 1.
Schematic diagram of the treatments used for 13C/15N labelling of L. terrestris and A. caliginosa. An unlabelled control was set up for each treatment (not shown in this diagram).
Treatments with a seven day soil incubation were prepared by filling 200 g sieved and sterilized soil into polypropylene bags, adding (i) 100 mg 15NH4NO3 and 400 mg unlabelled glucose dissolved in 4 ml deionized water (treatment “once + incub”), or (ii) 100 mg 15NH4NO3 and 400 mg unlabelled glucose dissolved in 4 ml deionized water and 20 g ground oat flakes (particle size <1 mm; treatment “once + incub + oats”), or (iii) 25 mg 15NH4NO3 and 400 mg unlabelled glucose dissolved in 4 ml deionized water (treatment “staggered + incub”). These mixtures were incubated in the dark at 15 °C for seven days. To ensure aerobic conditions and a homogeneous 15N distribution, soil was stirred daily. Treatments that did not include soil incubation were prepared seven days later (Fig. 1). Here, soil was enriched with (iv) 100 mg 15NH4NO3 and 400 mg unlabelled glucose dissolved in 4 ml deionized water (treatment “once + no incub”) or (v) 25 mg 15NH4NO3 and 400 mg unlabelled glucose dissolved in 4 ml deionized water (treatment “staggered + no incub”).
Afterwards, the 15N labelled soil was transferred into polypropylene boxes (volume 500 ml) and 100 mg 13C-glucose dissolved in 2.5 ml deionized water were added to the treatments “once + incub”, “once + incub + oats” and “once no incub”. In treatments “staggered + incub” and “staggered no incub”, 25 mg 13C-glucose dissolved in 2.5 ml deionized water were added. On days 2, 3 and 4 of the labelling period (see next section), 25 mg 15NH4NO3, 400 mg unlabelled glucose and 25 mg 13C-glucose dissolved in 2.5 ml deionized water were added to treatment with staggered isotope labelling (Fig. 1). Overall, all treatments received the same total amount of ammonium nitrate (equals 183 mg N kg−1 soil), glucose (equals 200 mg C kg−1 soil), and water (6.5 ml) during the experiment.
Labelling of earthworms and casts
To label the earthworms, five individuals of L. terrestris or A. caliginosa, respectively, were held in polypropylene boxes (volume 500 ml) each containing 200 g soil treated and labelled as described above. Boxes with earthworms were stored in the dark at 15 °C. We used adult individuals of L. terrestris obtained from two commercial suppliers (R. Pechmann, Langenzersdorf, Austria; Denu's Würmer Stuttgart, Germany), with a mean initial biomass of 3684 ± 365 mg. Adult and semi-adult individuals of A. caliginosa, with a mean initial biomass of 705 ± 54 mg were collected by hand-sorting from a garden soil south of Vienna, Austria in March 2008. After four days in the labelled soil, earthworms were transferred into new boxes containing 200 g unlabelled and sterilized moist soil. Boxes were again stored in the dark at 15 °C and re-randomized daily.
On days 1, 3, 7, 14 and 21 after transferring the earthworms into unlabelled soil, a pooled sample of casts (a small portion of all casts present in a box taken with a laboratory scoop's point) and one earthworm were collected from each replicate and analysed (see below).
Long-term storage of isotopically enriched earthworm casts
Since we planned to use labelled casts of L. terrestris for a subsequent experiment, we wanted to test how the isotopic enrichment would be affected by storage. Therefore, after the last worm was taken out of the boxes on day 21 of the above described sampling period, labelled L. terrestris casts from treatment “once + incub” were stored in two different ways. First, three boxes containing the labelled casts were stored in the dark at 15 °C in a conditioning cabinet with no additional moisture being added throughout the storage period. Second, six cast samples from each box were packed separately in plastic tissue capsules with grid openings on each side (volume ca. 3 ml; Histosette I, Simport, Beloeil, QC, Canada) and buried at a depth of 30 cm in a pot filled with field soil (volume 40 l) in a greenhouse (mean temperature during storing period: 14.5 ± 3.1 °C). A pooled cast sample of each box and a plastic tissue capsule corresponding to each box were taken every two weeks over a period of 105 days and prepared for analyses.
Isotopic analysis, calculations and statistics
The earthworm cast samples were dried at 60° for 24 h and homogenized with a ball mill. The earthworms taken from the boxes were rinsed individually with water, dried on tissue paper, weighed and deep-frozen (−20 °C). Later on they were dissected and cleaned of internal organs including intestines by rinsing with a fine stream of distilled water. Only the anterior 15 segments of the frozen earthworms were used to avoid contamination from intestinal contents. Earthworm tissue was dried for 24 h at 60 °C and pulverized manually using a mortar and pestle. Earthworm casts and earthworm tissues were analysed for 13C and 15N by continuous flow isotope ratio mass spectrometry (CF-IRMS).
For calculations, isotopic enrichment was expressed in atom % excess (APE), where APE is the difference in atom % between the sample and the natural abundance level of 13C and 15N in the worm tissue or casts from control treatments (L. terrestris: tissue 1.080 ± 0.002 at.% 13C, 0.369 ± 0.0004 at.% 15N, casts 1.096 ± 0.001 at.% 13C, 0.379 ± 0.006 at.% 15N; A. caliginosa: tissue 1.080 ± 0.001 at.% 13C, 0.370 ± 0.001 at.% 15N, casts 1.096 ± 0.001 at.% 13C, 0.378 ± 0.007 at.% 15N).
Since data on isotopic enrichments in tissue and casts of both earthworm species were not normally distributed (not even after transformations), we mainly used non-parametric methods in the statistical analysis. We used Kruskal–Wallis-tests to compare all treatments and Mann–Whitney-U-tests for two-sample comparisons (i.e., comparisons of species and of sampling dates; pairwise treatment comparisons). Relationships between isotopic enrichments in tissue and casts were tested using Spearman correlations when data were not normally distributed, otherwise Pearson correlations were used. For regression analyses (earthworm biomass vs. enrichment) data were log-transformed to achieve a normal distribution. Enrichment data of tissue and casts are given as the mean ± one standard deviation (SD). Statistical analyses were conducted with SPSS 15 for Windows (SPSS Inc., Chicago, IL, USA).
Results
In all tissue and cast samples from L. terrestris and A. caliginosa taken from any of the five treatments, an enrichment of 15N and 13C compared to the control treatments was found (Table 1, Fig. 2).
Table 1.
Enrichment levels (atom % excess, APE, means ± SD) with stable isotopes (13C and 15N) in earthworm tissue and casts (Lumbricus terrestris, Aporrectodea caliginosa) after feeding four days on labelled soil. Results of Kruskal–Wallis–tests comparing five different labelling treatments (for details see Materials and methods section) are provided.
| Treatments |
Kruskall–Wallis (df = 4) |
||||||
|---|---|---|---|---|---|---|---|
| Once + incub | Once + incub + oat | Staggered + incub | Once + no incub | Staggered + no incub | χ2 | P | |
| Tissue – 13C APE | |||||||
| L. terrestris | 0.146 ± 0.063 | 0.021 ± 0.009 | 0.029 ± 0.016 | 0.012 ± 0.004 | 0.026 ± 0.015 | 36.13 | <0.001 |
| A. caliginosa | 0.062 ± 0.075 | 0.019 ± 0.012 | 0.066 ± 0.036 | 0.054 ± 0.044 | 0.058 ± 0.042 | 15.32 | 0.004 |
| Tissue – 15N APE | |||||||
| L. terrestris | 0.556 ± 0.138 | 0.049 ± 0.024 | 0.160 ± 0.079 | 0.082 ± 0.066 | 0.146 ± 0.056 | 41.26 | <0.001 |
| A. caliginosa | 0.352 ± 0.113 | 0.050 ± 0.035 | 0.309 ± 0.138 | 0.363 ± 0.185 | 0.475 ± 0.176 | 29.33 | <0.001 |
| Casts (days 7–21) – 13C APE | |||||||
| L. terrestris | 0.012 ± 0.008 | 0.007 ± 0.003 | 0.002 ± 0.001 | 0.002 ± 0.001 | 0.005 ± 0.005 | 15.89 | 0.003 |
| A. caliginosa | 0.008 ± 0.005 | 0.003 ± 0.003 | 0.007 ± 0.003 | 0.004 ± 0.002 | 0.007 ± 0.004 | 9.03 | 0.060 |
| Casts (days 7–21) – 15N APE | |||||||
| L. terrestris | 0.706 ± 0.069 | 0.080 ± 0.038 | 0.578 ± 0.074 | 0.464 ± 0.154 | 0.513 ± 0.144 | 21.96 | <0.001 |
| A. caliginosa | 0.664 ± 0.168 | 0.110 ± 0.029 | 0.373 ± 0.024 | 0.113 ± 0.038 | 0.426 ± 0.094 | 20.62 | <0.001 |
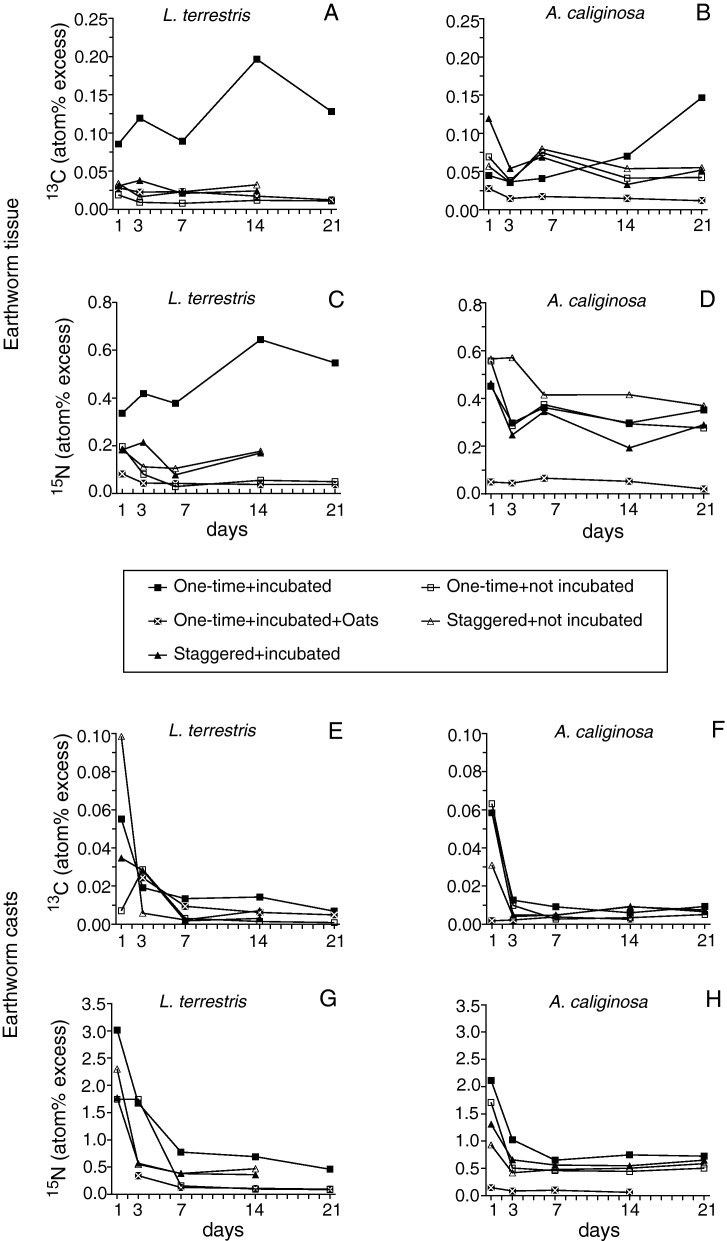
Fig. 2.
Duration of 13C and 15N enrichment of earthworm tissue (A–D) and earthworm casts (E–H) over 21 days after labelling (means, n = 3).
Tissue enrichment levels for 15N and 13C differed significantly between treatments in both earthworm species (Kruskal–Wallis-tests; Table 1). In L. terrestris one treatment (once + incub) resulted in higher enrichment levels than all other treatments (Fig. 2A and C); in A. caliginosa one treatment (once + incub + oat) showed considerable lower APE values than the other treatments (Fig. 2B and D). The addition of oat flakes did not improve the results, but enrichment levels tended to be even lower than in the treatment without oat flakes (once + incub). For 15N in A. caliginosa casts (P = 0.016) and for 15N and 13C in L. terrestris tissue (P < 0.001) these differences were significant (Mann–Whitney-U-tests). For all but one treatment (once + incub + oat), the tissue isotopic enrichment differed between the species (Mann–Whitney-U-tests, P ≤ 0.025). Enrichments in A. caliginosa exceeded values in L. terrestris and in only in one treatment (once + incub) did L. terrestris have a higher enrichment than A. caliginosa. Isotopic enrichment did not decrease significantly from day 1 to day 21 (Mann–Whitney-U-test, P > 0.05); except for 15N APE in A. caliginosa (Mann–Whitney-U-test, P = 0.040).
In earthworm casts, 15N enrichments differed significantly between treatments in both species (Kruskal–Wallis, P < 0.001) while 13C enrichments did not (P ≥ 0.050). Since enrichment levels were obviously higher on the first two sampling dates (Fig. 2E–H), treatments were also compared from day 7 on, which revealed significant differences between treatments in 15N and 13C enrichments in L. terrestris and A. caliginosa (Kruskal–Wallis, Table 1). Overall the treatment “once + incub” had the highest and the treatment “once + incub + oat” the lowest APE values in almost all cases (Fig. 2E–H). The 15N and 13C enrichment of casts showed a similar exponential decline for both species in all treatments during the first three days but stayed approximately at the same level from day 7 to day 21 (Fig. 2E–H). Enrichment levels differed significantly between day 1 and day 21 for 15N as well as for 13C in both species (Mann–Whitney-U-tests, P ≤ 0.003), but not between days 7 and 21 (Mann–Whitney-U-tests, P ≥ 0.050). Generally, species did not differ significantly in 15N and 13C enrichment in their casts (Mann–Whitney-U-test, P ≥ 0.500), except for the treatment “once + incub + oat” in which L. terrestris casts showed significantly higher APE values than those observed in A. caliginosa (Mann–Whitney-U-test, P = 0.004).
The 15N enrichment in casts stored in the climate chamber was significantly higher over the whole course of the storage period than in the soil stored casts in the greenhouse (Mann–Whitney-U-test, P = 0.005; Fig. 3A); no such difference was observed for 13C (Mann–Whitney-U-test, P = 0.074; Fig. 3B). After 90 days enrichment levels had not decreased significantly compared to the start of the storage period on day 35 (Mann–Whitney-U-test, P ≥ 0.500).
Fig. 3.
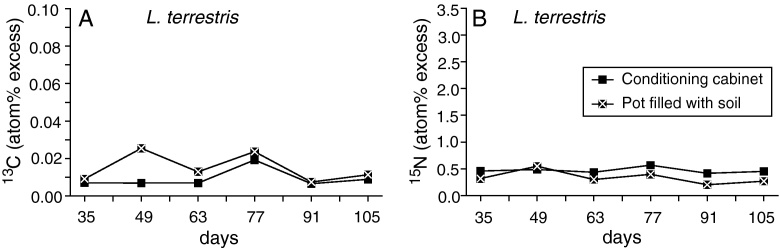
Duration of 13C (A) and 15N (B) enrichment of labelled L. terrestris casts from the treatment once + incub stored in an conditioning cabinet at constant temperature (15 °C) or in soil in a greenhouse (mean temperature 15 °C) from day 35 to day 105 after labelling (for details see Materials and methods section; means, n = 3).
The 15N and 13C enrichments were positively correlated in the tissue as well as in the casts in both species (Table 2); similarly, the enrichments in tissue and in the casts, respectively, were positively correlated for both stable isotopes, 15N and 13C (Table 2). For L. terrestris the 13C enrichment of casts was positively correlated with the initial earthworm biomass (r2 = 0.827, P < 0.01); no such correlation was found for 15N or between A. caliginosa biomass and the isotopic enrichment in their casts (P ≥ 0.050).
Table 2.
Spearman correlations between 15N and 13C APE for labelled tissue and casts of Lumbricus terrestris and Aporrectodea caliginosa.
|
L. terrestris |
A. caliginosa |
|||
|---|---|---|---|---|
| rs | P | rs | P | |
| 15N APE vs. 13C APE | ||||
| Tissue | 0.782 | <0.001 | 0.643 | <0.001 |
| Casts | 0.703 | <0.001 | 0.648 | <0.001 |
| Tissue vs. casts | ||||
| 15N | 0.757 | <0.001 | 0.512 | <0.001 |
| 13C | 0.462 | 0.001 | 0.424 | 0.001 |
Discussion
This is the first study attempting to isotopically label two different functional groups of earthworms using the same method. We could demonstrate that tissue and casts of adults of two different earthworm species can be isotopically labelled in a technically simple way by cultivating them in soil enriched with 15N and 13C for only four days. From the different variants studied, a one-time addition of isotopes resulted in higher enrichments than a staggered addition of isotopes. For both species, a higher enrichment in tissue always correlated with a higher enrichment in casts. We also demonstrated that isotopically labelled casts can be stored over a period of at least 105 days without significantly decreasing their isotopic signals. It is noteworthy that the method works equally well for earthworms belonging to different functional groups differing in their feeding habits (i.e., soil-feeding A. caliginosa vs. litter-feeding L. terrestris) (Curry and Schmidt 2007).
Isotopic enrichment in earthworm tissue
Although we found significant differences between the two earthworm species in isotopic tissue enrichment for certain treatments, the enrichment levels were comparable and no consistent patterns could be seen. Contrary to our expectations, the addition of oat flakes to the labelled soil did not improve the incorporation of isotopic labels into earthworm tissue. This indicates that (i) the method works equally well for earthworms that are not preferential soil-feeders and (ii) it is not necessary to feed L. terrestris additional plant litter, as Dyckmans et al. (2005) proposed for litter-feeding earthworms. In contrast, the finding that the addition of oat flakes affected A. caliginosa more than L. terrestris suggests that the endogeic species is better able to collect small highly palatable food particles than the anecic species. Furthermore, the uptake of non-labelled C and N from this additional food could actually dilute the isotopic signal.
The anecic species, L. terrestris, is one of the most active earthworm species in temperate soils but has never been investigated in this respect before and our results show that cultivating this species, as well as A. caliginosa, for four days in enriched soil can result in a stable signature in its tissue for at least 21 days. In the study by Dyckmans et al. (2005), tissue of A. caliginosa had isotopic enrichments about 20% higher for 15N and almost five times higher for 13C than in our study, although the amount of 15N and 13C added to the soil and the average A. caliginosa biomass were similar in both studies. However, isotopic incorporation can vary considerably between individuals due to differences in physiological condition, growth and protein turnover (Martinez del Rio et al. 2009). Similarly, Whalen and Janzen (2002) and Dyckmans et al. (2005) reported that differences in biomass cause enrichment variability.
In our study, we observed considerable differences in earthworm condition, between individuals as well as between boxes. Some earthworms were in suboptimal condition resulting in overall data variability, partly reduced activity and higher mortality (see missing data points in Fig. 2) that could be associated with low enrichment levels. L. terrestris had considerably higher enrichment in the “once + incub” treatment than in other treatments, but comparable to the highest enrichments in A. caliginosa. In contrast, enrichments in the treatment “once + incub + oat” in A. caliginosa were low compared to other treatments, but still at levels similar to some L. terrestris treatments.
Isotopic enrichment in earthworm casts
This study is the first to test the feasibility of dual-labelling earthworm casts with 15N and 13C in a technically simple way: feeding labelled soil to the earthworms and collecting their casts. The results show that even the simplest treatment, without incubation of the ammonium nitrate and with a one-time addition of glucose to the soil, resulted in casts being readily with stable isotopes. It is possible to store labelled casts over a period of 105 days without a significant loss of the labelling signal, which is very useful for planning and preparing experiments where labelled casts are needed. The strength of the isotopic signal in casts differed from that in earthworms tissue, especially between day one and day seven. The latter showed an exponential decrease during this period while the signal in tissue remained stable. This rapid loss during the first days indicates that earthworms still may have had labelled soil in their guts after the transfer to the unlabelled soil, which led to the high amount of label signal on day one. After day seven, the signal in the casts remained stable until day 21 although earthworms fed on unlabelled soil and would thus have diluted the isotopic signal. Dyckmans et al. (2005) found a similar pattern for mucus enrichment in A. caliginosa and suggested that two different pools of 15N and 13C with different turnover times might be responsible for this pattern. Further work would be needed to determine nutrient fluxes and turnover rates in earthworm tissue and casts.
Applications in ecological research
The primary aim of the current work was to test the possibility of producing isotopically labelled earthworms and casts that could be used as a tool in studying functional relationships between earthworms and associated organisms (Wurst and Jones, 2003, Wurst et al., 2004, Eisenhauer et al., 2009). Labelled casts could be used to study their utilisation by plants (Zaller and Arnone 1999b) and other organisms or to track the predation upon earthworms. The stable signal in casts would also enable longer-term experiments investigating the role of these nutrient-rich soil microsites for plant nutrition and competitive interactions in plant communities. A better understanding of plant–earthworm-interactions is needed since there is increasing evidence that potential global climate change will significantly affect interactions between plants and earthworms with consequences for ecosystem processes (elevated CO2: Yeates et al., 1997, Zaller and Arnone, 1997, Zaller and Arnone, 1999b; ultraviolet-B radiation and warming: Zaller et al. 2009).
Although our results did not clearly identify the best treatment, we recommend adding the labelled glucose and ammonium nitrate all at once and incubating the labelled substrate (once + incub) since this variant resulted in consistently good enrichment levels and was easy to prepare with no need for additional food for earthworms. In summary, the method presented in this study for producing isotopically labelled earthworm casts and tissue proved to be simple, effective and applicable both for soil-feeding and litter-feeding earthworms.
Acknowledgements
We are grateful to Lina Weissengruber, Lisa Kargl, Birgit Putz and Norbert Schuller for help in the laboratory. We thank Olaf Schmidt and two anonymous reviewers for their comments which helped to improve this manuscript. This research was supported by the Austrian Science Fund (grant no. P20171-B16).
References
- Barois I., Verdier B., Kaiser P., Mariotti A., Rangel P., Lavelle P. Influence of the tropical earthworm Pontoscolex corethrurus (Glososcolecidae) on the fixation and mineralization of nitrogen. In: Bonvicini Pagliai A.M., Omodeo P., editors. On Earthworms. Collana Unione Zoologica Italiana Selected Symposia and Monographs No. 2; Modena, Italy: 1987. pp. 151–158. [Google Scholar]
- Binet F., Trehen P. Experimental microcosm study of the role of Lumbricus terrestris (Oligochaeta, Lumbricidae) on nitrogen dynamics in cultivated soils. Soil Biol. Biochem. 1992;24:1501–1506. [Google Scholar]
- Bouché M.B. Strategies lombriciennes. In: Lohm U., Persson T., editors. Soil Organisms as Components of Ecosystems. Ecol. Bull. 25; Stockholm, Sweden: 1977. pp. 122–133. [Google Scholar]
- Brown G.G., Barois I., Lavelle P. Regulation of soil organic matter dynamics and microbial activity in the drilosphere and the role of interactions with other edaphic functional domains. Eur. J. Soil Biol. 2000;36:177–198. [Google Scholar]
- Chaoui H., Edwards C.A., Brick M., Lee S., Arancon N.Q. Proc. Brighton Crop Prot. Conf. Pests Diseases 2 (8B-3) 2002. Suppression of the plant disease pythium, rhizoctonia, and verticillium by vermicomposting; pp. 711–716. [Google Scholar]
- Curry J.P., Byrne D., Boyle K.E. The earthworm population of a winter cereal field and its effects on soil and nitrogen turnover. Biol. Fertil. Soils. 1995;19:166–172. [Google Scholar]
- Curry J.P., Schmidt O. The feeding ecology of earthworms – A review. Pedobiologia. 2007;50:463–477. [Google Scholar]
- Decaens T., Mariani L., Lavelle P. Soil surface macrofaunal communities associated with earthworm casts in grasslands of the Eastern Plains of Colombia. Appl. Soil Ecol. 1999;13:87–100. [Google Scholar]
- Doube B.M., Schmidt O., Killham K., Correll R. Influence of mineral soil on the palatability of organic matter for lumbricid earthworms: A simple food preference study. Soil Biol. Biochem. 1997;29:569–575. [Google Scholar]
- Dyckmans J., Scrimgeour C.M., Schmidt O. A simple and rapid method for labelling earthworms with 15N and 13C. Soil Biol. Biochem. 2005;37:989–993. [Google Scholar]
- Edwards C.A., Bohlen P.J. 3rd edn. Chapman & Hall; London: 1996. Biology and Ecology of Earthworms. [Google Scholar]
- Edwards C.A., Bohlen P.J., Linden D.R., Subler S. Earthworms in agroecosystems. In: Hendrix P.F., editor. Earthworm Ecology and Biogeography in North America. Lewis Publishers; MI, USA: 1995. pp. 185–213. [Google Scholar]
- Eisenhauer N., König S., Sabais A.C.W., Renker C., Buscot F., Scheu S. Impacts of earthworms and arbuscular mycorrhizal fungi (Glomus intraradices) on plant performance are not interrelated. Soil Biol. Biochem. 2009;41:561–567. [Google Scholar]
- Eisenhauer N., Milcu A., Sabais A.C.W., Scheu S. Animal ecosystem engineers modulate the diversity–invasibility relationship. PLoS One. 2008;3:e3489. doi: 10.1371/journal.pone.0003489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhauer N., Scheu S. Earthworms as drivers of the competition between grasses and legumes. Soil Biol. Biochem. 2008;40:2650–2659. [Google Scholar]
- Elfstrand S., Lagerlöf J., Hedlund K., Martensson A. Carbon routes from decomposing plant residues and living roots into soil food webs assessed with C-13 labelling. Soil Biol. Biochem. 2008;40:2530–2539. [Google Scholar]
- Hameed B., Bouché M.B., Cortez J. Etudes in situ des transferts d’azote d’origine lombricienne (Lumbricus terrestris L.) vers les plantes. Soil Biol. Biochem. 1994;26:495–501. [Google Scholar]
- James S.W. Soil, nitrogen, phosphorus, and organic matter processing by earthworms in Tallgrass Prairie. Ecology. 1991;72:2101–2109. [Google Scholar]
- Jones C.G., Lawton J.H., Shachak M. Organisms as ecosystem engineers. Oikos. 1994;69:373–386. [Google Scholar]
- Lavelle P. Earthworm activities and the soil system. Biol. Fertil. Soils. 1988;6:237–251. [Google Scholar]
- Martin A., Balesdent J., Mariotti A. Earthworm diet related to soil organic matter dynamics through 13C measurements. Oecologia. 1992;91:23–29. doi: 10.1007/BF00317236. [DOI] [PubMed] [Google Scholar]
- Martin A., Mariotti A., Balesdent J., Lavelle P. Soil organic matter assimilation by a geophagous tropical earthworm based on delta 13C measurement. Ecology. 1992;73:118–128. [Google Scholar]
- Martinez del Rio C., Wolf N., Carleton S.A., Gannes L.Z. Isotopic ecology ten years after a call for more laboratory experiments. Biol. Rev. 2009;84:91–111. doi: 10.1111/j.1469-185X.2008.00064.x. [DOI] [PubMed] [Google Scholar]
- McKenzie B.M., Dexter A.R. Physical properties of casts of the earthworm Aporrectodea rosea. Biol. Fertil. Soils. 1987;5:152–157. [Google Scholar]
- Scheu S. Microbial activity and nutrient dynamics in earthworm casts (Lumbricidae) Biol. Fertil. Soils. 1987;5:230–234. [Google Scholar]
- Scheu S. Mucus excretion and carbon turnover of endogeic earthworms. Biol. Fertil. Soils. 1991;12:217–220. [Google Scholar]
- Scheu S. There is an earthworm mobilizable nitrogen pool in soil. Pedobiologia. 1993;37:243–249. [Google Scholar]
- Scheu S., Falca M. The soil food web of two beech forests (Fagus sylvatica) of contrasting humus type: stable isotope analysis of a macro- and a mesofauna-dominated community. Oecologia. 2000;123:285–296. doi: 10.1007/s004420051015. [DOI] [PubMed] [Google Scholar]
- Schmidt O., Curry J.P., Dyckmans J., Rot E., Scrimgeour C.M. Dual stable isotope analysis (delta 13C and delta 15N) of soil invertebrates and their food sources. Pedobiologia. 2004;48:171–180. [Google Scholar]
- Schmidt O., Scrimgeour C.M., Handley L.L. Natural abundance of N-15 and C-13 in earthworms from a wheat and a wheat-clover field. Soil Biol. Biochem. 1997;29:1301–1308. [Google Scholar]
- Schrader S., Zhang H. Earthworm casting: stabilization or destabilization of soil structure? Soil Biol. Biochem. 1997;29:469–475. [Google Scholar]
- Seeber J., Langel R., Meyer E., Traugott M. Dwarf shrub litter as a food source for macro-decomposers in alpine pastureland. Appl. Soil Ecol. 2009;41:178–184. [Google Scholar]
- Spain A., Feuvre R.L. Stable C and N isotope values of selected components of a tropical Australian sugarcane ecosystem. Biol. Fertil. Soils. 1997;24:118–122. [Google Scholar]
- Spain A.V., Saffigna P.G., Wood A.W. Tissue carbon sources for Pontoscolex corethrurus (Oligocheta: Glossoscolecidae) in a sugarcane ecosystem. Soil Biol. Biochem. 1990;22:307–703. [Google Scholar]
- Whalen J.K., Janzen H.H. Labeling earthworms uniformly with 13C and 15N: implications for monitoring nutrient fluxes. Soil Biol. Biochem. 2002;34:1913–1918. [Google Scholar]
- Whalen J.K., Parmelee R.W., Subler S. Quantification of nitrogen excretion rates for three lumbricid earthworms using 15N. Biol. Fertil. Soils. 2000;32:347–352. [Google Scholar]
- Willems J.H., Huijsmans K.G.A. Vertical seed dispersal by earthworms: A quantitative approach. Ecography. 1994;17:124–130. [Google Scholar]
- Wurst S., Dugassa-Gobena D., Langel R., Bonkowski M., Scheu S. Combined effects of earthworms and vesicular–arbuscular mycorrhizas on plant and aphid performance. New Phytol. 2004;163:169–176. doi: 10.1111/j.1469-8137.2004.01106.x. [DOI] [PubMed] [Google Scholar]
- Wurst S., Jones T.H. Indirect effects of earthworms (Aporrectodea caliginosa) on an above-ground tritrophic interaction. Pedobiologia. 2003;47:91–97. [Google Scholar]
- Yeates G.W., Tate K.R., Newton P.C.D. Response of the fauna of a grassland soil to doubling of atmospheric carbon dioxide concentration. Biol. Fertil. Soils. 1997;25:307–315. [Google Scholar]
- Zaller J.G., Arnone J.A. Activity of surface-casting earthworms in a calcareous grassland under elevated atmospheric CO2. Oecologia. 1997;111:249–254. doi: 10.1007/PL00008817. [DOI] [PubMed] [Google Scholar]
- Zaller J.G., Arnone J.A. Earthworm responses to plant species’ loss and elevated CO2 in calcareous grassland. Plant Soil. 1999;208:1–8. [Google Scholar]
- Zaller J.G., Arnone J.A. Interactions between plant species and earthworm casts in a calcareous grassland under elevated CO2. Ecology. 1999;80:873–881. [Google Scholar]
- Zaller J.G., Caldwell M.M., Flint S.D., Ballaré C.L., Scopel A.L., Sala O.E. Solar UV-B and warming affect decomposition and earthworms in a fen ecosystem in Tierra del Fuego, Argentina. Global Change Biol. 2009;15:2493–2502. [Google Scholar]
- Zaller J.G., Saxler N. Selective vertical seed transport by earthworms: implications for the diversity of grassland ecosystems. Eur. J. Soil Biol. 2007;43:S86–S91. [Google Scholar]