Abstract
Cannabis remains one of the world’s most widely used substance of abuse amongst pregnant women. Trends of the last 50 years show an increase in popularity in child-bearing women together with a constant increase in cannabis potency. In addition, potent herbal “legal” highs containing synthetic cannabinoids that mimic the effects of cannabis with unknown pharmacological and toxicological effects have gained rapid popularity amongst young adults. Despite the surge in cannabis use during pregnancy, little is known about the neurobiological and psychological consequences in the exposed offspring. In this review, we emphasize the importance of maternal programming, defined as the intrauterine presentation of maternal stimuli to the foetus, in neurodevelopment. In particular, we focus on cannabis-mediated maternal adverse effects, resulting in direct central nervous system alteration or sensitization to late-onset chronic and neuropsychiatric disorders. We compare clinical and preclinical experimental studies on the effects of foetal cannabis exposure until early adulthood, to stress the importance of animal models that permit the fine control of environmental variables and allow the dissection of cannabis-mediated molecular cascades in the developing central nervous system. In sum, we conclude that preclinical experimental models confirm clinical studies and that cannabis exposure evokes significant molecular modifications to neurodevelopmental programs leading to neurophysiological and behavioural abnormalities.
Keywords: Foetal development, Endocannabinoid, THC, Neuropsychiatric disease
Introduction
In 2010, with an estimated annual prevalence ranging from 2.6 to 5 % of the adult population (between 119 million and 224 million estimated users aged 15–64), cannabis remains one of the world’s most widely used substance of abuse [1]. Recent data from the European Monitoring Centre for Drugs and Drug Addiction (2013) present that the global popularity of cannabis is stable, in contrast to the continuous decline of “hard drugs”, such as heroin and cocaine. Conversely, cannabis use in teenagers and young adults is rising [2]. Data from the 2013 European Drug Report indicate that European cannabis consumption has increased in a small number of countries (Bulgaria, Estonia, Finland and Sweden), while in others it has stabilized (e.g. eastern and south-eastern Europe) or decreased (e.g. Western and Central Europe) [1–3].
Despite its stable global prevalence, the social and political status of cannabis consumption changed remarkably during the last century. In Europe, as in the USA, the policy on cannabis possession for personal use is one of the most controversial political issues. While cannabis is classified as a narcotic drug, under the control of the United Nations and by all EU Member States from 1961 (United Nations Single Convention on Narcotic Drugs), the measures adopted to regulate it at a national level vary considerably. This has resulted in a heterogeneous “legal map” regarding cannabis offences. For example, in Belgium, Germany, Greece and Austria, “small amounts” for personal use are locally tolerated. Alternatively, a drug awareness course (stage de sensibilisation; France) may be ordered in cases of occasional consumption, while in the Netherlands the possession or sale in “coffee shops” up to 5 g is generally not penalized. Other countries apply administrative sanctions or fines (Denmark, Czech Republic, Estonia, Ireland, Italy Latvia, Luxemburg, Portugal, Slovenia, Finland, Sweden, Croatia and Norway); while still others apply penal sanctions (Cyprus, Hungary, Poland, Slovak Republic and UK) [4]. Beside the geographical fluctuation of the legal status of cannabis, the acceptance of cannabis consumption, either medical or recreational, increased during the last decade. At the time of this review, 21 states in the USA and an increasing number of countries in Europe allowed medical cannabis use under specified conditions. Medical cannabis, or purified THC (dronabinol), is used successfully in the clinic as an (1) anti-nausea and antiemetic, (2) antispasmodic and analgesic, (3) anti-inflammatory and anti-allergic and (4) anti-epilepsy drug [5]. In addition to the constant increase in European countries legalizing medical cannabinoid use, recreational cannabis consumption was recently legalized in Colorado and Washington states, supporting the notion that cannabis consumption is gaining acceptance.
However, together with its rising popularity amongst young adults [1], cannabis potency has gradually increased since selective cultivation boosted the level of its main psychoactive compound, Δ-9-Tetrahydrocannabinol (THC) [1, 2]. Depending on the strain and production site, the actual mean THC content of cannabis herb ranges from 3 to 17 % [2]. For example, the Netherlands, where cannabis is decriminalized, registered a sharp increase in THC concentration of up to 17 % in herb and 40 % in cannabis resin preparations in the last decade alone. In contrast, other countries, such as Germany, presented a more stable THC concentration (8 % for both herb and resin) [1, 2] stressing the need to recognize THC content when studying and comparing cannabis’ effect on physical and psychological health.
The criminalization of cannabis use led to the recent development of herbal “legal” high products (e.g. Spice), containing synthetic cannabinoids mimicking THC’s effects [6, 7]. These cannabinomimetics are of higher potency than THC and lead to a wide variety of negative effects compared with cannabis itself [8]. Despite the strict monitoring of synthetic cannabinoids at the European level in the Early Warning System (EWS) on new psychoactive substances, little is known about the pharmacology and toxicology of these cannabinomimetics in humans [9]. The 2012 US Monitoring the Future survey of students is the most robust prevalence dataset on the use of synthetic cannabinoids and reported an 8.8 and 11.3 % consumption for those aged 15/16 and 17/18 years, respectively [3].
While cannabis and synthetic cannabinoids remain most popular amongst males, studies demonstrate that cannabis use during pregnancy steadily increases. Recent population statistics reveal that over 10 % of pregnancies in the US and Europe are associated with maternal cannabis exposure [10]. Despite the high incidence of prenatal cannabis use in society, a limited set of data is available on the incidence of foetal cannabis exposure and its neurobiological and psychological consequences in the exposed offspring. Data from the 2012 National Survey on Drug Use and Health (NSDUH) in the USA clearly show a significant change in prevalence trends with the age of the consumer [11], from 5.9 % illicit drug users amongst pregnant women aged 15–44, up to 18.3 % amongst pregnant women aged 15–17 [11]. In addition to an increase in pregnancy rates [12], the treatment episode data set reports that 72.9 % of pregnant teen admissions to rehabilitation clinics used cannabis, which is relatively more than alcohol consumption (45.7 %) [13].
Despite the available wealth of population data regarding worldwide cannabis consumption, the literature focusing on cannabis-induced foetal developmental complications in humans is still lacking (for review see [14–17]), particularly when compared to studies on nicotine and alcohol [18–20]. With the increasing accessibility to cannabis, together with its increasing strength, the advent of potent synthetic mixtures and the growing number of cannabis users during pregnancy, the need arises to thoroughly address the prenatal consequences associated with foetal cannabis exposure. Therefore, we compared preclinical (animal models) and clinical longitudinal human studies on prenatal cannabis exposure and offspring outcome and summarized their most significant neurobiological effects at the molecular, cellular and systems neuroscience levels to provide an overview of cannabis-induced influences on brain development, offspring behaviour and late-life neurological disorders. We conclude that foetal cannabis exposure disturbs fine-tuned molecular signalling pathways and leads to altered brain circuit formation, underpinning long-lasting physiological and behavioural alterations.
Maternal drug-induced malprogramming and foetal development
The prenatal period plays a fundamental role in the proper development of the foetal brain [21]. In the past years, the scientific community increasingly recognizes the critical importance of the prenatal and early postnatal developmental period in chronic and psychiatric disease, highlighting a new scientific approach to medical issues [22] [23]. Here, we propose that maternal programming, defined as the in utero presentation of external stimuli to the foetus, is a major contributor to foetal development. External stimuli, including nutrients or stress hormones, can affect embryonic signalling systems, resulting in long-lasting or even permanent alterations to organogenesis, including brain circuit formation, leading to the in utero adaptation of the foetus to an anticipated non-physiological environment once born. However, in the case of substance abuse, such as alcohol and cannabis consumption, these stimuli can induce aberrant signalling events and lead to physiological complications and neuropsychiatric disease [15, 18, 24]. One such example is tobacco exposure during pregnancy, resulting in adverse effects to offspring health [25], spontaneous abortions [26] and reduced intrauterine growth [27]. In a recent study, foetal tobacco exposure was linked to alterations in brain morphology, in particular reduced brain volumes and cortical thinning, as well as increased measures of affective problems [28] confirming previous findings in rodents [29, 30].
In this review, we approach the source of developmental deficits through two different mechanisms originating from maternal programming. In utero stimuli, or the lack thereof through nutrient deficits, can result in (1) a direct pathological condition visible from the early ages (e.g. microcephaly in foetal alcohol exposure [31]) or (2) an alternative indirect non-symptomatic sensitivity of neuronal circuits. This latter “double hit” hypothesis involves the generation of an imbalanced brain circuit at sub-threshold levels (non-manifested first hit during foetal development) that can precipitate neurodevelopmental disease by otherwise sub-threshold stimuli (second hit) later in life. Here, we will employ these two types of maternal malprogramming to compare the neurodevelopmental alterations induced by in utero cannabis exposure between animal models and human longitudinal studies.
THC readily passes through the placenta [32, 33] and is correlated with direct physiological effects including human foetal distress and growth retardation [34–36]. Moreover, studies showing a significant contribution of early cannabis exposure to the susceptibility to neuropsychiatric disorders (e.g. schizophrenia) [37, 38] have reinforced the hypothesis that cannabis can act as an indirect “double hit” stressor to precipitate otherwise unrevealed diseases in later stages of life. Therefore, uncovering the signalling molecules that link maternal inputs to foetal development, especially in the case of maternal cannabis use, and revealing their mechanisms, sites of action and causal relationships to postnatal illnesses is imperative to efficiently deal with unwanted effects in the offspring.
Evidence from human longitudinal studies
Detrimental effects of prenatal cannabis exposure have been investigated in three major prospective longitudinal studies with follow-up data on the offspring beyond the early neonatal period (Fig. 1). (1) The Ottawa Prenatal Prospective Study (OPPS) from the late 1970s by Fried et al. (for detailed description, see [39, 40]). It included a low-risk, European–American, middle-class sample of pregnant women. (2) The Maternal Health Practices and Child Development Study (MHPCD) started in 1982 [35]. This study focused on high-risk pregnant women, with a low socioeconomic status of mixed ethnicity (57 % of African–American ethnicity). (3) The Generation R study started in 2001 and encompasses a multi-ethnic population-based prospective cohort study from foetal life until adulthood in the Netherlands (for details see [41, 42]). The mothers from the Generation R cohort were also studied in regard to the determinants for their cannabis use during pregnancy, which were strongly related to cannabis use by the biological father, being single, childhood trauma and delinquency, but not maternal age, ethnicity, psychopathology, family functioning and perceived stress [43].
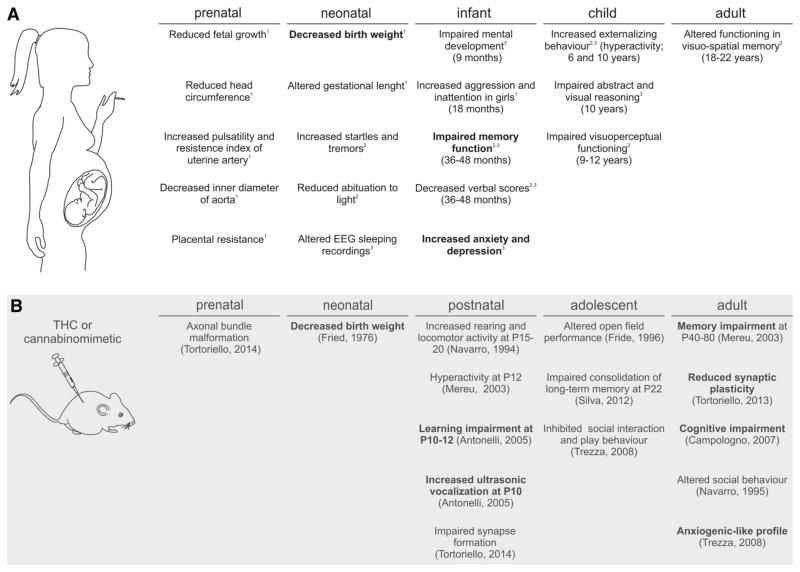
Fig. 1.
Main physiological effects of in utero cannabis exposure in human and animal studies. a Overview of the major physiological complications found in prenatal cannabis exposure from human longitudinal studies: (1) the Generation “R” study, (2) the OPPS study and (3) the MHPC study. b List of comparable animal studies directly (bold) or indirectly, reflecting physiological findings from human studies
While the first two longitudinal studies contain data spanning a large part of adolescent offspring (up to 22 and 14 years old, respectively), the recent initiation of the Generation R study only allows follow-up results until the first years of life (n = 9,778 mothers, 80 % follow-up rates until age 6 years). The results of these studies, of which the most significant ones are presented in this review, are clustered in neonatal findings (up to 10-month-old offspring) and child findings (up to 5- and 10-year-old offspring). Since cannabis is often combined with tobacco, cannabis-exposed offspring was compared with tobacco-exposed and non-exposed individuals to exclude the effects of tobacco in the studies described below. However, care should be taken to compare these studies since THC content steadily increased in cannabis preparations over the past years [1, 2]. Below, we summarize their main physiological and psychological findings correlated with foetal cannabis exposure.
Neonatal physical findings and behaviour
The MHPCD and OPPS studies assessed neonatal growth parameters within 48 h after birth [36], while the Generation R study examined foetal growth parameters [41, 44]. The Generation R study reports a lower birth weight and a reduction in the head circumference of the offspring [45] of mothers who used cannabis as compared to non-users and tobacco users, similar to earlier reports [33, 46]. Since it was hypothesized that cannabis exposure could provoke adaptations to the vascular system, foetal circulation variables were assessed in a subset of neonates in the Generation R study [14, 44]. Accordingly, an increase in foetal pulsatility index, described as the variability in blood velocity in a vessel, was shown. In addition, the resistance index of the uterine artery was found elevated after cannabis exposure, suggesting increased placental resistance during pregnancy [47]. This, together with data showing a reduced inner diameter of the aorta in cannabis-exposed foetuses [48], can explain foetal growth retardation due to a diminished accessibility of oxygen and nutrients. The lack of nutrients thus limits proper organogenesis and is detrimental for the development of the foetal nervous system. Despite the variability of the results in behavioural data obtained, there is evidence for foetal irritability, expressed as increased tremors, startles and altered sleep patterns in the offspring [39, 49]. However, to reach a definite conclusion, these data need adequate controls of potential confounding factors, including socioeconomics and ethnicity [50].
Child (<5 years) behaviour and cognitive development
Based on the MHPCD study, in addition to growth deficits, decreased mental scores, evaluated as sensory/perceptual acuities and response, memory, learning and vocalization parameters (noticeable up to 1 year of age) are evident in cannabis-exposed offspring that suggests impaired mental development [51]. By the age of 3–4 years, in addition to those in the MHPCD population, a small subgroup of the OPPS cohort indicated a negative association between prenatal cannabis exposure and verbal and memory functioning. While these cognitive deficits could not be reproduced in the larger and more recent Generation R study, there was evidence for a temporary effect on girls’ aggression and inattention levels [41]. Other human non-longitudinal studies have substantiated such sexual dimorphisms. For example, impaired dopamine receptor expression in amygdala regions is most evident in males in association with prenatal cannabis exposure [52] and 10-year-old boys are more susceptible to behavioural problems than girls [53].
Child (<10 years) behaviour and cognitive development
By now, the OPPS and MHPCD studies have analysed data of prenatally cannabis-exposed offspring beyond infancy. Externalizing behaviour symptoms (that include hyperactivity, inattention, impulsive symptoms and delinquency) were reported in children at ages 6 (OPPS and MHPCD) and 10 years (MHPCD) after prenatal cannabis exposure [54–56].
Moreover, prenatal cannabis exposure was found to be associated with cognitive behavioural aspects that fall in executive function domains [16, 40, 49, 57], which are higher-order cognitive functions including sustained and focused attention and planning and working memory [58]. Impairment in executive function, therefore, has a notable impact on daily life experiences, as the inability of planning, organizing, prioritizing, paying attention to and remembering details and controlling emotional reactions. In particular, prenatal cannabis exposure seems to affect attention/impulsivity and problem-solving situations that require integration and manipulation of basic visuoperceptual skills [16, 40, 49, 57]. Interestingly, these deficits in executive functions seem to be long lasting, since 18- to 22-year-old young adults with prenatal cannabis exposure demonstrate altered neuronal functioning during visuo-spatial working memory processing [59].
The MHPCD study also provided insights about the effect of prenatal cannabis exposure on school achievement. Exposure during the first trimester predicted deficits in reading and spelling scores, and lower child performance at 10 years of age [55]. Moreover, second-trimester cannabis use was significantly associated with reading comprehension and underachievement [53]. However, these findings have not yet been examined in the Generation R population, thus for now preventing the formation of definite conclusions regarding prenatal cannabis exposure on cognitive behaviour in early childhood [42].
The importance of animal studies
Environmental and genetic factors are difficult to control in human studies. The resulting variability increases the complexity of analysis aimed to make causal links between cannabis use during pregnancy and the offspring’s neurodevelopment. One strategy to evaluate this relationship, permitting a high level of control of genetics, environmental factors (such as dietary, disease and THC pharmacology), is the use of animal models. Therefore, a wide array of animal studies has been performed to dissect the molecular, physiological and behavioural effects of pre-natal cannabis exposure. For example, the behavioural alterations found in the OPPS and MHPCD studies matched earlier findings in rodent models from almost a decade ago. These studies demonstrated altered spontaneous locomotor and exploratory behaviours [60], as well as impairments in social interactions and behavioural responses to novelty [60]. In addition, prenatal cannabinoid exposure resulted in lower memory function and motor hyperactivity [61], similar to human studies [55]. A more recent study revealed that prenatal THC exposure causes an increased rate of ultrasonic vocalizations, a sign of distress and anxiety, in rat offspring, again similar to earlier human foetal distress [57]. This suggests an emotional reactivity that can be altered by THC exposure, resulting in effects on serotonin and dopamine release [62]. This can provide the background for the depressive symptoms found in children at age 10 in the MPHCD study (e.g. [53, 55]).
In addition, a recent study investigating transgenerational effects of parental germline exposure demonstrates that adolescent cannabis consumption can alter reproductive cells, leading to molecular abnormalities in the offspring’s striatum as well as increasing drug-seeking behaviour [63]. Thus, preclinical results reinforce that prenatal cannabis exposure, either through germline transmission or directly during pregnancy, may affect foetal developmental and behavioural outcomes. In particular, findings relating to diminished habituation and affected memory functioning corroborate findings of the OPPS and MHPCD studies (as discussed above, e.g. [49, 64]). In sum, animal models prove to be a valid and highly sensitive method to predict physiological and behavioural effects of prenatal cannabis exposure.
Endocannabinoid signalling in the foetal brain
The CB1 cannabinoid receptor (CB1R) is one of the most abundant G protein-coupled receptor (GPCR) in the adult brain [65]. It is the major target of the main psychoactive compound of cannabis, THC [66]. In addition, THC can also signal through the cannabinoid receptor type 2 (CB2R; [67] and G protein-coupled receptor 55 (GPR55) [68] identified in the human adult brain [69]. Traditional classifications posit CB1Rs as the major central nervous system cannabinoid receptor (“brain type”), while CB2Rs are mainly restricted to the periphery and involved in the modulation of immune responses (“spleen type”) [70]. However, this dogma has recently been challenged, since CB1Rs also seem to be involved in immunomodulatory responses [71] and functional CB2Rs have been localized to neurons [72, 73], increasing the complexity of this signalling system.
CB1R mRNA has been detected as early as the preimplantation period [74] and is involved in embryo implantation [75], embryonic growth [36] and neuronal development [76]. In the developing central nervous system of rodents, CB1R mRNA and receptor density gradually increase during foetal development [77, 78] from day 11 of gestation (comparable to 5/6 weeks in the human embryo). In human foetuses, CB1Rs were detected at week 14 of gestation with a similar developmental pattern during pre- and postnatal development as observed in rodents [52, 79].
The presence and distribution of THC-sensitive cannabinoid receptors in the developing and adult brain, argues for the existence of endogenous ligands. These endogenous ligands (Fig. 2a) have been discovered to be arachidonic acid derivatives with functional similarity to THC and are therefore termed as “endocannabinoids” [80]. The two major endocannabinoids are anandamide (AEA) [80] and 2-arachidonoyl glycerol (2-AG) [81]. The synthesis of AEA involves the recruitment of Ca2+-dependent N-acyl-phosphatidyl-ethanolamine-selective phospholipase D (NAPE-PLD) [82], while 2-AG is synthesized by sn-1-diacylglycerol lipases α and β (DAGLα/β) [78]. AEA and 2-AG are degraded by fatty-acid amide hydrolase (FAAH) [83] and monoacylglycerol lipase (MAGL) together with α/β hydrolase domain-containing protein 6 (ABHD6) [84], respectively.
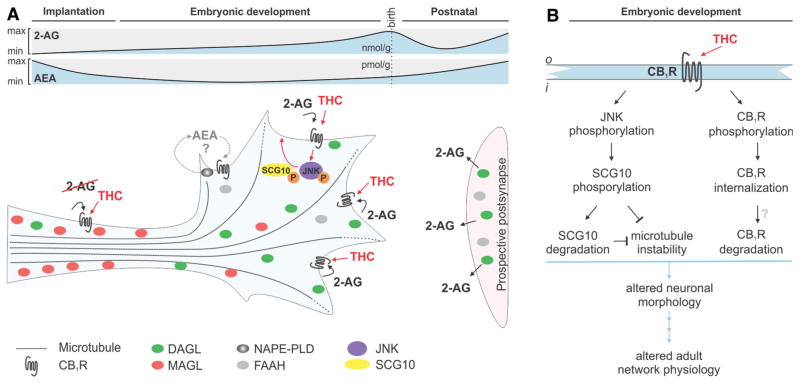
Fig. 2.
Molecular mechanisms of THC signalling in developing neurons. a Endocannabinoid signalling in developing neurons in relation to SCG10. The growth cone is a 2-AG-rich domain due to low levels of MAGL. Within the stabilized axon, MAGL accumulates and prevents excess 2-AG from engaging CB1Rs. However, THC can induce ectopic CB1R signalling since MAGL is unable to degrade it. Note that 2-AG can either be synthesized from DAGLs within the growth cone (autocrine) or be released from neighbouring cells (paracrine). Since the role of AEA signalling during axonal elongation and pathfinding is poorly understood, the AEA signalling machinery is colour-coded in grey [110]. b Exposure of THC to developing foetal neurons results in c-Jun terminal kinase (JNK) phosphorylation leading to SCG10 degradation and microtubule stabilization. This, together with the internalization of CB1Rs (leading to overall less signalling events) results in changes to neuronal morphology and eventually to altered brain circuit formation (i inside and o outside the cell membrane)
In the adult, endocannabinoids act as retrograde messengers by being released from postsynaptic sites, which contain endocannabinoid synthesis enzymes [85]. Upon release, endocannabinoids activate presynaptic CB1Rs resulting in the reduction in neurotransmitter release by the inhibition of voltage-gated calcium channels [86]. The catabolic enzymes are also mainly expressed in the presynaptic terminal to scavenge excess endocannabinoids to limit excess CB1R activation [78]. However, the distribution and levels of the components of the endocannabinoid-signalling cassette during development differ from adulthood (Fig. 2a).
In developing neurons, CB1Rs and 2-AG’s synthesis enzymes (DAGLα/β) coexist and are preferentially located in elongating neurites and their motile tips, the growth cones [76]. During development, the levels of AEA and 2-AG change considerably. 2-AG concentrations peak during the embryonic development, suggesting a significant role in foetal brain signalling [87]. AEA has a major role in embryo implantation [88] and exists in low concentrations in the brain at midgestation with its levels gradually increasing throughout the perinatal period [87]. Strikingly, both AEA and 2-AG were found as early as E10.5 in the developing mouse [89]. While the AEA synthesis machinery is present during development [90], data on the appearance and the distribution of its enzymes are still discussed. Therefore, we focus this review on 2-AG signalling since its enzymatic machinery and their localization are better understood.
Activation of CB1Rs in developing neurons leads to neurite outgrowth [91] and affects growth cone steering decisions [90]. The unique configuration of CB1Rs and 2-AG synthesis enzymes in the growth cone allows for a primarily autocrine activation of CB1Rs, as well as the paracrine signalling amongst neighbouring neuronal segments or growth cones advancing in parallel and coalescing into axonal bundles [92]. To prevent ectopic activation of CB1Rs, either expressed or transported along the axon, and consequently, unwanted neurite outgrowth or steering decisions, MAGL is expressed in the stabilized neurite segment to scavenge excess 2-AG [93]. When the growth cone reaches its postsynaptic target, the premature presynapse halts by adopting the “adult configuration” of 2-AG signalling by the redistribution of DAGLs and MAGL [76], through an as of yet unidentified molecular mechanism (Fig. 2a).
MAGL, or any other endocannabinoid-related catabolic enzyme known to date, is not able to degrade THC. Thus, introduction of THC to developing foetal circuits could result in the ectopic activation of CB1Rs, leading to unwanted directional neurite outgrowth, including synapse formation errors [94]. In addition, THC exposure increases the synthesis and release of endocannabinoids, particularly AEA, in a concentration-dependent manner through CB1Rs [95] and mobilizes phospholipase D (PLD) [96]. In response to chronic THC or synthetic cannabinoid agonist exposure, CB1Rs undergo downregulation and rapid desensitization in a regionally distinct manner with considerable magnitude [97]. In sum, besides out-of-place activation of CB1Rs, THC is able to reshape endocannabinoid signalling by directly affecting receptor and enzyme levels. The tightly regulated spatiotemporal expression of endocannabinoid-related enzymes and receptors during foetal development thus predicts sensitivity to prenatal cannabis exposure.
The sensitivity of the endocannabinoid system to disturbances is stressed by the phenotypes found in different knockout mouse models. For example, the lack of CB1Rs affects neural progenitor proliferation [98], induces an axon fasciculation phenotype, as well as alters synapse formation in the hippocampus leading to learning and memory problems [90, 99]. In addition, genetic deletion of DAGLα displays similar abnormalities in the innervation of the hippocampus [100, 101]. Thus, mouse models are refined tools for in-depth mechanistic analysis of the involvement of endocannabinoid signalling in developmental and adult functions.
The underlying molecular mechanisms of THC signalling
Despite the evidence that activation of CB1Rs, by either THC or synthetic cannabinoids, evokes significant modifications to neuronal differentiation [76] and synapse physiology by disrupting normal patterns of endocannabinoid signalling [101], little is known about the molecular mechanisms and signalling cascade requirements at the cellular level. Since advanced array technologies allow the molecular fingerprint of prenatal cannabis to be for a large part identified through unbiased genomic and/or proteomic analysis in animal models (for a detailed review see [76]), it is hoped that molecular sensitivities and checkpoints that can be exploited for therapeutic intervention will soon emerge.
The recently identified complex gene and protein networks affected by cannabis exposure, resulting from genome and proteome profiling in adults rodents confirms the powerful effect of THC on signalling systems. These profiling studies identify clusters of target molecules affected by cannabis exposure that can be classified as (1) the endocannabinoid system [102–104], (2) cytoskeletal instability [105, 106] and (3) neurotransmission [106–108]. However, the exact signalling cascades underlying these molecular changes are largely unknown.
Only one in-depth study investigating the molecular signalling pathways affected by prenatal cannabis exposure in the developing nervous system has been published so far [109]. This reports major alterations to the assembly of cortical networks and reproduces the axonal fasciculation phenotype found in CB1R knockouts (see above) suggesting that THC is able to negatively regulate CB1R signalling by either (1) desensitizing CB1R receptors, (2) outcompeting endocannabinoids and/or (3) downregulating receptor and ligand availability [109]. Notably, this study demonstrates for the first time a long-term functional modification to the cortical circuitry after prenatal cannabis exposure [109]. An impairment to long-term potentiation, a major cellular mechanisms involved in the formation of memories, was found by investigating synaptic plasticity in the hippocampus. This, together with an increased paired-pulse facilitation [109], indicative of increased presynaptic communication suggests deregulated presynaptic activity as well as reduced synaptic plasticity. These data are similar to the detrimental effects of a single THC injection on long-term potentiation in neonates, underlying learning and memory formation [109], as well as transgenerational effects on dopamine signalling [107]. Moreover, it links endocannabinoids signalling to epilepsy, such as febrile seizures, for which endocannabinoids are important [109], and the efficacy of CB1Rs to tune network excitability in the foetal and neonatal brain [109]. These data can thus provide, at least in part, an explanation for the impaired mental development and memory function found in the OPPS and MHPC studies [14, 55].
In addition, Tortoriello et al. [109] describe a molecular mechanism for THC-induced axonal growth deficits involving Superior Cervical Ganglion 10 (SCG10)/stathmin-2, a microtubule-binding protein, in the lack of instability of the neuronal cytoskeleton (Fig. 2b). These data were confirmed in human foetal tissues, with a reduction in SCG10 mRNA and protein levels corresponding to in utero cannabis exposure [109]. In sum, this study not only reveals some of the molecular mechanisms of THC-induced wiring deficits, but also provides a biological basis for the memory impairments found in longitudinal human studies.
Summary
Endocannabinoids have a broad range of physiological effects, depending on the enzymatic distribution of the endocannabinoid-signalling cassette, in foetal and adult brain function. The importance of proper endocannabinoid signalling is stressed by the phenotypes found in knockout mouse models and pharmacological studies and imply that endocannabinoids are a nexus in the positioning of neurons and wiring of brain circuitry in foetal development. Therefore, disrupted temporal and/or spatial precision of cannabinoid receptor activation, especially due to in utero cannabis exposure, can destabilize finely tuned signalling networks resulting in altered brain circuit formation and sensitivity to secondary insults. Here, we compared preclinical findings with human longitudinal studies to delineate the complications associated with maternal cannabis use. We find that human studies recapitulate results obtained from animal models, demonstrating that cannabis exposure evokes significant molecular modifications to neurodevelopmental programs leading to neurophysiological and behavioural abnormalities. These findings are especially relevant in light of the rising popularity of cannabis consumption in young mothers, together with an increased potency of cannabis mixtures and the availability of potent synthetic cannabinoid mixtures (e.g. Spice). However, data are lacking on the physiological effects of potent cannabis preparations, as well as synthetic cannabinoid exposure. Along these lines, detailed studies disentangling the consequences of foetal exposure to substances often associated with cannabis abuse (e.g. alcohol and tobacco use) are also lacking. At the time of this review, no data were available on early exposure to cannabis (with or without tobacco) in early neonatal life, either by breast-feeding or second-hand smoking. Moreover, only one study adequately addressed the underlying molecular signalling pathways of prenatal THC exposure resulting in brain wiring deficits [109]. Therefore, dissecting the potential consequences of cannabis-mediated cytoskeletal reorganization and the contribution of distinct subsets of neurons to brain circuit formation will allow us to understand in more details the molecular cascades underlying THC-induced foetal brain malformations. This, together with the recent changes in political acceptance and increase in medical cannabis consumption, raises awareness on the implications of cannabis consumption on foetal development. Similar to alcohol and nicotine exposure, it is increasingly evident that understanding the full impact of maternal cannabis use is required to prevent possible foetal complications with far-reaching effects into adulthood.
Acknowledgments
This work was supported by the Swedish Medical Research Council (T.H), Swedish Brain Foundation (“Hjärnfonden”; T.H.), Novo Nordisk Foundation (Nordic Endocrinology Research Initiative; T.H.), the Petrus & Augusta Hedlunds Foundation (T.H.) and the National Institutes of Health (DA230214, T.H. & Y.L.H.; DA033660 Y.L.H.).
Abbreviations
- 2-AG
2-Arachidonoylglycerol
- ABHD6
α/β Hydrolase domain-containing 6 serine hydrolase
- AEA
Anandamide
- CB1R
Type 1 cannabinoid receptor
- DAGLα/β
Sn-1-Diacylglycerol lipase isoforms α/β
- eCB
Endocannabinoid
- FAAH
Fatty acid amide hydrolase
- MAGL
Monoacylglycerol lipase
- NAPE
N-acyl phosphatidylethanolamine
- SCG10
Super cervical ganglion-10
- THC
Δ9-Tetrahydrocannabinol
Footnotes
Conflict of interest None.
Contributor Information
Daniela Calvigioni, Division of Molecular Neurobiology, Department of Medical Biochemistry and Biophysics, Karolinska Institutet, 17177 Stockholm, Sweden. Department of Molecular Neurosciences, Center for Brain Research, Medical University of Vienna, 1090 Vienna, Austria.
Yasmin L. Hurd, Department of Psychiatry and Pharmacology and Systems Therapeutics, Icahn School of Medicine at Mount Sinai, New York, NY 10029-6574, USA
Tibor Harkany, Email: Tibor.Harkany@meduniwien.ac.at, Division of Molecular Neurobiology, Department of Medical Biochemistry and Biophysics, Karolinska Institutet, 17177 Stockholm, Sweden. Department of Molecular Neurosciences, Center for Brain Research, Medical University of Vienna, 1090 Vienna, Austria.
Erik Keimpema, Department of Molecular Neurosciences, Center for Brain Research, Medical University of Vienna, 1090 Vienna, Austria.
References
- 1.UNODC. World Drug Report. 2011. [Google Scholar]
- 2.European Monitoring Centre for Drugs and Drug Addiction E. Trends and Development. 2013. European drug report. [Google Scholar]
- 3.European Monitoring Centre for Drugs and Drug Addiction E. Synthetic cannabinoids in Europe. 2013. [Google Scholar]
- 4.European Monitoring Centre for Drugs and Drug Addiction E. Legal topic overviews: possession of cannabis for personal use. 2012. [Google Scholar]
- 5.Grotenhermen F. Pharmakologie, Toxikologie und therapeutisches Potential. 2. Hans Huber; Göttingen: 2004. [Google Scholar]
- 6.Atwood BK, et al. JWH018, a common constituent of ‘Spice’ herbal blends, is a potent and efficacious cannabinoid CB receptor agonist. Br J Pharmacol. 2010;160(3):585–593. doi: 10.1111/j.1476-5381.2009.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atwood BK, et al. CP47, 497-C8 and JWH073, commonly found in ‘Spice’ herbal blends, are potent and efficacious CB(1) cannabinoid receptor agonists. Eur J Pharmacol. 2011;659(2–3):139–145. doi: 10.1016/j.ejphar.2011.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zawilska JB, Wojcieszak J. Spice/K2 drugs: more than innocent substitutes for marijuana. Int J Neuropsychopharmacol. 2013;17(3):509–525. doi: 10.1017/S1461145713001247. [DOI] [PubMed] [Google Scholar]
- 9.Fantegrossi WE, et al. Distinct pharmacology and metabolism of K2 synthetic cannabinoids compared to Delta-THC: mechanism underlying greater toxicity. Life Sci. 2013;97(1):45–54. doi: 10.1016/j.lfs.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.SAMHSA. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. 2011. [Google Scholar]
- 11.SAMHSA. 2012 National Survey on Drug Use and Health (NSDUH) 2013. [Google Scholar]
- 12.Wingo PA, et al. Recent changes in the trends of teen birth rates, 1981–2006. J Adolesc Health. 2011;48(3):281–288. doi: 10.1016/j.jadohealth.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 13.SAMHSA. Pregnant teen admissions to substance abuse treatment: 1992 and 2007. 2010. [Google Scholar]
- 14.Huizink AC. Prenatal cannabis exposure and infant outcomes: overview of studies. Prog Neuropsychopharmacol Biol Psychiatry. 2013;52:45–52. doi: 10.1016/j.pnpbp.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Jutras-Aswad D, et al. Neurobiological consequences of maternal cannabis on human fetal development and its neuropsychiatric outcome. Eur Arch Psychiatry Clin Neurosci. 2009;259(7):395–412. doi: 10.1007/s00406-009-0027-z. [DOI] [PubMed] [Google Scholar]
- 16.Fried PA. Conceptual issues in behavioral teratology and their application in determining long-term sequelae of prenatal marihuana exposure. J Child Psychol Psychiatry. 2002;43(1):81–102. doi: 10.1111/1469-7610.00005. [DOI] [PubMed] [Google Scholar]
- 17.Morris CV, et al. Molecular mechanisms of maternal cannabis and cigarette use on human neurodevelopment. Eur J Neurosci. 2011;34(10):1574–1583. doi: 10.1111/j.1460-9568.2011.07884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Driscoll CD, Streissguth AP, Riley EP. Prenatal alcohol exposure: comparability of effects in humans and animal models. Neurotoxicol Teratol. 1990;12(3):231–237. doi: 10.1016/0892-0362(90)90094-s. [DOI] [PubMed] [Google Scholar]
- 19.Knopik VS. Maternal smoking during pregnancy and child outcomes: real or spurious effect? Dev Neuropsychol. 2009;34(1):1–36. doi: 10.1080/87565640802564366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linnet KM, et al. Maternal lifestyle factors in pregnancy risk of attention deficit hyperactivity disorder and associated behaviors: review of the current evidence. Am J Psychiatry. 2003;160(6):1028–1040. doi: 10.1176/appi.ajp.160.6.1028. [DOI] [PubMed] [Google Scholar]
- 21.Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108(Suppl 3):511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gross C, Hen R. The developmental origins of anxiety. Nat Rev Neurosci. 2004;5(7):545–552. doi: 10.1038/nrn1429. [DOI] [PubMed] [Google Scholar]
- 23.Lyon M, et al. Fetal neural development and schizophrenia. Schizophr Bull. 1989;15(1):149–161. doi: 10.1093/schbul/15.1.149. [DOI] [PubMed] [Google Scholar]
- 24.Thompson BL, Levitt P, Stanwood GD. Prenatal exposure to drugs: effects on brain development and implications for policy and education. Nat Rev Neurosci. 2009;10(4):303–312. doi: 10.1038/nrn2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rogers JM. Tobacco and pregnancy. Reprod Toxicol. 2009;28(2):152–160. doi: 10.1016/j.reprotox.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen A, et al. Maternal smoking predicts the risk of spontaneous abortion. Acta Obstet Gynecol Scand. 2006;85(9):1057–1065. doi: 10.1080/00016340600589560. [DOI] [PubMed] [Google Scholar]
- 27.Abbott LC, Winzer-Serhan UH. Smoking during pregnancy: lessons learned from epidemiological studies and experimental studies using animal models. Crit Rev Toxicol. 2012;42(4):279–303. doi: 10.3109/10408444.2012.658506. [DOI] [PubMed] [Google Scholar]
- 28.El Marroun H, et al. Prenatal tobacco exposure and brain morphology: a prospective study in young children. Neuropsychopharmacology. 2014;39(4):792–800. doi: 10.1038/npp.2013.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roy TS, Sabherwal U. Effects of prenatal nicotine exposure on the morphogenesis of somatosensory cortex. Neurotoxicol Teratol. 1994;16(4):411–421. doi: 10.1016/0892-0362(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 30.Roy TS, et al. Nicotine evokes cell death in embryonic rat brain during neurulation. J Pharmacol Exp Ther. 1998;287(3):1136–1144. [PubMed] [Google Scholar]
- 31.Bell SH, et al. The remarkably high prevalence of epilepsy and seizure history in fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2010;34(6):1084–1089. doi: 10.1111/j.1530-0277.2010.01184.x. [DOI] [PubMed] [Google Scholar]
- 32.Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet. 2003;42(4):327–360. doi: 10.2165/00003088-200342040-00003. [DOI] [PubMed] [Google Scholar]
- 33.Hurd YL, et al. Marijuana impairs growth in midgestation fetuses. Neurotoxicol Teratol. 2005;27(2):221–229. doi: 10.1016/j.ntt.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 34.Dinieri JA, Hurd YL. Rat models of prenatal and adolescent cannabis exposure. Methods Mol Biol. 2012;829:231–242. doi: 10.1007/978-1-61779-458-2_14. [DOI] [PubMed] [Google Scholar]
- 35.Day NL, Richardson GA. Prenatal marijuana use: epidemiology, methodologic issues, and infant outcome. Clin Perinatol. 1991;18(1):77–91. [PubMed] [Google Scholar]
- 36.Fried PA, O’Connell CM. A comparison of the effects of prenatal exposure to tobacco, alcohol, cannabis and caffeine on birth size and subsequent growth. Neurotoxicol Teratol. 1987;9(2):79–85. doi: 10.1016/0892-0362(87)90082-1. [DOI] [PubMed] [Google Scholar]
- 37.Burns JK. Pathways from cannabis to psychosis: a review of the evidence. Front Psychiatry. 2013;4:128. doi: 10.3389/fpsyt.2013.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weiser M, Noy S. Interpreting the association between cannabis use and increased risk for schizophrenia. Dialogues Clin Neurosci. 2005;7(1):81–85. doi: 10.31887/DCNS.2005.7.1/mweiser. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fried PA. Marihuana use by pregnant women: neurobehavioral effects in neonates. Drug Alcohol Depend. 1980;6(6):415–424. doi: 10.1016/0376-8716(80)90023-x. [DOI] [PubMed] [Google Scholar]
- 40.Fried PA, Watkinson B, Gray R. Differential effects on cognitive functioning in 9- to 12-year olds prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol. 1998;20(3):293–306. doi: 10.1016/s0892-0362(97)00091-3. [DOI] [PubMed] [Google Scholar]
- 41.Hofman A, et al. Growth, development and health from early fetal life until young adulthood: the Generation R Study. Paediatr Perinat Epidemiol. 2004;18(1):61–72. doi: 10.1111/j.1365-3016.2003.00521.x. [DOI] [PubMed] [Google Scholar]
- 42.Jaddoe VW, et al. The generation R Study: design and cohort update 2012. Eur J Epidemiol. 2012;27(9):739–756. doi: 10.1007/s10654-012-9735-1. [DOI] [PubMed] [Google Scholar]
- 43.El Marroun H, et al. Demographic, emotional and social determinants of cannabis use in early pregnancy: the generation R study. Drug Alcohol Depend. 2008;98(3):218–226. doi: 10.1016/j.drugalcdep.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 44.El Marroun H, et al. Intrauterine cannabis exposure affects fetal growth trajectories: the generation R Study. J Am Acad Child Adolesc Psychiatry. 2009;48(12):1173–1181. doi: 10.1097/CHI.0b013e3181bfa8ee. [DOI] [PubMed] [Google Scholar]
- 45.Hadlock FP, et al. Sonographic estimation of fetal weight. The value of femur length in addition to head and abdomen measurements. Radiology. 1984;150(2):535–540. doi: 10.1148/radiology.150.2.6691115. [DOI] [PubMed] [Google Scholar]
- 46.Zuckerman B, et al. Effects of maternal marijuana and cocaine use on fetal growth. N Engl J Med. 1989;320(12):762–768. doi: 10.1056/NEJM198903233201203. [DOI] [PubMed] [Google Scholar]
- 47.Boito S, et al. Umbilical venous volume flow in the normally developing and growth-restricted human fetus. Ultrasound Obstet Gynecol. 2002;19(4):344–349. doi: 10.1046/j.1469-0705.2002.00671.x. [DOI] [PubMed] [Google Scholar]
- 48.El Marroun H, et al. A prospective study on intrauterine cannabis exposure and fetal blood flow. Early Hum Dev. 2010;86(4):231–236. doi: 10.1016/j.earlhumdev.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 49.Fried PA, Watkinson B. 36- and 48-month neurobehavioral follow-up of children prenatally exposed to marijuana, cigarettes, and alcohol. J Dev Behav Pediatr. 1990;11(2):49–58. [PubMed] [Google Scholar]
- 50.Huizink AC, Mulder EJ. Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. Neurosci Biobehav Rev. 2006;30(1):24–41. doi: 10.1016/j.neubiorev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 51.Richardson GA, Day NL, Goldschmidt L. Prenatal alcohol, marijuana, and tobacco use: infant mental and motor development. Neurotoxicol Teratol. 1995;17(4):479–487. doi: 10.1016/0892-0362(95)00006-d. [DOI] [PubMed] [Google Scholar]
- 52.Wang X, et al. In utero marijuana exposure associated with abnormal amygdala dopamine D2 gene expression in the human fetus. Biol Psychiatry. 2004;56(12):909–915. doi: 10.1016/j.biopsych.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 53.Goldschmidt L, et al. Prenatal marijuana and alcohol exposure and academic achievement at age 10. Neurotoxicol Teratol. 2004;26(4):521–532. doi: 10.1016/j.ntt.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 54.Fried PA, Watkinson B, Gray R. A follow-up study of attentional behavior in 6-year-old children exposed prenatally to marihuana, cigarettes, and alcohol. Neurotoxicol Teratol. 1992;14(5):299–311. doi: 10.1016/0892-0362(92)90036-a. [DOI] [PubMed] [Google Scholar]
- 55.Goldschmidt L, Day NL, Richardson GA. Effects of prenatal marijuana exposure on child behavior problems at age 10. Neurotoxicol Teratol. 2000;22(3):325–336. doi: 10.1016/s0892-0362(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 56.Leech SL, et al. Prenatal substance exposure: effects on attention and impulsivity of 6-year-olds. Neurotoxicol Teratol. 1999;21(2):109–118. doi: 10.1016/s0892-0362(98)00042-7. [DOI] [PubMed] [Google Scholar]
- 57.Trezza V, Cuomo V, Vanderschuren LJ. Cannabis and the developing brain: insights from behavior. Eur J Pharmacol. 2008;585(2–3):441–452. doi: 10.1016/j.ejphar.2008.01.058. [DOI] [PubMed] [Google Scholar]
- 58.Trivedi JK. Cognitive deficits in psychiatric disorders: current status. Indian J Psychiatry. 2006;48(1):10–20. doi: 10.4103/0019-5545.31613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith AM, et al. Effects of prenatal marijuana on visuo-spatial working memory: an fMRI study in young adults. Neurotoxicol Teratol. 2006;28(2):286–295. doi: 10.1016/j.ntt.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 60.Navarro M, Rubio P, de Fonseca FR. Behavioural consequences of maternal exposure to natural cannabinoids in rats. Psychopharmacology. 1995;122(1):1–14. doi: 10.1007/BF02246436. [DOI] [PubMed] [Google Scholar]
- 61.Mereu G, et al. Prenatal exposure to a cannabinoid agonist produces memory deficits linked to dysfunction in hippocampal long-term potentiation and glutamate release. Proc Natl Acad Sci USA. 2003;100(8):4915–4920. doi: 10.1073/pnas.0537849100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Trezza V, et al. Altering endocannabinoid neurotransmission at critical developmental ages: impact on rodent emotionality and cognitive performance. Front Behav Neurosci. 2012;6:2. doi: 10.3389/fnbeh.2012.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Szutorisz H, et al. Parental THC exposure leads to compulsive heroin-seeking and altered striatal synaptic plasticity in the subsequent generation. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2013.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Day NL, et al. Effect of prenatal marijuana exposure on the cognitive development of offspring at age three. Neurotoxicol Teratol. 1994;16(2):169–175. doi: 10.1016/0892-0362(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 65.Herkenham M. Cannabinoid receptor localization in brain: relationship to motor and reward systems. Ann N Y Acad Sci. 1992;654:19–32. doi: 10.1111/j.1749-6632.1992.tb25953.x. [DOI] [PubMed] [Google Scholar]
- 66.Matsuda LA, et al. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346(6284):561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 67.Morgan NH, Stanford IM, Woodhall GL. Functional CB2 type cannabinoid receptors at CNS synapses. Neuropharmacology. 2009;57(4):356–368. doi: 10.1016/j.neuropharm.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 68.Lauckner JE, et al. GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc Natl Acad Sci USA. 2008;105(7):2699–2704. doi: 10.1073/pnas.0711278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sawzdargo M, et al. Identification and cloning of three novel human G protein-coupled receptor genes GPR52, PsiGPR53 and GPR55: GPR55 is extensively expressed in human brain. Brain Res Mol Brain Res. 1999;64(2):193–198. doi: 10.1016/s0169-328x(98)00277-0. [DOI] [PubMed] [Google Scholar]
- 70.Cabral GA, et al. CB2 receptors in the brain: role in central immune function. Br J Pharmacol. 2008;153(2):240–251. doi: 10.1038/sj.bjp.0707584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kaplan BL. The role of CB1 in immune modulation by cannabinoids. Pharmacol Ther. 2013;137(3):365–374. doi: 10.1016/j.pharmthera.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 72.den Boon FS, et al. Excitability of prefrontal cortical pyramidal neurons is modulated by activation of intracellular type-2 cannabinoid receptors. Proc Natl Acad Sci USA. 2012;109(9):3534–3539. doi: 10.1073/pnas.1118167109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Van Sickle MD, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310(5746):329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- 74.Belue RC, et al. The ontogeny of cannabinoid receptors in the brain of postnatal and aging rats. Neurotoxicol Teratol. 1995;17(1):25–30. doi: 10.1016/0892-0362(94)00053-g. [DOI] [PubMed] [Google Scholar]
- 75.Oh HA, et al. Uncovering a role for endocannabinoid signaling in autophagy in preimplantation mouse embryos. Mol Hum Reprod. 2013;19(2):93–101. doi: 10.1093/molehr/gas049. [DOI] [PubMed] [Google Scholar]
- 76.Keimpema E, Mackie K, Harkany T. Molecular model of cannabis sensitivity in developing neuronal circuits. Trends Pharmacol Sci. 2011;32(9):551–561. doi: 10.1016/j.tips.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Berrendero F, et al. Localization of mRNA expression and activation of signal transduction mechanisms for cannabinoid receptor in rat brain during fetal development. Development. 1998;125(16):3179–3188. doi: 10.1242/dev.125.16.3179. [DOI] [PubMed] [Google Scholar]
- 78.Bisogno T, et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163(3):463–468. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang X, et al. Preferential limbic expression of the cannabinoid receptor mRNA in the human fetal brain. Neuroscience. 2003;118(3):681–694. doi: 10.1016/s0306-4522(03)00020-4. [DOI] [PubMed] [Google Scholar]
- 80.Devane WA, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258(5090):1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 81.Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388(6644):773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- 82.Hillard CJ, Campbell WB. Biochemistry and pharmacology of arachidonylethanolamide, a putative endogenous cannabinoid. J Lipid Res. 1997;38(12):2383–2398. [PubMed] [Google Scholar]
- 83.Cravatt BF, et al. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384(6604):83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- 84.Dinh TP, et al. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci USA. 2002;99(16):10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gulyas AI, et al. Segregation of two endocannabinoid-hydrolyzing enzymes into pre- and postsynaptic compartments in the rat hippocampus, cerebellum and amygdala. Eur J Neurosci. 2004;20(2):441–458. doi: 10.1111/j.1460-9568.2004.03428.x. [DOI] [PubMed] [Google Scholar]
- 86.Di Marzo V, et al. Endocannabinoids: endogenous cannabinoid receptor ligands with neuromodulatory action. Trends Neurosci. 1998;21(12):521–528. doi: 10.1016/s0166-2236(98)01283-1. [DOI] [PubMed] [Google Scholar]
- 87.Harkany T, et al. The emerging functions of endocannabinoid signaling during CNS development. Trends Pharmacol Sci. 2007;28(2):83–92. doi: 10.1016/j.tips.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 88.Paria BC, et al. Dysregulated cannabinoid signaling disrupts uterine receptivity for embryo implantation. J Biol Chem. 2001;276(23):20523–20528. doi: 10.1074/jbc.M100679200. [DOI] [PubMed] [Google Scholar]
- 89.Psychoyos D, et al. Cannabinoid receptor 1 signaling in embryo neurodevelopment. Birth Defects Res B Dev Reprod Toxicol. 2012;95(2):137–150. doi: 10.1002/bdrb.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Berghuis P, et al. Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science. 2007;316(5828):1212–1216. doi: 10.1126/science.1137406. [DOI] [PubMed] [Google Scholar]
- 91.Argaw A, et al. Concerted action of CB1 cannabinoid receptor and deleted in colorectal cancer in axon guidance. J Neurosci. 2011;31(4):1489–1499. doi: 10.1523/JNEUROSCI.4134-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Harkany T, et al. Endocannabinoid functions controlling neuronal specification during brain development. Mol Cell Endocrinol. 2008;286(1–2 Suppl 1):S84–S90. doi: 10.1016/j.mce.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 93.Keimpema E, et al. Differential subcellular recruitment of monoacylglycerol lipase generates spatial specificity of 2-arachidonoyl glycerol signaling during axonal pathfinding. J Neurosci. 2010;30(42):13992–14007. doi: 10.1523/JNEUROSCI.2126-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim D, Thayer SA. Cannabinoids inhibit the formation of new synapses between hippocampal neurons in culture. J Neurosci. 2001;21(10):RC146. doi: 10.1523/JNEUROSCI.21-10-j0004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Burstein S, et al. Phospholipase participation in cannabinoid-induced release of free arachidonic acid. Biochem Pharmacol. 1994;48(6):1253–1264. doi: 10.1016/0006-2952(94)90163-5. [DOI] [PubMed] [Google Scholar]
- 96.Bari M, et al. New insights into endocannabinoid degradation and its therapeutic potential. Mini Rev Med Chem. 2006;6(3):257–268. doi: 10.2174/138955706776073466. [DOI] [PubMed] [Google Scholar]
- 97.Coutts AA, et al. Agonist-induced internalization and trafficking of cannabinoid CB1 receptors in hippocampal neurons. J Neurosci. 2001;21(7):2425–2433. doi: 10.1523/JNEUROSCI.21-07-02425.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mulder J, et al. Endocannabinoid signaling controls pyramidal cell specification and long-range axon patterning. Proc Natl Acad Sci USA. 2008;105(25):8760–8765. doi: 10.1073/pnas.0803545105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Puighermanal E, et al. Cannabinoid modulation of hippocampal long-term memory is mediated by mTOR signaling. Nat Neurosci. 2009;12(9):1152–1158. doi: 10.1038/nn.2369. [DOI] [PubMed] [Google Scholar]
- 100.Tanimura A, et al. The endocannabinoid 2-arachidonoyl-glycerol produced by diacylglycerol lipase alpha mediates retrograde suppression of synaptic transmission. Neuron. 2010;65(3):320–327. doi: 10.1016/j.neuron.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 101.Keimpema E, et al. Nerve growth factor scales endocannabinoid signaling by regulating monoacylglycerol lipase turnover in developing cholinergic neurons. Proc Natl Acad Sci USA. 2013;110(5):1935–1940. doi: 10.1073/pnas.1212563110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kittler JT, et al. Large-scale analysis of gene expression changes during acute and chronic exposure to [Delta]9-THC in rats. Physiol Genomics. 2000;3(3):175–185. doi: 10.1152/physiolgenomics.2000.3.3.175. [DOI] [PubMed] [Google Scholar]
- 103.Grigorenko E, et al. Assessment of cannabinoid induced gene changes: tolerance and neuroprotection. Chem Phys Lipids. 2002;121(1–2):257–266. doi: 10.1016/s0009-3084(02)00161-5. [DOI] [PubMed] [Google Scholar]
- 104.Perez-Rosado A, et al. Prenatal Delta(9)-tetrahydrocannabinol exposure modifies proenkephalin gene expression in the fetal rat brain: sex-dependent differences. Brain Res Dev Brain Res. 2000;120(1):77–81. doi: 10.1016/s0165-3806(99)00170-4. [DOI] [PubMed] [Google Scholar]
- 105.Gomez M, Hernandez M, Fernandez-Ruiz J. The activation of cannabinoid receptors during early postnatal development reduces the expression of cell adhesion molecule L1 in the rat brain. Brain Res. 2007;1145:48–55. doi: 10.1016/j.brainres.2007.01.102. [DOI] [PubMed] [Google Scholar]
- 106.Quinn HR, et al. Adolescent rats find repeated Delta(9)-THC less aversive than adult rats but display greater residual cognitive deficits and changes in hippocampal protein expression following exposure. Neuropsychopharmacology. 2008;33(5):1113–1126. doi: 10.1038/sj.npp.1301475. [DOI] [PubMed] [Google Scholar]
- 107.DiNieri JA, et al. Maternal cannabis use alters ventral striatal dopamine D2 gene regulation in the offspring. Biol Psychiatry. 2011;70(8):763–769. doi: 10.1016/j.biopsych.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Campolongo P, et al. Perinatal exposure to delta-9-tetra-hydrocannabinol causes enduring cognitive deficits associated with alteration of cortical gene expression and neurotransmission in rats. Addict Biol. 2007;12(3–4):485–495. doi: 10.1111/j.1369-1600.2007.00074.x. [DOI] [PubMed] [Google Scholar]
- 109.Tortoriello G, et al. Miswiring the brain: Delta9-tetra-hydrocannabinol disrupts cortical development by inducing an SCG10/stathmin-2 degradation pathway. EMBO J. 2014;33(7):668–685. doi: 10.1002/embj.201386035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Morozov YM, Ben-Ari Y, Freund TF. The spatial and temporal pattern of fatty acid amide hydrolase expression in rat hippocampus during postnatal development. Eur J Neurosci. 2004;20(2):459–466. doi: 10.1111/j.1460-9568.2004.03507.x. [DOI] [PubMed] [Google Scholar]