Abstract
Conventional NK cells are well characterized in the mouse spleen and circulate in the blood. Less well described are NK cells found in organs such as the liver, thymus, and uterus. Recently we identified a tissue-resident NK (trNK) cell population in the liver, suggesting a potential diversity of trNK cells in other organs. In this review we compare and contrast the similarities and differences among the subpopulations of NK and innate lymphoid cells to the trNK cells in the liver.
I. Introduction
Several immune cell lineages migrate throughout the body via the circulatory system in search of detrimental insults provoked by pathogenic events, such as invading microorganisms or developing tumors. Once detected, the circulating immune cells stop and respond in secondary lymphoid organs such as the lymph nodes and spleen. What follows subsequently is an orchestrated host immune response, which controls the pathological process by recruiting relevant immune cells to the damaged tissue. In contrast to the well-studied circulating immune cells are tissue-resident immune cells, which already reside in selected organs where they appear to be armed and ready to rapidly respond. However, less is known about the properties of tissue-resident immune cells that seem to be closely related to their counterparts which re-circulate.
Conventional natural killer (cNK) cells are constituents of the innate arm of the immune system [1]. First described on the basis of their inherent capacity to directly kill tumor cells without prior sensitization, NK cells are now known to participate in a wide variety of immune responses, such as viral infections, stem cell transplantation, and pregnancy. In addition, they can respond to pro-inflammatory cytokines by producing interferon-γ (IFN-γ), their signature cytokine, which can impact adaptive immunity. Although classically studied in the mouse spleen, NK cells are also found in organs, such as the thymus and liver [1]. In the thymus, NK cells have been described which are phenotypically different from cNK cells [2]. In the liver, we recently showed that there are two populations of NK cells, one that resembles splenic cNK cells and that recirculates and another that is tissue-resident [3].
In this review we will discuss the developmental, phenotypic, and functional relationships between the splenic cNK, thymic NK cells, and tissue-resident NK (trNK) cells in the liver. We will highlight features of cNK cells that are relevant to understanding the other NK cell subpopulations and we will also describe NK cells found in other organs, such as the uterus, which may include trNK cells. Finally, we will discuss how these NK cells relate not only to one another but to the larger family of innate lymphoid cells (ILCs) [4, 5].
II. Developmental Requirements of cNK Cells
The bone marrow (BM) is the site of splenic cNK development and maturation. In the BM, the developmental stages are characterized by acquisition and loss of cytokine receptors, NK cell receptors, and integrins [6–8]. One of the late maturation markers, DX5 (α2 integrin), is expressed prior to exit out of the BM and is one of the markers of mature splenic cNK cells. Out in the periphery, mature splenic cNK cells can be further distinguished by a loss of CD27 expression [6, 9]. Thus, the maturation status of splenic cNK cells is closely related to the expression of defined developmental markers.
The family of cytokines, which uses the common receptor gamma chain (γc), a component of receptors for interleukin (IL)-2, IL-4, IL-7, IL-9, IL-15 and IL-21, has been classically defined as growth and survival factors for many immune cells spanning many cell lineages [10]. More specifically for NK cells, splenic cNK cells require IL-15 and its cognate receptor, IL-15R, for development [11–15]. In mice deficient in IL-15 or any chain of the trimeric IL-15R (α, β, γ) chains, splenic cNK cells are absent. While the exact stage of developmental arrest has not been clearly characterized, it is likely that immature NK cells at a very early stage of lineage commitment are affected because IL-2/15Rβ (CD122) is expressed even before other markers associated with NK cells in the BM. Interestingly, cNK cells can develop from precursors lacking expression of IL-15Rα, indicating that trans-presentation of IL-15 from a non-NK cell is sufficient for cNK cell development [16, 17]. Thus, IL-15 and its receptor are critical for cNK cell development.
The development of cNK cells requires certain transcription factors [18], in particular NFIL3 (nuclear factor, IL-3 regulated; also known as E4BP4), to date described as the NK cell-specification factor [19]. Mice deficient in NFIL3 have essentially no splenic cNK cells though other organs were not thoroughly examined [20–22]. The transcription factor Id2 (inhibitor of DNA binding 2) also is essential for the development and maturation of splenic cNK cells [23]. More specifically, Id2-deficient mice have a defect in mature splenic cNK cells while a normal immature cNK population is maintained in the BM, emphasizing that Id2 plays a later role in cNK cell differentiation [24]. Id2 in turn is regulated by the E protein, E2A. Tbet (Tbx21) and eomesodermin (Eomes), related t-box transcription factors, play more intricate roles in NK cell development [25, 26]. In the absence of Tbet, splenic NK cells display an immature phenotype, and a subpopulation of NK cells in the liver is absent, consistent with redundant and cooperative roles of Tbet and Eomes in NK cell development. Importantly, the Tbet and Eomes studies suggest that NK cell subsets in different tissues may be distinguishable from cNK cells based on the differential transcription factor requirements for their development in the various anatomical locations.
III. Thymic NK Cells
Among the lymphoid tissues, the thymus has NK cells with surface marker phenotypes resembling immature cNK cells [2]. Specifically, as compared to splenic cNK cells that are Ly49hi CD11bhi, thymic NK cells are Ly49low CD11blow, like immature BM cNK cells. However, thymic NK cells are CD127+ CD69high unlike resting splenic cNK cells and developing cNK cells in the BM. While the thymic NK cells have not been rigorously tested for selective tissue localization, their relatively unique phenotype as compared to cNK cells suggests they may be trNK cells in the thymus. On the other hand, cells with a thymic NK cell phenotype are enriched in LNs and are absent in GATA3-deficient mice while Id2 was dispensable [2]. Moreover, peripheral thymic NK cells require a thymus for development and can develop in vivo and in vitro from double negative (CD4− CD8−) 1 (DN1) subset of immature thymocytes [27], indicating that they do not develop directly in the BM, unlike cNK cells. Moreover, unlike immature cNK cells, thymic NK cells have cytotoxic capability and the ability to produce IFN-γ [2]. Thus, the thymus is both a site for development of thymic NK cell maturation and may be a “home” for thymic NK cells.
IV. Tissue-resident NK (trNK) Cells
In the liver, a population of NK cells appeared to be similar to immature cNK cells because they express similar surface markers such as NK1.1 and NKp46 and low levels of CD11b [3, 6, 8]. Moreover, they are DX5− and display high levels of TNF-related apoptosis-inducing ligand (TRAIL). This immature phenotype gave the impression that organs such as the liver contained a subpopulation of NK cells that was not fully differentiated. Our recent studies, however, indicate that these liver NK cells appeared to be distinct from immature BM cNK cells [3].
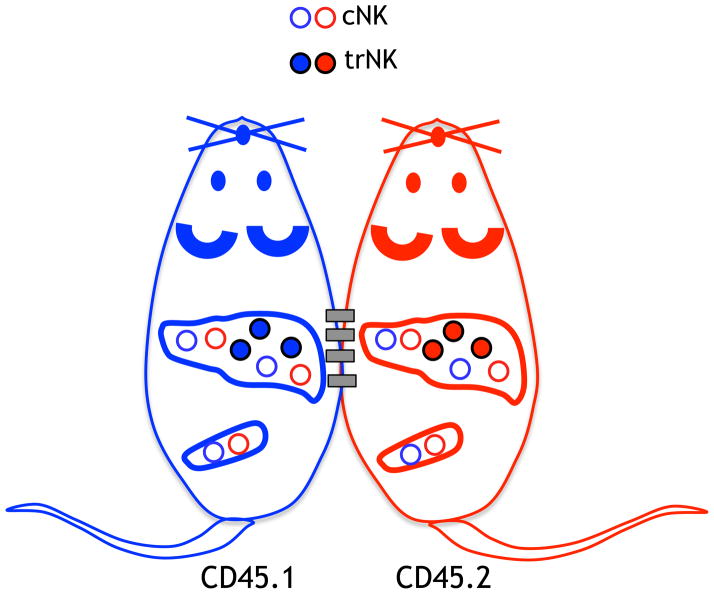
Detailed phenotypic analysis revealed that DX5 and CD49a are mutually exclusively expressed on liver NK cells, i.e., there are CD49a+ DX5− and CD49a− DX5+ NK cells in the liver [3]. There were no NK cells which were double-positive or double-negative for CD49a and DX5. Adoptive transfer of DX5− liver NK cells into congenically distinct hosts indicated selective migration of the cells to the liver. However, DX5+ liver NK cells could be found in both the liver and spleen after adoptive transfer. Based on other markers, CD49a+ DX5− were unlike splenic cNK cells whereas CD49a− DX5+ were very similar to splenic cNK cells. In parabiotic mice, the host liver contained CD49a+ DX5− NK cells primarily of host origin as well as CD49a− DX5+ NK cells derived from both the host and other parabiont (Figure 1). Thus, the CD49a+ DX5− NK cells in the liver are non-circulating trNK cells whereas the CD49a− DX5+ NK cells are circulating cNK cells.
Figure 1. trNK cells in the liver do not migrate.
Congenically marked animals were surgically parabiosed and on day 14 post-surgery the livers and spleens were analyzed for cNK cells and trNK cells, as schematically shown.
The liver receives blood from two afferent vessels, the hepatic artery which supplies oxygenated blood and the portal vein which supplies nutrient-rich blood from the intestines and spleen [28]. The blood mixes in the sinusoidal space, a low pressure, highly fenestrated vascular system. Immune cells such as NKT cells patrol sinusoidal endothelial cells by crawling in the sinusoidal space [29]. Selective sampling of the thoracic aorta (which ultimately feeds the hepatic artery), portal vein, and vena cava (which receives blood from the hepatic vein) showed only CD49a− DX5+ NK cells resembling cNK cells in the spleen [3]. However, ligation of the vasculature of the liver before excision, then flushing of the excised liver produced both CD49a+ DX5− and CD49a− DX5+ NK cells, similar to single cell suspensions from homogenized livers. Thus, the liver trNK cells selectively reside in the sinusoids.
The localization of liver trNK cells to the sinusoids highlights the difficulty in identifying NK cells in liver tissue sections. Examination of the liver parenchyma revealed essentially no NK cells though the tissue sections did not preserve sinusoidal blood [30]. Moreover, these findings are reminiscent of early electron microscopy studies of the liver sinusoids. The rat liver was found to contain “pit cells,” now known to be NK cells, in the sinusoidal space [31, 32]. These studies provide micro-anatomical relationships to sinusoidal cells. Usually adjacent to sinusoidal endothelial cells, pit cells were often found next to Kupffer cells, the macrophage of the liver. Although liver trNK cells have not been formally examined as pit cells, it seems likely that they are related, if not equivalent.
The discovery of trNK cells in the liver suggests that other organs may also contain subpopulations of tissue-resident cells as well as circulating cNK cells resembling those in the spleen. For example, NK cells are normally present in the non-pregnant uterus [33–35] but have been mostly studied after they expand at the site of embryo implantation during pregnancy [36, 37]. Some uterine NK (uNK) cells are phenotypically different from splenic cNK cells in that they lack the expression of the maturation marker DX5 [34]. Human NK cells found in the endometrium, prior to pregnancy, appear to be an immature population [38]. In the total uNK cell population, phenotypic differences therefore may be due to a trNK cell unique to the uterus and potentially distinguishable from cNK cells. The characteristics of the total uterine NK cell pool will then depend on the proportion of such putative uterine trNK cells and cNK cells circulating in the uterus. Furthermore, uNK cells accumulate at the site of embryo implantation and expand during pregnancy, and are responsible for spiral artery remodeling [37]. It will be of interest to determine if such properties are specifically due to a putative uterine trNK cell subpopulation.
Taken together with previous observations, our recent description of liver trNK cells suggest that trNK cells may be more diverse than currently recognized. If so, it will be of interest to determine if such putative trNK cells are distinct for each tissue of residence or are more closely related to each other than to cNK cells or thymic NK cells.
V. Similarities and Differences between NK Cell Subsets
Based on the detailed descriptions above, there appear to be several distinguishable populations of NK cells. Here we will highlight the major similarities and differences between these NK cell subpopulations in different anatomical locations (Table 1).
TABLE 1.
COMPARISON OF NK CELL SUBPOPULATIONS
| NK Cell Subpopulation | Transcription Factor Requirements | Cytokine Receptor Dependence | Functions | Location |
|---|---|---|---|---|
| cNK | NFIL3 (E4BP4) | IL-15R | Cytotoxicity, IFN-production | Spleen, blood, BM, liver |
| Thymic NK | GATA3 | IL-15R, IL-7R | Cytotoxicity, IFN-production | Thymus, LN |
| uNK | ? | IL-15R | Cytotoxicity, IFN-production | Uterus |
| trNK-liver | ? | ? | Cytotoxicity, IFN-production | Liver |
Although there are many distinguishing features between the aforementioned NK cell subpopulations, it is relevant to point out their commonalities. All of these NK cells do not depend on recombination activating genes (RAG) to rearrange antigen receptors, do not express CD3ε, and express the NK cell surface markers NK1.1 and NKp46. The cNK cells require IL-15 or IL-15Rα [14, 15] such that no NK1.1+ CD3− cells were identifiable in spleen of the respective knockout mice, though liver trNK cells have not yet been examined. Like the cNK cells in the spleen, thymic NK cells require IL-15 [2, 27, 39]. Similarly, IL-15-deficient mice lack NK1.1+ CD3− cells in the uterus [40, 41], indicating that potential trNK cells in the uterus should be IL-15Rα-dependent. Thus, NK cell subpopulations described thus far are IL-15-dependent, though the requirement for IL-15 has not been determined for liver trNK cells.
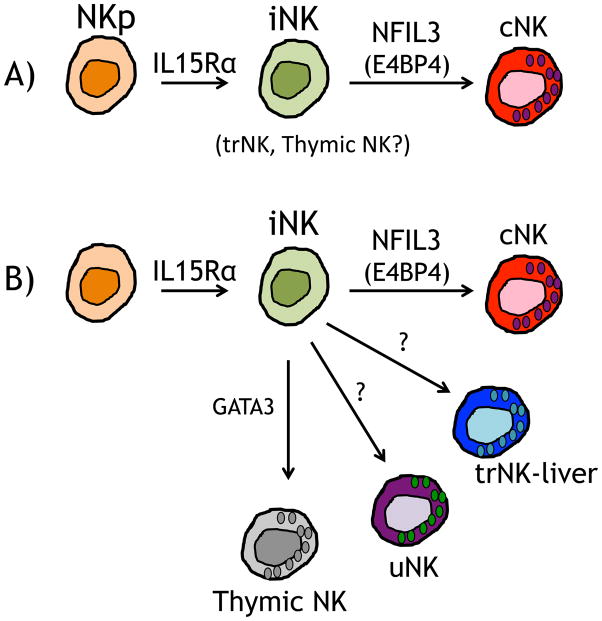
The trNK cells in the liver produce IFN-γ as do cNK cells and thymic NK cells. Much like the cNK cells and thymic NK cells that can respond to YAC-1 targets by degranulation and killing, the trNK cells in the liver also respond [3, 42, 43]. In addition, uNK are cytotoxic as they express perforin and granzymes, and they produce IFNγ [44, 45]. While more detailed analysis of the progenitors for NK cells will be required to establish the precise lineage relationship of cNK, thymic NK and trNK cells to each other (Figure 2), current data, particularly cytotoxic potential, cytokine production, and IL-15-dependence, support their intimate relationship (Table 1).
Figure 2. Potential models of NK cell development.
All NK precursors (NKp) cells that transition to an immature NK (iNK) cell depend on IL-15R. A) In a linear model cNK cells transition from iNK cells and is dependent on NFIL3 transcription factor. The cNK cells populate organs such at the spleen, liver and other organs. In this model, trNK and thymic NK cells may represent iNK cells. B) Alternatively the iNK cells could give rise to distinct NK cell subpopulations that populate the various organs and may depend on different transcription factors, as illustrated by thymic NK cells.
The trNK cells in the liver can be clearly distinguished from cNK cells by differential expression of CD49a and DX5 [3, 6]. The trNK cells stably express CD49a but not DX5, even after adoptive transfer [3], though these markers could change expression during inflammatory responses, an issue not yet examined. Moreover, trNK cells in the liver preferentially express TRAIL, and CXCR6, and higher levels of CD27 and CD51 than cNK cells in the liver [3]. Although NK cells in other organs have not been thoroughly examined with these markers, it is conceivable that such trNK cells may similarly display differential expression of these markers or potentially other markers that will distinguish them from cNK cells. Regardless, it is currently unclear how differential expression of CD49a and DX5 contribute to the different properties of trNK and cNK cells, such as tissue localization.
The cNK and trNK cells can be distinguished from thymic NK cells in several ways, particularly with regard to expression of CD127 (IL-7 receptor α). Both trNK and cNK cells do not express high levels of CD127 that is characteristic of thymic NK cells [2, 34]. Furthermore, peripheral NK cells resembling thymic NK cells are absent in athymic nude mice though it is not yet known if nude mice possess liver trNK cells. Finally, thymic NK cells appear to arise from early thymocyte precursors [27].
Thymic NK cells are absent in mice lacking transcription factor GATA3 that contains cNK cells in the spleen [2], though similar analysis is needed for trNK cells in the liver. Tbet-deficient mice possess varying numbers of cNK cells but a distinct subset of liver NK cells is absent [25, 26]. Interestingly, the absent liver NK cell subset resembles trNK cells but CD49a+ NK cells were not examined. By contrast, uNK cells express Eomes at high levels and are largely intact in Tbet-deficient mice [46].
Detailed analysis of the NK cell populations discussed here will be needed to further define the lineage relationships between them. Thymic NK, liver trNK cells, and uNK cells could represent immature intermediate cells in the development of mature splenic cNK cells from NK cell precursors, or they could represent distinct lineages of NK cells with differential cytokine and transcriptional requirements (Figure 2). How the potential diversity of trNK cells in other organs is related to these cells also will require analysis.
VI. Comparison of NK Cells and ILCs
The ILCs are a heterogeneous family of cells which belong to the innate immune system [4, 5]. ILCs reside in specialized submucosal areas as the gastrointestinal tract and bronchial pathway in the lungs, where they maintain epithelial integrity, and contain commensal bacteria, and inflammation. The ILCs require the Id2 transcription factor for development, and some express and require CD127 for their maintenance. The ILCs have recently been organized into three major groups (group 1, 2, and 3, i.e., ILC1, ILC2, and ILC3) based on shared functional characteristics though this area of research is very active with potentially more cells to be identified and additional properties to be discovered [4, 5]. While each group appears to have specific transcription factor requirements and respond to different cytokines which in turn leads to production of other distinctive cytokines, they all appear to arise from Id2+ precursor cells, like NK cells.
A distinguishing characteristic of the ILC1 group is the production of IFN-γ, signature cytokine produced by cNK cells, earning them a place in the group. Importantly, cNK cells can be discerned from other group 1 ILCs members by their cytotoxic function, dependence on IL-15, and general lack of IL-7Rα expression, features shared between cNK and trNK cells in the liver. Recently, a new IFN-γ-producing ILC1 cell was identified which demonstrated dependence on NFIL3, Tbet, and IL-15 but not IL-15Rα [47] which in some respects resembles cNK cells. Additional studies will be needed to further determine the relationship of this ILC1 cell with other NK cell subpopulations.
As the family of ILCs becomes increasingly more complex, these emerging studies suggest it may become difficult to distinguish between ILCs and NK cell subpopulations. Even thymic NK cells are difficult to define in the current ILC classification. Like the ILC2 and ILC3 groups, they are CD127+ but unlike ILCs in general, thymic NK cells are not dependent on Id2. Their other phenotypic markers suggest a relationship to cNK cells but markers such as NKp46 may not be reliable for lineage determinations. Indeed, NKp46, a receptor once thought to be exclusively expressed on cNK cells, is shared and expressed on group 3 ILCs that now can be distinguished from cNK cells [5, 48, 49].
A further perplexing issue is the different ILCs may not be terminally differentiated which leads to potential interconversion between the ILC groups. This lack of stability and apparent plasticity of ILCs [50, 51], demonstrates the increasing difficulty of definitively defining ILC populations and potentially distinguishing ILCs groups from other immune cells. If trNK cells are more closely related to ILCs, the trNK cells in solid organs such as the liver and potentially others are unique in location among currently identifiable ILCs. Additional microscopy studies will be needed to more finely localize the anatomic location of trNK cells in these tissues, work that will greatly benefit from the development of new tools to distinguish trNK cells from cNK cells and ILCs.
VIII. Conclusions
In summary, trNK cells in the liver are distinct from circulating cNK cells and thymic NK cells in their differential expression of NK cell receptors. Their tissue residency in the sinusoidal space of the liver potentially highlights a special feature that suggests that they have tissue-specific functions, raising the possibility that other organs, such as the uterus, have trNK cells. In addition, these NK cells can be distinguished from ILCs although these distinctions may be challenging as new data emerge. Our studies should prompt a re-examination of the countless roles of NK cells in immune responses in many organs that could be due to a diversity of trNK cells, rather than cNK cells.
Highlights.
Mouse splenic conventional natural killer (cNK) cells have been primarily studied
The thymus contains NK cells that can be distinguished from cNK cells
The liver has cNK cells and tissue-resident (trNK) NK cells
Other organs may also have trNK cells, adding their potential diversity
We review the features of these NK cells
Acknowledgments
We thank the Yokoyama lab for great discussions. Studies in the Yokoyama laboratory are supported by RO1-AI106561 from the National Institutes of Health. W.M.Y. is an Investigator of the Howard Hughes Medical Institute. D.K.S is supported by T32 CA009547 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yokoyama WM. Natural killer cells. In: Paul WE, editor. Fundamental immunology. 7. Chapter 17. Philadelphia: Lippincott Williams & Wilkins; 2013. pp. 395–431. [Google Scholar]
- 2.Vosshenrich CA, Garcia-Ojeda ME, Samson-Villeger SI, Pasqualetto V, Enault L, Richard-Le Goff O, et al. A thymic pathway of mouse natural killer cell development characterized by expression of GATA-3 and CD127. Nat Immunol. 2006;7:1217–1224. doi: 10.1038/ni1395. [DOI] [PubMed] [Google Scholar]
- 3.Peng H, Jiang X, Chen Y, Sojka DK, Wei H, Gao X, et al. Liver-resident NK cells confer adaptive immunity in skin-contact inflammation. The Journal of clinical investigation. 2013;123:1444–1456. doi: 10.1172/JCI66381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu Rev Immunol. 2012;30:647–675. doi: 10.1146/annurev-immunol-020711-075053. [DOI] [PubMed] [Google Scholar]
- 5.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 6.Kim S, Iizuka K, Kang HS, Dokun A, French AR, Greco S, et al. In vivo developmental stages in murine natural killer cell maturation. Nature Immunol. 2002;3:523–528. doi: 10.1038/ni796. [DOI] [PubMed] [Google Scholar]
- 7.Yokoyama WM, Kim S, French AR. The dynamic life of natural killer cells. Annu Rev Immunol. 2004;22:405–429. doi: 10.1146/annurev.immunol.22.012703.104711. [DOI] [PubMed] [Google Scholar]
- 8.Di Santo JP. Natural killer cell developmental pathways: a question of balance. Annu Rev Immunol. 2006;24:257–286. doi: 10.1146/annurev.immunol.24.021605.090700. [DOI] [PubMed] [Google Scholar]
- 9.Hayakawa Y, Smyth MJ. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol. 2006;176:1517–1524. doi: 10.4049/jimmunol.176.3.1517. [DOI] [PubMed] [Google Scholar]
- 10.Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol. 2009;9:480–490. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiSanto JP, Muller W, Guy-Grand D, Fischer A, Rajewsky K. Lymphoid development in mice with a targeted deletion of the interleukin 2 receptor gamma chain. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:377–381. doi: 10.1073/pnas.92.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao X, Shores EW, Hu-Li J, Anver MR, Kelsall BL, Russell SM, et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 1995;2:223–238. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki H, Duncan GS, Takimoto H, Mak TW. Abnormal development of intestinal intraepithelial lymphocytes and peripheral natural killer cells in mice lacking the IL-2 receptor beta chain. Journal of Experimental Medicine. 1997;185:499–505. doi: 10.1084/jem.185.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 16.Koka R, Burkett PR, Chien M, Chai S, Chan F, Lodolce JP, et al. Interleukin (IL)-15R[alpha]-deficient natural killer cells survive in normal but not IL-15R[alpha]-deficient mice. J Exp Med. 2003;197:977–984. doi: 10.1084/jem.20021836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prlic M, Blazar BR, Farrar MA, Jameson SC. In vivo survival and homeostatic proliferation of natural killer cells. J Exp Med. 2003;197:967–976. doi: 10.1084/jem.20021847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hesslein DG, Lanier LL. Transcriptional control of natural killer cell development and function. Adv Immunol. 2011;109:45–85. doi: 10.1016/B978-0-12-387664-5.00002-9. [DOI] [PubMed] [Google Scholar]
- 19.Di Santo JP. A defining factor for natural killer cell development. Nat Immunol. 2009;10:1051–1052. doi: 10.1038/ni1009-1051. [DOI] [PubMed] [Google Scholar]
- 20.Gascoyne DM, Long E, Veiga-Fernandes H, de Boer J, Williams O, Seddon B, et al. The basic leucine zipper transcription factor E4BP4 is essential for natural killer cell development. Nat Immunol. 2009;10:1118–1124. doi: 10.1038/ni.1787. [DOI] [PubMed] [Google Scholar]
- 21.Kamizono S, Duncan GS, Seidel MG, Morimoto A, Hamada K, Grosveld G, et al. Nfil3/E4bp4 is required for the development and maturation of NK cells in vivo. J Exp Med. 2009;206:2977–2986. doi: 10.1084/jem.20092176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kashiwada M, Levy DM, McKeag L, Murray K, Schroder AJ, Canfield SM, et al. IL-4-induced transcription factor NFIL3/E4BP4 controls IgE class switching. Proc Natl Acad Sci U S A. 2010;107:821–826. doi: 10.1073/pnas.0909235107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yokota Y, Mansouri A, Mori S, Sugawara S, Adachi S, Nishikawa S, et al. Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature. 1999;397:702–706. doi: 10.1038/17812. [DOI] [PubMed] [Google Scholar]
- 24.Boos MD, Yokota Y, Eberl G, Kee BL. Mature natural killer cell and lymphoid tissue-inducing cell development requires Id2-mediated suppression of E protein activity. J Exp Med. 2007;204:1119–1130. doi: 10.1084/jem.20061959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Townsend MJ, Weinmann AS, Matsuda J, Saloman R, Farnham P, Biron CA, et al. T-bet regulates the terminal maturation and homeostasis of NK and Va14i NKT cells. Immunity. 2004;20:477–494. doi: 10.1016/s1074-7613(04)00076-7. [DOI] [PubMed] [Google Scholar]
- 26.Gordon SM, Chaix J, Rupp LJ, Wu J, Madera S, Sun JC, et al. The transcription factors T-bet and Eomes control key checkpoints of natural killer cell maturation. Immunity. 2012;36:55–67. doi: 10.1016/j.immuni.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vargas CL, Poursine-Laurent J, Yang L, Yokoyama WM. Development of thymic NK cells from double-negative 1 thymocyte precursors. Blood. 2011;118:3570–3578. doi: 10.1182/blood-2011-06-359679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147–163. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- 29.Geissmann F, Cameron TO, Sidobre S, Manlongat N, Kronenberg M, Briskin MJ, et al. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol. 2005;3:e113. doi: 10.1371/journal.pbio.0030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dokun AO, Chu DT, Yang L, Bendelac AS, Yokoyama WM. Analysis of in situ NK cell responses during viral infection. J Immunol. 2001;167:5286–5293. doi: 10.4049/jimmunol.167.9.5286. [DOI] [PubMed] [Google Scholar]
- 31.Wisse E, van’t Noordende JM, van der Meulen J, Daems WT. The pit cell: description of a new type of cell occurring in rat liver sinusoids and peripheral blood. Cell Tissue Res. 1976;173:423–435. doi: 10.1007/BF00224305. [DOI] [PubMed] [Google Scholar]
- 32.Bouwens L, Remels L, Baekeland M, Van Bossuyt H, Wisse E. Large granular lymphocytes or “pit cells” from rat liver: isolation, ultrastructural characterization and natural killer activity. Eur J Immunol. 1987;17:37–42. doi: 10.1002/eji.1830170107. [DOI] [PubMed] [Google Scholar]
- 33.Parr EL, Parr MB, Zheng LM, Young JD. Mouse granulated metrial gland cells originate by local activation of uterine natural killer lymphocytes. Biol Reprod. 1991;44:834–841. doi: 10.1095/biolreprod44.5.834. [DOI] [PubMed] [Google Scholar]
- 34.Yadi H, Burke S, Madeja Z, Hemberger M, Moffett A, Colucci F. Unique receptor repertoire in mouse uterine NK cells. J Immunol. 2008;181:6140–6147. doi: 10.4049/jimmunol.181.9.6140. [DOI] [PubMed] [Google Scholar]
- 35.Mallidi TV, Craig LE, Schloemann SR, Riley JK. Murine endometrial and decidual NK1.1+ natural killer cells display a B220+CD11c+ cell surface phenotype. Biol Reprod. 2009;81:310–318. doi: 10.1095/biolreprod.109.076448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moffett A, Loke C. Immunology of placentation in eutherian mammals. Nat Rev Immunol. 2006;6:584–594. doi: 10.1038/nri1897. [DOI] [PubMed] [Google Scholar]
- 37.Hatta K, MacLeod RJ, Gerber SA, Croy BA. Emerging themes in uterine natural killer cell heterogeneity and function. Am J Reprod Immunol. 2012;68:282–289. doi: 10.1111/j.1600-0897.2012.01160.x. [DOI] [PubMed] [Google Scholar]
- 38.Manaster I, Mizrahi S, Goldman-Wohl D, Sela HY, Stern-Ginossar N, Lankry D, et al. Endometrial NK cells are special immature cells that await pregnancy. J Immunol. 2008;181:1869–1876. doi: 10.4049/jimmunol.181.3.1869. [DOI] [PubMed] [Google Scholar]
- 39.Ribeiro VS, Hasan M, Wilson A, Boucontet L, Pereira P, Lesjean-Pottier S, et al. Cutting edge: Thymic NK cells develop independently from T cell precursors. J Immunol. 2010;185:4993–4997. doi: 10.4049/jimmunol.1002273. [DOI] [PubMed] [Google Scholar]
- 40.Ashkar AA, Black GP, Wei Q, He H, Liang L, Head JR, et al. Assessment of requirements for IL-15 and IFN regulatory factors in uterine NK cell differentiation and function during pregnancy. J Immunol. 2003;171:2937–2944. doi: 10.4049/jimmunol.171.6.2937. [DOI] [PubMed] [Google Scholar]
- 41.Barber EM, Pollard JW. The uterine NK cell population requires IL-15 but these cells are not required for pregnancy nor the resolution of a Listeria monocytogenes infection. J Immunol. 2003;171:37–46. doi: 10.4049/jimmunol.171.1.37. [DOI] [PubMed] [Google Scholar]
- 42.Kiessling R, Klein E, Pross H, Wigzell H. Natural killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur J Immunol. 1975;5:117–121. doi: 10.1002/eji.1830050209. [DOI] [PubMed] [Google Scholar]
- 43.Linnemeyer PA, Pollack SB. Murine granulated metrial gland cells at uterine implantation sites are natural killer lineage cells. J Immunol. 1991;147:2530–2535. [PubMed] [Google Scholar]
- 44.Parr EL, Young LH, Parr MB, Young JD. Granulated metrial gland cells of pregnant mouse uterus are natural killer-like cells that contain perforin and serine esterases. Journal of Immunology. 1990;145:2365–2372. [PubMed] [Google Scholar]
- 45.Ashkar AA, Di Santo JP, Croy BA. Interferon gamma contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J Exp Med. 2000;192:259–270. doi: 10.1084/jem.192.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tayade C, Fang Y, Black GP, VAP, Erlebacher A, Croy BA. Differential transcription of Eomes and T-bet during maturation of mouse uterine natural killer cells. J Leukoc Biol. 2005;78:1347–1355. doi: 10.1189/jlb.0305142. [DOI] [PubMed] [Google Scholar]
- 47.Fuchs A, Vermi W, Lee JS, Lonardi S, Gilfillan S, Newberry RD, et al. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-gamma-producing cells. Immunity. 2013;38:769–781. doi: 10.1016/j.immuni.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walzer T, Blery M, Chaix J, Fuseri N, Chasson L, Robbins SH, et al. Identification, activation, and selective in vivo ablation of mouse NK cells via NKp46. Proc Natl Acad Sci U S A. 2007;104:3384–3389. doi: 10.1073/pnas.0609692104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cella M, Otero K, Colonna M. Expansion of human NK-22 cells with IL-7, IL-2, and IL-1beta reveals intrinsic functional plasticity. Proc Natl Acad Sci U S A. 2010;107:10961–10966. doi: 10.1073/pnas.1005641107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vonarbourg C, Mortha A, Bui VL, Hernandez PP, Kiss EA, Hoyler T, et al. Regulated expression of nuclear receptor RORgammat confers distinct functional fates to NK cell receptor-expressing RORgammat(+) innate lymphocytes. Immunity. 2010;33:736–751. doi: 10.1016/j.immuni.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]