Abstract
Nicotine addiction is most likely a result of a combination of factors including the rewarding effects of the drug; these effects, however, might be influenced by genetic background. Using a conditioned place preference (CPP) paradigm and 8 inbred mouse strains, we conducted an initial examination of the role of genetic background in the rewarding effects of nicotine. Following habituation and initial place preference test, inbred strains (A/J, BALB/cByJ, C3H/HeJ, C57BL/6J, CBA/J, DBA/1J, DBA/2J, and 129/SvEv) were trained and tested in CPP for nicotine (0.35 mg/kg). Although several strains (C57BL/6J, CBA/J, and 129/SvEv) showed nicotine-induced CPP, 1 strain (DBA/1J) showed conditioned place aversion (CPA), and other strains (A/J, BALB/cByJ, C3H/HeJ, and DBA/2J) did not show CPP. Overall, these results indicate that nicotine’s rewarding effects tested in CPP are differentially affected by the genetic background, and this trait has a relatively high heritability (42%–57%). This initial investigation lays the foundation for future studies examining the genetic substrates of nicotine reward.
Keywords: nicotine, conditioned place preference, genetics, reward, addiction
Although nicotine addiction remains a global health problem causing the largest number of preventable deaths in the United States (Center for Disease Control, 2007), the genetic substrates of nicotine addiction are still unknown. Nevertheless, previous studies have shown that some smoking-related phenotypes such as smoking initiation, smoking persistent, nicotine dependence, and number of cigarettes smoked are highly heritable (between 39% and 86%) in humans (Haberstick et al., 2007; Hardie, Moss, & Lynch, 2006; Li, Cheng, Ma, & Swan, 2003; Sullivan & Kendler, 1999; see Portugal & Gould, 2008, for review). Hence, understanding how genotypes contribute to nicotine addiction phenotypes may lead to better treatment methods for nicotine dependence.
One method to identify specific genotypes associated with behavioral characteristics of nicotine dependence is testing different inbred mice in animal models of nicotine dependence. Inbred mouse strains are genetically identical within each strain after 20 generations of breeding; therefore, differences in phenotypes between inbred strains can be attributed to differences in the genome (Lyon & Searle, 1989). Previously, genetic differences in nicotine phenotypes such as respiration, heart rate, locomotor activity, antinociception, and tolerance have been reported by several studies (Collins, Miner, & Marks, 1988; Damaj et al., 2007; Marks, Campbell, Romm, & Collins, 1991; Marks, Stitzel, & Collins, 1989). Overall, these results suggest that genetic differences between inbred mouse strains contribute to differences in nicotine phenotypes; however, phenotypic and genotypic differences in the rewarding effects of nicotine are not well studied. In this study, eight different inbred mouse strains (A/J, BALB/cByJ, C3H/HeJ, C57BL/6J, CBA/J, DBA/1J, DBA/2J, and 129/SvEv) were examined for nicotine conditioned place preference (CPP) to establish the associated genetic heritability.
Method
Subjects
Male A/J (A; n = 19), BALB/cByJ (BALB; n = 18), C3H/HeJ (C3H; n = 18), C57BL/6J (B6; n = 18), CBA/J (CBA; n = 18), DBA/1J (D1; n = 20), and DBA/2J (D2; n = 18) mice were obtained from Jackson Laboratory (Bar Harbor, ME). 129/SvEv (129) mice were obtained from Taconic Farms (Germantown, NY). These inbred strains were chosen because previous work from our lab found phenotype differences in these strains for the effects of nicotine on learning and memory (Portugal, Wilkinson, Kenney, Sullivan, & Gould, 2012). Mice were trained at 8–10 weeks of age. Mice were housed in groups of three per cage and maintained on a 12-hr light–dark cycle (lights on 7:00 a.m.) with unrestricted access to food and water. Experiments took place during the light phase. All behavioral and surgical procedures were approved by the Temple University Institutional Animal Care and Use Committee.
Apparatus
Three identical boxes (41.4 cm × 21.6 cm × 21.6 cm) were used for training and testing of nicotine CPP. Each box contained two distinct chambers (19.7 cm × 20.3 cm × 18.3 cm, each) with clear Plexiglas lids. The walls and floor of one chamber were made of gray Plexiglas, whereas the other chamber was made of gray Plexiglas walls with white stripes (1.3 cm-wide) and a stainless steel mesh floor. During training, a gray Plexiglas wall separated the two chambers. During preconditioning and testing, the gray wall was taken off so that mice could access both sides. Preconditioning and testing sessions were recorded, and the preference for each chamber was scored manually by persons blinded to the experimental conditions. Nicotine hydrogen tartrate salt (Sigma, St. Louis, MO) was dissolved in saline at 0.35 mg/kg (dose reported in freebase nicotine weight and based on prior work; Portugal & Gould, 2009) and administered subcutaneously (injection volume = 10 ml/kg).
Procedure
CPP followed the procedure performed by Portugal and Gould (2009). A habituation phase, in which mice were handled and habituated to the experimental room, was conducted before CPP experiments. Following the first habituation, each mouse was gently handled for ~1 min and held as if a subcutaneous injection was to be administered.
During the first day of training, a preconditioning session was run. Mice were randomly placed into a chamber and had free access to both chambers for 15 min. Entry into a chamber was defined as instances when the head and all four paws of the mouse crossed over into the chamber (Vastola, Douglas, Varlinskaya, & Spear, 2002). The amount of time spent in each chamber was measured, and nicotine was paired with the chamber least preferred by mice on the preconditioning session; nicotine CPP is more robust with the biased CPP procedure (Acquas, Carboni, Leone, & Di Chiara, 1989; Calcagnetti & Schechter, 1994; Le Foll & Goldberg, 2005). The CPP training occurred in two phases with the first phase starting the day after the preconditioning session and consisting of three conditioning sessions (Days 2–4). Mice in the nicotine CPP groups received a subcutaneous injection of either 0.35 mg/kg nicotine or saline and were immediately confined to a nicotine or saline-paired chamber, respectively, for 15 min. After 5 hr, mice were treated with the alternate drug condition and were immediately placed in the opposite chamber for 15 min. To control for order effects, the schedule of injections was counterbalanced within conditioning sessions so that half of the mice received saline and half of the mice received nicotine during the first conditioning trial. Mice that were assigned to saline CPP groups received subcutaneous injections of saline before being placed in both chambers for 15 min. The first testing day was on Day 5 (Test 1); mice were placed in the saline-paired chamber and were allowed to move freely between the two chambers for 15 min. The second phase of CPP training started on Day 6. During this phase, mice underwent four conditioning sessions on Days 6–9, following the same procedure as in previous conditioning sessions. A second test session (Test 2), similar to the first test session, was performed on Day 10.
Genetic heritability computation
Using Test 1 and Test 2 CPP preference scores for the nicotine groups, we conducted a one-way analysis of variance (ANOVA) to determine the within-and between-strain variance to estimate the heritability, the fraction of phenotypic variation that is due to genetic variation (H2), of nicotine place preference. Following previous studies (Owen, Christensen, Paylor, & Wehner, 1997; Rai et al., 2012) the heritability was calculated by dividing the variance among between ( ) by the sum of within-strain ( ) and between-strain variance: .
Statistical Analysis
Following Portugal and Gould (2009), preference scores were calculated by subtracting the difference between the amount of time spent in the nicotine-paired chamber during preconditioning from the amount of time spent in the same chamber during testing and analyzed using separate 2 (drug) × 8 (strain) ANOVAs (SPSS 16.0.3). Least significant difference (LSD) post hoc tests were used for between-group comparisons, and planned comparison t tests were used for within group comparisons at α = .05 level. Group sizes were indicated in the figure caption.
Results
Initial Preference
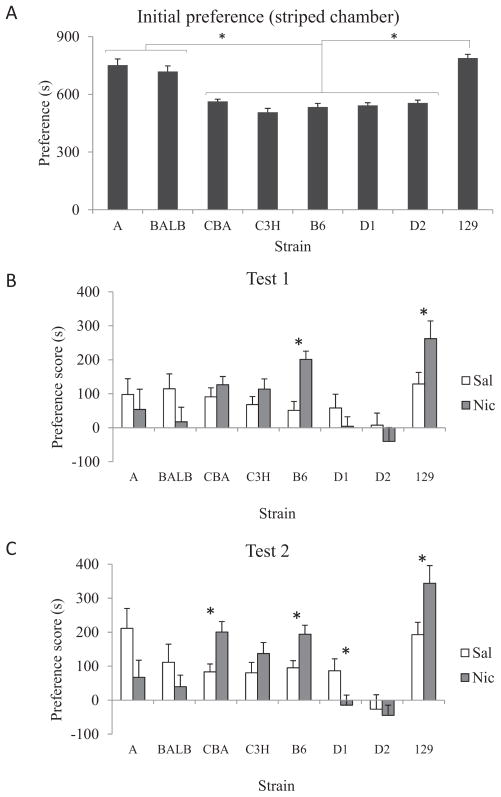
A significant one-way ANOVA suggested that mice showed strain-related differences on the initial preference for the striped chamber, F(7, 126) = 27.25, p < .01 (Figure 1A). Subsequent LSD post hoc tests showed that A, BALB, and 129 mice had a higher initial preference for the striped chamber than C3H, B6, CBA, D1, and D2 mice (ps < .01).
Figure 1.
A: Initial preference for the striped chamber in seconds among different inbred mouse strains. The inbred strains showed differential preference for the striped chamber during preconditioning. Error bars indicate SEM, and asterisks represent differences at p < .05. B: Preference scores from the A (saline n = 10, nicotine n = 9), BALB (saline n = 9, nicotine n = 9), CBA (saline n = 9, nicotine n = 9), C3H (saline n = 9, nicotine n = 9), B6 (saline n = 9, nicotine n = 9), D1 (saline n = 10, nicotine n = 10), D2 (saline n = 9, nicotine n = 9), and 129 (saline n = 9, nicotine n = 9) inbred mice during Test1. C: Preference scores from the inbred mice during Test 2. Error bars indicate SEM, and asterisks represent differences at p < .05.
Within-Strain Comparisons
Performance during nicotine CPP for Test 1 and Test 2 was evaluated for each of the eight inbred mice strains. Two separate 2 × 8 ANOVAs yielded that the drug–strain interaction was significant for both Test 1, F(7, 131) = 2.88, p < .05, and for Test 2, F(7, 131) = 4.16, p < .01. Planned comparison t tests were calculated to determine differences between mice in the saline or nicotine conditions for each strain. For Test 1 (Figure 1B), nicotine CPP designated by significant differences between nicotine and saline groups was seen for B6, t(16) = −4.22, p < .01, and 129 mice, t(16) = −2.14, p < .05, but not for the remaining strains (A, BALB, CBA, C3H, D1, and D2; ps > .05). For Test 2 (Figure 1C), significant differences between nicotine and saline groups for B6, t(16) = −2.96, p < .01, CBA, t(16) = −3.03, p < .05, and 129 mice, t(16) = 2.38, p < .01, were seen indicating nicotine CPP. In contrast, D1 mice showed significant conditioned place aversion for nicotine (CPA), t(18) = 2.21, p < .05. Nonsignificant effects were seen for the remaining strains (A, BALB, C3H, and D2; ps > .05). These results suggest that the effects of nicotine in the development of conditioned place preference or aversion in mice are modulated in part by genetic background and amount of training. B6 and 129 mice showed preference for the chamber paired with nicotine at both Test and Test 2, whereas CBA mice developed nicotine CPP only after seven training sessions. On the other hand, D1 mice showed nicotine CPA only after seven training sessions.
Between-Strain Comparisons: Saline-Condition Mice
Mice in the saline condition showed similar performance during Test 1, F(7, 66) = 1.14, p > .1 (see Figure 1B). For Test 2 (Figure 1C), saline mice showed strain-related performance differences, F(7, 66) = 4.180, p < .05. Post hoc LSD tests showed that saline-treated A mice had a higher preference scores than all the other strains except BALB and 129, whereas saline-treated D2 animals showed significantly lower preference scores than other groups except CBA and C3H. Together with initial preference scores, these results suggest that A, BALB, and 129 mice may have an innate tendency to prefer specific visual cues, which could affect CPP.
Genetic Heritability
The analysis of heritability showed that the nicotine-induced conditioned place preference during Test 1 was 42% and during Test 2 was 57% heritable, which suggests that this phenotype has relatively high heritability.
Discussion
Our results demonstrated that genetic background influenced nicotine CPP. Although B6, CBA, and 129 mice showed nicotine-induced CPP, and D1 mice showed CPA, other strains (A, BALB, C3H, and D2) did not show conditioned place preference for the dose of nicotine tested. Analysis of heritability for the tested dose suggests that conditioned place preference has a relatively high heritability (42%–57%) among inbred mouse strains. These results are in line with previous reports in humans that suggest nicotine addiction-associated behaviors are highly heritable (Haberstick et al., 2007; Hardie et al., 2006; Li et al., 2003; Sullivan & Kendler, 1999) and a study showing CPP in B6 mice but not D2 mice (Grabus, Martin, Brown, & Damaj, 2006).
Other nicotine-related phenotypes have also been demonstrated to vary between mouse strains with different genetic background (Crawley et al., 1997; Lad et al., 2010; Portugal et al., 2012; Park et al., 2014). For example, Portugal et al. (2012) investigated the effects of acute, chronic, and withdrawal from chronic nicotine on contextual fear conditioning in different inbred mouse strains. The results of the Portugal et al. study revealed that acute nicotine dose dependently enhanced contextual fear conditioning in 129, A, BALB, B6, D1, and D2 strains but not in C3H and CBA strains. Also, Portugal et al. found that nicotine withdrawal deficits in contextual fear conditioning in A, BALB, C3H, B6, and D1 strains not 129, CBA, and D2 strains. These results suggest that different subset of genes may be responsible for the acute nicotine and withdrawal deficit phenotypes. Furthermore, because different strains of mice show nicotine CPP (129, B6, and CBA), enhancement of learning by acute nicotine (129, A, BALB, B6, D1, and D2), and disruption of learning by nicotine withdrawal (A, BALB, C3H, B6, and D1), this suggests these nicotine-associated effects may be largely related to different genetic substrates. The conditioned-place aversion shown by the D1 mice is in line with the previous data showing high sensitivity to nicotine indicated by high levels of nicotine-induced seizures in this strain in comparison to other strains (Crawley et al., 1997). This suggests that D1 mice might be too sensitive for nicotine’s physical effects and therefore, the rewarding properties of nicotine might be prevented in this strain or only seen with substantially lower doses.
Using a biased CPP procedure, the results of this study may also reflect differences in anxiety-related behaviors between strains (Brielmaier et al., 2008). This is because the strain differences in exploring the different compartments used in the biased CPP apparatus, shown by initial preferences, might be an indicator of strain differences in anxiety-like behavior (Crawley, 1985). There is also evidence suggesting that anxiety affects CPP performance. For example, Shimosato and Watanabe (2003) found that increased latency to cross between compartments in the CPP apparatus, an indicator of increased anxiety, was associated with stronger cocaine CPP, which suggests that increased anxiety may be related to increased sensitivity to rewarding processing. Overall, our results suggest that nicotine CPP, whether it reflects hedonic properties or anxiolytic properties of nicotine (both of which could contribute to drug seeking behavior), is influenced by different genetic backgrounds.
One of the limitations of our study is the lack of multiple doses used for nicotine CPP. It is possible that strains in this study that did not show CPP would have shown CPP with a different dose. In support, a recent study found that nicotine CPP is altered by dose (Ise, Mori, Katayama, Suzuki, & Wang, 2014). It is possible that genetic differences in pharmacokinetics of nicotine could contribute to these effects. In support, strain differences have been shown in nicotine metabolism (Petersen, Norris, & Thompson, 1984) and nicotinic receptor binding in the brain (Marks, Stitzel, & Collins, 1986; Stevens et al., 1996).
In summary, our results indicate that the rewarding effects of nicotine tested in a CPP paradigm are differentially affected by genetic background and that this phenotype has a relatively high heritability. Identification of genes that are responsible for the rewarding, anxiolytic, and cognitive effects of nicotine will help us better understand nicotine dependence and develop treatment techniques that are tailored for phenotypes. Further studies are needed to examine the interaction between sex and genetic background and whether genetic background shifts the dose response for nicotine CPP.
Acknowledgments
This work was funded with grant support from the National Institute on Drug Abuse (DA017949).
Footnotes
We report no conflicts of interest.
References
- Acquas E, Carboni E, Leone P, Di Chiara G. SCH 23390 blocks drug-conditioned place-preference and place-aversion: Anhedonia (lack of reward) or apathy (lack of motivation) after dopamine-receptor blockade? Psychopharmacology. 1989;99:151–155. doi: 10.1007/BF00442800. http://dx.doi.org/10.1007/BF00442800. [DOI] [PubMed] [Google Scholar]
- Brielmaier JM, McDonald CG, Smith RF. Nicotine place preference in a biased conditioned place preference design. Pharmacology Biochemistry and Behavior. 2008;89:94–100. doi: 10.1016/j.pbb.2007.11.005. http://dx.doi.org/10.1016/j.pbb.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Calcagnetti DJ, Schechter MD. Nicotine place preference using the biased method of conditioning. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1994;18:925–933. doi: 10.1016/0278-5846(94)90108-2. http://dx.doi.org/10.1016/0278-5846(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention. Cigarette smoking among adults—United States, 2006. MMWR Weekly. 2007;56:1157–1161. [PubMed] [Google Scholar]
- Collins AC, Miner LL, Marks MJ. Genetic influences on acute responses to nicotine and nicotine tolerance in the mouse. Pharmacology Biochemistry and Behavior. 1988;30:269–278. doi: 10.1016/0091-3057(88)90455-8. http://dx.doi.org/10.1016/0091-3057(88)90455-8. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Exploratory behavior models of anxiety in mice. Neuroscience and Biobehavioral Reviews. 1985;9:37–44. doi: 10.1016/0149-7634(85)90030-2. http://dx.doi.org/10.1016/0149-7634(85)90030-2. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Paylor R. Behavioral phenotypes of inbred mouse strains: Implications and recommendations for molecular studies. Psychopharmacology. 1997;132:107–124. doi: 10.1007/s002130050327. http://dx.doi.org/10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Fonck C, Marks MJ, Deshpande P, Labarca C, Lester HA, Martin BR. Genetic approaches identify differential roles for alpha4beta2* nicotinic receptors in acute models of antinociception in mice. The Journal of Pharmacology and Experimental Therapeutics. 2007;321:1161–1169. doi: 10.1124/jpet.106.112649. http://dx.doi.org/10.1124/jpet.106.112649. [DOI] [PubMed] [Google Scholar]
- Grabus SD, Martin BR, Brown SE, Damaj MI. Nicotine place preference in the mouse: Influences of prior handling, dose and strain and attenuation by nicotinic receptor antagonists. Psychopharmacology. 2006;184:456–463. doi: 10.1007/s00213-006-0305-7. http://dx.doi.org/10.1007/s00213-006-0305-7. [DOI] [PubMed] [Google Scholar]
- Haberstick BC, Timberlake D, Ehringer MA, Lessem JM, Hopfer CJ, Smolen A, Hewitt JK. Genes, time to first cigarette and nicotine dependence in a general population sample of young adults. Addiction. 2007;102:655–665. doi: 10.1111/j.1360-0443.2007.01746.x. http://dx.doi.org/10.1111/j.1360-0443.2007.01746.x. [DOI] [PubMed] [Google Scholar]
- Hardie TL, Moss HB, Lynch KG. Genetic correlations between smoking initiation and smoking behaviors in a twin sample. Addictive Behaviors. 2006;31:2030–2037. doi: 10.1016/j.addbeh.2006.02.010. http://dx.doi.org/10.1016/j.addbeh.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Ise Y, Mori T, Katayama S, Suzuki T, Wang TC. Genetic background influences nicotine-induced conditioned place preference and place aversion in mice. Journal of Nippon Medical School. 2014;81:53–56. doi: 10.1272/jnms.81.53. http://dx.doi.org/10.1272/jnms.81.53. [DOI] [PubMed] [Google Scholar]
- Lad HV, Liu L, Paya-Cano JL, Parsons MJ, Kember R, Fernandes C, Schalkwyk LC. Behavioural battery testing: Evaluation and behavioural outcomes in 8 inbred mouse strains. Physiology & Behavior. 2010;99:301–316. doi: 10.1016/j.physbeh.2009.11.007. http://dx.doi.org/10.1016/j.physbeh.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Nicotine induces conditioned place preferences over a large range of doses in rats. Psychopharmacology. 2005;178:481–492. doi: 10.1007/s00213-004-2021-5. http://dx.doi.org/10.1007/s00213-004-2021-5. [DOI] [PubMed] [Google Scholar]
- Li MD, Cheng R, Ma JZ, Swan GE. A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction. 2003;98:23–31. doi: 10.1046/j.1360-0443.2003.00295.x. http://dx.doi.org/10.1046/j.1360-0443.2003.00295.x. [DOI] [PubMed] [Google Scholar]
- Lyon MF, Searle AG. Genetic variants and strains of the laboratory mouse. 2. New York, NY: Oxford University Press; 1989. [Google Scholar]
- Marks MJ, Campbell SM, Romm E, Collins AC. Genotype influences the development of tolerance to nicotine in the mouse. The Journal of Pharmacology and Experimental Therapeutics. 1991;259:392–402. [PubMed] [Google Scholar]
- Marks MJ, Stitzel JA, Collins AC. Dose-response analysis of nicotine tolerance and receptor changes in two inbred mouse strains. The Journal of Pharmacology and Experimental Therapeutics. 1986;239:358–364. [PubMed] [Google Scholar]
- Marks MJ, Stitzel JA, Collins AC. Genetic influences on nicotine responses. Pharmacology Biochemistry and Behavior. 1989;33:667–678. doi: 10.1016/0091-3057(89)90406-1. http://dx.doi.org/10.1016/0091-3057(89)90406-1. [DOI] [PubMed] [Google Scholar]
- Owen EH, Christensen SC, Paylor R, Wehner JM. Identification of quantitative trait loci involved in contextual and auditory-cued fear conditioning in BXD recombinant inbred strains. Behavioral Neuroscience. 1997;111:292–300. doi: 10.1037//0735-7044.111.2.292. http://dx.doi.org/10.1037/0735-7044.111.2.292. [DOI] [PubMed] [Google Scholar]
- Park CC, Gale GC, Lusis AJ, Smith DJ. Fear conditioning in males of 94 strains of mice in the Hybrid Mouse Diversity Panel (HMDP) Bar Harbor, ME: The Jackson Laboratory; 2014. Retreived from http://phenome.jax.org. [Google Scholar]
- Petersen DR, Norris KJ, Thompson JA. A comparative study of the disposition of nicotine and its metabolites in three inbred strains of mice. Drug Metabolism and Disposition: The Biological Fate of Chemicals. 1984;12:725–731. [PubMed] [Google Scholar]
- Portugal GS, Gould TJ. Genetic variability in nicotinic acetylcholine receptors and nicotine addiction: Converging evidence from human and animal research. Behavioural Brain Research. 2008;193:1–16. doi: 10.1016/j.bbr.2008.05.006. http://dx.doi.org/10.1016/j.bbr.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal GS, Gould TJ. Nicotine withdrawal disrupts new contextual learning. Pharmacology Biochemistry and Behavior. 2009;92:117–123. doi: 10.1016/j.pbb.2008.11.001. http://dx.doi.org/10.1016/j.pbb.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal GS, Wilkinson DS, Kenney JW, Sullivan C, Gould TJ. Strain-dependent effects of acute, chronic, and withdrawal from chronic nicotine on fear conditioning. Behavior Genetics. 2012;42:133–150. doi: 10.1007/s10519-011-9489-7. http://dx.doi.org/10.1007/s10519-011-9489-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai MF, Hashimoto S, Johnson EE, Janiszak KL, Fitzgerald J, Heber-Katz E, Sandell LJ. Heritability of articular cartilage regeneration and its association with ear wound healing in mice. Arthritis and Rheumatism. 2012;64:2300–2310. doi: 10.1002/art.34396. http://dx.doi.org/10.1002/art.34396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimosato K, Watanabe S. Concurrent evaluation of locomotor response to novelty and propensity toward cocaine conditioned place preference in mice. Journal of Neuroscience Methods. 2003;128:103–110. doi: 10.1016/s0165-0270(03)00153-5. http://dx.doi.org/10.1016/S0165-0270(03)00153-5. [DOI] [PubMed] [Google Scholar]
- Stevens KE, Freedman R, Collins AC, Hall M, Leonard S, Marks MJ, Rose GM. Genetic correlation of inhibitory gating of hippocampal auditory evoked response and α-bungarotoxin-binding nicotinic cholinergic receptors in inbred mouse strains. Neuropsychopharmacology. 1996;15:152–162. doi: 10.1016/0893-133X(95)00178-G. http://dx.doi.org/10.1016/0893-133X(95)00178-G. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Kendler KS. The genetic epidemiology of smoking. Nicotine & Tobacco Research. 1999;1(Suppl 2):S51–S57. doi: 10.1080/14622299050011811. http://dx.doi.org/10.1080/14622299050011811. [DOI] [PubMed] [Google Scholar]
- Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP. Nicotine-induced conditioned place preference in adolescent and adult rats. Physiology & Behavior. 2002;77:107–114. doi: 10.1016/s0031-9384(02)00818-1. http://dx.doi.org/10.1016/S0031-9384(02)00818-1. [DOI] [PubMed] [Google Scholar]