Abstract
Background
Endomyocardial biopsy (EMB) is of value in determining the underlying etiology of a cardiomyopathy. The sensitivity, however, for focal disorders, such as lymphocytic myocarditis and cardiac sarcoidosis (CS), is low. The sensitivity of routine fluoroscopically guided EMB is low. Abnormal intracardiac electrograms are seen at sites of myocardial disease. However, the exact value for electrogram-guided EMB is unknown.
Methods
We report eleven patients who underwent electrogram-guided EMB for evaluation of myocarditis and CS. We describe the method used to perform the procedure and correlate electrogram characteristics with pathologic and clinical outcomes.
Results
Of 40 total biopsy specimens taken from 11 patients, 19 had electrogram voltage <5mV, all of which resulted in histopathologic abnormality (100% specificity and positive predictive value). A voltage amplitude cutoff value of 5mV had substantially higher sensitivity (70% vs 26%) and negative predictive value (62%) than 1.5mV. Abnormal electrogram appearance at biopsy site had good sensitivity (67%) and specificity (92%) in predicting abnormal myocardium. Normal signals with voltage greater than 5 mV signified normal myocardium with no significant diagnostic yield. Biopsy results guided therapy in all patients including 5 with active myocarditis or CS, all of whom subsequently received immunosuppressive therapy. There were no procedural complications.
Conclusions
In patients with suspected myocarditis or CS, electrogram-guided EMB targeting sites with abnormal or low-amplitude electrograms may increase the diagnostic yield for detecting abnormal pathologic findings.
Keywords: Cardiomyopathy, Electrogram, Endomyocardial biopsy, Cardiac sarcoidosis, Myocarditis
Introduction
Endomyocardial biopsy (EMB) is a useful tool to define the etiology of heart failure when a specific diagnosis is suspected and if establishing a histopathological diagnosis would influence therapy (1,2). A major drawback of standard EMB with fluoroscopic or intracardiac echocardiographic guidance is the low sensitivity and high rate of false negatives, particularly in cardiomyopathies such as cardiac sarcoidosis (CS), lymphocytic myocarditis, and arrhythmogenic right ventricular cardiomyopathy (ARVC), which often only involve focal areas of the endomyocardium (3). Additionally, affected myocardium may closely approximate or involve critical cardiac structures, such as the conduction system or tricuspid valve cords, which may lead to complications such as ventricular and supraventricular arrhythmias, conduction abnormality, or tricuspid regurgitation if biopsied (4).
The utility of electroanatomic mapping to guide EMB in the evaluation of suspected CS, ARVC, and lymphocytic myocarditis has been described in case reports (5–8) but has not been well studied. We have been utilizing electrogram-guidance at our institution to target diseased tissue and therefore increase the diagnostic yield of EMB in certain suspected disease processes. As shown in Table 1, we hypothesize that areas with normal electrograms will unlikely harbor abnormal pathology, since they consist of normal myocardium. Meanwhile, we hypothesize that abnormal and low-voltage electrograms will signify underlying scar tissue and that areas with fragmented signals without isoelectric segments (noted in either sinus or a ventricular paced rhythm) are likely to show active disease. We believe that targeting sites with abnormal electrograms and fragmented signals with EMB will result in the highest diagnostic yield.
Table 1.
Predicted biopsy results based on electrogram amplitude and characteristics
| Electrogram amplitude | Predicted result |
| Normal amplitude (>5mV) | Normal myocardium |
| Low amplitude (<5mV) | Likely abnormal myocardium |
| Very low amplitude (<1.5mV) | Abnormal myocardium |
| Electrogram characteristics | Predicted result |
| Single component signal | Mild fibrosis; unlikely active disease |
| Multicomponent electrograms | Interspersed fibrosis; possible active disease |
| Fragmented signals | Active disease |
Methods
We correlated pre-biopsy electrogram characteristics with histopathologic findings and clinical outcomes in eleven sequential patients undergoing electrogram-guided EMB at our institution. Approval to conduct this study was granted by the Mayo Clinic Institutional Review Board.
Mapping and biopsy procedure
The general approach of electrogram-guided EMB at our institution involves establishing 8-French access in the right femoral vein, through which a Blazer 4mm mapping catheter (Boston Scientific; Natick, MA) may be inserted. In cases where ablation is planned following EMB, an ablation catheter is introduced instead. Seven-French access is established in the right internal jugular vein (preferably) or another groin access site in preparation for bioptome insertion. Under fluoroscopic guidance, areas of fractionated low-voltage electrograms are identified. Next, with the mapping catheter held at a site with abnormal electrograms to target, a flexible, modified Cordis bioptome (Cordis Corporation; Bridgewater, NJ) is advanced utilizing fluoroscopy (Figure 1) and intracardiac echocardiography. Multiple biopsies are taken as close to the catheter tip as possible from areas with abnormal electrograms. Additionally, specimens are taken from standard biopsy sites (e.g., mid-ventricular septum or apical septum) with the catheter positioned nearby to determine the electrogram characteristics in these locations. In cases where abnormal electrograms are identified along the right ventricular (RV) free wall, biopsies are not taken from these sites given the concern for possible cardiac perforation. After biopsy is performed, intracardiac ultrasound is used to evaluate for the presence of post-procedural pericardial effusion. Due to the concern for bleeding complications with EMB, care is taken to assure that the patient is not anticoagulated during the procedure. For those patients in whom ablation is also planned, intravenous heparin is commenced only after pericardial effusion has been ruled out following EMB.
Figure 1.
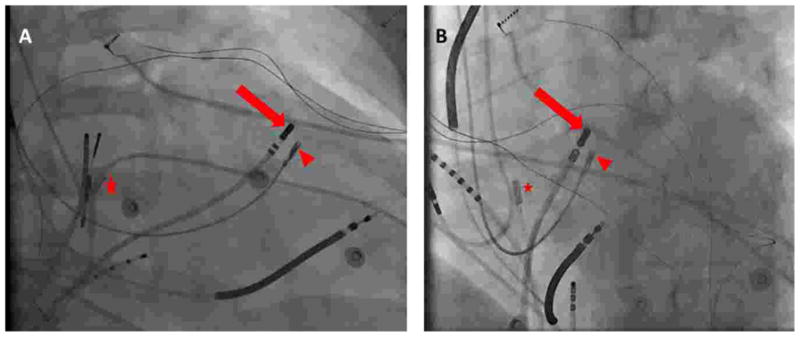
Left anterior oblique (A) and right anterior oblique (B) fluoroscopic views demonstrating intracardiac positioning of the mapping catheter (arrow) and bioptome (arrowhead) during electrogram-guided endomyocardial biopsy. An intracardiac echocardiography probe (star) is also present to confirm the proximity between the mapping catheter tip and bioptome.
Definitions of Abnormal versus Normal Electrogram Voltage and Morphology
Prior studies proposed a bipolar electrogram voltage amplitude of 1.5mV to be the lower-limit of normal in the RV and <0.5mV to suggest the presence of densely scarred endocardium (9–11) For this study, we arbitrarily defined electrogram amplitude as being “normal voltage” when >5mV, “low voltage” when <5mV, and “very low voltage” when <1.5mV.
We considered the morphology of RV bipolar endocardial electrograms to be normal when there was a single deflection with duration less than 70 msec. We defined the morphology of electrograms to be “abnormal” if they were either double/split potentials (two clearly separated deflections separated by at least 10 msec) or fractionated (polyphasic, primarily low-amplitude deflection) in appearance (Figure 2).
Figure 2.
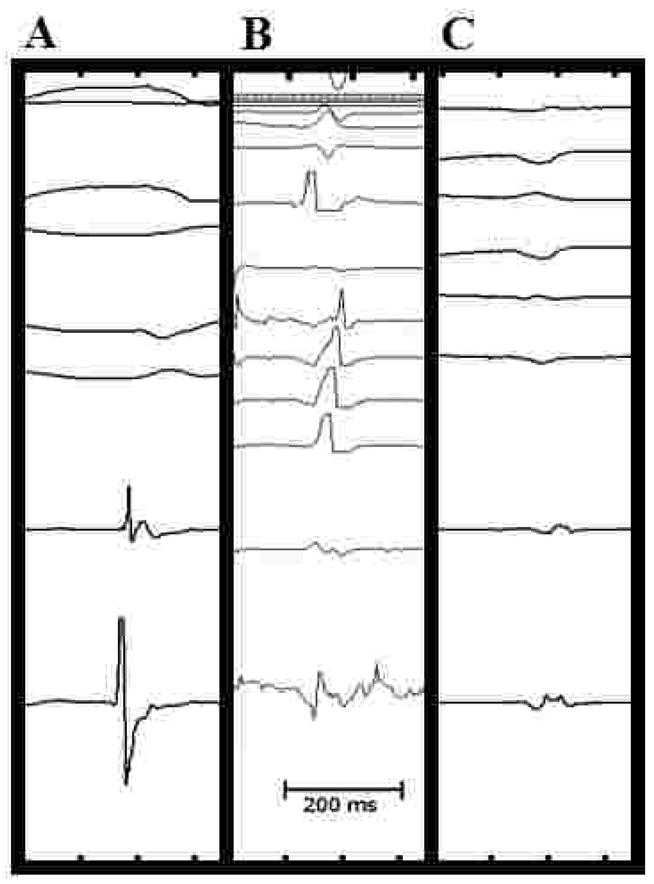
Classification of bipolar electrograms as recorded during sinus rhythm. Single component potentials (A) are characterized by a single narrow deflection of short duration. Split potentials (B) are usually >70 ms with two components clearly separated by a relatively isoelectric segment. Fractionated electrograms (C) show multiple fragmented potentials with multiple peaks and low-amplitude deflections resulting in prolonged duration (>70 ms).
Pathology review
Biopsy specimens were reviewed by cardiovascular pathologists (J.J.M and W.D.E). Results of pathologic analysis were considered for this study to be “abnormal” if they showed active myocardial inflammation or fibrosis. While nonspecific, we considered biopsy specimens with fibrosis as being “abnormal” for analysis, because despite not being diagnostic for active myocarditis, in this cohort of high-risk patients with suspected inflammation, fibrosis may have represented healed myocarditis or other cardiomyopathic processes and thus may be clinically significant. Biopsies demonstrating normal myocardium or only mild to moderate myocyte hypertrophy were considered to be “normal.”
Results
Eleven patients underwent electrogram-guided EMB for evaluation of suspected myocarditis or CS and 41 biopsy specimens were submitted for histopathologic evaluation. The corresponding electrogram for one specimen was not saved so it was excluded from the analysis.
Electrogram amplitude was <5mV at 19/40 (47.5%) biopsy sites and <1.5mV at 7/40 (17.5%) sites. Electrograms were considered “abnormal” in appearance in 19/40 (47.5%) biopsy sites. Overall, 27/40 (67.5%) of the biopsy specimens resulted in abnormal pathology, consistent with active myocarditis or fibrosis. Sensitivity and specificity as well as positive and negative predictive value based on voltage and/or abnormal electrogram appearance were calculated and are reported in Table 2.
Table 2.
Sensitivity, Specificity, Positive and Negative Predictive Values based on electrogram appearance and amplitude
| Electrogram characteristics | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| Very low amplitude (<1.5mV) | 7/27= 25.9% | 13/13= 100% | 7/7= 100% | 13/33= 39.4% |
| Low amplitude (<5mV) | 19/27= 70.4% | 13/13= 100% | 19/19= 100% | 13/21= 61.9% |
| Abnormal EGM | 18/27= 66.7% | 12/13= 92.3% | 18/19= 94.7% | 12/21= 57.1% |
| Abnormal EGM and <1.5mV | 7/27= 25.9% | 13/13= 100% | 7/7= 100% | 13/33= 39.4% |
| Abnormal EGM and <5mV | 18/27=66.7% | 12/13= 92.3% | 18/19= 94.7% | 12/21= 57.1% |
Very low (<1.5mV) and low (<5mV) electrogram amplitude had 100% specificity and positive predictive value for identifying abnormal myocardial tissue. Increasing the voltage cutoff from 1.5mV to 5mV decreased the sensitivity (70.4% down to 25.9%) and negative predictive value (61.9% down to 39.4%). The presence of abnormal electrogram appearance had a high specificity (92.3%) and positive predictive value (94.7%) for detecting abnormal myocardium. Of the 4 (11%) biopsy specimens taken at sites with severe fragmentation without a discernible electrogram, all exhibited histopathologic abnormality (2 showed active inflammation, 1 showed healed myocarditis, 1 showed severe focal fibrosis).
A diagnosis of active myocarditis or CS was made by electrogram-guided EMB in five patients (Supplement) and treatment was directed by electrogram-guided EMB results in all eleven patients. The relationships between electrogram characteristics and EMB results for all patients are documented in Table 3. Figure 3 shows the correlation between electrogram and biopsy histopathology in one patient in whom electrogram-guided biopsy led to the diagnosis of a mixed disease process consistent with both CS and giant cell myocarditis (GCM).
Table 3.
Relationships between electrogram characteristics and EMB pathologic results in patients undergoing electrogram-guided EMB
| Pt # | Age/sex | EGM appearance | EGM Amplitude (mV) | EMB location | Histopathologic Findings | Abnormal myocardium? |
|---|---|---|---|---|---|---|
| 1 | 48M | Fragmented, 20% cycle length | 2.7 | RV mid-septum | Active myocarditis | Y |
| Highly fragmented, >50% fragmentation | 1.7 | Unknown | Active myocarditis | Y | ||
| Normal | 7 | RV mid septum posterior | Mild myocyte hypertrophy | N | ||
| >50% fragmentation, neighboring tissue with double potential | 3 | RV mid septum posterior | Active myocarditis | Y | ||
| Highly fragmented, >50% cycle length | 2–3 | anterior septum distal to membranous septum, near right bundle branch | Active myocarditis | Y | ||
| 2 | 50F | Highly fragmented | No discernible EGM | RV mid septum | Non-necrotizing granulomatous myocarditis with eosinophilic infiltrate | Y |
| Fragmented, <20% cycle length | 3 | Posterior septal RVOT | Non-necrotizing granulomatous myocarditis with eosinophilic infiltrate | Y | ||
| No consistent EGMs | <0.5 | RV base near right bundle branch | Densely scarred endocardium | Y | ||
| Fragmented <10% | 4 | RV 3 cm proximal to the apex on the septum | Non-necrotizing granulomatous myocarditis with eosinophilic infiltrate | Y | ||
| 3 | 44M | Highly fragmented, >75% cycle length | No discernible EGM | Base of anterior septum | Active giant cell myocarditis, versus CS | Y |
| Single EGM | 11 | RV mid septum | Mild myocyte hypertrophy | N | ||
| Single EGM | 7 | Proximal RVOT | Mild interstitial fibrosis and myocyte hypertrophy | Y | ||
| Normal | 11 | RV mid septum | Normal myocardium | N | ||
| 4 | 50F | Double potential, no fragmentation | 4–5 | RV mid septum, 2 cm from annulus | Non-diagnostic but consistent with early giant cell formation and lymphocytic infiltration, possible CS | Y |
| Normal | 11 | RV mid septum | Mild interstitial fibrosis | Y | ||
| No fragmentation | 7.8 | RV apex | Mild replacement fibrosis, mild lymphocytic infiltrate | Y | ||
| Single EGM | 12 | 2 cm proximal to the apex, RV septum | Mild interstitial fibrosis | Y | ||
| 5 | 51F | Highly fragmented, >50% cycle length, neighboring double potential | 3 | Annular RV septum | Active inflammation, noncaseating granuloma consistent with CS | Y |
| Highly fragmented, >25% cycle length | 4 | RVOT, posterior septum at base of outflow tract | Suggestive of CS, inflammatory cells, suggestion of giant cells | Y | ||
| Single EGM, no fragmentation | 8 | RV apical septum | Mild myocyte hypertrophy | N | ||
| Normal | 9 | Proximal/mid apex on septum | Mild myocyte hypertrophy | N | ||
| Double potential to neighboring fragmented signals | 4 | RV mid septum | Consistent with CS | Y | ||
| 6 | 34F | Highly fragmented signals, no discernible EGM | No discernible EGM | RV septum | Focal interstitial fibrosis, no granuloma. Possible healed myocarditis | Y |
| Normal | 7 | RV septum | Moderate myocyte hypertrophy, mild focal interstitial fibrosis. Possible healed myocarditis | Y | ||
| 7 | 23F | Mildly abnormal EGMs, no fragmentation, no hypo potential | 7 | RV mid to low septum | Mild myocyte hypertrophy | N |
| Normal | 11 | RV mid to low septum | Mild myocyte hypertrophy | N | ||
| Normal | 11 | RV mid to low septum | Mild myocyte hypertrophy | N | ||
| Normal | 11 | RV apex on septum | Mild myocyte hypertrophy | N | ||
| 8 | 42F | Highly fragmented signal, no discernible EGM >75% of cycle length | No discernible EGM | RV mid septum | Severe focal fibrosis, generalized moderate myocyte hypertrophy and interstitial fibrosis | Y |
| Single EGM | 4 | RV apex | Mild fibrosis, mild myocyte hypertrophy | Y | ||
| 9 | 16F | Normal | 11 | RV apical septum | Mild focal interstitial fibrosis, mild hypertrophy | Y |
| Normal | 11 | RV mid septum | Normal myocardium, no active inflammation | N | ||
| Single EGM | 9–10 | RV mid septum | Mild hypertrophy, mild interstitial fibrosis | Y | ||
| 10 | 20F | Normal | >10 | RV annular septum | Myocyte hypertrophy, no inflammation | N |
| Single EGM | 6 | RV apical septum | Myocyte hypertrophy with fibrosis, no inflammation | Y | ||
| Single EGM | 9 | RV mid septum | Myocyte hypertrophy, no fibrosis, no inflammation | N | ||
| 11 | 52M | Fragmented signal, 140 msec, split potentials surrounding | 1.2 | Septal RVOT basal | Moderate interstitial fibrosis | Y |
| Normal | 9 | Mid-septum | Mild myocyte hypertrophy | N | ||
| Split potential, extensive fragmentation surrounding | 0.6 | Apical inferior RV septum | Mild interstitial fibrosis | Y | ||
| Split potential, fragmentation surrounding | 3.7 | Septal RVOT | Mild hypertrophy and mild interstitial fibrosis | Y |
Figure 3.
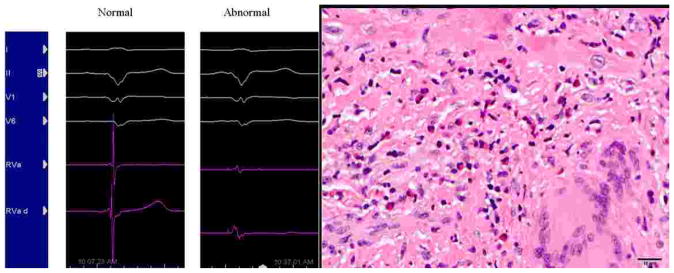
Normal intracardiac electrogram (A) and electrogram with fragmented signal (B) targeted for biopsy in Patient 2. Histologic image (C) from EMB tissue taken from area of fragmented signal in the patient demonstrating both non-necrotizing granulomas and eosinophils consistent with mixed pattern of CS and GCM.
In the six patients without active inflammation on microscopic evaluation, immunosuppression was withheld. In three of these patients without evidence of active inflammation, no fragmented signals were identified and all three remain well to date off immunosuppression. In one patient with fragmented electrogram signals, focal interstitial fibrosis and features of healed myocarditis were identified histopathologically, and immunosuppression was withheld. Three years later she was diagnosed clinically and radiographically with CS and commenced on immunosuppression.
There were no complications directly caused by electrogram-guided EMB in our series. However, one patient returned to the hospital after discharge with dyspnea and hypotension and was found to have anemia, a small right hemothorax, and a hemodynamically insignificant hemopericardium. Nevertheless, the complication was attributed to cardiac resynchronization therapy-defibrillator upgrade rather than the EMB procedure itself, since she had undergone placement of right atrial and coronary sinus leads after her uncomplicated EMB. She was managed medically, discharged after three days, and had no further complications.
Discussion
Although currently most commonly used for rejection surveillance monitoring after cardiac transplantation, EMB is an important diagnostic procedure in evaluation of selected patients with cardiomyopathy (1–3). Russell et al. recently reported their single-center 851-patient experience with EMB for unexplained heart failure, finding that EMB provided a diagnostic result in 25.5% of cases and changed clinical course in 22.6% (12). All of our patients fit “Clinical Scenario #3” from the 2007 AHA/ACCF/ESC Scientific Statement (1), which states that EMB is reasonable to perform in patients with unexplained heart failure of over three months duration when associated with dilated left ventricle (LV) and new ventricular arrhythmias, high-grade atrioventricular (AV) block, or failure to respond to normal heart failure therapy within 1–2 weeks. EMB is particularly helpful to diagnose conditions where management differs dramatically depending on the underlying process (i.e. immunosuppression for CS, lymphocytic myocarditis, and GCM versus screening of family members for ARVC).
Limitations of contemporary EMB
One of the major limitations of EMB is its low sensitivity in detection of certain disorders that do not diffusely involve the myocardium. Sensitivity with EMB in detection of lymphocytic myocarditis, which varies depending on duration of disease, may as low as 10–35% (1,13,14). For CS, EMB sensitivity ranges from 20–30% (1,15), while sensitivity tends to be much higher (80–85%) with fulminant giant cell myocarditis (16). In a study by Kandolin et al which examined 72 patients under age 55 who underwent pacemaker implantation for initially unexplained high-grade AV block, EMB later established the diagnoses of CS and GCM in 14 (19%) and 4 (6%) patients, respectively (17). The 25% positive biopsy rate in these patients with unexplained high-grade AV block suggests that EMB is reasonable in similarly presenting patients (18). Due to the low sensitivity of contemporary EMB, previous recommendations have suggested that only positive findings be considered diagnostic when lymphocytic myocarditis is suspected (19). Repeating EMB after negative results when suspicion for underlying process may increase the sensitivity, as has been reported with GCM (68% with single biopsy versus 93% after up to 2 repeat procedures) (20). This however, places patients at risk for procedural complications associated with each additional EMB.
Electrogram-guidance to increase diagnostic yield
EMB using an electrogram-guided approach may be beneficial in diagnosing certain disease processes. Areas of active inflammation or chronic fibrosis will have abnormal electrogram morphology and amplitude, allowing the operator to avoid biopsying normal myocardium, potentially increasing diagnostic yield. Furthermore, when biopsies taken from areas of low-voltage electrograms only demonstrate fibrosis without active inflammation, active disease can be more confidently ruled out allowing for aggressive immunosuppression to be withheld. While the addition of electrogram-guidance increases procedural and fluoroscopic time associated with EMB, it may increase the sensitivity and specificity of EMB, potentially curtailing the need for repeated biopsy procedures to establish a diagnosis in certain patients.
Successful EMB guided by electroanatomic mapping has been previously described in the diagnosis of ARVC (5,6) and isolated CS (7). Recently, Seizer et al. have described the diagnosis of acute lymphocytic myocarditis in a patient using site-directed EMB at the location of an abnormal electrogram in the LV via transeptal puncture, while RV septal biopsies from sites of normal electrograms were unremarkable in the same patient (8). CS and ARVC (particularly in early stages) may have focal cardiac involvement and electrogram-guidance may identify low-voltage areas of myocardium which have been replaced by fibrous or adipose tissue, allowing for targeted biopsy. CS classically involves the base of the heart so standard mid-ventricular or apical biopsies may be unrevealing. While it is technically more difficult to biopsy at the base (particularly near the outflow tract), the presence of basal fractionated signals should prompt the operator to target these areas for EMB. Chimenti and Frustaci recently reported that RV EMB alone has high diagnostic yield (96.5%) in cases where RV involvement was seen on pre-EMB imaging (21). However, in cases where abnormalities were limited to the LV on pre-EMB imaging, they found that LV EMB was safe when performed by skilled providers and significantly increased diagnostic yield compared to RV biopsy alone (97.8% vs 53%). Therefore, pre-procedural imaging to localize areas of inflammation should be used as an adjunctive tool, as it may prompt consideration for electrogram-guided biopsy of the LV or even epicardium.
Marchlinski et al. have described bipolar voltage amplitude to be >1.44mV in 95% of all RV signals recorded during sinus rhythm using the Carto system in patients with drug-refractory ventricular tachycardia (11). Based on their findings, an RV bipolar electrogram voltage amplitude of >1.5mV has since been widely accepted to be “normal,” while an amplitude <0.5mV has been correlated with “densely scarred” endomyocardium (9,10). However, the presence of abnormal myocardial pathology in 20/33 (60.6%) biopsy specimens at sites of electrogram voltage >1.5mV in our series suggests that abnormal myocardium can exist in areas of apparently higher voltage. Due to the low sensitivity and negative predictive value of such a low voltage cutoff, an amplitude of <5mV and/or the presence of abnormal electrogram appearance may more accurately predict abnormal myocardium at sites where EMB should be targeted. All biopsies taken at areas with low-voltage electrograms in our series exhibited histopathologic abnormality suggesting that electrogram-guidance is particularly helpful in identifying areas which would be low-yield and unlikely to harbor myocardium with active inflammation or fibrosis. While positive electrogram-guided biopsy findings demonstrating active myocarditis or CS is helpful in confirming a diagnosis, negative results do not completely rule out active disease.
Safety of electrogram-guided EMB
With contemporary EMB, complications related to sheath insertion and biopsy have been reported to occur in 1–6% of cases (1,4). The risks of cardiac perforation (0.2%), and vascular (1.2%) or embolic (0.4%) complications from catheter manipulation during electrophysiologic studies are low (22). Additionally, electrograms characteristic of the conduction system identify sites to avoid with the bioptome, thus minimizing electrical complications including arrhythmia and conduction abnormalities which have been previously reported to occur in 1% of cases with contemporary EMB (4).
Study Limitations
We acknowledge that one major limitation of our study was the small sample size, which make the results difficult to extrapolate to a larger population. Despite this, we believe we were able to show “proof-of-concept” of a promising electrogram-guided biopsy technique. We hope our findings will encourage other investigators to conduct larger population and prospective studies to determine the true benefit of electrogram guidance for EMB. While we attempted to achieve optimal approximation between the ablation catheter and bioptome, we had to assure that there was no direct contact between the ablation catheter and bioptome since that would result in noise, impairing electrogram interpretation. As such, it is possible that the region from which the biopsy was taken may not have corresponded exactly with the area from which the signal was recorded as small differences in location between the bioptome and ablation catheter may account for differences in location, particularly in focal diseases processes and along the border zones of abnormal tissues. Factors such as movement artifact and filtering may cause bipolar electrograms to appear fractionated in the absence of underlying diseased myocardium (23). Additionally, while bipolar recording is not as sensitive to remote activity compared to unipolar recording, remote activation may still interfere.
Conclusion
We describe eleven patients who successfully underwent electrogram-guided EMB without complication. In each patient, areas with abnormal electrograms were targeted for biopsy where present. In five patients, biopsies taken at areas with fragmented signal revealed active myocarditis, prompting treatment with aggressive immunosuppressive therapy. Areas with normal electrogram appearance and amplitude >5mV will likely harbor normal myocardium and biopsies should not be taken from these locations when the intent is to detect myocardial inflammation or fibrosis.
Normal voltage and electrogram appearance does not significantly yield results to identify abnormal myocardium. As a result, randomly acquired, fluoroscopically guided EMB where such sites may be sampled had suboptimal diagnostic yield. Further prospective studies, preferably randomized between electrogram-guided versus routine RV-septal biopsies, are needed to demonstrate the exact value for electrogram-guided biopsy.
Supplementary Material
Case descriptions of the five patients with active myocarditis or cardiac sarcoidosis diagnosed with electrogram-guided biopsy.
Acknowledgments
Funding Sources: None
Abbreviations
- EMB
endomyocardial biopsy
- CS
cardiac sarcoidosis
- ARVC
arrhythmogenic right ventricular cardiomyopathy
- RV
right ventricle
- GCM
giant cell myocarditis
- LV
left ventricle
- AV
atrioventricular
Footnotes
Disclosures: Samuel Asirvatham, MD:
Honoraria/Consulting (none significant): Abiomed, Atricure, Biotronik, Biosense Webster, Boston Scientific, Medtronic, Spectranetics, St. Jude, Sanofi-Aventis, Wolters Kluwer, Elsevier
Co-patent holder – may receive future royalties from: Aegis: Appendage ligation; ATP: Atrial fibrillation ablation and coagulum reduction during ablation; Nevro: Use of nerve signal modulation to treat central, autonomic, and peripheral nervous system disorders including pain; Sanovas: Lung ablation; Sorin Medical: Tricuspid valve project
All other authors report no relevant financial disclosures or relationships with industry.
Disclosures: None
References
- 1.Cooper LT, Baughman KL, Feldman AM, et al. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Circulation. 2007;116:2216–33. doi: 10.1161/CIRCULATIONAHA.107.186093. [DOI] [PubMed] [Google Scholar]
- 2.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 3.From AM, Maleszewski JJ, Rihal CS. Current status of endomyocardial biopsy. Mayo Clin Proc. 2011;86:1095–102. doi: 10.4065/mcp.2011.0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deckers JW, Hare JM, Baughman KL. Complications of transvenous right ventricular endomyocardial biopsy in adult patients with cardiomyopathy: A seven-year survey of 546 consecutive diagnostic procedures in a tertiary referral center. Journal of the American College of Cardiology. 1992;19:43–47. doi: 10.1016/0735-1097(92)90049-s. [DOI] [PubMed] [Google Scholar]
- 5.Avella A, Pappalardo A, d’Amati G, et al. Endomyocardial biopsy guided by electroanatomic voltage mapping in arrhythmogenic right ventricular cardiomyopathy: a case report. J Cardiovasc Electrophysiol. 2007;18:991–3. doi: 10.1111/j.1540-8167.2007.00807.x. [DOI] [PubMed] [Google Scholar]
- 6.Ejima K, Shoda M, Manaka T, Hagiwara N. Targeted endomyocardial biopsy using electroanatomical voltage mapping in the early stage of arrhythmogenic right ventricular cardiomyopathy. Europace. 2009;11:388–9. doi: 10.1093/europace/eun357. [DOI] [PubMed] [Google Scholar]
- 7.Nery PB, Keren A, Healey J, Leug E, Beanlands RS, Birnie DH. Isolated Cardiac Sarcoidosis: Establishing the Diagnosis With Electroanatomic Mapping-Guided Endomyocardial Biopsy. Can J Cardiol. 2012 doi: 10.1016/j.cjca.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Seizer P, Klingel K, Stickel J, et al. Left ventricular site-directed biopsy guided by left ventricular voltage mapping: A proof of principle. International Journal of Cardiology. 2013;168:3113–3114. doi: 10.1016/j.ijcard.2013.04.068. [DOI] [PubMed] [Google Scholar]
- 9.Kienzle MG, Miller J, Falcone RA, Harken A, Josephson ME. Intraoperative endocardial mapping during sinus rhythm: relationship to site of origin of ventricular tachycardia. Circulation. 1984;70:957–65. doi: 10.1161/01.cir.70.6.957. [DOI] [PubMed] [Google Scholar]
- 10.Cassidy DM, Vassallo JA, Miller JM, et al. Endocardial catheter mapping in patients in sinus rhythm: relationship to underlying heart disease and ventricular arrhythmias. Circulation. 1986;73:645–52. doi: 10.1161/01.cir.73.4.645. [DOI] [PubMed] [Google Scholar]
- 11.Marchlinski FE, Callans DJ, Gottlieb CD, Zado E. Linear Ablation Lesions for Control of Unmappable Ventricular Tachycardia in Patients With Ischemic and Nonischemic Cardiomyopathy. Circulation. 2000;101:1288–1296. doi: 10.1161/01.cir.101.11.1288. [DOI] [PubMed] [Google Scholar]
- 12.Bennett MK, Gilotra NA, Harrington C, et al. Evaluation of the Role of Endomyocardial Biopsy in 851 Patients With Unexplained Heart Failure From 2000–2009. Circulation: Heart Failure. 2013;6:676–684. doi: 10.1161/CIRCHEARTFAILURE.112.000087. [DOI] [PubMed] [Google Scholar]
- 13.Felker GM, Thompson RE, Hare JM, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342:1077–84. doi: 10.1056/NEJM200004133421502. [DOI] [PubMed] [Google Scholar]
- 14.Narula J, Khaw BA, Dec GW, et al. Diagnostic accuracy of antimyosin scintigraphy in suspected myocarditis. J Nucl Cardiol. 1996;3:371–81. doi: 10.1016/s1071-3581(96)90070-7. [DOI] [PubMed] [Google Scholar]
- 15.Uemura A, Morimoto S-i, Hiramitsu S, Kato Y, Ito T, Hishida H. Histologic diagnostic rate of cardiac sarcoidosis: Evaluation of endomyocardial biopsies. American Heart Journal. 1999;138:299–302. doi: 10.1016/s0002-8703(99)70115-8. [DOI] [PubMed] [Google Scholar]
- 16.Shields RC, Tazelaar HD, Berry GJ, Cooper LT., Jr The role of right ventricular endomyocardial biopsy for idiopathic giant cell myocarditis. J Card Fail. 2002;8:74–8. doi: 10.1054/jcaf.2002.32196. [DOI] [PubMed] [Google Scholar]
- 17.Kandolin R, Lehtonen J, Kupari M. Cardiac Sarcoidosis and Giant Cell Myocarditis as Causes of Atrioventricular Block in Young and Middle-Aged Adults. Circulation: Arrhythmia and Electrophysiology. 2011;4:303–309. doi: 10.1161/CIRCEP.110.959254. [DOI] [PubMed] [Google Scholar]
- 18.Cooper LT, Blauwet LA. When Should High-Grade Heart Block Trigger a Search for a Treatable Cardiomyopathy? Circulation: Arrhythmia and Electrophysiology. 2011;4:260–261. doi: 10.1161/CIRCEP.111.963249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hauck AJ, Kearney DL, Edwards WD. Evaluation of Postmortem Endomyocardial Biopsy Specimens From 38 Patients With Lymphocytic Myocarditis: Implications for Role of Sampling Error. Mayo Clinic Proceedings. 1989;64:1235–1245. doi: 10.1016/s0025-6196(12)61286-5. [DOI] [PubMed] [Google Scholar]
- 20.Kandolin R, Lehtonen J, Salmenkivi K, Räisänen-Sokolowski A, Lommi J, Kupari M. Diagnosis, Treatment, and Outcome of Giant-Cell Myocarditis in the Era of Combined Immunosuppression. Circulation: Heart Failure. 2013;6:15–22. doi: 10.1161/CIRCHEARTFAILURE.112.969261. [DOI] [PubMed] [Google Scholar]
- 21.Chimenti C, Frustaci A. Contribution and Risks of Left Ventricular Endomyocardial Biopsy in Patients With Cardiomyopathies: A Retrospective Study Over a 28-Year Period. Circulation. 2013;128:1531–1541. doi: 10.1161/CIRCULATIONAHA.13.001414. [DOI] [PubMed] [Google Scholar]
- 22.Horowitz LN, Kay HR, Kutalek SP, et al. Risks and complications of clinical cardiac electrophysiologic studies: A prospective analysis of 1,000 consecutive patients. Journal of the American College of Cardiology. 1987;9:1261–1268. doi: 10.1016/s0735-1097(87)80465-5. [DOI] [PubMed] [Google Scholar]
- 23.de Bakker JMT, Wittkampf FHM. The Pathophysiologic Basis of Fractionated and Complex Electrograms and the Impact of Recording Techniques on Their Detection and Interpretation. Circulation: Arrhythmia and Electrophysiology. 2010;3:204–213. doi: 10.1161/CIRCEP.109.904763. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Case descriptions of the five patients with active myocarditis or cardiac sarcoidosis diagnosed with electrogram-guided biopsy.


