Abstract
Chronic circadian dysfunction impairs declarative memory in humans but has little effect in common rodent models of arrhythmia caused by clock gene knockouts or surgical ablation of the suprachiasmatic nucleus (SCN). An important problem overlooked in these translational models is that human dysrhythmia occurs while SCN circuitry is genetically and neurologically intact. Siberian hamsters (Phodopus sungorus) are particularly well suited for translational studies because they can be made arrhythmic by a one-time photic treatment that severely impairs spatial and recognition memory. We found that once animals are made arrhythmic, subsequent SCN ablation completely rescues memory processing. These data suggest that the inhibitory effects of a malfunctioning SCN on cognition require preservation of circuitry between the SCN and downstream targets that are lost when these connections are severed.
Deficits in cognitive performance caused by disrupted circadian timing have become a growing concern among health care professionals (1). Recent clinical studies have found that age-related declines in circadian function can lead to mild cognitive impairment or dementia (2, 3). These memory deficits are not simply a consequence of poorer sleep, because reductions in circadian rhythm amplitude and robustness can accelerate progression of mild cognitive impairment or dementia even when sleep quality is maintained (2, 3). The observation that circadian timing is substantially weakened among people with Alzheimer's disease has raised the possibility that cognitive deficits might be treated by improving circadian function (4–6). However, there are no mouse or rat models of adult-onset circadian dysfunction in genetically and neurologically intact animals living in standard laboratory conditions.
Rodent models of chronic circadian arrhythmia such as clock gene knockouts or surgical ablation of the central circadian pacemaker, the suprachiasmatic nucleus (SCN), exhibit no or only modest deficits in declarative memory. Clock gene knockouts of cryptochrome 1 and 2 or period 1 and 2 are arrhythmic, and they exhibit normal spontaneous alternation behavior, long-term spatial memory for food rewards, and contextual memory for environments associated with foot shock (7, 8), although cryptochrome 1 and 2 knockouts do fail to learn time-place associations (7). Bmal1 knockouts are also arrhythmic and exhibit normal contextual fear conditioning and novel object recognition; however, they navigate poorly in the Morris water maze (9). In the instances where knockout mice exhibit performance deficits, it is unclear whether memory impairments are due to arrhythmia, to pleiotropic gene effects, or to abnormalities during brain development (10). Mice and rats with SCN lesions exhibit no substantial impairment in avoidance tasks, recognition memory, spatial learning, or reversal spatial learning but have modest deficits in contextual fear conditioning and in the water maze probe test (11–14). In some studies, SCN ablation actually improves task performance (11, 12).
The marginal effect of clock gene knockouts and SCN lesions on memory stands in stark contrast to the well-documented adverse effects of shift work and jet lag on cognition (15). One critical factor that often gets overlooked in relating animal circadian studies to human conditions is the fact that human dysrhythmia occurs while the SCN circuitry remains intact both genetically and structurally. We evaluated the possibility that intact SCN circuitry is necessary for circadian dysfunction to interfere with memory processing by using the Siberian hamster (Phodopus sungorus) model of circadian arrhythmia. This model has been used to study homeostatic sleep mechanisms, where its value compared to clock gene knockout and SCN lesion models of arrhythmia has been recognized (16).
Siberian hamsters exhibit phase-resetting responses to single light pulses that are typical of nocturnal rodents (Fig. 1A), yet their response to two light signals is quite different. When these animals are given a phase-advancing light signal on one night, followed by a phase-delaying light signal on the next night, circadian timing is completely abolished within a few days, even though each signal given alone does not disrupt their circadian organization (Fig. 1B). This disruptive phase shift (DPS) protocol causes arrhythmia by suppressing the amplitude of clock gene oscillations within the SCN to zero, thereby driving the clock to its singularity point (17, 18). Induction of arrhythmia at the genetic and behavioral levels occurs within a few days and lasts indefinitely despite the continued presence of a daily light-dark cycle (18, 19).
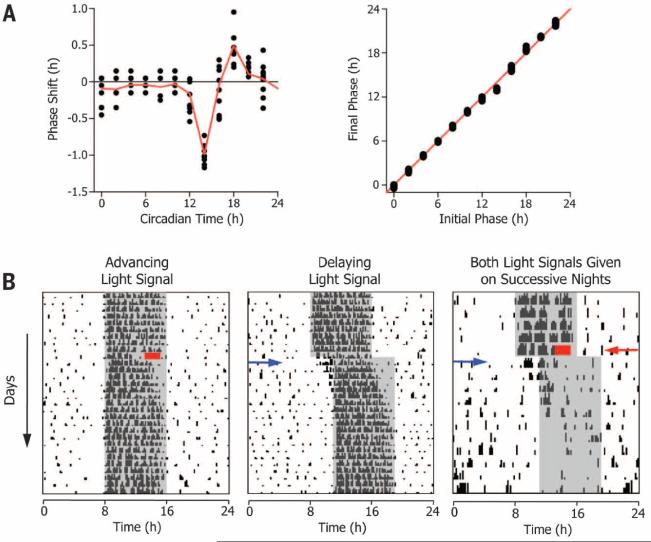
Fig. 1. Phase-resetting responses of the Siberian hamster circadian system.
(A) Phase response (left) and transition (right) curves are typical for nocturnal rodents. Hamsters were housed in 16 hours of light followed by 8 hours of dark. Each animal was given a single light pulse (300 lux, 30 min) at different phases of the first circadian cycle in constant darkness (22). Each filled black circle represents a single animal (n = 98); means of each time point are connected by the red line.The phase transition curve has a slope of 1, which indicates type 1 (i.e.,weak) phase resetting (27). (B) The DPS protocol. Actograms show daily locomotor activity on each line, with successive days plotted from top to bottom. A phase-advancing signal (2-hour light pulse, red rectangle), followed by a phase-delaying signal (3-hour delay of the light-dark cycle, blue arrow) on the next day drives the system into arrhythmia. Night is indicated by the gray shaded area. The progressive daily shortening of the nightly active phase (i.e., alpha compression) after DPS treatment reliably predicts arrhythmia (17).
The DPS protocol allowed us to evaluate recognition and spatial memory in arrhythmic Siberian hamsters with and without an intact SCN. Novel object recognition (NOR) was assessed as described (20), where the interval between the sample and test phases was 24 hours. Hamsters are assumed to have remembered the familiar object if they spend significantly more time investi gating the novel object during the test phase. Spatial working memory was assessed by spontaneous alternation (SA) behavior in a T maze using a continuous trials procedure (21).
In the first experiment, adult Siberian hamsters were made arrhythmic either by the DPS protocol or by bilateral surgical ablation of the SCN (Fig. 2C) (22). Arrhythmia was confirmed by chi-square periodogram analysis of locomotor activity rhythms in the animal's home cages during the 10 days before behavioral testing (Fig. 2A) (22). Lesions were confirmed by histological analysis of the SCN, which showed that sleep regulatory areas of the hypothalamus and the optic tract remained undamaged after the surgery (fig. S6). Hamsters were evaluated 4 to 6 weeks after surgery or after the DPS protocol. NOR testing was conducted over two consecutive days, followed by the SA test 3 days later. All tests were conducted within a 5-hour time window before dark onset in the colony room.
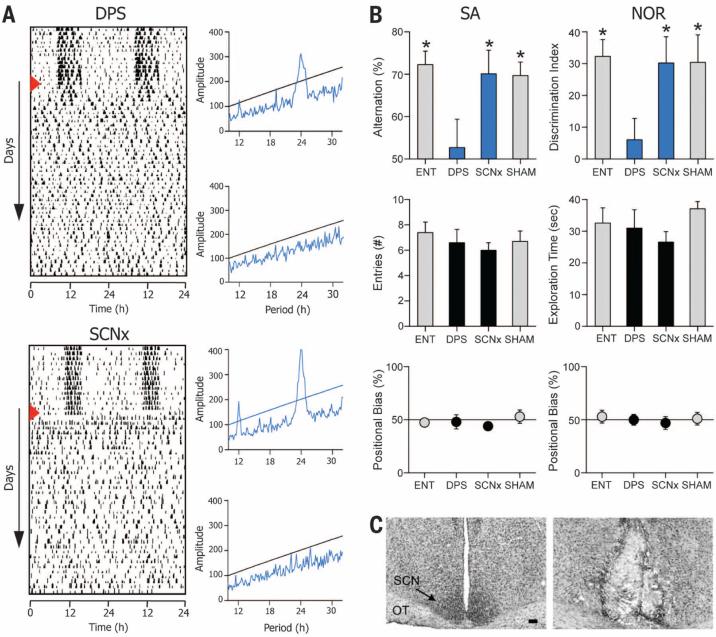
Fig. 2. An intact SCN is necessary for circadian arrhythmia to impair recognition and spatial memory.
(A) Representative actograms of DPS (top) and SCNx (bottom) animals; red triangle indicates day of treatment. Chi-square periodograms confirm robust rhythms during entrainment (blue peaks above black line are significant; P = 0.001) and arrhythmia. (B) SCNx animals (n = 11) performed significantly better than random chance (i.e., 50 for SA, 0 for NOR), whereas DPS-treated animals (n = 8) failed both memory tests (upper panels). ENT (n = 10) and sham-operated (SHAM) (n = 11) hamsters also performed significantly better than chance. Arrhythmic groups shown in blue. Scores significantly different from chance indicated by * (one-sample t test; 0.0001 < P < 0.01). For DPS animals, P > 0.05. Number of T-maze arm entries and NOR exploration times did not differ significantly among ENT, DPS, SCNx, and SHAM groups (P > 0.05; middle panels). No left-right positional biases were found in the Tmaze or NOR arena (P > 0.05; lower panels) (22). (C) Representative Nissl-stained tissue sections from intact (left) and SCNx (right) hamsters. Optic tracts are labeled OT. Black scale bar, 100 μm.
Consistent with studies done in mice and rats, hamsters made arrhythmic with complete bilateral ablation of the SCN (SCNx) performed just as well as normal entrained (ENT) animals in both NOR and SA tests (Fig. 2B and figs. S1A and S2A). By contrast, arrhythmic DPS hamsters did not perform better than chance in either test (Fig. 2B and figs. S1A and S2A). Surgery had no effect on the animal's engagement with the memory tests; exploration during the behavioral tests was the same for all groups (Fig. 2B and figs. S1A and S2A) (22). Because hippocampal lesions can cause perseverative responses in mazes (21), we also analyzed the data for left-right positional biases of SCNx hamsters in the open arena and the T maze, but none were found (Fig. 2B and figs. S1A and S2A) (22). We conclude that cognitive impairment caused by circadian dysfunction is predicated on maintaining circuit connections from the SCN to other areas of the brain involved with information processing.
The idea that the arrhythmic SCN could actively inhibit memory formation was evaluated by a series of sequential treatments in a single group of animals. Siberian hamsters were first tested for NOR and SA while they were entrained to the light-dark cycle to establish baseline performance and then subjected to the DPS protocol. Once arrhythmia was confirmed (Fig. 3A), they were tested a second time 4 to 6 weeks later and then subjected to SCN ablation or sham surgery. After 4 to 6 weeks of postsurgical recovery, these animals were tested a third and final time. SCN lesions sometimes reduce overall levels of locomotor activity (23). Because such reductions could potentially limit exploration time in the memory tests, we examined the data for any changes in locomotor activity during the various treatment phases, but none were found (one-way analysis of variance; P > 0.05) (Fig. 3B).
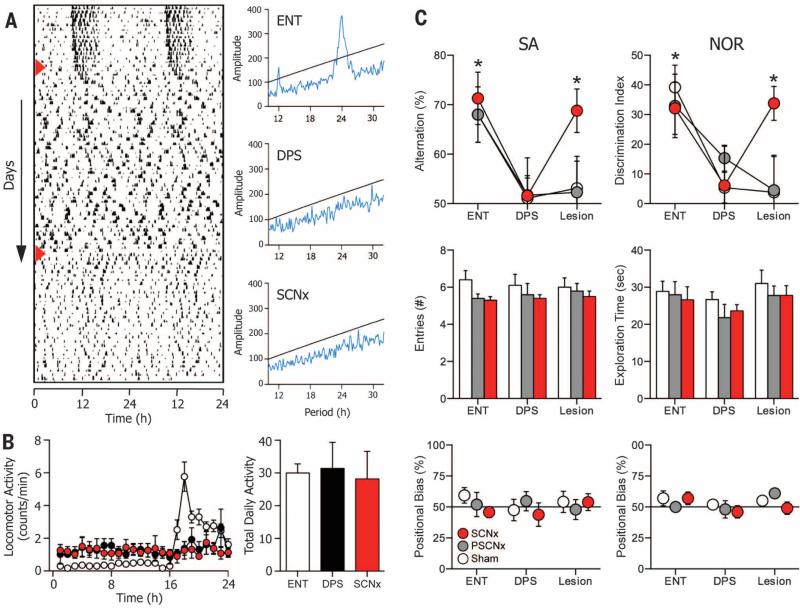
Fig. 3. SCN ablation rescues memory in arrhythmic animals.
(A) Representative actogram of a hamster during ENT, DPS, and SCNx phases of the experiment. Phases were separated by 4 to 6 weeks. Days of DPS and SCNx treatments are indicated (red arrowheads). Periodograms evaluated the last 10 days of data from each phase of the experiment. (B) Locomotor activity was redistributed across the 24-hour day when animals became arrhythmic (left), but total daily activity is conserved across all conditions (right). (C) Performance on both memory tests (upper panels) was normal while animals were entrained but was impaired after the DPS protocol made them arrhythmic. SCN ablation subsequently rescued test performance (Lesion; n = 11; red circles). Scores significantly different from chance indicated by * (one-sample t test; 0.0001 < P < 0.05). Memory did not improve in SHAM animals (Lesion; n = 8; white circles) or in animals with partial damage to the SCN (Lesion; n = 9; gray circles). Surgery did not alter the number of arm entries or exploration time (middle panels) among SCNx (red bars), PSCNx (gray bars), or SHAM (white bars) animals (22). (C) None of the treatment groups showed any positional bias in the T maze or NOR arena (lower panels) (22).
Animals successfully mastered the NOR and SA tests when they were entrained, but arrhythmia induced by the DPS protocol impaired their recognition and spatial memory (Fig. 3C and figs. S1B and S2B) (22). These memory deficits were completely reversed in hamsters that subsequently underwent SCN ablation surgery (Fig. 3C and figs. S1B and S2B). Bilateral ablation of the SCN improved test performance to levels observed during entrainment. Exploratory activity and positional responses during the tests remained unaffected by the DPS protocol or by SCN surgery (supplementary text and movies S1 to S3), and sham-operated animals did not improve their performance (Fig. 3C and figs. S1B and S2B). We also found that memory was not restored in hamsters that sustained only partial ablation of the SCN (PSCNx) (Fig. 3C and figs. S1B and S2B). In PSCNx hamsters, the extent of the ablation was restricted between the rostral and middle SCN and damaged only about 20 to 60% of the nucleus.
The present study demonstrates that chronic arrhythmia per se does not cause memory impairments in animals. Rather, an arrhythmic SCN is necessary to realize these deficits, even if only a subpopulation of SCN neurons remains, as was the case for PSCNx animals. Lesion studies have consistently shown that circadian rhythms persist even if as little as 10% of SCN neurons survive ablation surgery (24) (supplementary text). This also appears to be the case for SCN control of memory processing. A number of studies have proposed that cognitive impairments caused by circadian dysfunction in humans are due to SCN cell loss or degeneration (5), but data from PSCNx hamsters suggest that cell loss is not a direct cause of these impairments. Instead, gradual cell loss in humans might lead to changes in the remaining SCN circuitry that actively restrict the brain's ability to form new memory representations (25). The rescue of memory by SCN ablation seems to be due to a direct effect of SCN ablation rather than to indirect effects on sleep or damage to extra-SCN regions (figs. S3 to S6 and supplementary text).
These findings might bridge the gap between mechanistic neurobiological research in rodents and the understanding of human disorders of dysrhythmia because they open up the possibility that SCN connections to the medial temporal lobe, among other sites, might be targeted for pharmacotherapeutic treatments of cognitive impairment. Based on our past work showing that a γ-aminobutyric acid type A (GABAA) antagonist can rescue memory in DPS arrhythmic hamsters (19, 20), we proposed that an arrhythmic SCN might produce a steady GABAergic signal to the septal nuclei that attenuates cholinergic activation of the hippocampus, and septal activation is necessary for normal SA behavior (19). Thus, the present study supports the idea that the SCN influences memory through an inhibitory mechanism that impairs memory processing when SCN function is compromised.
Circadian dysregulation facilitates progression of clinically diagnosed mild cognitive impairment and Alzheimer's disease (4–6). Although counterintuitive, pharmacotherapies designed around silencing the SCN might also be applicable in treating memory problems associated with these disorders; however, potential adverse consequences of this approach must be carefully weighed (26).
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by a grant from the National Institute of Mental Health (#MH095837).
Footnotes
SUPPLEMENTARY MATERIALS
www.sciencemag.org/content/346/6211/854/suppl/DC1
Materials and Methods
Supplementary Text
Figs. S1 to S6
Movies S1 to S3
References (28–34)
REFERENCES AND NOTES
- 1.Rouch I, Wild P, Ansiau D, Marquié JC. Ergonomics. 2005;48:1282–1293. doi: 10.1080/00140130500241670. [DOI] [PubMed] [Google Scholar]
- 2.Tranah GJ, et al. Ann. Neurol. 2011;70:722–732. doi: 10.1002/ana.22468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schlosser Covell GE, et al. Neurologist. 2012;18:426–429. doi: 10.1097/NRL.0b013e318272f7ef. [DOI] [PubMed] [Google Scholar]
- 4.van Someren EJW, et al. Biol. Psychiatry. 1996;40:259–270. doi: 10.1016/0006-3223(95)00370-3. [DOI] [PubMed] [Google Scholar]
- 5.Coogan AN, et al. Biol. Psychiatry. 2013;74:333–339. doi: 10.1016/j.biopsych.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 6.Wu Y-H, Swaab DF. Sleep Med. 2007;8:623–636. doi: 10.1016/j.sleep.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Van der Zee EA, et al. Curr. Biol. 2008;18:844–848. doi: 10.1016/j.cub.2008.04.077. [DOI] [PubMed] [Google Scholar]
- 8.Mulder C, Van Der Zee EA, Hut RA, Gerkema MP, Biol J. Rhythms. 2013;28:367–379. doi: 10.1177/0748730413512958. [DOI] [PubMed] [Google Scholar]
- 9.Wardlaw SM, Phan TX, Saraf A, Chen X, Storm DR. Learn. Mem. 2014;21:417–423. doi: 10.1101/lm.035451.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu EA, Weaver DR. Aging. 2011;3:479–493. doi: 10.18632/aging.100323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stephan FK, Kovacevic NS. Behav. Biol. 1978;22:456–462. doi: 10.1016/s0091-6773(78)92565-8. [DOI] [PubMed] [Google Scholar]
- 12.Mistlberger RE, de Groot MH, Bossert JM, Marchant EG. Brain Res. 1996;739:12–18. doi: 10.1016/s0006-8993(96)00466-0. [DOI] [PubMed] [Google Scholar]
- 13.Cain SW, Ralph MR. Neurobiol. Learn. Mem. 2009;91:81–84. doi: 10.1016/j.nlm.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Phan TX, Chan GC, Sindreu CB, Eckel-Mahan KL, Storm DR. J. Neurosci. 2011;31:10640–10647. doi: 10.1523/JNEUROSCI.6535-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smarr BL, Jennings KJ, Driscoll JR, Kriegsfeld LJ. Behav. Neurosci. 2014;128:283–303. doi: 10.1037/a0035963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deboer T. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;287:R8–R9. doi: 10.1152/ajpregu.00125.2004. [DOI] [PubMed] [Google Scholar]
- 17.Steinlechner S, Stieglitz A, Ruf T. J. Biol. Rhythms. 2002;17:248–258. doi: 10.1177/074873040201700308. [DOI] [PubMed] [Google Scholar]
- 18.Grone BP, et al. J. Biol. Rhythms. 2011;26:78–81. doi: 10.1177/0748730410388404. [DOI] [PubMed] [Google Scholar]
- 19.Ruby NF, et al. Proc. Natl. Acad. Sci. U.S.A. 2008;105:15593–15598. doi: 10.1073/pnas.0808259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruby NF, et al. PLOS ONE. 2013;8:e72433. doi: 10.1371/journal.pone.0072433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deacon RM, Rawlins JN. Nat. Protoc. 2006;1:7–12. doi: 10.1038/nprot.2006.2. [DOI] [PubMed] [Google Scholar]
- 22.Materials and methods are available as supplementary materials on Science Online [Google Scholar]
- 23.Ko CH, McDonald RJ, Ralph MR. Biol. Rhythm Res. 2003;34:177–192. [Google Scholar]
- 24.Ruby NF, Dark J, Burns DE, Heller HC, Zucker I. J. Neurosci. 2002;22:357–364. doi: 10.1523/JNEUROSCI.22-01-00357.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harper DG, et al. Brain. 2008;131:1609–1617. doi: 10.1093/brain/awn049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turek FW. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;294:R1672–R1674. doi: 10.1152/ajpregu.00160.2008. [DOI] [PubMed] [Google Scholar]
- 27.Winfree AT. The Geometry of Biological Time. Springer-Verlag; New York: 1980. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.