Abstract
Experimental autoimmune uveitis (EAU) induced in mice by immunization with the retinal antigen IRBP is a model of human autoimmune uveitis. We examined whether T regulatory (Treg) cells found in uveitic eyes are (i) IRBP specific, (ii) functionally suppressive, and (iii) play a role in natural resolution of disease and in maintenance of remission. Progressive increase of Foxp3+ Treg to T effector (Teff) ratio in uveitic eyes correlated with resolution of disease. At peak disease, up to 20% of Treg (CD4+Foxp3+) and up to 60% of Teff (CD4+FoxP3−) cells were IRBP-specific, while in lymphoid organs retina-specific T cells were undetectable. Tregs isolated from eyes of mice with EAU efficiently suppressed IRBP-specific responses of Teff from the same eyes. Importantly, systemic depletion of Tregs at peak disease delayed resolution of EAU, and their depletion after resolution triggered a relapse. This could be partially duplicated by depletion of Tregs locally within the eye. Thus, the T cell infiltrate in uveitic eyes of normal mice with a polyclonal T cell repertoire is highly enriched in IRBP-specific Treg and Teff cells. Unlike what has been reported for Tregs in other inflammatory sites, Tregs from uveitic eyes appear unimpaired functionally. Finally, Foxp3+ Tregs play a role in the natural resolution of uveitis and in the maintenance of remission, which occurs at least in part through an effect that is local to the eye.
INTRODUCTION
Natural Tregs that are released from the thymus of normal animals soon after birth are essential for maintenance of tissue homeostasis (1, 2). Treg depletion in adulthood precipitates multi-organ autoimmune disease and death (3). Induced Tregs arise as a result of contact with antigen during adult life, but their ability to control active autoimmunity has been debated. While Tregs were shown to be present in the target organ affected by inflammation, isolated Tregs from inflammatory sites may be deficient functionally. Inflammatory cytokines have been implicated in this impairment of Treg function, possibly permitting ongoing and chronic inflammation in the target organ (4, 5).
In the case of the eye, the situation is colored by the unique needs and properties of the ocular tissues. Due to the necessity to preserve vision that might sustain collateral damage as a result of inflammation, the healthy eye is an immunoregulatory environment that resists inflammatory processes (6). By using T cells expressing a transgenic retina-specific TCR, we demonstrated directly that the living eye converts naïve retina-specific T cells into functionally competent Tregs (7). This process, which is part of the phenomenon known as ocular immune privilege, is promoted by high concentrations of TGF-β and retinoic acid that are constitutively present in the eye. Other studies indicated that a tiny population of resident Tregs is present in the healthy eye and may constitute part of ocular immune privilege (8). Nevertheless, the eye is subject to destructive inflammation precipitated by uveitogenic T cells activated outside the eye, which have acquired the ability to actively cross the blood-retinal barrier. Our previous data demonstrated that such committed effector T cells are impervious to the inhibitory ocular microenvironment, explaining why uveitis can be induced despite ocular immune privilege. However, even though the ocular microenvironment cannot prevent uveitis, the acute phase of EAU disease is typically of short duration and starts resolving spontaneously after about a week to 10 days. This self-limiting nature of EAU is in apparent contradiction with the demonstrated inability of the ocular microenvironment to directly control activated uveitogenic T cells (7).
Though the small population of Tregs within the healthy eye raises the threshold of development of retinal autoimmunity (8), their presence does not clarify the role of Tregs after this threshold has already been broken and autoimmunity has begun. The present study deals with this question. We examine the hypothesis that the spontaneous resolution of EAU and maintenance of remission involves the activity of functionally competent Tregs. Using gene-manipulated C57BL/6 mice in which Tregs can be identified by Foxp3-driven GFP expression and can also be deleted by Foxp3-driven diphtheria toxin receptor (DTR) expression, we examine the behavior of Tregs in the uveitic eye and the consequences of their depletion peripherally as well as locally. In complementary experiments using B10.RIII mice, where availability of MHC-antigen multimers and retina-specific TCR transgenic mice permit the identification and analysis of retina-specific T cells and their responses, we study the specificity of eye-infiltrating Tregs and their functional competence in an antigen-driven setting. Our data indicate that the T cell infiltrate in uveitic eyes of normal mice with a polyclonal T cell repertoire is highly enriched in interphotoreceptor retinoid-binding protein (IRBP)-specific Treg and Teff cells. Interestingly, unlike what has been reported for Treg in other inflammatory sites, Treg from uveitic eyes are able to inhibit not only naïve, but also bona fide uveitogenic effector T cells that are present in eyes during uveitis (9–12). Systemic and local Treg depletion studies support the notion that Foxp3+ Tregs play a role in the natural resolution of uveitis as well as in the maintenance of remission, and that this is achieved, at least in part, through local mechanisms.
MATERIALS AND METHODS
Mice
C57BL/6 and B10.RIII were purchased from The Jackson Laboratory (Bar Harbor, ME). C57BL/6 DEREG mice, which express a GFP-Diphtheria Toxin Receptor fusion protein under control of the FoxP3 promoter, were a kind gift from Dr. Tim Sparwasser (Hanover, Germany) (13, 14). GFP-FoxP3 reporter mice obtained from Dr. Alexander Y. Rudensky (Memorial Sloan-Kettering Cancer Center, New York, NY) (15) were crossed onto the B10.RIII background for 10 generations to produce FoxP3-GFP B10.RIII reporter mice. Some investigators reported an altered in vivo function of Treg cells expressing this construct, in which Foxp3-GFP is present as a fusion protein (16, 17). In our hands. However, in vivo studies in the EAU model did not reveal any evidence for changes in Treg function in the Rudensky mice crossed onto the B10.RIII background, as judged by EAU scores and frequency of CD4+Foxp3+ Tregs in their uveitic eye (Figure S1), so that this effect might be particular to the strain and disease model under study. IRBP TCR Tg mice (R161H) on the Rag2−/− B10.RIII background (18) were bred in-house. All strains of mice were tested and found to be free of the rd8 mutation of the crb1 gene, except for the DEREG mice on C57BL/6 background. The rd8 mutation manifests as focal areas of disorganization within the retina, without infiltration of inflammatory cells. Since the DEREG mice were compared to their own littermates as controls, and since the rd8 lesions cannot be confused with the lesions and inflammation typical of uveitis, presence of the rd8 gene does not affect the interpretation of our experiments. Mice were housed under pathogen-free conditions, fed standard laboratory chow ad libitum and used at 6–12 weeks of age. Treatment of animals was in compliance with institutional guidelines. The National Eye Institute Animal Care and Use Committee approved all animal study protocols.
Reagents
Human IRBP peptide residues 1–20 (H-2b restricted) and 161–180 (H-2r restricted) were synthesized by Anaspec (Fremont, CA). Whole IRBP was purified from bovine retinas by Concanavalin A-Sepharose affinity chromatography and high-performance liquid chromatography (19). IRBP-specific T cells were identified with the fluorescently labeled IRBP161–180– MHC class II dimer reagent (20). Tregs were identified by flow cytometry either by detection of GFP or by FoxP3 staining (eBioScience, San Diego, CA). All additional antibodies for flow cytometry and cell sorting were purchased from eBioscience or BioLegend (San Diego, CA).
EAU Induction and Scoring
EAU in FoxP3-GFP B10.RIII reporter mice was induced with 10–12 µg of human IRBP161–180 (21). Mice on the C57BL/6 background were immunized with a mixture of 150 µg of IRBP plus 300 µg IRBP1–20. As additional adjuvant, 0.2 µg of Bordetella pertussis toxin (PTX; List Biologicals, Campbell, CA) was given i.p. on day 0 and again on day 2. All IRBP antigens were emulsified 1:1 vol/vol in complete Freund’s adjuvant that had been supplemented with Mycobacterium tuberculosis strain H37RA (Sigma, St. Louis, MO) to 2.5 mg/ml. A total of 200 µl of emulsion was injected subcutaneously, divided among three sites: base of tail and both thighs. Inflammatory activity was evaluated in a masked fashion in anesthetized mice by fundus examination under a binocular microscope after dilation of the pupil. Clinical inflammation scores ranged from 0 to 4 according to the following criteria: 0 = No inflammation; 0.5 = trace disease; 1 = minimally active, localized; 2 = moderately active, multiple lesions; 3 = Active, multiple diffuse lesions; 4 = Very active, often with retinal detachment or hemorrhage. Histology scoring followed previously published criteria (22).
Isolation of T cells for sorting and analysis
To isolate T cells from eyes of uveitic mice, enucleated eyes were first trimmed of external tissue and then the globe was opened along the limbus to remove the lens. The remaining tissue was minced in HL-1 media (Lonza, Walkersville, MD). Cell/tissue separation was accomplished with 1.0 mg/ml collagenase D treatment (Roche, Indianapolis, IN) for 40 minutes at 37°C. After washing, tissue was dispersed through a 40 µm strainer, resuspended in PBS + 2% FBS + 2mM EDTA sorting buffer and then stained. For lymph nodes (LN), tissue was dispersed through a 40 µm strainer and then resuspended in sorting buffer. Cell populations were analyzed using a BD FACS Calibur (Becton Dickinson, San Jose, CA) and MACSQuant Analyzer (Miltenyi Biotec, Auburn, CA) or were sorted on a FACS Aria II (BD) to 99% purity. To determine the number of Tregs present in eyes of mice months after EAU challenge, eye cells were incubated with CD4+ isolation beads (Miltenyi Biotec) prior to staining and then passed through a magnetic column that was integrated into a MACSQuant Analyzer. Since the frequency of Tregs at these late time points was expected to be low, utilization of the column permitted the enrichment of the cells before analysis. All data were analyzed using FlowJo (TreeStar, Ashland, OR).
DNA methylation analysis of the TSDR in Tregs
Eyes from (male) FoxP3-GFP B10.RIII reporter mice were harvested 17 days after EAU immunization and were prepared for sorting as described above. The following populations were collected: IRBP Dimer+ CD4+GFP+, IRBP Dimer+ CD4+GFP−, IRBP Dimer−CD4+GFP+, and IRBP Dimer− CD4+GFP−. Methylation analysis followed the protocol of Kim with modifications (23). Briefly, the cells were processed through the following steps: genomic DNA extraction (QIAmp DNA Micro Kit), bisulfate conversion (EpiTect Bisulfite kit), preparation of PCR reaction (PyroMark PCR Kit), and then pyrosequencing (PyroMark Gold Q96) on a PyroMark ID 1.0 instrument (Biotage, Charlotte, NC). Analysis of 11 cytosine guanine dinucleotides (CpGs) of the mouse FoxP3 promoter Treg specific demethylation region (TSDR) was performed. The mean of all 11 CpGs of each sample constituted the percent methylation of each sample. All kits were from Qiagen (Germantown, MD).
IRBP specific Treg suppression assays
The suppressive ability of Tregs (CD4+GFP+) sorted from uveitic eyes on proliferation and cytokine production of target cells was tested in co-culture experiments using two types of responder cells: 1) Naïve (CD62Lhi CD44Low) sorted LN cells from R161H Rag2−/− mice and 2) Teffs (CD4+GFP−) sorted from uveitic eyes of FoxP3-GFP B10.RIII reporter mice 17 days post immunization. 5 ×104 responder cells were cultured in HL-1 media supplemented with 2% normal mouse serum plus 1×105 T cell-depleted syngeneic (irradiated) APC +/− 1.0 µg/ml IRBP161–180 as stimulant. Various numbers of Tregs sorted from the same uveitic eyes were added to the cultures. Cultures were incubated in round bottom plates for 60 h and proliferation was evaluated by (3H)-thymidine uptake in the last 18 h. IFNγ, IL-17, and GM-CSF levels were measured from 48 h co-culture supernatants by ELISA (R&D Systems, Minneapolis, MN). Each suppression experiment utilized 30–40 EAU-challenged mice. Nevertheless, only small numbers of cells could be sorted from the uveitic eyes so that only one replicate per dilution could be done. Therefore, reproducibility of the experiments was demonstrated in repeat experiments rather than intra-experiment replicates.
Systemic and local Treg depletion
FoxP3-expressing Tregs in DEREG mice were depleted systemically in mice during the peak of EAU (day 21) or, after disease resolution (day 64 or 88) by the administration of 0.5 µg of diphtheria toxin (DT, EMD Millipore, Billerica, MA) i.p. on two successive days. Experiments established that this depletion regimen eliminated systemic as well as eye-infiltrating Tregs for about a period of one week (Ref. 14, Fig. S2 and data not shown). Treg depletion in DEREG mice was verified in all experiments by assessment of GFP-expressing Tregs in the blood (tail bleeds) after administration of DT. Local Treg depletion in the eye was performed following the protocol of Lehmann et al. (24) with modifications. 25 ng of DT was injected in a volume of 1.5 µl into the anterior chamber (AC) of one eye and an equal volume of PBS was injected into the contralateral eye 3 times during one week, either starting during the peak (day 19) or, after the resolution of disease (day 69 or 77). To evaluate inflammation, eyes were harvested 36 h after the last AC treatment, prefixed in 4% phosphate-buffered glutaraldehyde for 1 h and then transferred to 10% phosphate-buffered formaldehyde. Fixed and dehydrated tissue was embedded in methacrylate, and 4- to 6-µm sections were stained with standard hematoxylin and eosin. Cellular infiltrates within the entire vitreous of the DT and PBS-injected eyes were enumerated from the histology sections in a masked fashion. In all systemic and local depletion experiments, both DEREG and WT littermates received DT treatment.
Statistics
Statistics were performed using the unpaired or paired t-test as indicated. Two-tailed p value of <0.05 was considered significant.
RESULTS
Foxp3+ Tregs accumulate in the eye during the course of EAU
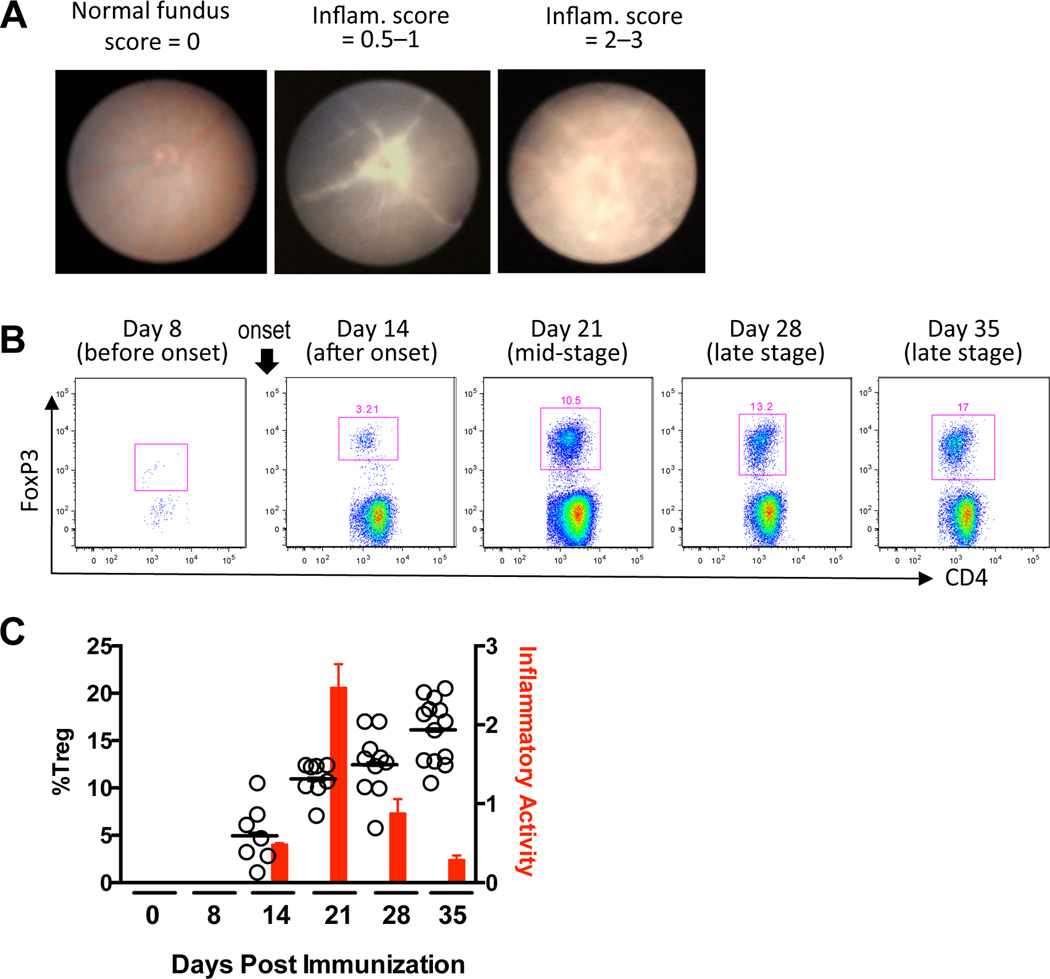
Immunization with retinal antigen gives rise to uveitogenic CD4+ Teffs in the periphery that are capable of penetrating the blood retinal barrier, migrating into the eye, and inducing uveitis with the aid of other recruited leukocytes. Inflammatory activity in the eye is defined by the degree of cellular infiltrate. To determine whether Tregs are a component of the infiltrating population of cells, C57BL/6 mice were challenged for EAU, and the eye infiltrate was examined for viable CD4+Foxp3+ Tregs at weekly intervals. The inflammatory activity was monitored by fundus exams at the same time points. Fig. 1A shows examples of inflammation visible in the fundus. C57BL/6 mice were chosen for this experiment because their disease development progresses to peak activity in an incremental fashion in the first 3 weeks after EAU challenge, in contrast to B10.RIII mice whose disease advances explosively, peaks early, and is accompanied by severe anterior inflammation which precludes fundus exams for 7–10 days after onset. Disease onset occurred about day 14 with inflammation peaking from days 19–21 and then rapidly resolving. The proportion of CD4+Foxp3+ Tregs increased progressively throughout the mid- and late stages of disease (Fig. 1B) and correlated with resolution of inflammation (Fig. 1C). In addition, the transcription factor Helios, which has been associated with T cell activation (25, 26), was expressed in ≥ 80% of Foxp3+ Tregs at all time points by intracellular staining (data not shown).
Figure 1. Accumulation of Tregs in the eye during the course of EAU is associated with resolution of disease.
C57BL/6 mice were challenged for EAU. At each time point, inflammatory activity was evaluated by fundus exam and eye-infiltrating cells were collected after perfusion for flow cytometry. A) Shown are examples of normal, mild and severe inflammation in the fundus of EAU-challenged mice. B) Percent viable CD4+FoxP3+ Tregs in the pooled eyes of one representative mouse at each time point. C) Bars: average inflammatory activity of mice at each time point. Circles: Percent viable CD4+FoxP3+ Tregs. Each circle represents one mouse (both eyes averaged). Shown are results from two repeat experiments combined.
IRBP-specific Tregs and Teffs are highly enriched in the inflamed eyes compared to LNs
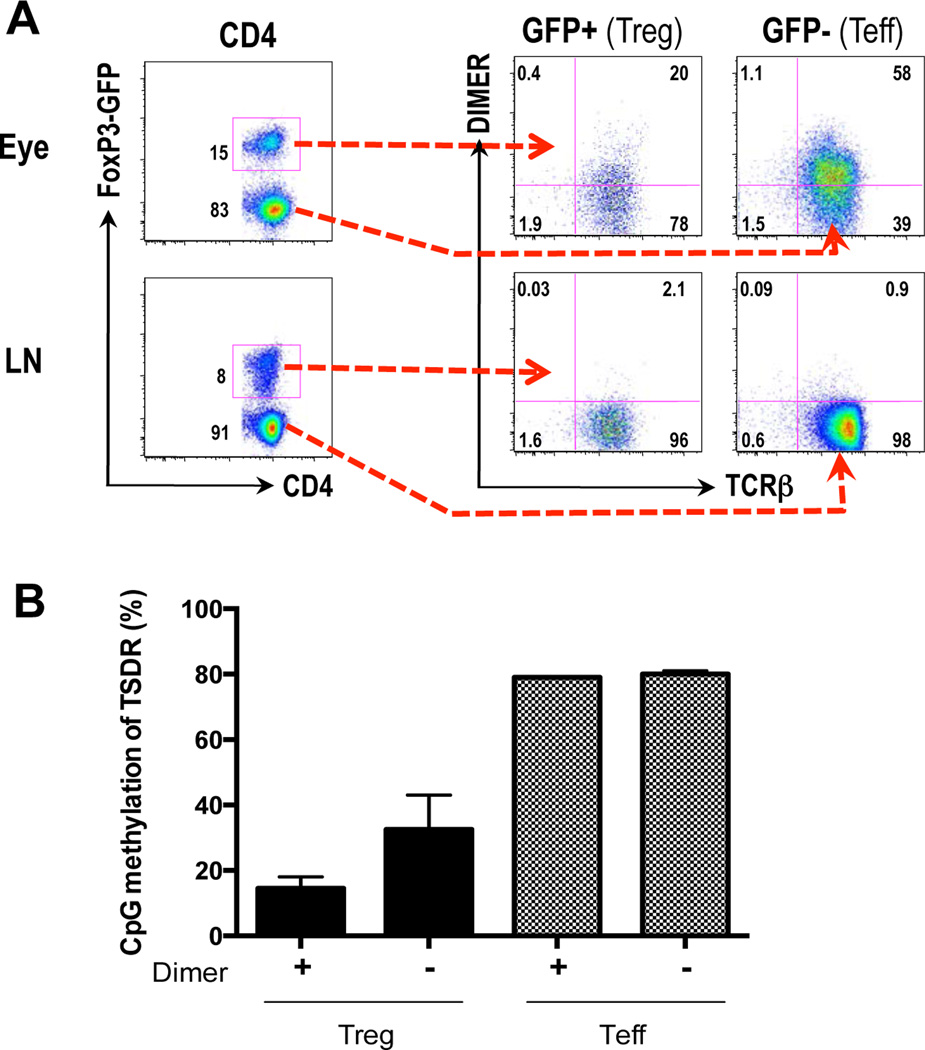
Since the expected frequency of IRBP-specific T cells in WT mice is initially very low, experiments were conducted to determine whether the T lymphocytes that accumulated in the uveitic eye and draining LNs were specific for IRBP. IRBP-specific cells were detected by flow cytometry after staining with labeled IRBP161–180 MHC class II dimer, which bind to cells bearing TCRs specific to IRBP (20). Ocular cells were harvested from FoxP3-GFP B10.RIII reporter mice 17 days post immunization. IRBP-specific Tregs (GFP+) and Teffs (GFP−) were highly enriched in the inflamed eyes compared to the LNs that drain the immunization site (Fig. 2A). Notably, 20% of the total CD4+GFP+ Treg population was specific for IRBP. Of the infiltrating GFP− population, 60% were IRBP-specific. Furthermore, these numbers are likely to represent an underestimation, as T cells exposed to their cognate antigen (present in the eye) downregulate TCR expression (27, 28). In contrast, only low numbers of IRBP-specific Tregs were found in the peripheral LNs that drain the site of immunization, and IRBP-specific effector cells in the periphery were below detection.
Figure 2. IRBP-specific Tregs are highly enriched in inflamed eyes and have a demethylated TSDR.
B10.RIII FoxP3-GFP reporter mice were challenged for EAU. A) 17 days post immunization, cells from the eyes and draining peripheral LNs (draining the immunization site) were harvested and stained with CD4 and the IRBP161-180 MHC class II dimer and analyzed by flow cytometry. Shown is one representative experiment of five. B) 17 days post-immunization, eye cells were stained and sorted for the designated populations. Methylation status of Foxp3 TSDR was assessed per Materials and Methods. Shown is a combination of 2 experiments.
The TSDR of IRBP-specific Tregs in eyes with uveitis is demethylated
It has been reported that epigenetics plays a role in FoxP3 gene expression. The Treg-specific demethylated region (TSDR) is an evolutionary conserved CpG-rich element in the FoxP3 locus. Previous studies reported that demethylation of this region is associated with stability of the FoxP3 phenotype. In contrast, this region in Tconv cells remains heavily methylated (29). We wanted to assess the phenotypic stability of the Tregs found in the uveitic eyes, in particular those that are IRBP-specific. We therefore examined the methylation status of the TSDR region in sorted Foxp3+ vs. Foxp3− T cells contained within the IRBP-specific vs. nonspecific CD4+ T cell populations sorted from the ocular inflammatory infiltrate of FoxP3-GFP B10RIII reporter mice with EAU on day 17 after immunization.
IRBP-specific Tregs (GFP+Dimer+) were demethylated (14.5%) indicative of a stable Treg phenotype (Fig. 2B). Tregs that were not IRBP-specific (GFP+ Dimer−) exhibited an intermediate methylation pattern (32.5%), suggesting that this population is a mixture of stable and unstable Tregs that may express FoxP3 transiently. Both IRBP-specific and non-specific Teffs (GFP−Dimer+ or GFP−Dimer−) were highly methylated (80%), a status characteristic of Tconv cells (Fig. 2B). These data suggest that at peak disease, the polyclonal T cell infiltrate of the eye does contain an IRBP-specific population of Tregs with stable FoxP3 expression, indicative of a fully differentiated and stable regulatory phenotype.
Tregs from inflamed eyes suppress IRBP-specific effector T cells
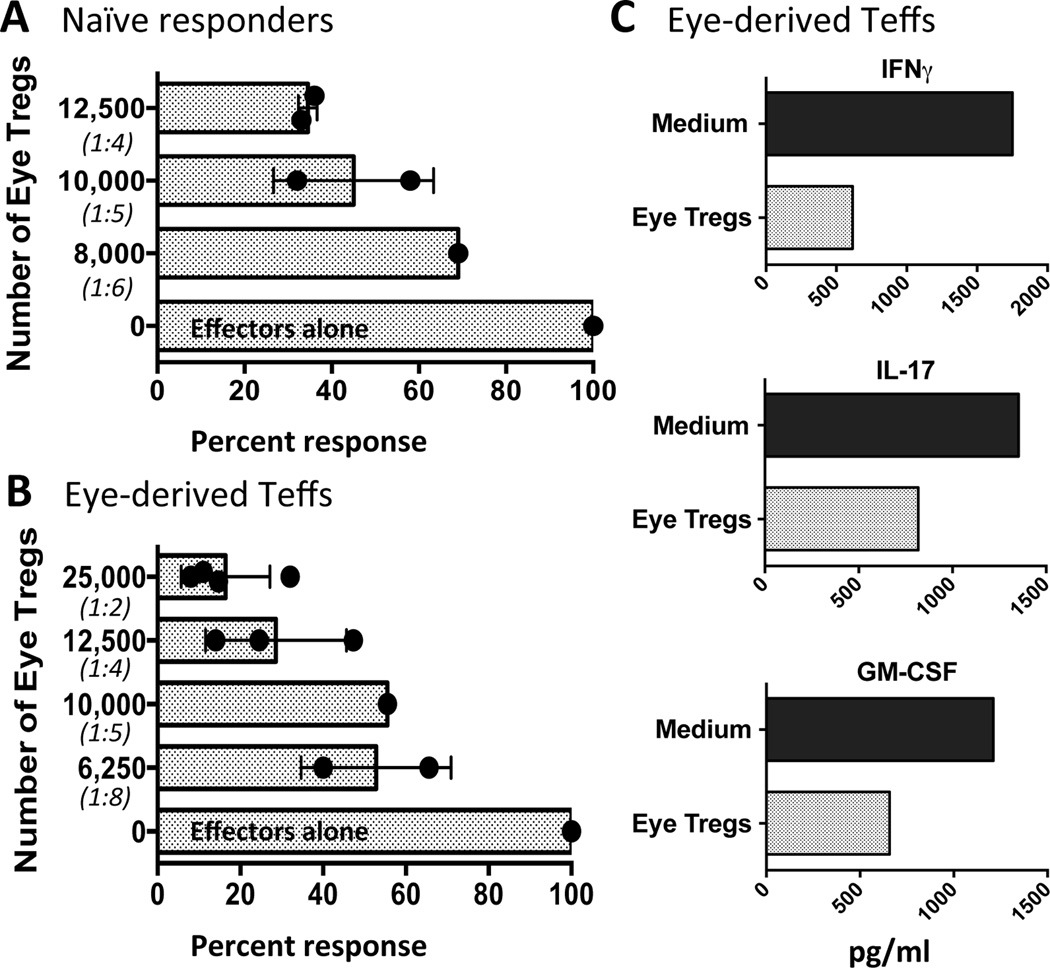
Considering that the methylation status of the IRBP-specific uveitic Tregs that are found in uveitic eyes implied a stable phenotype, and by inference, suppressive function, we next asked whether suppression by the eye-infiltrating Tregs could be demonstrated functionally. In order to maintain relevance to disease, which targets IRBP, we used an IRBP-specific suppression assay to examine the ability of eye-derived Tregs to inhibit naïve and effector T cells. Towards that end, Tregs sorted from eyes of uveitic FoxP3-GFP B10.RIII reporter mice were co-cultured with naïve IRBP-specific R161H T cells from Rag2−/− donors (18), or with CD4+Foxp3− T cells (Teffs) sorted from the same eyes, in the presence of APC (irradiated splenocytes) and IRBP161–180 antigen. The eye-derived Tregs were able to inhibit proliferation of 50,000 responder R161H T cells (Fig. 3A) dose dependently, with 65% inhibition achieved at 1:4 Treg/responder ratio and progressively less inhibition at lower ratios. More importantly, Tregs from inflamed eyes of the FoxP3-GFP B10.RIII reporter mice suppressed the IRBP161–180-specific proliferation of 50,000 Teff cells sorted from the same eyes (Fig. 3B). Again, a dose response was apparent where better than 80% and 70% suppression was achieved at 1:2 and 1:4 Treg/Teff ratio, respectively. Lower numbers of eye Tregs were also inhibitory.
Figure 3. Tregs from inflamed eyes are functionally competent to inhibit activation and effector function of IRBP-specific T cells.
Graded numbers of sorted CD4+GFP+ Tregs from pooled eyes of EAU-challenged B10.RIII FoxP3-GFP reporter mice, harvested 17 days after immunization, were cultured with 1×105 T-depleted APCs + 1.0 µg/ml IRBP161-180 + 5×104 sorted naïve (CD62LhiCD44low) R161H Rag2−/− responder cells (A), or CD4+GFP− Teffs sorted from eyes of the same EAU-challenged mice (B). Treg:Tresp ratio is shown in parentheses. Proliferation was measured by H3-thymidine uptake. Each circle is the result of one replicate at that cell dilution and each bar represents the average +/− SEM for all the experiments. (Responder cell CPM range for A: 12,495 to 25,492. Responder cell CPM range for B: 10,505 to 20,230). C) Cytokine content in supernatants from co-cultures described in B were measured by ELISA (Treg:Teff ratio = 1:5). Shown is one representative experiment of two.
The proinflammatory cytokines IFN-γ and IL-17 are produced in the eye by Th1 and Th17 effector cells, both of which infiltrate uveitic eyes and each of which constitutes a pathogenic effector cell type (30). Both of these cell types produce GM-CSF, which also has been shown to be a pathogenic cytokine in autoimmune disease (31, 32). We therefore measured the effect of eye-derived Tregs on IRBP-driven production of these three cytokines on supernatants from eye-derived effector T cells responding to antigen. Ten thousand eye-derived Tregs suppressed Ag-driven production of IFNγ, IL-17, and GM-CSF by 50,000 eye-derived effector T cells (Fig. 4C). Taken together, these data demonstrate that the Tregs from uveitic eyes have suppressive ability and can inhibit both activation and effector function of IRBP-specific T cells.
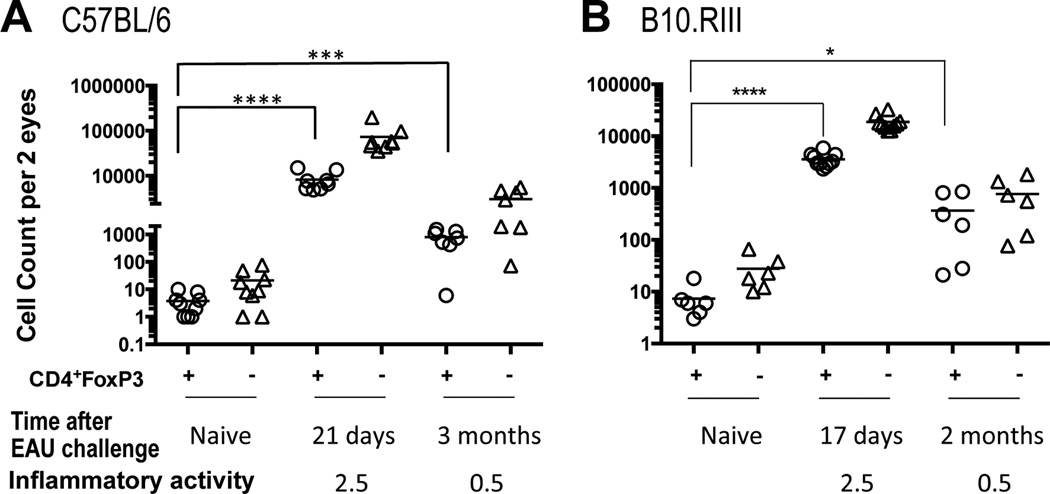
Figure 4. FoxP3+ Tregs persist in eyes of post-EAU mice long after resolution.
57BL/6 and B10.RIII mice were challenged for EAU. At each time point, the fundus was examined for inflammatory activity and then both eyes of each mouse were harvested after perfusion and pooled for cellular analysis. CD4+FoxP3+ Tregs (circle) and the CD4+FoxP3− Teffs (triangle) were enumerated by flow cytometry. Age-matched naive mice served to determine background counts. Number of CD4+Foxp3+ Tregs in challenged mice compared to background counts: A) C57BL/6: 21 days post-challenge, p<0.0001; 3 months post-challenge, p<0.0005. B) B10.RIII: 17 days post- challenge, p<0.0001; 2 months post-challenge, p<0.04. Statistical significance determined by unpaired t-test. Cell counts for all mice are shown in Table S1.
Tregs continue to persist in post-uveitic eyes after disease resolution
We next asked whether Tregs continued to persist in eyes of C57BL/6 and B10.RIII mice after resolution has occurred. Fundus exams showed that the high inflammatory activity that was present in both strains at peak disease had subsided to trace activity in the early months after disease induction (Fig. 4A, B). At each time point, both eyes of each mouse strain were pooled and the CD4+Foxp3+ Tregs and CD4+Fox P3− Teffs were enumerated by flow cytometry. Counts were compared to those of age-matched naïve controls. Though eyes were thoroughly perfused before harvesting, some cells may have adhered to the vasculature and may not have been flushed out. Therefore, the naïve cell counts were considered as background and averaged fewer than 80 cells for each T cell population. Cell numbers in individual animals were highly variable. Nevertheless, while at the peak of disease the average of total CD4+ infiltrating cells was in the tens of thousands per eye, with Tregs composing anywhere between 10–20% of the infiltrate, at 3 months the average of total infiltrating cells had diminished by an order of magnitude and Treg frequency nearly doubled on average (Fig. 4 and Supplementary Table 1). These data demonstrate that Tregs as well as Tconv cells continue to be present locally for several months after the acute inflammation has resolved, with the progressive increase of Treg-to-Teff ratio observed during the earlier stages of disease (Fig. 1) continuing to show the same trend.
Depletion of Tregs at the peak of uveitis delays resolution, and depletion after resolution precipitates relapse of disease
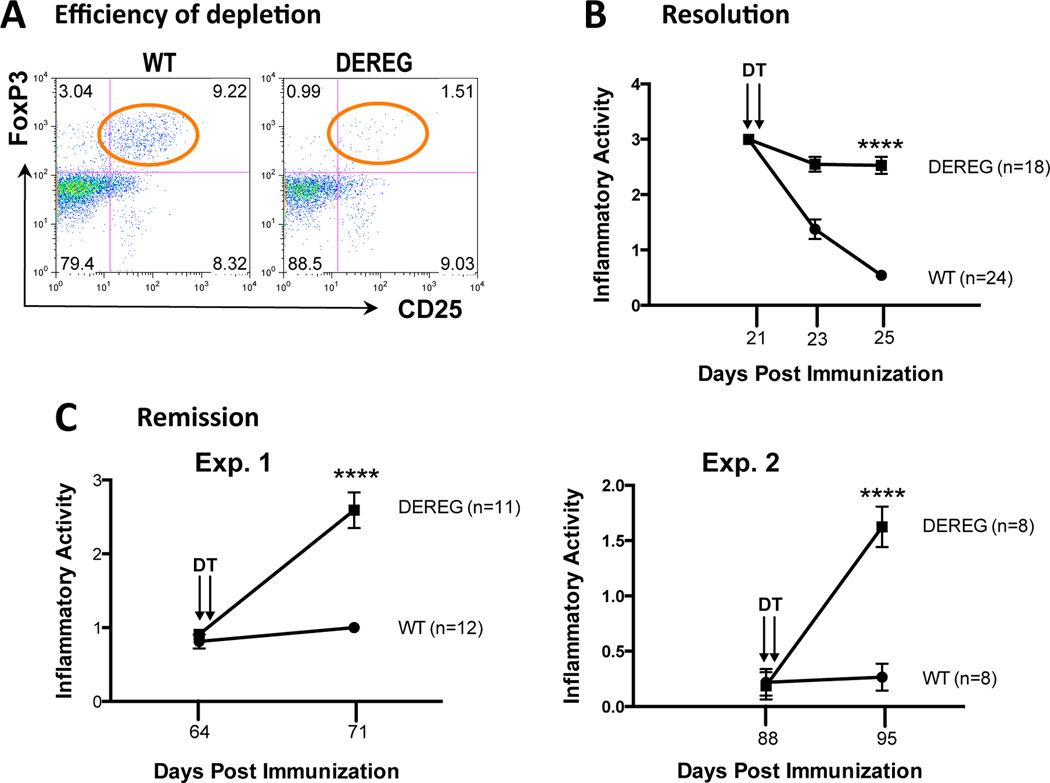
From the data described above, it was logical to hypothesize that Tregs play a role in remission of the disease. To examine this directly, we used mice expressing a diphtheria toxin receptor under the Foxp3 promoter (DEREG mice) (13, 14). Preliminary experiments established that systemic injection of DT depletes Tregs peripherally as well as in the eye (Fig 5A and S2). DEREG mice and their WT littermates were challenged for EAU. An initial fundus exam to determine inflammatory activity was performed at peak disease, which in the C57BL/6 strain is between day19–21. Afterwards, the Tregs were depleted by intraperitoneal administration of DT. Subsequent fundus exams showed that while the eye inflammation in the WT mice resolved rapidly, as expected, the inflammation continued to remain active in the DEREG mice whose Tregs had been depleted (Fig. 5B).
Figure 5. Systemic depletion of Tregs at the peak of EAU, or after resolution, has functional consequences on disease.
DEREG mice and their WT littermates were challenged for EAU. The average inflammatory activity of all mice +/− SEM is graphed at each time point. A) Systemic depletion efficiently depleted Tregs from the blood of DEREG mice 48 h after DT administration. B) Systemic depletion of Tregs at the peak of EAU delayed resolution. DT was administered i.p. on days 21 and 22. (unpaired t-test: p<0.0001 for day 25). Shown is a combination of 3 experiments. C) Systemic depletion of Tregs after EAU resolution reversed remission. DT was administered i.p. on days 64 and 65 (Exp. 1) or day 88 and 89 (Exp. 2) (unpaired t-test: p<0.0001 for day 71 (Exp. 1) and day 95 (Exp. 2).
We next investigated whether Treg depletion after resolution of disease would precipitate a relapse. Both DEREG and WT mice were immunized for EAU. Presence of full-blown uveitis was confirmed by fundus exam 3 weeks later. The mice were then allowed to recover from disease until day 64 in one experiment, and until day 88 in the second experiment. The mice continued to maintain a very low-grade chronic inflammation throughout this time period. After DT was administered, an immediate relapse of inflammation was observed in the eyes of mice whose Tregs were depleted (Fig. 5C). These data suggest that Tregs play a role not only in the resolution of disease but also in the maintenance of remission.
Treg activity to bring about and maintain resolution is in part due to local mechanisms
Because systemic injection of DT depletes Tregs peripherally as well as in the eyes (Fig 5A and S2), it was therefore relevant to examine whether Tregs controlled disease activity at least in part by acting locally. We used the same experimental paradigm as in the systemic depletion experiments described above, except that DT was injected into the anterior chamber (AC). This route was preferred to injecting into the vitreous in order to avoid the confounding effects of the microtrauma of injection at the site of inflammation. Small molecules can percolate from the anterior chamber into the back of the eye, although the efficiency of this process is difficult to estimate. DT was injected into one eye and PBS was injected into the contralateral eye to control for the nonspecific proinflammatory effects of the anterior chamber injection. The functional efficacy of such AC injection has been reported (8). Each mouse received 3 DT injections during a one-week period. Eyes were collected for analysis one day after the third injection.
We first confirmed that this AC regimen of DT administration reduced the Treg population only in the eye, but had no effect on peripheral Treg numbers (Figure S2). By flow cytometry, the AC depletion protocol depleted about 50% of Tregs in inflamed eyes of DEREG mice (Fig S2C). In WT mice with EAU, AC DT treatment had no effect (data not shown). Importantly, although Tregs were reduced by half in the DT injected eye of DEREG mice, no difference in the percent of CD4+Foxp3+ Tregs was observed in the (eye-draining) submandibular lymph nodes of the same mice (Fig. S2D). As well, there was no difference in percent Foxp3-GFP+ Tregs in the blood of individual mice before and 36 h after DT treatment (Fig. S2E).
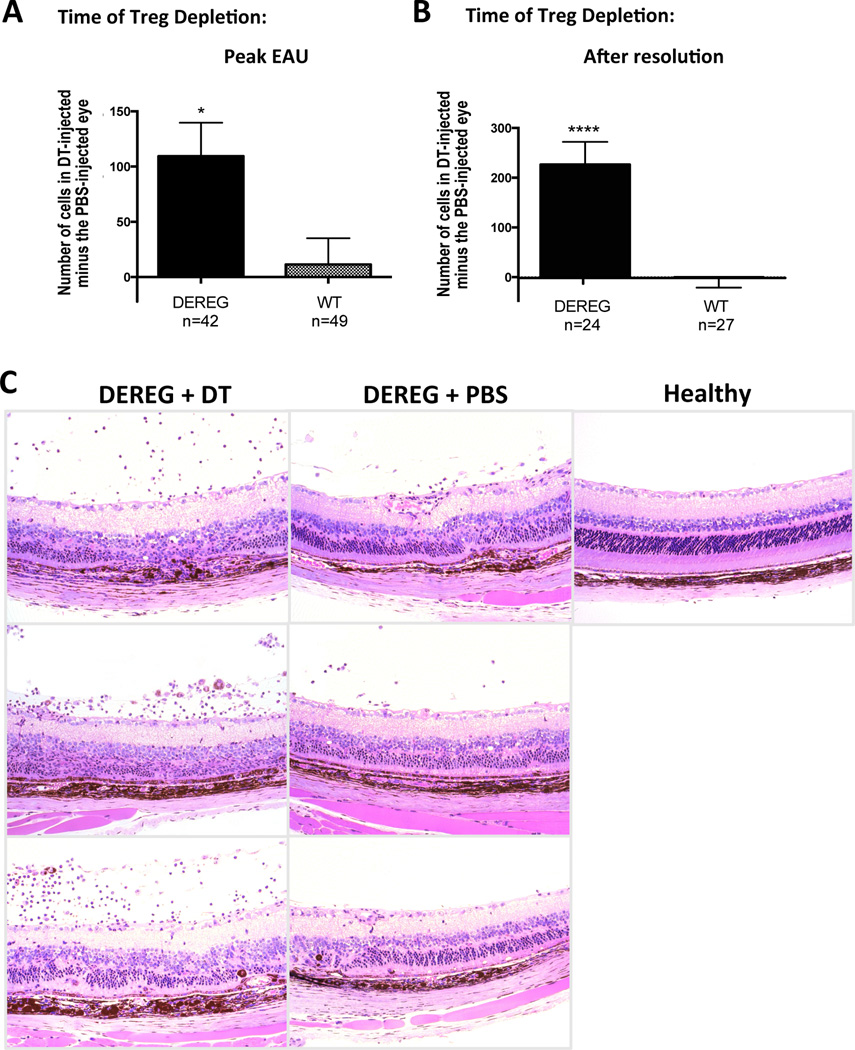
Because AC injections interfered with the ability to perform optimal fundus examination, inflammatory activity in the back of the eye was evaluated by counting the number of infiltrating cells in histology sections in multiple fields in a blind fashion. For each mouse, the total number of cells counted in the PBS-injected eye was subtracted from the total number of cells in the contralateral DT-injected eye. The differences for each mouse were averaged. It should be noted that the architectural damage is present in both the DT and the PBS injected eyes, as they have already undergone disease, and that even after resolution there is a residual infiltration in PBS treated eyes, which does not completely disappear for many months. The intensity of inflammation can, however, be quantitated by counting the number of inflammatory infiltrating cells. There was a significantly higher retention of infiltrating cells in eyes in which Tregs were depleted at the peak of EAU (Fig. 6A). When local Tregs were depleted from the eye after resolution, a significant increase in the number of infiltrating cells occurred (Fig. 6B, C). Table S2 displays individual cell counts for all mice, emphasizing that, while in DEREG mice there was a substantial increase in cells in the eye that received the DT injection, in eyes of WT mice the total number of cells in the two eyes was quite close. These data suggest that the effect of Tregs in bringing about and maintaining remission can at least in part be attributed to Tregs acting locally within the eye.
Figure 6. Local depletion of Tregs within the eye has functional consequences on disease.
DEREG mice and WT littermates were challenged for EAU. DT was injected into the AC of one eye and PBS was injected into the contralateral eye as described in Materials and Methods. Cellular infiltrates within the entire vitreous were counted from histology sections. The number of cells in the PBS-injected eye was subtracted from the number of cells in the DT-injected eye for the same mouse. Shown is the mean of all cell count differences for DEREG and WT mice +/− SEM. A) Local depletion of Tregs at the peak of EAU delayed resolution of inflammation. Eyes were treated with DT on days 19, 21, and 23 and harvested for histology on day 25. Shown is a combination of 4 experiments (unpaired t test: p<0.01). B) Local depletion of Tregs after resolution induced a relapse of inflammation. DT treatment was started 10 weeks after EAU-challenge and eyes were harvested for histology one week later. Shown is a combination of 2 experiments (unpaired t test: p<0.0001). C) Eye histology of DEREG mouse. AC of one eye was injected with DT and AC of the contralateral eye was injected with PBS. The pictures represent panel B.
DISCUSSION
The healthy eye has the ability to induce Tregs. This property has been postulated for a long time based on the ability of ocular fluids to induce Treg function in conventional T cells (6), and has recently been demonstrated directly to be an inherent property of the living eye by using mice that express a TCR recognizing an antigen present within the retina (7, 8). The question then arises, how can uveitis occur if the eye can convert conventional T cells to Tregs? Our previous data indicated that the ability of the eye to control already primed effector T cells, such as are involved in inducing uveitis, is quite limited compared to its ability to control naïve retina-specific T cells. Such T cells, which have been primed in the periphery and have acquired the ability to actively break down the blood-retinal barrier, cannot be converted to Tregs within the eye. Although recently evidence has been presented in favor of a tiny eye-resident population of Tregs that raise the threshold of susceptibility to uveitis (8), the activated effector T cells can clearly overcome local inhibitory mechanisms and induce disease. Furthermore, the inflamed eye is less efficient in converting even naïve T cells to Tregs (7). Therefore, we hypothesized that successful resolution of disease requires additional mechanisms, such as influx of Tregs from the periphery.
In keeping with the scenario that for EAU to develop, the Teffs must be the first cells on the scene, the percentage of CD4+Foxp3+ to CD4+Foxp3− cells among CD4+ T cells in eyes with developing uveitis starts out low. However, that proportion kept rising throughout the disease process, and when Foxp3+ T cells reached 10% of the total CD4+ cells in the eye on day 21, acute inflammation rapidly resolved. The mechanisms that act to progressively change the Teff to Treg ratio in the uveitic eye are unclear, and may involve additional cell types, such as myeloid-derived regulatory cells and regulatory B cells (33, 34). Nevertheless, the functional role of Tregs in the resolution process is upheld by failure of the disease to resolve during the period of observation if Tregs were depleted at peak disease by DT treatment. Conversely, depletion of Tregs after resolution precipitated a recurrence of inflammation. This led us to the conclusion that Foxp3+ Tregs are instrumental in bringing about spontaneous resolution and in maintaining remission.
In contrast to findings in some other experimental and clinical autoimmune diseases, where Tregs showed reduced ability to suppress Teffs from the site of infammation, Tregs isolated from uveitic eyes were competent in suppressing Teffs from the same location in an antigen-specific suppression assay. The reason for this apparent discrepancy is not clear, but it has been reported that high levels of inflammatory cytokines, particularly TNF-α and possibly IL-6, can compromise Treg function and reduce their ability to suppress (9, 11, 12). In this context, it might be of note that uveitogenic Teff cells isolated from the eye produced relatively little IL-6 and TNFα (data not shown).
In interpreting these experiments, it has to be taken into account that the expression of the DTR on Foxp3+ T cells in DEREG mice is “leaky” and there is a small population of Foxp3+ cells that do not express DTR. These cells are not depleted. They undergo homeostatic expansion and repopulate the mouse, leaving about a 1-week window when functional consequences of Treg depletion can be reliably measured (reference 14 and our unpublished data). This property of DEREG mice is both a limitation and an advantage. On the one hand, the “window of opportunity” in which biological effects can be observed is fairly short (but in our case, sufficient). On the other hand, the mice remain healthy and do not develop an autoimmune disorder that can confound the situation, as does another strain of Foxp3-DTR mice, where depletion of Foxp3+ Tregs is permanent and the mice progress to spontaneous systemic autoimmunity (3).
Our data demonstrate a novel finding that a very high proportion of Foxp3+ and an even higher proportion of Foxp3− CD4+ T cells in uveitic eyes of WT mice with a normal polyclonal repertoire are IRBP-specific. In contrast, outside the eye, including the eye-draining submandibular lymph nodes, the frequency of IRBP-specific dimer-binding cells was negligible. This could imply that IRBP-specific T cells are either retained in the eye, or, that they actively proliferate there. We and others have previously presented data indicating that retinal Ag recognition by uveitogenic T cells occurs in situ (and in fact is a prerequisite for elicitation of EAU) (35, 36). Furthermore, naïve IRBP-specific R161H T cells (but not polyclonal T cells) injected into the eye proliferate and dilute a T cell proliferation dye as they acquire Foxp3 expression, and acquire an antigen-experienced phenotype (7). This supports the notion that antigen recognition in the eye is accompanied by specific functional consequences, both for effector and for regulatory cells. The finding that local depletion of Tregs within the eye promptly re-ignites EAU suggests that at least some of the post-recovery homeostasis occurs within the eye and involves an active control of Teff cells by Tregs that may be present there.
To examine this hypothesis directly, we attempted to deplete Tregs locally within the eye, without affecting the peripheral Tregs, by injecting DT into the AC using a method described by Lehmann et al (24). Although AC administration of DT indeed had no demonstrable effects outside the eye, it did not deplete Tregs within the eye as effectively as did systemic DT administration. After 3 consecutive DT injections over the course of 1 week only 50% of Tregs within the eye appeared to have been depleted. It is unknown whether the amount of DT reaching the vitreous from the anterior chamber was insufficient to deplete all the Tregs, or whether they were rapidly replaced in the eye from the periphery, as has been proposed by a recent study (8). Nevertheless, the changes in inflammatory activity within the eye after local Treg depletion paralleled those seen after systemic administration. This supports the notion that the ameliorating effect of Tregs on uveitis was, at least in part, being exerted locally within the eye. Extrapolating these findings to human uveitis, which may relapse due to mechanisms that remain obscure, it is conceivable that one such mechanism could be the systemic or local failure of Tregs.
In summary, the present data identify Foxp3+ Tregs as an essential regulatory cell type in ocular autoimmunity. While presence of a resident Foxp3+ Treg population in the healthy eye has been suggested, any protective effects of such Treg cells are clearly overcome by retina-specific effector T cells that had been activated in the periphery and can enter the eye actively through the blood-retinal barrier. Thus, although Tregs are unable to prevent uveitis, they are nevertheless instrumental in bringing about resolution of the disease and in maintaining a state of remission. This appears to be achieved, at least in part, by Tregs acting locally within the eye to dampen acute inflammation and prevent its recurrence.
Supplementary Material
ACKNOWLEDGMENTS
The authors wish to recognize the expert contribution of the NEI Flow Cytometry Core to these experiments. We thank Dr. Dale Gregerson of the University of Minnesota and his staff for help with the anterior chamber injection technique. We thank Dr. Sadiye Rieder, NIAID, for assistance in measuring the TSDR methylation status. Dr. A. Hansen and W. McManigle in our lab helped to determine appropriate systemic doses of diphtheria toxin.
Grant support: NEI/NIH Intramural funding, Project # EY000184
REFERENCES
- 1.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Piccirillo CA, Shevach EM. Naturally-occurring CD4+CD25+ immunoregulatory T cells: central players in the arena of peripheral tolerance. Semin Immunol. 2004;16:81–88. doi: 10.1016/j.smim.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 4.Buckner JH. Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nat Rev Immunol. 2010;10:849–859. doi: 10.1038/nri2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sawant DV, Vignali DA. Once a Treg, always a Treg? Immunol Rev. 2014;259:173–191. doi: 10.1111/imr.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Streilein JW. Ocular immune privilege: the eye takes a dim but practical view of immunity and inflammation. Journal of leukocyte biology. 2003;74:179–185. doi: 10.1189/jlb.1102574. [DOI] [PubMed] [Google Scholar]
- 7.Zhou R, Horai R, Silver PB, Mattapallil MJ, Zarate-Blades CR, Chong WP, Chen J, Rigden RC, Villasmil R, Caspi RR. The living eye "disarms" uncommitted autoreactive T cells by converting them to Foxp3(+) regulatory cells following local antigen recognition. J Immunol. 2012;188:1742–1750. doi: 10.4049/jimmunol.1102415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McPherson SW, Heuss ND, Gregerson DS. Local "on-demand" generation and function of antigen-specific Foxp3+ regulatory T cells. J Immunol. 2013;190:4971–4981. doi: 10.4049/jimmunol.1202625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, Mauri C. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. The Journal of experimental medicine. 2004;200:277–285. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.You S, Belghith M, Cobbold S, Alyanakian MA, Gouarin C, Barriot S, Garcia C, Waldmann H, Bach JF, Chatenoud L. Autoimmune diabetes onset results from qualitative rather than quantitative age-dependent changes in pathogenic T-cells. Diabetes. 2005;54:1415–1422. doi: 10.2337/diabetes.54.5.1415. [DOI] [PubMed] [Google Scholar]
- 11.Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, Backstrom BT, Sobel RA, Wucherpfennig KW, Strom TB, Oukka M, Kuchroo VK. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valencia X, Stephens G, Goldbach-Mansky R, Wilson M, Shevach EM, Lipsky PE. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood. 2006;108:253–261. doi: 10.1182/blood-2005-11-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lahl K, Loddenkemper C, Drouin C, Freyer J, Arnason J, Eberl G, Hamann A, Wagner H, Huehn J, Sparwasser T. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J Exp Med. 2007;204:57–63. doi: 10.1084/jem.20061852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lahl K, Sparwasser T. In vivo depletion of FoxP3+ Tregs using the DEREG mouse model. Methods Mol Biol. 2011;707:157–172. doi: 10.1007/978-1-61737-979-6_10. [DOI] [PubMed] [Google Scholar]
- 15.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 16.Darce J, Rudra D, Li L, Nishio J, Cipolletta D, Rudensky AY, Mathis D, Benoist C. An N-terminal mutation of the Foxp3 transcription factor alleviates arthritis but exacerbates diabetes. Immunity. 2012;36:731–741. doi: 10.1016/j.immuni.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bettini ML, Pan F, Bettini M, Finkelstein D, Rehg JE, Floess S, Bell BD, Ziegler SF, Huehn J, Pardoll DM, Vignali DA. Loss of epigenetic modification driven by the Foxp3 transcription factor leads to regulatory T cell insufficiency. Immunity. 2012;36:717–730. doi: 10.1016/j.immuni.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horai R, Silver PB, Chen J, Agarwal RK, Chong WP, Jittayasothorn Y, Mattapallil MJ, Nguyen S, Natarajan K, Villasmil R, Wang P, Karabekian Z, Lytton SD, Chan CC, Caspi RR. Breakdown of immune privilege and spontaneous autoimmunity in mice expressing a transgenic T cell receptor specific for a retinal autoantigen. J Autoimmun. 2013;44:21–33. doi: 10.1016/j.jaut.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pepperberg DR, Okajima TL, Ripps H, Chader GJ, Wiggert B. Functional properties of interphotoreceptor retinoid-binding protein. Photochem Photobiol. 1991;54:1057–1060. doi: 10.1111/j.1751-1097.1991.tb02129.x. [DOI] [PubMed] [Google Scholar]
- 20.Karabekian Z, Lytton SD, Silver PB, Sergeev YV, Schneck JP, Caspi RR. Antigen/MHC class II/Ig dimers for study of uveitogenic T cells: IRBP p161-180 presented by both IA and IE molecules. Invest Ophthalmol Vis Sci. 2005;46:3769–3776. doi: 10.1167/iovs.05-0187. [DOI] [PubMed] [Google Scholar]
- 21.Agarwal RK, Silver PB, Caspi RR. Rodent models of experimental autoimmune uveitis. Methods Mol Biol. 2012;900:443–469. doi: 10.1007/978-1-60761-720-4_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caspi RR. Experimental autoimmune uveoretinitis in the rat and mouse. In: Coligan JE, editor. Curr Protoc Immunol. Chapter 15. 2003. p. Unit 15 16. [DOI] [PubMed] [Google Scholar]
- 23.Kim YC, Bhairavabhotla R, Yoon J, Golding A, Thornton AM, Tran DQ, Shevach EM. Oligodeoxynucleotides stabilize Helios-expressing Foxp3+ human T regulatory cells during in vitro expansion. Blood. 2012;119:2810–2818. doi: 10.1182/blood-2011-09-377895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lehmann U, Heuss ND, McPherson SW, Roehrich H, Gregerson DS. Dendritic cells are early responders to retinal injury. Neurobiol Dis. 2010;40:177–184. doi: 10.1016/j.nbd.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gottschalk RA, Corse E, Allison JP. Expression of Helios in peripherally induced Foxp3+ regulatory T cells. J Immunol. 2012;188:976–980. doi: 10.4049/jimmunol.1102964. [DOI] [PubMed] [Google Scholar]
- 26.Akimova T, Beier UH, Wang L, Levine MH, Hancock WW. Helios expression is a marker of T cell activation and proliferation. PLoS One. 2011;6:e24226. doi: 10.1371/journal.pone.0024226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai Z, Kishimoto H, Brunmark A, Jackson MR, Peterson PA, Sprent J. Requirements for peptide-induced T cell receptor downregulation on naive CD8+ T cells. J Exp Med. 1997;185:641–651. doi: 10.1084/jem.185.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niedergang F, Dautry-Varsat A, Alcover A. Peptide antigen or superantigen-induced down-regulation of TCRs involves both stimulated and unstimulated receptors. Journal of immunology. 1997;159:1703–1710. [PubMed] [Google Scholar]
- 29.Polansky JK, Kretschmer K, Freyer J, Floess S, Garbe A, Baron U, Olek S, Hamann A, von Boehmer H, Huehn J. DNA methylation controls Foxp3 gene expression. Eur J Immunol. 2008;38:1654–1663. doi: 10.1002/eji.200838105. [DOI] [PubMed] [Google Scholar]
- 30.Luger D, Silver PB, Tang J, Cua D, Chen Z, Iwakura Y, Bowman EP, Sgambellone NM, Chan CC, Caspi RR. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med. 2008;205:799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, Zhang GX, Dittel BN, Rostami A. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol. 2011;12:568–575. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Codarri L, Gyulveszi G, Tosevski V, Hesske L, Fontana A, Magnenat L, Suter T, Becher B. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011;12:560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 33.London A, Benhar I, Mattapallil MJ, Mack M, Caspi RR, Schwartz M. Functional macrophage heterogeneity in a mouse model of autoimmune central nervous system pathology. J Immunol. 2013;190:3570–3578. doi: 10.4049/jimmunol.1202076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang RX, Yu CR, Dambuza IM, Mahdi RM, Dolinska MB, Sergeev YV, Wingfield PT, Kim SH, Egwuagu CE. Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nat Med. 2014;20:633–641. doi: 10.1038/nm.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prendergast RA, Iliff CE, Coskuncan NM, Caspi RR, Sartani G, Tarrant TK, Lutty GA, McLeod DS. T cell traffic and the inflammatory response in experimental autoimmune uveoretinitis. Invest Ophthalmol Vis Sci. 1998;39:754–762. [PubMed] [Google Scholar]
- 36.Thurau SR, Mempel TR, Flugel A, Diedrichs-Mohring M, Krombach F, Kawakami N, Wildner G. The fate of autoreactive, GFP+ T cells in rat models of uveitis analyzed by intravital fluorescence microscopy and FACS. International Immunology. 2004;16:1573–1582. doi: 10.1093/intimm/dxh158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.