Abstract
It has been proposed that deep brain stimulation (DBS) of the subthalamic nucleus (STN DBS) and dopaminergic therapy ameliorate the symptoms of Parkinson's disease through similar functional mechanisms. We examined this notion using PET to compare the metabolic effects of these treatment approaches. Nine Parkinson's disease patients (age 61.7 ± 11.1 years) were scanned ON and OFF STN stimulation and nine others (age 60.0 ± 9.3 years) were scanned ON and OFF an individual titrated intravenous levodopa infusion. The two treatment groups were matched for baseline disease severity as well as clinical response to therapy. Similarities and differences in the effects of treatment on regional metabolism were assessed using statistical parametric mapping (SPM). In addition, we used network analysis to assess the effect of therapy on the expression of an abnormal Parkinson's disease-related spatial covariance pattern (PDRP). We found that both STN DBS and levodopa therapy were associated with significant (P < 0.001) metabolic reductions in the putamen/globus pallidus, sensorimotor cortex and cerebellar vermis, as well as increases in the precuneus (BA 7). The metabolic effects of the two interventions differed in the STN and medial prefrontal cortex, with relative increases with stimulation in the former structure and decreases in the latter. Network quantification disclosed reductions in PDRP activity with both interventions, which correlated with clinical improvement (P < 0.05). The degree of network modulation by therapy did not differ significantly for the two treatment approaches (P > 0.6). These findings support the results of previous imaging studies indicating that effective symptomatic therapies for Parkinson's disease involve a common mechanism. The modulation of pathological brain networks is a critical feature of the treatment response in parkinsonism.
Keywords: Parkinson s disease, subthalamic nucleus, deep brain stimulation, brain metabolism, PET
Introduction
The subthalamic nucleus (STN) plays a critical role in modulating the activity of cortico-striato-pallido-thalamocortical (CSPTC) loops and related pathways (Parent and Hazrati, 1995a, b; Hamani et al., 2004). The motor symptoms of Parkinson's disease have been associated with increased STN activity, resulting in excessive inhibitory outflow from the basal ganglia to the thalamus and brainstem (Wichmann and DeLong, 1996; Vitek and Giroux, 2000). A variety of stereotaxic surgical approaches, including high frequency deep brain stimulation (DBS), have been introduced to modulate STN neural activity and restore normal functioning to motor CSPTC circuitry (Benabid, 2003; Lozano and Mahant, 2004). Dopaminergic therapy also appears to suppress the activity of STN and its excitatory projections to the internal globus pallidus (GPi) and the pars reticulata of the substantia nigra (SNpr) (Lozano et al., 2000).
PET can provide useful information concerning the functional status of CSPTC pathways in Parkinson's disease and related disorders (Eidelberg et al., 2000; Carbon and Eidelberg, 2002). In particular, network analyses of regional PET data have consistently revealed the presence of an abnormal spatial covariance pattern associated with this disease (Eidelberg et al., 1994; Moeller et al., 1999; Lozza et al., 2004; Asanuma et al., 2005). This Parkinson's disease-related pattern (PDRP) is characterized by increased pallidothalamic and pontine metabolic activity associated with relative reductions in premotor and posterior parietal cortical regions. We have attributed this abnormal metabolic topography to overactivity of internal pallidal (GPi) afferents from STN and consequent increases in inhibitory pallidal outflow to the ventral thalamus and pons, in turn resulting in a reduction in thalamocortical afferent activity (Eidelberg et al., 1994, 1996, 1997). Indeed, STN lesioning has been found to produce a marked and sustained reduction in network activity in Parkinson's disease patients with advanced disease (Su et al., 2001; Trošt et al., 2003). Analogous changes in PDRP expression have also been observed during levodopa infusion, consistent with pharmacological suppression of the STN and its efferent projections (Feigin et al., 2001).
The activity of STN and its projection pathways can also be modulated by high frequency stimulation. Although the precise mechanism of DBS action is not known, a number of experimental studies have pointed to local inhibition as the major effect of this intervention in Parkinson's disease (Filali et al., 2004; Lozano and Mahant, 2004; McIntyre et al., 2004a, b). In a recent PET study comparing STN DBS and lesioning, we reported significant declines in PDRP expression with both interventions (Trošt et al., 2006). However, generalization based upon these findings was limited by the small number of subjects in each treatment group, the very advanced symptoms of the patients and the mainly unilateral nature of the stereotaxic procedures that were performed.
In the present study we applied a similar network-based approach to compare STN DBS with levodopa administration, two interventions for Parkinson's disease that are thought on clinical grounds to be mechanistically similar (Krack et al., 2002; Vingerhoets et al., 2002). To this end, we used PET to assess the metabolic consequences of this procedure in a larger, more typical cohort of moderately affected Parkinson's disease patients undergoing bilateral STN stimulation. These changes were compared with those observed in a separate group of patients who were scanned before and during intravenous levodopa infusion. Both treatment groups were matched for age and baseline disease severity, and achieved equivalent clinical responses with therapy. These imaging experiments allowed us to contrast the degree of network modulation achieved by STN DBS and levodopa, and also to rigorously assess commonalities and differences in the local metabolic effects of the two interventions.
Material and methods
Subjects
We studied nine Parkinson's disease patients with bilaterally implanted STN electrodes (7 men and 2 women, age 61.7 ± 11.1 years). Nine other Parkinson's disease patients (6 men and 3 women, age 60.0 ± 9.3 years) served as a comparison treatment group receiving intravenous levodopa infusion. In all subjects, a diagnosis of Parkinson's disease was made if the patients had ‘pure’ parkinsonism without a history of known causative factors such as encephalitis or neuroleptic treatment, and did not have dementia, supranuclear gaze abnormalities or ataxia. Subjects’ estimated pre-morbid IQ, based on the National Adult Reading Test (NART) (Nelson, 1982), was in the high average range (115.9 ± 12.3). The average depression rating (Beck, 1987) was within normal limits (5.8 ± 4.6). Before study entry, all patients demonstrated at least 20% improvement in the motor portion of the Unified Parkinson's Disease Rating Scale (UPDRS; Fahn and Elton, 1987) with treatment. (However, during the imaging experiments, one patient in each group demonstrated an improvement in the motor UPDRS in the 15–20% range.) The clinical response to treatment was highly significant in both treatment groups (P < 0.001).
Patient characteristics are presented in Table 1. Limited clinical and metabolic data from Patients 2–6 in the levodopa infusion group have appeared previously (Feigin et al., 2001). In addition to age, both groups were matched for baseline motor ratings (OFF state UPDRS 33.3 ± 12.1 and 28.9 ± 7.0 for the DBS and levodopa groups, P = 0.9) and for clinical response to treatment (change in motor UPDRS 39.1 and 38.3% for the respective treatment groups, P = 0.7).
Table 1.
Patient characteristics
| Patient | Age (year) | Sex | UPDRSa |
||
|---|---|---|---|---|---|
| OFF (baseline) | ON | Change (%) | |||
| (A) STN DBS | |||||
| 1 | 74 | F | 22 | 18 | –18.6 |
| 2 | 56 | M | 23 | 11 | –51.1 |
| 3 | 68 | M | 51 | 40 | –21.8 |
| 4 | 67 | M | 21 | 13 | –39.0 |
| 5 | 69 | M | 49 | 34 | –30.9 |
| 6 | 69 | M | 31 | 21 | –33.9 |
| 7 | 38 | F | 47 | 14 | –70.2 |
| 8 | 53 | M | 31 | 20 | –37.1 |
| 9 | 62 | M | 28 | 14 | –49.1 |
| Mean | 61.7 | 7 M/2 F | 33.3 | 20.2 | –39.1 |
| SD | 11.1 | 12.1 | 9.9 | 15.9 | |
| (B) Levodopa infusion | |||||
| 1 | 71 | M | 39 | 20 | –48.7 |
| 2 | 64 | M | 20 | 13 | –35.0 |
| 3 | 55 | F | 25 | 12 | –52.0 |
| 4 | 60 | M | 35 | 23 | –34.3 |
| 5 | 59 | M | 32 | 27 | –15.6 |
| 6 | 66 | F | 35 | 27 | –22.9 |
| 7 | 40 | F | 20 | 9 | –55.0 |
| 8 | 56 | M | 31 | 18 | –41.0 |
| 9 | 69 | M | 24 | 14 | –40.4 |
| Mean | 60.0 | 6 M/3 F | 28.9 | 18.1 | –38.3 |
| SD | 9.3 | 7.0 | 6.6 | 13.1 | |
Composite Unified Parkinson's Disease Rating Scale (UPDRS) motor ratings obtained 12 h following the cessation of anti-parkinsonian medication.
Study design
The PET studies were performed on two consecutive days. All anti-parkinsonian medications were held for at least 12 h before each day of testing. On one day, the patients were scanned off levodopa/DBS (OFF state). On the other day, the same patients were scanned either during bilateral STN stimulation or during an individually titrated levodopa infusion adjusted to achieve maximal clinical improvement without inducing dyskinesia (ON state). [The details of these procedures have been presented elsewhere (Feigin et al., 2001; Fukuda et al., 2001).] The order of the sessions was randomized. Subjects were rated according to UPDRS at each scanning session.
Subjects fasted overnight before imaging. Subjects were scanned with 18F-fluorodeoxyglucose (FDG) and PET using the GE Advance tomograph (General Electric Medical Systems, Milwaukee, WI) at North Shore University Hospital. This eight-ring bismuth germanate scanner provided 35 2D image planes with axial field view of 14.5 cm and transaxial resolution of 4.2 mm in all directions. In each PET session (ON and OFF), the patients were positioned in the scanner using a stereoadapter with three-dimensional laser alignment. All FDG PET studies were performed with the subject's eyes open in a dimly lit room with minimal auditory stimulation. Global and regional metabolic rates for glucose were calculated on a voxel basis as described previously (Fukuda et al., 2001).
All patients in the STN DBS treatment group and seven members of the levodopa treatment group completed a brief neuro-psychological assessment in both the ON and OFF conditions. Alternate forms of the following instruments were administered in all the examined subjects: brief test of attention (Schretlen, 1989); symbol digit modality test (SDMT) (Smith, 1982); controlled oral word association (Benton et al., 1994); and the Hopkins verbal learning test—revised (HVLT-R) (Brandt and Benedict, 2001).
Ethical permission for the studies was obtained from the Institutional Review Board of North Shore University Hospital. Written consent was obtained from each subject following detailed explanation of the procedures.
Data analysis
Data processing was performed using SPM99 (Wellcome Department of Cognitive Neurology, London, UK) implemented in MATLAB (Mathworks, Sherborn, MA). The scans from each subject were non-linearly warped into Talairach space (Talairach and Tournoux, 1988) and were smoothed with an isotropic Gaussian kernel (FWHM 10 mm for all directions) to improve the signal to noise ratio.
We used the paired t-test option in SPM99 to assess the regional metabolic changes induced by STN DBS and by levodopa infusion. The general linear model (GLM) was used to compare metabolic responses with treatment across interventions on a voxel-by-voxel basis. We first used conjunction analysis (Price and Friston, 1997) with multi-group and multi-condition options to identify regions in which the two interventions gave rise to similar metabolic changes (i.e. areas where both interventions led to either increases or decreases in regional glucose utilization). We then used a separate linear contrast to localize regions in which the treatment effects on metabolism differed across interventions (i.e. areas where metabolism increased with one treatment but decreased with the other, or vice versa).
In these SPM analyses we hypothesized that both STN DBS and levodopa would alter metabolic activity within CSPTC loops and related cerebello-cortical pathways. We therefore assessed treatment-induced changes and group differences within a set of voxels known to be abnormal in Parkinson's disease subjects on the basis of prior PET studies. This was accomplished by creating a mask defined by significant (P < 0.001) between-group metabolic differences in an independent population of 69 unmedicated Parkinson's disease patients and normal volunteer subjects (Fukuda et al., 2001). The mask included the basal ganglia, thalamus, pons and cerebellum, as well as the motor, premotor, anterior cingulate, prefrontal and posterior parietal cortical regions. Metabolic changes within this mask were considered part of the distributed network of regions altered in Parkinson's disease and not independent of each other. For hypothesis testing, changes within the mask were considered significant at P < 0.01, uncorrected for multiple regional comparisons. The effects outside the Parkinson's disease mask were considered to be hypothesis-generating at a threshold of P < 0.001 (uncorrected), and were considered significant if they survived correction for multiple comparisons at P < 0.05. Coordinates were reported in the standard anatomical space developed at the Montreal Neurological Institute. The cytoarchitectonic localization of each reported cluster was confirmed using the Talairach space utility (TSU, available at http://www.ihb.spb.ru/~pet_lab/TSU/TSUMain.html).
Network analysis
In all subjects, the expression of the PDRP network (Fig. 1) was quantified in the OFF and ON conditions using a fully automated voxel-based algorithm (Asanuma et al., 2005; Trošt et al., 2006, software available at http://neuroscience-nslij.org/Methods/software.html). All PDRP computations were performed blind to subject intervention, group (STN DBS or levodopa), treatment condition (OFF or ON) and clinical status (UPDRS motor ratings). As part of this analysis, we also quantified PDRP expression in 14 additional Parkinson's disease patients (age 63.5 ± 7.7 years; UPDRS motor ratings 24 ± 13.2) who were scanned twice with FDG PET in the same treatment condition. PDRP scores for this test–retest group served as control values for comparison with the two treatment groups. Baseline PDRP scores for the three groups (i.e. the OFF condition for the two treatment groups and the first scan for the test–retest group) were compared using one-way analysis of variance (ANOVA). These values were also correlated with baseline UPDRS motor ratings using pearson product moment correlation coefficients.
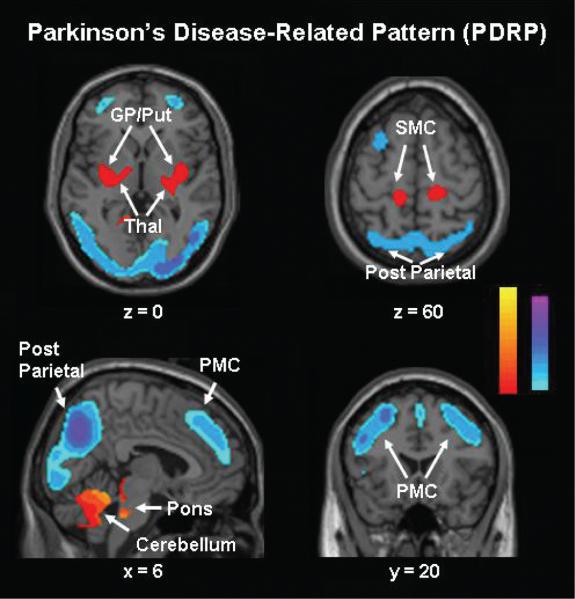
Fig. 1.
PDRP identified by spatial covariance analysis of FDG PET scans from 20 Parkinson's disease patients and 20 age-matched normal volunteer subjects (Asanuma et al., 2005). This pattern was characterized by relative metabolic increases in the pallidum and thalamus (upper left), in the pons and cerebellum (bottom left) and in sensorimotor cortex (SMC) (upper right). These changes covaried with metabolic decreases in the lateral premotor cortex (PMC) and in parieto-occipital association regions (bottom right). [The display represents voxels that contribute significantly to the network at P = 0.001, and that were demonstrated to be reliable (P < 0.001) on bootstrap estimation (Efron and Tibshirani, 1994). Voxels with positive region weights (metabolic increases) are colour coded from red to yellow; those with negative region weights (metabolic decreases) are colour coded from blue to purple.]
To determine group differences in treatment response, we performed a two-way ANOVA on the PDRP scores from the three groups with the treatment condition as the repeated measure. By examining the group × treatment interaction effect, this analysis allowed us to assess the change of PDRP expression following treatment in each intervention group relative to the change in PDRP expression in the test–retest reference group. If a significant group–treatment interaction effect was observed, post hoc Bonferroni pairwise comparisons were used to assess the change of PDRP expression within each group. If significant treatment effects were observed in both intervention groups relative to the reference group, we performed another two-way (group–treatment) ANOVA including only the two intervention groups in order to examine the comparability of the treatment effects between the two interventions. Additionally, we examined the correlation between the changes in PDRP scores and UPDRS motor ratings for each of the treatment groups (STN DBS or levodopa) by computing Pearson's correlation coefficients.
SPM and network computations were conducted on PCs running Windows 2000. ANOVA and correlation analyses were performed on these PCs using SPSS software (SPSS Inc., Chicago, IL); the results were considered significant at P < 0.05.
Results
Changes in regional glucose metabolism with treatment
STN DBS
Voxel-based contrast of ON and OFF scans (Table 2A) disclosed several areas with significant metabolic change during stimulation. With treatment, regional glucose metabolism declined in the right putamen, left GPi and prefrontal cortex (BA 10), and bilaterally in the sensorimotor cortex and cerebellar vermis. Stimulation was associated with increased glucose metabolism in a cluster of voxels spanning the subthalamic region and ventrolateral thalamus, and in the right medial posterior parietal cortex (precuneus, BA 7).
Table 2.
Brain regions with significant metabolic changes with treatment
| Brain region | Coordinatesa |
Z max | ||
|---|---|---|---|---|
| x | y | z | ||
| (A) STN DBS | ||||
| Metabolic decreases (ON < OFF) | ||||
| Cerebellar vermis, lobule Vb,* | 10 | –54 | –22 | –2.77 |
| –6 | –56 | –22 | –2.74 | |
| Putamen** | 36 | 18 | –6 | –3.48 |
| GPi* | –16 | 14 | –6 | –2.30 |
| Prefrontal gyrus, BA 10** | –16 | 60 | –6 | –3.41 |
| Precentral gyrus, BA 4** | –50 | –16 | 40 | –3.39 |
| 28 | –24 | 52 | –2.28 | |
| 36 | –32 | 62 | –2.50 | |
| Metabolic increases (ON > OFF) | ||||
| Ventrolateral thalamus/STN* | –10 | –14 | 8 | –2.48 |
| Precuneus, BA 7* | 14 | –74 | 32 | –2.58 |
| (B) Levodopa infusion | ||||
| Metabolic decreases (ON < OFF) | ||||
| Cerebellar vermis, lobule | 6 | – 46 | –22 | 4.05 |
| Putamen/GP/thalamus** | –34 | –14 | –6 | 3.83 |
| Precentral gyrus, BA 4† | –16 | –28 | 54 | 4.67 |
| 14 | –30 | 68 | 3.45 | |
| Metabolic increases (ON > OFF) | ||||
| Cerebellar hemisphere, lobule IV* | 42 | –54 | –26 | 2.68 |
| Prefrontal gyrus, BA 10, 11* | 28 | 58 | 10 | 2.86 |
| Precuneus, BA 7, 31* | 2 | –68 | 26 | 2.87 |
Montreal Neurological Institute (MNI) standard space.
According to the atlas of Schmahmann (Schmahmann et al., 2000).
P < 0.01
P < 0.001 (uncorrected, within hypothesis-testing mask)
P < 0.05 (corrected for multiple non-independent comparisons).
Levodopa infusion
Voxel analysis (Table 2B) revealed that this intervention was associated with significant metabolic reductions in the cerebellar vermis, in the left globus pallidus and ventral thalamus, and bilaterally in sensorimotor cortex. This intervention was associated with increased metabolism in the right cerebellar hemisphere, precuneus (BA 7) and prefrontal cortex (BA 10, 11).
Comparison of interventions
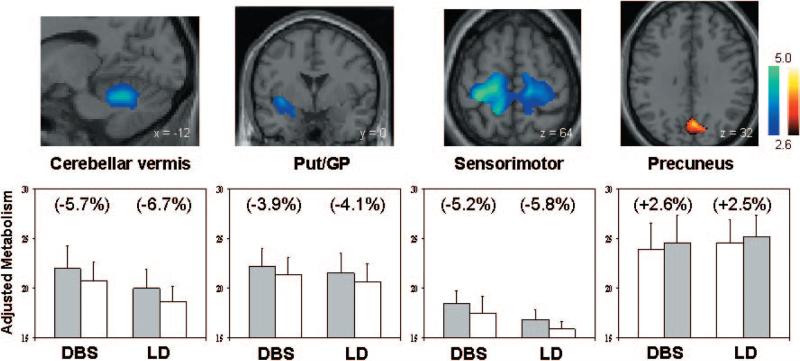
Voxel-wise comparison of the OFF scans from the STN DBS and levodopa cohorts revealed no significant group differences in regional metabolism at baseline. Conjunction analysis disclosed several local metabolic changes that were common to both interventions (Table 3A; Fig. 2). Metabolic declines in the putamen/globus pallidus, sensorimotor cortex (BA 4/3) and cerebellar vermis were shared features of STN DBS and levodopa infusion, as were metabolic increases in the precuneus. In contrast, the local metabolic response to treatment differed between interventions in only two brain regions (Table 3B; Fig. 3). In the ventromedial prefrontal cortex (BA 10, 11), STN stimulation and levodopa infusion were respectively associated with treatment-induced reductions and increases in local glucose utilization. The opposite metabolic effects were noted in a single cluster extending from the ventral thalamus to the subthalamic region, with local increases with stimulation and decreases with levodopa infusion.
Table 3.
Regional metabolic effects of treatment: commonalities and differences
| Brain region | Coordinatesa |
Z max | ||
|---|---|---|---|---|
| x | y | z | ||
| (A) Brain regions with shared metabolic effects in both interventions | ||||
| Metabolic decreases (ON < OFF) | ||||
| Cerebellar vermis, lobule Vb,† | 8 | –54 | –20 | 4.23 |
| Putamen/GP** | –34 | –14 | –6 | 3.51 |
| Precentral gyrus, BA 4† | –16 | –24 | 60 | 4.16 |
| 16 | –32 | 70 | 3.80 | |
| Metabolic increases (ON > OFF) | ||||
| Precuneus, BA 7** | 14 | –76 | 36 | 4.23 |
| (B) Brain regions with different metabolic effects across interventions | ||||
| STN DBS effects < levodopa effects | ||||
| Prefrontal gyrus, BA 10, 11** | 30 | 56 | 14 | 3.45 |
| –2 | 56 | –12 | 3.40 | |
| STN DBS effects > levodopa effects | ||||
| Ventral thalamus/STN** | –8 | –14 | 8 | 3.11 |
Montreal Neurological Institute (MNI) standard space.
According to the atlas of Schmahmann (Schmahmann et al., 2000).
* P < 0.01
P < 0.001 (uncorrected, within hypothesis-testing mask)
P < 0.05 (corrected for multiple non-independent comparisons).
Fig. 2.
(Top) Regions in which the metabolic effects of treatment were similar for STN DBS and levodopa infusion (see text). Both interventions were associated with metabolic reductions in the cerebellar vermis, putamen/globus pallidus (Put/GP) and sensorimotor cortex (left and middle). Shared metabolic increases with treatment were identified in the precuneus (right). [SPM {t} maps were superimposed on a single-subject MRI brain template. Treatment-mediated metabolic decreases (blue–green scale) and increases (yellow–red scale) were thresholded at t = 2.6, P = 0.01 within the hypothesis-testing mask]. (Bottom) Bar graphs illustrating rates of glucose utilization for each of the significant clusters displayed above. Metabolic values (mean ± SE) are presented for each treatment condition. [OFF (filled bars) and ON (open bars) correspond to the treatment conditions for the stimulation (DBS) and levodopa (LD) groups. Regional changes with intervention appear in parentheses.]
Fig. 3.
(Top) Regions in which the metabolic effects of treatment were different for STN stimulation and levodopa infusion. Metabolic increases with stimulation relative to levodopa (left) were present in a cluster of voxels in the thalamus, extending into the subthalamic region. Relative metabolic decreases with stimulation (right) were present in the ventromedial prefrontal cortex. [SPM {t} maps were superimposed on a single-subject MRI brain template. Treatment-induced metabolic differences across interventions (blue–green scale) were thresholded at t = 2.6, P = 0.01 within the hypothesis-testing mask]. (Bottom) Bar graphs illustrating rates of glucose utilization for the significant clusters displayed above. Metabolic values (mean ± SE) are presented for each treatment condition. [OFF (filled bars) and ON (open bars) correspond to the treatment conditions for the stimulation (DBS) and levodopa (LD) groups. Regional changes with intervention appear in parentheses.]
Changes in network expression with treatment
PDRP scores were computed for the ON and OFF conditions of the two treatment groups and for the test–retest scan pairs of the reference group (Fig. 4). One-way ANOVA revealed significant differences in baseline PDRP expression across groups [F(2,29) = 4.9, P < 0.02]. Post hoc pairwise testing showed higher baseline network activity (P < 0.05) in the STN DBS group relative to the other groups. Baseline PDRP scores correlated with UPDRS motor ratings (r = 0.37, P < 0.05) across the three groups.
Fig. 4.
Plot of mean values (±6SE) for the expression of the PDRP. Network scores were computed on an individual basis in the OFF and ON conditions for the STN stimulation (DBS) and the levodopa (LD) treatment groups, and in a test–retest control group (CN) (see text). Similar reductions in network expression were present with both interventions; the change in the control group was not significant. [Asterisks represent P-values with respect to baseline. *P < 0.05; **P < 0.01).]
We also found that the treatment effects (ON versus OFF) differed for the three groups as supported by the results of two-way ANOVA, which revealed a significant group–treatment interaction [F(2,29) = 4.8, P < 0.02]. Post hoc pairwise testing showed significant reductions in PDRP expression in the STN DBS (P < 0.005) and the levodopa (P < 0.02) groups, but not in the reference group (P > 0.6). This indicates that each of the two interventions is associated with PDRP network suppression relative to the test–retest cohort. Subsequent analysis of the STN DBS and levodopa infusion groups revealed no significant interaction effect between the two interventions and the treatment conditions [F(1,16) = 0.2; P > 0.6]. This is consistent with the observation that the two interventions were associated with similar degrees of network suppression (Fig. 5, left panel). In both treatment groups, reductions in PDRP expression with treatment were found to correlate with the percent improvement in UPDRS motor ratings (STN DBS: r = 0.79, P < 0.01; levodopa infusion: r = 0.59, P < 0.05).
Fig. 5.
Bar graph illustrating treatment-mediated changes (±SE) in the expression of the PDRP metabolic brain network. Left panel: Difference values from the levodopa infusion (LD; dotted), STN stimulation (DBS; black), and the test-retest control (CN; white) groups from the current study are presented. Changes in PDRP expression for two patients scanned while receiving both levodopa and STN stimulation are represented by a filled square and triangle (see text). In one of these subjects, the change in PDRP expression was also computed from a separate set of scans performed off and on levodopa infusion without STN DBS (open triangle). Right panel: These measures were compared with those computed on the basis of previous PET data (grey) from patient cohorts undergoing GPi and STN lesioning (Eidelberg et al., 1996; Su et al., 2001) and stimulation (Fukuda et al., 2001; Trošt et al., 2006). [The changes in PDRP expression were computed on a hemisphere-by-hemisphere basis for all treatment groups. For the unilateral surgical interventions, these values reflect changes in network activity in the operated hemispheres. For the bilateral therapies, including levodopa infusion, PDRP changes were computed for each hemisphere and averaged (Fukuda et al., 2001). [Asterisks represent p values with respect to the untreated condition (paired Student's t-test). *p<0.05, **p<0.01].
Effects of treatment on neuropsychological tests
The means and standard deviations for each test in the ON and OFF conditions for the two treatment groups are presented in Table 4. For each of the neuropsychological tests, we assessed ON/OFF changes in performance in the two treatment groups using a 2 × 2 repeated measures ANOVA. A significant interaction (group × treatment) was identified [F(1,14) = 5.33, P < 0.05] for the recognition component (hits – false positives) of the HVLT-R. Post hoc testing indicated an improvement in performance in the levodopa group (t = –2.42; P < 0.05), with stable performance in the STN DBS group (t = 0.48; P = 0.65). We also found improved performance for delayed recall [F(1,14) = 9.8; P < 0.01), percent retention [F(1,14) = 10.1; P < 0.01] and SDMT [F(1,14) = 10.6; P < 0.01] in both treatment groups. No significant treatment effects were found for the other tests.
Table 4.
Neuropsychological test performance ON and OFF treatment
| Test | STN DBS [mean (± SD)] |
Levodopa [mean (± SD)] |
Reference levela | ||
|---|---|---|---|---|---|
| OFF | ON | OFF | ON | ||
| Brief test of attention | 6.3 (1.9) | 6.3 (2.4) | 6.9 (2.4) | 7.0 (2.4) | 7.9 (1.6) |
| Symbol digit modality test* | 26.9 (6.5) | 29.3 (6.6) | 39.4 (6.6) | 42.0 (7.1) | 41.5 (8.6) |
| Controlled oral word association | 34.1 (16.6) | 34.9 (17.3) | 40.4 (9.0) | 38.1 (11.2) | 35.6 (12.5) |
| Hopkins verbal learning test | |||||
| Trials 1–3 | 20.0 (5.8) | 19.8 (4.6) | 23.4 (6.2) | 24.7 (3.7) | 27.7 (4.3) |
| Delay* | 4.3 (3.9) | 5.9 (1.5) | 6.9 (2.1) | 9.0 (1.0) | 9.9 (1.9) |
| % Retention* | 48.9 (37.5) | 79.1 (15.7) | 73.7 (18.8) | 88.6 (11.9) | 92.4 (14.4) |
| Recognition/discrimination | 8.3 (2.9) | 7.9 (1.4) | 8.0 (2.8) | 11.1 (0.9) | 10.8 (1.3) |
Scores with significant interactions are given in boldface (P < 0.05; see text).
Reference based on average age of study participants.
Significant main effect of treatment (P < 0.01).
Discussion
In this direct comparison of the metabolic effects associated with STN DBS and levodopa infusion, we found that the two interventions achieve clinical benefit through similar mechanisms. Indeed, both treatment approaches were associated with comparable reductions in PDRP network activity, which correlated with the clinical improvement that was achieved. Overall, the findings support the notion that modulation of pathological network activity is the basis for therapeutic benefit in Parkinson's disease.
Metabolic effects of STN DBS
In this study we found that bilateral STN stimulation was associated with reduced resting glucose utilization in the putamen, globus pallidus, primary motor and prefrontal cortex, and cerebellar vermis. The finding of discrete metabolic reductions in motor cortical and cerebellar areas distant from stimulation site accord with prior studies showing analogous changes in resting regional cerebral blood flow and metabolism during STN stimulation (Limousin et al., 1997; Ceballos-Baumann et al., 1999; Hershey et al., 2003; Payoux et al., 2004; Trošt et al., 2006). The observed stimulation-induced declines in metabolism in the sensori-motor cortex and cerebellar vermis may reflect a reduction in the abnormal synchronized oscillation of motor cortical neuronal activity that has been proposed to occur with anti-parkinsonian treatment (cf. Goldberg et al., 2002; Levy et al., 2002; Haslinger et al., 2005). Alternatively, these changes may be secondary consequences of reduced proprioceptive input following reductions in tremor and/or rigidity. Likewise, metabolic declines in the globus pallidus and putamen may be attributable to reductions in the synaptic activity of STN projections to neighbouring structures (Parent and Hazrati, 1995b). Indeed, we have noted similar changes in previous FDG PET studies of patients undergoing STN lesioning (Su et al., 2001; Trošt et al., 2003, 2006).
We also observed that STN DBS increased metabolism in the vicinity of the subthalamic target site extending rostrally into the ventral thalamus. Similar findings have been reported with 15O-labelled water and PET during STN stimulation (Hershey et al., 2003). These changes can be attributed to the direct effects of electrical current on the cell membrane by depolarization block (Beurrier et al., 2001), by increasing inhibitory afferents from the external globus pallidus to STN (Dostrovsky et al., 2000) or by a combination of the two mechanisms. The presence of stimulation-induced metabolic increases in the ventrolateral thalamus with STN DBS has also been described previously (Hilker et al., 2004). This effect may be the result of activation of fibre pathways to the thalamus in the vicinity of the STN (Parent et al., 2000).
Stimulation-induced increases in regional metabolism were also observed in the precuneus. This posterior parietal region is known to receive afferents from prefrontal regions, sensory cortex and multiple thalamic relay nuclei (Yeterian and Pandya, 1985; Neal et al., 1990; Taktakishvili et al., 2002). As suggested by prior PET studies, metabolic increases in the precuneus with treatment may be a downstream effect of functional changes in any of these areas (Hilker et al., 2004; Vafaee et al., 2004; Goerendt et al., 2006; Trošt et al., 2006). Given the stimulation-induced metabolic reductions in the sensorimotor and prefrontal regions seen in this study, it is likely that the increases in the precuneus were related to changes in the activity of afferent projections from the ventral thalamus rather than from other cortical fields. We note that the metabolic effects of stimulation involved preferentially the left STN/ventral thalamus (increases) as well as the left pallidum and prefrontal cortex (decreases). The apparent unilaterality of the metabolic response to bilateral STN DBS can be attributed to the stringent statistical threshold that was employed. At a more liberal hypothesis-testing threshold (P = 0.05, within mask), we identified bilateral responses in these regions. The magnitude of the metabolic response was however greater on the left for these structures, perhaps as a consequence of the marginally higher amplitudes of stimulation used on that side.
Comparison of the metabolic effects of STN DBS and levodopa infusion
In this study we also compared the metabolic effects of STN DBS with those induced by levodopa. Although ideally this comparison should have been performed using a within-subject design, dosimetric limitations necessitated an alternative cross-sectional approach. In this design, the subjects undergoing levodopa infusion were matched for both age and baseline disease severity with the STN DBS group. The comparison of changes in regional metabolism across interventions was further facilitated by selecting patient cohorts with nearly identical clinical responses to treatment. Indeed, voxel-wise analysis disclosed shared metabolic reductions in the putamen/globus pallidus, sensorimotor cortex and cerebellar vermis in the two interventions. These findings are compatible with prior studies describing metabolic decreases in the primary motor area and cerebellar vermis with levodopa administration (Feigin et al., 2001; Hilker et al., 2002), and suggest a common final pathway for the therapeutic actions of dopaminergic therapy and STN stimulation. Although reductions in putamen and pallido-thalamic hypermetabolism have been noted with levodopa therapy (Blesa et al., 1991; Feigin et al., 2001), these local effects have not been reported in prior resting state comparisons of the ON and OFF STN stimulation conditions (Ceballos-Baumann et al., 1999; Hilker et al., 2002; Payoux et al., 2004). Subtle changes in GPi metabolism with STN stimulation have however been detected using conjunction analysis (Trošt et al., 2006), which can capture such small effects if they are shared across interventions. Interestingly, as with STN DBS, the metabolic response to levodopa was asymmetrical, with greater treatment-induced subcortical changes on the left. This finding is consistent with similar left-sided asymmetries in cerebral blood flow observed in parkinsonian primates and in Parkinson's disease patients treated with levodopa (Hershey et al., 2000, 2003; Goerendt et al., 2006). These observations have been linked to structural asymmetries in the nigrostriatal system.
We also found that both interventions were associated with metabolic increases in the precuneus. Similar changes in the posterior parietal cortex have been described in previous PET studies of STN stimulation (Hilker et al., 2004; Vafaee et al., 2004; Trošt et al., 2006) and dopaminergic therapy (Goerendt et al., 2006). The precise mechanism underlying the observed enhancement of neural activity in this region by anti-parkinsonian therapy is unclear. As discussed above, STN DBS may increase glucose utilization in parietal association regions by potentiating afferents from prefrontal cortex and/or thalamic relay nuclei. These cortical regions also receive dopaminergic input (Lewis and Sesack, 1997) and may be directly responsive to pharmacologic repletion of this neurotransmitter (Mattay et al., 2002; Carbon et al., 2003b). Irrespective of mechanism, the degree of local metabolic enhancement that was observed in the precuneus was similar for both interventions. This suggests that STN DBS and levodopa may have comparable effects on neuropsychological tests associated with the functioning of this region.
In contrast, the two interventions differed in their effects on prefrontal metabolism. Specifically, compared to levodopa, STN stimulation was associated with decreased metabolism in the ventromedial prefrontal cortex. This effect may be mediated via the mediodorsal thalamus or through antidromic modulation of prefrontal-STN pathways (Maurice et al., 1998; Middleton and Strick, 2002; cf. Chudasama et al., 2003). This relative suppression of medial prefrontal activity may explain aspects of the neurobehavioural effects of therapy that are specific for STN stimulation (Pillon et al., 2000; Saint-Cyr et al., 2000; Funkiewiez et al., 2004). Indeed, analysis of the neuro-psychological data revealed significant effects of treatment in several of the tests that were administered. Improvement in the SDMT was noted for both treatments. This is expected given the substantial motor component of this task. In contrast, the different components of the memory test (HVLT-R) varied between treatments. Improvements with both interventions were found for the delayed recall components of this task, as was previously observed with STN DBS (Hilker et al., 2004). However, the effect of treatment on recognition memory appears to be more complex, in that performance is improved by levodopa and not STN stimulation. Recognition memory has previously been shown to relate to the integrity of the prefrontal cortex, particularly ventrally (Grady et al., 2005). Our behavioural findings are consistent with the PET data showing a difference in the metabolic effects of the two interventions in this dopaminoceptive region (Lewis and Sesack, 1997; Kaasinen et al., 2000; Seamans and Yang, 2004). It is conceivable that levodopa enhances dopamine signalling locally, thereby improving both prefrontal functioning and task performance. This effect appears not to be present with STN DBS, in keeping with the variable metabolic responses reported in the pre-frontal area and other cortical regions with this intervention (Hershey et al., 2003; cf. Hilker et al., 2003).
Network modulation: a common feature of anti-parkinsonian therapy
Using an automated approach for quantifying network activity in single scans, we found that both STN DBS and levodopa were associated with downward modulation of the PDRP, a previously validated disease-related spatial covariance pattern. The suppression of abnormal PDRP activity by treatment is consistent with the results of the SPM analysis, which localized the metabolic effects of both interventions to discrete nodes within this pathological brain network. Indeed, many of the regional metabolic changes observed during treatment for the motor manifestations of Parkinson's disease can be interpreted in the context of the PDRP topography. Both STN DBS and levodopa are associated with declines in pallidal metabolism, as well as concomitant downstream reductions in the activity of pallidofugal inhibitory afferents to the ventral thalamus and pons (cf. Eidelberg et al., 1997). (In the case of STN DBS, there may also be a direct effect on ventrolateral thalamic activity via local fibre bundles surrounding the target.) It appears that changes in thalamic activity with treatment can give rise to two separate effects on cortical functioning within the PDRP. Brain regions like the premotor cortex (PMC) and posterior parietal cortex are relatively hypometabolic in untreated Parkinson's disease (Eidelberg et al., 1994). Treatment with levodopa or STN DBS gives rise to metabolic increases in these areas, presumably by increasing excitatory afferent activity from the thalamus. In contrast, as discussed above, the sensorimotor cortex is metabolically overactive at baseline. Metabolism in this region is reduced by treatment, perhaps through the potentiation of inhibitory pathways involving the thalamic reticular nucleus (Benazzouz and Hallett, 2000). In both interventions, the decline in metabolism in the sensorimotor cortex is associated with concomitant reductions in the activity of its efferent projections to the putamen and cerebellar vermis.
The relevance of the treatment-mediated reduction in PDRP expression is supported by the presence of significant correlations between the network changes and clinical measures of motor improvement. Furthermore, STN DBS and levodopa produced comparable degrees of PDRP suppression, consistent with their similar treatment effects. These findings suggest that a common functional/anatomical pathway underlies the therapeutic effects of both STN DBS and dopaminergic therapy. This notion is consistent with the clinical experience that oral levodopa can be substantially reduced or discontinued following STN stimulation (Vingerhoets et al., 2002). However, regional differences in the metabolic effects of the two interventions were also identified, raising the possibility of synergism (Peppe et al., 2004). It is noteworthy that divergent effects of therapy were identified in the ventromedial prefrontal cortex, a region lying outside the boundaries of the PDRP. As mentioned above, these metabolic changes may be more relevant to behavioural/cognitive aspects of the therapeutic response rather than to motor symptoms.
We found that baseline PDRP expression was significantly greater in the DBS cohort, despite the fact that the two treatment groups did not differ with respect to the corresponding UPDRS motor ratings. While network values significantly correlated with UPDRS ratings at baseline, the magnitude of this effect was small (R2 < 15%). Indeed, in a recent PET study (Huang et al., 2005), we found that the relationship between longitudinal changes in these two parameters was also rather weak. Thus, although correlated with the UPDRS, subject expression of PDRP network is likely to represent a different feature of disease progression than standardized clinical motor ratings.
It is also important to note that although PDRP suppression is a common feature of both STN DBS and levodopa therapy, this effect is not necessarily additive. We have recently measured PDRP changes in two subjects scanned while receiving both STN stimulation and levodopa infusion as the ON condition. In both cases, the decline in PDRP expression induced by both interventions in combination was within the range of values observed with stimulation alone (see Fig. 5, left panel; filled symbols). In one of these subjects, this ON/OFF difference in PDRP expression (filled triangle) was compared with that obtained in the same individual during a separate 2-day scanning session performed OFF and ON levodopa infusion alone, without STN stimulation (open triangle). Despite nearly identical baseline PDRP scores for both treatment sessions, the addition of STN stimulation to levodopa reduced PDRP expression only by an additional 20% (OFF/ON PDRP scores: 9.8/5.2 and 9.9/4.6 for levodopa and combined therapy, respectively). It is likely that therapeutic modulation of PDRP activity is limited by a floor effect defined roughly by the degree of network expression that can be achieved by STN lesioning (see Fig. 5, right panel).
Modulation of abnormal PDRP activity is a feature of other clinically effective approaches for the management of Parkinson's disease symptoms. Our computational approach allowed us to assess changes in network expression across multiple treatment groups in a single, comprehensive analysis. These data (Fig. 5), based upon the current experiments and our prior FDG PET studies (Eidelberg et al., 1996; Fukuda et al., 2001; Su et al., 2001; Trošt et al., 2006) indicate that although PDRP suppression is a feature of all the studied interventions, the magnitude of this effect is dependent upon the surgical target (GPi or STN) and mode of treatment (lesion or stimulation). In agreement with the general clinical impression that STN is superior to GPi as a treatment target (Hamani et al., 2004; Volkmann, 2004), the magnitude of network modulation was greater for interventions involving the former, more proximal structure. Given the possibility of superimposed excitatory effects with DBS (Hashimoto et al., 2003; McIntyre et al., 2004a, b), treatment-mediated reductions in PDRP activity were found to be relatively smaller for stimulation than for lesioning of either the STN or GPi (cf. Trošt et al., 2006). Moreover, for each intervention, changes in PDRP expression with treatment correlated significantly with objective measures of clinical improvement (Carbon et al., 2003a; Trošt et al., 2006). Overall, these results suggest that assessing treatment-mediated changes in the expression of Parkinson's disease-related brain networks may be a useful means of evaluating new therapeutic options for this disease.
Acknowledgements
This work was supported by NIH NINDS R01 35069 (D.E.) and by the General Clinical Research Center of the North Shore-Long Island Jewish Health System (M01 RR018535). The authors wish to thank Ms Toni Flanagan for valuable editorial assistance.
Abbreviations
- CSPTC
cortico-striato-pallido-thalamocortical
- DBS
deep brain stimulation
- FDG
18F-fluorodeoxyglucose
- GPi
internal globus pallidus
- HVLT-R
Hopkins verbal learning test—revised
- NART
National Adult Reading Test
- PDRP
Parkinson's disease-related pattern
- SDMT
symbol digit modality test
- STN
subthalamic nucleus
- UPDRS
Unified Parkinson's Disease Rating Scale
References
- Asanuma K, Ma Y, Huang C, Carbon M, Edwards C, Raymond D, et al. The metabolic pathology of dopa-responsive dystonia. Ann Neurol. 2005;57:596–600. doi: 10.1002/ana.20442. [DOI] [PubMed] [Google Scholar]
- Beck A. Beck depression inventory: manual. Psychological Corporation; San Antonio: 1987. [Google Scholar]
- Benabid AL. Deep brain stimulation for Parkinson's disease. Curr Opin Neurobiol. 2003;13:696–706. doi: 10.1016/j.conb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Benazzouz A, Hallett M. Mechanism of action of deep brain stimulation. Neurology. 2000;55:S13–6. [PubMed] [Google Scholar]
- Benton A, Hamsher K, Sivan A. Multilingual aphasia examination. 3rd edn. AJA Associates; Iowa City: 1994. [Google Scholar]
- Beurrier C, Bioulac B, Audin J, Hammond C. High-frequency stimulation produces a transient blockade of voltage-gated currents in subthalamic neurons. J Neurophysiol. 2001;85:1351–56. doi: 10.1152/jn.2001.85.4.1351. [DOI] [PubMed] [Google Scholar]
- Blesa R, Blin J, Miletich R. Levodopa-reduced glucose metabolism in striatopallido-thalamocortical circuit in Parkinson's disease. Neurology. 1991;441:359. [Google Scholar]
- Brandt J, Benedict R. Hopkins Verbal Learning Test—Revised Manual. Psychological Assessment Resources; Odessa: 2001. [Google Scholar]
- Carbon M, Eidelberg D. Modulation of regional brain function by deep brain stimulation: studies with positron emission tomography. Curr Opin Neurol. 2002;15:451–5. doi: 10.1097/00019052-200208000-00008. [DOI] [PubMed] [Google Scholar]
- Carbon M, Edwards C, Eidelberg D. Functional brain imaging in Parkinson's disease. Adv Neurol. 2003a;91:175–81. [PubMed] [Google Scholar]
- Carbon M, Ghilardi MF, Feigin A, Fukuda M, Silvestri G, Mentis MJ, et al. Learning networks in health and Parkinson's disease: reproducibility and treatment effects. Hum Brain Mapp. 2003b;19:197–211. doi: 10.1002/hbm.10115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceballos-Baumann AO, Boecker H, Bartenstein P, von Falkenhayn I, Riescher H, Conrad B, et al. A positron emission tomographic study of subthalamic nucleus stimulation in Parkinson disease: enhanced movement-related activity of motor-association cortex and decreased motor cortex resting activity. Arch Neurol. 1999;56:997–1003. doi: 10.1001/archneur.56.8.997. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Baunez C, Robbins TW. Functional disconnection of the medial prefrontal cortex and subthalamic nucleus in attentional performance: evidence for corticosubthalamic interaction. J Neurosci. 2003;23:5477–85. doi: 10.1523/JNEUROSCI.23-13-05477.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostrovsky JO, Levy R, Wu JP, Hutchison WD, Tasker RR, Lozano AM. Microstimulation-induced inhibition of neuronal firing in human globus pallidus. J Neurophysiol. 2000;84:570–4. doi: 10.1152/jn.2000.84.1.570. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani R. An introduction to the bootstrap. CRC Press, LLC; New York: 1994. [Google Scholar]
- Eidelberg D, Moeller JR, Dhawan V, Spetsieris P, Takikawa S, Ishikawa T, et al. The metabolic topography of parkinsonism. J Cereb Blood Flow Metab. 1994;14:783–801. doi: 10.1038/jcbfm.1994.99. [DOI] [PubMed] [Google Scholar]
- Eidelberg D, Moeller JR, Ishikawa T, Dhawan V, Spetsieris P, Silbersweig D, et al. Regional metabolic correlates of surgical outcome following unilateral pallidotomy for Parkinson's disease. Ann Neurol. 1996;39:450–9. doi: 10.1002/ana.410390407. [DOI] [PubMed] [Google Scholar]
- Eidelberg D, Moeller JR, Kazumata K, Antonini A, Sterio D, Dhawan V, et al. Metabolic correlates of pallidal neuronal activity in Parkinson's disease. Brain. 1997;120:1315–24. doi: 10.1093/brain/120.8.1315. [DOI] [PubMed] [Google Scholar]
- Eidelberg D, Edwards C, Mentis M, Dhawan V, Moeller JR. Movement disorders: Parkinson's disease. In: Mazziotta JC, Toga AW, Frackowiak RSJ, editors. Brain mapping: the disorders. Academic Press; San Diego: 2000. pp. 241–61. [Google Scholar]
- Fahn S, Elton RL. Unified Parkinson's Disease Rating Scale. In: Fahn S, Marsden CD, Calne DB, editors. Recent Developments in Parkinson's Disease. MacMillan Health Care Information; Florham Park: 1987. pp. 153–63. [Google Scholar]
- Feigin A, Fukuda M, Dhawan V, Przedborski S, Jackson-Lewis V, Mentis MJ, et al. Metabolic correlates of levodopa response in Parkinson's disease. Neurology. 2001;57:2083–8. doi: 10.1212/wnl.57.11.2083. [DOI] [PubMed] [Google Scholar]
- Filali M, Hutchinson WD, Palter VN, Lozano AM, Dostrovsky J. Stimulation-induced inhibition of neuronal firing in human subthalamic nucleus. Exp Brain Res. 2004;156:274–81. doi: 10.1007/s00221-003-1784-y. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Mentis MJ, Ma Y, Dhawan V, Antonini A, Lang AE, et al. Networks mediating the clinical effects of pallidal brain stimulation for Parkinson's disease: a PET study of resting-state glucose metabolism. Brain. 2001;124:1601–9. doi: 10.1093/brain/124.8.1601. [DOI] [PubMed] [Google Scholar]
- Funkiewiez A, Ardouin C, Caputo E, Krack P, Fraix V, Klinger H, et al. Long term effects of bilateral subthalamic nucleus stimulation on cognitive function, mood, and behaviour in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2004;75:834–9. doi: 10.1136/jnnp.2002.009803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goerendt IK, Lawrence AD, Mehta MA, Stern JS, Odin P, Brooks DJ. Distributed neural actions of anti-parkinsonian therapies as revealed by PET. J Neural Transm. 2006;113:75–86. doi: 10.1007/s00702-005-0305-5. [DOI] [PubMed] [Google Scholar]
- Goldberg JA, Boraud T, Maraton S, Haber SN, Vaadia E, Bergman H. Enhanced synchrony among primary motor cortex neurons in the 1-methyl-4-phenyl-12,3,6-tetrahydropyridine primate model of Parkinson's disease. J Neurosci. 2002;22:4639–53. doi: 10.1523/JNEUROSCI.22-11-04639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Craik FI. Task-related activity in prefrontal cortex and its relation to recognition memory performance in young and old adults. Neuropsychologia. 2005;43:1466–81. doi: 10.1016/j.neuropsychologia.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Hamani C, Saint-Cyr JA, Fraser J, Kaplitt M, Lozano AM. The subthalamic nucleus in the context of movement disorders. Brain. 2004;127:4–20. doi: 10.1093/brain/awh029. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Elder CM, Okun MS, Patrick SK, Vitek JL. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J Neurosci. 2003;23:1916–23. doi: 10.1523/JNEUROSCI.23-05-01916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslinger B, Kalteis K, Boecker H, Alesch F, Ceballos-Baumann AO. Frequency-correlated decreases of motor cortex activity associated with subthalamic nucleus stimulation in Parkinson's disease. Neuroimage. 2005;28:598–606. doi: 10.1016/j.neuroimage.2005.06.034. [DOI] [PubMed] [Google Scholar]
- Hershey T, Black KJ, Carl JL, Perlmutter JS. Dopa-induced blood flow responses in nonhuman primates. Exp Neurol. 2000;166:342–9. doi: 10.1006/exnr.2000.7522. [DOI] [PubMed] [Google Scholar]
- Hershey T, Revilla FJ, Wernle AR, McGee-Minnich L, Antenor JV, Videen TO, et al. Cortical and subcortical blood flow effects of subthalamic nucleus stimulation in PD. Neurology. 2003;61:816–21. doi: 10.1212/01.wnl.0000083991.81859.73. [DOI] [PubMed] [Google Scholar]
- Hilker R, Voges J, Thiel A, Ghaemi M, Herholz K, Sturm V, et al. Deep brain stimulation of the subthalamic nucleus versus levodopa challenge in Parkinson's disease: measuring the on- and off-conditions with FDG-PET. J Neural Transm. 2002;109:1257–64. doi: 10.1007/s00702-002-0696-5. [DOI] [PubMed] [Google Scholar]
- Hilker R, Voges J, Ghaemi M, Lehrke R, Rudolf J, Koulousakis A, et al. Deep brain stimulation of the subthalamic nucleus does not increase the striatal dopamine concentration in parkinsonian humans. Mov Disord. 2003;18:41–8. doi: 10.1002/mds.10297. [DOI] [PubMed] [Google Scholar]
- Hilker R, Voges J, Weisenbach S, Kalbe E, Burghaus L, Ghaemi M, et al. Subthalamic nucleus stimulation restores glucose metabolism in associative and limbic cortices and in cerebellum: evidence from a FDG-PET study in advanced Parkinson's disease. J Cereb Blood Flow Metab. 2004;24:7–16. doi: 10.1097/01.WCB.0000092831.44769.09. [DOI] [PubMed] [Google Scholar]
- Huang C, Feigin A, Ma Y, Eidelberg D. Imaging measures of longitudinal change in Parkinson's disease. Neurology. 2005;64:A235. [Google Scholar]
- Kaasinen V, Nagren K, Hietala J, Oikonen V, Vilkman H, Farde L, et al. Extrastriatal dopamine D2 and D3 receptors in early and advanced Parkinson's disease. Neurology. 2000;54:1482–7. doi: 10.1212/wnl.54.7.1482. [DOI] [PubMed] [Google Scholar]
- Krack P, Fraix V, Mendes A, Benabid AL, Pollak P. Postoperative management of subthalamic nucleus stimulation for Parkinson's disease. Mov Disord. 2002;17:188–97. doi: 10.1002/mds.10163. [DOI] [PubMed] [Google Scholar]
- Levy R, Ashby P, Hutchison WD, Lang AE, Lozano AM, Dostrovsky JO. Dependence of subthalamic nucleus oscillations on movement and dopamine in Parkinson's disease. Brain. 2002;125:1196–209. doi: 10.1093/brain/awf128. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Sesack SR. Dopamine systems in the primate brain. In: Bloom FE, Bjorklund A, Hokfelt T, editors. Handbook of chemical neuroanatomy, the primate nervous system, Pt I. Elsevier Science; New York: 1997. pp. 261–373. [Google Scholar]
- Limousin P, Greene J, Pollak P, Rothwell J, Benabid AL, Frackowiak R. Changes in cerebral activity pattern due to subthalamic nucleus or internal pallidum stimulation in Parkinson's disease. Ann Neurol. 1997;42:283–91. doi: 10.1002/ana.410420303. [DOI] [PubMed] [Google Scholar]
- Lozano AM, Mahant N. Deep brain stimulation surgery for Parkinson's disease: mechanisms and consequences. Parkinsonism Relat Disord. 2004;10(Suppl 1):S49–57. doi: 10.1016/j.parkreldis.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Lozano AM, Lang AE, Levy R, Hutchison W, Dostrovsky J. Neuronal recordings in Parkinson's disease patients with dyskinesias induced by apomorphine. Ann Neurol. 2000;47:S141–6. [PubMed] [Google Scholar]
- Lozza C, Baron JC, Eidelberg D, Mentis MJ, Carbon M, Marie RM. Executive processes in Parkinson's disease: FDG-PET and network analysis. Hum Brain Mapp. 2004;22:236–45. doi: 10.1002/hbm.20033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore A, Hariri AR, Das S, Callicott JH, et al. Neurophysiological correlates of age-related changes in human motor function. Neurology. 2002;58:630–5. doi: 10.1212/wnl.58.4.630. [DOI] [PubMed] [Google Scholar]
- Maurice N, Deniau JM, Glowinski J, Thierry AM. Relationships between the prefrontal cortex and the basal ganglia in the rat: physiology of the corticosubthalamic circuits. J Neurosci. 1998;18:9539–46. doi: 10.1523/JNEUROSCI.18-22-09539.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre CC, Savasta M, Kerkerian-Le Goff L, Vitek JL. Uncovering the mechanism(s) of action of deep brain stimulation: activation, inhibition, or both. Clin Neurophysiol. 2004a;115:1239–48. doi: 10.1016/j.clinph.2003.12.024. [DOI] [PubMed] [Google Scholar]
- McIntyre CC, Savasta M, Walter BL, Vitek JL. How does deep brain stimulation work? Present understanding and future questions. J Clin Neurophysiol. 2004b;21:40–50. doi: 10.1097/00004691-200401000-00006. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal-ganglia ‘projections’ to the prefrontal cortex of the primate. Cereb Cortex. 2002;12:926–35. doi: 10.1093/cercor/12.9.926. [DOI] [PubMed] [Google Scholar]
- Moeller JR, Nakamura T, Mentis MJ, Dhawan V, Spetsieres P, Antonini A, et al. Reproducibility of regional metabolic covariance patterns: comparison of four populations. J Nucl Med. 1999;40:1264–9. [PubMed] [Google Scholar]
- Neal JW, Pearson RC, Powell TP. The ipsilateral corticocortical connections of area 7 with the frontal lobe in the monkey. Brain Res. 1990;509:31–40. doi: 10.1016/0006-8993(90)90305-u. [DOI] [PubMed] [Google Scholar]
- Nelson HE. The National Adult Reading Test (NART): Test Manual. NFER-Nelson; Windsor, UK: 1982. [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Brain Res Rev. 1995a;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Res Brain Res Rev. 1995b;20:128–54. doi: 10.1016/0165-0173(94)00008-d. [DOI] [PubMed] [Google Scholar]
- Parent A, Cossete M, Levesque M. Anatomical considerations in basal ganglia surgery. In: Lozano AM, editor. Movement disorder surgery. Progress in neurological surgery. Vol. 15. Karger; Basel: 2000. pp. 21–30. [Google Scholar]
- Payoux P, Remy P, Damier P, Miloudi M, Loubinoux I, Pidoux B, et al. Subthalamic nucleus stimulation reduces abnormal motor cortical overactivity in Parkinson disease. Arch Neurol. 2004;61:1307–13. doi: 10.1001/archneur.61.8.1307. [DOI] [PubMed] [Google Scholar]
- Peppe A, Pierantozzi M, Bassi A, Altibrandi MG, Brusa L, Stefani A, et al. Stimulation of the subthalamic nucleus compared with the globus pallidus internus in patients with Parkinson disease. J Neurosurg. 2004;101:195–200. doi: 10.3171/jns.2004.101.2.0195. [DOI] [PubMed] [Google Scholar]
- Pillon B, Ardouin C, Damier P, Krack P, Houeto JL, Klinger H, et al. Neuropsychological changes between ‘off’ and ‘on’ STN or GPi stimulation in Parkinson's disease. Neurology. 2000;55:411–8. doi: 10.1212/wnl.55.3.411. [DOI] [PubMed] [Google Scholar]
- Price CJ, Friston KJ. Cognitive conjunction: a new approach to brain activation experiments. Neuroimage. 1997;5:261–70. doi: 10.1006/nimg.1997.0269. [DOI] [PubMed] [Google Scholar]
- Saint-Cyr JA, Trepanier LL, Kumar R, Lozano AM, Lang AE. Neuropsychological consequences of chronic bilateral stimulation of the subthalamic nucleus in Parkinson's disease. Brain. 2000;123:2091–108. doi: 10.1093/brain/123.10.2091. [DOI] [PubMed] [Google Scholar]
- Schretlen D. Brief Test of Attention. Psychological Assessment Resources; Odessa: 1989. [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Smith A. Symbol Digit Modalities Test (SDMT): Manual (Revised) Western Psychological Services; Los Angeles: 1982. [Google Scholar]
- Su PC, Ma Y, Fukuda M, Mentis MJ, Tseng HM, Yen RF, et al. Metabolic changes following subthalamotomy for advanced Parkinson's disease. Ann Neurol. 2001;50:514–20. doi: 10.1002/ana.1232. [DOI] [PubMed] [Google Scholar]
- Taktakishvili O, Sivan-Loukianova E, Kultas-Ilinksky K, Ilinsky IA. Posterior parietal cortex projections to the ventral lateral and some association thalamic nuclei in Macaca mulatta. Brain Res Bull. 2002;59:135–50. doi: 10.1016/s0361-9230(02)00857-2. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Coplanar stereotaxic atlas of the human brain. Thieme Medical; New York: 1988. [Google Scholar]
- Trošt M, Su PC, Barnes A, Su SL, Yen RF, Tseng HM, et al. Evolving metabolic changes during the first postoperative year after subthalamotomy. J Neurosurg. 2003;99:872–8. doi: 10.3171/jns.2003.99.5.0872. [DOI] [PubMed] [Google Scholar]
- Trošt M, Su S, Su PC, Yen R-F, Tseng H-M, Barnes A, et al. Network modulation by the subthalamic nucleus in the treatment of Parkinson's disease. Neuroimage. 2006;31:301–7. doi: 10.1016/j.neuroimage.2005.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vafaee MS, K OS, Sunde N, Gjedde A, Dupont E, Cumming P. Focal changes of oxygen consumption in cerebral cortex of patients with Parkinson's disease during subthalamic stimulation. Neuroimage. 2004;22:966–74. doi: 10.1016/j.neuroimage.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Vingerhoets FJ, Villemure JG, Temperli P, Pollo C, Pralong E, Ghika J. Subthalamic DBS replaces levodopa in Parkinson's disease: two-year follow-up. Neurology. 2002;58:396–401. doi: 10.1212/wnl.58.3.396. [DOI] [PubMed] [Google Scholar]
- Vitek JL, Giroux M. Physiology of hypokinetic and hyperkinetic movement disorders: model for dyskinesia. Ann Neurol. 2000;47:S131–40. [PubMed] [Google Scholar]
- Volkmann J. Deep brain stimulation for the treatment of Parkinson's disease. J Clin Neurophysiol. 2004;21:6–17. doi: 10.1097/00004691-200401000-00003. [DOI] [PubMed] [Google Scholar]
- Wichmann T, DeLong MR. Functional and pathophysiological models of the basal ganglia. Curr Opin Neurobiol. 1996;6:751–8. doi: 10.1016/s0959-4388(96)80024-9. [DOI] [PubMed] [Google Scholar]
- Yeterian EH, Pandya DN. Corticothalamic connections of the posterior parietal cortex in the rhesus monkey. J Comp Neurol. 1985;237:408–26. doi: 10.1002/cne.902370309. [DOI] [PubMed] [Google Scholar]