Abstract
Purpose
The global incidence of oropharyngeal squamous cell carcinoma (OPSCC) has been increasing, and it has been proposed that a rising rate of human papillomavirus (HPV) associated cancers is driving the observed changes in OPSCC incidence. We carried out this systematic review to further examine the prevalence of HPV in OPSCC over time worldwide.
Methods
A systematic literature search was performed to identify all articles through January 31, 2014 that reported on the prevalence of HPV in OPSCC. Articles that met inclusion criteria were divided into four time frames (pre-1995, 1995—1999, 2000—2004, and 2005—present) based on the median year of the study's sample collection period. Employing a weighted analysis of variance (ANOVA) model, we examined the trends of HPV-positivity over time worldwide, in North America, and in Europe.
Results
Our literature search identified 699 unique articles. 175 underwent review of the entire study and 105 met inclusion criteria. These 105 articles reported on the HPV prevalence in 9541 OPSCC specimens across 23 nations. We demonstrated significant increases in the percentage change of HPV-positive OPSCCs from pre-1995 to present: 20.6% worldwide (p-value for trend: p<0.001), 21.6% in North America (p=0.013) and 21.5% in Europe (p=0.033).
Discussion
Interestingly, while in Europe there was a steady increase in HPV prevalence across all time frames, reaching nearly 50% most recently, in North America HPV prevalence appears to have plateaued over the past decade at about 65%. These findings may have important implications regarding predictions for the future incidence of OPSCC.
Keywords: HPV, Human papillomavirus, Oropharyngeal squamous cell carcinoma, Oropharyngeal cancer, OPSCC
Introduction
Head and neck squamous cell carcinoma (HNSCC) represents the eighth most common cancer worldwide.1 The oropharynx (tonsils, soft palate, base of tongue and lateral/posterior pharyngeal walls) is an important region of the head and neck. Recently, numerous groups have revealed a rising incidence of oropharyngeal squamous cell carcinoma (OPSCC) in their respective country, particularly in white men.2-7 Chaturvedi et al. confirmed OPSCC incidence has increased globally, and they also showed there has been a decrease in the incidence of other oral cavity cancers among men, likely due to declines in alcohol and tobacco abuse.8 With the emergence of this data, there has been a drive to understand why the incidence of OPSCC is rising while other cancers of the head and neck are decreasing.
Since human papillomavirus (HPV) is a causal factor in a subset of OPSCCs,9 it has been proposed that a steadily rising rate of HPV-associated cancers has led to the changes in OPSCC incidence. Several groups have compared the percentage of HPV-positive OPSCCs within different time periods for patient populations in their country. Across the Netherlands the prevalence of HPV in OPSCC rose from 5% to 29% between 1990 and 2010.10 Hong et al. demonstrated an increase from 19% in 1987—1990 to 47% for 2001—2005 in Australia.11 In the United States, Chaturvedi et al. showed a significant increase from 16.3% for 1984—1989 to 72.7% by 2000—2004.12 Additional studies with patients from other countries also revealed a rise in the frequency of HPV-positive OPSCCs over time.13-18 Based on this data, it appears that a rising prevalence of HPV-positive OPSCCs could be driving the observed changes in the incidence of OPSCC. However, the vast majority of studies that have evaluated the HPV status of OPSCCs derive their samples from a relatively short time period and a single region within one country.
We performed this systematic review to analyze changes in the worldwide prevalence of HPV in OPSCC. Several groups have recently published pooled analyses describing HPV in head and neck cancer.19, 20 Ndiaye et al. described the global burden of HPV in multiple head and neck subsites (oropharynx, hypopharynx/larynx and oral cavity).19 They compared various HPV detection techniques and demonstrated a high correlation between p16 immunohistochemistry (IHC) and HPV DNA polymerase chain reaction (PCR) based approaches for identifying HPV-positive oropharyngeal cancers. Significantly, this relationship did not exist among the non-oropharyngeal cancers. Mehanna and colleagues described changes in the prevalence of HPV-positive OPSCCs through the year 2010.20 However, they included multiple studies with overlapping patients, so in an effort to avoid this bias, we systematically excluded articles analyzing the same patient population. Moreover, our study includes 38 articles published after 2010 describing almost 5,000 patients that would not have been available to Mehanna et al.
Overall, in this work we examine time trends in the worldwide prevalence of HPV-positive OPSCCs. By employing a statistical model, we combined data from all available articles to determine whether the frequency of these cancers has truly increased over the past few decades. HPV-positive OPSCC is a distinct disease entity with an improved prognosis,21, 22 and the results of this study may have important implications regarding predictions for the future incidence of OPSCC and in the planning of future studies related to this disease.
Methods
We followed the PRISMA guidelines throughout our analysis and manuscript preparation.23 Detailed methods are provided in the supplemental information document.
Article selection
A systematic literature review was performed using the NIH PubMed search engine to identify articles published through January 31, 2014 reporting on the prevalence of HPV in OPSCC by PCR, in situ hybridization (ISH), p16 IHC, or other molecular methods. Studies that met these initial criteria were selected for detailed evaluation of the entire study. Reviewing the reference sections of this initial cohort of articles identified additional relevant papers.
To be included in our analysis, all articles needed to present the first and last years that their samples derived from (sample collection period), number of HPV-positive OPSCCs, total number of OPSCCs analyzed, and the country/region where the samples originated. Eighteen articles did not report the sample collection period and three failed to present the number of HPV-positive OPSCCs detected. Before excluding these articles, we contacted the authors to gather the missing information (16 authors provided us with the necessary data). Finally, we carefully examined all articles in order to exclude ones with overlapping patient populations (included article reporting on the larger number of patients) (Supplemental Table S1).
Data abstraction and organization
Data extracted from each manuscript is documented in Supplemental Table S2. We stratified the head and neck cancers reported by each study into oropharyngeal and non-oropharyngeal subgroups and recorded the number of HPV-positive OPSCCs out of the total number of OPSCCs evaluated. Studies were separated into distinct geographical regions: North America, Europe, and Other (included Asia, Australia, South America and International due to the small number of articles from these regions). To assess changes in the prevalence of HPV-positive OPSCCs over time, we used the median year of each article's sample collection period (rounded down to the nearest year) to separate articles into four discrete time frames: pre-1995, 1995—1999, 2000—2004 and 2005—present. For example, an article with samples derived from the years 1998—2004 would have a median year of 2001, and thus the data from that article would be categorized into the 2000—2004 time frame. Finally, the cut-off years for the time frames were chosen since they provided the most even distribution of articles across the four time frames and thereby maximized the potential of our statistical analyses.
Statistical analysis
Due to the small number of articles for individual countries, we could not examine trends over time for each nation (aside from the United States24). Therefore, we examined broader regions and determined if HPV prevalence changed over time worldwide, in North America and throughout Europe. There were not sufficient articles from specific geographic regions such as Asia, Australia or South America to perform informative analyses on these individual areas.
Additionally, all articles within a particular region were combined together into a single analysis regardless of the detection method utilized (PCR, ISH, p16, etc). The number of articles employing methods other than PCR was too small to perform relevant statistical analyses assessing trends over time for each distinct detection method.
HPV prevalence was calculated for each article by dividing the number of patients with HPV-positive OPSCCs by the total number of OPSCCs analyzed. An analysis of variance (ANOVA) model with time frame used as a four-level categorical variable and weighted by the total number of OPSCCs analyzed in each paper was utilized to model the time trends of HPV in OPSCC. Two-sided tests and p-values less than 0.05 were used to determine statistical significance.
Results
Article selection and characteristics
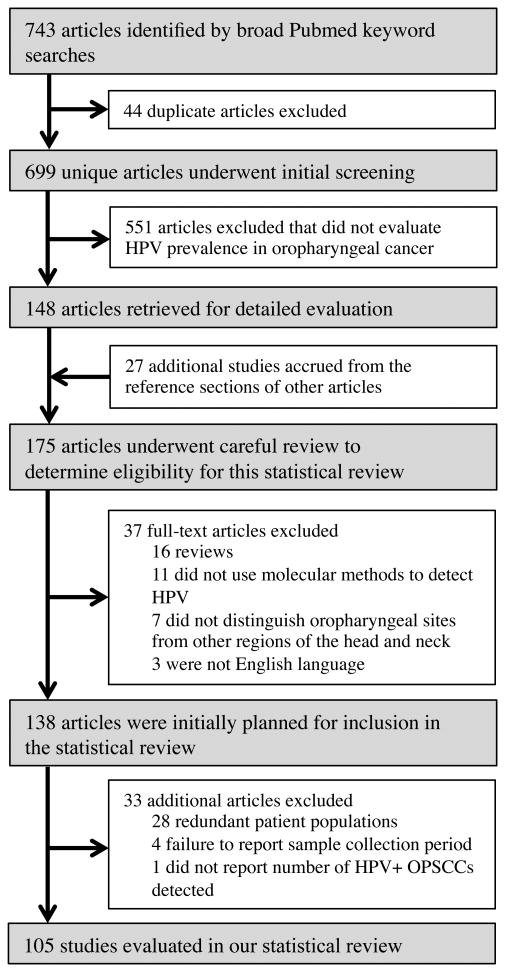
Based on our broad PubMed searches, 699 unique articles were identified. Following careful article evaluation and the described exclusion/inclusion process, 105 articles were included in our analysis (Figure 1). These 105 articles evaluated 9541 OPSCC specimens for the presence of HPV. Each article presented data for a median of 65 patients (range 5—711). 72 studies employed PCR-based methods, 12 ISH, nine p16 IHC with PCR, six p16 IHC alone, and six used other methods (two p16 IHC+ISH+PCR, two PCR with flight mass spectroscopy, one ISH+PCR, and one Southern blot). The majority of articles analyzed formalin-fixed, paraffin embedded (FFPE) tumors (n=72), followed by fresh frozen tissue (n=20), a combination of both FFPE and fresh frozen (n=12), and one article did not report the tissue type analyzed. Finally, studies derived from 23 different countries spanning five continents (Supplemental Table S3).
Figure 1. Flow chart of article inclusion and exclusion.
Diagram depicting the article accrual process as well as the inclusion and exclusion of studies from this systematic review.
HPV prevalence in OPSCC by region
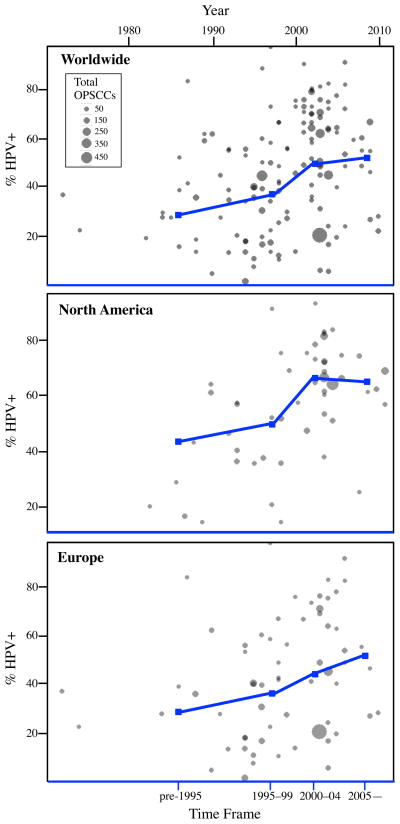
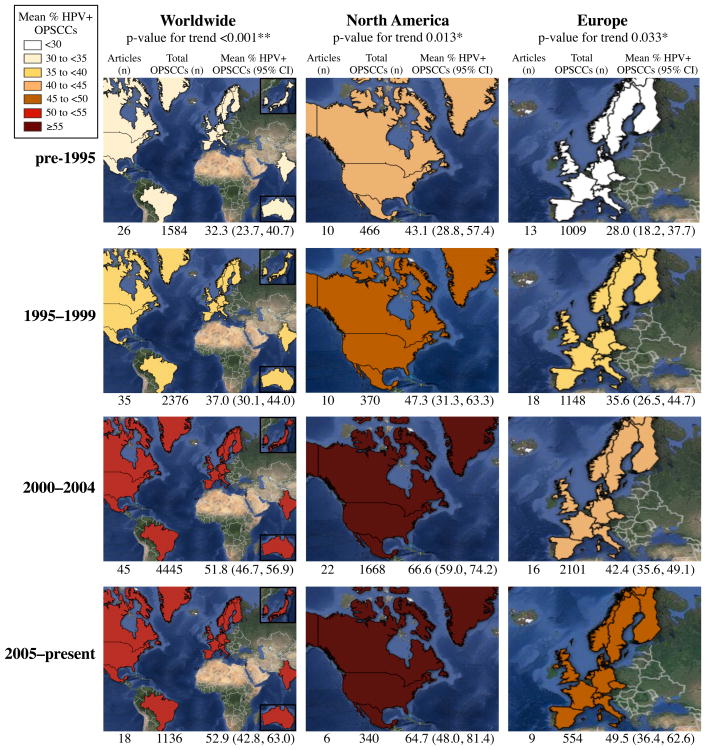
To assess changes over time related to the prevalence of HPV in OPSCC, we evaluated three geographic regions: worldwide, North America and Europe and separated articles into four time frames: pre-1995, 1995—1999, 2000—2004, and 2005—present based on each study's median year of sample collection (Figures 2 and 3). We demonstrated a significant increasing trend of HPV-positive OPSCCs over time worldwide (p<0.001). The mean prevalence (95% CI) was 32.3% (23.7%, 40.7%) for pre-1995, 37.0% (30.1%, 44.0%) in 1995—1999, 51.8% (46.7%, 56.9%) for 2000—2004, and 52.9% (42.8%, 63.0%) by 2005—present. Across North America there was also a significant trend (p=0.013), and the mean prevalence was 43.1% (28.8%, 57.4%) in pre-1995, 47.3% (31.3%, 63.3%) from 1995—1999, 66.6% (59.0%, 74.2%) by 2000—2004 and 64.7% (48.0%, 81.4%) for 2005—present. Finally, data from Europe revealed a significant trend (p=0.033), with the prevalence rising from 28.0% (18.2%, 37.7%) for pre-1995, to 35.6% (26.5%, 44.7%) in 1995—1999, 42.4% (35.6%, 49.1%) within 2000—2004 and eventually to 49.5% (36.4%, 62.6%) by 2005—present.
Figure 2. HPV prevalence in OPSCC by region.
Scatterplots depicting the raw HPV prevalence values for each article within the three main regions analyzed: worldwide, North America and Europe. The relative size of each grey circle is dependent on the number of OPSCCs evaluated by the study. The year for each article represents the median year of sample collection. The blue line plot represents the mean values for HPV prevalence in OPSCC generated by our statistical model for each region across four different time frames: pre-1995, 1995—1999, 2000—2004, and 2005—present.
Figure 3. Frequency of HPV-positive OPSCCs worldwide, in North America and across Europe.
Shaded maps representing the prevalence of HPV-positive OPSCCs in each region within a specified time frame. As the map's color shifts from yellow to orange to red, the prevalence of HPV is increasing. Documented below each map is the number of articles analyzed, total number of OPSCCs evaluated and the pooled mean HPV prevalence value and associated 95% confidence interval for that specific region and time frame. *p<0.05, **p<0.01.
The mean prevalence (95% CI) of HPV-positive OPSCCs in North America increased by 4.2% (-17.3%, 25.7%) from pre-1995 to 1995—1999, rose by 19.3% (1.6%, 37.0%) between the periods 1995—1999 to 2000—2004, and then decreased by 1.9% (-16.5%, 20.3%) from 2000—2004 to 2005—present. Across Europe there appears to be a steady increase in mean prevalence of HPV-positive cancers: 7.6% (-5.6%, 21.0%) from pre-1995 to 1995—1999, 6.8% (-4.6%, 18.1%) from 1995—1999 to 2000—2004 and 7.1% (-7.6%, 21.8%) from 2000—2004 to 2005—present (Table 1).
Table 1.
Pairwise comparisons for consecutive time frames in North America and Europe.
| Time Frames | Prevalence Difference (95% CI) | p-value |
|---|---|---|
| North America | ||
| 1995—1999 vs. pre-1995 | 4.2 (-17.3, 25.7) | 0.702 |
| 2000—2004 vs. 1995—1999 | 19.3 (1.6, 37.0) | 0.033* |
| 2005—present vs. 2000—2004 | -1.9 (-20.3, 16.5) | 0.838 |
| Europe | ||
| 1995—1999 vs. pre-1995 | 7.6 (-5.6, 21.0) | 0.255 |
| 2000—2004 vs. 1995—1999 | 6.8 (-4.6, 18.1) | 0.241 |
| 2005—present vs. 2000—2004 | 7.1 (-7.6, 21.8) | 0.342 |
p<0.05
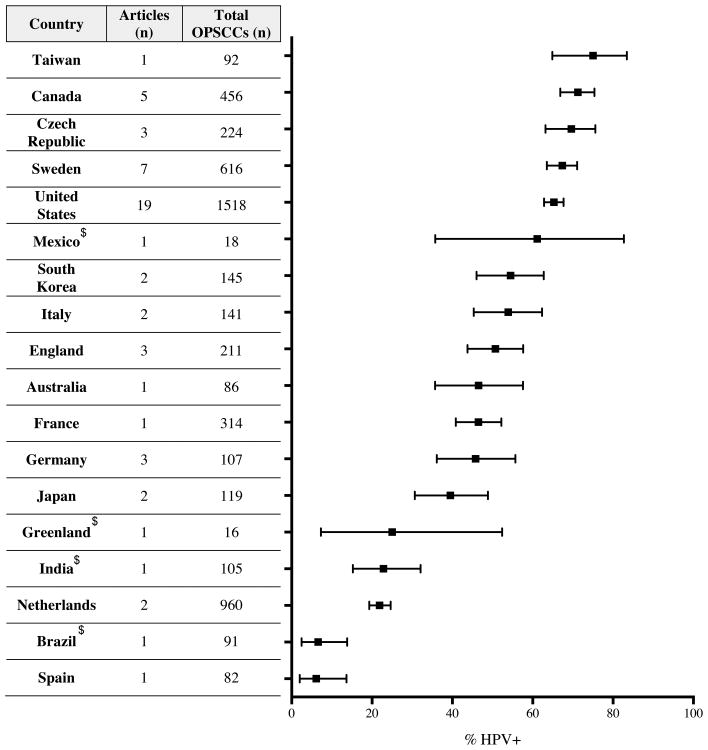
Next, we determined the present day frequency of HPV-positive OPSCCs by country (2000—present time frame, Figure 4). Thirteen of 18 countries had values between 39.5% and 75.0%. Taiwan was at the high end with 75.0% (95% CI: 64.9%, 83.4%) and Spain had the lowest value at 6.1% (2.0%, 13.7%). At 6.1% and 21.9% (19.3%, 24.6%), Spain and the Netherlands, respectively, had lower HPV-positive frequencies than Europe as a whole. Within North America the United States, Canada and Mexico all had prevalence values between 61.1—71.3% while a study from Greenland reported 25.0% (7.3%, 52.4%). Due to the limited number of articles available for this analysis from many of the countries, these results must be interpreted with caution.
Figure 4. Prevalence of HPV in OPSCC for individual countries.
All studies with a median year for their sample collection period from 2000—present were included in this analysis. The total number of OPSCCs and HPV-positive OPSCCs were summed from all articles within an individual nation. These values were used to determine the proportion of HPV-associated cancers along with a 95% confidence interval, which is depicted for each country in this plot. Counties not classified as advanced economies by the International Monetary Fund are indicated by a $.
HPV subtypes
58 articles presented HPV subtype information on a total of 2363 HPV-positive OPSCCs. Kreimer et al. previously revealed HPV16 was the most prevalent subtype in OPSCC followed by 18, 33, and 35,25 so we focused on documenting the distribution of these four subtypes. Examining all 58 articles demonstrated a HPV16 prevalence of 93.1%, followed by HPV33 at 1.4%, HPV18 at 1.3%, HPV35 at 0.5%, and all other genotypes had a combined prevalence of 4.2%. The same analysis was carried out for North America, Europe and Other. All three regions demonstrated a similar trend with at least 90.0% of HPV-positive cancers represented by HPV16 along with much lower prevalence values for subtypes 18, 33 and 35 (Table 2).
Table 2.
Distribution of HPV subtypes by region.
| Worldwide 58 Articles 2363 HPV+ OPSCCs |
North America 24 Articles 1062 HPV+ OPSCCs |
Europe 24 Articles 914 HPV+ OPSCCs |
Other 10 Articles 387 HPV+ OPSCCs |
|||||
|---|---|---|---|---|---|---|---|---|
| HPV Subtype | Positive Cases (n) | Prevalence | Positive Cases (n) | Prevalence | Positive Cases (n) | Prevalence | Positive Cases (n) | Prevalence |
| 16 | 2200 | 93.1% | 1016 | 95.7% | 831 | 90.9% | 353 | 91.2% |
| 18 | 30 | 1.3% | 6 | 0.6% | 9 | 1.0% | 15 | 3.9% |
| 33 | 34 | 1.4% | 6 | 0.6% | 28 | 3.1% | 0 | 0.0% |
| 35 | 12 | 0.5% | 5 | 0.5% | 7 | 0.8% | 0 | 0.0% |
| §Other | 100 | 4.2% | 29 | 2.7% | 42 | 4.6% | 29 | 7.5% |
Other HPV subtypes include 6, 11, 31, 32, 52, 53, 56, 57, 58, 59, 73, 81 and 82
Prevalence values within an individual column may sum to greater than 100% due to the presence of dual infections in some cancers
Analysis of potential confounding variables
We carried out a series of analyses to address potential confounding factors, including degradation of earlier collected tissue samples, changes in HPV detection method sensitivities over time, and differences in preservation and detection techniques. To assess sample degradation, we identified articles with a greater than 10 year gap between the publication year and the first year of sample collection. 65 articles fell into this group. 48 of the 65 articles carried out DNA integrity measures by amplifying beta-globin, actin, GAPDH or another housekeeping gene prior to performing HPV detection. To ensure the 17 articles that failed to carry out these measures did not influence our results, we excluded them and repeated the primary analysis for the worldwide articles. As shown in Table 3, the means and overall trend appear unchanged from the initial analysis.
Table 3.
Summary of analyses performed to evaluate for any potential confounding variables.
| Articles (n) | Total OPSCCs (n) | Mean % HPV+ OPSCCs (95% CI) | p-value | |
|---|---|---|---|---|
| §Evaluating the potential influence of the degradation of earlier samples | ||||
| pre-1995 | 22 | 1401 | 31.0 (21.6, 40.5) | <0.001** (for trend) |
| 1995—1999 | 30 | 1962 | 35.3 (27.3, 43.2) | |
| 2000—2004 | 37 | 3983 | 51.8 (46.3, 57.4) | |
| 2005—present | 18 | 1136 | 52.9 (42.5, 63.4) | |
| Changes in the sensitivity of HPV detection methods over time (articles with ‡median year pre-1995) | ||||
| Published pre-2000 | 9 | 220 | 35.9 (15.0, 56.8) | 0.701 |
| Published 2000—present | 17 | 1364 | 31.7 (23.3, 40.1) | |
| ISH versus PCR for HPV detection (articles with ‡median year 2000—2004) | ||||
| ISH | 7 | 682 | 62.2 (49.4, 74.9) | 0.615 |
| PCR | 27 | 1968 | 58.5 (51.0, 66.0) | |
| Fresh frozen versus FFPE samples (articles with ‡median year 1995—1999) | ||||
| Fresh frozen | 9 | 291 | 31.6 (16.2, 47.0) | 0.319 |
| FFPE | 23 | 1896 | 39.8 (33.8, 45.9) | |
For this analysis, 17 articles were excluded that did not carry out DNA integrity measures and had greater than a 10 year gap between publication year and first year of sample collection. Then, we repeated the same statistical analysis that was previously carried out for the worldwide articles (Figure 3). The p-value for this analysis represents the trend in the percentage of HPV-positive OPSCCs over the four time frames.
p<0.01;
Median year: Median year of each study's sample collection period
Next, improvements in HPV detection sensitivity could lead to an artificial increase in prevalence over time. We examined articles with a median year of sample collection in the pre-1995 time frame and stratified them into two groups: those published before 2000 (potentially using older methods) and articles published 2000—present (likely employing newer detection techniques). We calculated and compared the mean HPV prevalence for these two groups (Table 3), and found no statistically significant difference between them (p=0.701). Then, we evaluated if the type of detection method had any impact by comparing PCR and ISH. We selected articles with a sample collection median year in the 2000—2004 time frame as this era contained the greatest number of articles performing ISH. There was no difference in the mean HPV prevalence value determined by these two methods (p=0.615, Table 3). We could not carry out statistical analyses comparing p16 to PCR or ISH due to the small number of articles performing p16 alone. Importantly, a recent analysis demonstrated that 86.7% of HPV DNA PCR positive OPSCCs are also p16 positive.19 Finally, we compared the HPV-positive prevalence for FFPE specimens versus fresh frozen tumors. We examined studies with median year of sample collection within the 1995—1999 time frame since this era had the largest number of articles utilizing fresh frozen tissue. We did not find any difference when comparing the frequency of HPV-positive OPSCCs for FFPE samples to fresh frozen tissue (p=0.319, Table 3).
Discussion
In order to elucidate the current worldwide trends for HPV prevalence in OPSCC, we undertook this systematic review and employed a statistical model to examine all published literature through January 31, 2014 that documented the percentage of HPV-positive OPSCCs from patient populations across the world. We found there to be a significant increase in the prevalence of HPV in OPSCC worldwide, in North America and across Europe. This information provides further evidence that HPV likely contributed to the rising incidence of OPSCC observed in recent years.
Interestingly, the prevalence of HPV-positive OPSCC in North America increased in the 1990s, rose dramatically at the turn of the twenty-first century, and has apparently plateaued during the last decade at about 65% (Figure 2). In contrast, Europe demonstrated a gradual increase in HPV prevalence that has continued to the present day. The fact that HPV prevalence may have plateaued in North America could impact future projections related to the overall incidence of OPSCC. Chaturvedi et al. used a statistical model to predict future incidence of OPSCC in the United States and determined the number of cases of HPV-positive OPSCC would continue to increase and eventually surpass the number of cervical carcinoma cases by 2020.12 However, this prediction assumed that the current observed increases in incidence for OPSCC would continue into the future. But if HPV-associated cancers were driving the incidence of OPSCC and if they have now reached a plateau in North America, then we might expect to see a leveling off of total OPSCC incidence as well. The potential plateau we have observed in the prevalence of HPV in OPSCC across North America represents an interesting finding that has not been reported by other groups evaluating patient populations within North America. Importantly, a recent analysis by Nasman et al. revealed a leveling off of the incidence of HPV-positive tonsillar squamous cell carcinoma in Sweden since 2008.26
An understanding of the epidemiology of both HPV-negative and HPV-positive OPSCC is required to explore the possible reasons behind our results. Patients with HPV-negative OPSCC are generally older and have a history of substantial alcohol and tobacco abuse. Conversely, patients with HPV-positive cancers tend to be younger and have distinct risk factors including a high number of sexual partners, early age of first sexual encounter, and prior history of sexually transmitted infections.27, 28 Owing to these unique risk factor profiles, it has been suggested that changing sexual practices, in particular increasing oral sexual behavior, may have led to higher rates of oral HPV infection and ultimately HPV-positive OPSCCs. The United States is the only country with significant studies reporting time-based trends of oral sexual behavior, and studies spanning from the 1940s to the present day appear to support the notion of increasing oral sexual behavior. The number of men who ever engaged in oral sex rose from 10% in the 1940s/1950s to approximately 50% by the 1970s/1980s29 and continued to rise to 75% by 199130 and 85% by 2010.31 Moreover, men exhibit greater rates of oral HPV infection as compared to women,32 suggesting there may be gender specific factors promoting this interaction or more efficient viral transmission from women to men.31
These changing sexual practices could help explain the observed trends in the prevalence of HPV-positive OPSCCs in North America and Europe. Specifically, as there is likely a 20 to 30 year time lag between initial infection, tumorigenic progression and emergence of cancer, the steep rise in HPV-positive OPSCCs seen at the turn of the twenty-first century in North America could reflect a more dramatic increase in oral sexual behavior in the 1970s/1980s. In comparison, these practices may be changing more gradually across many nations in Europe, leading to the more steady increase in HPV-positive OPSCCs. With respect to the plateau observed in North America, this could be a reflection of oral sexual behavior achieving a new norm in this region of the world. Additionally, studies from Canada and the United States have revealed a decrease in smoking in both men and women since the 1950s/1960s.33, 34 At least in the United States, the decline in smoking has started to slow down in recent years.33 In this manner, it is possible that the major changes in sexual behavior and smoking habits occurred about 20 to 30 years ago across North America, and as a result HPV-negative (primarily smoking related) and HPV-positive OPSCCs have now reached a new balance in the population.
Although we observed a plateau in HPV-positive OPSCCs in North America, we still anticipate prevalence values to remain high in this part of the world for a minimum of 20 to 30 years because oral sexual behavior remains common,28 vaccination rates against HPV remain low in women and more so in men,35 and people developing oral HPV infections today likely will not present with OPSCC until the year 2030 or 2040. As countries in Europe such as the Netherlands and Spain see higher levels of HPV-positive OPSCCs,10, 17 we anticipate this cancer will continue to increase steadily in Europe. In this manner, HPV-positive OPSCC will remain a significant health burden worldwide for at least the next few decades. Therefore, this cancer represents an important disease for clinicians, scientists, epidemiologists and public health advocates to be aware of and understand.
One potential way to reduce the future burden of HPV-positive OPSCCs would be to prevent the initial infection in young men and women by vaccinating against HPV. Currently, Gardasil (targets HPV6, 11, 16, and 18) and Cervarix (targets HPV16 and 18) represent the two available vaccines. Both have lowered the rates of cervical intraepithelial neoplasia in large, randomized trials.36, 37 Unfortunately, minimal information exists regarding the efficacy of these vaccines against OPSCC. However, a recent trial revealed a significant decrease in oral HPV infection in women receiving the vaccine versus control.38 This trial, coupled with the fact that greater than 90% of HPV-positive OPSCCs are due to HPV16 and 18, suggests both vaccines could effectively prevent OPSCC.
As of 2012 both vaccines were licensed in over 100 countries, and HPV vaccination had been incorporated into the national immunization programs of more than 40 countries.39 In the majority of these countries vaccine recommendations apply solely to females. Based on our detailed literature review, it appears Australia,40 Canada41 and the United States42 are the only countries with recommendations for vaccinating males. Only the United States has documented the vaccine coverage for young men, and in 2010 1.4% of males aged 13—17 received at least one dose of the vaccine.43 Although this rose to 20.8% by 2012, only 6.8% of these males completed the recommended three dose vaccine series.35 The high worldwide prevalence of HPV-positive OPSCC combined with likely protection conferred by vaccination against this and other HPV-related diseases should encourage all countries to implement and promote vaccination programs for both men and women.
We acknowledge several important limitations to this work. It is possible we have introduced publication bias since we focused solely on published literature. Moreover, our data was collected from a heterogeneous population of studies derived from different countries. There existed variability in the anatomic regions the authors evaluated as OPSCCs in their respective studies, which could have lead to differences in the HPV prevalence values reported as a result of the original study design. Since patients with HPV-positive OPSCCs carry unique clinical and demographic characteristics, it is possible that our data is impacted by the fact that most of the studies we evaluated were drawn from academic medical centers, which could lead to an enriched dataset. As can be appreciated from Supplemental Table S3, the number of articles and patients varied across countries. This resulted in the majority of articles deriving from North America (specifically the United States and Canada) and Europe (mainly Western Europe), with much lower representation from countries in Asia, Australia, South America and Central America and no information from Africa. Based on this fact, it is important to understand that the data for the worldwide articles is driven primarily by the North America and Europe data. Certainly, it would be important moving forward for research groups in other regions of the world to evaluate the proportion of HPV-positive OPSCCs in their respective country to obtain a more accurate depiction of the prevalence of this cancer worldwide. The majority of articles retrospectively analyzed their OPSCC specimens from a multi-year period without providing year-by-year results, so we utilized the median year of each article's sample collection period in order to separate the data from each article into distinct time frames. It is possible this methodology could have impacted our results depending on the number of OPSCCs that each article analyzed from earlier versus later years as well as the range of years from which the samples were derived. Lastly, each article employed different criteria for patient inclusion/exclusion and utilized different detection methods for defining HPV-positivity.
Indeed the wide array of available HPV detection methods warrants discussion of the advantages and disadvantages of certain approaches. In this systematic review, the majority of studies employed HPV DNA PCR-based methods. Multiple primer sets exist for PCR such as L1 consensus primers that detect numerous HPV types, type specific primers for high-risk HPVs, and commercial kits targeting a wide range of HPV sequences. In general, PCR is highly sensitive for detecting HPV DNA, but it cannot determine if the HPV originated in tumor cells versus adjacent non-tumor tissue. ISH represents the second most frequently used method in our cohort of articles. Multiple probes are available for ISH including the Ventana INFORM HPV ISH assay (detects 12 high risk HPVs) and HPV16 specific probes. In contrast to PCR, ISH reveals the cellular localization of the HPV. An important shortcoming of PCR and ISH-based techniques is they solely demonstrate the presence of HPV without determining viral activity. One clinically validated method that identifies OPSCCs with transcriptionally active HPV is p16 IHC.44-46 p16 is inhibited in vivo by the tumor suppressor retinoblastoma (Rb). Classically, the described mechanism of p16 overexpression in HPV-positive cancers has been attributed to one of HPV's oncogenes, E7, degrading Rb and thereby releasing p16 from its negative regulator.47 However, McLaughlin-Drubin et al. have also shown that p16 overexpression arises independently from Rb degradation in HPV-positive cervical carcinoma cell lines.48 Recently, a two-step approach combining a highly sensitive test (p16 IHC) with a specific one (HPV DNA ISH) has demonstrated efficacy in identifying HPV-positive tumors.49, 50 Other molecular methods for identifying HPV-positive cancers include ones directly identifying HPV oncogenes E6 and E7, such as the newly validated E6/E7 RNA ISH assay.51, 52 Indeed the diversity among methods for determining HPV-positivity demonstrates the need for a consensus committee to review the current techniques and develop recommendations for clinicians and scientists regarding best practices for identifying HPV-positive cancers. We believe moving forward that validated algorithms should demonstrate both the transcriptional activity and presence of HPV within tumor cells.
Considering the diversity of available detection methods, it would have been interesting to evaluate and compare HPV-positivity in OPSCC over time by detection method. Unfortunately, the number of articles utilizing methods other than PCR was too small to stratify our data by detection method. Moreover, numerous different primer pairs were utilized for PCR testing by these studies, further limiting the ability to perform detection specific analyses. In this manner, the most significant limitation of our study is that the results from all detection methods from the different articles were combined into a single analysis, and the majority of studies employed PCR based methods, which as described above only demonstrates HPV presence and not activity. Importantly, Ndiaye et al. revealed that approximately 87% of HPV DNA PCR positive OPSCCs were also positive for either p16 or E6/E7 mRNA.19 Owing to the high level of correlation between these different tests in OPSCCs, our results should still maintain the temporal relationships demonstrated even if the articles had employed p16 or E6/E7 mRNA in combination with (or in place of) PCR.
In this study, we report the results of a comprehensive systematic review analyzing all published literature through January 2014 reporting on the prevalence of HPV in OPSCC. As expected, we demonstrated a significant increase in the prevalence of HPV-positive OPSCCs over time worldwide, which is consistent with HPV playing an important role in the observed increases in OPSCC incidence in recent years. Surprisingly, we also showed that HPV-positive OPSCCs have reached an apparent plateau in North America over the past decade while the prevalence in Europe continues to rise steadily. Certainly, further data is needed to determine if these trends truly manifest themselves moving forward, but this information could have important implications regarding future predictions related to the incidence of OPSCC.
Supplementary Material
Acknowledgments
We acknowledge Drs. Anil Chaturvedi, James Lewis, Maura Gillison, Francis Worden, James Rocco, Anthony Nichols, Bernard Fortin, Denis Soulieres, John Field, Jens Klussman, Aldo Venuti, Peter Snijders, Ruud Brakenhoff, Barbara Rose, Angela Hong, Rekha Kumar, Hiroyuki Mineta and Kiyoshi Misawa for corresponding with us about their articles and providing additional information for our systematic review.
This study was supported by grants from the NIH (CA022443 and CA160639) and the University of Wisconsin Carbone Cancer Center/Wisconsin Institutes of Discovery/Morgridge Institute.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Chaturvedi AK, Engels EA, Anderson WF, et al. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26:612–9. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 3.Forte T, Niu J, Lockwood GA, et al. Incidence trends in head and neck cancers and human papillomavirus (HPV)-associated oropharyngeal cancer in Canada, 1992-2009. Cancer Causes Control. 2012;23:1343–8. doi: 10.1007/s10552-012-0013-z. [DOI] [PubMed] [Google Scholar]
- 4.Braakhuis BJ, Visser O, Leemans CR. Oral and oropharyngeal cancer in The Netherlands between 1989 and 2006: Increasing incidence, but not in young adults. Oral Oncol. 2009;45:e85–9. doi: 10.1016/j.oraloncology.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Hammarstedt L, Dahlstrand H, Lindquist D, et al. The incidence of tonsillar cancer in Sweden is increasing. Acta Otolaryngol. 2007;127:988–92. doi: 10.1080/00016480601110170. [DOI] [PubMed] [Google Scholar]
- 6.Reddy VM, Cundall-Curry D, Bridger MW. Trends in the incidence rates of tonsil and base of tongue cancer in England, 1985-2006. Ann R Coll Surg Engl. 2010;92:655–9. doi: 10.1308/003588410X12699663904871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hocking JS, Stein A, Conway EL, et al. Head and neck cancer in Australia between 1982 and 2005 show increasing incidence of potentially HPV-associated oropharyngeal cancers. Br J Cancer. 2011;104:886–91. doi: 10.1038/sj.bjc.6606091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaturvedi AK, Anderson WF, Lortet-Tieulent J, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol. 2013;31:4550–9. doi: 10.1200/JCO.2013.50.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans: Human papillomaviruses. IARC Monogr Eval Carcinog Risks Hum. 2007;90:637. [PMC free article] [PubMed] [Google Scholar]
- 10.Rietbergen MM, Leemans CR, Bloemena E, et al. Increasing prevalence rates of HPV attributable oropharyngeal squamous cell carcinomas in the Netherlands as assessed by a validated test algorithm. Int J Cancer. 2013;132:1565–71. doi: 10.1002/ijc.27821. [DOI] [PubMed] [Google Scholar]
- 11.Hong AM, Grulich AE, Jones D, et al. Squamous cell carcinoma of the oropharynx in Australian males induced by human papillomavirus vaccine targets. Vaccine. 2010;28:3269–72. doi: 10.1016/j.vaccine.2010.02.098. [DOI] [PubMed] [Google Scholar]
- 12.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nichols AC, Palma DA, Dhaliwal SS, et al. The epidemic of human papillomavirus and oropharyngeal cancer in a Canadian population. Curr Oncol. 2013;20:212–9. doi: 10.3747/co.20.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avnstorp MB, Jensen RG, Garnaes E, et al. Human papillomavirus and oropharyngeal cancer in Greenland in 1994-2010. Int J Circumpolar Health. 2013;72:22386. doi: 10.3402/ijch.v72i0.22386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schache AG, Liloglou T, Risk JM, et al. Evaluation of human papilloma virus diagnostic testing in oropharyngeal squamous cell carcinoma: sensitivity, specificity, and prognostic discrimination. Clin Cancer Res. 2011;17:6262–71. doi: 10.1158/1078-0432.CCR-11-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hannisdal K, Schjolberg A, De Angelis PM, et al. Human papillomavirus (HPV)-positive tonsillar carcinomas are frequent and have a favourable prognosis in males in Norway. Acta Otolaryngol. 2010;130:293–9. doi: 10.3109/00016480903071377. [DOI] [PubMed] [Google Scholar]
- 17.Rodrigo JP, Heideman DA, Garcia-Pedrero JM, et al. Time trends in the prevalence of HPV in oropharyngeal squamous cell carcinomas in northern Spain (1990-2009) Int J Cancer. 2014;134:487–92. doi: 10.1002/ijc.28355. [DOI] [PubMed] [Google Scholar]
- 18.Nasman A, Attner P, Hammarstedt L, et al. Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: an epidemic of viral-induced carcinoma? Int J Cancer. 2009;125:362–6. doi: 10.1002/ijc.24339. [DOI] [PubMed] [Google Scholar]
- 19.Ndiaye C, Mena M, Alemany L, et al. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: a systematic review and meta-analysis. The Lancet Oncology. 2014;15:1319–31. doi: 10.1016/S1470-2045(14)70471-1. [DOI] [PubMed] [Google Scholar]
- 20.Mehanna H, Beech T, Nicholson T, et al. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer--systematic review and meta-analysis of trends by time and region. Head Neck. 2013;35:747–55. doi: 10.1002/hed.22015. [DOI] [PubMed] [Google Scholar]
- 21.Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–20. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 22.Panwar A, Batra R, Lydiatt WM, et al. Human papilloma virus positive oropharyngeal squamous cell carcinoma: a growing epidemic. Cancer Treat Rev. 2014;40:215–9. doi: 10.1016/j.ctrv.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stein AP, Saha S, Yu M, et al. Prevalence of Human Papillomavirus in Oropharyngeal Squamous Cell Carcinoma in the United States Across Time. Chem Res Toxicol. 2014 doi: 10.1021/tx500034c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kreimer AR, Clifford GM, Boyle P, et al. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14:467–75. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 26.Nasman A, Nordfors C, Holzhauser S, et al. Incidence of human papillomavirus positive tonsillar and base of tongue carcinoma: a stabilisation of an epidemic of viral induced carcinoma? Eur J Cancer. 2015;51:55–61. doi: 10.1016/j.ejca.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 27.Gillison ML, D'Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100:407–20. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 28.D'Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–56. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 29.Newcomer SF, Udry JR. Oral sex in an adolescent population. Arch Sex Behav. 1985;14:41–6. doi: 10.1007/BF01541351. [DOI] [PubMed] [Google Scholar]
- 30.Billy JO, Tanfer K, Grady WR, et al. The sexual behavior of men in the United States. Fam Plann Perspect. 1993;25:52–60. [PubMed] [Google Scholar]
- 31.D'Souza G, Cullen K, Bowie J, et al. Differences in oral sexual behaviors by gender, age, and race explain observed differences in prevalence of oral human papillomavirus infection. PLoS One. 2014;9:e86023. doi: 10.1371/journal.pone.0086023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gillison ML, Broutian T, Pickard RK, et al. Prevalence of oral HPV infection in the United States, 2009-2010. JAMA. 2012;307:693–703. doi: 10.1001/jama.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Centers for Disease C, Prevention. Cigarette smoking among adults--United States, 2006. MMWR Morb Mortal Wkly Rep. 2007;56:1157–61. [PubMed] [Google Scholar]
- 34.Corsi DJ, Boyle MH, Lear SA, et al. Trends in smoking in Canada from 1950 to 2011: progression of the tobacco epidemic according to socioeconomic status and geography. Cancer Causes Control. 2014;25:45–57. doi: 10.1007/s10552-013-0307-9. [DOI] [PubMed] [Google Scholar]
- 35.Centers for Disease C, Prevention. National and state vaccination coverage among adolescents aged 13-17 years--United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:685–93. [PMC free article] [PubMed] [Google Scholar]
- 36.Paavonen J, Naud P, Salmeron J, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301–14. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 37.Group FIS. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915–27. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 38.Herrero R, Quint W, Hildesheim A, et al. Reduced prevalence of oral human papillomavirus (HPV) 4 years after bivalent HPV vaccination in a randomized clinical trial in Costa Rica. PLoS One. 2013;8:e68329. doi: 10.1371/journal.pone.0068329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Markowitz LE, Tsu V, Deeks SL, et al. Human papillomavirus vaccine introduction--the first five years. Vaccine. 2012;30(Suppl 5):F139–48. doi: 10.1016/j.vaccine.2012.05.039. [DOI] [PubMed] [Google Scholar]
- 40.Australian Government Department of Health: Immunise Australia Program. 2014 [Google Scholar]
- 41.Public Health Agency of Canada: Update On Human Papillomavirus (HPV) Vaccines. 2012 [Google Scholar]
- 42.Centers for Disease C, Prevention. Recommendations on the use of quadrivalent human papillomavirus vaccine in males--Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1705–8. [PubMed] [Google Scholar]
- 43.Centers for Disease C, Prevention. National and state vaccination coverage among adolescents aged 13 through 17 years--United States, 2010. MMWR Morb Mortal Wkly Rep. 2011;60:1117–23. [PubMed] [Google Scholar]
- 44.Dok R, Kalev P, Van Limbergen EJ, et al. p16INK4a Impairs Homologous Recombination-Mediated DNA Repair in Human Papillomavirus-Positive Head and Neck Tumors. Cancer Res. 2014;74:1739–51. doi: 10.1158/0008-5472.CAN-13-2479. [DOI] [PubMed] [Google Scholar]
- 45.Hoffmann M, Ihloff AS, Gorogh T, et al. p16(INK4a) overexpression predicts translational active human papillomavirus infection in tonsillar cancer. Int J Cancer. 2010;127:1595–602. doi: 10.1002/ijc.25174. [DOI] [PubMed] [Google Scholar]
- 46.El-Naggar AK, Westra WH. p16 expression as a surrogate marker for HPV-related oropharyngeal carcinoma: a guide for interpretative relevance and consistency. Head Neck. 2012;34:459–61. doi: 10.1002/hed.21974. [DOI] [PubMed] [Google Scholar]
- 47.Klaes R, Friedrich T, Spitkovsky D, et al. Overexpression of p16(INK4A) as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri. Int J Cancer. 2001;92:276–84. doi: 10.1002/ijc.1174. [DOI] [PubMed] [Google Scholar]
- 48.McLaughlin-Drubin ME, Park D, Munger K. Tumor suppressor p16INK4A is necessary for survival of cervical carcinoma cell lines. Proc Natl Acad Sci U S A. 2013;110:16175–80. doi: 10.1073/pnas.1310432110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singhi AD, Westra WH. Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer. 2010;116:2166–73. doi: 10.1002/cncr.25033. [DOI] [PubMed] [Google Scholar]
- 50.Jordan RC, Lingen MW, Perez-Ordonez B, et al. Validation of methods for oropharyngeal cancer HPV status determination in US cooperative group trials. Am J Surg Pathol. 2012;36:945–54. doi: 10.1097/PAS.0b013e318253a2d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ukpo OC, Flanagan JJ, Ma XJ, et al. High-risk human papillomavirus E6/E7 mRNA detection by a novel in situ hybridization assay strongly correlates with p16 expression and patient outcomes in oropharyngeal squamous cell carcinoma. Am J Surg Pathol. 2011;35:1343–50. doi: 10.1097/PAS.0b013e318220e59d. [DOI] [PubMed] [Google Scholar]
- 52.Schache AG, Liloglou T, Risk JM, et al. Validation of a novel diagnostic standard in HPV-positive oropharyngeal squamous cell carcinoma. Br J Cancer. 2013;108:1332–9. doi: 10.1038/bjc.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.