Abstract
Each year, millions of individuals undergo cancer surgery that is intended to be curative or at least a necessary component of a curative regimen. Particularly for those patients whose cancer harbors cells that are resistant to chemotherapy or radiation, the extent of surgery often defines whether they will be a survivor or casualty of the disease. For many solid tumor types, the difference in survival between patients who undergo gross total resection and those who have residual bulky disease is often profound. With surgery being central to cancer survivorship, it is stunning how few resources have been invested in improving surgical outcomes, particularly in comparison to chemotherapeutic research and discovery. This article reviews recent advances related to developing targeted fluorescent agents to guide surgeons during cancer removal. The goal of these drugs and devices is to clearly distinguish cancer from normal tissue to improve surgical outcome for cancer patients.
The unmet clinical need
In certain cases, distinguishing between cancer and normal tissue affects not only survival, but quality of life for those who survive. For patients with cancer of the tongue, esophagus, or anus, preserving key normal tissues while completely resecting the cancer impacts the most basic functions of daily life.
In certain brain tumors, complete resection of non-metastatic tumors has significant clinical value resulting in improved survival and reduced requirement for radiation or chemotherapy. In pediatric ependymoma patients where is it possible to achieve gross total resection of posterior fossa lesions, patients experience an 80% event free survival (EFS), and over 90% overall survival at 5 years even without subsequent radiation or chemotherapy [1]. By contrast, children with ependymomas and residual bulky disease post-surgery surgery require chemotherapy and radiation and experience a less than 50% 5 year EFS [1]. The situation is similar in young medulloblastoma patients where gross total resection (GTR) followed by 18 Gy craniospinal radiation experience approximately 75% 5 year EFS. By contrast, patients with incompletely resected medulloblastomas and bulky residual disease require 36 Gy craniospinal radiation and experience only approximately 60% 5 year EFS [2]. The additional radiation and chemotherapy required in these cases frequently results in severe neurocognitive damage and other toxicities [3, 4].
The potential benefit of image guided tumor resection for patients with glioblastoma multiforme (GBM), the most aggressive and common adult brain tumor, is more controversial. Skeptics correctly point out that diffuse invasion of glioma cells occur well beyond any reasonable surgical margin, and can even be found on the contralateral side of the brain at the time of diagnosis. However, it is also well known that surgical resection is the most effective therapy known to increase survival, and several studies have shown a clear improvement in survival with increased percent of resection [5, 6]. Further, fluorescent compounds that mark the tumor intraoperatively, such as 5-ALA, have been used to enhance the percent of tumor resected resulting in improved median survival, but increased deficits [7]. Likewise, multi-institutional trials of pediatric glioma patients show long-term survival of pediatric high-grade glioma patients depends on GTR [8]. Presumably radiation therapy and chemotherapy are more effective for microscopic residual disease than for bulky disease due to the mechanisms of therapy resistance that flourish within the tumor microenvironment.
Limiting the frequency of second surgeries is a key goal of many investigators. Nearly 25% of women who undergo breast conservation surgery for breast cancer [9] and 20 % of patients with prostate cancer [10] learn that the margins of their cancer were positive days to weeks after the surgery has been completed. Gaining insight into whether margins are free of detectable disease in real time during the initial surgery could reduce second surgeries for patients and ultimately result in significant cost savings to healthcare and insurance systems. Similarly, pathologists may be able to use benchtop imaging devices to identify the margins of a tumor that are most likely to be positive for cancer cells, thus focusing their time and effort on high payoff regions rather than potentially missing key regions because there is simply too much tissue to evaluate microscopically. Furthermore, some cancer cells may appear identical or nearly identical to non-neoplastic cells by light microscopy, even with immunohistochemical stains. While it will need to be determined for each probe and each tumor type, it is conceivable that image guided pathology will reveal microscopic disease that is undetectable by current methods.
Informing adjuvant therapy
In some instances, fluorescence imaging for cancer can guide more than surgery. In cases where cancer is thought to be localized to a single location based on CT or MRI scans, fluorescence imaging can illuminate microscopic tumor studding that is below the limit of detection for other modalities. A recent study with fluorescein-labeled folate revealed that surgeons observed an average of seven tumor loci per ovarian cancer patient without the imaging agent and observed an average of 34 tumor loci per patient with fluorescence guidance [11]. Clearly it would be futile to surgically remove dozens of microscopic cancer foci and clearly these patients require adjuvant therapy even if CT scans reveal a single site of disease and surgeons achieve a complete resection of that locus with clean surgical margins.
The National Cancer Institute created a devoted community of investigators that are advancing image guided surgery strategies. The US Food and Drug Administration (FDA) and its European counterpart have issued clear and helpful guidance documents to assist those who intend to create clinical candidates [12–16]. While reporting all progress in this rapidly evolving field is beyond the scope of a single review article, the following sections attempt to provide a high-level overview of progress and considerations related to ligands and devices for image guided resection.
Emerging image guidance modalities
The use of imaging to guide tumor resection encompasses everything from simple white light illumination of a surgical field to the sophisticated neuronavigation systems that enable neurosurgeons to orient the surgical field to a pre-operative MRI scan. Recent innovation in this field, driven by the need for better real-time intraoperative tools, has taken the form of optical, primarily fluorescence, imaging in real or near-real time. Approaches across all surgical oncology indications include systems that measure differences in endogenous tissue properties, and systems that depend on an exogenous imaging agent.
Systems that measure changes in endogenous tissue properties, including autofluorescence, exploit cellular characteristics that may be altered in neoplastic tissue. These include differences in DNA, amino acid, collagen, and glycolipid composition, porphyrin levels, hemoglobin content and saturation, oxidation, cellular architecture, or angiogenesis (for reviews, see [17], [18], and [19]). These changes are inherently subtle, but consistent enough to offer additional information that supports lesion localization and margin assessment. Clinically they have been applied, for example, in bedside margin assessment for breast cancer [18], and in indications such as head & neck cancer [17], esophageal cancer [20], and lung cancer [19]. More recently, mass spectrometry has been applied to analyze lipid profiles in aerosolized samples [21, 22].
Non-targeted imaging agents
The use of an exogenous contrast agent may enhance differences between tumor and non-tumor tissue, and thereby simplify the distinction. Exogenous agents can be either non-targeted or targeted to antigens that are preferentially expressed in tumor, tumor vasculature, or microenvironment. Non-targeted agents provide contrast due general properties of tumors, rather than presence of a specific antigen, and they include unconjugated fluorescent dyes and a subset of nanoparticles. Unconjugated fluorescent dyes such as fluorescein [23], methylene blue [24], or indocyanine green [23, 25] can be delivered locally or systemically, and accumulate in areas of tumor tissue that have disrupted or altered tumor vasculature and drainage.
The metabolic agent 5-aminolevulinic acid (5-ALA) induces production of fluorescent protoporphyrin IX (PpIX) in tumor cells. 5-ALA has been used in a variety of clinical settings, including photochemotherapy in the dermatology setting, detection of bladder tumors following interstitial delivery, and detection of glioma following systemic delivery. A randomized Phase 3 clinical trial in adult glioma showed a significant increase in “complete” resections compared to conventional surgery (65% vs. 36%), with complete resection significantly influencing progression free survival [26]. A prospective comparison with intraoperative MRI has shown that the use of 5-ALA can improve detection of tumor, particularly in infiltrative zones [27]. Unfortunately, many low-grade and pediatric brain tumors do not produce clinically useful fluorescence following 5-ALA administration [28, 29]. Due to its excitation in the ultraviolet and emission in the visible spectrum, visualization of PpIX fluorescence is restricted to within a few millimeters of the tissue surface. Normal brain tissue can obscure 5-ALA – induced fluorescence, in some cases resulting in residual tumor being left behind and repeat surgery [30].
Nanoparticles
A variety of nanoparticle formats are being investigated for fluorescence-guided resection as well as diagnostic imaging. Non-targeted nanoparticles, like unconjugated dyes, can enter and be retained within tumors due to EPR (enhanced permeability and retention), an effect of tumor vasculature that is non-specific and varies with the size and type of lesion. Because they have large surface areas and can be functionalized, nanoparticles can be targeted to antigens as well as carry multiple “payloads,” including fluorescent dyes, radioactive tags for imaging or therapeutic utility, and agents for photodynamic or photothermal therapy. The utility of near-infrared (NIR) fluorescent dyes is well-recognized in the field [31], and many nanoparticle-NIR dye conjugates are being developed. While these conjugates can provide very intense labeling and good contrast, they face significant technical and regulatory hurdles for clinical applications. Nanoparticles intended solely for imaging will have a different risk-benefit profile as compared to those used for therapeutic purposes [32], requiring them to be safer. Early clinical development has been initiated with C-dots, an integrin-targeting nanoparticle that is used for pre-operative PET imaging as well as intraoperative fluorescence imaging [33].
Targeted imaging agents
Other targeted imaging agents have been constructed using dye conjugates with antibodies, peptides, or natural ligands to tumor targets. A number of RGD-containing peptides are being developed to target integrins on tumor vasculature [34]. These probes will highlight tumor vasculature and in some cases tumor cells themselves. Another approach that focuses on the tumor microenvironment uses tumor associated enzymes (e.g., caspase) to cleave imaging pro-drugs into fluorescing metabolites [35, 36]. There have been several preclinical studies and a few clinical trials of approved therapeutic antibodies conjugated to NIR fluorophores; these include cetuximab and panitumimab (EGFR), trastuzumab (HER-2), and bevacizumab (VEGF) [37–39]. Due to their large size, antibody conjugates may penetrate less effectively into tumor tissue compared with small molecule, aptamer, or peptide conjugates. Small molecule conjugates are being developed that target folate receptor alpha and PSMA [40]. As noted above, a folate-FITC conjugate was evaluated for intraoperative imaging during ovarian cancer resection, where fluorescence imaging resulted in identification of peritoneal metastases that were not visible to surgeons using white light alone [11]. While offering high specificity for tumors expressing the targeted antigen, these conjugates are not likely to be broadly applicable across multiple tumor types.
The cationic peptide chlorotoxin is internalized by cancer cells in a wide variety of solid tumor indications [41, 42]. A radiolabeled chlorotoxin peptide validated these findings in humans [43–45]. Furthermore, BLZ-100, a variant of chlorotoxin conjugated to a form of indocyanine green, is able to distinguish very small foci of brain tumor cells from normal brain (Fig 1). Compared to non-targeted agents that rely on disrupted blood brain barrier and EPR, molecules that specifically target cells and are internalized have the potential to distinguish cancer from normal tissue on a near cell by cell basis provided that high resolution detection devices are used.
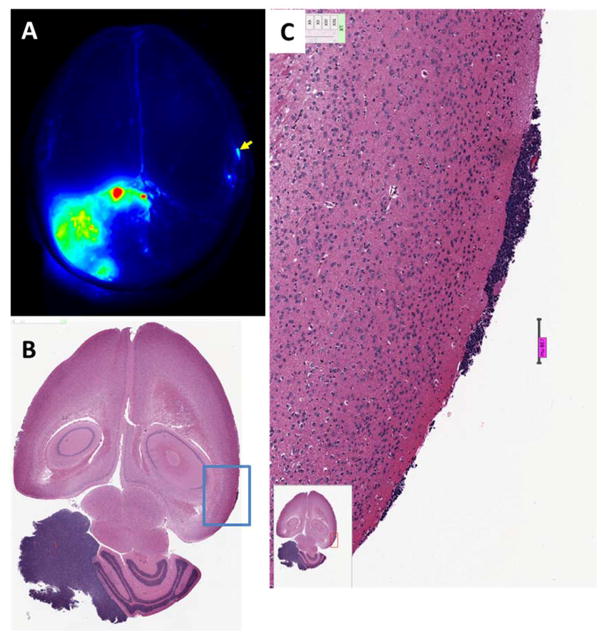
Figure 1. Detection of tumors in a mouse medulloblastoma model using BLZ-100 fluorescence.
(A) Odyssey image of whole brain. Fluorescence from the gross tumor is evident in the lower left quadrant. Signal in tumor was about 20-fold higher than in normal brain. A fluorescent spot that was well above background was noted (arrow). (B) H&E stained sections showed a small tumor lesion in this area of the brain. (C) High resolution image of the area delineated by the blue box in panel B. Scale bar = 138 microns.
Exploiting more general properties of cancer cells is a strategy that may lead to other imaging agents that can be applied to multiple tumor types. Examples of such approaches include a phospholipid ether analog that targets lipid raft structures [46], and probes that are cleaved by extracellular matrix enzymes overexpressed at tumor borders, such as cathepsins and matrix metalloproteinase [47]. One example of this technology is a cell-penetrating peptide that is protected until the probe is cleaved or processed by the matrix metalloproteinase enzymes, after which the peptide penetrates nearby cells [48]. This approach ensures that the activated probe is retained in the tissue surrounding the site of activation, which is important for maintenance of distinct tumor boundary imaging throughout the time frame of a surgery.
Targeting agent considerations
The observation that some fluorescent molecules preferentially distribute to some cancers compared to adjacent tissue was first made over 50 years ago. These agents have not advanced to human clinical use primarily because the disrupted barriers in tumors are heterogeneous, so some regions of cancer are not detected. Also, some non-neoplastic tissues surrounding the tumor non-specifically take up the agents as well. Hence both sensitivity and specificity are problems and these are the two parameters that are critical for providing useful information to surgeons. For this reason, many investigators and clinicians favor targeted agents, particularly those that bind directly to tumor cells with sufficient affinity or avidity that tumor cell rather than interstitial or peri-tumoral fluid is marked by fluorescent signal.
Device considerations
Most new fluorescent imaging agents are being developed with probes that emit in the near infrared (NIR) range because normal tissues have little autofluorescence at these wavelengths and because the light passes through small amounts of tissue that might lie between the camera and the fluorescent tissue (Fig. 2). The devices for detecting NIR fluorescence are evolving rapidly and include endoscopes or exoscopes, surgical microscopes, and robotic systems, most incorporating CCD camera technology [49, 50]. Devices that collect and integrate images over short periods of time may have superior sensitivity compared to microscopes, but devices that can display high-definition images in real time will likely be preferred. For surgical margin evaluation, particularly in deep pockets during tumor debulking, flexible devices that can “see” around corners will provide an advantage over cameras or microscopes that are suspended over the patient.
Figure 2. Imaging BLZ-100 fluorescence in a mouse orthotopic glioma xenograft model.
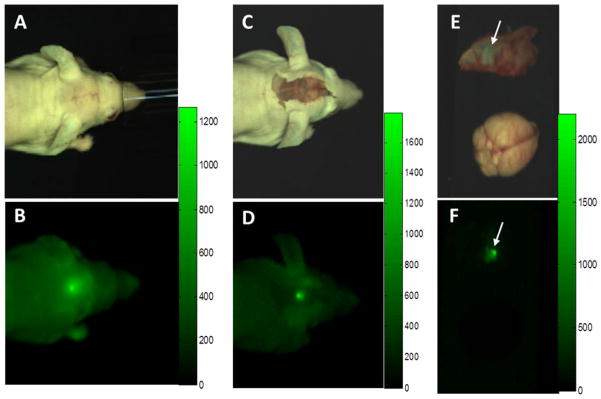
(A, B) White light and NIR images prior to exposure of tumor. Lesion is visible through skin and skull. (C, D) White light and NIR images following removal of overlying skin. Note the increase in brightness, contrast, and sharpness. (E, F) White light and NIR images of fully exposed tumor (which had adhered to the inside of the skull) and normal brain. Fluorescence scale bars are shown to the right of each NIR image. NIR images were captured using a prototype open imaging system [51] by PerkinElmer.
Economic considerations of developing molecular imaging agents
The FDA regulates imaging agents as drugs. Published guidance documents and regulatory precedent focus on safety and the predictive value of the agents for accurately distinguishing cancer from normal tissue. As such, the cost of non-clinical studies, manufacturing, early stage clinical and registration trials will often exceed $100M US dollars regardless of the market potential. Unlike chemotherapeutic agents that are prescribed for months or years, imaging agents are often used only once per patient so the potential to recover research and development costs is challenging.
Targeting molecules that bind to many types of cancer have potential to help more patients than those developed for a narrow subclass of cancer patients and may be more economically viable to develop. That said, there are certain instances in which a target is very highly expressed on a specific type of cancer cell and in which residual microscopic disease cannot be adequately addressed by adjuvant therapy due to inherent resistance to chemotherapy or radiation therapy. In these situations, agents with exceptional tumor to background ratio coupled with exquisitely sensitive devices could benefit patients and still be economically viable.
Conclusion
It seems to be only a matter of time before image guided surgery is commonplace and surgeons will reminisce about trying to distinguish cancer from normal tissue using their eyes, fingers and thumbs. In the meantime, the race is on among device designers and manufacturers. Clinical proof of concept experiments with intriguing molecular imaging agents reveal the potential power of this approach and increasingly candidate agents are being tested in larger numbers of cancer patients (Table 1). The evolution of image guidance will likely be to surgeons and pathologists what the CT or MRI scan was to radiologists. Importantly, these imaging modalities provide new information to clinicians rather than absolute diagnostics. Thus, medical judgment, experience, and expertise will continue to play a key role to minimize both undertreatment and overtreatment of cancer patients following image guided tumor resection.
Table 1.
Selected current clinical trials of fluorescent imaging agents for tumor resection
| Agent | Imaging system | Phase | Indication | # of subjects |
Design | Primary endpoints | NCT number (clinicaltrials.gov) |
|---|---|---|---|---|---|---|---|
| Targeted Agents | |||||||
| Bevacizumab-IRDye800 | MSOT FDOT |
1 | Breast cancer | 30 | Single arm | Uptake in cancer, LN, surrounding tissue | NCT01508572 |
| Bevacizumab-IRDye800 | NIR endoscope | 1 | Esophageal cancer Dysplasia |
10 | Single arm | Fluorescence intensity | NCT02129933 |
| Bevacizumab-IRDye800 | NIR endoscope | 1 | Familial adenomatous polyposis | 15 | Non-randomized (time point groups) | Number of fluorescent adenomatous polyps | NCT02113202 |
| BLZ-100 | Fluobeam 800 | 1 | Glioma | 21 | Single arm | Safety | NCT02234297 |
| BLZ-100 | Fluobeam 800 | 1 | Skin neoplasms | 30 | Single arm | Safety | NCT02097875 |
| Cetuximab-IRDye800 | 1 | Head and neck cancer | 15 | Single arm | Safety | NCT01987375 | |
| cRGDY-PEG-Cy5.5-C dots | Artemis | 0 | Multiple solid tumor types | 30 | Single arm | Feasibility for SLN mapping | NCT02106598 |
| Folate-FITC | 1 | Breast cancer | 10 | Single arm | Feasibility | NCT01994369 | |
| Folate-FITC | 1 | Lung cancer | 48 | Single arm | Feasibility | NCT01778920 | |
| Folate-FITC | 1 | Ovarian cancer | 10 | Single arm | Feasibility | NCT02000778 | |
| Folate-FITC | 0 | Renal cell carcinoma | 10 | Single arm | Feasibility | NCT01778933 | |
| LUM015 | 1 | Sarcoma Breast cancer |
15 | Single arm | Safety, dose | NCT01626066 | |
| MDX1201-A488 | Prostate cancer | 30 | Single arm | Fluorescence intensity | NCT02048150 | ||
| OTL38 | 2 | Ovarian cancer | 45 | Single arm | Safety Tumor to background ratio |
NCT02317705 | |
| Non-Targeted Agents | |||||||
| 5-ALA | 2 | Brain cancer | 120 | Randomized (dose groups) | Fluorescence intensity | NCT00752323 | |
| 5-ALA | 1/2 | Brain cancer | 15 | Single arm | Safety | NCT01128218 | |
| 5-ALA | 2 | Brain cancer | 108 | Randomized (5-ALA vs 5-ALA + iMRI) | Extent of resection | NCT01798771 | |
| 5-ALA | 1 | Brain cancer | 540 | Single arm | Fluorescence correlation with histopathology | NCT02191488 | |
| 5-ALA | 1 | Brain tumors | 234 | Single arm | Correlation of fluorescence with conventional imaging data | NCT00870779 | |
| 5-ALA | PRODIGI | Breast cancer | 45 | Controlled (No agent vs 5-ALA dose groups) | Sensitivity, specificity | NCT01837225 | |
| 5-ALA | 2 | Glioblastoma | 60 | Single arm | Safety in combination with Gliadel wafers | NCT01310868 | |
| 5-ALA | 3 | Glioblastoma | 204 | Randomized (5-ALA vs placebo) | Rate of complete resections | NCT01811121 | |
| 5-ALA | Modified neurosurgical microscope | 2 | Glioma | 300 | Single arm | Efficacy | NCT01116661 |
| 5-ALA | 2 | Glioma | 83 | Single arm | Extent of resection | NCT01445691 | |
| 5-ALA | Confocal microscopy | 3 | Glioma | 192 | Randomized (5-ALA vs placebo) | Extent of resection | NCT01502280 |
| 5-ALA | 1/2 | Pediatric CNS tumors | 100 | Single arm | Safety Sensitivity |
NCT02050243 | |
| 5-ALA | Recurrent glioma | 60 | Single arm | Fluorescence in tumor Comparison with pathology |
NCT02119338 | ||
| Fluorescein | CellVizio | Rectal cancer | 70 | Single arm | Margin identification Concordance with histopathology |
NCT01887509 | |
| ICG | 0 | Breast cancer | 10 | Single arm | Proportion of tumors identified by ICG uptake | NCT01796041 | |
| ICG | PDE | 2 | Breast cancer | 20 | Single arm | Fluorescence in tumor | NCT02027818 |
| ICG | PDE | 2 | Breast cancer | 20 | Single arm | Fluorescence intensity following neoadjuvant therapy | NCT02032563 |
| ICG | 1/2 | Breast cancer | 20 | Single arm | Non-palpable lesion localization rate | NCT02172989 | |
| ICG | PDE | 2 | Colorectal cancer peritoneal carcinomatosis | 10 | Single arm | Fluorescence contrast | NCT02032485 |
| ICG | 2 | Head and neck cancer | 10 | Single arm | Fluorescence in tumor and cervical LN | NCT02027831 | |
| ICG | Fluobeam 800 | 1/2 | Liver cancer | 46 | Single arm | Feasibility Rate of lesion detection |
NCT01738217 |
| ICG | 1 | Lung cancer | 10 | Single arm | Feasibility | NCT01335893 | |
| ICG | SPY thoracoscope | 2 | Lung cancer | 20 | Single arm | Safety and feasibility | NCT02090660 |
| ICG | 3 | Prostate cancer | 37 | Single arm | Sensitivity compared to histopathology | NCT02260349 | |
| ICG | da Vinci | 1 | Renal cell carcinoma | 50 | Single arm | Dose finding | NCT01281488 |
| ICG | Solid tumor | 50 | Single arm | Contrast ratio | NCT01884584 | ||
| ICG | 1 | Solid tumor | 48 | Single arm | Detection by imaging system | NCT02280954 | |
Acknowledgments
This work was supported by NIH contract HHSN261201200054C and NIH grant R01CA135491.
Contributor Information
Julia Parrish-Novak, Email: Julie.Novak@blazebioscience.com, VP of Research and Project Management, Blaze Bioscience, Inc., 530 Fairview Ave N, Suite 1400, Seattle, WA 98109, Office (206) 535-8144, Fax (206) 257-5924.
Eric C. Holland, Email: eholland@fredhutch.org, Senior VP and Director, Human Biology Division, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, Mailstop C3-168, Seattle, WA 98109, Office (206) 667-6117, Fax (206) 667-6022.
James M. Olson, Email: jolson@fredhutch.org, Member, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, Mailstop D4-100, Seattle, WA 98109, Office (206) 667-7955, Fax (206) 667-2917.
References
- 1.Krieger MD, I, Bowen E. Effects of surgical resection and adjuvant therapy on pediatric intracranial ependymomas. Expert Rev Neurother. 2005;5(4):465–71. doi: 10.1586/14737175.5.4.465. [DOI] [PubMed] [Google Scholar]
- 2.Johnston DL, et al. Medulloblastoma in children under the age of three years: a retrospective Canadian review. J Neurooncol. 2009;94(1):51–6. doi: 10.1007/s11060-009-9799-2. [DOI] [PubMed] [Google Scholar]
- 3.Mulhern RK, et al. Neurocognitive consequences of risk-adapted therapy for childhood medulloblastoma. J Clin Oncol. 2005;23(24):5511–9. doi: 10.1200/JCO.2005.00.703. [DOI] [PubMed] [Google Scholar]
- 4.Moxon-Emre I, et al. Impact of craniospinal dose, boost volume, and neurologic complications on intellectual outcome in patients with medulloblastoma. J Clin Oncol. 2014;32(17):1760–8. doi: 10.1200/JCO.2013.52.3290. [DOI] [PubMed] [Google Scholar]
- 5.Lacroix M, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95(2):190–8. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- 6.Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;62(4):753–64. doi: 10.1227/01.neu.0000318159.21731.cf. discussion 264–6. [DOI] [PubMed] [Google Scholar]
- 7.Zhao S, et al. Intraoperative fluorescence-guided resection of high-grade malignant gliomas using 5-aminolevulinic acid-induced porphyrins: a systematic review and meta-analysis of prospective studies. PLoS One. 2013;8(5):e63682. doi: 10.1371/journal.pone.0063682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wisoff JH, et al. Current neurosurgical management and the impact of the extent of resection in the treatment of malignant gliomas of childhood: a report of the Children’s Cancer Group trial no. CCG-945. J Neurosurg. 1998;89(1):52–9. doi: 10.3171/jns.1998.89.1.0052. [DOI] [PubMed] [Google Scholar]
- 9.Wilke LG, et al. Repeat surgery after breast conservation for the treatment of stage 0 to II breast carcinoma: a report from the National Cancer Data Base, 2004–2010. JAMA Surg. 2014;149(12):1296–305. doi: 10.1001/jamasurg.2014.926. [DOI] [PubMed] [Google Scholar]
- 10.Ramsay C, et al. Systematic review and economic modelling of the relative clinical benefit and cost-effectiveness of laparoscopic surgery and robotic surgery for removal of the prostate in men with localised prostate cancer. Health Technol Assess. 2012;16(41):1–313. doi: 10.3310/hta16410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Dam GM, et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-alpha targeting: first in-human results. Nat Med. 2011;17(10):1315–9. doi: 10.1038/nm.2472. [DOI] [PubMed] [Google Scholar]
- 12.Services, U.S.D.o.H.a.H., . Developing Medical Imaging Drug and Biological Products; Part 2: Clinical Indications. Clinical Medical. 2004 [Google Scholar]
- 13.Services, U.S.D.o.H.a.H., . Developing Medical Imaging Drug and Biological Products; Part 3: Design, Analysis, and Interpretation of Clinical Studies. Clinical Medical. 2004 [Google Scholar]
- 14.Services, U.S.D.o.H.a.H., . Developing Medical Imaging Drug and Biological Products; Part 1: Conducting Safety Assessments. Clinical Medical. 2004 [Google Scholar]
- 15.Use, C.f.M.P.f.H. Pre-Authorisation Evaluation of Medicines for Human Use; Guideline on Clinical Evaluation of Diagnostic Agents. European Medicines Agency; 2009. [Google Scholar]
- 16.Use, C.f.M.P.f.H. Pre-Authorisation Evaluation of Medicines for Human Use; Appendix 1 to the Guideline on Clinical Evaluation of Diagnostic Agents. European Medicines Agency; 2009. [Google Scholar]
- 17.Green B, et al. Optical diagnostic techniques for use in lesions of the head and neck: review of the latest developments. Br J Oral Maxillofac Surg. 2014;52(8):675–80. doi: 10.1016/j.bjoms.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Thill M, Baumann K, Barinoff J. Intraoperative assessment of margins in breast conservative surgery--still in use? J Surg Oncol. 2014;110(1):15–20. doi: 10.1002/jso.23634. [DOI] [PubMed] [Google Scholar]
- 19.Nakajima T, Yasufuku K. Early lung cancer: methods for detection. Clin Chest Med. 2013;34(3):373–83. doi: 10.1016/j.ccm.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi M, et al. Endoscopic diagnosis of early neoplasia of the esophagus with narrow band imaging: correlations among background coloration and iodine staining findings. J Gastroenterol Hepatol. 2014;29(4):762–8. doi: 10.1111/jgh.12477. [DOI] [PubMed] [Google Scholar]
- 21.Balog J, et al. Intraoperative tissue identification using rapid evaporative ionization mass spectrometry. Sci Transl Med. 2013;5(194):194ra93. doi: 10.1126/scitranslmed.3005623. [DOI] [PubMed] [Google Scholar]
- 22.Eberlin LS, et al. Molecular assessment of surgical-resection margins of gastric cancer by mass-spectrometric imaging. Proc Natl Acad Sci U S A. 2014;111(7):2436–41. doi: 10.1073/pnas.1400274111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zehri AH, et al. Neurosurgical confocal endomicroscopy: A review of contrast agents, confocal systems, and future imaging modalities. Surg Neurol Int. 2014;5:60. doi: 10.4103/2152-7806.131638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tummers QR, et al. Real-time intraoperative detection of breast cancer using near-infrared fluorescence imaging and Methylene Blue. Eur J Surg Oncol. 2014;40(7):850–8. doi: 10.1016/j.ejso.2014.02.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schaafsma BE, et al. The clinical use of indocyanine green as a near-infrared fluorescent contrast agent for image-guided oncologic surgery. J Surg Oncol. 2011;104(3):323–32. doi: 10.1002/jso.21943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stummer W, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncology. 2006;7:392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- 27.Coburger J, et al. Tumor detection with 5-aminolevulinic acid fluorescence and Gd-DTPA-enhanced intraoperative MRI at the border of contrast-enhancing lesions: a prospective study based on histopathological assessment. Neurosurg Focus. 2014;36(2):E3. doi: 10.3171/2013.11.FOCUS13463. [DOI] [PubMed] [Google Scholar]
- 28.Marbacher S, et al. Use of fluorescence to guide resection or biopsy of primary brain tumors and brain metastases. Neurosurg Focus. 2014;36(2):E10. doi: 10.3171/2013.12.FOCUS13464. [DOI] [PubMed] [Google Scholar]
- 29.Stummer W, et al. Predicting the “usefulness” of 5-ALA-derived tumor fluorescence for fluorescence-guided resections in pediatric brain tumors: a European survey. Acta Neurochir (Wien) 2014;156(12):2315–24. doi: 10.1007/s00701-014-2234-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schucht P, et al. Early re-do surgery for glioblastoma is a feasible and safe strategy to achieve complete resection of enhancing tumor. PLoS One. 2013;8(11):e79846. doi: 10.1371/journal.pone.0079846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vahrmeijer AL, et al. Image-guided cancer surgery using near-infrared fluorescence. Nat Rev Clin Oncol. 2013;10(9):507–18. doi: 10.1038/nrclinonc.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Meel R, et al. Ligand-targeted particulate nanomedicines undergoing clinical evaluation: current status. Adv Drug Deliv Rev. 2013;65(10):1284–98. doi: 10.1016/j.addr.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 33.Phillips E, et al. Clinical translation of an ultrasmall inorganic optical-PET imaging nanoparticle probe. Sci Transl Med. 2014;6(260):260ra149. doi: 10.1126/scitranslmed.3009524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Danhier F, Le Breton A, Preat V. RGD-based strategies to target alpha(v) beta(3) integrin in cancer therapy and diagnosis. Mol Pharm. 2012;9(11):2961–73. doi: 10.1021/mp3002733. [DOI] [PubMed] [Google Scholar]
- 35.Johnson JR, et al. Caspase-activated cell-penetrating peptides reveal temporal coupling between endosomal release and apoptosis in an RGC-5 cell model. Bioconjug Chem. 2012;23(9):1783–93. doi: 10.1021/bc300036z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hensley H, et al. Evaluating new therapies in gastrointestinal stromal tumor using in vivo molecular optical imaging. Cancer Biol Ther. 2014;15(7):911–8. doi: 10.4161/cbt.28880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Day KE, et al. Fluorescently labeled therapeutic antibodies for detection of microscopic melanoma. Laryngoscope. 2013;123(11):2681–9. doi: 10.1002/lary.24102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heath CH, et al. Use of panitumumab-IRDye800 to image microscopic head and neck cancer in an orthotopic surgical model. Ann Surg Oncol. 2012;19(12):3879–87. doi: 10.1245/s10434-012-2435-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang RE, et al. Antibody-based imaging of HER-2: moving into the clinic. Curr Mol Med. 2013;13(10):1523–37. doi: 10.2174/1566524013666131111120951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelderhouse LE, et al. Development of tumor-targeted near infrared probes for fluorescence guided surgery. Bioconjug Chem. 2013;24(6):1075–80. doi: 10.1021/bc400131a. [DOI] [PubMed] [Google Scholar]
- 41.Veiseh M, et al. Tumor Paint: A chlorotoxin:Cy5.5 bioconjugate for intraoperative visualization of cancer foci. Cancer Research. 2007;67(14):6882–6888. doi: 10.1158/0008-5472.CAN-06-3948. [DOI] [PubMed] [Google Scholar]
- 42.Stroud MR, Hansen SJ, Olson JM. In vivo bio-imaging using chlorotoxin-based conjugates. Current Pharmaceutical Design. 2011;17(38):4362–4371. doi: 10.2174/138161211798999375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mamelak AN, et al. Phase I single-dose study of intracavitary-administered iodine-131-TM-601 in adults with recurrent high-grade glioma. J Clin Oncol. 2006;24(22):3644–50. doi: 10.1200/JCO.2005.05.4569. [DOI] [PubMed] [Google Scholar]
- 44.Hockaday DC, et al. Imaging glioma extent with 131I-TM-601. The Journal of Nuclear Medicine. 2005;46(4):580–586. [PubMed] [Google Scholar]
- 45.Gribbin T, et al. A phase I evaluation of intravenous (IV) ^131 I-chlorotoxin delivery to solid peripheral and intracranial tumors. J Clin Oncol. 2009;27(suppl):abstr e14507. [Google Scholar]
- 46.Deming DA, et al. Phospholipid ether analogs for the detection of colorectal tumors. PLoS One. 2014;9(10):e109668. doi: 10.1371/journal.pone.0109668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bu L, Shen B, Cheng Z. Fluorescent imaging of cancerous tissues for targeted surgery. Adv Drug Deliv Rev. 2014;76:21–38. doi: 10.1016/j.addr.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hussain T, Nguyen QT. Molecular imaging for cancer diagnosis and surgery. Adv Drug Deliv Rev. 2014;66:90–100. doi: 10.1016/j.addr.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chi C, et al. Intraoperative imaging-guided cancer surgery: from current fluorescence molecular imaging methods to future multi-modality imaging technology. Theranostics. 2014;4(11):1072–84. doi: 10.7150/thno.9899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Butte PV, et al. Near-infrared imaging of brain tumors using the Tumor Paint BLZ-100 to achieve near-complete resection of brain tumors. Neurosurgical Focus. 2014;36(2):E1. doi: 10.3171/2013.11.FOCUS13497. [DOI] [PubMed] [Google Scholar]
- 51.Konecky SD, et al. Advanced fluorescence imaging system for clinical translation. Mol Imaging Biol. 2013;15(1 Suppl):S984–S985. [Google Scholar]