Abstract
Purpose of review
A feature of the innate immune response that is conserved across kingdoms is the induction of cell death. In this review, we discuss the direct and indirect effects of increased inflammatory cell death, including pyroptosis, a caspase-1-dependent cell death, and necroptosis, a RIPK3/MLKL-dependent, caspase-independent cell death, on emergency hematopoiesis.
Recent findings
Activation of non-apoptotic cell death pathways during infection can trigger release of cytokines and/or damage-associated molecular patterns (DAMPs) such as IL-1α, IL-1β, IL-18, IL-33, HMGB1 and mtDNA to promote emergency hematopoiesis. During systemic infection, pyroptosis and necroptosis can directly kill hematopoietic stem and progenitor cells, which results in impaired hematopoiesis, cytopenia and immunosuppression. Although originally described as discrete entities, there now appears to be more intimate connections between the non-apoptotic and death receptor signaling pathways.
Summary
The choice to undergo pyroptotic and necroptotic cell death constitutes a rapid response system serving to eliminate infected cells, including hematopoietic stem and progenitor cells. This system has the potential to be detrimental to emergency hematopoiesis during severe infection. We discuss the potential of pharmacological intervention for the pyroptosis and necroptosis pathways that may be beneficial during periods of infection and emergency hematopoiesis.
Keywords: pyroptosis, necroptosis, emergency hematopoiesis, hematopoietic stem and progenitor cells, inflammation
Introduction
In the battle of microbes and mammalian cells, the fight or flight (die) decision is emerging as a central factor contributing to resistance to infection. Since the work of Bradley and Metcalf in the 1960’s on colony-stimulating factors that regulate hematopoiesis [1,2], we have gained a deep understanding of soluble factors and downstream signaling pathways controlling hematopoiesis at steady state and following infection. Changes in the induction of inflammatory cytokines and hematopoietic growth factors in response to microbes has been intensively studied as a mechanism to explain abnormal responses to infection and the development of immune suppression. Despite this effort, few immunological studies explain the common occurrence of multi-lineage suppression of hematopoiesis in patients with life-threatening systemic infections.
A hematological hallmark of septic shock patients is peripheral blood cytopenia [3]. This persistent cytopenia commonly affects myeloid, lymphoid and erythroid lineages resulting in immunosuppression and is a key prognostic indicator for survival. The Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) network revealed that non-survivors of influenza infection appear to have defective emergency hematopoiesis and were therefore profoundly pan-cytopenic and had frequently developed a bacterial superinfection [4]. However, a convincing mechanism to explain this failure of emergency hematopoiesis has not been proposed. Numerous viral and bacterial pathogens, including HIV, LCMV, cytomegalovirus, human herpesvirus-6, human herpesvirus-7, vaccinia virus (VACV), Bartonella and parvovirus B19 are known to infect hematopoietic stem and progenitor cells (HSPC) and in some cases, remain dormant in HSPC [5–12]. Recently, it was revealed that abortive HIV infection of T cells induces a caspase-1-dependent cell death, known as pyroptosis [13,14]. HIV infection can infect hematopoietic progenitor cells and induce cytopenia, and numerous studies demonstrate that infection of CD34+ HSPC with HIV induces cell death and impairs reconstitution in humanized mouse models [6,11,15–17].
One possibility to explain defects in emergency hematopoiesis during systemic infection is the inappropriate activation of cell death, a hypothesis proposed by Hotchkiss and colleagues in 1999 using data collected from mice and humans [18,19]. Alternatively, suppression of hematopoietic stem and progenitor cell proliferation, differentiation and self-renewal can also explain these clinical syndromes. Recent findings demonstrating that hematopoietic progenitor cells drive hematopoiesis at steady state, rather than long-term HSC, suggest that the response of the progenitor cell compartment to intracellular infection and inflammatory cytokines may be central to an effective immune response [20•,21•].
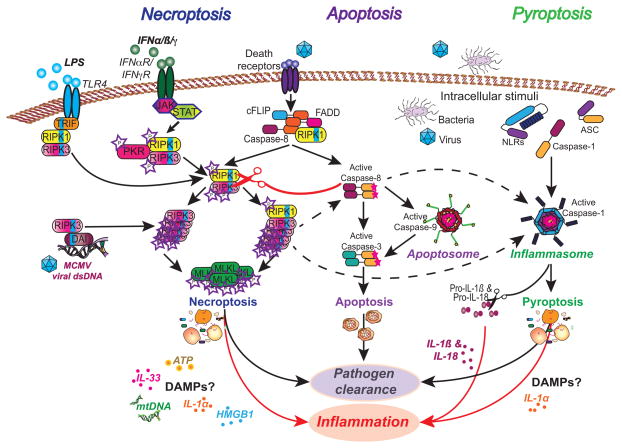
Since 1972, apoptotic and necrotic cell death has dominated the literature as two forms of cell death with distinct effects on the immune system [22]. The discovery of genes regulating apoptosis, most notably Bcl-2 [23], has driven major scientific and clinical advances in the field of cell death. Alternative non-apoptotic modes of programmed cell death have been recently recognized to exist, including pyroptosis, a caspase-1-dependent cell death, and necroptosis, a RIPK3/MLKL-dependent, caspase-independent cell death (Figure 1).
Fig. 1. Apoptosis and the inflammatory cell death pathways, pyroptosis and necroptosis.
Non-apoptotic cell death pathways can be triggered by numerous factors including inflammasome activation, death receptor ligation and intracellular pathogens. When caspase activity is blocked, TLR ligation and a cytoplasmic complex comprising RIPK1 can drive necroptotic death. Phosphorylation and oligomerization of RIPK3 activates necroptosis by inducing phosphorylation of MLKL and its translocation to the cell membrane. The cellular contents released from necroptotic cells can serve as DAMPs to further induce inflammation. Pyroptosis is induced by the formation of the inflammasome complex to induce caspase-1 activation and IL-1β and IL-18 maturation. Pathogen clearance and inflammation must be balanced to prevent a chronic inflammatory response.
There are a multitude of intracellular proteins acting as cellular sentinels that monitor for signs of infection. When triggered, they move swiftly to induce the release of inflammatory cytokines and/or to induce an inflammatory form of cell death, both of which can drive emergency hematopoiesis. During pyroptosis or necroptosis, emergency hematopoiesis can be potently influenced by the programmed release of inflammatory cytokines. The release of host-derived damage-associated molecular patterns (DAMPs) such as mitochondrial DNA [24] and HMGB1 [25] further induces cytokine production and influences emergency hematopoiesis [26••,27] (Figure 2). These forms of cell death contrast to the immunologically-silent apoptotic forms of cell death [28]. How cells choose the fight or die option during infection remains enigmatic: is it a binary switch controlling both cytokine production and non-apoptotic cell death? Or does this depend on the cell type and pathway recruited? What are the crucial intracellular targets of these cell death pathways that culminate in the demise of the cell? And what are the specific DAMPs that activate the surrounding immune cells to drive inflammation and emergency hematopoiesis? Here we will focus on the role of inflammatory cell death including pyroptosis and necroptosis as key mechanisms controlling emergency hematopoiesis. We will discuss recent advances that demonstrate that non-apoptotic inflammatory cell death can regulate emergency hematopoiesis.
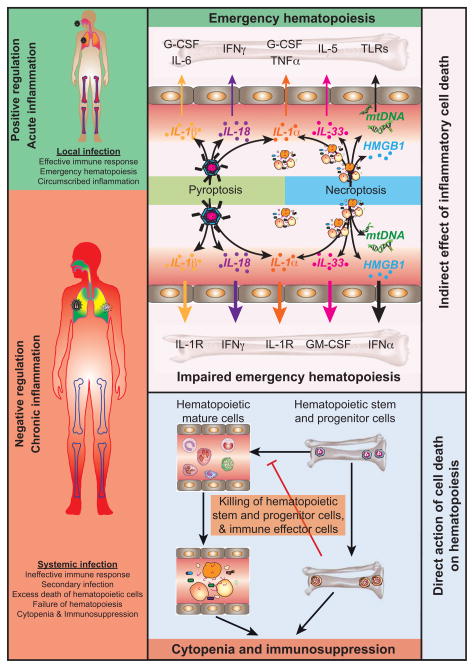
Fig. 2. Direct and indirect effects of pyroptosis and necroptosis on emergency hematopoiesis.
The non-apoptotic and inflammatory forms of cell death, pyroptosis and necroptosis, have direct and indirect effects on emergency hematopoiesis. These modalities of cell death release inflammatory cytokines and DAMPs that stimulate emergency hematopoiesis. In chronic settings, including prolonged periods of infectious stress that may be characterized by secondary infection or systemic infection, these inflammatory factors may impair hematopoiesis. Pyroptosis and necroptosis can also exert a strong influence on hematopoiesis by directly killing HSPC.
Defining the forms of inflammatory cell death
The Nomenclature Committee on Cell Death has prepared guidelines to define different forms of cell death including intrinsic and extrinsic apoptosis, mitotic catastrophe and necroptosis [29•]. Here we will extend the definitions of pyroptosis to include recent data and to acknowledge the significant differences in morphology of pyroptotic cells [30].
The dependence on receptor interacting protein kinase 3 (RIPK3) and the pseudo-kinase mixed lineage kinase domain-like protein (MLKL) provides the best definition of necroptosis [31]. The common use of necrostatin-1 (Nec-1) and necrostatin-1s, chemical inhibitors of RIPK1, to define necroptotic death can be useful, however RIPK1-independent, RIPK3-dependent forms of death are now well recognized [32••,33••,34••]. Furthermore Nec-1 may inhibit inflammatory cell responses that are not linked to cell death [35,36••]. Like apoptosis, where a multitude of inputs and cellular stresses can trigger the apoptotic cascade, necroptosis can be engaged via numerous upstream pathways. These include the ligation of death receptors, Toll-like receptors (TLRs) and intracellular receptors such as DAI [37–39] (Figure 1). Negative regulators are also now well defined: RIPK1, caspase-8, cFLIP and FADD all play key roles in restricting the activity of the necroptotic pathway [32–34,40,41•,42–43]. Combined, these upstream regulators form an important arm of the innate immune system, acting as sentinels for pathogens or other pathophysiological processes that aim to subvert the apoptotic machinery.
Pyroptosis is defined by the dependence on caspase-1 or caspase-11 in mice and either caspase-1 or caspase-4 in humans [30,44]. Caspase-1 activation is dependent on the formation of a macromolecular complex, termed the inflammasome. Numerous inputs and cellular stresses can trigger the formation of the inflammasome complex, often via the adaptor protein ASC, which bridges the interaction of the ‘sensing’ proteins (NLRs, RIG, AIM2) and caspase-1, leading to auto-cleavage and activation of caspase-1 [45]. Pyroptosis induces inflammation via the release of the active form of the pro-inflammatory cytokines IL-1α, IL-1β and IL-18 [46] (Figure 1). Necroptosis can induce inflammation and drive emergency hematopoiesis by releasing IL-1α, IL-33 and damage-associated host factors [47] (Figure 1 and Figure 2). Here we will consider scenarios where these processes are subverted by pathogens and abnormalities in host biochemical pathways.
Apoptosis, necroptosis and pyroptosis crosstalk
An emerging theme in the field of inflammatory cell death is that the apoptotic and non-apoptotic cell death pathways communicate extensively. Vince and colleagues, demonstrate that when inhibitor of apoptosis proteins (IAPs) are depleted, RIPK3 activation triggers caspase-8 and NLRP3/Caspase-1 activation leading to ROS production and IL-1β secretion independently of RIPK3 kinase activity [48] and MLKL [49•]. Interestingly cIAPs where shown to regulate cytokine production and myelopoiesis in a RIPK1- and RIPK3- dependent manner that was independent of necroptosis [36].
Dimerization of RIPK1 and RIPK3 activates cell death however the precise pathway taken to cell death remains unclear. Evidence suggests that RIPK3 can drive MLKL-dependent necroptosis or caspase-1-dependent pyroptosis. Mutations affecting the kinase domain of RIPK3 unexpectedly induce caspase-8-dependent lethality but not MLKL-mediated cell death [50•]. Subsequent studies showed that the mutation likely induced a conformational change in RIPK3 exposing the RHIM domain rather than inhibiting the kinase activity per se [51•]. Cook and colleagues propose that the availability of substrates, namely caspase-8, MLKL and FADD, determine the outcome to RIPK1 and RIPK3 dimerization [52]. A RIPK3 construct that can be induced to dimerize was used to show that the kinase domain of RIPK3 drives MLKL-mediated necroptosis in the absence of RIPK1, caspase-8 and FADD. In contrast, in the absence of MLKL, dimerized RIPK3 induces caspase-8-dependent apoptosis, and cleavage of caspase-3 and PARP. This process is enhanced by RIPK1 and occurs independently of RIPK3 kinase function. These studies have revealed significant interactions between pyroptosis, necroptosis and caspase-8-dependent apoptosis. How these interactions alter the morphological and biochemical changes associated with cell death, the kinetics of cell death, and the pathophysiological outcome of inflammatory cell death awaits further study.
Controversial killing by MLKL
MLKL has recently emerged as a central player in the execution of RIPK3-dependent necroptotic death but its precise role in this cell death pathway is highly controversial [31]. Phosphorylation of the activation loop of MLKL induces a conformational change disrupting an auto-inhibitory interaction between the pseudokinase domain and the four-helix bundle. This promotes an interaction with phosphatidylinositol phosphates promoting membrane localization [53,54•]. It is controversial if this membrane localization and pore formation represents the end of this pathway [55], or if additional players such as PGAM5, the mitochondrial fission factor Drp1, or transient receptor potential melastatin related 7 (TRPM7) exist downstream to induce mitochondrial fragmentation and calcium current across the plasma membrane to kill the cell [55–57]. Future in vivo studies using cells deficient in these proteins in parallel with RIPK3-deficient cells will be helpful to determine the specific nature of RIPK3-dependent cell death.
Inflammatory cell death drives emergency hematopoiesis
The archetypal example of a non-apoptotic form of cell death driving emergency hematopoiesis comes from work on caspase-1 and IL-1β. IL-1β, the most commonly studied product of the pyroptotic cell death pathway, is a potent inducer of granulocyte colony-stimulating factor (G-CSF) and IL-6, both of which drive granulopoiesis [58] (Figure 2). The other major cytokine processed by active caspase-1, IL-18, [59••] can induce IFNγ which is known to regulate HSC self-renewal, repopulation and proliferation during infection and aplastic anemia [60–62] (Figure 2).
IL-1α, a necroptotic [47] and pyroptotic DAMP [63], can also influence G-CSF and IL-6 production to drive emergency hematopoiesis (Figure 2). IL-33, which is an inflammatory DAMP released during necroptosis [34], can influence emergency hematopoiesis and eosinophil production. IL-33 can induce HSPC mobilization in a CCR2-dependent manner to fight fungal infection [64•]. IL-33 also promotes IL-5 production and thereby can cause systemic eosinophilia in vivo [65] (Figure 2). Eosinophils are now known to express cell surface receptors, PIR-A and PIR-B, that modulate eosinophil cell death activation to enable IL-5-mediated expansion, demonstrating the complexity of cell death pathways operating discrete cell types during emergency hematopoiesis [66•]. IL-33 also promotes the differentiation of bone marrow Lineage-Sca1+c-Kit-CD25+ cells to type-2 innate lymphoid cells (ILC2) cells [67]. The number of natural ILC2 (nILC2) also increases following IL-33 treatment in vivo and these cells play key roles during Helminth infection [68•].
Negative regulation of emergency hematopoiesis by inflammatory cell death
Two scenarios exist that can account for effects of non-apoptotic inflammatory cell death on emergency hematopoiesis: the first, an indirect effect of chronic inflammation on HSPC activity; and secondly, direct killing of HSPC (Figure 2).
Indirect negative feedback of emergency hematopoiesis
Hematopoietic growth factors are induced to high levels during infection and they promote emergency hematopoiesis. Several inflammatory cytokines counteract the actions of hematopoietic growth factors to perturb hematopoiesis. Type-I interferon drastically reduces the number of hematopoietic progenitor cells following LCMV infection [5]. Its mechanism of action is not understood but may act by licensing these cells to undergo non-apoptotic cell death [33,69–72,73•,74] or they may interfere with the proliferation and differentiation of HSPC [75]. IL-1β production during pyroptosis may interfere with emergency hematopoiesis because treatment of mice with recombinant IL-1 receptor antagonist supports hematopoiesis and reduces mortality following chemotherapy [76,77]. Consistently, G-CSF treatment increases the expression of the IL-1R antagonist and supports engraftment of donor hematopoietic stem cells [78].
Another factor released from pyroptotic cells, IL-18 [59], synergizes with IL-12 to upregulate IFNγ which perturbs HSC proliferation, differentiation and self-renewal at steady state, during infection with Mycobacterium avium or Ehrlichia muris [60,61] and when chronically expressed can lead to aplastic anemia [62] (Figure 2). IFNγ pretreatment of HSPC also reduces the engraftment potential of donor cells suggesting that these are cell-intrinsic changes [79].
DAMPs are released during pyroptosis and necroptosis, and they have diverse effects on hematopoiesis. For example, HMGB1 and mtDNA are potent inducers of type-I interferon and they have the capacity to negatively regulate emergency hematopoiesis [24,25,80]. IL-33, also considered a DAMP, can antagonise eosinophil production by inducing GM-CSF production, in contrast to its positive role in IL-5 production [65] (Figure 2). Additional studies will be required to establish the physiological role of IL-33 in regulation of eosinophil production via its effects on IL-5 and GM-CSF.
Direct negative feedback
Killing of hematopoietic stem and progenitor cells is hypothesized to be an efficient means of removing infected cells, thereby eliminating the risk of spread to progeny cells [30]. Both pyroptotic and necroptotic machinery exists in HSPC and both pathways have now been shown to have the capability to kill HSPC. NLRP1-dependent caspase-1-dependent pyroptosis can kill HSPC during hematopoietic stress induced by viral infection or chemotherapy, causing cytopenia, immunosuppression and bone marrow failure [59] (Figure 2). Recently it was shown that deletion of caspase1/11 improved the survival of neonatal mice following bacterial challenge. This reduction in mortality was associated with elevated numbers of HSC in the bone marrow and spleen [81••]. Likewise, RIPK3-dependent necroptotic cell death limits the “self-renewal” capacity of RIPK1-deficient LT-HSC in lethally-irradiated recipient mice in a TNF-dependent process suggesting that the necroptotic machinery can exert selective pressure on LT-HSC [34,82••] (Figure 2).
Targeting cell death to modulate emergency hematopoiesis
Pharmacological inhibition of pyroptosis and necroptosis may have therapeutic value in diverse clinical settings including infection, auto-immunity and hematopoietic stress. For example, an NLRP3-specific inhibitor has been shown to protect against experimental autoimmune encephalomyelitis (EAE) and murine neonatal lethality caused by mutations equivalent to human cryopyrin-associated periodic syndrome (CAPS) [83]. Caspase-1 and caspase-4 inhibitors have not been tested in the clinic in the setting of infection but extensive mouse data suggests that inhibitors will be beneficial for patients with systemic inflammatory disease and infection-triggered cytopenias [59,81,84,85].
Genetic or pharmacological inhibition of the necroptotic machinery, namely RIPK1 and RIPK3, alleviates cerulean-induced pancreatitis [86,87], TNF-induced inflammation in mice [35,50,88,89], atherosclerosis in Apoe and Ldlr mutant mice [90], retinal degeneration [91,92], ischemia-reperfusion injury of the kidneys [93,94], myocardial infarction [95], steatohepatitis and hepatic injury induced by ethanol [96–98]. Furthermore, necroptosis was first described to play a role in viral defense during VACV infection, a virus that can inhibit apoptosis [99,100] and pyroptosis [101]. Chan et al showed that RIPK1 was required for TNF-induced necroptosis of VACV-infected cells [102]. Subsequently VACV-induced necroptosis was shown to require RIPK3 [103]. Treatment with the RIPK1 inhibitor, Nec-1, or the human MLKL inhibitor NSA inhibits the cytopathic effects of the HIV-1 virus [104]. HSV-1 and HSV-2 induce a RIPK3/MLKL-dependent death of mouse embryonic fibroblasts and. RIPK3-deficient mice are susceptible to HSV-1 and display high viral titers [105,106]. In contrast, work in human cell lines demonstrates that the ICP6 and ICP10 proteins from HSV-1 and HSV-2, respectively, can inhibit TNF-induced necroptosis indicating cell type and possibly species-specific differences in necroptosis pathways [106,107]. Exacerbations in necroptosis and pyroptosis have therefore been demonstrated in a variety of mouse models but their contribution to human disease is only now being tested in clinical trials for chronic inflammatory disease.
The lethality caused by loss of RIPK1 and the normal development of RIPK3-deficient mice suggested that RIPK3 would be ideal for drug development. However, mice with a RIPK3 D161N mutation display increased caspase-8 and RIPK1 activity leading to apoptosis. Compounds targeting the kinase activity of RIPK3 can at high concentrations also induce apoptosis by promoting a conformational change in RIPK3 that drives RHIM interactions with RIPK1 and activation of caspase-8 [51]. Mutations of other residues of RIPK3 (D161G, D143N, K51A) inhibit necroptosis in response to TLR3, TLR4, TNFR1, DAI and IFNβ but do not trigger the lethality seen in the RIPK3 D161N mutants [51]. These data suggest that RIPK3 antagonists may be valuable for inhibition of necroptosis but are likely to promote induction of apoptosis in some settings, a feature that may be useful in the setting of cancer chemotherapy.
Conclusion
As we now appreciate the complex interplay between the non-apoptotic and apoptotic cell death cascades, and the appearance of discrete features of apoptosis in response to non-apoptotic stimuli, more rigorous genetic, biochemical and morphological information will be required to characterize, refine and integrate the many forms of cell death that are now known to exist. Characterization of additional DAMPs generated during non-apoptotic cell death will shed light on the indirect modulators of emergency hematopoiesis. Recent findings have also unveiled non-apoptotic cell death as a key biological process restricting HSPC “self-renewal”, and could have clinical implications in the setting of systemic infection, and also for improving the engraftment potential of HSPC in transplantation settings using bone marrow, G-CSF-mobilized peripheral blood stem cells, umbilical cord blood units, and for gene therapy clinical trials.
Key points.
Pyroptosis and necroptosis are non-apoptotic inflammatory forms of death
Pyroptosis and necroptosis can kill HSC and progenitor cells
Apoptotic and non-apoptotic pathways cross-talk
Systemic activation of cell death can inhibit hematopoiesis
Acknowledgments
Financial support and sponsorship
This work was supported by NIH Grant 1R01HL124209-01A1 (BAC) and JS has an NHMRC fellowship (541901).
Abbreviations
- ASC
apoptosis associated speck-like protein containing a CARD
- DAMPs
damage-associated molecular patterns
- G-CSF
granulocyte colony-stimulating factor
- GM-CSF
granulocyte/macrophage colony-stimulating factor
- HMGB1
high-mobility group protein B1
- HSC
hematopoietic stem cells
- HSPC
hematopoietic stem and progenitor cells
- IAPs
inhibitor of apoptosis proteins
- IL
interleukin
- ILC2
type-2 innate lymphoid cells
- LT-HSC
long term HSC
- MLKL
mixed lineage kinase domain-like protein
- mtDNA
mitochondrial DNA
- Nec-1
necrostatin-1
- NLRP
nucleotide-binding oligomerization domain, leucine-rich repeat and pyrin domain-containing protein
- NLRs
nucleotide-binding domain and leucine-rich repeat containing gene family
- RHIM
receptor interacting protein homotypic interaction motif
- RIPK
receptor interacting protein kinase
- ROS
reactive oxygen species
- TLRs
Toll-like receptors
- TNFα
Tumor necrosis factor alpha
Footnotes
Conflicts of interest
The authors have no conflicts of interest.
References and recommended reading
Papers of particular interest, published within the annual period of review, (18 months/2014–2015) have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Bradley TR, Metcalf D. The growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci. 1966;44:287–299. doi: 10.1038/icb.1966.28. [DOI] [PubMed] [Google Scholar]
- 2.Bradley TR, Robinson W, Metcalf D. Colony production in vitro by normal polycythaemic and anaemic bone marrow. Nature. 1967;214:511. doi: 10.1038/214511a0. [DOI] [PubMed] [Google Scholar]
- 3.Venet F, Davin F, Guignant C, Larue A, Cazalis MA, Darbon R, Allombert C, Mougin B, Malcus C, Poitevin-Later F, et al. Early assessment of leukocyte alterations at diagnosis of septic shock. Shock. 2010;34:358–363. doi: 10.1097/SHK.0b013e3181dc0977. [DOI] [PubMed] [Google Scholar]
- 4.Hall MW, Geyer SM, Guo CY, Panoskaltsis-Mortari A, Jouvet P, Ferdinands J, Shay DK, Nateri J, Greathouse K, Sullivan R, et al. Innate immune function and mortality in critically ill children with influenza: a multicenter study. Crit Care Med. 2013;41:224–236. doi: 10.1097/CCM.0b013e318267633c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Binder D, Fehr J, Hengartner H, Zinkernagel RM. Virus-induced transient bone marrow aplasia: major role of interferon-alpha/beta during acute infection with the noncytopathic lymphocytic choriomeningitis virus. J Exp Med. 1997;185:517–530. doi: 10.1084/jem.185.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter CC, Onafuwa-Nuga A, McNamara LA, Riddell Jt, Bixby D, Savona MR, Collins KL. HIV-1 infects multipotent progenitor cells causing cell death and establishing latent cellular reservoirs. Nat Med. 2010;16:446–451. doi: 10.1038/nm.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slobedman B, Mocarski ES. Quantitative analysis of latent human cytomegalovirus. J Virol. 1999;73:4806–4812. doi: 10.1128/jvi.73.6.4806-4812.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slobedman B, Stern JL, Cunningham AL, Abendroth A, Abate DA, Mocarski ES. Impact of human cytomegalovirus latent infection on myeloid progenitor cell gene expression. J Virol. 2004;78:4054–4062. doi: 10.1128/JVI.78.8.4054-4062.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kondo K, Kaneshima H, Mocarski ES. Human cytomegalovirus latent infection of granulocyte-macrophage progenitors. Proc Natl Acad Sci U S A. 1994;91:11879–11883. doi: 10.1073/pnas.91.25.11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Minton EJ, Tysoe C, Sinclair JH, Sissons JG. Human cytomegalovirus infection of the monocyte/macrophage lineage in bone marrow. J Virol. 1994;68:4017–4021. doi: 10.1128/jvi.68.6.4017-4021.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nixon CC, Vatakis DN, Reichelderfer SN, Dixit D, Kim SG, Uittenbogaart CH, Zack JA. HIV-1 infection of hematopoietic progenitor cells in vivo in humanized mice. Blood. 2013;122:2195–2204. doi: 10.1182/blood-2013-04-496950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mandle T, Einsele H, Schaller M, Neumann D, Vogel W, Autenrieth IB, Kempf VA. Infection of human CD34+ progenitor cells with Bartonella henselae results in intraerythrocytic presence of B. henselae. Blood. 2005;106:1215–1222. doi: 10.1182/blood-2004-12-4670. [DOI] [PubMed] [Google Scholar]
- 13.Doitsh G, Galloway NL, Geng X, Yang Z, Monroe KM, Zepeda O, Hunt PW, Hatano H, Sowinski S, Munoz-Arias I, et al. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature. 2014;505:509–514. doi: 10.1038/nature12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monroe KM, Yang Z, Johnson JR, Geng X, Doitsh G, Krogan NJ, Greene WC. IFI16 DNA sensor is required for death of lymphoid CD4 T cells abortively infected with HIV. Science. 2014;343:428–432. doi: 10.1126/science.1243640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zauli G, Vitale M, Re MC, Furlini G, Zamai L, Falcieri E, Gibellini D, Visani G, Davis BR, Capitani S, et al. In vitro exposure to human immunodeficiency virus type 1 induces apoptotic cell death of the factor-dependent TF-1 hematopoietic cell line. Blood. 1994;83:167–175. [PubMed] [Google Scholar]
- 16.Banda NK, Tomczak JA, Shpall EJ, Sipple J, Akkina RK, Steimer KS, Hami L, Curiel TJ, Singer Harrison G. HIV-gp120 induced cell death in hematopoietic progenitor CD34+ cells. Apoptosis. 1997;2:61–68. doi: 10.1023/a:1026439726053. [DOI] [PubMed] [Google Scholar]
- 17.Aillet F, Masutani H, Elbim C, Raoul H, Chene L, Nugeyre MT, Paya C, Barre-Sinoussi F, Gougerot-Pocidalo MA, Israel N. Human immunodeficiency virus induces a dual regulation of Bcl-2, resulting in persistent infection of CD4(+) T- or monocytic cell lines. J Virol. 1998;72:9698–9705. doi: 10.1128/jvi.72.12.9698-9705.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hotchkiss RS, Tinsley KW, Swanson PE, Chang KC, Cobb JP, Buchman TG, Korsmeyer SJ, Karl IE. Prevention of lymphocyte cell death in sepsis improves survival in mice. Proc Natl Acad Sci U S A. 1999;96:14541–14546. doi: 10.1073/pnas.96.25.14541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hotchkiss RS, Tinsley KW, Swanson PE, Schmieg RE, Jr, Hui JJ, Chang KC, Osborne DF, Freeman BD, Cobb JP, Buchman TG, et al. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J Immunol. 2001;166:6952–6963. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- •20.Sun J, Ramos A, Chapman B, Johnnidis JB, Le L, Ho YJ, Klein A, Hofmann O, Camargo FD. Clonal dynamics of native haematopoiesis. Nature. 2014;514:322–327. doi: 10.1038/nature13824. This study suggests that long-lived hematopoietic progenitor cells supply demand for immune cells throughout life and that depletion of this population will impact emergency hematopoiesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •21.Busch K, Klapproth K, Barile M, Flossdorf M, Holland-Letz T, Schlenner SM, Reth M, Hofer T, Rodewald HR. Fundamental properties of unperturbed haematopoiesis from stem cells in vivo. Nature. 2015 doi: 10.1038/nature14242. This study suggests that ST-HSC and MPP supply the majority of demand for immune cell production but that LT-HSC are still ultimately responsible for maintaining life-long hematopoiesis and immunity. [DOI] [PubMed] [Google Scholar]
- 22.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yanai H, Ban T, Wang Z, Choi MK, Kawamura T, Negishi H, Nakasato M, Lu Y, Hangai S, Koshiba R, et al. HMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responses. Nature. 2009;462:99–103. doi: 10.1038/nature08512. [DOI] [PubMed] [Google Scholar]
- ••26.Li Y, Esain V, Teng L, Xu J, Kwan W, Frost IM, Yzaguirre AD, Cai X, Cortes M, Maijenburg MW, et al. Inflammatory signaling regulates embryonic hematopoietic stem and progenitor cell production. Genes Dev. 2014;28:2597–2612. doi: 10.1101/gad.253302.114. A study suggesting that sterile DAMPs cause inflammation and therby influence hematopoiesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schuettpelz LG, Link DC. Regulation of hematopoietic stem cell activity by inflammation. Front Immunol. 2013;4:204. doi: 10.3389/fimmu.2013.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: signals for a good meal. Nat Rev Immunol. 2007;7:964–974. doi: 10.1038/nri2214. [DOI] [PubMed] [Google Scholar]
- •29.Galluzzi L, Bravo-San Pedro JM, Vitale I, Aaronson SA, Abrams JM, Adam D, Alnemri ES, Altucci L, Andrews D, Annicchiarico-Petruzzelli M, et al. Essential versus accessory aspects of cell death: recommendations of the NCCD 2015. Cell Death Differ. 2015;22:58–73. doi: 10.1038/cdd.2014.137. Criteria for classifying different forms of cell death. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Croker BA, O’Donnell JA, Gerlic M. Pyroptotic death storms and cytopenia. Curr Opin Immunol. 2014;26:128–137. doi: 10.1016/j.coi.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- ••32.Dillon CP, Weinlich R, Rodriguez DA, Cripps JG, Quarato G, Gurung P, Verbist KC, Brewer TL, Llambi F, Gong YN, et al. RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3. Cell. 2014;157:1189–1202. doi: 10.1016/j.cell.2014.04.018. RIPK1 negatively regulates caspase-8-mediated apoptosis and RIPK3-dependent necroptosis, thereby preventing DAMP release and inflammation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••33.Kaiser WJ, Daley-Bauer LP, Thapa RJ, Mandal P, Berger SB, Huang C, Sundararajan A, Guo H, Roback L, Speck SH, et al. RIP1 suppresses innate immune necrotic as well as apoptotic cell death during mammalian parturition. Proc Natl Acad Sci U S A. 2014;111:7753–7758. doi: 10.1073/pnas.1401857111. RIPK1 negatively regulates caspase-8-mediated apoptosis and RIPK3-dependent necroptosis, thereby preventing DAMP release and inflammation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••34.Rickard JA, O’Donnell JA, Evans JM, Lalaoui N, Poh AR, Rogers T, Vince JE, Lawlor KE, Ninnis RL, Anderton H, et al. RIPK1 regulates RIPK3-MLKL-driven systemic inflammation and emergency hematopoiesis. Cell. 2014;157:1175–1188. doi: 10.1016/j.cell.2014.04.019. RIPK1 negatively regulates caspase-8-mediated apoptosis and RIPK3-dependent necroptosis, thereby preventing DAMP release and inflammation and further show the importance of this regulation in HSPC following transplantation. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi N, Duprez L, Grootjans S, Cauwels A, Nerinckx W, DuHadaway JB, Goossens V, Roelandt R, Van Hauwermeiren F, Libert C, et al. Necrostatin-1 analogues: critical issues on the specificity, activity and in vivo use in experimental disease models. Cell Death Dis. 2012;3:e437. doi: 10.1038/cddis.2012.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••36.Wong WW, Vince JE, Lalaoui N, Lawlor KE, Chau D, Bankovacki A, Anderton H, Metcalf D, O’Reilly L, Jost PJ, et al. cIAPs and XIAP regulate myelopoiesis through cytokine production in an RIPK1- and RIPK3-dependent manner. Blood. 2014;123:2562–2572. doi: 10.1182/blood-2013-06-510743. A study showing that cIAPs regulate myelopoiesis by impairing RIPK3-dependent cytokine production prior to necroptosis. [DOI] [PubMed] [Google Scholar]
- 37.Kaiser WJ, Sridharan H, Huang C, Mandal P, Upton JW, Gough PJ, Sehon CA, Marquis RW, Bertin J, Mocarski ES. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem. 2013;288:31268–31279. doi: 10.1074/jbc.M113.462341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Upton JW, Kaiser WJ, Mocarski ES. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe. 2012;11:290–297. doi: 10.1016/j.chom.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stanger BZ, Leder P, Lee TH, Kim E, Seed B. RIP: a novel protein containing a death domain that interacts with Fas/APO-1 (CD95) in yeast and causes cell death. Cell. 1995;81:513–523. doi: 10.1016/0092-8674(95)90072-1. [DOI] [PubMed] [Google Scholar]
- 40.Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, Hakem R, Salvesen GS, Green DR. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •41.Dannappel M, Vlantis K, Kumari S, Polykratis A, Kim C, Wachsmuth L, Eftychi C, Lin J, Corona T, Hermance N, et al. RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis. Nature. 2014;513:90–94. doi: 10.1038/nature13608. This study confirms that RIPK1 negatively regulates caspase-8-mediated apoptosis and RIPK3-dependent necroptosis using conditional KOs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang H, Zhou X, McQuade T, Li J, Chan FK, Zhang J. Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature. 2011;471:373–376. doi: 10.1038/nature09878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, Caspary T, Mocarski ES. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–372. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, Hu L, Shao F. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514:187–192. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- 45.Proell M, Gerlic M, Mace PD, Reed JC, Riedl SJ. The CARD plays a critical role in ASC foci formation and inflammasome signalling. Biochem J. 2013;449:613–621. doi: 10.1042/BJ20121198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bauernfeind F, Hornung V. Of inflammasomes and pathogens--sensing of microbes by the inflammasome. EMBO Mol Med. 2013;5:814–826. doi: 10.1002/emmm.201201771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaczmarek A, Vandenabeele P, Krysko DV. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity. 2013;38:209–223. doi: 10.1016/j.immuni.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 48.Vince JE, Wong WW, Gentle I, Lawlor KE, Allam R, O’Reilly L, Mason K, Gross O, Ma S, Guarda G, et al. Inhibitor of apoptosis proteins limit RIP3 kinase-dependent interleukin-1 activation. Immunity. 2012;36:215–227. doi: 10.1016/j.immuni.2012.01.012. [DOI] [PubMed] [Google Scholar]
- •49.Lawlor KE, Khan N, Mildenhall A, Gerlic M, Croker BA, D’Cruz AA, Hall C, Kaur Spall S, Anderton H, Masters SL, et al. RIPK3 promotes cell death and NLRP3 inflammasome activation in the absence of MLKL. Nat Commun. 2015;6:6282. doi: 10.1038/ncomms7282. This research shows extensive crosstalk between the necroptotic and pyroptotic pathways. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •50.Newton K, Dugger DL, Wickliffe KE, Kapoor N, de Almagro MC, Vucic D, Komuves L, Ferrando RE, French DM, Webster J, et al. Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis. Science. 2014;343:1357–1360. doi: 10.1126/science.1249361. One of two studies to show that RIPK3 can signal to caspase-8. [DOI] [PubMed] [Google Scholar]
- •51.Mandal P, Berger SB, Pillay S, Moriwaki K, Huang C, Guo H, Lich JD, Finger J, Kasparcova V, Votta B, et al. RIP3 induces apoptosis independent of pronecrotic kinase activity. Mol Cell. 2014;56:481–495. doi: 10.1016/j.molcel.2014.10.021. One of two studies to show that RIPK3 can signal to caspase-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cook WD, Moujalled DM, Ralph TJ, Lock P, Young SN, Murphy JM, Vaux DL. RIPK1- and RIPK3-induced cell death mode is determined by target availability. Cell Death Differ. 2014;21:1600–1612. doi: 10.1038/cdd.2014.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hildebrand JM, Tanzer MC, Lucet IS, Young SN, Spall SK, Sharma P, Pierotti C, Garnier JM, Dobson RC, Webb AI, et al. Activation of the pseudokinase MLKL unleashes the four-helix bundle domain to induce membrane localization and necroptotic cell death. Proc Natl Acad Sci U S A. 2014;111:15072–15077. doi: 10.1073/pnas.1408987111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •54.Dondelinger Y, Declercq W, Montessuit S, Roelandt R, Goncalves A, Bruggeman I, Hulpiau P, Weber K, Sehon CA, Marquis RW, et al. MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep. 2014;7:971–981. doi: 10.1016/j.celrep.2014.04.026. A study supporting a direct role for MLKL in pore formation and cell lysis. [DOI] [PubMed] [Google Scholar]
- 55.Cai Z, Jitkaew S, Zhao J, Chiang HC, Choksi S, Liu J, Ward Y, Wu LG, Liu ZG. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol. 2014;16:55–65. doi: 10.1038/ncb2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Z, Jiang H, Chen S, Du F, Wang X. The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell. 2012;148:228–243. doi: 10.1016/j.cell.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 57.Moujalled DM, Cook WD, Murphy JM, Vaux DL. Necroptosis induced by RIPK3 requires MLKL but not Drp1. Cell Death Dis. 2014;5:e1086. doi: 10.1038/cddis.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dubois CM, Neta R, Keller JR, Jacobsen SE, Oppenheim JJ, Ruscetti F. Hematopoietic growth factors and glucocorticoids synergize to mimic the effects of IL-1 on granulocyte differentiation and IL-1 receptor induction on bone marrow cells in vivo. Exp Hematol. 1993;21:303–310. [PubMed] [Google Scholar]
- ••59.Masters SL, Gerlic M, Metcalf D, Preston S, Pellegrini M, O’Donnell JA, McArthur K, Baldwin TM, Chevrier S, Nowell CJ, et al. NLRP1 inflammasome activation induces pyroptosis of hematopoietic progenitor cells. Immunity. 2012;37:1009–1023. doi: 10.1016/j.immuni.2012.08.027. This study shows the consequences of pyroptosis in HSPC, leading to cytopenia and bone marrow failure after chemotherapy or infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baldridge MT, King KY, Boles NC, Weksberg DC, Goodell MA. Quiescent haematopoietic stem cells are activated by IFN-gamma in response to chronic infection. Nature. 2010;465:793–797. doi: 10.1038/nature09135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.MacNamara KC, Jones M, Martin O, Winslow GM. Transient activation of hematopoietic stem and progenitor cells by IFNgamma during acute bacterial infection. PLoS One. 2011;6:e28669. doi: 10.1371/journal.pone.0028669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lin FC, Karwan M, Saleh B, Hodge DL, Chan T, Boelte KC, Keller JR, Young HA. IFN-gamma causes aplastic anemia by altering hematopoietic stem/progenitor cell composition and disrupting lineage differentiation. Blood. 2014;124:3699–3708. doi: 10.1182/blood-2014-01-549527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gross O, Yazdi AS, Thomas CJ, Masin M, Heinz LX, Guarda G, Quadroni M, Drexler SK, Tschopp J. Inflammasome activators induce interleukin-1alpha secretion via distinct pathways with differential requirement for the protease function of caspase-1. Immunity. 2012;36:388–400. doi: 10.1016/j.immuni.2012.01.018. [DOI] [PubMed] [Google Scholar]
- •64.Kim J, Kim W, Le HT, Moon UJ, Tran VG, Kim HJ, Jung S, Nguyen QT, Kim BS, Jun JB, et al. IL-33-induced hematopoietic stem and progenitor cell mobilization depends upon CCR2. J Immunol. 2014;193:3792–3802. doi: 10.4049/jimmunol.1400176. A study showing IL-33 can promote HSPC mobilization. [DOI] [PubMed] [Google Scholar]
- 65.Dyer KD, Percopo CM, Rosenberg HF. IL-33 promotes eosinophilia in vivo and antagonizes IL-5-dependent eosinophil hematopoiesis ex vivo. Immunol Lett. 2013;150:41–47. doi: 10.1016/j.imlet.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •66.Ben Baruch-Morgenstern N, Shik D, Moshkovits I, Itan M, Karo-Atar D, Bouffi C, Fulkerson PC, Rashkovan D, Jung S, Rothenberg ME, et al. Paired immunoglobulin-like receptor A is an intrinsic, self-limiting suppressor of IL-5-induced eosinophil development. Nat Immunol. 2014;15:36–44. doi: 10.1038/ni.2757. This study reveals that eosinophils express a constitutively active kill switch that needs to be suppressed to allow for full expansion in response to IL-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brickshawana A, Shapiro VS, Kita H, Pease LR. Lineage(−)Sca1+c-Kit(−)CD25+ cells are IL-33-responsive type 2 innate cells in the mouse bone marrow. J Immunol. 2011;187:5795–5804. doi: 10.4049/jimmunol.1102242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •68.Huang Y, Guo L, Qiu J, Chen X, Hu-Li J, Siebenlist U, Williamson PR, Urban JF, Jr, Paul WE. IL-25-responsive, lineage-negative KLRG1(hi) cells are multipotential ‘inflammatory’ type 2 innate lymphoid cells. Nat Immunol. 2015;16:161–169. doi: 10.1038/ni.3078. A novel immune effector cell that differentiates in response to IL-33, a necroptotic DAMP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thapa RJ, Nogusa S, Chen P, Maki JL, Lerro A, Andrake M, Rall GF, Degterev A, Balachandran S. Interferon-induced RIP1/RIP3-mediated necrosis requires PKR and is licensed by FADD and caspases. Proc Natl Acad Sci U S A. 2013;110:E3109–3118. doi: 10.1073/pnas.1301218110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McComb S, Cessford E, Alturki NA, Joseph J, Shutinoski B, Startek JB, Gamero AM, Mossman KL, Sad S. Type-I interferon signaling through ISGF3 complex is required for sustained Rip3 activation and necroptosis in macrophages. Proc Natl Acad Sci U S A. 2014;111:E3206–3213. doi: 10.1073/pnas.1407068111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rathinam VA, Vanaja SK, Waggoner L, Sokolovska A, Becker C, Stuart LM, Leong JM, Fitzgerald KA. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell. 2012;150:606–619. doi: 10.1016/j.cell.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Case CL, Kohler LJ, Lima JB, Strowig T, de Zoete MR, Flavell RA, Zamboni DS, Roy CR. Caspase-11 stimulates rapid flagellin-independent pyroptosis in response to Legionella pneumophila. Proc Natl Acad Sci U S A. 2013;110:1851–1856. doi: 10.1073/pnas.1211521110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •73.Meunier E, Dick MS, Dreier RF, Schurmann N, Kenzelmann Broz D, Warming S, Roose-Girma M, Bumann D, Kayagaki N, Takeda K, et al. Caspase-11 activation requires lysis of pathogen-containing vacuoles by IFN-induced GTPases. Nature. 2014;509:366–370. doi: 10.1038/nature13157. A study indicating that caspase-11 responds to intracellular Gram-negative bacteria to induce pyroptosis. [DOI] [PubMed] [Google Scholar]
- 74.Pilla DM, Hagar JA, Haldar AK, Mason AK, Degrandi D, Pfeffer K, Ernst RK, Yamamoto M, Miao EA, Coers J. Guanylate binding proteins promote caspase-11-dependent pyroptosis in response to cytoplasmic LPS. Proc Natl Acad Sci U S A. 2014;111:6046–6051. doi: 10.1073/pnas.1321700111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Essers MA, Offner S, Blanco-Bose WE, Waibler Z, Kalinke U, Duchosal MA, Trumpp A. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 2009;458:904–908. doi: 10.1038/nature07815. [DOI] [PubMed] [Google Scholar]
- 76.Qian L, Xiang D, Zhang J, Zhu S, Gao J, Wang X, Gao J, Zhang Y, Shen J, Yu Y, et al. Recombinant human interleukin-1 receptor antagonist reduces acute lethal toxicity and protects hematopoiesis from chemotoxicity in vivo. Biomed Pharmacother. 2013;67:108–115. doi: 10.1016/j.biopha.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 77.Zhang J, Xiang D, Zhu S, Mao W, Lu H, Wu M, Wang Q, Yu Y, Herbst KD, Han W. Interleukin 1 receptor antagonist inhibits normal hematopoiesis and reduces lethality and bone marrow toxicity of 5-fluouracil in mouse. Biomed Pharmacother. 2009;63:501–508. doi: 10.1016/j.biopha.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 78.Schwabe M, Hartert AM, Bertz H, Finke J. Treatment with granulocyte colony-stimulating factor increases interleukin-1 receptor antagonist levels during engraftment following allogeneic stem-cell transplantation. Eur J Clin Invest. 2004;34:759–765. doi: 10.1111/j.1365-2362.2004.01421.x. [DOI] [PubMed] [Google Scholar]
- 79.Yang L, Dybedal I, Bryder D, Nilsson L, Sitnicka E, Sasaki Y, Jacobsen SE. IFN-gamma negatively modulates self-renewal of repopulating human hemopoietic stem cells. J Immunol. 2005;174:752–757. doi: 10.4049/jimmunol.174.2.752. [DOI] [PubMed] [Google Scholar]
- 80.White MJ, McArthur K, Metcalf D, Lane RM, Cambier JC, Herold MJ, van Delft MF, Bedoui S, Lessene G, Ritchie ME, et al. Apoptotic caspases suppress mtDNA-induced STING-mediated type I IFN production. Cell. 2014;159:1549–1562. doi: 10.1016/j.cell.2014.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••81.Gentile LF, Cuenca AL, Cuenca AG, Nacionales DC, Ungaro R, Efron PA, Moldawer LL, Larson SD. Improved Emergency Myelopoiesis and Survival in Neonatal Sepsis by Caspase-1/11 Ablation. Immunology. 2015 doi: 10.1111/imm.12450. A study examining the correlation between caspase1/11-deficiency, hematopoiesis and mortality. This supports the clinical administration of caspase-1 inhibitors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••82.Roderick JE, Hermance N, Zelic M, Simmons MJ, Polykratis A, Pasparakis M, Kelliher MA. Hematopoietic RIPK1 deficiency results in bone marrow failure caused by apoptosis and RIPK3-mediated necroptosis. Proc Natl Acad Sci U S A. 2014;111:14436–14441. doi: 10.1073/pnas.1409389111. This study confirms that loss of RIPK1 induces bone marrow failure due to apoptosis and necroptosis of HSPC using conditional KOs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Coll RC, Robertson AA, Chae JJ, Higgins SC, Munoz-Planillo R, Inserra MC, Vetter I, Dungan LS, Monks BG, Stutz A, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015 doi: 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brydges SD, Mueller JL, McGeough MD, Pena CA, Misaghi A, Gandhi C, Putnam CD, Boyle DL, Firestein GS, Horner AA, et al. Inflammasome-mediated disease animal models reveal roles for innate but not adaptive immunity. Immunity. 2009;30:875–887. doi: 10.1016/j.immuni.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Meng G, Zhang F, Fuss I, Kitani A, Strober W. A mutation in the Nlrp3 gene causing inflammasome hyperactivation potentiates Th17 cell-dominant immune responses. Immunity. 2009;30:860–874. doi: 10.1016/j.immuni.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 87.Wu J, Huang Z, Ren J, Zhang Z, He P, Li Y, Ma J, Chen W, Zhang Y, Zhou X, et al. Mlkl knockout mice demonstrate the indispensable role of Mlkl in necroptosis. Cell Res. 2013;23:994–1006. doi: 10.1038/cr.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Linkermann A, Brasen JH, De Zen F, Weinlich R, Schwendener RA, Green DR, Kunzendorf U, Krautwald S. Dichotomy between RIP1- and RIP3-mediated necroptosis in tumor necrosis factor-alpha-induced shock. Mol Med. 2012;18:577–586. doi: 10.2119/molmed.2011.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Duprez L, Takahashi N, Van Hauwermeiren F, Vandendriessche B, Goossens V, Vanden Berghe T, Declercq W, Libert C, Cauwels A, Vandenabeele P. RIP kinase-dependent necrosis drives lethal systemic inflammatory response syndrome. Immunity. 2011;35:908–918. doi: 10.1016/j.immuni.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 90.Lin J, Li H, Yang M, Ren J, Huang Z, Han F, Huang J, Ma J, Zhang D, Zhang Z, et al. A role of RIP3-mediated macrophage necrosis in atherosclerosis development. Cell Rep. 2013;3:200–210. doi: 10.1016/j.celrep.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 91.Murakami Y, Matsumoto H, Roh M, Giani A, Kataoka K, Morizane Y, Kayama M, Thanos A, Nakatake S, Notomi S, et al. Programmed necrosis, not apoptosis, is a key mediator of cell loss and DAMP-mediated inflammation in dsRNA-induced retinal degeneration. Cell Death Differ. 2014;21:270–277. doi: 10.1038/cdd.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Trichonas G, Murakami Y, Thanos A, Morizane Y, Kayama M, Debouck CM, Hisatomi T, Miller JW, Vavvas DG. Receptor interacting protein kinases mediate retinal detachment-induced photoreceptor necrosis and compensate for inhibition of apoptosis. Proc Natl Acad Sci U S A. 2010;107:21695–21700. doi: 10.1073/pnas.1009179107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Linkermann A, Brasen JH, Himmerkus N, Liu S, Huber TB, Kunzendorf U, Krautwald S. Rip1 (receptor-interacting protein kinase 1) mediates necroptosis and contributes to renal ischemia/reperfusion injury. Kidney Int. 2012;81:751–761. doi: 10.1038/ki.2011.450. [DOI] [PubMed] [Google Scholar]
- 94.Lau A, Wang S, Jiang J, Haig A, Pavlosky A, Linkermann A, Zhang ZX, Jevnikar AM. RIPK3-mediated necroptosis promotes donor kidney inflammatory injury and reduces allograft survival. Am J Transplant. 2013;13:2805–2818. doi: 10.1111/ajt.12447. [DOI] [PubMed] [Google Scholar]
- 95.Oerlemans MI, Liu J, Arslan F, den Ouden K, van Middelaar BJ, Doevendans PA, Sluijter JP. Inhibition of RIP1-dependent necrosis prevents adverse cardiac remodeling after myocardial ischemia-reperfusion in vivo. Basic Res Cardiol. 2012;107:270. doi: 10.1007/s00395-012-0270-8. [DOI] [PubMed] [Google Scholar]
- 96.Roychowdhury S, Chiang DJ, Mandal P, McMullen MR, Liu X, Cohen JI, Pollard J, Feldstein AE, Nagy LE. Inhibition of apoptosis protects mice from ethanol-mediated acceleration of early markers of CCl4 -induced fibrosis but not steatosis or inflammation. Alcohol Clin Exp Res. 2012;36:1139–1147. doi: 10.1111/j.1530-0277.2011.01720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Roychowdhury S, McMullen MR, Pisano SG, Liu X, Nagy LE. Absence of receptor interacting protein kinase 3 prevents ethanol-induced liver injury. Hepatology. 2013;57:1773–1783. doi: 10.1002/hep.26200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vucur M, Reisinger F, Gautheron J, Janssen J, Roderburg C, Cardenas DV, Kreggenwinkel K, Koppe C, Hammerich L, Hakem R, et al. RIP3 inhibits inflammatory hepatocarcinogenesis but promotes cholestasis by controlling caspase-8- and JNK-dependent compensatory cell proliferation. Cell Rep. 2013;4:776–790. doi: 10.1016/j.celrep.2013.07.035. [DOI] [PubMed] [Google Scholar]
- 99.Dobbelstein M, Shenk T. Protection against apoptosis by the vaccinia virus SPI-2 (B13R) gene product. J Virol. 1996;70:6479–6485. doi: 10.1128/jvi.70.9.6479-6485.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wasilenko ST, Stewart TL, Meyers AF, Barry M. Vaccinia virus encodes a previously uncharacterized mitochondrial-associated inhibitor of apoptosis. Proc Natl Acad Sci U S A. 2003;100:14345–14350. doi: 10.1073/pnas.2235583100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gerlic M, Faustin B, Postigo A, Yu EC, Proell M, Gombosuren N, Krajewska M, Flynn R, Croft M, Way M, et al. Vaccinia virus F1L protein promotes virulence by inhibiting inflammasome activation. Proc Natl Acad Sci U S A. 2013;110:7808–7813. doi: 10.1073/pnas.1215995110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chan FK, Shisler J, Bixby JG, Felices M, Zheng L, Appel M, Orenstein J, Moss B, Lenardo MJ. A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. J Biol Chem. 2003;278:51613–51621. doi: 10.1074/jbc.M305633200. [DOI] [PubMed] [Google Scholar]
- 103.Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pan T, Wu S, He X, Luo H, Zhang Y, Fan M, Geng G, Ruiz VC, Zhang J, Mills L, et al. Necroptosis takes place in human immunodeficiency virus type-1 (HIV-1)-infected CD4+ T lymphocytes. PLoS One. 2014;9:e93944. doi: 10.1371/journal.pone.0093944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang X, Li Y, Liu S, Yu X, Li L, Shi C, He W, Li J, Xu L, Hu Z, et al. Direct activation of RIP3/MLKL-dependent necrosis by herpes simplex virus 1 (HSV-1) protein ICP6 triggers host antiviral defense. Proc Natl Acad Sci U S A. 2014;111:15438–15443. doi: 10.1073/pnas.1412767111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huang Z, Wu SQ, Liang Y, Zhou X, Chen W, Li L, Wu J, Zhuang Q, Chen C, Li J, et al. RIP1/RIP3 Binding to HSV-1 ICP6 Initiates Necroptosis to Restrict Virus Propagation in Mice. Cell Host Microbe. 2015;17:229–242. doi: 10.1016/j.chom.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 107.Guo H, Omoto S, Harris PA, Finger JN, Bertin J, Gough PJ, Kaiser WJ, Mocarski ES. Herpes simplex virus suppresses necroptosis in human cells. Cell Host Microbe. 2015;17:243–251. doi: 10.1016/j.chom.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]