Abstract
Background: The brain is highly vascular and richly perfused, and dependent on continuous flow for normal function. Although confined within the skull, pressure within the brain is usually less than 15 mmHg, and shows small pulsations related to arterial pulse under normal circumstances. Pulsatile arterial hemodynamics in the brain have been studied before, but are still inadequately understood, especially during changes of intracranial pressure (ICP) after head injury.
Method: In seeking cohesive explanations, we measured ICP and radial artery pressure (RAP) invasively with high-fidelity manometer systems, together with middle cerebral artery flow velocity (MCAFV) (transcranial Doppler) and central aortic pressure (CAP) generated from RAP, using a generalized transfer function technique, in eight young unconscious, ventilated adults following closed head trauma. We focused on vascular effects of spontaneous rises of ICP (‘plateau waves’).
Results: A rise in mean ICP from 29 to 53 mmHg caused no consistent change in pressure outside the cranium, or in heart rate, but ICP pulsations increased in amplitude from 8 to 20 mmHg, and ICP waveform came to resemble that in the aorta. Cerebral perfusion pressure (=central aortic pressure – ICP), which equates with transmural pressure, fell from 61 to 36 mmHg. Mean MCAFV fell from 53 to 40 cm/s, whereas pulsatile MCAFV increased from 77 to 98 cm/s. These significant changes (all P < 0.01) may be explained using the Monro–Kellie doctrine, because of compression of the brain, as occurs in a limb when external pressure is applied.
Conclusion: The findings emphasize importance of reducing ICP, when raised, and on the additional benefits of reducing wave reflection from the lower body.
Keywords: aortic pressure, intracranial pressure, plateau waves
INTRODUCTION
Modern neurosurgical intensive care activities include monitoring of intracranial pressure (ICP), together with pulsatile blood flow in cerebral arteries (transcranial Doppler) and systemic arterial pressure, usually in the radial artery [1–4]. Explanations of data often conflict with approaches used by cardiovascular physiologists and physicians for flow in other parts of the body [5–7], because neurosurgical intensivists must consider variations in ICP within the cranium resulting from intracranial disease and cannot measure pressure in intracerebral arteries unless a catheter is fed up through a systemic artery into the cranium.
The brain's location in a box (the skull), with limited connection to the exterior and the rest of the circulation, presents complexities [8,9]. Such are compounded in the presence of disease with which neurosurgeons have to deal. This has been recognized for over 200 years when Alexander Monro, an Edinburgh anatomist, introduced a concept leading to the present view (known as the Monro–Kellie doctrine) of hemodynamic implications arising from a fixed intracranial/vertebral space occupied by brain, cerebrospinal fluid (CSF), and blood [10–13].
Bearing in mind the simple logic and importance of this doctrine that increase in one intracranial component – brain, CSF, venous blood, or arterial blood – must reduce volume of another component, we sought explanations from physiological principles that apply in other situations in which external pressure modifies pressure and flow waveforms. We examined the effects of raised ICP on flow patterns in cerebral arteries and on pressure patterns within the skull and in arteries entering the skull to supply the brain. We also sought relationships between ICP, cerebral artery perfusion pressure (CPP), cerebral artery transmural pressure (CTP), and middle cerebral artery flow velocity (MCAFV) patterns that may help to understand its ill effects, when elevated.
METHODS
Data were obtained from eight patients (six males and two females), selected retrospectively from a database of closed head trauma patients, aged 19–36 (mean 27) years treated between 1992 and 1998 in the Neurocritical Care Ward of Addenbrooke's Hospital, Cambridge, UK [1,2]. Data were selected by M.C. and P.S. as being representative of closed head trauma and showing episodes of marked spontaneous increase of ICP (‘plateau waves’) (Fig. 1). Brain monitoring in presented configuration [ICP, MCAFV, and radial artery pressure (RAP)] is a standard clinical practice of care for patients after severe traumatic brain injury in Cambridge. Data were analyzed anonymously (deidentified) as agreed with the Neurocritical Care Users Committee as a part of routine clinical audit.
FIGURE 1.
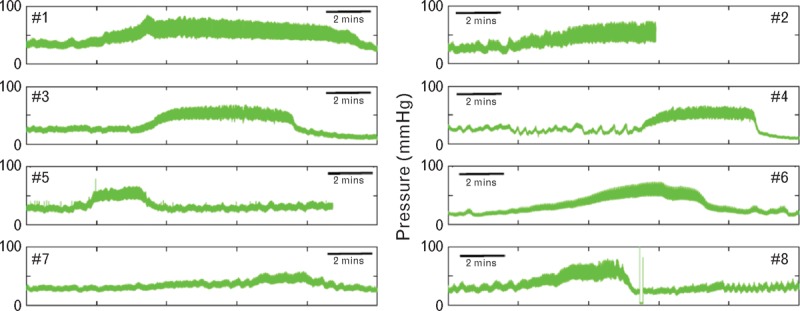
Spontaneous increases in intracranial pressure (‘plateau waves’) recorded in eight young adult unconscious patients with severe closed head injury during treatment in a neurosurgical intensive care ward at Addenbrooke's Hospital, Cambridge University, Cambridge, UK.
Patients were unconscious but sedated and ventilated. Details of procedures, protocols, and ethical issues are provided elsewhere in previous descriptions of this cohort [1,2]. ICP was monitored using an intraparenchymal probe (Codman ICP MicroSensor; Codman & Shurtleff Inc., Raynham, Massachusetts, USA). RAP was directly measured from the radial artery (Baxter Healthcare Corp. CardioVascular Group, Irvine, California, USA) and with zero pressure set at the heart level (with the patient recumbent). We used a generalized transfer function (GTF) technique, validated to the US Food and Drug Administration requirements [14,15], to generate the central aortic pressure (CAP) wave from the RAP wave. The principal differences between RAP and CAP waveform corrected by GTF use were lower amplitude of the aortic wave, later systolic peak, and near-exponential decay of pressure from the time of aortic valve closure [13].
MCAFV was recorded using transcranial Doppler (TCD) with a 2-MHz probe (model PCDop 842; Scimed, Bristol, UK) in this study. These pressure and flow velocity signals were digitized using analog-to-digital converter (DT9801; Data Translation, Marlboro, Massachusetts, USA), sampled at a frequency of 50 Hz. Before analysis, signal artifacts such as arterial line flushing and repositioning of Doppler probes were removed. The data acquisition and analysis was done using a computer running ICM+ software (http://www.neurosurg.cam.ac.uk/icmplus) [16].
RESULTS
Intracranial pressure
All eight patients showed elevation of ICP under basal conditions and were undergoing treatment according to the existing protocol [1,2]. Despite therapy, spontaneous unprovoked ‘plateau waves’ of high ICP occurred in all (Fig. 1). These were not associated with any change in heart rate or measured systolic or diastolic RAP or calculated CAP. In the unconscious, ventilated patients, their presence was not apparent except for concomitant changes of the measured mean ICP, its pulsations, and amplitude of the TCD flow velocity wave (Fig. 2).
FIGURE 2.
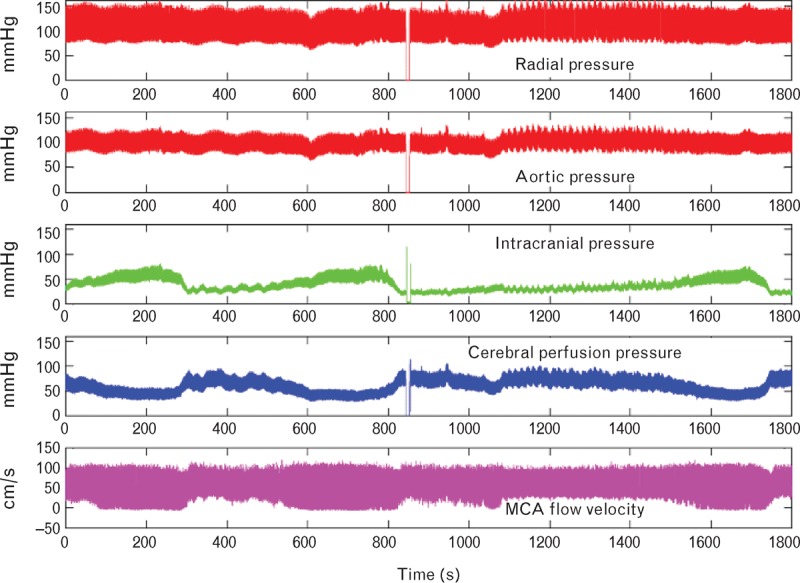
Data as recorded over a 30-min period in one patient (no. 6). From top down: radial artery pressure (RAP) recorded invasively (orange), central aortic pressure (CAP) synthesized from RAP using SphygmoCor (red), intracranial pressure (ICP) (green), cerebral perfusion pressure (=CAP − ICP) (blue), and right middle cerebral artery flow velocity (Doppler technique) (purple).
Data from all patients are summarized for pressure and TCD MCAFV in Tables 1 and 2, respectively. The amplitude of the RAP wave was greater than that synthesized for the CAP, but mean pressure and changes with respiration were the same at the wrist and the ascending aorta. Differences between the two were best exposed and explained on examination of individual waveforms, where it is apparent that the radial waveform was delayed (by 40–60 ms) and amplified (by 20–40%) [13–15]. Application of the GTF corrected for the distortion, delay, and amplification of the RAP waveform, so that foot and upstroke of the synthesized CAP waves coincided with the foot and upstroke of ICP and MCAFV waveforms (so accounting for delay of peripheral pulse in relation to the central pulse) [13–15]. The peak of the RAP was always apparent in early systole, some 100 ms after the foot of the wave, whereas the CAP peak was lower, broader, and seen later, in mid-to-late systole for all of the eight relatively young patients studied.
TABLE 1.
Data from all patients summarized for pressure (mmHg)
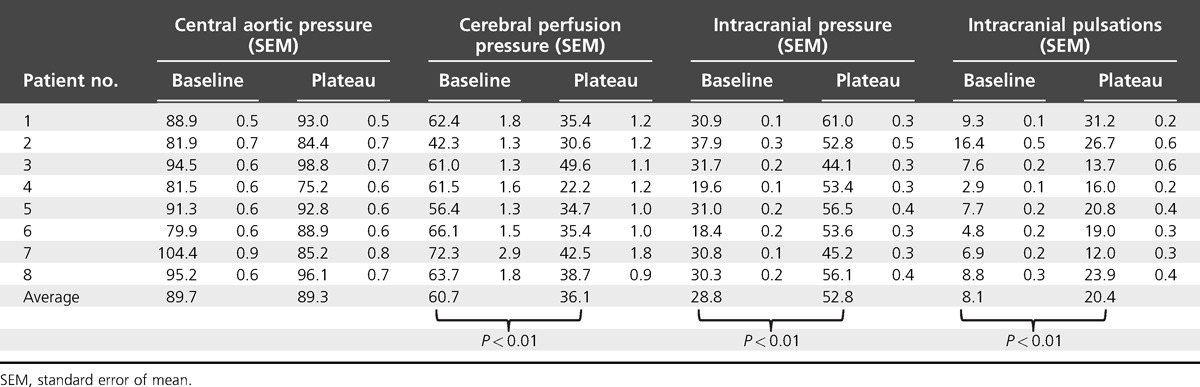
TABLE 2.
Data from all patients summarized for transcranial Doppler middle cerebral artery flow velocity (cm/s)

Pulsations of ICP were dependent on the level of ICP, in all patients, increasing with rise in ICP to the peak of the plateau wave, and decreasing with its fall (Figs 2–3). For individual waves, it was apparent that timing of the late peak of the ICP waveform corresponded with the peak of the corresponding CAP wave when ICP was elevated. Although amplitude of the ICP waveforms was dependent on the level of mean ICP (Fig. 3), amplitude of the CAP and RAP wave did not vary with ICP (Fig. 2). Changes in ICP were quite independent also of systemic arterial pressure at radial or aortic sites (Fig. 2) and heart rate, which were 118 bpm (standard error of mean 10) for baseline and 117 bpm (standard error of mean 10) for plateau peak.
FIGURE 3.
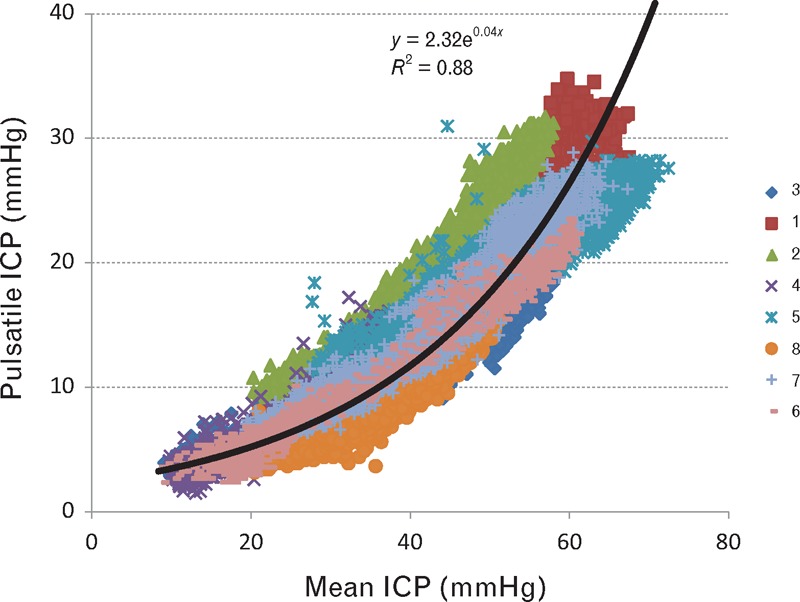
Relationship between mean and pulsatile intracranial pressure (ICP) at baseline and with elevation of ICP during ‘plateau waves’ of all eight patients. Pressure pulsations were directly related to mean ICP. The relationship is seen down to normal levels where mean ICP and pressure fluctuations are small and less than 10 mmHg.
The spontaneous increase in ICP in these eight patients was associated with reciprocal reduction in CPP, which is displayed by subtracting ICP from CAP (Fig. 2). Although there were no changes in CAP or RAP during the plateau waves, there were consistent and highly significant differences in ICP pulsations (Table 1), and in CPP. CPP is taken to measure pressure gradient across the cerebral vascular bed, and so is the generator of pulsatile middle cerebral artery flow. CPP also is a measure of the distending pressure (CTP) across the middle cerebral artery (MCA) wall throughout the cardiac cycle, and so the generator of the pulsatile diameter changes in MCA and other cerebral arteries. Hence, ICP pulsations are dependent on amplitude and contour of CPP. Although changes in pulsatile ICP were most marked at the peak of plateau waves, their relationship persisted at baseline, and when ICP was rising to and falling from its peak (Figs 2 and 3).
Transcranial Doppler flow velocity
Flow waves, recorded with headband-fixed Doppler probes, did not have the same reproducibility as the cannulated intraarterial pressure measurements, but the patterns of flow amplitude were consistent in relation to pressure change during the plateau waves, showing increase in amplitude as ICP rose to its peak and decrease in amplitude as ICP fell subsequently. Peak flow velocity did not always increase, nor did the nadir always fall, but amplitude of the flow wave (foot to peak) had a consistent direct relationship with ICP pulsation (Table 2). With respect to mean flow velocity, however, the relationship was inverse. As mean ICP rose, despite increase in flow velocity pulsations, mean flow velocity actually decreased (Table 2).
Cerebral vascular impedance
Cerebral vascular impedance is shown in Fig. 4, measured at baseline and the summit of the plateau waves – that is, with ICP moderately elevated (29 mmHg) and more definitely elevated (53 mmHg). Also shown is impedance determined using the same technique in a group of normal individuals [13]. The resistive component of impedance was highest (at zero frequency) during the plateau waves (4.7 × 103 ± 1.7 dyne.s.cm−3) than during baseline, that is, before or between plateau waves (3.7 × 103 ± 1.3 dyne.s.cm−3), whereas characteristic impedance (determined as impedance modulus between 5 and 10 Hz) was similar in both (1.0 × 103 ± 0.3 and 1.3 × 103 ± 0.5 dyne.s.cm−3, respectively). Patterns of impedance modulus were similar in both, and in a control group [13], showing a steady fall from zero frequency to a minimal value at 4–6 Hz before rising again at higher frequencies. Impedance phase approached or passed through zero at 4–6 Hz as was seen in a normal control group, but at a lower frequency in the patients with elevated ICP.
FIGURE 4.
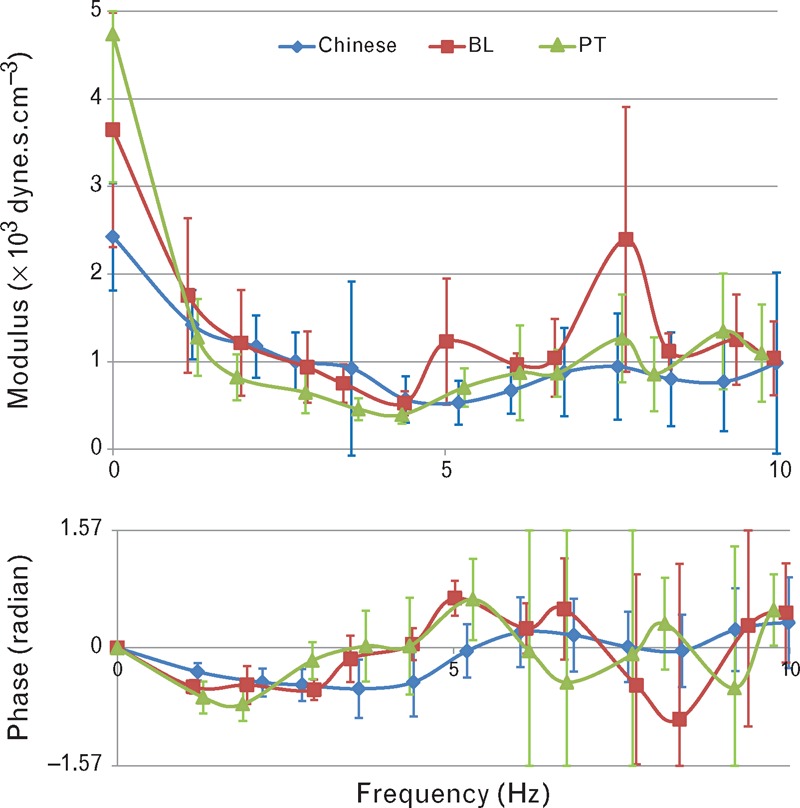
Vascular impedance [modulus (above) and phase (below)] calculated as ratio between central aortic pressure and middle cerebral artery flow velocity waveform harmonics and phase delay between pressure and flow. Modulus and phase of impedance were similar at baseline (red) and during the plateau wave (green), and to that reported in a group of normal Chinese individuals (blue) [13]. Values are mean ± 1 standard deviation.
DISCUSSION
Pressure/flow relationship: explanations and challenges; reconciliations with peripheral pressure and flow
The challenge these data provide with increase in ICP is to explain the major findings that are as follows.
Pulsations of ICP are synchronous with the heartbeat; they increase during plateau wave to approach the amplitude of the CAP pulse and take on the shape of the CAP wave with peak in late systole. With subsequent fall in ICP, these changes reverse (data not shown).
TCD MCAFV pulsations increase progressively with increase in ICP pulsations, but mean flow velocity decreases so that peak flow velocity may or may not increase. When peak flow velocity does not increase, the nadir of the flow velocity wave does decrease, so that amplitude of the flow waveform almost always increases, in company with ICP pulsations.
Impedance modulus and phase are similar with elevation of ICP under normal conditions.
Models of the intracranial circulation derived from the peripheral circulation and its investigations
Circulation in the brain within the cranium can be modeled on the basis of instruments used in physiology and medicine, where a limb or part thereof is enclosed in a device, with pressure applied from without. In all of these devices, pressure from without, applied through the skin to tissues beneath, uncovers pulsation of the arteries as well as compressing the veins and restricting flow through these.
Venous occlusion plethysmography
Venous occlusion plethysmography (Fig. 5) measures flow into a limb, through enclosing the limb in an airtight box, then preventing venous egress through application of a tourniquet around the proximal part of the limb. Entry of arterial blood into the limb in a box causes increase in pressure within the box, which can be calibrated to calculate mean flow, using the initial part of the output tracing. A problem with this method is the presence of arterial pulsations, which increase with time as blood in the arm and pressure in the box increase. This reduces distending pressure across the arterial wall and increases pulsations. Such pulsations can interfere with determination of change in mean pressure (and so flow entry) into the box.
FIGURE 5.
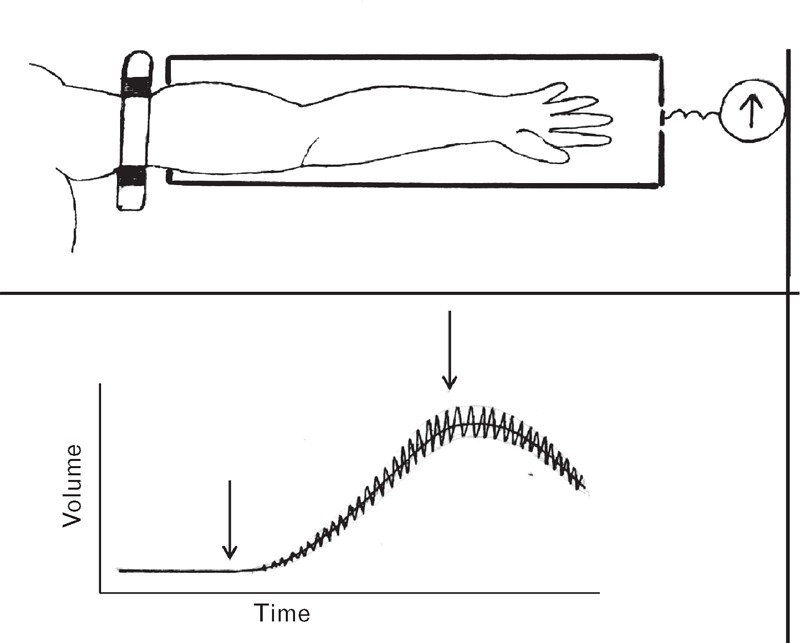
Method of venous occlusion plethysmography. The arm is enclosed in a rigid airtight box, sealed at junction of arm and box. A tourniquet is applied to the arm outside the box, sufficient to prevent blood passing out, but insufficient to prevent blood passing in. The manometer within is calibrated to generate flow from pressure change in the box. Before inflating the tourniquet, pressure is atmospheric and there is no pulsation of pressure. After tourniquet inflation, pressure rises in the box and shows marked pulsations that correspond with the arterial pulse.
Finger photoplethysmography
Digital photoplethysmography is widely used in anesthetic and medical practice to confirm mechanical action of the heart throughout a surgical procedure and most useful when surgical drapes interfere with observation of the patient. The device also provides a good measure of oxygen saturation of blood in small vessels. It is used instead of the cuff sphygmomanometer and electrocardiogram in many simple operative procedures. Although arterial pulsations are a source of noise in venous occlusion plethysmography, they are the objects of scrutiny in finger photoplethysmography. As in volume plethysmography, no pulsatile signal is obtained until arterial blood vessels in the finger are compressed. Pressure is applied by the device to maximize the pulsatile signal, which can then be recorded for hours. The pulsatile signal summates all pulsatile volume change in arterial vessels between light emitter and light sensor, and its waveform is similar to the carotid rather than the RAP pulse. The pulsations of the device are utilized for monitoring pulsatile cardiac activity.
Oscillometric sphygmomanometry
The oscillometric sphygmomanometer is increasingly used in medical practice for the measurement of arterial pressure over the brachial or radial artery and has all but completely replaced the mercury sphygmomanometer and Korotkov sound technique. Pressure is applied to the artery beneath through a cuff that encircles the upper limb above the elbow, and measurements of systolic and diastolic pressure are gained from proprietary algorithms that are based on change in the pressure pulsations within the cuff as this is inflated to suprasystolic pressure and then allowed to drop to infradiastolic pressure. The mean pressure is taken to be the pressure in the cuff when pulsations are maximal [17], and systolic pressure is estimated up and diastolic pressure down with proprietary algorithms. Above systolic pressure and below diastolic pressure, pulsations in the cuff disappear.
Palpation of the pulse, applanation tonometry
The principle of arterial pressure unloading is widely applied in medical practice. For palpation of the radial artery at the wrist, one needs to exert gentle pressure over the artery. No pulsation is apparent when the finger of the examiner is applied lightly to the skin over the radial artery. In applanation tonometry, the round wall of an artery needs to be applanated (flattened) to record an accurate pressure signal of intraarterial pressure [18].
Intracranial circulation and its physiological model
Changes in pressure and flow within the cranium need be considered on the basis of the well established Monro–Kellie doctrine. This (Fig. 6) states that because the skull and spinal canal space is fixed, and its contents are incompressible, any change in one component (brain, CSF, venous blood, or arterial blood) must entail reciprocal change in the volume of other components. The history of this concept is fascinating and based on relatively good state of the brain, apparently protected within the skull in persons who had died violently [8,10,11]. It is also based on venous drainage from the cranium that is easy and free, when a person is standing, sitting, or lying with head slightly elevated (as customary in intensive care), with the jugular vein in the neck semicollapsed and at atmospheric pressure.
FIGURE 6.
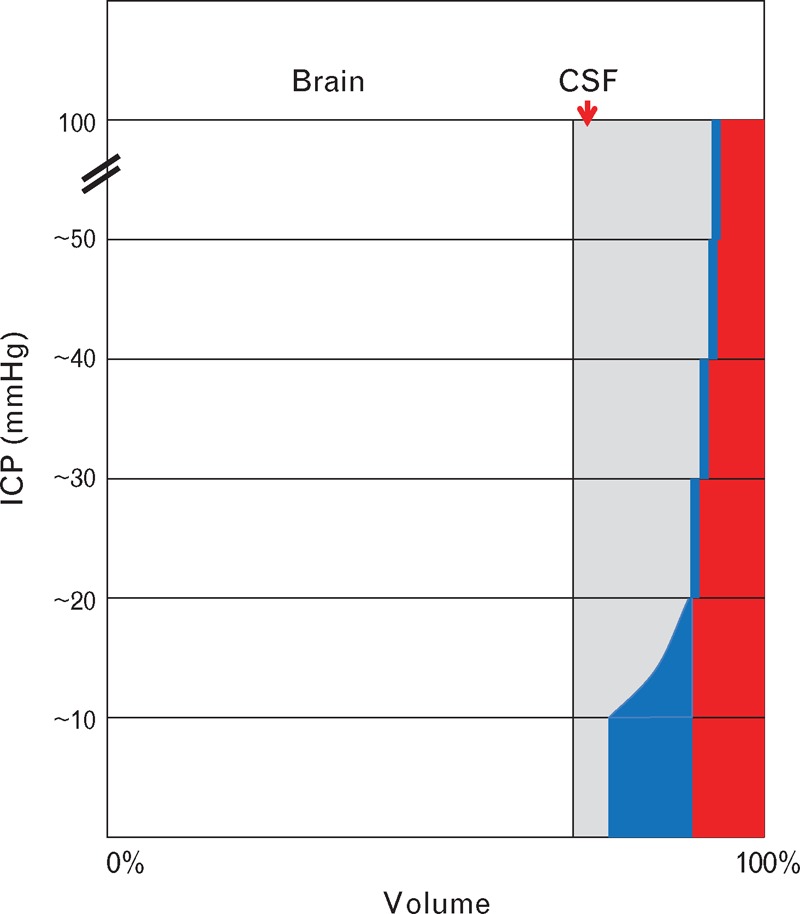
Illustration of Monro–Kellie principle. The cranium (and its extension into the spinal canal) has a fixed volume and contains four components: brain (and its extension as the spinal cord), cerebrospinal fluid (CSF), venous blood (blue), and arterial blood (red). If one component increases, another must decrease. Under normal conditions, intracranial pressure (ICP) is near atmospheric. If volume of a component rises (here CSF, but could be brain), there is progressive decrease in venous blood through venting from venous sinuses into the jugular vein in the neck. As pressure rises further, the veins are flattened and arteries are progressively narrowed.
When for any reason, pressure begins to rise in the cranium, venous blood from its small veins and sinuses is first displaced, mostly through the jugular foramen into the neck and down into the right atrium. With walls of the veins and sinuses lax, and the CSF and ventricles likewise, pressure is now still near normal with respect to all parts of the brain except the arteries of supply.
As pressure continues to rise in the cranium beyond around 15–25 mmHg, a large amount, possibly most, of the venous blood is displaced into the jugular veins. From this point, ICP would be expected to rise steeply, because mechanical compensation (loss of venous blood from the cranium) would be near complete. The high-pressure arteries are next to be compromised, because there is no way out of the cranium and spinal canal for CSF or other brain contents. The arteries are narrowed from compression by ICP and transmural pressure is decreased, and is now represented by CPP (= CAP – ICP), which is also CTP. Arteries outside the cranium are not compressed.
Decrease in arterial pressure within the skull, relative to ICP has three ill effects:
to decrease CPP (and mean flow – if decrease in CPP is reaching below lower limit of autoregulation) by an amount equal to ICP (i.e. CPP = CAP − ICP),
to unload the arterial wall, so that its CTP is decreased (to CPP from CAP) – and the artery pulsates more over the cardiac cycle with each beat of the heart. Every intracranial artery behaves as though it is more distensible (Fig. 7), and
to cause increased pulsations of flow velocity in the MCA and other arteries from compression and narrowing of these large low-resistance arteries. These explanations account for the observation in the eight patients (Figs 1–4, Table 1). The increase in ICP pulsations are explicable on the basis of greater pulsatility of all intracranial arteries, with increase in lateral expansion with each beat of the heart transmitted into and throughout the brain, and apparent as increase in ICP pulsations (Fig. 3).
FIGURE 7.
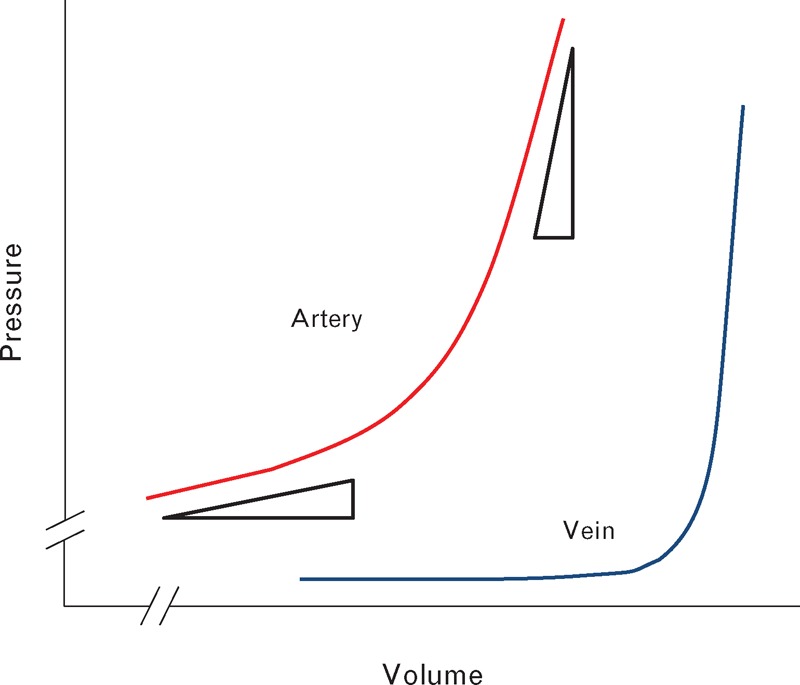
Theoretical pressure/volume relation in a vein and an artery to explain phenomena observed. At low pressure, veins (blue) have high volume and accommodate more or less blood with little change in transmural pressure. Arteries (red) are round and always distended, and at higher pressure. Change in volume of an artery at low mean pressure is much larger than change in volume at a higher mean pressure. The artery shows greater volume pulsation at a given change of pressure across its wall than at a higher mean pressure.
Figure 3 shows data from all eight patients combined. The logarithmic regression line for the patients resembles the pressure/diameter relationship established for exposed systemic arteries (Fig. 7). Similarity indicates a common mechanism. In Fig. 7, the mean pressure appears on the ordinate and volume on the abscissa, whereas in Fig. 3, the amplitude of ICP pulse appears on the ordinate and mean ICP on the abscissa. Findings for Fig. 3 show increasing pulsation with increasing mean ICP, whereas Fig. 7 shows the inverse – increasing arterial pulsation with decreasing arterial CTP. This relationship between mean and pulsatile ICP (Fig. 3) corresponds to that described as ‘intracranial compliance’, when measured as the increase in ICP, achieved by infusion of fluid into the subarachnoid space [19,20]. With total intracranial volume constant (Monro–Kellie doctrine) (Fig. 6), fluid infused can only displace blood from the cranium and so decrease arterial volume and create higher arterial pulsations as seen in Fig. 7.
The exponential relationship between pressure and volume in exposed arteries (with atmospheric pressure outside) is attributable to the two-phase (elastin and collagen) structure of elastic arteries, with elastin fibers bearing most of the load at low or normal pressures, but with collagen elements being progressively recruited as pressure within the lumen rises [15].
Clinical implications
Ill effects of high cerebral artery flow pulsations are most apparent in children with sickle cell anemia. In such children, at high MCAFV pulsations (with peak >180 cm/s), stroke is more likely than at lower flow velocities [21], and can be prevented by reducing high flow pulsations through blood transfusion [22]. Cerebral blood flow velocities in children are generally greater than in adults, that is, higher than 100 cm/s peak, but in adults, high cerebral flow pulsations do carry the same ominous prognosis (in some cases on account of accompanying but unrecognized high ICP). In adults with subarachnoid hemorrhage, survival after aneurysm clipping or coiling is better in patients with lower pulse pressure [23]. Outcome after stroke is also better in patients with low central pulse pressure and augmentation index [24]. Ill effects of high pressure and flow pulsations – tearing of medial muscle and dislodgment of endothelial cells, respectively – have been established by Byrom [25] and Russell [26] in acute and chronic hypertension for humans and animals, and by Fry at the US National Institutes of Health in animals [27,28].
Byrom [25], in relation to acute cerebral hemorrhagic lesions, stated ‘... These facts suggest very strongly that the essential lesion is simply local overstretching or tearing of medial muscle fibres which may simultaneously kill the muscle and turn the vessel wall into a semi-permeable, permeable or visibly punctured membrane which allows fluid of varying colloid content to escape into the tissue while tending itself to become saturated with concentrated plasma protein or blood ... (but) the underlying morbid process is essentially reversible ...’.
Fry [27], in relation to endothelial shedding and thrombotic lesions, said ‘... When the endothelial surface is exposed to shearing stresses in excess of some critical value ... the cell appears to become mechanically unstable and is washed away from its moorings to the basement membrane ... Continued exposure appears to produce erosion of the subjacent intrafibrillar matrix ... and deposition of thrombotic material ... ’; he continued [28] ‘... In some ways the vascular surface may be thought of as a thatched roof. Very high winds will cause erosion and evulsion of the thatch, producing a leaky roof. Less severe unidirectional winds will simply cause orientation of the thatch in the direction of the wind making a more waterproof roof. Winds that are constantly changing direction will cause the thatch to become disordered, again producing a leaky roof ...’.
Limitations
We did not measure arterial pressure directly within the cranium. We did generate the CAP waveform, which is an advance over reliance on radial pressure. The CAP waveform is variably amplified and distorted in transmission through the upper limb [29]. We did assume, as have many others, that CAP is equivalent to (i.e., a surrogate of) carotid artery pressure in the terminal branches of the internal carotid (middle and internal carotid arteries). Because these arteries have similar vascular beds, are close together with few branches, and have similar flow velocities measurable within [30], this assumption would seem reasonable. We were not always confident with the stable position of TCD probes, but the changes with ICP and associations with arterial pressure were consistent and significant in data analyzed (Tables 1 and 2). We assumed that raised ICP during ‘plateau waves’ was representative of raised ICP from other causes, at least with respect to its effects on cerebral pressure and flow patterns. We recognize that this is not always the case and that brief increases in ICP, such as shown in Fig. 2, do not carry the same ominous prognosis as prolonged rises in ICP that persist over hours or days, and do not respond to treatment. Hemodynamic and ICP changes were continuous and explicable by the same mechanism, and appeared to be due to the physical effects of change in ICP within and outside the normal range.
Possible future therapeutic applications
The central pulsatile hemodynamic indices measured here do have relationships with ICP, and could provide ways to estimate this noninvasively through continuous monitoring in the intensive care ward. We have attempted this previously [31] with attention focused on Gosling's pulsatility index [2], and with some success. We did not, at the time, consider differences in pressure and flow waveforms as seen at different ages nor the association with other indices that is predictable from theoretic principles. We need to consider that indices of ICP pulsations may be more important than mean ICP level [23]. We will pursue these issues.
Extensions of this work could lead to a generalized change in therapy of closed head injury and stroke. At present, there is considerable debate on the value of decompressive craniectomy in treatment of closed head injury and major thrombotic stroke [32], and of thrombolysis or angioplasty and stenting in acute stroke [33]. These treatments carry high morbidity and mortality, but the diseases they are used to treat carry more risk, as suggested by the classic surgical aphorism ‘Diseases desperate grown, by desperate measures are relieved, or not at all’ (anonymous). If high pressure and/or flow pulsations in cerebral arteries are the cause of acute thrombotic stroke, as in subarachnoid hemorrhage [23] and children with sickle cell anemia [19], and if such pulsations can be suppressed, then measures such as extracorporeal membrane oxygenation (ECMO), which can create nonpulsatile flow, or use of nonpulsatile mechanical pumps for cardiac bypass [34] could be considered. Treatments such as ECMO are increasingly used for a period of days to weeks in patients who are potentially recoverable from acute cardiac or respiratory failure [35].
Short of ECMO and cardiac bypass treatment, other measures can be used to reduce pulsatility of pressure (and flow) that enters the cranium through the cerebral arteries [33,34]. Such measures include use of nitrates and calcium channel inhibitors – not for their effects on the cerebral arteries themselves, but for decreasing the damaging effects of early wave reflection from the lower body [36,37]. Wave reflection is the cause of pressure augmentation, and apparent in the data presented here, as a secondary late systolic boost to pressure and flow waves in the cerebral vasculature, and is even more marked in ICP waveforms.
ACKNOWLEDGEMENTS
This study was supported by the National Institute of Health Research, the Biomedical Research Centre (Neuroscience Theme), and the Medical Research Council (Grants G0600986 and G9439390). J.D.P. has received the NIHR Investigator Awards. M.O.K. is sponsored by an Australian Postgraduate Awards Industrial Linkage Grant from the Australian Research Council (LP0884094), with AtCor Medical Australia as the collaborating organization.
Conflicts of interest
M.F.O. is a founding director with AtCor Medical P/L, manufacturer of pulse wave analysis system, SphygmoCor, and Aortic Wrap P/L, developer of methods to reduce aortic stiffness, and a consultant with Novartis and Merck. P.S. and M.C. are inventors of ICM+ software, licensed by Cambridge Enterprise Ltd, UK. They both have a share in licensing fees. The other authors have no disclosure.
Reviewers’ Summary Evaluations
Reviewer 2
The paper carefully describes the simultaneous changes of intracerebral, radial and aortic pressure, more than 200 years after the enunciation of the Monro-Kellie doctrine on the hemodynamic implications of fixed intracranial space occupied by brain, cerebrospinal fluid and blood. Its strength is the new and clear demonstration of how the changes of intracerebral pressure (ICP) affect blood flow patterns within the brain. Its weakness, however, is the poor demonstration of the cause-effect relationships between ICP fluctuation and arterial wave reflection that may be relevant for the generation of ICP waves and for therapeutic purposes.
Reviewer 3
This study shows the relationship between simultaneously recorded central aortic pressure and middle cerebral artery flow waveforms, showing a similar contour of both during an elevation of intra-cranial pressure. High pressure and flow pulsation in large and small vessels are implicated in development of secondary brain damage after closed head injury. The results of this study emphasize the importance of reducing intra-cranial pressure, when raised, and aim to improve the management of patients in neurosurgical intensive care to prevent secondary brain damage following closed head trauma, ruptured intracranial aneurysm and other neurosurgical procedures.
Footnotes
Abbreviations: CAP, central aortic pressure; CPP, cerebral perfusion pressure; CSF, cerebrospinal fluid; CTP, cerebral artery transmural pressure; ECMO, extracorporeal membrane oxygenation; GTF, generalized transfer function; ICP, intracranial pressure; MCA, middle cerebral artery; MCAFV, middle cerebral artery flow velocity; RAP, radial artery pressure; SAH, subarachnoid hemorrhage; SEM, standard error of mean; TCD, transcranial Doppler; FDA, Food and Drug Administration
REFERENCES
- 1.Castellani G, Zweifel C, Kim DJ, Carrera C, Radolovich DK, Smielewski PK, et al. Plateau waves in head-injured patients requiring neurocritical care. Neurocrit Care 2009; 11:143–150. [DOI] [PubMed] [Google Scholar]
- 2.Zweifel C, Czosnyka M, Carrera E, de Riva N, Pickard JD, Smielewski PK. Reliability of the blood flow velocity Pulsatility Index for assessment of intracranial and cerebral perfusion pressures in head-injured patients. Neurosurgery 2012; 71:853–861. [DOI] [PubMed] [Google Scholar]
- 3.Kim DJ, Kasparowicz M, Carrera E, Castellani G, Zweifel C, Lavinio A, et al. The monitoring of relative changes in compartmental compliances of brain. Physiol Meas 2009; 30:647–659. [DOI] [PubMed] [Google Scholar]
- 4.Wagshul ME, Eide PK, Madsen JR. The pulsating brain: a review of experimental and clinical studies of intracranial pulsatility. Fluids Barriers CNS 2011; 8:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Folkow B, Gaskell P, Waaler B. Blood flow through limb muscles during heavy rhythmic exercise. Acta Physiol Scand 1970; 80:61–72. [DOI] [PubMed] [Google Scholar]
- 6.O’Rourke MF. Vascular impedance in studies of arterial and cardiac function. Physiol Rev 1982; 62:570–623. [DOI] [PubMed] [Google Scholar]
- 7.Folkow B, Svanborg A. Physiology of cardiovascular aging. Physiol Rev 1993; 73:725–764. [DOI] [PubMed] [Google Scholar]
- 8.Moore W. The knife man. New York: Broadway; 2005. [Google Scholar]
- 9.Kety S. Fishman RP, Richards DW. The Cerebral Circulation. Circulation of the blood: men and ideas. New York: Oxford University Press; 1964. 703–742. [Google Scholar]
- 10.Monro A. Observations on structure and function of the nervous system. Edinburgh: Creech and Johnson; 1783. [Google Scholar]
- 11.Kellie G. Appearance observed in the dissection of two individuals; death from cold and congestion of the brain. Trans Med Chir Soc Edinburgh 1824; 1:84. [PMC free article] [PubMed] [Google Scholar]
- 12.Mokri B. The Monro-Kellie hypothesis: applications in CSF volume depletion. Neurology 2001; 56:1746–1748. [DOI] [PubMed] [Google Scholar]
- 13.Kim MO, Li Y, Wei F, Wang J, O’Rourke MF, Avolio A. Relationship between pressure and flow waveforms in cerebral arteries of 1020 subjects: establishing normal values. Circulation 2014; 130:A12309. [Google Scholar]
- 14.O’Rourke MF, Safar ME, Dzau V. Arterial vasodilation: mechanisms and therapy. Edinburgh: Lea and Febiger; 1993. [Google Scholar]
- 15.Pauca A, O’Rourke M, Kon N. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension 2001; 38:932–937. [DOI] [PubMed] [Google Scholar]
- 16.Smielewski P, Czosnyka M, Steiner L, Belestri M, Piechnik S, Pickard JD. ICM+: software for on-line analysis of bedside monitoring data after severe head trauma. Acta Neurochir Suppl 2005; 95:43–49. [DOI] [PubMed] [Google Scholar]
- 17.Oliver G. Pulse-gauging: a clinical study of radial measurement and pulse-pressure. London: HK Lewis; 1895. [Google Scholar]
- 18.Drzewiecki GM, Melbin J, Noordergraaf A. Arterial tonometry: review and analysis. J Biomech 1983; 16:141–152. [DOI] [PubMed] [Google Scholar]
- 19.Marmarou A. A theoretical and experimental evaluation of cerebral fluid system [thesis]. Philadelphia, PA: Drexer University; 1973. [Google Scholar]
- 20.The Hague, Avezaat CJJ, van Eijndhoven JHM. Cerebrospinal fluid pulse pressure and craniospinal dynamics: a theoretical, clinical and experimental study. 1984; Jongbloed, [Google Scholar]
- 21.Kwiatkowski JL, Granger S, Brambilla DJ, Brown RC, Miller ST, Adams RJ. For the STOP Trial Investigators. Elevated blood flow velocity in the anterior cerebral artery and stroke risk in sickle cell disease: extended analysis from the STOP trial. Br J Haematol 2006; 134:333–339. [DOI] [PubMed] [Google Scholar]
- 22.DeBaun MR, Gordon M, McKinstry RC, Noetzel MJ, White DA, Sarnaik SA, et al. Controlled trial of transfusions for silent cerebral infarcts in sickle cell anemia. N Engl J Med 2014; 371:699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eide PK, Bentsen G, Sorteberg A, Marthinsen PB, Stubhaug A, Sorteberg W. A randomized and blinded single-center trial comparing the effect of intracranial pressure and intracranial pressure wave amplitude-guided intensive care management on early clinical state and 12-month outcome in patients with aneurysmal subarachnoid hemorrhage. Neurosurgery 2011; 69:1105–1115. [DOI] [PubMed] [Google Scholar]
- 24.Soiza RL, Davie MM, Williams DJ. Use of the augmentation index to predict short-term outcome after acute ischemic stroke. Am J Hypertens 2010; 23:737–742. [DOI] [PubMed] [Google Scholar]
- 25.Byrom FB. The hypertensive vascular crisis. London: Heinemann; 1969. [Google Scholar]
- 26.Russell RW. How does blood pressure cause stroke? Lancet 1975; 2:1283–1285. [DOI] [PubMed] [Google Scholar]
- 27.Fry DL. Certain histologic and chemical responses of the vascular interface to acutely induced mechanical stress in the aorta of the dog. Circ Res 1969; 24:93–108. [DOI] [PubMed] [Google Scholar]
- 28.Fry DL. Responses of the arterial wall to certain physical factors. Ciba Foundation Symposium; Amsterdam, The Netherlands; 1973. pp. 93–125. [Google Scholar]
- 29.Adji A, O’Rourke MF. Brachial artery tonometry and the Popeye phenomenon: explanation of anomalies in generating central from upper limb pressure waveforms. J Hypertens 2012; 30:1540–1551. [DOI] [PubMed] [Google Scholar]
- 30.Nichols WW, O’Rourke MF, Vlachopoulos C. McDonald's blood flow in arteries. 6th ed.London: Hodder Arnold; 2011. [Google Scholar]
- 31.Kim MO, Butlin M, Li Y, Wei F, Wang J, O’Rourke MF, Avolio AP. Aortic, but not radial pressure, gives a model independent estimate of cerebral artery critical closing pressure. Artery Res 2013; 7:119. [Google Scholar]
- 32.Cooper DJ, Rosenfeld JV, Murray L, Arabi YM, Davies AR, D’Urso P, et al. DECRA Trial Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group. Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med 2011; 364:1493–1502. [DOI] [PubMed] [Google Scholar]
- 33.Kirkman MA, Citerio G, Smith M. The intensive care management of acute ischemic stroke: an overview. Intensive Care Med 2014; 40:640–653. [DOI] [PubMed] [Google Scholar]
- 34.Gupta S, Woldendorp K, Muthiah K, Robson D, Prichard R, Macdonald PS, et al. Normalisation of haemodynamics in patients with end-stage heart failure with continuous-flow left ventricular assist device therapy. Heart Lung Circ 2014; 23:963–969. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt M, Bailey M, Sheldrake J, Hodgson C, Aubron C, Rycus PT, et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am J Respir Crit Care Med 2014; 189:1374–1382. [DOI] [PubMed] [Google Scholar]
- 36.Pauca AL, Kon ND, O’Rourke MF. Benefits of nitroglycerine on arterial stiffness is directly due to effects on peripheral arteries. Heart 2005; 91:1428–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirata K, O’Rourke MF, Momura A. Favourable effect of sublingual nitrate on carotid flow and pressure augmentation index. J Clin Hypertens 2007; 9 Suppl A:A29. [Google Scholar]


