Abstract
Recent work shows that depression is intimately associated with changes in cognitive functioning, including memory, attention, verbal fluency, and other aspects of higher-order cognitive processing. Changes in cognitive functioning are more likely to occur when depressive episodes are recurrent and to abate to some degree during periods of remission. However, with accumulating frequency and duration of depressive episodes, cognitive deficits can become enduring, being evident even when mood improves. Such changes in cognitive functioning give depression links to mild cognitive impairment and thereby with neurodegenerative conditions, including Alzheimer’s disease, Parkinson’s disease, schizophrenia, and multiple sclerosis. Depression may then be conceptualized on a dimension of depression – mild cognitive impairment – dementia. The biological underpinnings of depression have substantial overlaps with those of neurodegenerative conditions, including reduced neurogenesis, increased apoptosis, reactive oxygen species, tryptophan catabolites, autoimmunity, and immune-inflammatory processes, as well as decreased antioxidant defenses. These evolving changes over the course of depressive episodes drive the association of depression with neurodegenerative conditions. As such, the changes in cognitive functioning in depression have important consequences for the treatment of depression and in reconceptualizing the role of depression in wider neuroprogressive conditions. Here we review the data on changes in cognitive functioning in recurrent major depression and their association with other central conditions.
Keywords: Cognition, Depression, Inflammation, Neurogenesis, Oxidants
Background
The symptoms of recurrent depressive disorders (rDD) affect four spheres of human functioning: emotional (depressed mood, discouragement, feelings of helplessness and hopelessness), cognitive (negative opinions of oneself, the surrounding world and the future, and cognitive dysfunctions), behavioral/motivational (lack of motivations, withdrawal from social activity, loss of interests) and physical/somatic (circadian rhythm changes, lack of appetite, physical fatigue). Mood and neurocognitive dysfunctions may be manifestations of shared structural, biochemical or immune-related mechanisms, thereby producing biological overlaps in the regulation of emotional states and memory [1].
According to Panza and colleagues [2], depression may form a continuum with the dementias, along the gradient depression – mild cognitive impairment (MCI) – dementia. An episode of severe depression increases the risk of progression from MCI to Alzheimer’s disease fourfold [2]. In a study of 436 women, aged 70–79, Rosenberg and colleagues demonstrated that symptoms of rDD positively correlated with an increased incidence of MCI among individuals in whom, prior to a diagnosis of depression, no cognitive deterioration was evident (Rosenberg et al., 2010). Other work likewise shows depression to increase the shift from MCI to dementia [3], with the number of depressive episodes increasing dementia susceptibility.
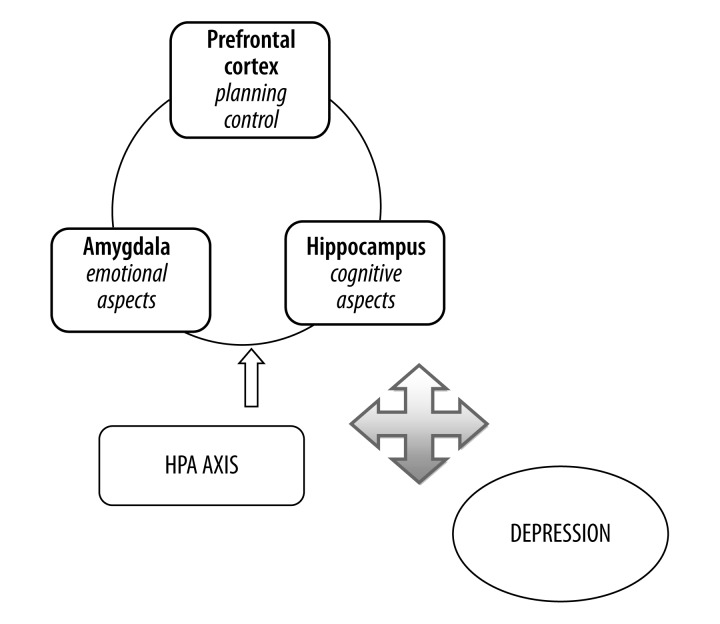
The course of cognitive dysfunctions in rDD is varied, partly dependent upon clinical features (depression subtype, disease duration, numbers of episodes) and therapy administered [4]. Measuring cognitive deficits may be difficult in patients of depression due to differences in cognitive tests used, as well as variations in socio-demographic parameters, such as age, gender (Table 1). Despite the difficulties in study comparisons that such factors introduce, this review analyzes the data pertaining to cognitive functioning in depressed patients. The interactions of cognition with mood may be a two way process, with some cognitive dysfunctions increasing the susceptibility to rDD, as well as decreased mood driving deficits in cognitive functioning (Figure 1). This hypothesis is supported by data on the profile of selected deficits in people with rDD (working memory, executive functions, episodic memory) and in their first-degree relatives [5].
Table 1.
Overview of studies related to neuropsychological deficits in depression.
| Authors | Patients N | Population | Age | Neuropsychological examination |
|---|---|---|---|---|
| Borkowska and Rybakowski, 2001. [124] | 30 | rDD + | – | Wechsler Adult Intelligence Scale-Revised (WAIS-R) |
| 15 | Bipolar depression | Trail Making Test (TMT) | ||
| Stroop test | ||||
| Verbal fluency test (VFT) | ||||
| Wisconsin Card Sorting Test (WCST) | ||||
|
| ||||
| Vinkers et al., 2004. [11] | 500 | Population based study | >85 | Mini-Mental State Examination (MMSE) |
| Stroop test | ||||
| Auditory Verbal Learning Test (AVLT) | ||||
| Letter digit coding test | ||||
|
| ||||
| Talarowska et al., 2010. [23] | 30 | rDD | 18–60 | AVLT |
| 57 | Healthy controls + | Stroop test | ||
|
| ||||
| Castaneda et al., 2010. [12] | 126 | rDD | 21–35 | Verbal and visual short-term memory |
| 71 | health controls + | Verbal long-term memory and learning | ||
| Attention | ||||
| Processing Speer | ||||
| Executive functions | ||||
|
| ||||
| Schaub et al., 2013. [13] | 74 | Major depression + | – | Verbal and visual short-term memory |
| 38 | Schizophrenia | Verbal fluency | ||
| Visual-motor coordination | ||||
| Information processing | ||||
| Selective attention | ||||
Figure 1.
Circle of depression.
Areas of Cognitive Dysfunction in rDD Patients
Cognitive dysfunctions in rDD
Cognitive deterioration in depressed patients can be heterogeneous, being selective, specific and mild in some instances, but general and severe in others [6]. Some work shows only a slight decrease in intellectual functioning in people with mild depressive states, as compared to healthy controls. However, a more severe depressive episode is more likely to be associated with decrements in specific cognitive functions, namely verbal memory and verbal fluency, even after sustained remission. This is exemplified in a study by Neu and colleagues, where 27 depressed patients were assessed by a series of neuropsychological tests at the beginning of the depressive episode and again after a euthymic phase of 6 months following treatment [7].
Typical cognitive symptoms include: decrements in memory, learning, attention, spatial visualization, visual-motor coordination, verbal fluency and psychomotor retardation, as well as decrements in executive functions, such as planning, problem solving and behavioral inhibition [4]. Deterioration in one or several essential cognitive processes may unfavorably influence other cognitive abilities.
Attention
Attention is a process that determines the scope and quality of information processing. Structures in the upper brain stem, as well as the reticular system of the mesencephalon, are intimately involved in the regulation of elementary forms of attention, in part via the maintenance of the awake state. Higher (selective) forms of attention to a given stimulus, with associated inhibition of peripheral stimuli, requires the participation of the brain structures associated with many higher order processes, and include: the limbic cortex (hippocampus, amygdala, caudate nucleus) and the frontal and parietal lobes of the cerebral cortex (the frontal callosal gyrus, frontal orbital cortex, dorsolateral prefrontal cortex), as well as parts of the basal nuclei and the thalamus [8]. Dysfunctions in the prefrontal cortex and the callosal gyrus found in imaging studies, are thought to be primarily responsible for attentional deficits in rDD patients, as exemplified in a study of 10 elderly people diagnosed, according to the DSM-IV criteria, with major depression of mild to moderate severity, as assessed by the Digit Span and Stroop tests [9].
Problems with both autopsychic (attention to self/own mind) and/or allopsychic (attention to outside world) cognition can occur in depressed patients, regardless of age. During cognitive testing, answers to questions may be delayed, including under the influence of an incentive, seemingly as a consequence of an additional effort from depressed patients. Attentional dysfunctions in depressed patients are at least partly driven by difficulties in ignoring negative information and inhibiting negative associations [10]. Subjectively, depressed patients report difficulties in maintaining concentration and attention, as well as problems in dividing and shifting attention [9]. Attentional deficits may be preceded by depression symptoms, as found in depressed patients over 85 years of age [11]. Similarly, in a group of 21depressed teenagers (aged 14–17), attentional deficits occurred over the course of a severe depressive episode, a finding replicated in 126 depressed patients (aged 21–35), where attentional dysregulation was accompanied by deficits in short-term visual memory [12]. Overall, attentional deficits accompany depression, sometimes preceding depression, across different age groups.
Short-term memory
Memory disorders are a common cognitive deficit in depressed patients, including short-term memory, especially when difficulty levels are increased. As a consequence primacy and recency effects, remembering words from the beginning and end of a list, may be relatively heightened in depressed patients during verbal list learning [13]. Difficulties in short-term memory may be driven by attention disorders, proactive inhibition and difficulties in information coding [14]. Even though anti-depressive drugs may have clinical efficacy, they may not adequately alleviate the accompanying cognitive deficits [15]. This might indicate a distinct pattern of cognitive deficits in patients with depression. Some of the deficits in memory, including short-term memory, in depressed patients may be a consequence of decreased metabolism in the hippocampus. Cigarette smoking, by the actions of nicotine on mitochondria, as well as on nicotinic receptors [16], is associated with improved cognitive performance in depressed smokers, leading to cigarette smoking being proposed as a form of self-medication for suboptimal cognition in depressed patients.
Long-term memory
Decrements in declarative memory may be particularly evident in depressed patients. The weakening of explicit memory efficiency is preceded by a diagnosis of depression and may be considered a pre-disease marker [14]. According to McBride [17], patients with rDD record worse results in tasks requiring identification of material previously learned, possibly indicating encoding difficulties in rDD memory deficits.
Depressive disorders also have an influence on the quality of reconstructed contents. Patients with depression find it easier to recall words, situations and facts of a negative rather than neutral valance, remembering better the tasks which ended in failure. This contrasts with dementia patients, who are more inclined to confabulate or fail to generate a response [17]. Moreover, in the course of rDD, new learning deteriorates in association with decreased work-rate and energy efficiency. Decrements in long-term memory may also precede the first occurrence of depressive symptomatology [11].
Temporal lobe damage, especially in the hippocampus of the dominant hemisphere, impairs learning, information storage and the identification of verbal material previously exposed visually or acoustically. Hippocampal volume reduction, commonly evident in depressed patients, is likely to contribute to the deterioration in declarative memory, in part via decreased neurogenesis [18]. In contrast, resection of the temporal lobe of the non-dominating hemisphere creates memory difficulties for non-verbal material, both visual and auditory, with the extent of the deficit dependent on the level of the structural damage in the medial surface of the temporal lobes [19]. Declarative memory requires the participation of the prefrontal cortex, hippocampus, fornix, mammillary bodies, thalamus, and callosal gyrus. In contrast, non-declarative memory requires the participation of the basal ganglia and cortical association areas of the parietal, occipital and frontal lobe. Neural systems underlying declarative memory may also participate in non-declarative memory, suggesting interactions between the different memory types [20], ultimately connecting via the striatum to behavioral outputs. The amygdala, altered in imaging studies in depression and closely linked to many CNS regions, is classically associated with the regulation of emotion, including emotion’s co-ordination with memory related processing [21] and emotion linked behavioral outputs. Data in rodents shows that the amygdala can over-ride the hippocampal and cortex inputs into the striatum [22], suggesting that changes in affective processing in depressed patients may alter the influence of higher order cognitive processes on motivated behavioral outputs and their accompanying thought outputs. This is one route by which the amygdala may contribute to the changes occurring in depression and requires investigation in rDD.
In a study by Talarowska and colleagues [23] on 30 rDD patients versus controls, rDD patients performed significantly poorer on a verbal learning task (auditory verbal learning test, AVLT, ten words), which allows the testing of both short- and long-term memory. rDD patients performed more poorly on both memory measures, even after 8 weeks of a pharmacological therapy with selective serotonin reuptake inhibitors (SSRIs). This suggests that cognitive decrements occurring in rDD are longer lasting than mood dysregulation and may provide the basis for the association of a continuum involving depression, MCI and dementia.
Operational memory and executive functions
The magnitude of frontal dysfunction in bipolar disorders in the depressed phase and in patients with MCI is significantly greater than in unipolar depression. Serious frontal dysfunction in bipolar disorder continues after the remission of a depressive phase, whilst in rDD a significant improvement may be observed [24]. In both conditions, cognitive deficits are comprised of decreased cognitive flexibility and deterioration in thinking efficiency [25]. Heightened frontal dysfunction in rDD patients is linked to hypofrontality, evident in neuroimaging studies. During the N-back test, a measure of frontal activity, depressed patients showed significant differences in prefrontal cortex activation, versus healthy controls [26]. As a physiological substrate for higher order cognitive functioning, the frontal cortex shows significant changes in activity in mood disordered patients that overlaps with the changes seen in MCI patients.
Verbal operational memory decrements are also observed in rDD patients when compared to controls, including deficits auditory and verbal operational memory [23]. Despite an improvement in mood following 8 weeks of SSRI treatment, these auditory and verbal operational memory deficits were still evident. Similar results were obtained by Joormann and colleagues [27], using a procedure based on the principles of the N-back test, which is supported by other work. Likewise, using an arithmetic based task, Hugdahl and colleagues also showed frontal operational deficits in depressed patients [28]. Overall, deficits in inhibitory frontal functioning and activity are common in depressed patients, which would be expected to contribute to heightened amygdala activity and amygdala influence on central processing, given that the frontal cortex forms a negative feedback loop with the amygdala. As such, frontal dysfunction would be expected to contribute to the susceptibility to recurrent depressive periods.
Operational memory and executive dysfunctions are also present in depressed children and teenagers, with frontal dysfunction more widely evident among healthy relatives of depressed patients. Children and adolescents of families with a history of depression also show perturbations in amygdala functioning, which is proposed to contribute to increased depression susceptibility [29]. As suggested above, this may be due to the prefrontal cortex and callosal gyrus regulating not only cognitive processes, but also mood, behavior and social relations, thereby contributing to deficits in mentalizing and humor processing (mind theory) [30]. Such higher order cognitive dysfunction may also be linked to decreased intra-cortex connectivity in depressed patients. Such changes in frontal cortex activity and its interactions with amygdala functioning means that operational memory decrements in rDD patients associates not only with difficulties in the planning of actions, undertaking a purposeful activity or reduction of mental plasticity, but also increases sensitivity to negative feedback, with difficulties, via aberrant amygdala emotional processing, in inhibiting and avoiding such types of information [31].
Executive dysfunction is especially evident in elderly depressed patients, aged above 61 years, although it is also present in younger adult patients [32]. Problem solving is hampered by difficulties in systematically generating possible solutions, due to deficits in planning, as well as the making and verifying of hypotheses. To some extent this is due to decrements in abstract thinking coupled to a cognitive bias that increases generalization and prototype formation at the expense of more specific exemplars [33]. This is thought to be underpinned by suboptimal functioning of the ventral lateral prefrontal cortex (PFC) [34].
Psychomotor speed and spatial abilities
Psychomotor retardation is a common depression symptom, which is thought to contribute to decrements in cognitive functioning, certainly in the case of timed tests. Spatial visualization function deficits may covary with the extent of depression severity [6], perhaps especially in patients with psychotic versus non-psychotic depression [35]. The importance of an adequate analysis of spatial relations in the perception and understanding of contextual information and linguistic communication should be emphasized, as well as in the recognition of emotional states of other people, as evident in deficits in recognition of facial expression, a deficit evident in adult [36], as well as adolescent depressed patients [37].
Verbal fluency
Verbal fluency disorders may take a variety of forms, including perseveration, the repeating of words or the formation of words that do not belong in a required category. Deficits in verbal fluency tests are generally a good diagnostic indicator of frontal lobe dysfunction, especially of the left hemisphere. However, a suboptimal verbal fluency test may also indicate alterations or dysfunctions of the left temporal cortex and/or PFC [38].
In depressed patients, the structure and content of statements may be relatively minimally impaired, yet distinct speech retardation can be evident [39]. Herrmann and colleagues showed that verbal fluency impairment in depressed patients may be due to a reduced level of oxyhemoglobin around their frontal lobes versus healthy controls [40]. Similarly, reduced blood flow and metabolic activity may be a link between cognitive changes and depression, especially in the elderly [41]. Other work links decreased verbal fluency to suboptimal neuronal activity around the caudate nucleus and the frontal part of the callosal gyrus in the left hemisphere [42]. In patients who showed high self-reporting of depression compared to psychiatric evaluation, decrements in verbal fluency predict suicide risk [43], highlighting the importance of changes underlying verbal fluency deficits in the course of depression.
Physiological Underpinnings of Cognitive Dysfunctions in rDD
Circadian rhythm disorders
Desynchronized biological rhythms of physiological and metabolic functions may play a role in depression recurrence, with different alleles in circadian genes conferring an increased depression risk. Deficits in circadian synchronization can impact on many fundamental processes, including those that condition the adaptation to changes taking place in the surrounding world [44]. However, the most common circadian dysregulation studied in depression has been alterations in sleep. Sleep disorders are evident in approximately 80% of hospitalized depressed patients and in about 50% of community dwelling patients. Decreased sleep continuity and reduced sleep duration are most common. Additionally, people suffering from depressive disorders enter the first phase of rapid eye movement (REM) sleep after 60 minutes of sleep onset, which is 15–20 minutes sooner than the general population. The pattern of sleep stages is also altered in depressed patients, who show relatively increased REM sleep and reduced REM latency in the first hours after sleep onset [45]. The genetic influence on depression susceptibility is heightened in both excessively long and short duration sleepers [46], highlighting the importance of the circadian rhythm in the etiology and course of depression. Sleep has diverse physiological roles that overlap with the pathways that lead to neuroprogression in depression, including pathways involved in the regulation of inflammation, oxidative repair and neurogenesis [47].
As a consequence of this, melatonin (N-acetyl-5-methoxytryptamine) has been proposed to play a significant role in the etiology, course and management of depression. Decreased melatonin levels are commonly observed in depressed patients, referred to as “low melatonin syndrome” [48]. Melatonin may modulate depression by a number of means. Low melatonin secretion may be associated with a genetic marker, which also contributes to a higher depression risk. However, dysregulated circadian melatonin secretion evidenced by elevated morning melatonin may also be associated with depression and, according to some authors, both significantly low and high melatonin concentrations may associate with depression [49]. Besides sleep induction and circadian rhythm regulation, melatonin regulates other physiological functions, including immune responses, antioxidative defenses, mitochondrial metabolic oxidative phosphorylation and memory. A number of studies show melatonin significantly improves cognitive function [50].
Physiologically, depression is classically associated with dysregulated serotonin, which is also the necessary precursor for the melatonergic pathway. Serotonin, a known positive modulator of cognition, is the immediate precursor of N-acetylserotonin (NAS), which is then catalyzed by N-Acetylserotonin O-methyltransferase (ASMT), also known as hydroxyindole-O-methyltransferase (HIOMT) [51], to form melatonin. ASMT is the rate-limiting enzyme in melatonin synthesis [52]. The ASMT gene is located in the pseudoautosomal region of the X chromosome, which is a candidate region for the development of mental illness [53].
A decrease in ASMT mRNA and protein have been significantly associated with cognitive impairment in 236 rDD patients, as measured by the Trail Making Test, part A (TMT) and verbal fluency tests [54]. Reduced ASMT gene expression in rDD patients is therefore related to suboptimal functioning in the frontal lobes and hippocampus, areas that underpin working memory, attention and verbal fluency. These results are in line with the data obtained in animal models [55].
In line with a dimension of depression-MCI-dementia, melatonin has been found to be useful in the therapy of some neurodegenerative conditions [56]. A combination of melatonin with phototherapy improved cognitive functioning, mood, sleep, and behavior in 189 dementia patients (mean age 85.8 years) [57], resulting in a slower cognitive decline and less diminution of autonomy. The efficacy of melatonin is likely mediated by many processes, including its circadian regulatory, antioxidant, anti-inflammatory, and mitochondrial oxidative phosphorylation boosting effects [58]. Melatonin also regulates other factors known to modulate cognition, including neural cell adhesion molecule (NCAM). Melatonin may also be involved in the structural remodeling of synaptic connections during memory and learning processes, as well as hippocampal neurogenesis during normal aging in rodents [59]. Neurodegenerative diseases, such as Alzheimer’s disease or Parkinson’s disease are often associated with sleep disturbances [60], which can be an early symptom in these conditions that in turn contributes to cognitive and motor symptoms. Chronic administration of melatonin significantly reduces lipid peroxidation and restores the decreased glutathione levels induced by chronic hyperhomocysteinemia in mice [61]. These authors also found that learning and memory deficits were reversed by chronic melatonin treatment.
It should also be noted that not all melatonin effects will occur via pineal release and circadian regulation. Melatonin is produced by possibly all mitochondria containing cells [62], with data showing its non-circadian production by astrocytes, where its synthesis is regulated by the Alzheimer’s susceptibility gene apolipoprotein (Apo) E4 [63]. The activational phenotype of macrophages is determined by the autocrine effects of melatonin [64], suggesting that variations in melatonergic genes and receptors may significantly modulate the nature of the immune response. As such, non-circadian melatonin release is likely to be important in both mood and neurodegenerative disorder processes, as well as to their overlaps with cognitive dysfunction.
Activity of the hypothalamic-pituitary-adrenal axis
The hypothalamic-pituitary-adrenal axis (HPA) plays a fundamental role in the body’s response to psychological and physical stress. Acute stress has its own natural negative feedback loop, which is blunted under conditions of chronic stress [65], where heightened HPA-axis and autonomic system activity negatively impact on mood and cognitive functioning. Heightened HPA activity alters immune responses, increases cholesterol concentrations and arterial blood pressure, as well as inhibiting sex hormone production. Moreover, chronic cortisol elevation reduces feedback sensitivity, in part by decreasing the number of glucocorticoid receptors [66]. Additionally, long-lasting HPA-axis activation results in excessive influx of calcium ions to hippocampal neurons, sensitizing neurons to apoptosis; elevated intracellular calcium being an established biomarker in a diverse array of neuropsychiatric disorders. As a consequence, the mechanisms in charge of regulating stress reactions are impaired, thereby further contributing to impaired negative feedback [67].
Pathophysiologically, depressive disorders resemble chronic stress. HPA-axis dysregulation is evident in 50% to 75% of depressed patients [68], as indicated by the following symptoms: increased concentration of glucocorticoids in plasma, urine and cerebrospinal fluid; changes in the daily profile of glucocorticoids secretion with more frequent and longer periods of secretion; increased secretion of glucocorticoids in response to adrenocorticotropic hormone (ACTH); and increased volume of the hypophysis and adrenal glands [68]. Additionally, increased cerebrospinal fluid corticotrophin releasing factor (CRF) and arginine vasopressin (AVP) are evident in depressed patients, often coupled to sympathetic system hyperactivity. Importantly, HPA-axis dysregulation is significantly more prominent in depressed patients with recurrent episodes and longer duration [69], representing a possible biomarker of different stages over depressive episodes. Increased morning salivary cortisol in depressed patients correlates with decreased executive functions and memory processes [70]. The hippocampus is particularly susceptible to stress-induced functional changes and HPA-axis dysregulation, leading to a drop in the expression of brain-derived neurotrophic factor (BDNF), deterioration of long-term potentiation and the inhibition of neurogenesis in the dentate gyrus [71]. These stress associated changes are highly prevalent in depressed patients.
It is of note that chronic stress/cortisol, but not acute, increases monoamine oxidase that increases the degradation of monoamines, including serotonin [72,73]. This may contribute to how stress contributes to driving the decreased serotonin that is commonly found in depressed patients. This also suggests that chronic stress/cortisol will decrease serotonin availability for NAS and melatonin synthesis, in turn decreasing the release of melatonin from astrocytes and other cells, thereby altering the nature of the immune response both centrally and systemically, as indicated above. NAS, the immediate melatonin precursor, is a BDNF mimic, activating its receptor, tyrosine receptor kinase B (TrkB). Both NAS and melatonin increase neurogenesis, suggesting that neurogenesis deficits in depressed patients may be mediated by the lack of availability of serotonin for NAS and melatonin synthesis [73]. As such, the regulation of the serotonin-NAS-melatonin pathway by chronic stress may contribute to many of the changes seen in depression, including decreased neurogenesis, decreased antioxidants, increased ROS, decreased mitochondrial functioning and altered immune responsivity. These changes are likely to contribute to decreases in mood and cognition, as well as to the changes occurring over the course of depression, termed neuroprogression [73].
Neuroprogression
Neuroprogression is the term used to describe changes in many psychiatric conditions that overlap these conditions with neurodegenerative disorders. Neuroprogression includes decreases in neurogenesis and neuroplasticity as well as increases in apoptotic or neurotoxic susceptibility. The brain structural changes most evident in depressed patients include: reduced volume in frontal lobes, prefrontal orbital cortex, frontal callosal gyrus, hippocampus and amygdala [74]. Moreover, hyperintense foci in the cortex and in white matter are common in depressed patients [75], with severely depressed patients showing decreased white matter integrity in the limbic system and frontal cortex. These changes are found early in the course of depression, being evident in adolescents and at first hospital presentation. Microstructural changes in cortical and subcortical white matter contribute to neuronal dysregulation and alterations in inter-area connectivity. It is of note that both NAS and melatonin decrease white matter loss and increase re-myelination, suggesting that stress-induced decreases in local white matter serotonin may contribute to white matter alterations, in part via decreased NAS and melatonin synthesis. This may be especially important in multiple sclerosis, another neurodegenerative condition that is highly associated with depression [76].
Orbitofrontal cortex (OFC) deficits may have an important role in the etiology of rDD, which has direct and indirect links with structures important in emotion regulation and executive functions, including the hippocampus, amygdala, ventral part of the striatum, frontal region of the callosal gyrus, hypothalamus and medial part of the frontal lobe. Recent work has shown that decreased OFC volume is evident in patients with severe depression [77].
Gradual reductions in hippocampal volume occur with successive depressive episodes, averaging an 8% decrease in the left hemisphere and 10% decrease in the right hemisphere. Young and non-treated patients in the families of depressed patients also show reduced hippocampus volume versus controls, suggesting a genetic link to decreased hippocampal volume in depression [78]. Hypercortisolemia and its associated impacts are also likely to modulate hippocampal volume [79], driving some of the changes occurring in neuroprogression. Animal models of stress and depression also highlight an important role for neuroprogressive changes in hippocampal sub-regions, including decreased dentate gyrus neurogenesis, as well as reduced length and number of apical dendrite branches in pyramidal cells of the CA1 and CA3 hippocampal regions [80]. The observed changes are linked with the length of depression episodes, number of episodes and a weaker therapeutic response to antidepressants.
The role of the right and left hemisphere of the brain
Damage to the brain’s right hemisphere makes facial and wider emotion identification in others difficult. Reduced negative emotion recognition is associated with an unfavorable prognosis over the course of depression. Right hemisphere damage is also associated with difficulties in recognizing non-verbal emotional stimuli, with characteristics that resemble alexithymia [81]. The right hemisphere, especially the dorsolateral area, plays a significant role in the regulation of negative emotions [82], thereby playing an important role in appropriate attentional processes, as well as in the nature of emotional responses during the course of depression.
During periods of remission, as compared to a depression episode, metabolism is increased in the right hemisphere dorsolateral PFC, lower parietal and dorsal cortex, frontal callosal gyrus. This is accompanied by a reduction in metabolism in the ventral limbic system (ventral callosal gyrus, as well as medial and posterior part of the insula) [83]. On the basis of the work highlighted above, mood changes in depressed patients may be a consequence of altered reciprocal interactions of the PFC, responsible for some cognitive processes, and structures of the limbic system. As indicated previously changes in the negative feedback of regions of the frontal and PFC on the amygdala may be of particular relevance, perhaps especially in the right hemisphere. Reduction in the thickness of the right cortex is also linked with a reduced ability during social interaction to notice, decode and remember emotional signals sent by other people [84].
The OFC is particularly important in balancing the information that determines emotional reaction and how appropriately to respond. Some work shows that both hemispheres of the OFC show increased activation in depressed patients [85]. Everyday life stressors correlate with an increased activity in the ventrolateral prefrontal cortex (VLPFC). This area is linked with a number of processes, including the cognitive control of emotions. Increased activation in the VLPFC may therefore represent increased effort to regulate emotions and/or be indicative of deficits in this area [86].
Activation of immune-inflammatory pathways
Over many decades, work has shown the brain to be a relatively “immunologically isolated” organ [87]. The CNS has been viewed as having its own immune system, which works relatively independently of the peripheral immune system. However, the peripheral immune system and the central immune-type cells, especially microglia and astrocytes are in continual communication [88]. Many experiments show that a systemic inflammation drives and/or intensifies a CNS immune response, thereby contributing to cognitive deficits [89]. As such, terms such as the “brain-gut axis” and “renal-brain axis” have become part of everyday medical conceptualizations of central-periphery interactions, with communication being not only neuronal but also via cytokines and variations in immune cell effluxes [90]. Astrocytes, by virtue of their enwrapment of brain endothelial cells are well placed to be important coordinators of the brain response to systemic inflammation.
The immune system changes functionally with age [88]. The activity of monocytes and macrophages decreases, Toll-like receptor expression goes down, secretion of oxygen radicals, nitric oxides (NO), chemokines and cytokines changes, thereby modifying the reaction of innate non-specific immunity [87]. Moreover, released inflammatory factors cause blood-brain barrier damage and increased central permeability, in turn increasing the extravasation of immunologically competent peripheral cells [89].
Proinflammatory cytokines, mainly tumor necrosis factor-alpha (TNF-α) and the interleukins (IL), are effluxed from both CNS and peripheral-derived immune cells and play a powerful role in the deterioration of cognitive processes (Bossù et al., 2012). In animal models, increased central proinflammatory cytokines are evident, especially in the hippocampus and PFC, in response to endotoxin injection (bacterial lipopolysaccharide; LPS). Such increases in proinflammatory cytokines are maintained for a period of up to 10 months after LPS administration. Additionally, proinflammatory cytokines intensify HPA-axis activation, with TNF-α playing an important role in the development of wider depression associated conditions such as atherosclerosis. Under conditions of systemic inflammation, TNF-α may drive down pineal melatonin production, in turn contributing to circadian dysregulation [92].
Oxidative and nitrosative stress
Oxidative stress is a state of relatively increased activity of reactive oxygen species (ROS). It develops as a consequence of an imbalance between the production and elimination of toxic derivatives of oxygen. A significant increase in the ratio of oxidizing agents over antioxidants may lead to irreversible changes in the organism that damages tissues and cellular processes, common in different pathological states [93]. While ROS have important signaling properties, excess ROS plays an important role in driving chronic inflammatory reactions, if the antioxidant response is not adequate. In physiological conditions, low levels of free radicals are maintained inside cells or may be transiently induced in a controlled manner as part of cellular plasticity or intercellular communication. However, ROS are also released by phagocytic cells to kill pathogenic organisms [94]. Stress caused by ROS leads not only to an inflammatory reaction, but also activates inflammatory gene transcriptions. Heightened or prolonged ROS leads to the inactivation of proteins containing thiol groups, inhibition of glycolysis by inactivation of glyecraldohyde-3-phosphate dehydrogenase, and DNA damage. ROS-driven protein oxidation results in their chemical modification, driving lipid peroxidation resulting in damage to membranes and their function [95].
Oxidative stress plays a significant role in the pathogenesis of neurodegenerative diseases and rDD [95]. The brain is particularly susceptible to oxidative damage, resulting from its high utilization of oxygen and from the presence of high levels of cellular lipids, including non-saturated fatty acids, with which free radicals easily react. Moreover, some regions of the human brain contain substantial amounts of metal ions, especially iron (Fe2/3+), copper (Cu2+), and zinc (Zn2+), which are conducive to ROS production. Additionally, lower concentrations of antioxidants are found in the CNS as compared to other organs [96].
ROS are therefore a powerful driver of neuroprogressive changes that drive down neurogenesis and are part of the pathophysiological bridge between depression and neurodegenerative processes [97]. Heightened central immune-inflammatory processes in association with increased ROS/antioxidant ratio mediate the inhibition of dentate gyrus neurogenesis as well as reducing hippocampal volume, with both processes consequently contributing to memory deficits [98]. A number of pro-inflammatory cytokines and their receptors drive this, including IL-1, IL-2, IL-6 and TNF-α. As with ROS, cytokines are an integral part of normal physiological functioning and plasticity at low concentrations, including IL-1, IL-6 and TNF-α, which are continuously produced in small concentrations by neurons and glia. CNS neurotransmitters also affect the synthesis and release of cytokines. Noradrenaline stimulates secretion of IL-6 from astrocytes, while receptors for the neurotransmitters (noradrenaline, dopamine, adrenaline, acetylcholine, serotonin, gamma-aminobutyric acid and endorphins) are located on the surface of immune system cells [99]. As such, ROS driven changes in neurotransmitter release are another mechanism whereby ROS and immune-inflammatory processes reciprocally interact.
Increased ROS/antioxidant ratio in conjunction with central inflammatory reactions are increasingly recognized to play an important role in the pathogenesis of a growing number of diseases, including many CNS diseases, such as Alzheimer’s disease, Parkinson disease, multiple sclerosis and stroke. Their role in the etiology and course of milder disorders of cognitive function is now recognized [100].
Cognitive dysfunction, often in conjunction with depression, also occurs in the course of somatic diseases including: chronic hepatitis C (CHC), systemic lupus erythematosus (SLE), circulatory system diseases [101], chronic obstructive pulmonary disease (COPD) [102], chronic fatigue syndrome (CFS), metabolic syndrome, type 1 and 2 diabetes [103] and influenza infections. The participation of inflammatory processes and oxidative stress has been shown in the etiology and course of each of these disorders, often co-existent with depression. In such disorders, an individual’s response to even moderate systemic inflammation may drive an increased CNS immune reaction.
We have previously shown that decreased operational memory (spatial visualization and auditory-verbal), declarative memory and verbal fluency in depressed patients were correlated with increased malondialdehyde (MDA) concentrations [104], nitrous oxide (NO) [105] and thiol protein groups (TPGs) [106], as well as decreased total antioxidant status (TAS) levels [107]. Such work highlights the relevance of oxidant changes to alterations in cognition that are evident in depressed patients.
Factors Affecting Patients’ Cognitive Functions in the Course of Recurrent Depressive Disorders
Intensification of clinical symptoms of depression and neurocognition
Cognitive deficits in the patients with rDD are in part dependent on the degree of mood symptoms intensity, with mood symptom remission usually associated with some improvement in cognitive functioning [108]. Han and colleagues [109], compared intensity of depressive symptoms (using the Hamilton Depression Rating Scale, HDRS) with cognition evaluated using the Mini-Mental State Examination (MMSE). Increases in HDRS scale scores were linked with poorer performance on the MMSE scale. In a study of 4392 people age over 65, every additional depressive symptom on the 10-item Center for Epidemiologic Studies Depression scale (CES-D) increased the risk of cognitive function deterioration by 5% over a five-year period [110]. This association was still maintained after excluding people with mild disorders of cognitive functions or chronic diseases. Moreover, increased HDRS rated depression negatively correlates with the auditory memory [111], operational memory efficiency [112], verbal fluency and attention processes. Again this links to data showing that increased levels of depression heighten the risk of dementia, with cognitive deficits in depressed patients also predicting the subjectively evaluated quality of physical, mental and social life [113]. This is likely to reflect a reciprocal relationship, whereby increased depression and cognitive symptoms modulate each other, at least in part via the overlaps in the underlying biological processes.
It is likely that the biological processes underpinning such ROS and immuno-inflammatory driven changes in mood and cognition lead to other biological processes that further decrease mood and cognition. Important wider processes include the pro-inflammatory cytokine induction of indoleamine 2,3-dioxygenase (IDO) and cortisol upregulation of tryptophan 2,3-dioxygenase (TDO) [114]. Both IDO and TDO take tryptophan away from serotonin synthesis and drive it down the kynurenine pathways to the formation of tryptophan catabolites (TRYCATs), such as the neurotoxic quinolinic acid and kynurenine. TRYCATs are also important regulators of neuronal activity, thereby allowing decreased serotonin and melatonin availability to be synchronized with alterations in neuronal patterning. As such, ROS, cytokines and cortisol have impacts both directly on neuronal and glia functioning, but also indirect effects via the differential induction of neuroregulatory TRYCATs.
Disease duration, staging and neurocognition
Decreased activity in the frontal callosal gyrus during a verbal fluency test differentiated elderly patients with rDD versus those with only one depressive episode [115]. In a study looking at changes in cognition in depressed patients, disease duration significantly negatively correlated with short- and long-term auditory memory, visual short-term memory and visual-motor coordination [116]. In this study, the number of depression episodes had the most prominent influence on the patients’ cognitive functions, correlating not only with short- and long-term auditory memory and with short-term visual memory, but also with verbal and visual-spatial operational memory and cognitive functions, as well as with reduced visual-motor coordination. A number of factors possibly contribute to this, including incomplete remission, variations in treatments used and permanent changes in the brain driven by the biological processes underpinning depression [79]. The latter is the essence of clinical staging in depression and the biological foundation of neuroprogression. Such changes in the nature of depression over time arise as a consequence of ROS and immuno-inflammatory processes, with the cellular damages that ensure further contributing to staging and neuroprogression, including via the induction of autoimmunity. Changes such as increased autoimmunity, will further contribute to alterations in cognition.
Remission of clinical symptoms and neurocognition
In the majority of cases, the remission of depressive symptoms leads to a significant improvement of cognitive functions [117], although not always. A number of studies have highlighted ongoing cognitive decrements during remission, including in verbal memory and verbal fluency after a 6-month remission period [118]. Other studies also support enduring cognitive deficits, including in episodic memory [108], attention and spatial visualization functions. Other work in remitted rDD shows decreased callosal gyrus activation during a verbal fluency test [119] and operational memory dysfunction, which deteriorates with successive depressive episodes. Decrements in executive functioning during periods of remission have also been shown, including in the elderly [120], as well in adults of working age. Some of the cognitive deficits in depression can be viewed as state-dependent. However, more persistent deficits in episodic memory and frontal functions are evident. As highlighted above, this is likely to be mediated by the anatomical and functional changes observed in the fields of the hippocampus and the frontal lobes in depressed patients.
Recently we have shown that successful treatment of rDD with SSRI’s for 8 weeks resulted in a number of cognitive improvements, including in the Stroop test (Naming Color of Words Different) and both parts of the Trail Making Test [111], indicative of improvements in spatial visualization, operational auditory and verbal memory, as well as psychomotor performance. As such, cognitive functioning deficits can improve with successful treatment in some rDD patients, but not in all. The precise nature of how an individual will acquire specific deficits and as to whether recovery occurs require further research, including how specific deficits correlate with the developing understanding of the biological underpinnings of depression. It should be emphasized that depression does not seem to be a static condition, but, via staging and neuroprogression, changes over recurrent episodes for a given individual. As such, different cognitive processes are likely to be differentially impaired as different stages and associated biological processes emerge, with therapy then having to be adapted accordingly. Such work should better clarify the association of depression with neurodegenerative disorders, such as Parkinson’s Disease, where cognition also changes over the course of the disease [121].
Conclusions
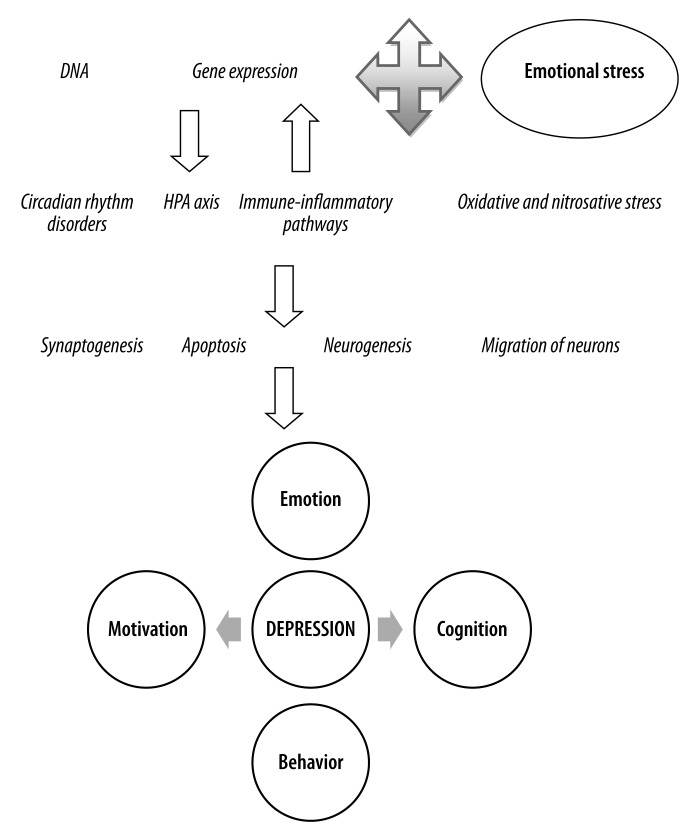
The data above highlights that depression is intimately associated with changes in cognitive functioning and thereby with a spectrum of neurodegenerative and neuroprogressive conditions. In some individuals, depression is an early manifestation of dementia, allowing a dimensional conceptualization of depression-MCI-dementia, especially in the elderly [122]. Recent conceptualizations of depression have emphasized the changing nature of its biological underpinnings over time, incorporating changes in TRYCATs, ROS, autoimmunity, apoptosis, neurogenesis, and immuno-inflammatory processes that form the foundation of a neuroprogressive process, which readily links to the biological factors driving neurodegenerative conditions such as Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis (Figure 2). It also highlights potential pathways that have the potential to be targeted therapeutically [123].
Figure 2.
Development of depression
Abbreviations
- ACTH
adrenocorticotropic hormone
- AD
Alzheimer’s disease
- APOE
apolipoprotein E4
- ASMT
N-Acetylserotonin O-methyltransferase
- AVLT
auditory verbal learning test
- AVP
arginine vasopressin
- BDNF
brain-derived neurotrophic factor
- CES-D
Center for Epidemiologic Studies Depression scale
- CFS
chronic fatigue syndrome
- CHC
chronic hepatitis C
- CNS
central nervous system
- COPD
chronic obstructive pulmonary disease
- CRF
corticotrophin releasing factor
- HDRS
Hamilton Depression Rating Scale
- HIOMT
hydroxyindole-O-methyltransferase
- HPA
hypothalamic-pituitary-adrenal axis
- IDO
indoleamine 2,3-dioxygenase
- IL
interleukin
- LPS
bacterial lipopolysaccharide
- MCI
mild cognitive impairment
- MDA
malondialdehyde
- MMSE
Mini-Mental State Examination
- N-acetyl-5-methoxytryptamine
melatonin
- NAS
N-acetylserotonin
- NCAM
neural cell adhesion molecule
- NCWd
Naming Color of Words Different
- NO
nitric oxide
- OFC
orbitofrontal cortex
- PD
Parkinson’s disease
- PFC
prefrontal cortex
- rDD
recurrent depressive disorders
- REM
rapid eye movement
- ROS
reactive oxygen species
- SLE
systemic lupus erythematosus
- SSRIs
selective serotonin reuptake inhibitors
- TAS
total antioxidant status
- TDO
tryptophan 2,3-dioxygenase
- TMT
Trail Making Test
- TNF-α
tumor necrosis factor-alpha
- TPGs
thiol protein groups
- TrkB
tyrosine receptor kinase B
- TRYCATs
tryptophan catabolites
- VLPFC
ventrolateral prefrontal cortex
Footnotes
Source of support: Michael Berk is supported by a NHMRC Senior Principal Research Fellowship 1059660. Michael Maes is supported by a CNPq (Conselho Nacional de Desenvolvimento Cientifico e Technologia) PVE fellowship and the Health Sciences Graduate Program fellowship, Londrina State University (UEL). Piotr Gałecki, Monika Talarowska – this study was supported with scientific research grants from the National Science Centre (no. 2011/01/D/HS6/05484 and no. 2012/05/B/NZ5/01452)
References
- 1.Rosenberg PB, Mielke MM, Xue QL, Carlson MC. Depressive symptoms predict incident cognitive impairment in cognitive healthy older women. Am J Ger Psych. 2010;18:204–11. doi: 10.1097/JGP.0b013e3181c53487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Panza F, Frisardi V, Capurso C, et al. Late-life depression, mild cognitive impairment, and dementia: possible continuum? Am J Geriatr Psychiatry. 2010;18(2):98–116. doi: 10.1097/JGP.0b013e3181b0fa13. [DOI] [PubMed] [Google Scholar]
- 3.Leonard BE. Inflammation, depression and dementia: are they connected? Neurochem Res. 2007;32(10):1749–56. doi: 10.1007/s11064-007-9385-y. [DOI] [PubMed] [Google Scholar]
- 4.Papakostas GI. Cognitive symptoms in patients with major depressive disorder and their implications for clinical practice. J Clin Psychiatry. 2014;75(1):8–14. doi: 10.4088/JCP.13r08710. [DOI] [PubMed] [Google Scholar]
- 5.Modrego PJ, Ferrández J. Depression in patients with mild cognitive impairment increases the risk of developing dementia of Alzheimer type: a prospective cohort study. Arch Neurol. 2004;61:1290–93. doi: 10.1001/archneur.61.8.1290. [DOI] [PubMed] [Google Scholar]
- 6.Gualtieri T, Johnson L, Benedict K. Neurocognition in depression: patients on and off medication versus healthy comparison subjects. J Neuropsychiatry Clin Neurosci. 2006;18(2):217–26. doi: 10.1176/jnp.2006.18.2.217. [DOI] [PubMed] [Google Scholar]
- 7.Neu P, Bajbouj M, Schilling A, et al. Cognitive function over the treatment course of depression in middle-aged patients: correlation with brain MRI signal hyperintensities. J Psychiatr Res. 2005;39:129–35. doi: 10.1016/j.jpsychires.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Funderud I, Løvstad M, Lindgren M, et al. Preparatory attention after lesions to the lateral or orbital prefrontal cortex an event related potentials study. Brain Res. 2013;1527:174–88. doi: 10.1016/j.brainres.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vasques PE, Moraes H, Silveira H, et al. Acute exercise improves cognition in the depressed elderly: the effect of dual-tasks. Clinics (Sao Paulo) 2011;66(9):1553–57. doi: 10.1590/S1807-59322011000900008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ng J, Chan HY, Schlaghecken F. Dissociating effects of subclinical anxiety and depression on cognitive control. Adv Cogn Psychol. 2012;8(1):38–49. doi: 10.2478/v10053-008-0100-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vinkers D, Gussekloo J, Stek M, et al. Temporal relation between depression and cognitive impairment in old age: prospective population based study. BMJ. 2004;329:881–91. doi: 10.1136/bmj.38216.604664.DE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castaneda AE, Marttunen M, Suvisaari J, et al. The effect of psychiatric co-morbidity on cognitive functioning in a population-based sample of depressed young adults. Psychol Med. 2010;40:29–39. doi: 10.1017/S0033291709005959. [DOI] [PubMed] [Google Scholar]
- 13.Schaub A, Neubauer N, Mueser KT, et al. Neuropsychological functioning in inpatients with major depression or schizophrenia. BMC Psychiatry. 2013;13:203. doi: 10.1186/1471-244X-13-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Airaksinen E, Wahlin Å, Larsson M, Forsell Y. Cognitive and social functioning in recovery from depression: results from a population-based three-year follow-up. Journal Affect Disord. 2006;96:107–10. doi: 10.1016/j.jad.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Luo LL, Chen X, Chai Y, et al. A distinct pattern of memory and attention deficiency in patients with depression. Chin Med J (Engl) 2013;126(6):1144–49. [PubMed] [Google Scholar]
- 16.Wang J, Kim JM, Donovan DM, et al. Significant modulation of mitochondrial electron transport system by nicotine in various rat brain regions. Mitochondrion. 2009;9(3):186–95. doi: 10.1016/j.mito.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McBride M. I’m not crazy, I’m just depressed: differential diagnosis of dementia vs. depression. Kans Nurse. 2006;81(9):4–7. [PubMed] [Google Scholar]
- 18.Anderson G, Maes M. Reconceptualizing adult neurogenesis: role for sphingosine-1-phosphate and fibroblast growth factor-1 in co-ordinating astrocyte-neuronal precursor interactions. CNS Neurol Disord Drug Targets. 2014;13(1):126–36. doi: 10.2174/18715273113126660132. [DOI] [PubMed] [Google Scholar]
- 19.Andersson C, Lindau M, Almkvist O, et al. Identifying patients at high and low risk of cognitive decline using Rey Auditory Verbal Learning Test among middle-aged memory clinic outpatients. Dement Geriatr Cogn Disord. 2006;21(4):251–59. doi: 10.1159/000091398. [DOI] [PubMed] [Google Scholar]
- 20.Bird C, Burgess N. The hippocampus and memory: insights from spatial processing. Nature Reviews. Nat Rev Neurosci. 2008;9(3):182–94. doi: 10.1038/nrn2335. [DOI] [PubMed] [Google Scholar]
- 21.Rutishauser U, Schuman E, Mamelak A. Activity of human hippocampal and amygdala neurons during retrieval of declarative memories. Proc Natl Acad Sci USA. 2008;105(1):329–34. doi: 10.1073/pnas.0706015105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGinty VB, Grace AA. Activity-dependent depression of medial prefrontal cortex inputs to accumbens neurons by the basolateral amygdala. Neuroscience. 2009;162(4):1429–36. doi: 10.1016/j.neuroscience.2009.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Talarowska M, Florkowski A, Zboralski K, et al. Auditory-verbal declarative and operating memory among patients suffering from depressive disorders – preliminary study. Adv Med Sci. 2010;55(2):317–27. doi: 10.2478/v10039-010-0053-0. [DOI] [PubMed] [Google Scholar]
- 24.Martinez-Aran A, Vieta E, Reinares M, et al. Cognitive functions across manic or hypomanic, depressed, and euthymic states in bipolar disorder. Am J Psychiatry. 2004;161:262–70. doi: 10.1176/appi.ajp.161.2.262. [DOI] [PubMed] [Google Scholar]
- 25.Kałwa A. Cognitive dysfunctions in bipolar disorders. Psychiatr Pol. 2011;XLV(6):901–10. [PubMed] [Google Scholar]
- 26.Harvey PO, Fossati P, Pochon JB, et al. Cognitive control and brain resources in major depression: an fMRI study using the n-back task. Neuroim. 2005;26:860–69. doi: 10.1016/j.neuroimage.2005.02.048. [DOI] [PubMed] [Google Scholar]
- 27.Joormann J, Gotlib IH. Updating the contents of working memory in depression: interference from irrelevant negative material. J Abnorm Psychol. 2008;117(1):182–92. doi: 10.1037/0021-843X.117.1.182. [DOI] [PubMed] [Google Scholar]
- 28.Hugdahl K, Rund BR, Lund A, et al. Brain activation measured with fMRI during a mental arithmetic task in schizophrenia and major depression. Am J Psychiatry. 2004;161:286–93. doi: 10.1176/appi.ajp.161.2.286. [DOI] [PubMed] [Google Scholar]
- 29.Pilhatsch M, Vetter NC, Hübner T, et al. Amygdala-function perturbations in healthy mid-adolescents with familial liability for depression. J Am Acad Child Adolesc Psychiatry. 2014;53(5):559–68. doi: 10.1016/j.jaac.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 30.Uekermann J, Abdel-Hamid M, Lehmkämper C, et al. Perception of affective prosody in major depression: A link to executive functions? J Int Neuropsychol Soc. 2008;14:552–61. doi: 10.1017/S1355617708080740. [DOI] [PubMed] [Google Scholar]
- 31.Schlosser N, Mensebach C, Rullkötter N, et al. Selective attention in depression: influence of emotionality and personal relevance. J Nerv Ment Dis. 2011;199(9):696–702. doi: 10.1097/NMD.0b013e318229d6cf. [DOI] [PubMed] [Google Scholar]
- 32.Watkins E, Brown R. Rumination and executive function in depression: an experimental study. J Neurol Neurosurg Psychiatry. 2002;72(3):400–3. doi: 10.1136/jnnp.72.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Talarowska M, Florkowski A, Gałecki P, et al. Cognitive functions and depression. Psychiatr Pol. 2009;43(1):31–40. [PubMed] [Google Scholar]
- 34.Badre D, Poldrack RA, Paré-Blagoev EJ, et al. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47(6):907–18. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 35.Jeste D, Heaton S. Clinical and neuropsychological comparison of psychotic depression with nonpsychotic depression and schizophrenia. Am J Psychiatry. 1996;153(4):490–96. doi: 10.1176/ajp.153.4.490. [DOI] [PubMed] [Google Scholar]
- 36.Kucharska-Pietura K, David AS. The perception of emotional chimeric faces in patients with depression, mania and unilateral brain damage. Psychol Med. 2003;33:739–45. doi: 10.1017/s0033291702007316. [DOI] [PubMed] [Google Scholar]
- 37.Jenness JL, Hankin BL, Young JF, Gibb BE. Misclassification and Identification of Emotional Facial Expressions in Depressed Youth: A Preliminary Study. J Clin Child Adolesc Psychol. 2014;14:1–7. doi: 10.1080/15374416.2014.891226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lanting S, Haugrud N, Crossley M. The effect of age and sex on clustering and switching during speeded verbal fluency tasks. J Int Neuropsychol Soc. 2009;15:196–204. doi: 10.1017/S1355617709090237. [DOI] [PubMed] [Google Scholar]
- 39.Lavender A, Watkins E. Rumination and future thinking in depression. Br J Clin Psychol. 2004;43:129–42. doi: 10.1348/014466504323088015. [DOI] [PubMed] [Google Scholar]
- 40.Herrmann LL, Goodwin GM, Ebmeier KP. The cognitive neuropsychology of depression in the elderly. Psychol Med. 2007;37(12):1693–702. doi: 10.1017/S0033291707001134. [DOI] [PubMed] [Google Scholar]
- 41.Alosco ML, Spitznagel MB, Cohen R, et al. Reduced cerebral perfusion predicts greater depressive symptoms and cognitive dysfunction at a 1-year follow-up in patients with heart failure. Int J Geriatr Psychiatry. 2014;29(4):428–36. doi: 10.1002/gps.4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okada G, Okamoto Y, Morinobu S, et al. Attenuated left prefrontal activation during a verbal fluency task in patients with depression. Neuropsychobiology. 2003;47(1):21–26. doi: 10.1159/000068871. [DOI] [PubMed] [Google Scholar]
- 43.Tsujii N, Akashi H, Mikawa W, et al. Discrepancy between self- and observer-rated depression severities as a predictor of vulnerability to suicide in patients with mild depression. J Affect Disord. 2014;161:144–49. doi: 10.1016/j.jad.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 44.Birchler-Pedross A, Frey S, Chellappa SL, et al. Higher frontal EEG synchronization in young women with major depression: a marker for increased homeostatic sleep pressure? Sleep. 2011;34(12):1699–706. doi: 10.5665/sleep.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Germain A, Kupfer DJ. Circadian rhythm disturbances in depression. Hum Psychopharmacol. 2008;23:571–85. doi: 10.1002/hup.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watson NF, Harden KP, Buchwald D, et al. Sleep duration and depressive symptom ms: a gene-environment interaction. Sleep. 2014;37(2):351–58. doi: 10.5665/sleep.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao Q, Peng C, Wu X, et al. Maternal Sleep Deprivation Inhibits Hippocampal Neurogenesis Associated with Inflammatory Response in Young Offspring Rats. Neurobiol Dis. 2014;68:57–65. doi: 10.1016/j.nbd.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 48.Wetterberg L. The relationship between the pineal gland and the pituitary-adrenal axis in health, endocrine and psychiatric conditions. Psychoneuroendocrinology. 1983;8(1):75–80. doi: 10.1016/0306-4530(83)90042-2. [DOI] [PubMed] [Google Scholar]
- 49.Hansen MV, Madsen MT, Hageman I, et al. The effect of MELatOnin on Depression, anxietY, cognitive function and sleep disturbances in patients with breast cancer. The MELODY trial: protocol for a randomised, placebo-controlled, double-blinded trial. BMJ Open. 2012;2(1):000647. doi: 10.1136/bmjopen-2011-000647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharma AK, Mehta AK, Rathor N, et al. Melatonin attenuates cognitive dysfunction and reduces neural oxidative stress induced by phosphamidon. Fundam Clin Pharmacol. 2013;27(2):146–51. doi: 10.1111/j.1472-8206.2011.00977.x. [DOI] [PubMed] [Google Scholar]
- 51.Sugden D, Ceña V, Klein DC. Hydroxyindole O-methyltransferase. Hydroxyindole O-methyltransferase. Methods Enzymol. 1987;142:590–96. doi: 10.1016/s0076-6879(87)42070-3. [DOI] [PubMed] [Google Scholar]
- 52.Reiter RJ, Tan DX, Terron MP, et al. Melatonin and its metabolites: new findings regarding their production and their radical scavenging actions. Acta Biochim Pol. 2007;54(1):1–9. [PubMed] [Google Scholar]
- 53.Müller DJ, Schulze TG, Jahnes E, et al. Association between a polymorphism in the pseudoautosomal X-linked gene SYBL1 and bipolar affective disorder. Am J Med Genet. 2002;114(1):74–78. doi: 10.1002/ajmg.10115. [DOI] [PubMed] [Google Scholar]
- 54.Talarowska M, Szemraj J, Zajączkowska M, Gałecki P. ASMT gene expression correlates with cognitive impairment in patients with recurrent depressive disorder. Med Sci Monit. 2014;20:905–12. doi: 10.12659/MSM.890160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nedzvetsky VS, Nerush PA, Kirichenko SV. Effect of Melatonin on Cognitive Ability of Rats and Expression of NCAM in Brain Structures in Streptozotocin-Induced Diabetes. Neurophysiol. 2003;35(6):422–27. [Google Scholar]
- 56.Polimeni G, Esposito E, Bevelacqua V, et al. Role of melatonin supplementation in neurodegenerative disorders. Front Biosci (Landmark Ed) 2014;19:429–46. doi: 10.2741/4217. [DOI] [PubMed] [Google Scholar]
- 57.Laborie S. Effect of bright light and melatonin on cognitive and non-cognitive function. Cah Année Gérontol. 2010;2:194–98. [Google Scholar]
- 58.Anderson G, Maes M. Local Melatonin Regulates Inflammation Resolution: A Common Factor in Neurodegenerative, Psychiatric and Systemic Inflammatory Disorders. CNS Neuro Dis-Dr Tar. doi: 10.2174/1871527313666140711091400. [in press] [DOI] [PubMed] [Google Scholar]
- 59.Ramírez-Rodríguez G, Vega-Rivera NM, Benítez-King G, et al. Melatonin supplementation delays the decline of adult hippocampal neurogenesis during normal aging of mice. Neurosci Lett. 2012;530(1):53–58. doi: 10.1016/j.neulet.2012.09.045. [DOI] [PubMed] [Google Scholar]
- 60.Rothman SM, Mattson MP. Sleep Disturbances in Alzheimer’s and Parkinson’s Diseases. Neuromol Med. 2012;14:194–204. doi: 10.1007/s12017-012-8181-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baydas G, Ozer M, Yasar A, et al. Melatonin improves learning and memory performances impaired by hyperhomocysteinemia in rats. Brain Res. 2005;1046(1–2):187–94. doi: 10.1016/j.brainres.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 62.Anderson G, Maes M. Multiple Sclerosis: The Role of Melatonin and N-acetylserotonin. Multiple Sclerosis & Related Disorders. 2014 doi: 10.1016/j.msard.2014.12.001. [in press] [DOI] [PubMed] [Google Scholar]
- 63.Liu YJ, Meng FT, Wang LL, et al. Apolipoprotein E influences melatonin biosynthesis by regulating NAT and MAOA expression in C6 cells. J Pineal Res. 2012;52(4):397–402. doi: 10.1111/j.1600-079X.2011.00954.x. [DOI] [PubMed] [Google Scholar]
- 64.Muxel SM, Pires-Lapa MA, Monteiro AW, et al. NF-κB drives the synthesis of melatonin in RAW 264.7 macrophages by inducing the transcription of the arylalkylamine-N-acetyltransferase (AA-NAT) gene. PLoS One. 2012;7(12):e52010. doi: 10.1371/journal.pone.0052010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Girdler SS, Klatzkin R. Neurosteroids in the context of stress: implications for depressive disorders. Pharmacol Ther. 2007;116(1):125–39. doi: 10.1016/j.pharmthera.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Patten SB, Wang JL, Williams JV, et al. Predictors of the longitudinal course of major depression in a Canadian population sample. Can J Psychiatry. 2010;55(10):669–76. doi: 10.1177/070674371005501006. [DOI] [PubMed] [Google Scholar]
- 67.Whittle S, Yap MB, Sheeber L, et al. Hippocampal volume and sensitivity to maternal aggressive behavior: A prospective study of adolescent depressive symptoms. Dev Psychopathol. 2011;23(1):115–29. doi: 10.1017/S0954579410000684. [DOI] [PubMed] [Google Scholar]
- 68.Vreeburg SA, Hoogendijk WJ, van Pelt J, et al. Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: Results from a large cohort study. Arch Gen Psychiatry. 2009;66(6):617–26. doi: 10.1001/archgenpsychiatry.2009.50. [DOI] [PubMed] [Google Scholar]
- 69.Porter RJ, Gallagher P. Abnormalities of the HPA axis in affective disorders: Clinical subtypes and potential treatments. Acta Neuropsychiatr. 2006;18:193–209. doi: 10.1111/j.1601-5215.2006.00152.x. [DOI] [PubMed] [Google Scholar]
- 70.Egeland J, Lund A, Landrø NI, et al. Cortisol level predicts executive and memory function in depression, symptom level predicts psychomotor speed. Acta Psychiatr Scand. 2005;112(6):434–41. doi: 10.1111/j.1600-0447.2005.00599.x. [DOI] [PubMed] [Google Scholar]
- 71.Pavlides C, Nivon LG, McEwen BS. Effects of chronic stress on hippocampal long-term potentiation. Hippocampus. 2002;12:245–57. doi: 10.1002/hipo.1116. [DOI] [PubMed] [Google Scholar]
- 72.Lee HY, Lee GH, Marahatta A, et al. The protective role of Bax inhibitor-1 against chronic mild stress through the inhibition of monoamine oxidase A. Sci Rep. 2013;3:3398. doi: 10.1038/srep03398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anderson G, Kubera M, Duda W, et al. Increased IL-6 trans-signaling in depression: focus on the tryptophan catabolite pathway, melatonin and neuroprogression. Pharmacol Rep. 2013;65(6):1647–54. doi: 10.1016/s1734-1140(13)71526-3. [DOI] [PubMed] [Google Scholar]
- 74.Konarski JZ, McIntyre RS, Kennedy SH, et al. Volumetric neuroimaging investigations in mood disorders: bipolar disorder versus major depressive disorder. Bipolar Disord. 2008;10:1–37. doi: 10.1111/j.1399-5618.2008.00435.x. [DOI] [PubMed] [Google Scholar]
- 75.Monkul ES, Malhi GS, Soares JC. Mood disorders – review of structural MRI studies. Acta Neuropsychiatr. 2003;15:368–80. doi: 10.1046/j.1601-5215.2003.00052.x. [DOI] [PubMed] [Google Scholar]
- 76.Anderson G, Rodriguez M. Multiple sclerosis, seizures, and antiepileptics: role of IL-18, IDO, and melatonin. Eur J Neurol. 18(5):680–85. doi: 10.1111/j.1468-1331.2010.03257.x. 201. [DOI] [PubMed] [Google Scholar]
- 77.Drevets WC. Orbitofrontal Cortex Function and Structure in Depression. Ann NY Acad Sci. 2007;1121:499–527. doi: 10.1196/annals.1401.029. [DOI] [PubMed] [Google Scholar]
- 78.MacMaster FP, Mirza Y, Szeszko PR, et al. Amygdala and hippocampal volumes in familial early onset major depressive disorder. Biol Psychiatry. 2008;63:385–90. doi: 10.1016/j.biopsych.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ebmeier KP, Donaghey C, Steele JD. Recent developments and current controversies in depression. Lancet. 2006;367:153–67. doi: 10.1016/S0140-6736(06)67964-6. [DOI] [PubMed] [Google Scholar]
- 80.Fuchs E, Flügge G. Social stress in tree shrews: effect on physiology, brain function and behavior of subordinate individuals. Pharmacol Biochem Behav. 2002;73:247–58. doi: 10.1016/s0091-3057(02)00795-5. [DOI] [PubMed] [Google Scholar]
- 81.Spalletta G, Guida G, Caltagirone C. Is left stroke a risk-factor for selective serotonin reuptake inhibitor antidepressant treatment resistance? J Neurol. 2003;250:449–55. doi: 10.1007/s00415-003-1023-2. [DOI] [PubMed] [Google Scholar]
- 82.Noesselt T, Driver J, Heinze HJ, Dolan R. Asymmetrical activation in the human brain during processing of fearful faces. Curr Biol. 2005;15:424–29. doi: 10.1016/j.cub.2004.12.075. [DOI] [PubMed] [Google Scholar]
- 83.Mayberg HS, Liotti M, Brannan SK, et al. Reciprocal limbic-cortical function and negative mood: Converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–82. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 84.Peterson BS, Warner V, Bansal R, Hongtu Z. Cortical thinning in persons at increased familial risk for major depression. Proc Natl Acad Sci USA. 2009;15:6273–78. doi: 10.1073/pnas.0805311106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hsu DT, Langenecker SA, Kennedy SE, et al. fMRI BOLD responses to negative stimuli in the prefrontal cortex are dependent on levels of recent negative life stress in major depressive disorder. Psychiatry Res. 2010;183(3):202–8. doi: 10.1016/j.pscychresns.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Langenecker SA, Kennedy SE, Guidotti LM, et al. Frontal and limbic activation during inhibitory control predicts treatment response in major depressive disorder. Biol Psychiatry. 2007;62:1272–80. doi: 10.1016/j.biopsych.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zabłocka A. Alzheimer’s disease as neurodegenerative disorder. Postepy Hig Med Dosw. 2006;60:209–16. [PubMed] [Google Scholar]
- 88.Alexopoulos GS, Morimoto SS. The inflammation hypothesis in geriatric depression. Int J Geriatr Psychiatry. 2011;26(11):1109–18. doi: 10.1002/gps.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Canon ME, Crimmins EM. Sex differences in the association between muscle quality, inflammatory markers, and cognitive decline. J Nutr Health Aging. 2011;15(8):695–98. doi: 10.1007/s12603-011-0340-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maes M, Kubera M, Leunis JC, et al. In depression, bacterial translocation may drive inflammatory responses, oxidative and nitrosative stress (O&NS), and autoimmune responses directed against O&NS-damaged neoepitopes. Acta Psychiatr Scand. 2013;127(5):344–54. doi: 10.1111/j.1600-0447.2012.01908.x. [DOI] [PubMed] [Google Scholar]
- 91.Bossù P, Cutuli D, Palladino I, et al. A single intraperitoneal injection of endotoxin in rats induces long-lasting modifications in behavior and brain protein levels of TNF-alpha and IL-18. J Neuroinflammation. 2012;9(1):101. doi: 10.1186/1742-2094-9-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pontes GN, Cardoso EC, Carneiro-Sampaio MM, Markus RP. Pineal melatonin and the innate immune response: the TNF-alpha increase after cesarean section suppress nocturnal melatonin production. J Pineal Res. 2007;43:365–71. doi: 10.1111/j.1600-079X.2007.00487.x. [DOI] [PubMed] [Google Scholar]
- 93.Insel KC, Moore IM, Vidrine AN, Montgomery DW. Biomarkers for cognitive aging-part II: oxidative stress, cognitive assessments, and medication adherence. Biol Res Nurs. 2012;14(2):133–38. doi: 10.1177/1099800411406527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Maes M, Berk M, Goehler L, et al. Depression and sickness behavior are Janus-faced responses to shared inflammatory pathways. BMC Med. 2012;10:66. doi: 10.1186/1741-7015-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gałecki P, Florkowski A, Bobińska K, et al. Functional polymorphismof the myeloperoxidase gene (G-463A) in depressive patients. Acta Neuropsychiatr. 2010;5:218–22. doi: 10.1111/j.1601-5215.2010.00483.x. [DOI] [PubMed] [Google Scholar]
- 96.Sarandol A, Sarandol E, Eker SS, et al. Major depressive disorder is accompanied with oxidative stress: short-term antidepressant treatment does not alter oxidative-antioxidative systems. Hum Psychopharmacol. 2007;22:67–73. doi: 10.1002/hup.829. [DOI] [PubMed] [Google Scholar]
- 97.Catena-Dell’Osso M, Bellantuono C, Consoli G, et al. Inflammatory and neurodegenerative pathways in depression: a new avenue for antidepressant development? Curr Med Chem. 2011;18(2):245–55. doi: 10.2174/092986711794088353. [DOI] [PubMed] [Google Scholar]
- 98.Marsland AE, Gianaros PJ, Abramowitch SM, et al. Interleukin- 6 covaries inversely with hippocampal grey matter volume in middle-aged adults. Biol Psychiatry. 2008;64:484–90. doi: 10.1016/j.biopsych.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cubała WJ, Godlewska B, Trzonkowski P, Landowski J. Indicators of the persistent pro-inflammatory activation of the immune system in depression. Psychiatr Pol. 2006;40(3):431–44. [PubMed] [Google Scholar]
- 100.Padurariu M, Ciobica A, Hritcu L, et al. Changes of some oxidative stress markers in the serum of patients with mild cognitive impairment and Alzheimer’s disease. Neurosci Lett. 2010;469(1):6–10. doi: 10.1016/j.neulet.2009.11.033. [DOI] [PubMed] [Google Scholar]
- 101.Baune BT, Unwin SJ, Quirk F, Golledge J. Neuropsychiatric symptoms in patients with aortic aneurysms. PLoS One. 2011;6(7):e22632. doi: 10.1371/journal.pone.0022632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Talarowska M, Florkowski A, Gałecki P, et al. The impact of psychological variables on the presentation and progress of asthma and patient’s cognitive functions. Pneumonol Alergol Pol. 2009;77:554–59. [in Polish] [PubMed] [Google Scholar]
- 103.Talarowska M, Florkowski A, Zboralski K, Gałecki P. Cognitive functions and clinical features among diabetic patients in Polish population. Cent Eur J Med. 2009;4(4):467–75. [Google Scholar]
- 104.Talarowska M, Gałecki P, Maes M, et al. Malondialdehyde plasma concentration correlates with declarative and working memory in patients with recurrent depressive disorder. Mol Biol Rep. 2012;39(5):5359–66. doi: 10.1007/s11033-011-1335-8. [DOI] [PubMed] [Google Scholar]
- 105.Talarowska M, Gałecki P, Maes M, et al. Nitric oxide plasma concentration associated with cognitive impairment in patients with recurrent depressive disorder. Neurosci Lett. 2012;510(2):127–31. doi: 10.1016/j.neulet.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 106.Gałecki P, Talarowska M, Bobińska K, et al. Thiol protein groups correlate with cognitive impairment in patients with recurrent depressive disorder. Neuro Endocrinol Lett. 2013;34(8):780–86. [PubMed] [Google Scholar]
- 107.Talarowska M, Gałecki P, Maes M, et al. Total antioxidant status correlates with cognitive impairment in patients with recurrent depressive disorder. Neurochem Res. 2012;37(8):1761–67. doi: 10.1007/s11064-012-0788-z. [DOI] [PubMed] [Google Scholar]
- 108.Airaksinen E, Wahlin Å, Forsell Y, Larsson M. Low episodic memory performance as a premorbid marker of depression: evidence from a 3-year follow-up. Acta Psychiatr Scand. 2007;115(6):458–65. doi: 10.1111/j.1600-0447.2006.00932.x. [DOI] [PubMed] [Google Scholar]
- 109.Han L, McCusker J, Abrahamowicz M, et al. The temporal relationship between depression symptoms and cognitive functioning in older medical patients-prospective or concurrent? J Geront. 2006;61A:1319–23. doi: 10.1093/gerona/61.12.1319. [DOI] [PubMed] [Google Scholar]
- 110.Wilson R, Mendes de Leon C, Bennett D, et al. Depressive symptoms and cognitive decline in a community population of older persons. J Neurol Neurosurg Psychiatry. 2004;75:126–29. [PMC free article] [PubMed] [Google Scholar]
- 111.Talarowska M, Zboralski K, Gałecki P. Correlations between working memory effectiveness and depression levels after pharmacological therapy. Psychiatr Pol. 2013;47(2):255–67. [PubMed] [Google Scholar]
- 112.Harvey PO, Le Bastard G, Pochon JB, et al. Executive functions and updating of the contents of working memory in unipolar depression. J Psychiatr Res. 2004;38:567–76. doi: 10.1016/j.jpsychires.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 113.Naismith SL, Longley WA, Scott EM, Hickie IB. Disability in major depression related to self-rated and objectively-measured cognitive deficits: a preliminary study. BMC Psychiatry. 2007;7:32–40. doi: 10.1186/1471-244X-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Anderson G, Maes M. Neurodegeneration in Parkinson’s disease: interactions of oxidative stress, tryptophan catabolites and depression with mitochondria and sirtuins. Mol Neurobiol. 2014;49(2):771–83. doi: 10.1007/s12035-013-8554-z. [DOI] [PubMed] [Google Scholar]
- 115.Takami H, Okamoto Y, Yamashita H, et al. Attenuated anterior cingulate activation during a verbal fluency task in elderly patients with a history of multiple-episode depression. Am J Geriatr Psychiatry. 2007;15(7):594–603. doi: 10.1097/01.JGP.0b013e31802ea919. [DOI] [PubMed] [Google Scholar]
- 116.Talarowska M, Florkowski A, Zboralski K, Gałecki P. Cognitive functions impairment depending on clinical traits of unipolar recurrent depression – preliminary rsults. Psychiatria Psychoterapia. 2010;6(1):11–18. [Google Scholar]
- 117.Neu P, Kiesslinger U, Schlattmann P, Reischies FM. Time-related cognitive deficiency in four different types of depression. Psychiatry Res. 2001;103(2–3):237–47. doi: 10.1016/s0165-1781(01)00286-4. [DOI] [PubMed] [Google Scholar]
- 118.Biringer E, Lundervold A, Stordal K, et al. Executive function improvement upon remission of recurrent unipolar depression. Eur Arch Psychiatry Clin Neurosci. 2005;255:373–80. doi: 10.1007/s00406-005-0577-7. [DOI] [PubMed] [Google Scholar]
- 119.Okada G, Okamoto Y, Yamashita H, et al. Attenuated prefrontal activation during a verbal fluency task in remitted major depression. Psychiatry Clin Neurosci. 2009;63(3):423–25. doi: 10.1111/j.1440-1819.2009.01952.x. [DOI] [PubMed] [Google Scholar]
- 120.Alexopoulos GS, Kiosses DN, Heo M, et al. Executive dysfunction and the course of geriatric depression. Biol Psychiatry. 2005;58:204–10. doi: 10.1016/j.biopsych.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 121.Anderson G, Maes M. Oxidative/Nitrosative Stress and Immuno-inflammatory Pathways in Depression: Treatment Implications. Curr Pharm Des. 2013;20(23):3812–47. doi: 10.2174/13816128113196660738. [DOI] [PubMed] [Google Scholar]
- 122.Tanaka M, Ishii A, Yamano E, et al. Cognitive dysfunction in elderly females with depressive symptoms. Med Sci Monit. 2012;18(12):CR706–11. doi: 10.12659/MSM.883596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Morris G, Anderson G, Dean O, et al. The glutathione system: a new drug target in neuroimmune disorders. Mol Neurobiol. 2014;50(3):1059–84. doi: 10.1007/s12035-014-8705-x. [DOI] [PubMed] [Google Scholar]
- 124.Borkowska A, Rybakowski JK. Neuropsychological frontal lobe tests indicate that bipolar depressed patients are more impaired than unipolar. Bipolar Dis. 2001;3:88–94. doi: 10.1034/j.1399-5618.2001.030207.x. [DOI] [PubMed] [Google Scholar]