Abstract
Background
MiR-133 expression is dysregulated in postmenopausal osteoporosis. However, its role in postmenopausal osteoporosis is still not well understood. In the current study, we explore how estrogen deficiency affects miR-133 expression and how miR-133 is involved in osteogenic differentiation of mesenchymal stem cells (MSCs).
Material/Methods
qRT-PCR analysis was performed to assess miR-133 expression in MSCs isolated from bone marrow of an ovariectomized (OVX) animal model and postmenopausal osteoporosis patients (PMOP) and their corresponding controls. The binding between miR-133 and predicted target SLC39A1 was verified using dual luciferase assay and Western blot analysis. The effect of miR-133 and SLC39A1 on osteogenic differentiation of MSCs was assessed through measuring alkaline phosphatase (ALP), mineralization nodules, and osteoblast-specific genes Runx2 and Osterix expression.
Results
miR-133 expression is significantly enhanced as a result of estrogen deficiency. Its overexpression is negatively correlated to osteogenic differentiation of hMSCs. SLC39A1 showed an inverse expression trend to miR-133 during the differentiation. miR-133 can directly target 3′UTR of SLC39A1 and thereby modulate its expression in hMSCs. The miR-133-SLC39A1 axis might play an important role in osteogenic differentiation of hMSCs. SLC39A1 can promote ALP activity and formation of mineralization nodules. In addition, SLC39A1 expression level is also positively correlated with RUNX2 and Osterix.
Conclusions
Estrogen deficiency is associated with miR-133 overexpression. MiR-133 can induce postmenopausal osteoporosis by weakening osteogenic differentiation of hMSCs, at least partly through repressing SLC39A1 expression.
Keywords: Mesenchymal Stromal Cells; MicroRNAs; Osteoporosis, Postmenopausal
Background
Postmenopausal osteoporosis is a common bone disease characterized as unbalanced bone resorption by osteoclasts and bone formation by osteoblasts [1]. Estrogen deficiency is closely related to excessive bone resorption and inadequate bone formation [2,3]. However, the detailed mechanism of estrogen deficiency in postmenopausal osteoporosis is not fully understood.
Mesenchymal stem cells (MSCs) can differentiate into osteoblasts and osteocytes, which are important sources of bone formation. Previous studies reported that estrogen deficiency can affect the differentiation of MSCs [4,5]. Directing mesenchymal stem cells to bone to augment bone formation was proposed as a strategy to deal with impairment of osteogenesis due to estrogen deficiency [6]. But how estrogen deficiency modulates osteogenic differentiation of MSCs is still beyond our current knowledge. Some recent studies reported that miRNAs can regulate the differentiation of MSCs. For example, miR-20 can promote, while miR-141-3p and miR-138 can inhibit, osteogenic differentiation of human MSCs (hMSCs) in vivo [7–9]. MiR-210 is involved in the regulation of postmenopausal osteoporosis by promoting VEGF expression and osteoblast differentiation [10]. Postmenopausal estrogen deficiency resulted in downregulated miR-21 expression, which acts a promoter of osteoblast differentiation of MSCs by repressing Spry1 [11]. Several other miRNAs were also dysregulated in postmenopausal osteoporosis, such as miR-133a, miR-503, miR-29, miR-124, and miR-155 [12]. However, their role in postmenopausal osteoporosis is still not well understood.
In the current study, we explored how estrogen deficiency affects miR-133 expression and how miR-133 is involved in osteogenic differentiation of hMSCs. This study showed that miR-133 is overexpressed in postmenopausal osteoporosis and can directly targets SLC39A1, leading to weakened osteogenic differentiation of hMSCs.
Material and Methods
Animal model
To generate ovariectomized (OVX) animal model, 12-week-old wild-type (WT) C57BL/6J mice were treated with bilateral ovariectomy under general anesthesia by the dorsal approach.
Mesenchymal stem cell culture
Human MSCs (hMSCs) were derived from bone marrow samples from 5 healthy premenopausal women (41–46 years old) and 5 women with postmenopausal osteoporosis (51–59 years old). Informed consent was obtained from each participant as a donor of bone marrow. Osteoporosis was diagnosed using the World Health Organization parameters. The hMSCs were purified from the bone marrow samples according to the Percoll density gradient centrifugation method (GE Healthcare Life Sciences). The primary mouse bone marrow cells were collected from the tibia and femur by crushing the bones, then the mouse MSCs (mMSCs) were isolated and purified using EasySep™ Mouse Mesenchymal Stem/Progenitor Cell Enrichment Kit (StemCell). Both hMSCs and mMSCs were cultured in a modified essential medium (a-MEM) supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, 100 U/ml penicillin, and 100 mg/mL streptomycin in an incubator with a humidified atmosphere and 5% CO2 at 37°C. The hMSCs and mMSCs, which were positive (>95%) for CD29, CD44, CD90, and CD105 but were negative (<5%) for hematopoietic markers CD34, CD14, and CD45, were used for further study.
HEK-293T cells were obtained from ATCC and were cultured in DMEM medium supplemented with 10% FBS, 2 mM L-glutamine, 100 μg/mL streptomycin, and 100 U/mL penicillin in an incubator with a humidified atmosphere and 5% CO2 at 37°C.
Cell transfection
The miR-133 mimics and the negative control (miR-NC), miR-133 inhibitor (anti-miR-133), and miR-133 inhibitor negative control (anti-miR-NC) were purchased from Ribobio (China). The cells were transfected with 50 nM miR-133 mimics, miR-NC, anti-miR-133, or anti-miR-NC by using the siPORT NeoFX transfection agent (Ambion, Cat. No. AM4511) according to the manufacturer’s instructions.
Human SLC39A1 lentiviral vector (Lenti-SLC39A1) without 3′-UTR were purchased from GENECHEM (China). The viral particles were cotransfected with the corresponding packaging mix into HEK-293T cells to generate pseudotyped lentivirus. The viral supernatant was filtered through a 0.45-μm filter. hMSCs cells were treated with the viral supernatant with 8 μg/ml Polybrene (Sigma-Aldrich).
Osteogenic differentiation
To induce osteogenic differentiation, the hMSCs were cultured in α-MEM supplemented with 10% FBS, 10 nM dexamethasone, 50 μg/ml ascorbic acid, and 5 mM β-glycerophosphate. The mMSCs were cultured in α-MEM supplemented 10% FBS, 100 μg/ml ascorbic acid, 10 mM β-glycerophosphate, and 10 nM dexamethasone. The media were changed every 2 days.
qRT-PCR analysis of miR-133a and SLC39A1 mRNA expression
TRIzol reagent (Invitrogen, USA) was used to extract total RNA from cell samples. Reverse transcription was performed to get miRNA-specific cDNA using TaqMan MicroRNA Reverse Transcription Kit and to get all mRNA corresponding cDNA by using PrimeScript RT reagent kit (TaKaRa, China). To determine miR-133a expression levels, qRT-PCR was performed using TaqMan MicroRNA Assays (Applied Biosystems), with U6 snRNA as the internal control. To detect SLC39A1 mRNA expression, amplification was performed using the SYBR Premix Ex Taq II kit (TaKaRa) with SLC39A1 specific primers: (F) 5′-GTCACCTCTGGAGGAAACAAG-3′ and (R) 5′-CAGGGAGAACACCAGTACACAG-3′. β-actin was used as endogenous normalization control.
ALP staining and Alizarin red S staining
ALP staining was performed by using 5-bromo-4-chloro-3-indolylphosphate/nitro blue tetrazolium alkaline phosphatase color development kit (Beyotime, China) according to the manufacturer’s protocol. Mineralization nodules were visualized by Alizarin red S staining by using the calcium colorimetric assay kit (BioVision, USA) according to the manufacturer’s protocol.
Western blot analysis off RUNX2 and Osterix expression
After transfection, cells were collected and lysed with lysis buffer (Beyotime, China). The extract was separated on 10% SDS-PAGE gel and then transferred to NC membrane for a conventional Western blotting analysis. The membranes were probed with primary antibodies to RUNX2 (1:500, ab76956, Abcam), Osterix (1:1000, ab187158, Abcam), SLC39A1 (1:1000, ab83950, Abcam), GAPDH (1:1000, ab37168, Abcam), and tubulin (1:1000, ab59680, Abcam) and then incubated with corresponding HRP-conjugated secondary antibody (anti-mouse IgG, HRP, 1:5000, ab6728, or anti-rabbit IgG, HRP, 1:5000, ab6721, Abcam). Signals were visualize by using an ECL kit (Pierce, IL, USA). The band intensity was quantified by using Image-J software. Experiments were performed in triplicate.
Dual luciferase assay
The possible pairing between miR-133 and human SLC39A1 mRNA were predicted in TargetScan 6.2. Then the wide-type 92-114 of SLC39A1 3′ UTR with the miR-133-specific binding sites and the mutant sequence without the binding sites were chemically synthesized and cloned into pGL3 promoter vector to construct recombinant reporters designed as miR-133-SLC39A1-wt and miR-133-SLC39A1-mut, respectively. HEK-293T cells were co-transfected with miR-133-SLC39A1-wt or miR-133-SLC39A1-mut and miR-133 mimics. At 18 h after transfection, firefly luciferase activity was measured and normalized to that of Renilla luciferase using the Dual-Luciferase Reporter Assay System (Promega, USA).
Results
MiR-133 is upregulated in estrogen deficiency induced osteoporosis
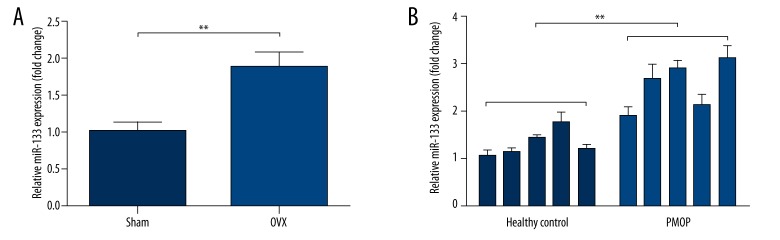
Previous studies reported that miR-133 is one of the miRNAs dysregulated in postmenopausal osteoporosis. We decided to further explore its expression profile in MSCs of estrogen deficiency animal model and in estrogen deficiency-induced osteoporosis. MiR-133 expression in MSCs derived from bone marrow of an OVX mouse model was significantly higher than that of the sham group (Figure 1A), suggesting estrogen deficiency could affect miR-133 expression. In addition, we also observed that miR-133 expression in MSCs isolated from bone marrow of postmenopausal osteoporosis patients (PMOP) was significantly higher than that of the healthy controls (Figure 1B). These results suggest that miR-133 is upregulated in estrogen deficiency-induced osteoporosis.
Figure 1.
MiR-133 is upregulated in estrogen deficiency induced osteoporosis. (A) qRT-PCR analysis of miR-133 expression in mMSCs derived from bone marrow of OVX mouse model and sham mice. (B) qRT-PCR analysis of miR-133 expression in hMSCs isolated from bone marrow of PMOP and healthy controls. Data are shown as mean ±S.D by 3 independent experiments. * P<0.05, ** P<0.01, *** P<0.001, NS P>0.5.
MiR-133 is downregulated during osteogenic differentiation of MSCs
To explore the role of miR-133 in osteogenic differentiation of MSCs, we first measured its expression in both mMSCs and hMSCs at different time points during osteogenic differentiation. In both of the cells, miR-133 was downregulated and reached the lowest point at day 12 after differentiation (Figure 2A, 2B). Then, we detected how miR-133 overexpression and knockdown could affect the bone formation markers. miR-133 overexpression could significantly decrease alkaline phosphatase (ALP) (Figure 2C) and also decrease mineralization nodules (Figure 2D). However, its downregulation was associated with increased ALP and mineralization nodules (Figure 2C, 2D). Western blot analysis showed that osteoblast-specific genes Runx2 and Osterix expression were inhibited due to miR-133 overexpression (Figure 2E, 2F), but were significantly promoted when miR-133 was knocked-down (Figure 2E, 2F). These results suggest that miR-133 is downregulated and can modulate osteogenic differentiation of MSCs.
Figure 2.
MiR-133 is downregulated during osteogenic differentiation of MSCs. (A, B) qRT-PCR analysis of miR-133 expression in mMSCs derived from bone marrow of OVX mouse model (A) and hMSCs derived from bone marrow of PMOP (B) at different time points during osteogenic differentiation. (C, D) ALP staining (C) and Alizarin red S staining (D) were performed to determine the osteogenic differentiation of hMSCs with miR-133 overexpression or knockdown (E, F) Western blot analysis of RUNX2 (E) and Osterix (F) expression in hMSCs with miR-133 overexpression or knockdown. Data are shown as mean ±S.D by 3 independent experiments. * P<0.05, ** P<0.01, *** P<0.001, NS P>0.5.
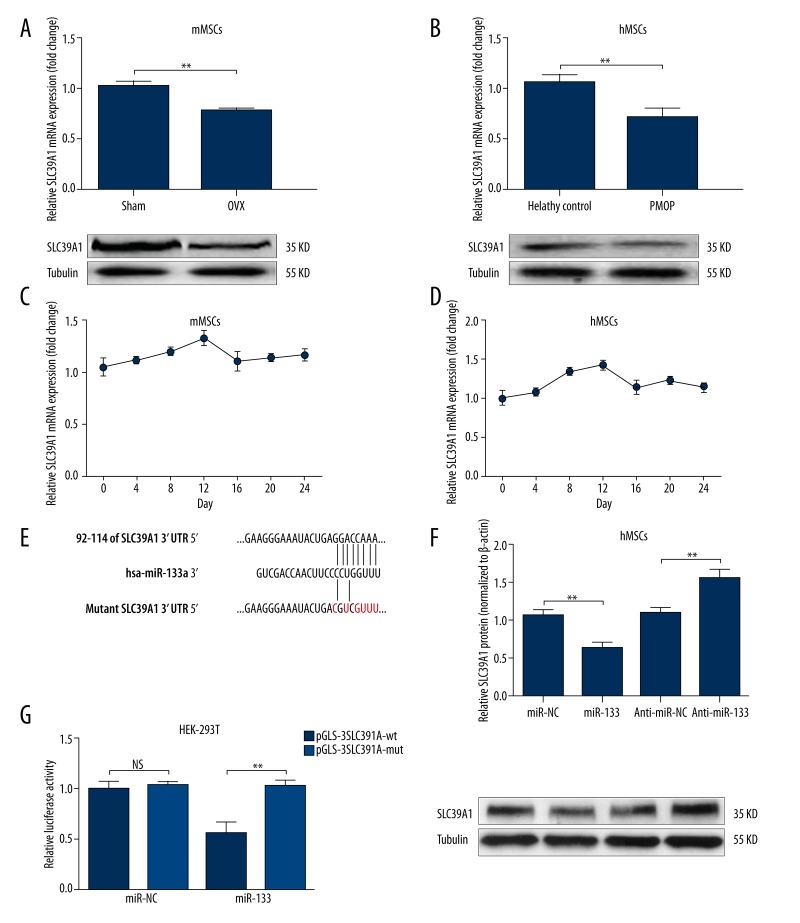
miR-133 can directly target SLC39A1 and modulate its expression
Since miR-133 can modulate osteogenic differentiation of MSCs, we decided to further explore its downstream regulation network. A previous study reported that SLC39A1 is a direct target of miR-133. Interestingly, in the current study, we also observed that SLC39A1 was significantly downregulated in both mMSCs from OVX mouse model and hMSCs isolated from bone marrow of PMOP (Figure 3A, 3B). In addition, we also found that the expression of SLC39A1 during osteogenic differentiation of both mMSCs and hMSCs showed an inverse trend to miR-133. Its expression gradually increased after differentiation and reached a peak point at day 12 (Figure 3C, 3D). Considering the possible regulative role of miR-133 over SLC39A1, we then verified whether miR-133 could directly target SLC39A1 and modulate its expression. Dual luciferase assay confirmed the putative binding between miR-133 and SLC39A1 (Figure 3E, 3G). In hMSCs, upregulation of miR-133 led to decreased SLC39A1 expression, while knockdown of miR-133 resulted in increased SLC39A1 expression (Figure 3F).
Figure 3.
miR-133 can directly target SLC39A1 and modulate its expression. (A, B) qRT-PCR and Western blot analysis of SLC39A1 expression in mMSCs derived from bone marrow of OVX mouse model (A) and hMSCs derived from bone marrow of PMOP (B). (C, D) qRT-PCR analysis of SLC39A1 expression in mMSCs derived from bone marrow of OVX mouse model (C) and hMSCs derived from bone marrow of PMOP (D) at different time points during osteogenic differentiation. (E) Predicted pairing and mutant sequence between SLC39A1 3′UTR and miR-133. (F) Western blot analysis of SLC39A1 expression in hMSCs with miR-133 overexpression or knockdown. (G) Dual luciferase assay of the relative luciferase activity in HEK-293T cells co-transfected with 150-ng reporter plasmids and 50-M miR-133 mimics. At 18 h after transfection, both firefly and Renilla luciferase activities were measured and the firefly luciferase activity was normalized to the Renilla luciferase activity. Data are shown as mean ±S.D by 3 independent experiments. * P<0.05, ** P<0.01, *** P<0.001, NS P>0.5.
MiR-133 modulates osteogenic differentiation of MSCs through SLC39A1
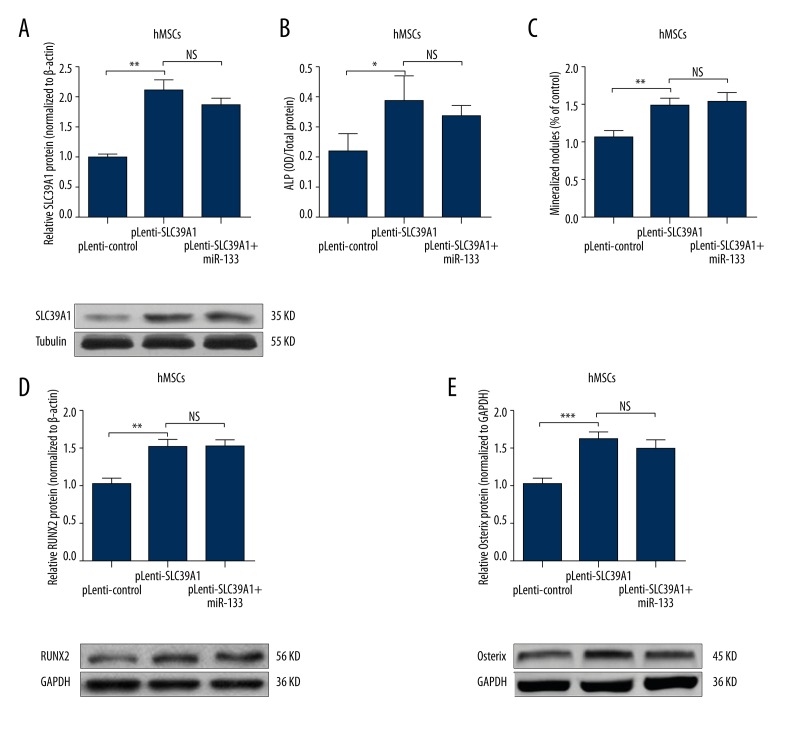
Since miR-133 can directly regulate SLC39A1 expression, we further explored the role of miR-133-SLC39A1 axis on osteogenic differentiation of MSCs. We first overexpressed SLC39A1 (without 3′ UTR) in hMSCs. MiR-133 could not affect its expression (Figure 4A). Overexpression of CLS39A1 could directly increase ALP activity and mineralization nodules (Figure 4B, 4C). In addition, its overexpression also associated with increased RUNX2 (Figure 4D) and Osterix (Figure 4E) level. These results suggest that SLC39A1 is an important protein modulating osteogenic differentiation.
Figure 4.
MiR-133 modulates osteogenic differentiation of MSCs through SLC39A1. (A) Western blot analysis of SLC39A1 expression in hMSCs transfected with pLenti-SLC39A1 (without 3′ UTR) or co-transfected with pLenti-SLC39A1 (without 3′ UTR) and miR-133 mimics. (B, C) ALP staining (B) and Alizarin red S staining (C) were performed to determine the osteogenic differentiation of hMSCs transfected with pLenti-SLC39A1 (without 3′ UTR) or co-transfected with pLenti-SLC39A1 (without 3′ UTR) and miR-133 mimics. (D, E) Western blot analysis of RUNX2 (D) and Osterix (E) expression in hMSCs transfected with pLenti-SLC39A1 (without 3′ UTR) or co-transfected with pLenti-SLC39A1 (without 3′ UTR) and miR-133 mimics. Data are shown as mean ±S.D by 3 independent experiments. * P<0.05, ** P<0.01, *** P<0.001, NS P>0.5.
Discussion
Postmenopausal woman are highly vulnerable to osteoporosis, partly due to estrogen deficiency-associated excessive bone resorption and inadequate bone formation [2,3]. However, the exact role of estrogen deficiency in postmenopausal osteoporosis is not quite clear. MSCs are progenitor cells of osteoblasts and osteocytes, which have an important role in bone metabolism. Reduced differentiation of MSCs into osteoblasts may contribute to osteoporosis.
Some recent studies reported that estrogen deficiency is associated with dysregulated miRNAs expression, which is involved in osteogenic differentiation of MSCs. In fact, miRNAs can be either inhibitors or promoters of MSCs osteoblast differentiation, depending on their downstream targets. For example, miR-20 can promote MSCs osteoblast differentiation in a co-regulatory pattern by targeting PPARγ, Bambi, and Crim1, the negative regulators of BMP signaling [8]. MiR-210 can ameliorate the estrogen deficiency-caused postmenopausal osteoporosis by enhancing VEGF expression [10]. MiR-21 can act as a promoter of osteoblast differentiation of MSCs by repressing Spry1 [11]. In contrast, miR-705 and miR-3077-5p can inhibit MSCs osteoblast differentiation and promote adipocyte differentiation through targeting HOXA10 and RUNX2 mRNA separately [13]. miR-141-3p can inhibit the differentiation by targeting cell division cycle 25A (CDC25A) [9]. miR-138 can also inhibit osteogenic differentiation of hMSCs in vivo through modulating the focal adhesion kinase, a kinase playing a central role in promoting osteoblast differentiation [7].
MiR-133 is also a miRNA dysregulated in postmenopausal osteoporosis [14,15]. It is negatively correlated with bone mineral density and therefore is considered as a potential biomarker associated with postmenopausal osteoporosis [14,15]. Previous study also found 3 potential osteoclast-related targets of miR-133 – CXCL11, CXCR3, and SLC39A1 [14]. However, the detailed mechanism of miR-133 in postmenopausal osteoporosis is not quite clear. In the current study, we found that miR-133 expression is significantly enhanced as a result of estrogen deficiency. Its overexpression is negatively correlated with osteogenic differentiation of hMSCs. Interestingly, we also observed that SLC39A1 had an inverse expression trend to miR-133 during the differentiation. Considering the possible regulative effect of miR-133 over SLC39A1, we decided to further verify this axis in hMSCs and to explore its role.
SLC39A1 encodes Zinc transporter ZIP1, which is responsible for the active transport of zinc into cells [16]. Zinc is an essential trace element in both the proliferation and differentiation of osteoblast-like cells [17]. A previous study suggested that this protein might play an important role in the initiation of an osteogenic lineage from MSCs [18]. However, how its expression is regulated in MSCs is not clear. In the current study, we demonstrated that miR-133 can directly target 3′UTR of SLC39A1 and thereby modulate its expression in hMSCs. More importantly, we revealed that the miR-133-SLC39A1 axis might play an important role in osteogenic differentiation of hMSCs. SLC39A1 can promote ALP activity and formation of mineralization nodules. In addition, SLC39A1 expression level is also positively correlated with RUNX2 and Osterix, which are 2 important osteoblast-specific genes. These findings suggest that estrogen deficiency-related miR-133 overexpression induces osteoporosis at least partly through repressing SLC39A1 expression.
Conclusions
Estrogen deficiency is associated with miR-133 overexpression. MiR-133 can induce postmenopausal osteoporosis by weakening osteogenic differentiation of hMSCs at least partly through repressing SLC39A1 expression.
Footnotes
Source of support: Departmental sources
Conflict of interest
The authors had no conflict of interest.
References
- 1.Khosla S, Melton LJ, III, Riggs BL. The unitary model for estrogen deficiency and the pathogenesis of osteoporosis: Is a revision needed? J Bone Miner Res. 2011;26:441–51. doi: 10.1002/jbmr.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Epstein S. Update of current therapeutic options for the treatment of postmenopausal osteoporosis. Clin Ther. 2006;28:151–73. doi: 10.1016/j.clinthera.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Heiss C, Govindarajan P, Schlewitz G, et al. : Induction of osteoporosis with its influence on osteoporotic determinants and their interrelationships in rats by dexa. Med Sci Monit. 2012;18(6):BR199–207. doi: 10.12659/MSM.882895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao JW, Gao ZL, Mei H, et al. Differentiation of human mesenchymal stem cells: The potential mechanism for estrogen-induced preferential osteoblast versus adipocyte differentiation. Am J Med Sci. 2011;341:460–68. doi: 10.1097/MAJ.0b013e31820865d5. [DOI] [PubMed] [Google Scholar]
- 5.Yao W, Guan M, Jia J, et al. Reversing bone loss by directing mesenchymal stem cells to bone. Stem Cells. 2013;31:2003–14. doi: 10.1002/stem.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan M, Yao W, Liu R, et al. Directing mesenchymal stem cells to bone to augment bone formation and increase bone mass. Nat Med. 2012;18:456–62. doi: 10.1038/nm.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eskildsen T, Taipaleenmaki H, Stenvang J, et al. Microrna-138 regulates osteogenic differentiation of human stromal (mesenchymal) stem cells in vivo. Proc Natl Acad Sci USA. 2011;108:6139–44. doi: 10.1073/pnas.1016758108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang JF, Fu WM, He ML, et al. Mirna-20a promotes osteogenic differentiation of human mesenchymal stem cells by co-regulating bmp signaling. RNA Biol. 2011;8:829–38. doi: 10.4161/rna.8.5.16043. [DOI] [PubMed] [Google Scholar]
- 9.Qiu W, Kassem M. Mir-141-3p inhibits human stromal (mesenchymal) stem cell proliferation and differentiation. Biochim Biophys Acta. 2014;1843:2114–21. doi: 10.1016/j.bbamcr.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Liu XD, Cai F, Liu L, et al. Microrna-210 is involved in the regulation of postmenopausal osteoporosis through promotion of vegf expression and osteoblast differentiation. Biol Chem. 2015;396:339–47. doi: 10.1515/hsz-2014-0268. [DOI] [PubMed] [Google Scholar]
- 11.Yang N, Wang G, Hu C, et al. Tumor necrosis factor alpha suppresses the mesenchymal stem cell osteogenesis promoter mir-21 in estrogen deficiency-induced osteoporosis. J Bone Miner Res. 2013;28:559–73. doi: 10.1002/jbmr.1798. [DOI] [PubMed] [Google Scholar]
- 12.Tang P, Xiong Q, Ge W, Zhang L. The role of micrornas in osteoclasts and osteoporosis. RNA Biol. 2014;11:1355–63. doi: 10.1080/15476286.2014.996462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liao L, Yang X, Su X, et al. Redundant mir-3077-5p and mir-705 mediate the shift of mesenchymal stem cell lineage commitment to adipocyte in osteoporosis bone marrow. Cell Death Dis. 2013;4:e600. doi: 10.1038/cddis.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Li L, Moore BT, et al. Mir-133a in human circulating monocytes: A potential biomarker associated with postmenopausal osteoporosis. PloS One. 2012;7:e34641. doi: 10.1371/journal.pone.0034641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li H, Wang Z, Fu Q, Zhang J. Plasma mirna levels correlate with sensitivity to bone mineral density in postmenopausal osteoporosis patients. Biomarkers. 2014;19:553–56. doi: 10.3109/1354750X.2014.935957. [DOI] [PubMed] [Google Scholar]
- 16.Gaither LA, Eide DJ. Functional expression of the human hzip2 zinc transporter. J Biol Chem. 2000;275:5560–64. doi: 10.1074/jbc.275.8.5560. [DOI] [PubMed] [Google Scholar]
- 17.Eberle J, Schmidmayer S, Erben RG, et al. Skeletal effects of zinc deficiency in growing rats. J Trace Elem Med Biol. 1999;13:21–26. doi: 10.1016/S0946-672X(99)80019-4. [DOI] [PubMed] [Google Scholar]
- 18.Tang Z, Sahu SN, Khadeer MA, et al. Overexpression of the zip1 zinc transporter induces an osteogenic phenotype in mesenchymal stem cells. Bone. 2006;38:181–98. doi: 10.1016/j.bone.2005.08.010. [DOI] [PubMed] [Google Scholar]