Abstract
Purpose
We investigate how type 2 diabetes (T2DM) and diabetic retinopathy (DR) affect color vision (CV) and mfERG implicit time (IT), whether CV and IT are correlated, and whether CV and IT abnormality classifications agree.
Methods
Adams desaturated D-15 color test, mfERG, and fundus photographs were examined in 37 controls, 22 T2DM patients without DR (NoRet group), and 25 T2DM patients with DR (Ret group). Color confusion score (CCS) was calculated. ITs were averaged within the central 7 hexagons (central IT; ≥4.5°) and outside this area (peripheral IT; ≤4.5°). DR was within (DRIN) or outside (DROUT) of the central 7 hexagons. Group differences, percentages of abnormalities, correlations, and agreement were determined.
Results
CCS was greater in the NoRet (P = 0.002) and Ret (P < 0.0001) groups than in control group. CCS was abnormal in 3, 41, and 48 % of eyes in the control, NoRet, and Ret groups, respectively. Ret group CV abnormalities were more frequent in DRIN than in DROUT subgroups (71 vs. 18 %, respectively; P < 0.0001). CCS and IT were correlated only in the Ret group, in both retinal zones (P ≥ 0.028). Only in the Ret group did CCS and peripheral IT abnormality classifications agree (72 %; P < 0.05).
Conclusion
CV is affected in patients with T2DM, even without DR. Central DR increases the likelihood of a CV deficit compared with non-central DR. mfERG IT averaged across central or peripheral retinal locations is less frequently abnormal than CV in the absence of DR, and these two measures are correlated only when DR is present.
Keywords: Color vision, Adams desaturated D-15, Multifocal, mfERG, Diabetes, Diabetic retinopathy
Introduction
In the USA, 25.8 million people (8.3 % of the total population) have diabetes and another 79 million are prediabetic [1]. Diabetic retinopathy is the leading cause of blindness in the working-age population, with 4.4 %of those with diabetes having vision-threatening retinopathy [2]. The financial cost in the USA is significant as well; $245 billion in total medical expenses in 2012 [3]. With such high costs to health and finances, there is value in learning further how to detect diabetes-related vision changes early in the disease process. This could allow for therapeutic trials to target the disease at earlier stages before vision is altered or threatened [4, 5].
Diabetic retinopathy (DR—see Table 1 for a list of most abbreviations used) is no longer considered to have only a vascular cause detected through fundoscopy, but also a neurologic etiology. Reduced trophic factor signaling, altered glutamate excitation, oxidative stress, and neuroinflammation are likely causes of both vascular and neural cell death [5–7]. Neural changes in the retina begin before clinically visible vascular changes; these can be detected by sensitive structural and functional tests. Changes in corneal nerve fibers prior to diabetic retinopathy onset have also been described [8–10]. In terms of vision function, previous studies have shown that contrast sensitivity, color vision, multifocal visually evoked potential (mfVEP), and multifocal electroretinogram (mfERG) are affected by diabetes, both with and without DR [11–27]. mfERG implicit time (IT) delays in patients without DR have been shown to be highly predictive of nonproliferative diabetic retinopathy (NPDR) [12, 28–30]. Also, IT delays in combination with mfERG amplitude have been shown to be predictive of the development of diabetic macular edema (DME) in patients with NPDR [31]. The mfERG has significant value because of these capabilities.
Table 1.
Abbreviations
| CV | Color vision |
| CCS | Color confusion score |
| mfERG | Multifocal electroretinogram |
| IT | Implicit time |
| Central IT | Central 7 mfERG hexagons average IT |
| Peripheral IT | Outer 96 mfERG hexagons average IT |
| T2DM | Type 2 diabetes mellitus |
| DR | Diabetic retinopathy |
| DRIN | Diabetic retinopathy within the central 7 hexagons |
| DROUT | Diabetic retinopathy outside of the central 7 hexagons |
In clinical practice, it would be more convenient to have a vision test that is equally or more sensitive, more efficient, less costly, and simpler to administer than the mfERG. Early research on color vision testing in diabetes performed from the 1950s through the 1970s by several investigators including Zanen, Verriest, Kinnear, Lakowski, and Barca revealed alterations in color vision in diabetes [21, 23–27]. Many of these studies did not specify the type of diabetes. Diabetics with and without retinopathy had elevated FM 100-hue error scores [21, 24]. Verriest showed that diabetics tend to have blue–yellow defects, a finding that has been replicated many times [25]. In the 1980s, Bresnick showed that macular edema exacerbates these color vision defects and that the magnitude of the color defect increased with edema severity and proximity to the fovea [32]. To our knowledge, the dependence of color vision on retinopathic signs within or near the fovea, versus signs located more peripherally, has not been examined.
The Adams desaturated D-15, a desaturated version of the Farnsworth D-15 dichotomous color vision test, has been shown to be useful in detecting color vision abnormalities in a range of ocular conditions, including glaucoma and diabetes (with and without DR) [11, 20, 33–35]. It is different from the standard D-15 by the reduction of the Munsell chroma of each color cap by 2 units. This makes the test more difficult to pass and more sensitive for the detection of subtle color vision abnormalities [33].
The present study addresses three questions: (1) To what extent do diabetes and diabetic retinopathy impact color vision as compared to mfERG IT? (2) Are color vision and mfERG IT correlated? (3) Is there agreement between classification of abnormalities on the two tests? No previous study has examined how results of simple color vision tests compare to those of the mfERG IT. These are clinically relevant questions because the Adams desaturated D-15 is clinically practical—inexpensive and rapid to administer— whereas the multifocal electroretinogram, though it has excellent local sensitivity to diabetes-related functional changes, requires expensive equipment, pupil dilation, and greater test duration, making it less suitable for use in routine eye care.
Materials and methods
Subjects
Three subject groups were included: 37 visually normal adults (control group); 22 patients with type 2 diabetes without DR (NoRet group); and 25 type 2 diabetic patients with DR (Ret group). The mean age of the control group was 42.2 ± 12.6 years. The NoRet group had a mean age of 53.7 ± 9.5 years and a mean duration of diabetes of 9.6 ± 5.7 years. The Ret group’s mean age was 55.2 ± 9.3 years with a mean duration of 12.6 ± 7.4 years. See Table 2. Inclusion factors were the following: age between 25 and 65 years; refractive error between ± 6 diopters; no ocular disease (aside from DR for the Ret group); no systemic disease (aside from diabetes and hypertension for the Ret and NoRet groups); no visually compromising lenticular opacification; no pseudophakia, visual acuity of 20/25 (log MAR 0.1) or better; no congenital color vision defects by self report; and no neurologic diseases or medications known to affect color vision or mfERG. mfERG data from two of the 84 subjects have been included in a previous publication [31]. None of the color vision data have been previously published. Also, no comparison of the performance of the mfERG and Adams desaturated D-15 test has been previously presented.
Table 2.
Subject characteristics (mean ± SD)
| Subject group (N) | Age (years) | Gender (M/F) | Diabetes duration (years) | HbA1c (%) |
|---|---|---|---|---|
| Control (37) | 42.2 ± 12.6 | 10/27 | N/A | 5.3 ± 0.3 |
| NoRet (22) | 53.7 ± 9.5 | 9/13 | 9.6 ± 5.7 | 7.4 ± 1.7 |
| Ret (25) | 55.2 ± 9.3 | 16/9 | 12.6 ± 7.4 | 8.0 ± 1.2 |
| DRIN (14) | 55.8 ± 8.5 | 10/4 | 12.9 ± 7.5 | 8.1 ± 1.2 |
| DROUT (11) | 54.4 ± 10.5 | 6/5 | 12.2 ± 7.6 | 7.9 ± 1.3 |
Fundus photographs covering the central 50° (Carl Zeiss Meditec, Dublin, CA) were graded by a retina specialist to determine retinopathy status. The ophthalmologist was masked to all other findings. Fourteen eyes of the Ret group had retinopathy signs within (and outside in some cases) the region corresponding to the central 7 elements of the mfERG stimulus (DRIN subgroup). In the other 11 eyes of the Ret group, retinopathy was confined to regions outside the central 7 mfERG stimulus elements (DROUT subgroup). The severity of retinopathy for both DRIN and DROUT groups is shown in Table 3.
Table 3.
Retinopathy type, location, and frequency
| Retinopathy level | DRIN | DROUT |
|---|---|---|
| Mild NPDR | 3 | 6 |
| Moderate NPDR | 9 | 5 |
| Severe NPDR | 1 | 0 |
| PDR | 1 | 0 |
| CSME (from above eyes) | 6 | 0 |
The nature of the study and any potential consequences of study participation were explained to the subjects, and written informed consent was obtained from all subjects before data collection. Procedures complied with the tenets of the Declaration of Helsinki, and the University of California Committee for Protection of Human Subjects approved the study protocol.
Color vision
The Adams desaturated D-15 test was administered under standard illuminant C with a MacBeth easel lamp [35]. Patients performed the test monocularly with full near correction. For each cap, patients were instructed to “Find the color among the remaining caps most like the last one in the box.” Patients were instructed to work through the color panel as efficiently as possible and take a final look at the end to make sure they were satisfied with their cap ordering, i.e., that the caps made a gradual progression in color. The order of the caps was recorded.
mfERG
mfERG testing followed color vision testing. Subjects’ pupils were dilated with 1.0 % tropicamide and 2.5 % phenylephrine. To anesthetize the eye for placement of a Burian-Allen bipolar contact lens electrode (LKC Technologies, Gaithersburg, MD), 0.5 %proparacaine was used. The non-tested eye was occluded, and a ground electrode was placed on the patient’s right ear lobe. mfERG was recorded with a commercial system (VERIS 5.2; EDI Redwood City, CA). The stimulus consisted of 103 scaled hexagonal elements covering a 45° field on the retina. A small fixation target in the center of the stimulus was used during the 8-min recording session, broken into 16 30-s segments. Each hexagon alternated between black and white throughout a 215–1 binary m-sequence. Room lighting provided an average luminance equal to that of the mean luminance of the stimulus (100 cd/m2). Bandpass filtering of 10–100 Hz with 100 K gain was used. Quality was monitored in real time through infrared fixation monitoring and waveform stability. Contaminated recording segments were discarded and repeated. See Ng et al. [30] for further details on this procedure.
Data analysis
The results obtained from the right eyes of the study participants were analyzed. Color confusion score (CCS), which is the percentage of color space traveled beyond that of a perfect arrangement, was calculated [11, 36]. A perfect arrangement yields a CCS of 0. For those with abnormal CCS, the type of color vision defect (blue–yellow, red–green, or non-selective), i.e., the angle (axis) of the cap order, was also determined.
Local first-order kernel mfERG IT was measured using a template-scaling method [37]. Response templates at each test location were constructed from the average local waveforms of 50 normal eyes from previous recordings. IT was measured as the time from focal flash onset to the first positive peak (P1).
In each eye, ITs were averaged within the central 7 hexagons (central IT), approximately 0°–4.5° eccentricity. ITs were also averaged outside of the central 7 hexagons (peripheral IT) from approximately 4.5°– 23° eccentricity. Our laboratory has not previously analyzed the mfERG in this way.
Abnormalities were defined as values exceeding the average of the two greatest (worst) control CCS and mfERG IT values, both corresponding to a specificity of 97.3 %. The criteria for abnormality were determined to be >20.5 for CCS, >32.6 ms for central IT, and >32.7 ms for peripheral IT. The CCS criterion of 20.5 reflects multiple single-place errors or 2 or more crossings in color space. This criterion improves repeatability of testing over more strict criteria [34]. Differences in the rate of abnormalities were examined using binomial probabilities.
In addition to analyses based on abnormalities, differences between subject groups were examined, first using the Kruskal–Wallis test followed by the Mann–Whitney U test. For all comparisons, the Kruskal–Wallis test showed a P value < 0.0001, indicating that the three groups differed. Therefore, only the Mann–Whitney U test results will be presented in the results section. Agreement between CCS and mfERG IT in classifying eyes as either normal or abnormal was assessed using the Fisher exact test. Correlations between CCS and mfERG IT were determined by calculating Spearman rank-order correlation coefficients. This nonparametric test was used because the color vision data have a non-normal distribution, and we wanted to determine the “relative” (rank-order) correlations between CCS and mfERG IT rather than “absolute value” correlations between the two measures. In other words, we wanted to determine whether CCS and mfERG IT order their relative deficits in significantly similar ways.
Results
Color vision
The medians and ranges of the CCS results are reported in column 2 of Table 4. Also in Table 4 are the significance levels of comparisons between pairs of groups. Significant differences in CCS were observed between the control and NoRet groups (P = 0.0002), and between the control and Ret groups (P < 0.0001). The NoRet and Ret groups did not significantly differ, however (P = 0.08). The DRIN and DROUT subgroups of the Ret group were marginally significantly different in CCS (P = 0.049), with the DRIN group having higher CCS.
Table 4.
P values of group comparisons
| Subject group | CCS | Central IT | Peripheral IT | |||
|---|---|---|---|---|---|---|
| Median/range | P value | Mean ± SD | P value | Mean ± SD | P value | |
| Control NoRet | 0/26.9 | 0.0002 | 31.2 ± 0.8 | 0.35 | 30.6 ± 0.8 | 0.0016 |
| 13.9/63.9 | 31.5 ± 0.9 | 31.2 ± 0.6 | ||||
| Control Ret | 0/26.9 | < 0.0001 | 31.2 ± 0.8 | < 0.0001 | 30.6 ± 0.8 | < 0.0001 |
| 18.6/121.3 | 32.7 ± 1.2 | 32.3 ± 1.1 | ||||
| NoRet Ret | 13.9/63.9 | 0.08 | 31.5 ± 0.9 | 0.0002 | 31.2 ± 0.6 | 0.0006 |
| 18.6/121.3 | 32.7 ± 1.2 | 32.3 ± 1.1 | ||||
| DRIN DROUT | 38.0/110.9 | 0.049 | 33.1 ± 1.3 | 0.208 | 32.3 ± 1.0 | 0.57 |
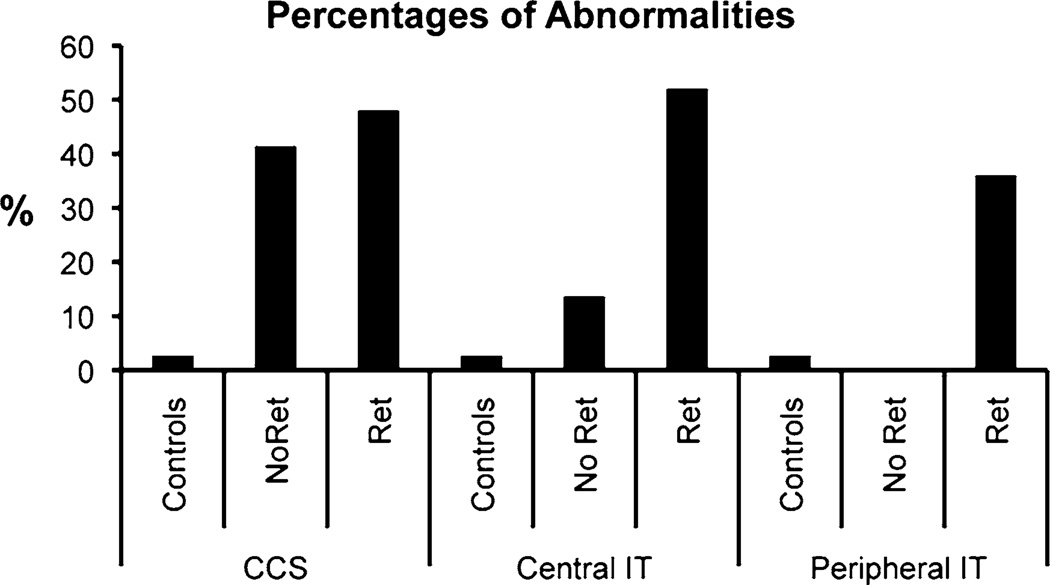
The percentage of eyes with abnormal CCS results were set at 2.7 % for controls and found to be 40.9 and 48.0 % for the NoRet and Ret groups, respectively (Fig. 1). The rates of abnormality were significantly different between control and NoRet groups, and also between the control and Ret groups (both P < 0.0001). The abnormality rates of the NoRet and Ret groups did not differ, however (P = 0.30). The color vision defects were predominantly blue–yellow in nature. For the NoRet and Ret Groups, 67 and 85 %, respectively, were blue–yellow, while the rest were non-selective.
Fig. 1.
CCS, central, and peripheral IT ≥ 20.51, 32.56, and 32.74 ms, respectively, are considered abnormal
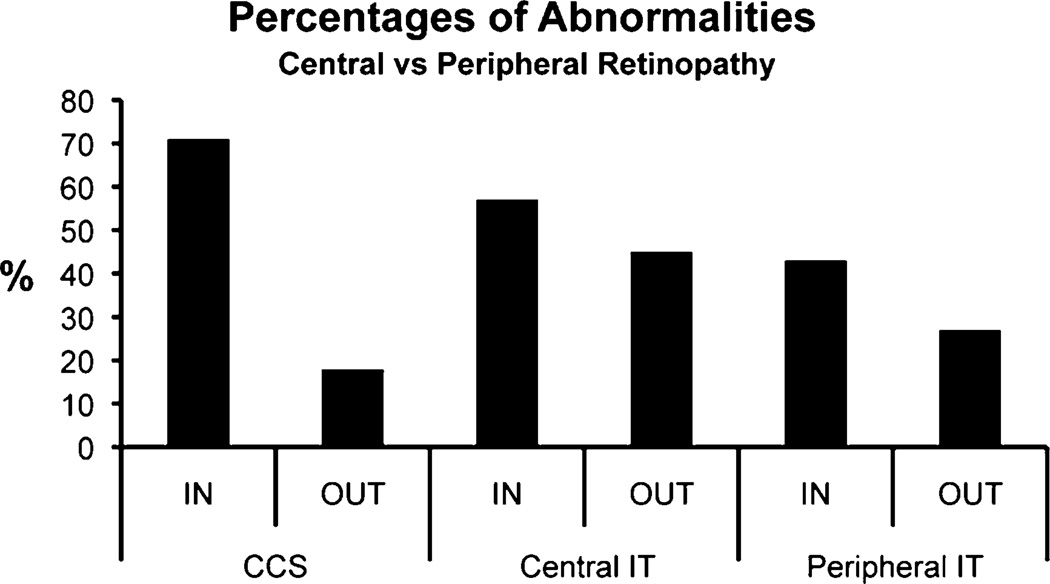
In the DRIN subgroup, 71 % had CCS abnormalities, while 18 % of the DROUT subgroup had abnormalities (Fig. 2). Thus, retinopathy near the fovea resulted in color vision defects more often than retinopathy restricted to more peripheral locations. The difference in these frequencies of color vision abnormality was significant (P < 0.0001).
Fig. 2.
Comparison of percentages of abnormalities in eyes with central retinopathy (DRIN) versus eyes with peripheral retinopathy (DROUT)
mfERG
The mean and standard deviations of the central and peripheral mfERG IT are reported in Table 4, as are the comparisons between subject groups. Significant differences in central IT were observed between the control and Ret groups, and also between the NoRet and Ret groups (both P < 0.0002), with the Ret group having the greatest mean IT. However, the central IT in the control and NoRet groups did not differ (P = 0.35). In contrast, all groups were significantly different with respect to peripheral IT (P < 0.002), with the Ret group again having the longest IT. Unlike the CCS results, neither central IT nor peripheral IT differed significantly between the DRIN and DROUT subgroups (P > 0.20).
The percentages of eyes with abnormal central IT were set at 2.7 % for controls and found to be 13.6 and 52.0 % for the NoRet and Ret groups, respectively (Fig. 1). The percentages of eyes with abnormal peripheral IT were also set at 2.7 % for controls and found to be 0 and 36 % for the NoRet and Ret groups, respectively (Fig. 1). The IT abnormality rates of all of these groups differed significantly (P < 0.0001) except the control and NoRet groups which did not differ significantly for mean central or peripheral IT. Interestingly, the rate of abnormality of both NoRet central and peripheral IT groups were significantly lower than the 40.9 % found for CCS in the NoRet eyes.
Central IT abnormalities are slightly, but not significantly, more common in eyes with DRIN than DROUT (Fig. 2). Peripheral IT abnormalities are also slightly, but not significantly, more common in DRIN than DROUT.
Associations and agreement
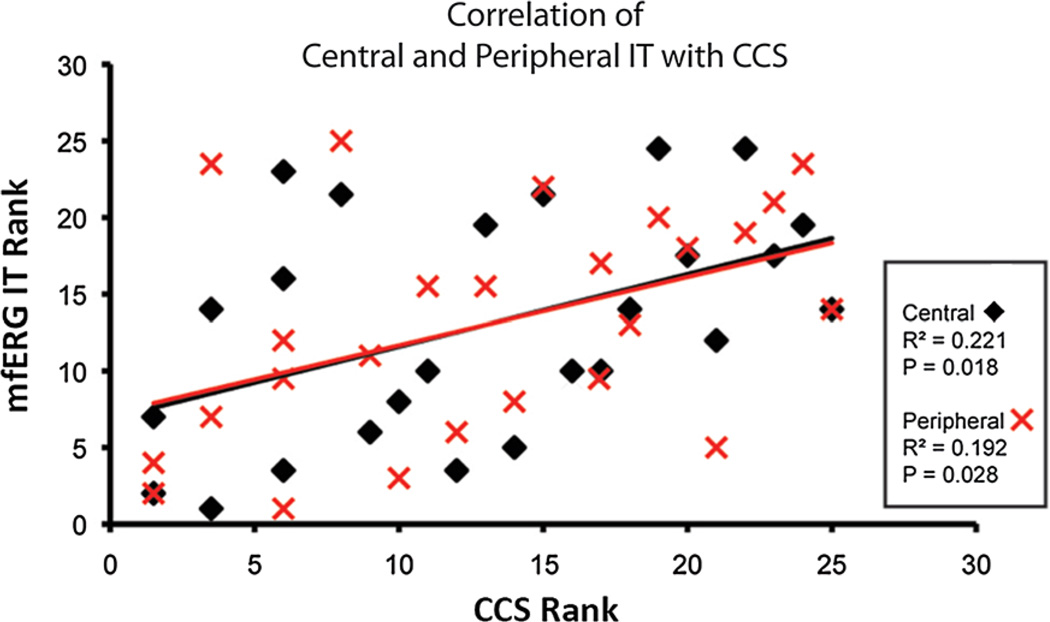
CCS and mfERG IT were not significantly correlated except in eyes with retinopathy. CCS in the Ret group was correlated with both central IT (P < 0.02) and peripheral IT (P < 0.03) (Fig. 3). However, as Table 5 shows, classification of abnormal eyes in the two patient groups by CCS and mfERG IT did not show significant agreement, except for CCS versus peripheral IT in the Ret group (72.0 % agreement; P < 0.041).
Fig. 3.
Spearman rank-order correlation coefficients. A significant association between both central and peripheral mfERG IT with CCS is found in diabetics with retinopathy
Table 5.
Agreement of normal/abnormal categorization
| Group | CCS versus central IT | CCS versus peripheral IT | ||
|---|---|---|---|---|
| Agreement (%) | P value | Agreement (%) | P value | |
| NoRet | 72.7 | 0.055 | 59.1 | 1.000 |
| Ret | 64 | 0.238 | 72 | 0.041 |
Discussion
This study is in agreement with previous reports that the Adams desaturated D-15 is often abnormal in diabetes patients without and with retinopathy [11, 20, 34]. In eyes without retinopathy, CCS was more often abnormal than either central or peripheral mfERG IT. This difference in rate of abnormality is statistically significant, showing that color vision measurement with a desaturated test may detect diabetes-related retinal changes more frequently than mfERG IT obtained from a central location of a retinopathy-free retina. While this may represent greater sensitivity of CCS to retinal changes, it is also possible that this greater percentage of abnormalities may be present because color vision, unlike the mfERG, is potentially influenced not only by diabetes-related changes within the retina, but also at other locations along the visual pathway. More likely, the apparent greater sensitivity of CV is attributable to the averaging of mfERG ITs across fairly large regions, which would wash out any local abnormalities. We have previously shown that IT abnormalities are present in eyes without retinopathy [38]. A potential limitation of using color vision testing that our results suggest is that patients without retinopathy behave like patients with retinopathy, making CCS less useful for alerting clinicians of the likely the presence of diabetic retinopathy.
Not surprisingly, the rates of color vision abnormality depend on the location of retinopathy. When retinopathy was present within the central 4.5°, the rate of CCS abnormality was 71 %, compared with 18 % when retinopathy was only present outside of the central 4.5°. It is possible that, in our study, color vision appeared more affected by central than peripheral retinopathy not only because the retinopathy location coincided more closely on the retina with the area used for color vision testing, but also because the severity of retinopathy was somewhat greater in DRIN eyes.
Color vision and mfERG IT were correlated only when retinopathy was present. This is not surprising, given that the rate of abnormalities on the two measures were only similar in the Ret groups. This appears similar to Han and colleagues’ finding of correlation of mfERG IT with short-wavelength automated perimetry (SWAP) only when retinopathy was present [39]. SWAP tests the function of the S-cone pathway, whereas the mfERG primarily tests the L- and M-cone pathway prior to the ganglion cell layer. As those authors suggested, once retinopathy is present, all cone pathways might be affected such that a correlation exists between the presence of predominantly blue–yellow color defects and mfERG IT [39]. Even with all cone pathways affected when retinopathy is present, as indicated by the mfERG, red–green color defects were not found in this and previous studies, and non-selective defects, which may reflect the presence of both red–green and blue–yellow abnormalities, were seen in only 15 % of eyes with retinopathy. Thus, color vision testing may be less sensitive to L- and M-cone-driven retinal dysfunction than the mfERG.
A significant rank-order correlation with CCS exists in the Ret group for both central and peripheral IT. This implies that, in eyes with retinopathy, when centrally tested color vision shows a reduction, mfERG IT will show increased delay regardless of test location. However, a categorical agreement of abnormality was only found with the peripheral IT, and only in the Ret group.
Hardy et al. [40] also explored the relative sensitivities of and association between color vision testing, using the FM 100-hue test and the oscillatory potentials (OP) of the full-field ERG in eyes of patients with type 1 diabetes without retinopathy. They also reported superior sensitivity of the color vision test over the electrophysiological measures. No correlation was seen between OP latency and performance on the 100 hue. Our study provides new information with regard to a simpler color vision test, the Adams D-15, in comparison with an electrophysiology measure that can better localize abnormalities, the mfERG, in type 2 diabetes.
It should be noted that the control group (mean age 42.2 years) was younger than the NoRet and Ret groups by averages of 11.5 and 13 years, respectively, raising the possibility that the age difference contributed to the poorer color vision and the presence of blue–yellow defects in the diabetic groups. However, Arden and Wolf [41] reported only an insignificant (r2 = 0.1) association between tritan contrast thresholds and age in normal, healthy subjects. Measuring color contrast thresholds, Arden and colleagues report that there was no correlation between age and central color vision thresholds across ages from 6 to 71 years [42]. Further, Roy et al. [43] report no change in error score of the L′Anthony desaturated D-15 test (a test similar to the Adams test) and age between 40 and 55 in normal observers with no lens changes. Much of the decline in performance and increase in frequency of blue–yellow defects with age is attributable to changes in the color and clarity of the ocular media, and in particular, the lens of the eye [44]. It has been demonstrated that older pseudophakic patients’ color vision resembles that of younger observers [44]. Though our subjects were not pseudophakic, all clinically significant cataracts were an exclusion criterion for our study for all subject groups. We see no association between color vision performance and age among our control group. Therefore, it is unlikely that the differences in age underlie the observed differences in color vision performance between those with diabetes and the normal control group.
In conclusion, as has been previously reported, color vision measured with the Adams desaturated D-15 is affected by type 2 diabetes, even in the absence of retinopathy. The presence of retinopathy did not significantly increase the severity of color defects in this sample, which showed predominantly mild or moderate retinopathy. Not surprisingly, the presence of a color vision defect depended significantly on the location of retinopathy; eyes with centrally located retinopathy have color defects significantly more often than eyes with peripheral retinopathy. Furthermore, in this study, color vision was significantly more frequently abnormal than mfERG IT, averaged across retinal locations, in eyes of patients without retinopathy. Finally, we found results from color vision assessment (CCS) and mfERG IT were correlated only when retinopathy was present. Color vision testing appears to be of value as a test to detect early visual dysfunction in patients with diabetes during routine eye care. Further studies are needed to assess whether color vision abnormalities in eyes without retinopathy can predict future retinopathy, as has been demonstrated with mfERG IT.
Acknowledgments
This research was funded by NIH EY021811 (MES) and NIH EY02271 (AJA). The authors thank Ken Huie for implementing the software for scoring the Adams desaturated D-15 data.
Footnotes
This study was presented at a conference of the American Academy of Optometry, 2010, San Francisco, California.
Conflict of interest I certify that there is no actual or potential conflict of interest in relation to this article.
Contributor Information
B. E. Wolff, Email: bewolff1@berkeley.edu, School of Optometry, University of California, Berkeley, Berkeley, CA 94720-2020, USA.
M. A. Bearse, Jr, School of Optometry, University of California, Berkeley, Berkeley, CA 94720-2020, USA.
M. E. Schneck, School of Optometry, University of California, Berkeley, Berkeley, CA 94720-2020, USA
K. Dhamdhere, School of Optometry, University of California, Berkeley, Berkeley, CA 94720-2020, USA
W. W. Harrison, Arizona College of Optometry, Midwestern University, Glendale, AZ, USA
S. Barez, School of Optometry, University of California, Berkeley, Berkeley, CA 94720-2020, USA
A. J. Adams, School of Optometry, University of California, Berkeley, Berkeley, CA 94720-2020, USA
References
- 1.Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 2.Zhang X, Saaddine JB, Chou CF, Cotch MF, Cheng YJ, Geiss LS, Gregg EW, Albright AL, Klein BE, Klein R. Prevalence of diabetic retinopathy in the United States, 2005–2008. J Am Med Assoc (JAMA) 2010;304(6):649–656. doi: 10.1001/jama.2010.1111. doi: 10.1001/jama.2010.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Diabetes Association. The cost of diabetes. [Accessed 12 Feb 2014];2013 http://www.diabetes.org/advocate/resources/cost-of-diabetes.html. [Google Scholar]
- 4.Ng JS. Specific medical intervention for diabetic retinopathy. Med Hypotheses. 2009;73(2):158–160. doi: 10.1016/j.mehy.2009.02.030. doi:10.1016/j.mehy.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 5.Zhang X, Wang N, Barile GR, Bao S, Gillies M. Diabetic retinopathy: neuron protection as a therapeutic target. Int J Biochem Cell Biol. 2013;45(7):1525–1529. doi: 10.1016/j.biocel.2013.03.002. doi:10.1016/j.biocel.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012;366(13):1227–1239. doi: 10.1056/NEJMra1005073. doi:10.1056/NEJMra1005073. [DOI] [PubMed] [Google Scholar]
- 7.Barber AJ, Gardner TW, Abcouwer SF. The significance of vascular and neural apoptosis to the pathology of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2011;52(2):1156–1163. doi: 10.1167/iovs.10-6293. doi:10.1167/iovs.10-6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pritchard N, Edwards K, Shahidi AM, Sampson GP, Russell AW, Malik RA, Efron N. Corneal markers of diabetic neuropathy. Ocul Surf. 2011;9(1):17–28. doi: 10.1016/s1542-0124(11)70006-4. [DOI] [PubMed] [Google Scholar]
- 9.Ziegler D, Papanas N, Zhivov A, Allgeier S, Winter K, Ziegler I, Bruggemann J, Strom A, Peschel S, Kohler B, Stachs O, Guthoff RF, Roden M. Early detection of nerve fiber loss by corneal confocal microscopy and skin biopsy in recently diagnosed type 2 diabetes. Diabetes. 2014;63(7):2454–2463. doi: 10.2337/db13-1819. doi:10.2337/db13-1819. [DOI] [PubMed] [Google Scholar]
- 10.Bitirgen G, Ozkagnici A, Malik RA, Kerimoglu H. Corneal nerve fibre damage precedes diabetic retinopathy in patients with type 2 diabetes mellitus. Diabetic Med J Br Diabetic Assoc. 2014;31(4):431–438. doi: 10.1111/dme.12324. doi:10.1111/dme.12324. [DOI] [PubMed] [Google Scholar]
- 11.Adams AJ, Haegerstrom-Portnoy G. Color Deficiency. In: Amos JF, editor. Diagnosis and management in vision care. Vol. 1. Stoneham: Butterworth-Heinemann; 1987. pp. 671–709. [Google Scholar]
- 12.Bearse MA, Jr, Adams AJ, Han Y, Schneck ME, Ng J, Bronson-Castain K, Barez S. A multifocal electroretinogram model predicting the development of diabetic retinopathy. Prog Retin Eye Res. 2006;25(5):425–448. doi: 10.1016/j.preteyeres.2006.07.001. doi:10.1016/j.preteyeres.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhamdhere KP, Bearse MA, Jr, Wolff BE, Harrison WW, Cardenas M, Barez S, Schneck ME, Adams AJ. Associations between contrast sensitivity and multifocal electroretinograms in Type2 diabetes. Paper presented at the ARVO; 05/02/2011; Fort Lauderdale, Florida. 2011. [Google Scholar]
- 14.Ewing FM, Deary IJ, Strachan MW, Frier BM. Seeing beyond retinopathy in diabetes: electrophysiological and psychophysical abnormalities and alterations in vision. Endocr Rev. 1998;19(4):462–476. doi: 10.1210/edrv.19.4.0340. [DOI] [PubMed] [Google Scholar]
- 15.Feitosa-Santana C, Paramei GV, Nishi M, Gualtieri M, Costa MF, Ventura DF. Color vision impairment in type 2 diabetes assessed by the D-15d test and the Cambridge colour test. Ophthalmic Physiol Opt. 2010;30(5):717–723. doi: 10.1111/j.1475-1313.2010.00776.x. doi:10.1111/j.1475-1313.2010.00776.x. [DOI] [PubMed] [Google Scholar]
- 16.Peduzzi M, Longanesi L, Ascari A, Cascione S, Galletti M, Roncaia R, Pacchioni C, Maione M. Screening of early color vision loss in diabetic patients. J Fr Ophtalmol. 1989;12(11):791–796. [PubMed] [Google Scholar]
- 17.Rodgers M, Hodges R, Hawkins J, Hollingworth W, Duffy S, McKibbin M, Mansfield M, Harbord R, Sterne J, Glasziou P, Whiting P, Westwood M. Colour vision testing for diabetic retinopathy: a systematic review of diagnostic accuracy and economic evaluation. Health Technol Assess. 2009;13(60):1–160. doi: 10.3310/hta13600. doi:10.3310/hta13600. [DOI] [PubMed] [Google Scholar]
- 18.Sukha AY, Rubin A. High, medium, and low contrast visual acuities in diabetic retinal disease. Optom Vis Sci Off Publ Am Acad Optom. 2009;86(9):1086–1095. doi: 10.1097/OPX.0b013e3181b48635. doi:10.1097/OPX.0b013e3181b48635. [DOI] [PubMed] [Google Scholar]
- 19.Wolff BE, Bearse MA, Jr, Schneck ME, Barez S, Adams AJ. Multifocal VEP (mfVEP) reveals abnormal neuronal delays in diabetes. Doc Ophthalmol Adv Ophthalmol. 2010;121(3):189–196. doi: 10.1007/s10633-010-9245-y. doi:10.1007/s10633-010-9245-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adams AJ, et al. Chromaticity and luminosity changes in glaucoma and diabetes. Doc Ophthalmol Proc Ser. 1982;33:413–418. [Google Scholar]
- 21.Barca L, Vaccari G. On the impairment of color discrimination in diabetic retinopathy, a report of 24 cases. Atti Fond G Ronchi. 1977;32:635–640. [Google Scholar]
- 22.Birch JM, Chisholm I, Kinnear P, Marre M, Pinckers AJLG, Pokorny J, Smith VC, Verriest G. Acquired color vision defects. In: Pokory J, Smith VC, Verriest G, Pinckers AJLG, editors. Congenital and Acquired Color Vision Defects. New York: Grune and Stratton Inc; 1979. pp. 282–284. [Google Scholar]
- 23.Kinnear PR, Aspinall PA, Lakowski R. The diabetic eye and colour vision. Trans Ophthalmol Soc U K. 1972;92:69–78. [PubMed] [Google Scholar]
- 24.Lakowski R, Aspinall PA, Kinnear PR. Association between colour vision losses and diabetes melitus. Ophthalmic Res. 1973;4:145–159. [Google Scholar]
- 25.Verriest G. Acquired Color Perception Defects. Memoires de l’Academie royale de medecine de Belgique. 1964;18:35–327. [PubMed] [Google Scholar]
- 26.Zanen J. [Introduction to the study of acquired central retinal dyschromatopsias] Bull Soc Belge Ophtalmol. 1953;103:3–144. discussion, 144–148. [PubMed] [Google Scholar]
- 27.Zanen J, Szucs S, Pirart J. Achromatic & chromatic thresholds in diabetes. Bull Soc Belge Ophtalmol. 1957;115(Pt 2):210–219. [PubMed] [Google Scholar]
- 28.Han Y, Bearse MA, Jr, Schneck ME, Barez S, Jacobsen CH, Adams AJ. Multifocal electroretinogram delays predict sites of subsequent diabetic retinopathy. Invest Ophthalmol Vis Sci. 2004;45(3):948–954. doi: 10.1167/iovs.03-1101. [DOI] [PubMed] [Google Scholar]
- 29.Harrison WW, Bearse MA, Jr, Ng JS, Jewell NP, Barez S, Burger D, Schneck ME, Adams AJ. Multifocal electroretinograms predict onset of diabetic retinopathy in adult patients with diabetes. Invest Ophthalmol Vis Sci. 2011;52(2):772–777. doi: 10.1167/iovs.10-5931. doi:10.1167/iovs.10-5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng JS, Bearse MA, Jr, Schneck ME, Barez S, Adams AJ. Local diabetic retinopathy prediction by multifocal ERG delays over 3 years. Invest Ophthalmol Vis Sci. 2008;49(4):1622–1628. doi: 10.1167/iovs.07-1157. doi:10.1167/iovs.07-1157. [DOI] [PubMed] [Google Scholar]
- 31.Harrison WW, Bearse MA, Jr, Schneck ME, Wolff BE, Jewell NP, Barez S, Mick AB, Dolan BJ, Adams AJ. Prediction, by retinal location, of the onset of diabetic edema in patients with nonproliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2011;52(9):6825–6831. doi: 10.1167/iovs.11-7533. doi:10.1167/iovs11-7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bresnick GH, Condit RS, Palta M, Korth K, Groo A, Syrjala S. Association of hue discrimination loss and diabetic retinopathy. Arch Ophthalmol. 1985;103(9):1317–1324. doi: 10.1001/archopht.1985.01050090069034. [DOI] [PubMed] [Google Scholar]
- 33.Adams AJ, Rodic R, Husted R, Stamper R. Spectral sensitivity and color discrimination changes in glaucoma and glaucoma-suspect patients. Invest Ophthalmol Vis Sci. 1982;23(4):516–524. [PubMed] [Google Scholar]
- 34.Hovis JK, Ramaswamy S, Anderson M. Repeatability indices for the Adams D-15 test for colour-normal and colour-defective adults. Clin Exp Optom J Aust Optom Assoc. 2004;87(4–5):326–333. doi: 10.1111/j.1444-0938.2004.tb05062.x. [DOI] [PubMed] [Google Scholar]
- 35.Adams AJ, Rodic R. Use of desaturated and saturated versions of the D-15 test in glaucoma and glaucoma-suspect patients. In: Verriest G, editor. Colour vision deficiencies IV. Doc Ophthalmol Proc Series. Vol. 33. The Hague: Dr W Junk; 1982. pp. 419–424. [Google Scholar]
- 36.Vingrys AJ, King-Smith PE. A quantitative scoring technique for panel tests of color vision. Invest Ophthalmol Vis Sci. 1988;29(1):50–63. [PubMed] [Google Scholar]
- 37.Hood DCLJ. A technique for measuring individual multifocal ERG records: non-invasive assessment of the visual system. Trends Opt Photon. 1997;11:33–41. [Google Scholar]
- 38.Fortune B, Schneck ME, Adams AJ. Multifocal electroretinogram delays reveal local retinal dysfunction in early diabetic retinopathy. Invest Ophthalmol Vis Sci. 1999;40(11):2638–2651. [PubMed] [Google Scholar]
- 39.Han Y, Adams AJ, Bearse MA, Jr, Schneck ME. Multifocal electroretinogram and short-wavelength automated perimetry measures in diabetic eyes with little or no retinopathy. Arch Ophthalmol. 2004;122(12):1809–1815. doi: 10.1001/archopht.122.12.1809. doi:10.1001/archopht.122.12.1809. [DOI] [PubMed] [Google Scholar]
- 40.Hardy KJ, Fisher C, Heath P, Foster DH, Scarpello JH. Comparison of colour discrimination and electroretinography in evaluation of visual pathway dysfunction in aretinopathic IDDM patients. Br J Ophthalmol. 1995;79(1):35–37. doi: 10.1136/bjo.79.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arden GB, Wolf JE. Colour vision testing as an aid to diagnosis and management of age related maculopathy. Br J Ophthalmol. 2004;88(9):1180–1185. doi: 10.1136/bjo.2003.033480. doi:10.1136/bjo.2003.033480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berninger T, Drobner B, Hogg C, Rudolph G, Arden GB, Kampik A. Color vision in relation to age: a study of normal values. Klin Monatsbl Augenheilkd. 1999;215(1):37–42. doi: 10.1055/s-2008-1034667. doi:10.1055/s-2008-1034667. [DOI] [PubMed] [Google Scholar]
- 43.Roy MS, Podgor MJ, Collier B, Gunkel RD. Color vision and age in a normal North American population. Graefe’s Arch Clin Exp Ophthalmol (Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie) 1991;229(2):139–144. doi: 10.1007/BF00170545. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen-Tri D, Overbury O, Faubert J. The role of lenticular senescence in age-related color vision changes. Invest Ophthalmol Vis Sci. 2003;44(8):3698–3704. doi: 10.1167/iovs.02-1191. [DOI] [PubMed] [Google Scholar]