On their way to the brain, optic nerves from the two eyes in several animal species pass through the striking anatomical formation called the optic chiasm. Interest in the optic chiasm can be traced at least as far back as Galen, who in the 1st century AD described the structure as a resembling the letter chi. Until the 17th century, it was believed (most notably by Descartes) that although the two optic nerves came close at the chiasm, they did not actually cross over (figure 1). A more accurate understanding of the chiasm began with Isaac Newton (Sweeney, 1984). Although there is no record of Newton ever having performed any dissections of the chiasm, he correctly predicted that some nerves from the two eyes should cross over to the other side at the chiasm to subserve binocular vision. Precisely how this crossing is accomplished has been a topic of great interest in recent years. A large body of research has explored the cellular and molecular biology of chiasm development (reviewed in Jeffery, 2001).
Figure 1.
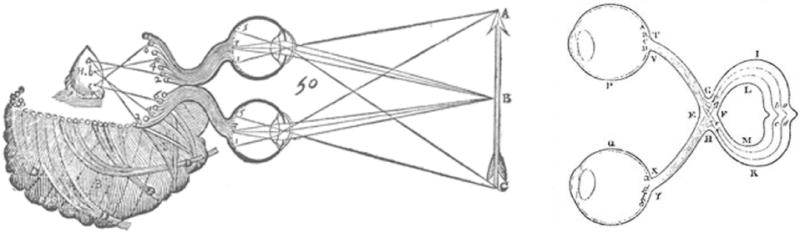
Structure of the optic chiasm as proposed by Descartes (left panel) and Isaac Newton (right panel). The former believed that the optic nerves came close together, but did not cross at the chiasm. Newton correctly hypothesized, based on a theoretical analysis of requirements for binocular vision, that there is a partial cross-over of optic nerves at the chiasm (technically referred to as a partial decussation of the fibers). In rare cases of achiasma, optic fibers behave as in Descartes’ conception.
For the vast majority of humans and many other animals, Newton’s prediction holds true. At the chiasm, nerve fibers carrying information from the nasal retina cross over to the contralateral side. This cross-over enables information from the left and right halves of the visual field to be channeled to the lateral geniculate nucleus and thence to the primary visual cortex in the contralateral cerebral hemisphere. At a finer grain, projections from the LGN are organized in such a way as to bring together information from cells that have roughly overlapping receptive fields, a pre-requisite, as Newton intuited, for binocular perception.
In rare cases, anatomy deviates from this schema. In a condition referred to as ‘achiasma’, the full complement of nerve fibers from an eye terminate only in the ipsilateral LGN, which then projects to the corresponding half of the primary visual cortex. V1 in each hemisphere thus receives information about both left and right visual fields. This brings up an obvious question: How does neuronal organization in the cortex change in response to this drastic alteration in the nature of the input?
There are various facets to this question. How is full visual space mapped onto a cortical surface that under normal circumstances is designed to handle only a hemi-field? What is the structure of V1 receptive fields in achiasma? Are connectivity patterns between the hemispheres altered by changes in their afferents? Answers to these questions can yield interesting clues about the extent and locus of reorganization possible in the visual system. In this regard, studies of achiasma are similar to those that have explored cortical reorganization following changes in sensory afferents as in blindness or deafness (see review in Merabet and Pascual-Leone, 2010). However, unlike the latter where a rich body of results has accumulated, little is known about cortical organization in achiasma due primarily to the rarity of the condition.
Hoffmann et al. in this issue help alleviate some of the dearth of knowledge about this condition. Before we describe their findings, let us provide some context by considering a few options that outline the space of possibilities for their results. We focus specifically on the issue of how the visual field in achiasma might be mapped onto V1’s surface.
Field restriction: The neural resources of V1 in each hemisphere are normally intended to process only one hemi-field’s worth of data. ‘Hardware’ limitations might restrict the extent of area within the full visual field that can be analyzed by V1 in either hemisphere. Furthermore, the visual field restriction can be different for the contra- and ipsi-lateral hemi-fields.
Contiguous full-field representation: V1 in each hemisphere may be remapped to represent the entire visual field, with the two hemi-fields placed side by side on the cortical surface.
Disrupted retinotopy: The drastic change in visual input might lead to a disruption of systematic retinotopic maps and no coherent spatial organization may be evident in V1.
Overlapped fields: Retinotopic maps for both hemi-fields might be spatially superimposed over the extent of V1 in each hemisphere.
Which of these possibilities actually holds in human achiasmic individuals? Working with two subjects, Hoffmann et al. present compelling fMRI results in support of the fourth option. There is no evidence of any field restriction either behaviorally or in imaging. V1 in each hemisphere displays systematic retinotopic maps for both fields that are precisely superimposed over each other. It is as if the visual world were folded in half along the midline and mapped onto the cortical surface. What this implies is that a given section in V1 would receive information from two very different regions in visual space arranged in a mirror-symmetric manner about the vertical midline. This is indeed what the authors find using an elegant population receptive field (pRF) mapping technique (Dumoulin and Wandell, 2008). In addition to retinotopy and pRF mapping, the authors also examine white matter connectivity patterns. They find no notable differences in the DTI results obtained from normal subjects relative to those in achiasma.
How can these results be explained? In light of a prior study that had examined LGN organization in achiasma (Williams et al., 1994), the account for the current results is appealingly straightforward. As the authors describe it, the results point to conserved connectivity patterns from LGN to V1 and beyond. In other words, these results suggest that there is no large-scale neural reorganization beyond the thalamus, despite the change in input connectivity from the eyes to the thalamus. Figure 2 illustrates the basic idea. Williams et al. (1994) have shown that in achiasma, the LGN layers, normally devoted to ipsi and contra eyes for the same hemifield (Kandel et al., 2000) get repurposed for the different hemifields instead. If nothing about the optic radiation from the LGN to V1 changes, then V1 units that normally would have received inputs from corresponding regions in space from the two eyes will end up receiving inputs from potentially widely separated regions, mirrored across the vertical midline, from the same eye.
Figure 2.
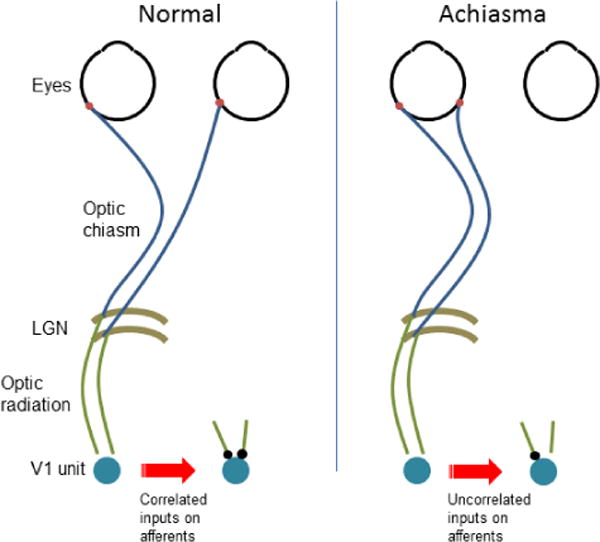
Schematic wiring diagrams for the eyes, LGN and V1 in normal and achiasmic individuals (left and right panels, respectively). V1 units in the normal brain receive inputs from similar regions of visual space. In achiasma, on the other hand, the inputs correspond to very different regions. The much higher temporal correlation of inputs in the first case will be expected, via classical Hebb-like learning mechanisms, to yield binocular V1 units in the normal brain, but not in the achiasmic one.
While this explanation accounts for the basic findings from Hoffmann et al., it leaves open the issue of fine-scale reorganization. What is the nature of response properties at the level of single V1 units? The pRFs show a bi-lobed structure straddling the midline, but does this hold true for individual neurons as well? Let us consider a potential behavioral consequence of this possibility. If every neuron were unable to distinguish between two mirror-imaged locations (and for every lobe position pair, the ambiguity was the same for all neurons sensitive to either of those two locations, i.e. every neuron that has an rf lobe at location ‘A’ necessarily has another lobe at the mirror-symmetric location ‘B’), then the ambiguity is unresolvable and will become manifest at the level of behavior, i.e. a person with such an rf organization will confuse left and right. However, as Hoffmann et al. and earlier researchers (Victor et al., 2000) report, no such confusions are apparent, suggesting that neurons do code for specific locations unambiguously. The observed bilobed structure of pRFs may be caused by the clustering of neurons with unilobed rfs at one or the other mirror-symmetric positions.
A classical Hebbian learning based account (Hebb, 1949) also argues for unilobed rfs at the level of individual neurons in achiasma. In the normal visual pathway, with appropriate decussation of optic fibers at the chiasm, a given neuron in V1 would receive inputs from the two eyes from spatially close (or even identical) locations in the visual field. This proximity would lead to a temporal pattern of stimulation ideally suited for Hebbian reinforcement of connections (since the inputs from the two locations would be temporally highly correlated). A binocular V1 cell would be the result.
However, in achiasma, the same fibers from LGN co-incident on any location in V1 carry information from two very disparate parts of the visual field. The temporal activity in these fibers is likely to be largely uncorrelated and provide no support for coupling via Hebbian reinforcement. Individual V1 neurons, therefore, would be driven by one or the other of these fibers but not by both, leading to single-lobed rfs. It would be important for future neurophysiological studies to empirically verify this theoretical prediction. In animal models of achiasma, do individual neurons in the primary visual cortex exhibit unilobed receptive fields and, furthermore, for nearby V1 neurons, are the locations of these lobes in visual space mirrored about the vertical mid-line?
Beyond characterizing the receptive field properties of individual V1 neurons in achiasma, it would also be interesting elucidate the spatial arrangement of small populations of neurons responding to inputs from the two hemifields. Are these neurons inter-digitated randomly or are there macro-patterns, akin to ocular dominance columns that they are organized in? In a related vein, what do hypercolumns look like in achiasma? Answers here might provide clues regarding the factors governing the genesis of medium-scale spatial organization in the visual cortex.
Several additional interesting questions about achiasma await behavioral and neurophysiological investigation. Some of these can potentially help understand feedforward, horizontal, and feedback circuits of cortical organization. For instance, would adaptation to contrast, orientation or motion transfer from one eye to the other, or from one hemifield to the other at corresponding locations? Would flanking stimuli laterally inhibit or facilitate detection of a probe at the corresponding mirror location (Adini et al., 1997)? And, would a peripheral cue lead to attentional priming at the corresponding mirror location (Posner and Petersen, 1990)?
Anatomically, although the work in achiasma so far has focused on the projections to and from the LGN, it would also be interesting to work out projections to the superior colliculus (SC). Is the topographic mapping in the SC changed in this condition? This question has both basic and applied significance. The SC is intimately involved in eye-movements (Wurtz and Goldberg, 1971; -), and is implicated in some disorders of ocular movement (Schiller et al., 1980; Keating and Gooley, 1988). Intriguingly, achiasma is seen to be accompanied by nystagmus, even though most other aspects of vision are quite normal (Apkarian et al., 1994). Are any abnormalities in the topographic mapping within the SC responsible for the nystagmus observed in cases of achiasma?
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
In the rare condition of achiasma, the visual cortex in each hemisphere receives information from both halves of the visual field. How is this ‘doubling’ of information accommodated in V1?
Hoffmann et al. in this issue investigate the cortical consequences of this anomaly.
References
- Adini Y, Sagi D, Tsodyks M. Excitatory-Inhibitory Network in the Visual Cortex: Psychophysical Evidence. Proceedings of the National Academy of Sciences USA. 1997;94:10426–10431. doi: 10.1073/pnas.94.19.10426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian P, Bour L, Barth PG. A unique achiasmatic anomaly detected in non-albinos with misrouted retinal-fugal projections. The European Journal of Neuroscience. 1994;6:501–507. doi: 10.1111/j.1460-9568.1994.tb00293.x. [DOI] [PubMed] [Google Scholar]
- Dumoulin SO, Wandell BA. Population receptive field estimates in human visual cortex. NeuroImage. 2008;39:647–660. doi: 10.1016/j.neuroimage.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb DO. The organization of behavior. New York: Wiley & Sons; 1949. [Google Scholar]
- Hoffmann MB, Kaule FR, et al. Plasticity and stability of the visual system in human achiasma. Neuron. 2012 doi: 10.1016/j.neuron.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery G. Architecture of the optic chiasm and the mechanisms that sculpt its development. Physiological Reviews. 2001;81(4):1393–1414. doi: 10.1152/physrev.2001.81.4.1393. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science. 4. McGraw-Hill; New York: 2000. [Google Scholar]
- Keating EG, Gooley SG. Saccadic disorders caused by cooling the superiorcolliculus or the frontal eye field, or from combined lesions of both structures. Brain Research. 1988;438(1–2):247–255. doi: 10.1016/0006-8993(88)91343-1. [DOI] [PubMed] [Google Scholar]
- Merabet LB, Pascual-Leone A. Neural reorganization following sensory loss: the opportunity of change. Nat Rev Neurosci. 2010;11(1):44–52. doi: 10.1038/nrn2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Schiller PH, True SD, Conway JL. Deficits in eye movements following frontal eye-field and superior colliculus ablations. J Neurophysiology. 1980;44(6):1175–1189. doi: 10.1152/jn.1980.44.6.1175. [DOI] [PubMed] [Google Scholar]
- Sweeney PJ. Isaac Newton and the optic chiasm. Neurology. 1984;34:309. [Google Scholar]
- Victor JD, Apkarian P, Hirsch J, Conte MM, Packard M, Relkin NR, Kim KH, Shapley RM. Visual function and brain organization in non-decussating retinal-fugal fibre syndrome. Cerebral Cortex. 2000;10:2–22. doi: 10.1093/cercor/10.1.2. [DOI] [PubMed] [Google Scholar]
- Williams RW, Hogan D, Garraghty PE. Target recognition and visual maps in the thalamus of achiasmatic dogs. Nature. 1994;367:637–639. doi: 10.1038/367637a0. [DOI] [PubMed] [Google Scholar]
- Wurtz RH, Goldberg ME. Superior Colliculus Cell Responses Related to Eye Movements in Awake Monkeys. Science. 1971;171(3966):82–84. doi: 10.1126/science.171.3966.82. [DOI] [PubMed] [Google Scholar]


