Abstract
The thalamus is a critical component of the frontal cortical-basal ganglia-thalamic circuits that mediate motivation and emotional drive, planning and cognition for the development and expression of goal-directed behaviors. Each functional region of the frontal cortex is connected with specific areas of each basal ganglia (BG) structure and of the thalamus. In addition, the thalamus sends a massive, topographically organized projection directly to the striatum. Tract-tracing and physiological experiments have indicated a general topographic organization of the cortical-BG-thalamic loops and supported a model of BG function based on parallel and segregated pathways. However, the learning and execution of appropriate behavioral responses require integration of inputs related to emotional, cognitive, and motor cortical functions. Our recent data indicate that integration may occur via non-reciprocal connections between the striatum and substantia nigra and within “hot spots” of convergence between cortico-striatal projections from different functional regions. Similarly, integration may exist in the thalamus. There are non-reciprocal connections between the thalamus and cortex via thalamocortical projections that terminate in the superficial and deep cortical layers. These terminals can influence different functional cortical areas that, in turn, project to the striatum and back to the thalamus. In addition, a non-reciprocal corticothalamic projection terminates in thalamic regions that are parts of other circuits. Finally, ‘hot spots’ of convergence between terminals from different cortical regions may also occur in the thalamus as is seen in the striatum. Thus, via several different pathways, the thalamus may serve as an important center of integration of networks that underlie the ability to modulate behaviors.
Introduction
Traditionally the basal ganglia (BG) have been considered to be primarily involved in the control of motor function [1] [2]. However, our knowledge of BG function has dramatically changed in the last 20 years. These structures are now considered to be particularly critical for learning of new complex behaviors [3] [4]. Thus, in addition to their involvement in the expression of goal-directed behaviors through movement, the BG are also involved in the processes that lead to movement, including emotions, motivation, and cognition [5] [6]. The BG are linked to the frontal cortex. The functional components of the frontal-BG-thalamic circuits that mediate motivation and emotional drive, planning and cognition, and movement, are reflected in the connections between specific areas of frontal cortex [7]. The thalamus is a critical piece of this circuitry. The thalamus is also organized into functional regions based on their connections to cortex [8] [9]. Thus, different frontal cortical areas and the corresponding BG and thalamic regions are involved in various aspects of motivation, cognition, and motor control.
Topographic organization of pathways
Functional cortico-BG loops
Each functional region of the frontal cortex is connected with specific areas in each BG structure. The anterior cingulate cortex (ACC) and orbital frontal cortex (OFC) mediate different aspects of reward-based behaviors [10] [11]. Projections from the ACC/OFC terminate primarily in the rostral, ventro-medial striatum [12] [13]. This striatal region, commonly referred to as the ventral striatum (VS), projects to the ventral pallidum (VP) and ventral tegmental area (VTA), and to the medial part of substantia nigra (SN) [14]. The dorsolateral prefrontal cortex (DLPFC) is engaged when tasks involve strategic planning and working memor [15]. In primates, projections from this prefrontal cortical region terminate primarily in the rostral and dorsal caudate n. [16] [17], which, in turns, project to the central part of the globus pallidum (GP) and SN [18]. The dorsal and rostral premotor cortex (PMdr) is involved with monitoring and planning action and its projections terminate in the dorsal and lateral caudate n. [19, 20] [16]. This striatal region sends afferent input to the central and dorsal GP and ventral SN [18] [21].
Thalamo-striatal connections
In addition to these general cortico-BG loops, there is a dense thalamic projection to the striatum. This projection also maintains a general functional topography. The midline and intralaminar nuclei are the source of the most widely reported thalamostriatal projections [22] [23–27]. These nuclei are further subdivided and associated with specific functions related to their cortical connections. In rodents and primates, the midline and medial intralaminar nuclei project to medial prefrontal areas, the amygdala and hippocampus, and, as such, are considered the limbic-related thalamic nuclear groups [28] [29] [30]. The intralaminar nuclei central medial (CM) and parafascicularis (Pf) have connections with association areas. The lateral CM nucleus projects to both the primary motor (M1) and sensory cortices and, therefore, is considered to be related to motor control [9] [31]. These midline and intralaminar thalamic nuclei project topographically to the striatum such that the midline and medial Pf nuclei project mainly to ventral (limbic) striatal areas, whereas the more lateral intralaminar nuclei have connections with the dorsolateral (association-sensorimotor) caudate and putamen [23] [32, 33]. In this way, the midline and intralaminar thalamic nuclei project to striatal areas that are consistent with the cortical area they are connected to, thus maintaining the functional distinction of different striatal regions.
In addition to intralaminar thalamic projections, in primates there is an equally large input to the dorsal striatum from the “specific” thalamic BG relay nuclei, the medialis dorsalis nuclues (MD), ventralis anterior (VA) and ventralis lateralis (VL) nuclei [33, 34]. These thalamic nuclei are also intimately connected with specific frontal cortical areas [8, 35–38]. Thus, different regions of the ventral motor nuclei (VA-VL) have reciprocal projections with specific premotor, motor and cingulate cortices. Different parts of the MD nucleus are linked to specific prefrontal areas. As with the midline and intralaminar nuclei, interconnected ventral VA/VL MD n and cortical areas project to the same region of the striatum [33, 39] (Diagram 1). For example, the ventralis posterior lateralis pars oralis nucleus (VPLo) projects to M1 and the caudal premotor areas. These areas issue dense projections to the dorsolateral putamen. The pars oralis of the VL nucleus (VLo) projects densely to the supplementary motor area (SMA) and to the caudal cingulate motor area (CMAc). VLo and SMA projections converge in the dorsal and central putamen. The pars caudalis of the VL nucleus (VLc) is predominately connected with PMdr and caudal dorsal premotor cortex (PMdc), but also has connections with ventral premotor areas (PMv) and the ventral part of CMAc. VLc and PMdr projections mainly target the dorsolateral caudate nucleus. Rostral motor areas, including PMdr, PreSMA, and rostral cingulate motor area (CMAr), receive inputs from the parvicellular part of VA (VApc). Striatal projections from these areas converge within the putamen and dorsolateral caudate (Diagram 1). Thus, there is a tight, anatomical and functional triad of basal ganglia input and output structures, involving the frontal motor cortices, the striatum, and the thalamic relay nuclei. These anatomical connections indicate a dual role for the VA/VL and MD relay nuclei: relaying basal ganglia output to frontal cortical areas and providing direct feedback to the striatum.
The thalamic link to cortex
The thalamus represents the final basal ganglia link back to cortex. This afferent projection also maintains a general functional topography. In primates, the ventral lateral thalamic complex and the MD nucleus receive the bulk of basal ganglia output (from the GP and SN reticulata/SNr) [40] [41]. Each subdivision of the VA and VL nuclei receives input from different pallidal and nigral regions. The magnocellular part of the VA nucleus (VAmc) receives nigral inputs, whereas the VApc and VLo nuclei receive pallidal inputs. The MD nucleus receives input from the medial SNr and the ventral GP [40, 42]. Regions of VL receiving the main output from motor regions of GP project to motor and premotor areas of frontal cortex [7] [35]. Likewise, VA is associated with the rostral premotor cortex and DLPFC, and the MD nucleus is linked to the DLPFC, OFC and ACC [36] [38].
Thus, the functional topography of cortex is maintained from cortical connections to the striatum, from the striatum to the GP/SNr, from these BG output structures to the thalamus, and finally back to cortex. Taken together, distinct pathways from cortex through the striatum, GP/SNr, thalamus, and back to cortex that mediate specific functions, such as motor control, cognition and emotion occupy different regions within each of the cortico-basal ganglia-thalamic structures. In addition, as mentioned above, the thalamus sends a massive, topographically organized projection directly to the striatum. This general topographic organization of the cortical-BG-thalamic loops has lead to a model of BG function based on parallel and segregated pathways operating through discrete motor, cognitive and limbic channels [43]. Moreover, it has been proposed that there are sub-circuits within each functional area [44].
Integrative processing of cortico-BG circuits
A major problem with the concept that functional information is processed solely through parallel and segregated circuits is that it does not address how information might flow between circuits, which is critical for developing new learned behaviors, or modifying old ones. Learning requires integration of inputs related to emotional, cognitive, and motor cortical functions. The development and execution of appropriate behavioral responses to environmental stimuli require continual updating and learning. Thus, response learning is unlikely to be contained within separate motor, cognitive and motivational neural circuits, rather it is likely to be the result of integration of several functions that together form smoothly executed, goal-directed behaviors. While the anatomical pathways appear to be generally topographic from cortex through BG circuits, there is an emerging literature supporting the idea that information from separate cortico-basal ganglia loops can influence each other [21, 45–49]. First, while projections from cortex terminate in a general topography through the BG structures, the dendrites and axons of cells within each functional region often cross-functional boundaries. An example is the dendritic arbors in the GP that extend beyond functional domains. In this way, distal dendrites from one functional region can invade an adjacent one. Second, there is convergence of terminals from functionally adjacent fields or regions onto progressively smaller BG structures [49, 50]. The fact that these adjacent regions overlap in function is not surprising. Many cortical areas are tightly linked to the immediately adjacent cortex. Thus, ‘edges’ of functionally identified regions are likely to process mixed signals. Moreover, the interface between functional circuits increases with the complexity of interconnections within the intrinsic BG circuitry and with the compression of pathways to successively smaller structures.
There are two additional important mechanisms that serve to integrate information between different functional regions that extend beyond integration at the edges of terminal fields. First, there are complex non-reciprocal arrangements between structures [21]. This arrangement provides a directional flow of information between regions. For example, the idea that the limbic striatum could influence motor output was first demonstrated in rodents via a striato-nigro-striatal (SNS) pathway, in which the ventral striatum could influence the dorsal striatum via the midbrain dopamine neurons, the cell group associated with reward-based learning [47, 51]. This system is more complex in primates. There is an inverse dorsal-ventral topographic organization to the midbrain striatal projection. The dorsal and medial dopamine cells project to the ventral and medial parts of the striatum, while the ventral and lateral cells project to the dorsal and lateral parts of the striatum [52]. Projections from the striatum to the midbrain are also arranged in an inverse dorsal-ventral topography; the dorsal aspects of the striatum terminate in the ventral regions of the midbrain, while the ventral areas terminate dorsally [53].
The ascending and descending projections for each functional area of the striatum differs in their proportional projections. The ventral striatum receives a limited midbrain input, but projects to a large SN region. In contrast, the dorsolateral striatum receives a wide input, but projects to a limited SN region. Thus the ventral striatum influences a wide region across dopamine neurons, but is influenced itself by a relatively limited group of dopamine cells. In contrast, the dorsolateral striatum influences a limited midbrain region, but is affected by a relatively large midbrain region. Moreover, for each striatal region there is one reciprocal and two non-reciprocal connections with the midbrain. Dorsal to the reciprocal connection lies a group of cells that project to the striatal region, but does not receive projections from it. Ventral to the reciprocal component lie efferent terminals without an ascending reciprocal connection. Finally, these three components for each striato- nigra-striatal projection system occupy a different position within the midbrain. The ventral striatum system lies dorsomedially, the dorsolateral striatum system lies ventrolaterally, and the central striatum system is positioned between the two [21]. With this arrangement, information from the limbic system can reach the motor system through a series of connections (spiral nigra diagram). The ventral striatum, which receives input from the ACC/OFC, projects to the midbrain terminating, in part, in the region that projects to the central (or associative) striatum. The central striatum terminates, in part, in the SN region that, in turn, projects to the dorsolateral (or motor) striatum. Taken together, information can thus be channeled from the ventral striatum, to the central striatum, and finally to the dorsolateral striatum. In this way, information flows from limbic to cognitive to motor circuits [21] (spiral nigra diagram).
The second mechanism by which anatomical integration occurs is through specific regions within a structure in which terminals from different functional areas converge, referred to here as “hot spots”. This was demonstrated within the corticostriatal pathway that showed two patterns of integrative connectivity [13] [16]. First, corticostriatal pathways are composed of well-described, focal, circumscribed projections. While these projections are organized in a general topographic manner, there is some convergence between terminal fields from different functional regions. This includes specific areas in which focal projections from cognitive- and reward-related prefrontal areas converge. In addition, focal projections from cognitive- and motor control-related areas also converge in specific striatal regions. These “hot spots” of interface between focal projections from different frontal cortical regions may be important zones for dynamic restructuring of neural ensembles fundamental to learning and habit formation. Thus, the corticostriatal network constitutes a system in which cortex exploits the striatum for additional processing to modulate learning and decision making leading to the development of goal-directed behaviors and habit formation. The second pattern of integrative connectivity is based on cortical projections that terminate in the striatum outside the focal terminal fields. These clusters of fibers, referred to as a diffuse projection, are widespread in the striatum and extend the area of potential interactions between cortical projections. The diffuse projections that penetrate into other functional regions at some distance may serve a separate integrative function from the convergence between focal projections (i.e., a broadcast vs. focal information processing). Taken together, these features of the corticostriatal frontal pathways suggest a potential integrative striatal network to help learning and flexible update of behavioral schemes. These findings raised the question of whether similar features of integration may also occur via the thalamus.
The thalamo-cortico-thalamic component of the cortico-basal ganglia circuits
The thalamic-cortical pathway is the last link in the circuit and is often treated as a simple ‘one-way relay’ back to cortex. However this pathway does not transfer information passively, but rather plays a key role in regulating cortical ensembles of neurons through its non-reciprocal connections with cortex. This occurs in two ways. First, the thalamus projects to different cortical layers. Therefore, while the thalamus receives input from the deep cortical layers, the thalamic projection to cortex, from the BG relay nuclei, terminates in superficial, middle, and deep layers (layers I/II, III/IV, and V, respectively) [39]. Projections that terminate in layer V form both direct thalamo-corticothalamic and thalamo-corticostriatal loops, thus sustaining information processing from the thalamus through each specific cortico-BG circuit. Thalamo-cortical projections to the superficial layers play a key role in corticocortical processing. In particular, thalamic projections to layers I/II may have a more global recruiting action response effecting wide networks affecting a wider network of cortical activity. In contrast to the topographically-specific thalamo-cortical projections to middle layers, the more widespread, diffuse terminals in layer I are in a position to modulate neuronal activity from all cortical layers with apical dendrites ascending into layer I. Moreover, this projection can provide an important mechanism for cross-communication between basal ganglia circuits. Projections to superficial layers interface with corticocortical connections. These cortical regions, in turn, send axons to the striatum, thereby potentially modulating a different loop.
Second, while corticothalamic projections to specific relay nuclei are thought to follow a general rule of reciprocity, corticothalamic projections to VA/VL and central MD sites, as seen in other thalamocortical systems, are more extensive than thalamocortical projections [9, 39, 54–58]. Furthermore, they are derived from areas not innervated by the same thalamic region, indicating non-reciprocal corticothalamic projections to specific basal ganglia relay nuclei [39]. Although each thalamic nucleus completes the cortico-BG segregated circuit, the non-reciprocal component is derived from a functionally distinct frontal cortical area. For example, the central MD has reciprocal connections with the lateral and orbital prefrontal areas and also a non-reciprocal input from medial prefrontal areas; VA has reciprocal connections with dorsal premotor areas and caudal DLPFC and also a non-reciprocal connection from medial prefrontal areas; and VLo has reciprocal connections with caudal motor areas along with a non-reciprocal connection from rostral motor regions. The potential for relaying information between circuits through thalamic connections, therefore, is accomplished both through the organization of projections to different layers and through the non-reciprocal corticothalamic pathways. Thus, the BG-recipient-thalamic nuclei appear to mediate information flow from higher cortical “association” areas of the prefrontal cortex to rostral motor areas involved in “cognitive” or integrative aspects of motor control to primary motor areas that direct movement execution. Therefore, similar to the striato-nigro-striatal projection system and the corticostriatal projection system, the thalamus also has a non-reciprocal and overlapping connectional arrangement with the cortex. In addition, preliminary evidence also demonstrates that, like the corticostriatal projections, cortical projections from different functional regions may also converge in specific thalamic regions [59].
A role for both parallel circuit and integrative cortico-BG-thalamic networks
Within each connected cortico-BG-thalamic structure, there are reciprocal connections linking up regions associated with similar functions (maintaining parallel networks). However, in addition, there are non-reciprocal connections linking regions that are associated with different cortical-BG-thalamic circuits (figure 1). The development and modification of goal-directed behaviors require continual processing of complex chains of events, which is reflected in the feed-forward organization of both the striato-nigral connections and the thalamo-cortical connections. Information can thus be channeled from limbic, to cognitive, to motor circuits, to produce decision-making processes that integrate different functional information, allowing the individual to respond appropriately to environmental cues. Parallel circuits and integrative circuits must work together, allowing coordinated behaviors to be maintained and focused (via parallel networks), but also to be modified and changed according to the appropriate external and internal stimuli (via integrative networks). Indeed, both the inability to maintain and to focus in the execution of specific behaviors, as well as the inability to adapt appropriately to external and internal cues, are key deficits in BG diseases which affect these aspects of motor control, cognition and motivation. Taken together, it is clear that the thalamus not only relays information back to cortex, but may also serve as an important center of integration of networks that underlie the ability to modulate behaviors.
Fig. 1.
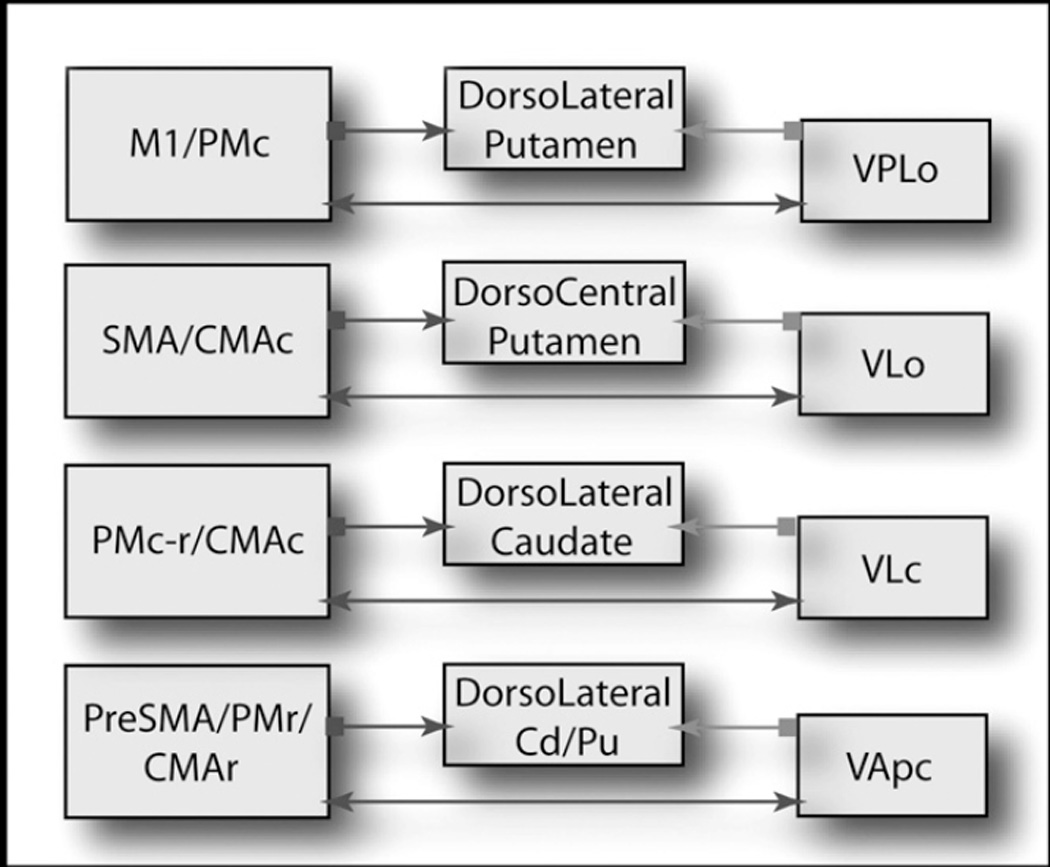
Diagram illustrates functionally similar cortical and thalamic regions projection to the same striatal area. Note the cortico-thalamic projection is reciprocal. The caudal motor-related areas of the frontal lobe (primary motor area, M1; caudal premotor cortex, PMc; supplementary motor area, SMA; and caudal cingulate motor area, CMAc) are primarily connected with the ventral thalamic nuclei VPLo and VLo. These cortical and thalamic regions send converging efferents projections to the dorsolateral and dorsocentral putamen. In contrast, the rostral motor-related areas of the frontal lobe (rostral premotor cortex, PMr; pre-supplementary motor area, PreSMA; and rostral cingulate motor area, CMAr) are primarily connected with the ventral thalamic nucleus VApc. These cortical areas and the VApc send converging efferents projections to the dorsolateral caudate nucleus and to the rostral putamen. Both rostral and caudal premotor areas, and CMAc are connected with the ventral thalamic nucleus VLc. These cortical areas and the VLc send efferents projections to the dorsolateral caudate n.
Fig. 2.
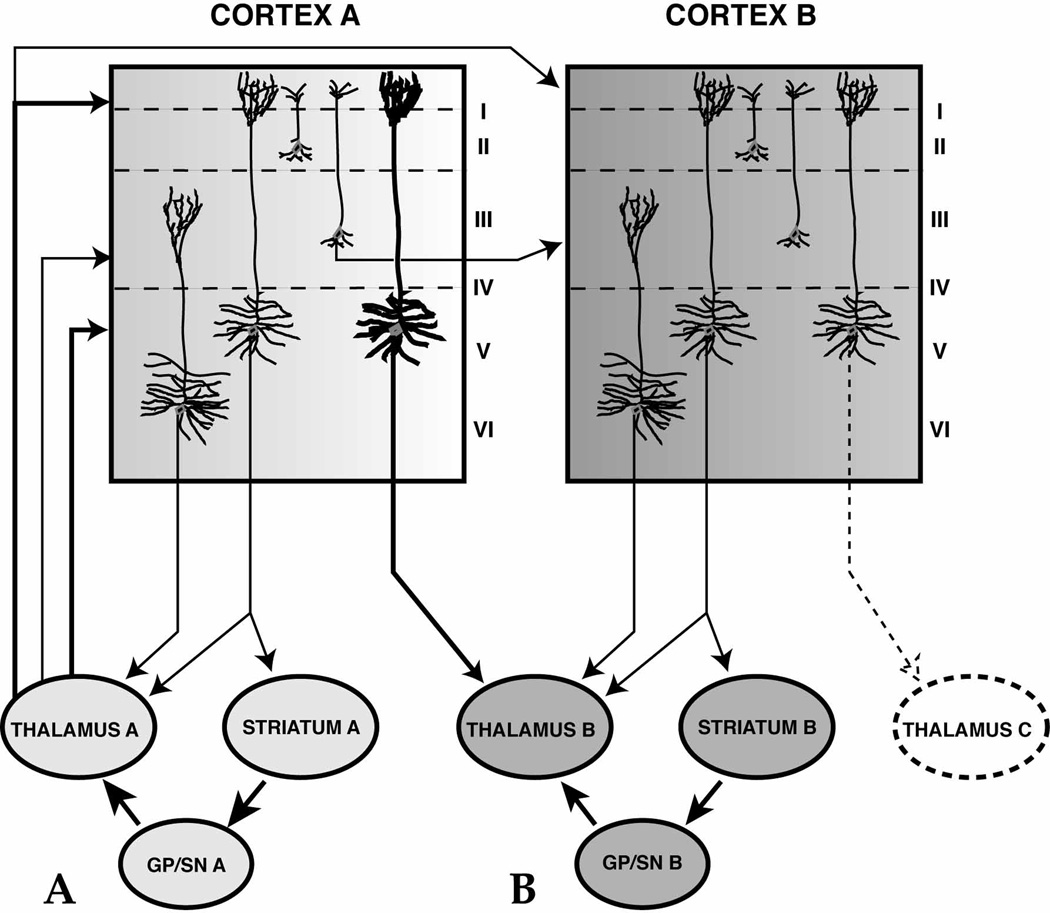
Summary of thalamic terminal organization in cortical layers. Projections to the deep layers may interact with neurons that, in turn, project back to both the thalamus and striatum. These terminals can directly reinforce corticothalamic and corticostriatal inputs to specific cortico-BG circuits (A). In addition, through the non-reciprocal corticothalamic projection, terminals in layer V may also interface with other cortico-BG circuits via projection to a thalamic region that is part of another circuit system (B). Thalamocortical projections to the superficial layers may have a similar dual function. These projections may interact with the apical dendrites of layer V cells, further reinforcing each parallel circuit. In addition, through corticocortical projections from layer III, these terminals may influence adjacent circuits (modified from J. Neurosci., 2002, 22:8117–8132).
Acknowledgments
This work was supported by NIH grants NS22511 and MH45573.
Abbreviations
- ACC
anterior cingulate cortex
- BG
basal ganglia
- CM
central medial nucleus
- CMAc
caudal cingulate motor area
- CMAr
rostral cingulate motor area
- DLPFC
dorsolateral prefrontal cortex
- GP
globus pallidum
- MD
medialis dorsalis nucleus
- M1
primary motor cortex
- OFC
orbital frontal cortex
- Pf
parafascicularis nucleus
- PFC
prefrontal cortex
- PMdc
dorsal and caudal premotor cortex
- PMdr
dorsal and rostral premotor cortex
- PMv
ventral premotor cortex
- preSMA
pre-supplementary motor area
- SMA
supplementary motor area
- SN
substantia nigra
- SNr
substantia nigra pars reticulata
- SNS
striato-nigro-striatal pathway
- VA
ventralis anterior nucleus
- VAmc
ventralis anterior, pars magnocellularis nucleus
- VApc
ventralis anterior, pars parvocellularis nucleus
- VL
ventral lateral nucleus
- VLc
ventralis lateralis caudalis nucleus
- VLo
ventralis lateralis oralis nucleus
- vmPFC
ventral medial prefrontal cortex
- VPLo
ventralis posterior lateralis, pars oralis nucleus
- VP
ventral pallidum
- VS
ventral striatum
- VTA
ventral tegmental area
References
- 1.Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Progress. Neurobiology. 1996;50(4):381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- 2.Hikosaka O, et al. Procedural Learning in the Monkey. In: Kimura M, Graybiel AM, editors. Functions of the Cortico-Basal Ganglia Loop. New York: Springer-Verlag; 1995. pp. 18–30. [Google Scholar]
- 3.Pasupathy A, Miller EK. Different time courses of learning-related activity in the prefrontal cortex and striatum. Nature. 2005;433(7028):873–876. doi: 10.1038/nature03287. [DOI] [PubMed] [Google Scholar]
- 4.Graybiel AM. The basal ganglia: learning new tricks and loving it. Curr Opin Neurobiol. 2005;15(6):638–644. doi: 10.1016/j.conb.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Shatner M, et al. Cognitive Decision Processes And Functional Characteristics Of The Basal Ganglia Reward System. In: Kitai S, DeLong M, Graybiel A, editors. The Basal Ganglia VI. Plenum Press; 2002. pp. 303–309. [Google Scholar]
- 6.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8(11):1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 7.Strick PL, Dum RP, Mushiake H. Basal Ganglia "Loops" with the Cerebral Cortex. In: Kimura M, Graybiel AM, editors. Functions of the Cortico-Basal Ganglia Loop. New York: Springer-Verlag; 1995. pp. 106–124. [Google Scholar]
- 8.Goldman-Rakic PS, Porrino LJ. The primate mediodorsal (MD) nucleus and its projection to the frontal lobe. J. Comp. Neurol. 1985;242:535–560. doi: 10.1002/cne.902420406. [DOI] [PubMed] [Google Scholar]
- 9.Jones EG. The thalamus of primates. In: Bloom FE, Björklund A, Hökfelt T, editors. The Primate Nervous System, Part II. Amsterdam: Elsevier Science; 1998. pp. 1–298. [Google Scholar]
- 10.Hadland KA, et al. The anterior cingulate and reward-guided selection of actions. J Neurophysiol. 2003;89(2):1161–1164. doi: 10.1152/jn.00634.2002. [DOI] [PubMed] [Google Scholar]
- 11.Roesch MR, Olson CR. Neuronal activity related to reward value and motivation in primate frontal cortex. Science. 2004;304(5668):307–310. doi: 10.1126/science.1093223. [DOI] [PubMed] [Google Scholar]
- 12.Carmichael ST, Price JL. Connectional networks within the orbital and medial prefrontal cortex of Macaque monkeys. Journal of Comparative Neurology. 1996;371:179–207. doi: 10.1002/(SICI)1096-9861(19960722)371:2<179::AID-CNE1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 13.Haber SN, et al. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical inputs, providing a substrate for incentive-based learning. J Neurosci. 2006;26(32):8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haber SN, et al. The orbital and medial prefrontal circuit through the primate basal ganglia. J. Neurosci. 1995;15:4851–4867. doi: 10.1523/JNEUROSCI.15-07-04851.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Constantinidis C, Franowicz MN, Goldman-Rakic PS. The sensory nature of mnemonic representation in the primate prefrontal cortex. Nat Neurosci. 2001;4(3):311–316. doi: 10.1038/85179. [DOI] [PubMed] [Google Scholar]
- 16.Calzavara R, Mailly P, Haber SN. Relationship between the corticostriatal terminals from areas 9 and 46, and those from area 8A, dorsal and rostral premotor cortex and area 24c: an anatomical substrate for cognition to action. Eur J Neurosci. 2007;26(7):2005–2024. doi: 10.1111/j.1460-9568.2007.05825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Selemon LD, Goldman-Rakic PS. Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. J. Neurosci. 1985;5:776–794. doi: 10.1523/JNEUROSCI.05-03-00776.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Selemon LD, Goldman-Rakic PS. Topographic intermingling of striatonigral and striatopallidal neurons in the rhesus monkey. J. Comp. Neurol. 1990;297:359–376. doi: 10.1002/cne.902970304. [DOI] [PubMed] [Google Scholar]
- 19.Hoshi E, Tanji J. Integration of target and body-part information in the premotor cortex when planning action. Nature. 2000;408(6811):466–470. doi: 10.1038/35044075. [DOI] [PubMed] [Google Scholar]
- 20.Wallis JD, Miller EK. From rule to response: neuronal processes in the premotor and prefrontal cortex. J Neurophysiol. 2003;90(3):1790–1806. doi: 10.1152/jn.00086.2003. [DOI] [PubMed] [Google Scholar]
- 21.Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. Journal of Neuroscience. 2000;20(6):2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith Y, et al. The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends Neurosci. 2004;27(9):520–527. doi: 10.1016/j.tins.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Sadikot AF, Parent A, Francois C. Efferent connections of the centromedian and parafascicular thalamic nuclei in the squirrel monkey: A PHA-L study of subcortical projections. J. Comp. Neurol. 1992;315:137–159. doi: 10.1002/cne.903150203. [DOI] [PubMed] [Google Scholar]
- 24.Giménez-Amaya JM, et al. Organization of thalamic projections to the ventral striatum in the primate. J. Comp. Neurol. 1995;354:127–149. doi: 10.1002/cne.903540109. [DOI] [PubMed] [Google Scholar]
- 25.Dubé L, Smith AD, Bolam JP. Identification of synaptic terminals of thalamic or cortical origin in contact with distinct medium-size spiny neurons in the rat neurostriatum. J. Comp. Neurol. 1988;267:455–471. doi: 10.1002/cne.902670402. [DOI] [PubMed] [Google Scholar]
- 26.Fenelon G, et al. Topographic distribution of the neurons of the central complex (Centre median-parafascicular complex) and of other thalamic neurons projecting to the striatum in macaques. Neuroscience. 1991;45(2):495–510. doi: 10.1016/0306-4522(91)90244-i. [DOI] [PubMed] [Google Scholar]
- 27.Nakano K, et al. Topographical projections from the thalamus, subthalamic nucleus and pedunculopontine tegmental nucleus to the striatum in the Japanese monkey, Macaca fuscata. Brain Res. 1990;537:54–68. doi: 10.1016/0006-8993(90)90339-d. [DOI] [PubMed] [Google Scholar]
- 28.Groenewegen HJ, Berendse HW. The specificity of the 'nonspecific' midline and intralaminar thalamic nuclei. Trends Neurosci. 1994;17:50. doi: 10.1016/0166-2236(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 29.Amaral DG, Cowan WM. Subcortical afferents to the hippocampal formation in the monkey. J. Comp. Neurol. 1980;189:573–591. doi: 10.1002/cne.901890402. [DOI] [PubMed] [Google Scholar]
- 30.Norita M, Kawamura K. Subcortical afferents to the monkey amygdala: an HRP study. Brain Res. 1980;190:225–230. doi: 10.1016/0006-8993(80)91171-3. [DOI] [PubMed] [Google Scholar]
- 31.Herkenham M. New perspectives on the organization and evolution of nonspecific thalamochortical projections. In: Jones EG, Peters A, editors. Cerebral Cortex: Sensory-Motor Areas and Aspects of Cortical Connectivity. New York: Plenum Press; 1986. pp. 403–445. [Google Scholar]
- 32.Francois C, et al. Topography of the projection from the central complex of the thalamus to the sensorimotor striatal territory in monkeys. J. Comp. Neurol. 1991;305:17–34. doi: 10.1002/cne.903050104. [DOI] [PubMed] [Google Scholar]
- 33.McFarland NR, Haber SN. Convergent inputs from thalamic motor nuclei and frontal cortical areas to the dorsal striatum in the primate. Journal of Neuroscience. 2000;20(10):3798–3813. doi: 10.1523/JNEUROSCI.20-10-03798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McFarland NR, Haber SN. Organization of thalamostriatal terminals from the ventral motor nuclei in the macaque. Journal of Comparative Neurology. 2001;429:321–336. doi: 10.1002/1096-9861(20000108)429:2<321::aid-cne11>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 35.Matelli M, et al. Thalamic input to inferior area 6 and area 4 in the macaque monkey. J. Comp. Neurol. 1989;280:468–488. doi: 10.1002/cne.902800311. [DOI] [PubMed] [Google Scholar]
- 36.Matelli M, Luppino G. Thalamic input to mesial and superior area 6 in the Macaque monkey. J. Comp. Neurol. 1996;372:59–87. doi: 10.1002/(SICI)1096-9861(19960812)372:1<59::AID-CNE6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 37.Wiesendanger R, Wiesendanger M. The thalamic connections with medial area 6 (supplementary motor cortex) in the monkey (macaca fascicularis) Exp. Brain Res. 1985;59:91–104. doi: 10.1007/BF00237670. [DOI] [PubMed] [Google Scholar]
- 38.Giguere M, Goldman-Rakic PS. Mediodorsal nucleus: area 1 laminar and tangential distribution of afferents and efferents in the frontal lobe of rhesus monkeys. J. Comp. Neurol. 1988;277(2):195–213. doi: 10.1002/cne.902770204. [DOI] [PubMed] [Google Scholar]
- 39.McFarland NR, Haber SN. Thalamic relay nuclei of the basal ganglia form both reciprocal and nonreciprocal cortical connections, linking multiple frontal cortical areas. The Journal of Neuroscience. 2002;22(18):8117–8132. doi: 10.1523/JNEUROSCI.22-18-08117.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ilinsky IA, Jouandet ML, Goldman-Rakic PS. Organization of the nigrothalamocortical system in the rhesus monkey. J. Comp. Neurol. 1985;236:315–330. doi: 10.1002/cne.902360304. [DOI] [PubMed] [Google Scholar]
- 41.Percheron G, et al. The primate motor thalamus. Brain Research - Brain Research Reviews. 1996;22(2):93–181. [PubMed] [Google Scholar]
- 42.Haber SN, et al. Efferent connections of the ventral pallidum. Evidence of a dual striatopallidofugal pathway. J. Comp. Neurol. 1985;235:322–335. doi: 10.1002/cne.902350304. [DOI] [PubMed] [Google Scholar]
- 43.Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- 44.Middleton FA, Strick PL. Basal-ganglia 'projections' to the prefrontal cortex of the primate. Cereb Cortex. 2002;12(9):926–935. doi: 10.1093/cercor/12.9.926. [DOI] [PubMed] [Google Scholar]
- 45.Kolomiets BP, et al. Segregation and convergence of information flow through the cortico-subthalamic pathways. Journal of Neuroscience. 2001;21(15):5764–5772. doi: 10.1523/JNEUROSCI.21-15-05764.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bevan MD, Clarke NP, Bolam JP. Synaptic integration of functionally diverse pallidal information in the entopeduncular nucleus and subthalamic nucleus in the rat. J. Neurosci. 1997;17:308–324. doi: 10.1523/JNEUROSCI.17-01-00308.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Somogyi P, et al. Monosynaptic input from the nucleus accumbens-ventral striatum region to retrogradely labelled nigrostriatal neurones. Brain Res. 1981;217:245–263. doi: 10.1016/0006-8993(81)90002-0. [DOI] [PubMed] [Google Scholar]
- 48.Francois C, et al. Topographic distribution of the axonal endings from the sensorimotor and associative striatum in the macaque pallidum and substantia nigra. Exp. Brain Res. 1994;102:305–318. doi: 10.1007/BF00227517. [DOI] [PubMed] [Google Scholar]
- 49.Percheron G, Filion M. Parallel processing in the basal ganglia: Up to a point. Trends Neurosci. 1991;14:55–59. doi: 10.1016/0166-2236(91)90020-u. [DOI] [PubMed] [Google Scholar]
- 50.Yelnik J. Functional anatomy of the basal ganglia. Mov Disord. 2002;17(Suppl 3):S15–S21. doi: 10.1002/mds.10138. [DOI] [PubMed] [Google Scholar]
- 51.Nauta WJH, et al. Efferent connections and nigral afferents of the nucleus accumbens septi in the rat. Neuroscience. 1978;3:385–401. doi: 10.1016/0306-4522(78)90041-6. [DOI] [PubMed] [Google Scholar]
- 52.Lynd-Balta E, Haber SN. The organization of midbrain projections to the striatum in the primate: Sensorimotor-related striatum versus ventral striatum. Neuroscience. 1994;59:625–640. doi: 10.1016/0306-4522(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 53.Lynd-Balta E, Haber SN. Primate striatonigral projections: A comparison of the sensorimotor-related striatum and the ventral striatum. J Comp Neurol. 1994;345(4):562–578. doi: 10.1002/cne.903450407. [DOI] [PubMed] [Google Scholar]
- 54.Hoogland PV, Welker E, Van der Loos H. Organization of the projections from barrel cortex to thalamus in mice studied with Phaseolus vulgaris-leucoagglutinin and HRP. Experimental Brain Research. 1987;68(1):73–87. doi: 10.1007/BF00255235. [DOI] [PubMed] [Google Scholar]
- 55.Catsman-Berrevoets CE, Kuypers HG. Differential laminar distribution of corticothalamic neurons projecting to the VL and the center median. An HRP study in the cynomolgus monkey. Brain Research. 1978;154(2):359–365. doi: 10.1016/0006-8993(78)90706-0. [DOI] [PubMed] [Google Scholar]
- 56.Deschenes M, Veinante P, Zhang ZW. The organization of corticothalamic projections: reciprocity versus parity. Brain Research - Brain Research Reviews. 1998;28(3):286–308. doi: 10.1016/s0165-0173(98)00017-4. [DOI] [PubMed] [Google Scholar]
- 57.Darian-Smith C, Tan A, Edwards S. Comparing thalamocortical and corticothalamic microstructure and spatial reciprocity in the macaque ventral posterolateral nucleus (VPLc) and medial pulvinar. Journal of Comparative Neurology. 1999;410(2):211–234. [PubMed] [Google Scholar]
- 58.Sherman SM, Guillery RW. Functional organization of thalamocortical relays. Journal of Neurophysiology. 1996;76(3):1367–1395. doi: 10.1152/jn.1996.76.3.1367. [DOI] [PubMed] [Google Scholar]
- 59.Calzavara R, et al. Society for Neuroscience. Washington, DC; 2005. 3D reconstructions demonstrate interface between cognitive andn motor terminal fields in the primate striatum and thalamus. [Google Scholar]