Abstract
The current study was designed to examine the sulfation of eight opioid drugs, morphine, hydromorphone, oxymorphone, butorphanol, nalbuphine, levorphanol, nalorphine, and naltrexone, in HepG2 human hepatoma cells and human organ samples (lung, liver, kidney, and small intestine) and to identify the human SULT(s) responsible for their sulfation. Analysis of the spent media of HepG2 cells, metabolically labeled with [35S]sulfate in the presence of each of the eight opioid drugs, showed the generation and release of corresponding [35S]sulfated derivatives. Five of the eight opioid drugs, hydromorphone, oxymorphone, butorphanol, nalorphine, and naltrexone, appeared to be more strongly sulfated in HepG2 cells than were the other three, morphine, nalbuphine, and levorphanol. Differential sulfating activities toward the opioid drugs were detected in cytosol or S9 fractions of human lung, liver, small intestine, and kidney, with the highest activities being found for the liver sample. A systematic analysis using eleven known human SULTs and kinetic experiment revealed SULT1A1 as the major responsible SULTs for the sulfation of oxymorphone, nalbuphine, nalorphine, and naltrexone, SULT1A3 for the sulfation of morphine and hydromorphone, and SULT2A1 for the sulfation of butorphanol and levorphanol. Collectively, the results obtained imply that sulfation may play a significant role in the metabolism of the tested opioid drugs in vivo.
Keywords: Cytosolic sulfotransferase, SULT, sulfation, opioids, drugs
Graphical Abstract

1. Introduction
Opioid drugs such as buprenorphine and morphine act on the opioid receptors located in cells of peripheral and central nervous systems and are mainly used for the treatment of acute and chronic pain (Ballantyne and Shin; 2008; Inturrisi, 2002; Krenzischek et al., 2008; Pasternak, 1993). The antagonist type of opioids including naloxone and naltrexone is in use for the treatment of opioid-overdose and alcohol-dependence (Chamberlain and Klein, 1994; Krystal et al., 2001; Martin, 1967). The opioid antagonists are also used in combination medications consisting of opioid agonist and antagonist, such as Suboxone (buprenorphine and naloxone) and Embeda (morphine and naltrexone), in order to mitigate potential opioid abuse (Fudala and Johnson, 2006; Helm et al., 2008; Orman and Keating, 2009; Smith, 2011). The metabolism of these opioid drugs, taking place primarily in the liver, had been shown to involve the Phase I cytochromes P450 (CYPs) as well as the Phase II UDP-glucuronosyltransferases (UGTs) (Coffman et al., 1998; Holmquist, 2009; King et al., 2000; Smith, 2009; Vallejo et al., 2011). Opioid drugs are generally subjected by N-demethylation, 3-O-demethylation, and 6-O-hydroxylation by CYPs, O-glucuronidation by UGTs, and O-sulfation by SULTs (Coffman et al., 1998; Frölich et al., 2011; Holmquist, 2009; King et al., 2000; Skarke and Lötsch, 2002; Smith, 2009; Vallejo et al., 2011). For example, morphine undergoes the N-demethylation to generate normorphine, the glucuronidation to generate morphine-3-O-glucuronide and morphine-6-O-glucuronide, and sulfation to generate morphine-3-O-sulfate (Frölich et al., 2011, Skarke and Lötsch, 2002). The metabolism of opioid drugs has been known to show the species differences. In human, rabbit, and rat, the glucuronide metabolites are the major metabolite and the sulfate metabolite is a minor metabolite which accounts for ~2% of the metabolites from urine (Frölich et al., 2011, Smith et al., 1973, Skarke and Lötsch, 2002). In contrast, in cat and chicken, the sulfated metabolite is a major metabolite which accounts for ~20% of morphine and its metabolite from urine instead of the glucuronide metabolite (Fujimoto and Haarstad, 1969, Smith et al., 1973). Previous studies have demonstrated that the sulfated metabolites of hydromorphone, levorphanol, nalorphine, and naloxone have been also identified from urine and/or bile in certain species including rat, dog, cat, and human (Leinweber et al., 1981; Yeh and Woods, 1971; Zheng et al., 2002). Nevertheless, the metabolism of those three opioid drugs through sulfation has not been well studied in human and the responsible SULT(s) for the sulfation of those three opioid drugs as well as morphine even still remained to be clarified. Recently, we have demonstrated the sulfation of opioid drugs, buprenorphine, pentazocine, and naloxone using human hepatocyte and tissue cytosols, and determined the responsible SULT enzymes (Kurogi et al., 2012). It is therefore possible that SULT-mediated sulfation may be involved in the metabolism of not only morphine, hydromorphone, levorphanol, nalorphine, but also other opioid drugs including oxymorphone, butorphanol, nalbuphine, nalorphine, and naltrexone.
Sulfation as catalyzed by the SULTs is a key process that serves for the biotransformation and homeostasis of endogenous steroid/thyroid hormones, catecholamines, and bile acids, as well as for the detoxification of xenobiotics including drug compounds (Falany and Roth, 1994; Mulder and Jakoby, 1990; Weinshilboum and Otterness, 1994; Glatt et al., 2000; Coughtrie, 2002; Pacifici, 2005; Runge-Morris and Kocarek, 2009). The SULTs catalyze the transfer of a sulfonate group from the active sulfate, 3’-phosphoadenosine 5’-phosphosulfate (PAPS), to acceptor substrate compounds containing either hydroxyl or amino group (Lipmann, 1958). Sulfate conjugation generally leads to the inactivation of biologically active compounds and the increase in their water-solubility, thereby facilitating their removal from the body (Falany and Roth, 1994; Mulder and Jakoby, 1990; Weinshilboum and Otterness, 1994; Glatt et al., 2000; Coughtrie, 2002; Pacifici, 2005; Runge-Morris and Kocarek, 2009). In humans, eleven SULTs that fall into three distinct gene families have been identified and characterized (Gamage et al., 2006; Glatt et al., 2001). Previous studies have demonstrated the sulfation of a variety of drug compounds (Falany, 1997; Falany et al., 2004; Meloche et al., 2002; Sugahara et al., 2003). SULT1A subfamily, especially SULT1A1and SULT1A3, is mainly involved in the sulfation of phenolic drugs such as acetaminophen, dobutamine, isoproterenol, and salbutamol (Falany, 1997; Sugahara et al., 2003). In contrast, SULT2A1 is capable of catalyzing the sulfation of steroid drug compounds such as budesonide and tibolone (Falany et al., 2004; Meloche et al., 2002). In a recent study, SULT2A1 and SULT1A3 displayed the sulfating activities toward buprenorphine and pentazocine, respectively, while several SULTs, particularly SULT1A1, showed strong sulfating activity toward naloxone (Kurogi et al., 2012).
In this paper, we report the sulfation of eight opioid drugs, morphine, hydromorphone, oxymorphone, butorphanol, levorphanol, nalorphine, and naltrexone, and an active metabolite of naltrexone, 6β-naltrexol, by metabolic labeling experiment which allows to monitor the generation of radioactive [35S]sulfated products within the whole cell and enzymatic assay using radioactive sulfonate donor, PAP[35S], to detect the sulfating activity. To date, these in vitro assay systems have revealed the sulfation of numerous compounds including tyrosine, monoamine neurotransmitters, environmental hormones as well as three opioid drugs, buprenorphine, pentazocine, and naloxone (Kurogi et al., 2012, Liu et al., 2007, Sakakibara et al., 1994, Suiko et al., 2000). HepG2 human hepatoma cell line was used as a model of hepatocyte for the metabolic labeling experiment. Cytosol or S9 fractions prepared from human lung, liver, small intestine, and kidney were examined to verify the presence of opioid drugs-sulfating activity in vivo. A systematic study using eleven known human SULTs, previously expressed and purified, was carried out to identify the one(s) that is(are) responsible for the sulfation of each of the eight opioid drugs as well as 6β-naltrexol. Moreover, the kinetics of the sulfation of these opioid compounds as well as buprenorphine, pentazocine, and naloxone by the relevant human SULTs were analyzed.
2. Materials and Methods
2.1. Materials
Morphine, hydromorphone, oxymorphone, butorphanol, levorphanol, nalbuphine, nalorphine, naltrexone, 6β-naltrexol, buprenorphine, pentazocine, and naloxone, were purchased from Cerilliant. Adenosine 5’-triphosphate (ATP), sodium dodecyl sulfate (SDS), dithiothreitol (DTT), dimethyl sulfoxide (DMSO), 3-(N-morpholino)propanesulfonic acid (Mops), Trizma base (Tris), isopropyl-β-D-thiogalactopyranoside (IPTG), and silica gel thin-layer chromatography (TLC) plates were from Sigma Chemical Company. Polyethyleneimine (PEI) cellulose TLC plates were from Brinkmann. Ecolume scintillation cocktail was purchased from MP Biomedical. Carrier-free sodium [35S]sulfate was from PerkinElmer. HepG2 human hepatoma cell line (ATCC HB-8065) was obtained from American Type Culture Collection. Pooled human lung S9 fraction, liver cytosol, small intestine (duodenum and jejunum) S9 fraction, and kidney S9 fraction were purchased from XenoTech, LLC. All other chemicals were of the highest grade commercially available.
2.2. Metabolic labeling of cultured HepG2 human hepatoma cells
HepG2 cells were routinely maintained, under a 5% CO2 atmosphere at 37oC in MEM supplemented with 10% fetal bovine serum (FBS), penicillin G (30 µg/ml), and streptomycin sulfate (50 µg/ml). Confluent cells, grown in individual wells of a 24-well culture plate, preincubated in sulfate-free (prepared by omitting streptomycin sulfate and replacing magnesium sulfate with magnesium chloride) MEM for four hours, were labeled with 0.25 ml aliquots of the same medium containing [35S]sulfate (0.3 mCi/ml), and different concentrations (ranging from 1 to 50 µM) of morphine, hydromorphone, oxymorphone, butorphanol, nalbuphine, levorphanol, nalorphine, or naltrexone. After a 21-hour labeling, the labeling media were collected and spin-filtered. Afterwards, each 2 µl of the filtrate was subjected to the analysis of [35S]sulfated products by silica gel thin-layer chromatography (TLC) with acetonitrile/n-butanol/isopropanol/formic acid/H2O (2:8:2:1:1; by volume) as the solvent system for morphine, hydromorphone, and oxymorphone or with acetonitrile/n-butanol (2:3; by volume) as the solvent system for the other opioid drugs tested. Upon completion of TLC, the plate was air-dried and an autoradiograph was taken from the TLC plate to reveal radioactive spots corresponding to the [35S]sulfated derivatives of the tested opioid drug compounds.
2.3. Preparation of purified human SULTs
Recombinant human P-form (SULT1A1 and SULT1A2) and M-form (SULT1A3) phenol SULTs, thyroid hormone SULT (SULT1B1), two SULT1Cs (SULT1C2 and SULT1C4), estrogen SULT (SULT1E1), dehydroepiandrosterone (DHEA) SULT (SULT2A1), two SULT2B1s (designated a and b), and a neuronal SULT (SULT4A1) were prepared as previously described (Pai et al., 2002; Sakakibara et al., 1998; Suiko et al., 2000). Briefly, for SULT enzymes other than SULT2B1s, GST-fusion SULTs expressed using pGEX-2TK vector in competent BL21 (DE3) cells, following induction with IPTG, were purified using Glutathione-Sepharose, followed by thrombin digestion to release the recombinant SULTs (Sakakibara et al., 1998; Suiko et al., 2000). In contrast, recombinant SULT2B1s expressed using pET23c vector in competent BL21 (DE3) cells, following induction with IPTG, were purified using Bio-gel hydroxyapatite column chromatography followed by ATP-agarose affinity column chromatography (Pai et al., 2002).
2.4. Enzymatic assay
The sulfating activity of purified recombinant human SULTs was assayed using PAP[35S] as the sulfonate group donor. The standard assay mixture, with a final volume of 20 µl, contained 50 mM Mops buffer at pH 7.5, 14 µM PAP[35S] (15 Ci/mmol), 1 mM DTT, and 50 µM substrate. The substrates tested were prepared in DMSO. Control with DMSO, in place of substrate, was also prepared. The reaction was started by the addition of 1.0 µg enzyme, allowed to proceed for 10 min at 37°C, and terminated by heating at 100°C for 3 min. The precipitates formed were cleared by centrifugation at 16,000 × g for 3 min, and 2 µl of the supernatant was subjected to the analysis of [35S]sulfated product by TLC with acetonitrile/n-butanol/isopropanol/formic acid/H2O (2:8:2:1:1; by volume) as the solvent system for [35S]sulfated morphine, hydromorphone, and oxymorphone, and with acetonitrile/n-butanol/ (2:3; by volume) as the solvent system for the [35S]sulfated derivatives of other opioid drugs. Upon completion of the TLC, the plate was air-dried and the radioactive spots were located by autoradiography, excised from the plate, eluted in 0.5 ml H2O in a glass vial. 4.5 ml of Ecolume scintillation liquid was added to each vial, mixed thoroughly, and counted for [35S]radioactivity using a liquid scintillation counter. Controls were installed to ensure that the [35S]sulfated product was not destructed during heat treatment, lost to precipitates upon centrifugation at the end of the enzymatic assay, or incompletely eluted from TLC plate into water. The cpm count obtained was calculated into nmol of sulfated product formed based on the specific activity of the PAP[35S] prepared for the corresponding reactions and expressed in nmol of sulfated product/min/mg protein. The quantitative detection limit was ~0.01 nmol/min/mg. To assay for opioid drug-sulfating activity of human tissue cytosol or S9 fraction, the reaction mixture mentioned above was supplemented with 50 mM NaF (a phosphatase inhibitor) and used in the assay. The reaction was started by the addition of the cytosol or S9 fraction (~50 µg), and allowed to proceed for 20 min, followed by the TLC analysis described above.
2.5. Kinetic analysis
In the kinetic studies on the sulfation of opioid drugs, the sulfation assays were carried out using varying concentrations of these substrate compounds and 50 mM MOPS at pH 7.5 according to the procedure described above. Data obtained were analyzed based on Michaelis-Menten kinetics using GraphPad Prism5 software and non-linear regression. Eadie-Hofstee plots were concomitantly analyzed, by plotting the velocity (nmol/min/mg)/ substrate concentration (µM) (v/[S]) against the velocity (nmol/min/mg) (v). The kinetic parameters, Km (µM) and Vmax (nmol/min/mg), were calculated based on the Michaelis-Menten equation.
2.6. Miscellaneous methods
PAP[35S] was synthesized from ATP and carrier-free [35S]sulfate using the recombinant human bifunctional PAPS synthase and its purity was determined as previously described (Yanagisawa et al., 1998). Briefly, the PAPS synthesis reaction mixture contained 50 mM Tris-HCl buffer at pH 8.0, 20 mM MgCl2, 10 mM ATP, 10 mM NaF, 2 mM DTT, 73.5 U/ml pyrophosphate phosphatase, and 50 mCi/ml [35S]sulfate. The reaction was started by addition of PAPS synthase-expressing cell lysate and incubated at 37C for 45 min. Thereafter the reaction mixture was spin-filtered to remove macromolecules including PAPS synthase. To analyze the radioactive PAP[35S], the filtrate was subjected to TLC analysis using a PEI-cellulose TLC plate with 0.75 M Trizma base/0.45 M HCl/0.5 M LiCl as the solvent system. The PAP[35S] synthesized was adjusted to the required concentration and a specific activity of 15 Ci/mmol at 1.4 mM by the addition of unlabelled PAPS. SDS-polyacrylamide gel electrophoresis (SDS-PAGE) was performed on 12% polyacrylamide gels using the method of Laemmli (Laemmli, 1970). Protein determination was based on the method of Bradford with bovine serum albumin as the standard (Bradford, 1976).
3. Results
3.1. Generation and release of [35S]sulfated metabolites by HepG2 cells labeled with [35S]sulfate in the presence of opioid drugs
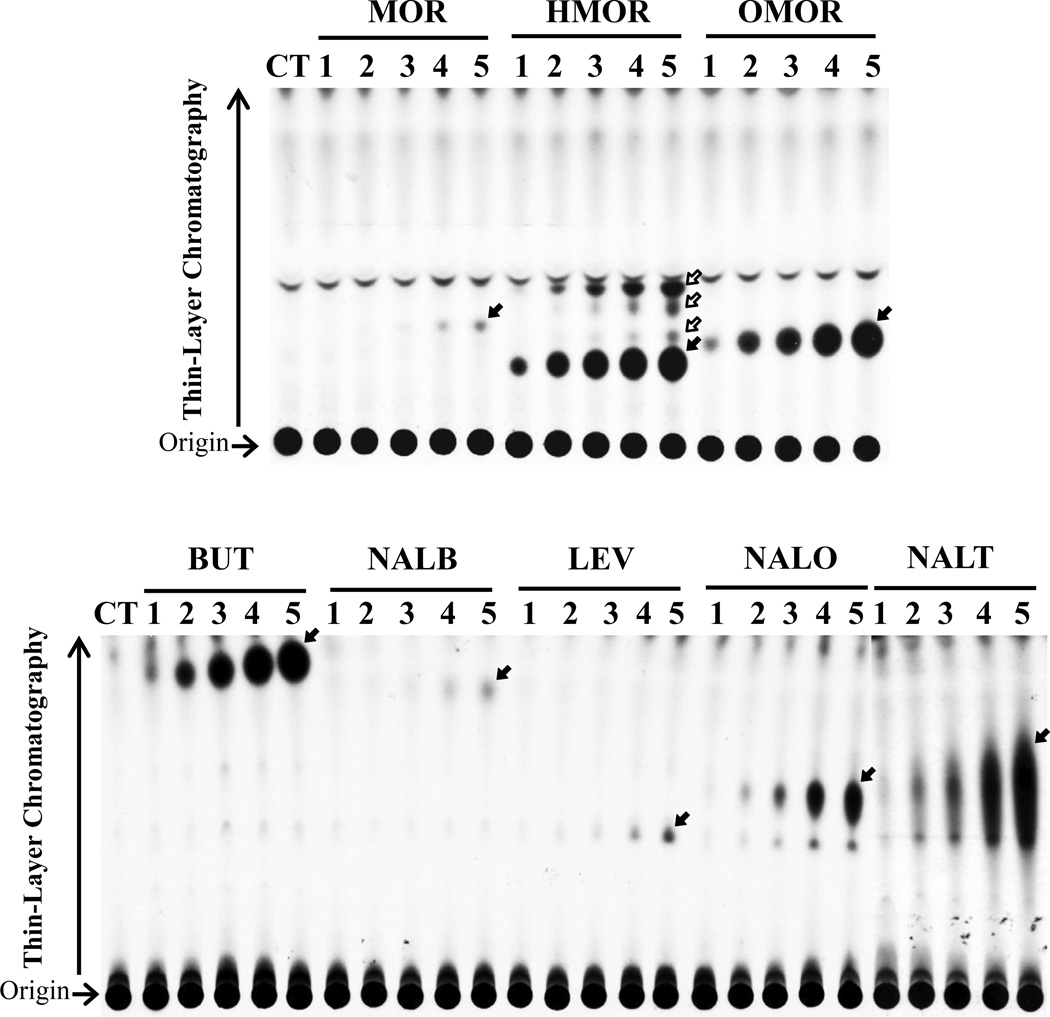
Confluent HepG2 cells grown in individual wells of a 24-well plate were labeled with [35S]sulfate in sulfate-free medium containing 0, 1, 5, 10, 25, and 50 µM of individual opioid drugs. Thin-layer chromatography analysis of the labeling media collected at the end of a 21-hour labeling period revealed the generation and release of [35S]sulfated derivatives of tested opioid drugs in a dose-dependent manner (Figure 1). Among the eight opioid drugs tested, HepG2 cells appeared to sulfate hydromorphone, oxymorphone, butorphanol, nalorphine, and naltrexone more efficiently than the other three opioid drugs. For hydromorphone, oxymorphone, and butorphanol, [35S]sulfated derivatives were detected when these two drugs were added to the labeling media at concentrations as low as 1 µM. For nalorphine and naltrexone, their [35S]sulfated derivatives were detected when these opioid drugs were added to the labeling media at concentrations of 5 µM or higher. For morphine, nalbuphine, and levorphanol, their [35S]sulfated derivatives were detected when these opioid drugs were added to the labeling media at concentrations higher than 25 µM. Interestingly, multiple [35S]sulfated derivatives (as indicated by arrows) were generated and released by HepG2 cells in the case of hydromorphone. These results indicated clearly that HepG2 cells are capable of metabolizing tested opioid drugs via sulfation under a metabolic setting.
Figure 1.
Analysis of [35S]sulfated products generated and released by HepG2 human hepatoma cells labeled with [35S]sulfate in the presence of different drug compounds. The figure shows the autoradiograph taken from the plate at the end of the TLC analysis. Confluent cells were incubated in labeling media containing, respectively, 1, 5, 10, 25, 50 µM (corresponding to lanes 1 to 5) of morphine (MOR), hydromorphone (HMOR), oxymorphone (OMOR), butorphanol (BUT), nalbuphine (NALB), levorphanol (LEV), nalorphine (NALO), and naltrexone (NALT) for 21 h. CT refers to the control labeling medium without added compounds. The arrows indicate the sulfated derivatives of each of the eight drugs.
3.2. Sulfation of opioid drugs by human organ samples
To obtain evidence for the presence of opioid drugs-sulfating activity in human tissues, enzymatic assays were performed using cytosol or S9 fraction prepared from human lung, liver, small intestine, or kidney. Activity data obtained are compiled in Table 1. Liver cytosol exhibited sulfating activities toward all opioid drugs tested, with considerably stronger activities detected with oxymorphone and naltrexone (24.9 and 19.3 pmol/min/mg protein) than with other opioid drugs. In contrast, S9 fractions prepared from lung, small intestine, and kidney showed significant sulfating activities toward only four of the eight opioid drugs, hydromorphone, oxymorphone, nalorphine, and naltrexone. Of the four organ samples, small intestine displayed the strongest activity toward hydromorphone (40.0 pmol/min/mg) than did the other three organ samples. These results indicated that different opioid drugs may be differentially metabolized through sulfation in different organs in human body.
Table 1.
Sulfating activities of human lung, liver, kidney, and small intestine cytosol fractions toward opioid drugs as substratesa)
| Substrate | Specific activity (pmol/min/mg) |
|||
|---|---|---|---|---|
| Lung | Liver | Kidney | Small Intestine | |
| Morphine | NDb) | 0.2 ± 0.1 | ND | ND |
| Hydromorphone | 6.3 ± 0.2 | 7.1 ± 1.1 | 3.2 ± 0.5 | 40.0 ± 0.7 |
| Oxymorphone | 3.7 ± 0.1 | 24.9 ± 0.9 | 5.5 ± 0.5 | 13.1 ± 0.9 |
| Butorphanol | ND | 1.3 ± 0.1 | ND | ND |
| Nalbuphine | ND | 0.3 ± 0.1 | ND | ND |
| Levorphanol | ND | 0.1 ± 0.1 | ND | ND |
| Nalorphine | 0.6 ± 0.1 | 3.7 ± 0.1 | 0.4 ± 0.1 | 3.6 ± 0.2 |
| Naltrexone | 2.5 ± 0.1 | 19.3 ± 0.2 | 1.5 ± 0.1 | 10.0 ± 0.3 |
| 6βNaltrexone | ND | 0.4 ± 0.2 | ND | ND |
Specific activity refers to nmol substrate sulfated/min/mg purified enzyme. Data represent mean ± S.D. derived from three determinations. The concentration of the substrate used in the assay mixture was 50 µM.
ND refers to activity not detected. Specific activity determined was lower than the detection limit (estimated to be ~0.1 pmol/min/mg protein).
3.3. Differential sulfating activities of the human SULTs toward opioid drugs
To identify the enzyme(s) that is(are) responsible for the sulfation of the eight opioid drugs, as well as 6β-naltrexol, an active metabolite of naltrexone, a systematic study using eleven purified human SULTs was carried out. Six of the eleven SULTs, SULT1A1, SULT1A2, SULT1A3, SULT1C4, SULT1E1, SULT2A1, exhibited differential sulfating activities toward the opioid drugs tested (Table 2). The sulfating activities of the relevant SULTs toward each of eight opioid drugs were linear with time from 5 to 30 min and enzyme amount from 0.2 to 1.0 µg in 20 µl reaction mixture. (data not shown). The other five SULTs (SULT1B1, SULT1C2, SULT2B1a, SULT2B1b, SULT4A1) showed no activities toward any of the tested drug compounds. Among the six SULTs that showed opioid drug-sulfating activity, SULT1A1 displayed strong activities (at 25.72 and 26.35 nmol/min/mg enzyme, respectively) toward oxymorphone and naltrexone and moderate activities toward hydromorphone and nalorphine. SULT1A2 showed the sulfating activity pattern comparable to but weaker than SULT1A1. SULT1A3 and SULT1C4 displayed strong sulfating activities (at 21.52 and 8.59 nmol/min/mg enzyme, respectively) toward hydromorphone and moderate activities toward oxymorphone. In contrast, SULT1E1 and SULT2A1 showed only weak sulfating activity toward some of the eight opioid drugs. Collectively, these results indicated that SULT1A1 is likely the major enzyme responsible for the sulfation of oxymorphone, nalorphine, and naltrexone. SULT1A3 is most capable of catalyzing the sulfation of hydromorphone, and SULT2A1 is primarily responsible for the sulfation of butorphanol. On the other hand, morphine, levorphanol, nalbuphine, and 6β-naltrexol, were each significantly (compared with the detection limit of the assay) but weakly sulfated by several different human SULTs, with morphine being more strongly sulfated by SULT1A3, levorphanol being sulfated by SULT1A3 and SULT2A1, and nalbuphine and 6-βnaltrexol being more strongly sulfated by SULT1A1.
Table 2.
Specific activities of the human SULTs toward drug compounds as substratesa)
| Substrate | Specific activity (nmol/min/mg) |
|||||
|---|---|---|---|---|---|---|
| SULT1A1 | SULT1A2 | SULT1A3 | SULT1C4 | SULT1E1 | SULT2A1 | |
| Morphine | 0.04 ± 0.02 | 0.15 ± 0.01 | 0.25 ± 0.02 | 0.11 ± 0.05 | NDb) | ND |
| Hydromorphone | 9.04 ± 0.05 | 1.04 ± 0.12 | 21.52 ± 0.09 | 8.59 ± 0.09 | 0.18 ± 0.03 | ND |
| Oxymorphone | 25.72 ± 0.50 | 5.26 ± 0.14 | 3.69 ± 0.02 | 5.84 ± 0.09 | 0.46 ± 0.03 | ND |
| Butorphanol | ND | ND | ND | ND | 0.03 ± 0.01 | 0.76 ± 0.02 |
| Nalbuphine | 0.23 ± 0.01 | 0.13 ± 0.01 | ND | 0.05 ± 0.01 | ND | 0.06 ± 0.01 |
| Levorphanol | ND | ND | 0.06 ± 0.01 | ND | ND | 0.08 ± 0.01 |
| Nalorphine | 6.11 ± 0.14 | 1.28 ± 0.04 | 0.34 ± 0.01 | 0.54 ± 0.04 | ND | 0.16 ± 0.01 |
| Naltrexone | 26.35 ± 1.01 | 8.78 ± 0.39 | 0.44 ± 0.01 | 0.79 ± 0.01 | 0.94 ± 0.02 | ND |
| 6βNaltrexol | 0.21 ± 0.03 | 0.08 ± 0.01 | ND | ND | ND | ND |
| Standardc) | 36.54 ± 0.21 | 21.93 ± 0.13 | 34.55 ± 0.55 | 33.53 ± 0.76 | 45.83 ± 0.30 | 47.70 ± 0.50 |
Specific activity refers to nmol substrate sulfated/min/mg purified enzyme. Data represent mean ± S.D. derived from three determinations. The concentration of the substrate used in the assay mixture was 50 µM.
ND refers to activity not detected. Specific activity determined was lower than the detection limit (estimated to be ~0.01 nmol/min/mg protein).
Standard refers to activity toward each representative substrate at the 10 µM for six SULTs. p-Nitrophenol was used for SULT1A1, SULT1A2, and SULT1C4, dopamine for SULT1A3, 17 β-estradiol for SULT1E1, and dehydroepiandrosterone for SULT2A1.
3.4. Kinetics of the sulfation of opioid drugs by the relevant human SULTs
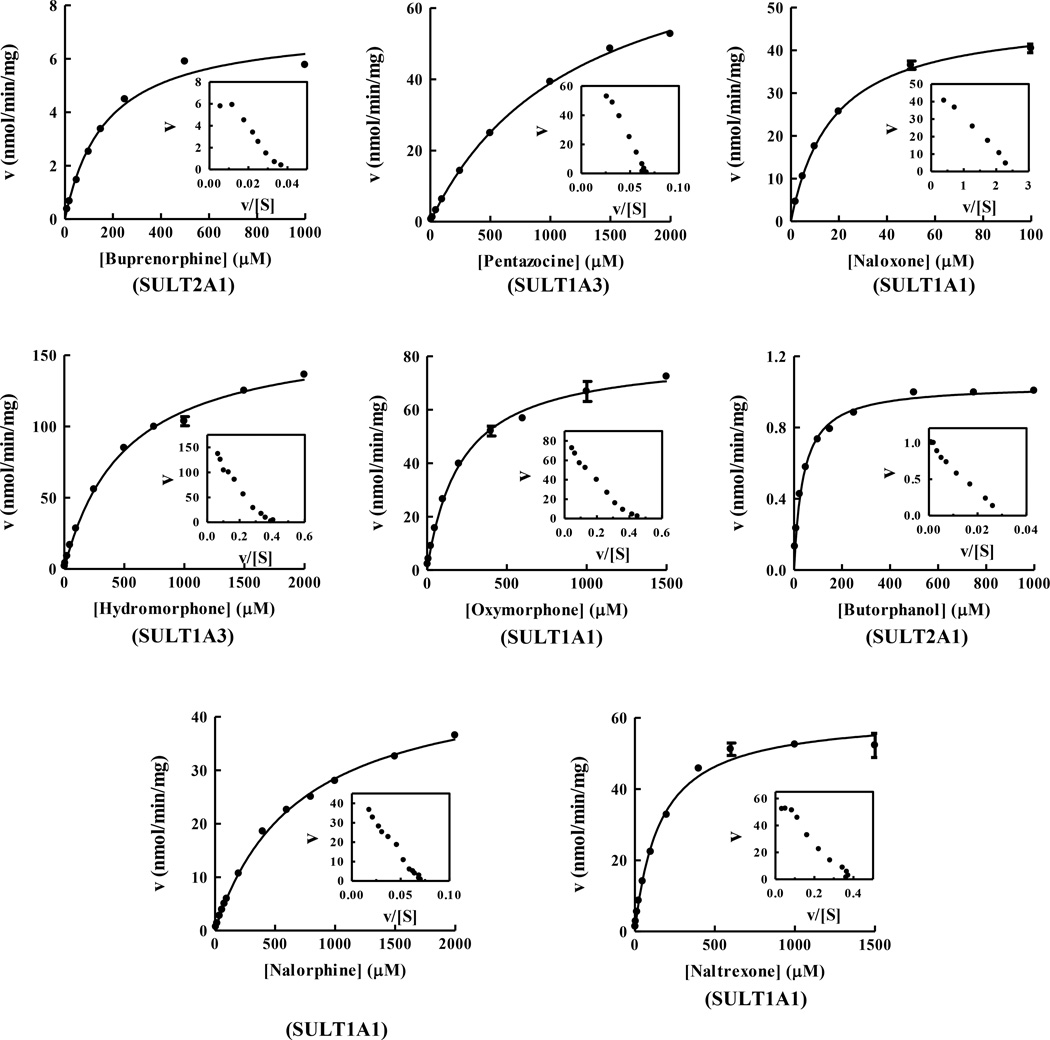
To investigate further the sulfation of each of eight opioid drugs by the relevant human SULTs, the kinetic analyses were carried out. Since previous study demonstrated that the sulfation of buprenorphine, pentazocine, and naloxone were mainly sulfated by, respectively, SULT2A1, SULT1A3, and SULT1A1 (Kurogi et al., 2012), the kinetics of the sulfation of those three opioid compounds were also analyzed in this study. To obtain the kinetic data, saturation curve analyses were examined using non-linear regression. All the sulfation of opioid drugs by the relevant human SULTs was fitted to hyperbolic kinetic curves (Michaelis-Menten kinetics), which was further confirmed by a linear Eadie-Hofstee plot. The Michaelis-Menten kinetic curves of buprenorphine and butorphanol by human SULT2A1, pentazocine and hydromorphone by SULT1A3, and naloxone, oxymorphone, nalorphine, and naltrexone by SULT1A1, are shown in Figure 2. As shown in Table 3, SULT1A1 showed the lower Km values toward naloxone (17.47 µM), oxymorphone (212.6 µM), nalorphine (694.1 µM), and naltrexone (157.5 µM) than those of other SULTs tested. For hydromorphone, SULT1A3 showed the lowest Km value, 4999 µM, among four SULTs tested. Based on the calculated Vmax/Km values, the catalytic efficiency for the sulfation of naloxone by SULT1A1 appeared to be much stronger than those for the sulfation of other opioid compounds. For oxymorphone and naltrexone, SULT1A1 also showed the highest catalytic efficiency among human SULTs tested. In contrast, SULT1A3 displayed the highest catalytic efficiency toward hydromorphone among human SULTs tested. For levorphanol, SULT2A1 showed lower Km value and higher catalytic efficiency than did SULT1A3, leading that SULT2A1 is the major responsible SULT for the sulfation of levorphanol. These results indicate that those three human SULTs, SULT1A1, SULT1A3, and SULT2A1, are mainly and differentially involved in the metabolism of opioid drugs.
Figure 2.
Kinetic analyses for the sulfation of opioid drugs by the relevant human SULTs. The fitting curves were generated using Michaelis-Menten kinetics. Eadie-Hofstee plots are inserted under each fitting curve. The fitting curves for the sulfation of eight opioid drugs, buprenorphine, pentazocine, naloxone, hydromorphone, oxymorphone, butorphanol, nolorphine, and naltrexone, represent the result of representative SULT for each substrate. Data shown represent calculated mean standard deviation derived from three experiments.
Table 3.
Kinetic constant of the human SULTs responsible for the sulfation of opioid drugsa)
| Substrate/SULT | Km (µM) |
Vmax (nmol/min/mg) |
Vmax / Km |
|---|---|---|---|
| Buprenorphine | |||
| /SULT2A1 | 169.9 ± 12.62 | 7.23 ± 0.19 | 0.043 |
| Pentazocine | |||
| /SULT1A3 | 1222 ± 40 | 86.36 ± 1.40 | 0.071 |
| Naloxone | |||
| /SULT1A1 | 17.47 ± 0.55 | 96.38 ± 1.05 | 5.517 |
| /SULT1A2 | 93.01 ± 2.89 | 115.1 ± 2.1 | 1.238 |
| /SULT1A3 | 1102 ± 45 | 57.57 ± 1.43 | 0.052 |
| /SULT1C4 | 419.2 ± 25.5 | 137.1 ± 5.1 | 0.327 |
| Morphine | |||
| /SULT1A3 | > 4000 | > 3.00 | - |
| Hydromorphone | |||
| /SULT1A1 | 670.9 ± 1.0 | 62.52 ± 29.28 | 0.093 |
| /SULT1A2 | 1146 ± 59 | 23.08 ± 0.58 | 0.020 |
| /SULT1A3 | 499.9 ± 19.6 | 166.2 ± 2.33 | 0.332 |
| /SULT1C4 | 826.7 ± 23.3 | 86.5 ± 1.09 | 0.105 |
| Oxymorphone | |||
| /SULT1A1 | 212.6 ± 8.4 | 80.71 ± 0.98 | 0.380 |
| /SULT1A2 | 1313 ± 58 | 76.47 ± 1.77 | 0.058 |
| /SULT1A3 | 1782 ± 76 | 73.96 ± 1.85 | 0.042 |
| /SULT1C4 | 1543 ± 62 | 93.78 ± 2.09 | 0.061 |
| Butorphanol | |||
| /SULT2A1 | 38.95 ± 1.21 | 1.04 ± 0.01 | 0.027 |
| Nalbuphine | |||
| /SULT1A1 | 4070 ± 536 | 7.37 ± 0.70 | 0.002 |
| Levorphanol | |||
| /SULT1A3 | 948.3 ± 119.9 | 0.57 ± 0.04 | 0.0006 |
| /SULT2A1 | 599.0 ± 60.7 | 0.32 ± 0.02 | 0.001 |
| Nalorphine | |||
| /SULT1A1 | 694.1 ± 17.1 | 48.22 ± 0.51 | 0.070 |
| /SULT1A2 | 3203 ± 223.4 | 61.23 ± 2.95 | 0.019 |
| Naltrexone | |||
| /SULT1A1 | 157.5 ± 8.3 | 60.81 ± 0.91 | 0.386 |
| /SULT1A2 | 758.6 ± 40.3 | 78.86 ± 1.89 | 0.104 |
Results represent means ± S.D. derived from three determinations.
4. Discussion
Sulfation as mediated by the SULTs is known to be critically involved in the metabolism of a number of drugs (Falany, 1997, 1991; Falany and Roth, 1994; Mulder and Jakoby, 1990; Weinshilboum and Otterness; 1994). Although previous studies have demonstrated the sulfated metabolites of several opioid drugs, morphine, hydromorphone, levorphanol, nalorphine, and naloxone in vivo, the metabolism of those opioid drugs through sulfation has not been well investigated. (Leinweber et al., 1981; Yeh and Woods, 1971; Zheng et al., 2002). The current study aimed to investigate whether opioid drugs may be metabolized through sulfation and, if so, which of the eleven human SULTs is(are) involved in the sulfation of different opioid drugs tested. In this study, not only morphine, hydromorphone, nalbuphine, and levorphanol whose sulfated metabolite have been reported in vivo but also other opioid drugs including oxymorphone, butorphanol, nalorphine, and naltrexone. Oxymorphone and butorphanol are commonly used morphinan-type analgesics such as morphine, hydromorphone, and buprenorphine being sulfated by SULT2A1, nalorphine and naltrexone are opioid antagonists like naloxone being considerably sulfated by SULT1A1, SULT1A2, and SULT1C4, so that those four opioid drugs were concomitantly analyzed in this study (Fudala et al., 2006, Heel et al., 1978, Kurogi et al., 2012, Martin, 1967, Sloan et al., 2005).
Previous studies have demonstrated that most of the SULTs present in the human liver, including SULT1A1, SULT1A2, SULT1A3, SULT1E1, and SULT2A1, are expressed in HepG2 human hepatoma cells, a human liver-derived cell line, so that the cells are widely used in the investigation of drug metabolism (Chen et al., 2005; Dooley et al., 2000; Miyano et al., 2005; Riches et al., 2009; Shwed et al., 1992; Westerink and Schoonen, 2007). Therefore, we opted to first use the HepG2 cells in this study as a model liver cell line to investigate the metabolism of opioid drugs through sulfation. A metabolic labeling study showed the generation and release of sulfated morphine, hydromorphone, oxymorphone, butorphanol, nalbuphine, levorphanol, nalorphine, and naltrexone (Figure 1), indicating that all these eight drugs can be taken up by HepG2 cells and subjected to the sulfation by the SULT enzyme(s) therein. A systematic survey of the eleven recombinant human SULTs revealed that SULT1A1 is the major enzyme responsible for the sulfation of oxymorphone, nalbuphine, nalorphine, and naltrexone (Table 2). SULT1A3, on the other hand, is the major sulfating enzyme for morphine and hydromorphone, whereas SULT2A1 is the major responsible enzyme for the sulfation of butorphanol and levorphanol. The identities of [35S]sulfated derivatives of the eight opioid drugs, as indicated by solid arrows, were confirmed by their co-migration, upon TLC, with [35S]sulfated product of corresponding opioid drugs enzymatically synthesized by relevant human SULTs (SULT1A3 for morphine and hydromorphone, SULT1A1 for oxymorphone, nalbuphine, nalorphine, and naltrexone, and SULT2A1 for butorphanol and levorphanol; data not shown). Among eight opioid drugs tested, hydromorphone, oxymorphone, butorphanol, nalorphine, and naltrexone were more markedly subjected to the sulfation than other three opioid drugs. It is possibly due to the expression of the SULTs showing the strong opioid drug-sulfating activities and the affinity toward a specific substrate. For example, hydromorphone, which appeared the prominent [35S]sulfated derivative at throughout concentrations, even as low as 1 µM concentration, was subjected to the sulfation by a number of SULTs (SULT1A1, SULT1A2, SULT1A3, SULT1E1, and SULT1C4), specifically SULT1A1 and SULT1A3 are strongly expressed in HepG2 and capable of sulfating the hydromorphone with high activities, 9.04 and 21.52 nmol/min/mg, respectively. For butorphanol, although the only SULT2A1 showed the moderate sulfating activity, the Km value toward butorphanol as a substrate is lowest among those of eight opioid drugs tested by the relevant SULTs. It is therefore possible that HepG2 could markedly generate and release the [35S]sulfated derivatives of those opioid drugs. In contrast, since no SULT enzyme showed the strong sulfating activity and low Km value toward morphine, nalbuphine, and levorphanol, HepG2 may have appeared the [35S]sulfated derivatives of weak intensity. It was also noted that, different from the other seven opioid drugs tested, several species of [35S]sulfated derivatives of hydromorphone were produced and released by HepG2 cells. The major [35S]sulfated derivative (as indicated by solid arrow), nevertheless, co-migrated with [35S]sulfated hydromorphone enzymatically synthesized using SULT1A3. It is noted that previous studies demonstrated the presence of a number of hydromorphone metabolites, including dihydromorphine, dihydroisomorphine, and norhydromorphone, in human urine (Benetton et al., 2004; Zheng et al., 2002). Since these hydromorphone metabolites all contain hydroxyl group(s), it is possible that they may also be sulfated by human SULT enzyme(s). It is therefore likely that the three less intensive, yet specific, [35S]sulfated species (as indicated by the empty arrows) detected in the labeling media of HepG2 cells labeled in the presence of hydromorphone may correspond to [35S]sulfated derivatives of hydromorphone metabolites.
An important issue is with regard to the tissues/organs capable of metabolizing opioid drugs through sulfation. Since human SULTs are widely expressed in the human body (Dooley et al., 2000; Riches et al., 2009), it is possible that organs other than the liver, from which HepG2 cells are believed to be derived, may also be capable of sulfating opioid drugs. Indeed, the sulfating activities toward opioid drugs were detected in the cytosol or S9 fractions of the human lung, kidney, small intestine, as well as liver (Table 1). It is not surprising that liver showed the strongest sulfating activities toward opioid drugs among the four human organs tested since a number of SULT enzymes have been shown to be strongly expressed in the liver (Dooley et al., 2000; Riches et al., 2009). It is noted, however, that small intestine displayed stronger sulfating activity toward hydromorphone than did liver. This could have been due to the differential expression of the human SULT enzymes in liver vs. small intestine. Previous studies had demonstrated that SULT1A1 and SULT2A1 were strongly expressed in liver and SULT1A3 was strongly expressed in gastrointestinal track (Dooley et al., 2000; Riches et al., 2009; Teubner et al., 2007). The tissue distributions of those enzymes are in line with stronger sulfating activity toward hydromorphone in small intestine and stronger sulfating activity toward oxymorphone, butorphanol, nalbuphine, and naltrexone in liver, among the four human organs tested. It is noteworthy that these opioid drugs tested may be administered through various routes including oral, intravenous, and intramuscular (Fudala and Johnson, 2006; Gonzalez and Brogden, 1988; Krenzischek et al., 2008; Prommer, 2005; Sarhill et al., 2001; Smith, 2011). It is possible that the administration route may influence the metabolic pattern of opioid drugs. In the case of hydromorphone, for example, it may be subjected to the sulfation to a greater extent upon oral administration than with parenteral administration.
Kinetic studies were performed to gain insight into the mechanism of the sulfation of opioid drugs by the human relevant SULTs. The substrate saturation curve and Eadie-Hofstee plot analyses revealed that the sulfation of each of the eleven opioid drugs followed the Michaelis-Menten kinetics (Figure 2). SULT1A1 showed the lowest Km values with naloxone (17.47 µM), oxymorphone (212.6 µM), and naltrexone (157.5 µM) among the relevant human SULTs tested. SULT2A1 showed the Km values with buprenorphine (169.9 µM) and butorphanol (38.95 µM). In contrast, with hydromorphone, while SULT1A3 had the lowest Km value (499.9 µM) among the relevant human SULTs tested, the affinity seems to be lower than those of SULT1A1 and SULT2A1 with naloxone and butorphanol, respectively. Therefore, among eleven opioid drugs tested, naloxone and butorphanol exert substrates with being the highest affinity for the relevant human SULT enzymes, SULT1A1 and SULT2A1, respectively. UGT-glucuronosyltransferases (UGTs) have been accepted to the major Phase II enzyme in the metabolism of opioid drugs (Coffman et al., 1998; Holmquist, 2009; King et al., 2000; Smith, 2009; Vallejo et al., 2011). Several isoforms, UGT1A1, UGT1A3, UGT1A8, and UGT2B7, are involved in the glucuronidation of opioid drugs, particularly UGT2B7 has been recognized as the major responsible for the glucuronidation of those compounds Coffman et al., 1998; King et al., 2000; Smith, 2009). It should be pointed out that the Km values of the UGT2B7 with opioid drugs are relatively comparable to those of the relevant SULTs. Previous studies have reported that the Km values of UGT2B7 with hydromorphone, oxymorphone, naloxone, and naltrexone are ranged into 360–1400 µM, 540–1360 µM, 40–60 µM, and 50–200 µM (Coffman et al., 1998; Green et al., 1997). These results implicate that SULTs may play a role on the metabolism of those opioid drugs as much as the contribution of UGTs to the metabolism. Inversely, the Km value of sulfation of morphine by SULT1A3 and nalbuphine by SULT1A1 were over 4000 µM, which implicating that morphine and nalbuphine may be not preferable opioid substrate toward SULTs and the sulfation may less contribute to the metabolism of these two opioid drugs. Indeed, previous studies have shown the morphine-sulfate as minor metabolite (~2% of metabolite) in contrast to the major metabolite, morphine-glucuronide (60–70% of metabolite), in human (Frölich et al., 2011, Skarke and Lötsch, 2002). Another interesting issue is the subcellular localizations of SULTs and UGTs. Since SULTs are localized in cytosol and UGTs are in endoplasmic reticulum (ER), the SULTs capable of sulfating the opioid drugs may contribute to the metabolism of opioid drug in cytosol with affinities comparable to those of UGTs.
Another important issue is the clinical and pharmacological relevance of the opioid drug sulfation. Sulfate conjugation regulates the pharmacological activity of drugs and generally leads to the inactivation (Falany and Roth, 1994; Mulder and Jakoby, 1990; Weinshilboum and Otterness, 1994; Glatt et al., 2000; Coughtrie, 2002; Pacifici, 2005; Runge-Morris and Kocarek, 2009). Previous studies have shown that, although morphine-3-sulfate has low affinity toward opioid receptors and low capability to activate the receptors, morphine-6-sulfate retains the affinity and ligand activity comparable to those of morphine and shows a greater analgesic effect than does morphine (Brown et al., 1985, Frölich et al., 2011, Zuckerman et al., 1999). It is therefore possible that sulfation may play two roles, inactivation and reinforcement of analgesic activity, in the metabolism of opioid drugs. The sulfate metabolites are poorly able to cross the blood–brain barrier from the circulatory system due to the negatively charged sulfate group, so that the sulfate derivatives of opioid drugs could not pass through the blood-brain barrier (Kishimoto and Hoshi, 1972, Guazzo et al., 1996). In contrast, most of opioid drugs are capable of crossing the blood-brain barrier, activate the dopaminergic reward pathway, and produce the feeling of euphoria, which result in the opioid drug abuse (Boström et al., 2006, Tunblad et al., 2003, Xie and Hammarlund-Udenaes, 1998). Since the sulfated metabolite of opioid drugs with being opioid activity, such as morphine-6-sulfate, may only act on the opioid receptors at peripheral nerve system, the sulfation may also play another role, reducing a euphoric effect of opioid drugs, in the pharmacological action of opioid drugs and may be a good target to develop the peripheral analgesic drugs. Further work will be required to better understand the clinical and pharmacological relevance of the opioid drug sulfation.
5. Conclusion
The present study demonstrated clearly the occurrences of sulfation of eight opioid drugs, morphine, hydromorphone, oxymorphone, butorphanol, nalbuphine, levorphanol, nalorphine, and neltrexone, in HepG2 hepatoma cells. Differential sulfating activities toward the eight drugs tested were detected in cytosol or S9 fractions of human lung, liver, kidney, and small intestine. A systematic enzymatic analysis revealed the major responsible SULTs being SULT1A1 for oxymorphone, nalbuphine, nalorphine, and naltrexone, SULT1A3 for morphine and hydromorphone, and SULT2A1 for butorphanol and levorphanol. Further work is warranted to fully elucidate the pharmacological relevance of the sulfation of opioid drugs.
Acknowledgments
This work was supported in part by a grant from National Institutes of Health (Grant # R03HD071146).
Abbreviations
- SULT
cytosolic sulfotransferase
- PAPS
3’-phosphoadenosine-5’-phosphosulfate
- TLC
thin-layer chromatography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ballantyne JC, Shin NS. Efficacy of opioids for chronic pain: a review of the evidence. Clin. J. Pain. 2008;24:469–478. doi: 10.1097/AJP.0b013e31816b2f26. [DOI] [PubMed] [Google Scholar]
- Benetton SA, Borges VM, Chang TK, McErlane KM. Role of individual human cytochrome P450 enzymes in the in vitro metabolism of hydromorphone. Xenobiotica. 2004;34:335–344. doi: 10.1080/00498250310001657559. [DOI] [PubMed] [Google Scholar]
- Boström E, Simonsson US, Hammarlund-Udenaes M. In vivo blood-brain barrier transport of oxycodone in the rat: indications for active influx and implications for pharmacokinetics/pharmacodynamics. Drug Metab. Dispos. 2006;34:1624–1631. doi: 10.1124/dmd.106.009746. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding, Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown CE, Roerig SC, Burger VT, Cody RB, Jr, Fujimoto JM. Analgesic potencies of morphine 3- and 6-sulfates after intracerebroventricular administration in mice: relationship to structural characteristics defined by mass spectrometry and nuclear magnetic resonance. J. Pharm. Sci. 1985;74:821–824. doi: 10.1002/jps.2600740804. [DOI] [PubMed] [Google Scholar]
- Chamberlain JM, Klein BL. A comprehensive review of naloxone for the emergency physician. Am. J. Emerg. Med. 1994;12:650–660. doi: 10.1016/0735-6757(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Chen X, Baker SM, Chen G. Methotrexate induction of human sulfotransferases in HepG2 and Caco-2 cells. J. Appl. Toxicol. 2005;25:354–360. doi: 10.1002/jat.1071. [DOI] [PubMed] [Google Scholar]
- Coffman BL, King CD, Rios GR, Tephly TR. The glucuronidation of opioids, other xenobiotics, and androgens by human UGT2B7Y(268) and UGT2B7H(268) Drug Metab. Dispos. 1998;26:73–77. [PubMed] [Google Scholar]
- Coughtrie MW. Sulfation through the looking glass--recent advances in sulfotransferase research for the curious. Pharmacogenomics J. 2002;2:297–308. doi: 10.1038/sj.tpj.6500117. [DOI] [PubMed] [Google Scholar]
- Dooley TP, Haldeman-Cahill R, Joiner J, Wilborn TW. Expression profiling of human sulfotransferase and sulfatase gene superfamilies in epithelial tissues and cultured cells. Biochem. Biophys. Res. Commun. 2000;277:236–245. doi: 10.1006/bbrc.2000.3643. [DOI] [PubMed] [Google Scholar]
- Falany CN. Molecular enzymology of human liver cytosolic sulfotransferases. Trends Pharmacol. Sci. 1991;12:255–259. doi: 10.1016/0165-6147(91)90566-b. [DOI] [PubMed] [Google Scholar]
- Falany CN, Roth JA. Properties of human cytosolic sulfotransferases involved in drug metabolism. In: Jeffery EH, editor. Human Drug Metabolism; from Molecular Biology to Man. Boca Raton: CRC Press; 1994. pp. 101–115. [Google Scholar]
- Falany CN. Enzymology of human cytosolic sulfotransferases. FASEB J. 1997;11:206–216. doi: 10.1096/fasebj.11.4.9068609. [DOI] [PubMed] [Google Scholar]
- Falany JL, Macrina N, Falany CN. Sulfation of tibolone and tibolone metabolites by expressed human cytosolic sulfotransferases. J. Steroid Biochem. Mol. Biol. 2004;88:383–391. doi: 10.1016/j.jsbmb.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Frölich N, Dees C, Paetz C, Ren X, Lohse MJ, Nikolaev VO, Zenk MH. Distinct pharmacological properties of morphine metabolites at G(i)-protein and β-arrestin signaling pathways activated by the human µ-opioid receptor. Biochem Pharmacol. 2011;81:1248–1254. doi: 10.1016/j.bcp.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Fudala PJ, Johnson RE. Development of opioid formulations with limited diversion and abuse potential. Drug Alcohol Depend. 2006;1:S40–S47. doi: 10.1016/j.drugalcdep.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Fujimoto JM, Haarstad VB. The isolation of morphine ethereal sulfate from urine of the chicken and cat. J. Pharmacol. Exp. Ther. 1969;165:45–51. [PubMed] [Google Scholar]
- Gamage N, Barnett A, Hempel N, Duggleby RG, Windmill KF, Martin JL, McManus ME. Human sulfotransferases and their role in chemical metabolism. Toxicol. Sci. 2006;90:5–22. doi: 10.1093/toxsci/kfj061. [DOI] [PubMed] [Google Scholar]
- Glatt H, Engelke CE, Pabel U, Teubner W, Jones AL, Coughtrie MW, Andrae U, Falany CN, Meinl W. Sulfotransferases: genetics and role in toxicology. Toxicol. Lett. 2000;112–113:341–348. doi: 10.1016/s0378-4274(99)00214-3. [DOI] [PubMed] [Google Scholar]
- Glatt H, Boeing H, Engelke CE, Ma L, Kuhlow A, Pabel U, Pomplun D, Teubner W, Meinl W. Human cytosolic sulphotransferases: genetics, characteristics, toxicological aspects. Mutat. Res. 2001;482:27–40. doi: 10.1016/s0027-5107(01)00207-x. [DOI] [PubMed] [Google Scholar]
- Gonzalez JP, Brogden RN. Naltrexone. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy in the management of opioid dependence. Drugs. 1988;35:192–213. doi: 10.2165/00003495-198835030-00002. [DOI] [PubMed] [Google Scholar]
- Green MD, Bélanger G, Hum DW, Bélanger A, Tephly TR. Glucuronidation of opioids, carboxylic acid-containing drugs, and hydroxylated xenobiotics catalyzed by expressed monkey UDP-glucuronosyltransferase 2B9 protein. Drug Metab. Dispos. 1997;25:1389–1394. [PubMed] [Google Scholar]
- Guazzo EP, Kirkpatrick PJ, Goodyer IM, Shiers HM, Herbert J. Cortisol, dehydroepiandrosterone (DHEA), and DHEA sulfate in the cerebrospinal fluid of man: relation to blood levels and the effects of age. J. Clin. Endocrinol. Metab. 1996;81:3951–3960. doi: 10.1210/jcem.81.11.8923843. [DOI] [PubMed] [Google Scholar]
- Heel RC, Brogden RN, Speight TM, Avery GS. Butorphanol: a review of its pharmacological properties and therapeutic efficacy. Drugs. 1978;16:473–505. doi: 10.2165/00003495-197816060-00001. [DOI] [PubMed] [Google Scholar]
- Helm S, Trescot AM, Colson J, Sehgal N, Silverman S. Opioid antagonists, partial agonists, and agonists/antagonists: the role of office-based detoxification. Pain Physician. 2008;11:225–235. [PubMed] [Google Scholar]
- Holmquist GL. Opioid metabolism and effects of cytochrome P450. Pain Med. 2009;10:S20–S29. [Google Scholar]
- Inturrisi CE. Clinical pharmacology of opioids for pain. Clin. J. Pain. 2002;18:S3–S13. doi: 10.1097/00002508-200207001-00002. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y, Hoshi M. Dehydroepiandrosterone sulphate in rat brain: incorporation from blood and metabolism in vivo. J. Neurochem. 1972;19:2207–2215. doi: 10.1111/j.1471-4159.1972.tb05129.x. [DOI] [PubMed] [Google Scholar]
- King CD, Rios GR, Green MD, Tephly TR. UDP-glucuronosyltransferases. Curr. Drug Metab. 2000;1:143–161. doi: 10.2174/1389200003339171. [DOI] [PubMed] [Google Scholar]
- Krenzischek DA, Dunwoody CJ, Polomano RC, Rathmell JP. Pharmacotherapy for acute pain: implications for practice. J. Perianesth. Nurs. 2008;23:S28–S42. doi: 10.1016/j.jopan.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Cramer JA, Krol WF, Kirk GF, Rosenheck RA. Naltrexone in the treatment of alcohol dependence. N. Engl. J. Med. 2001;345:1734–1739. doi: 10.1056/NEJMoa011127. [DOI] [PubMed] [Google Scholar]
- Kurogi K, Lee Y, Chen M, Shi B, Yang T, Liu MY, Sakakibara Y, Suiko M, Liu M-C. Sulfation of buprenorphine, pentazocine, and naloxone by human cytosolic sulfotransferases. Drug Metab. Lett. 2012 Aug 31; in press [Epub adead of print] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leinweber FJ, Szuna AJ, Williams TH, Sasso GJ, DeBarbieri BA. The metabolism of (−)-3-phenoxy-N-methylmorphinan in dogs. Drug Metab. Dispos. 1981;9:284–291. [PubMed] [Google Scholar]
- Lipmann F. Biological sulfate activation and transfer. Science. 1958;128:575–580. doi: 10.1126/science.128.3324.575. [DOI] [PubMed] [Google Scholar]
- Liu M-C, Yasuda S, Idell S. Sulfation of nitrotyrosine: biochemistry and functional implications. IUBMB Life. 2007;59:622–627. doi: 10.1080/15216540701589320. [DOI] [PubMed] [Google Scholar]
- Martin WR. Opioid antagonists. Pharmacol. Rev. 1967;19:463–521. [PubMed] [Google Scholar]
- Meloche CA, Sharma V, Swedmark S, Andersson P, Falany CN. Sulfation of budesonide by human cytosolic sulfotransferase, dehydroepiandrosterone-sulfotransferase (DHEA-ST) Drug Metab. Dispos. 2002;30:582–585. doi: 10.1124/dmd.30.5.582. [DOI] [PubMed] [Google Scholar]
- Miyano J, Yamamoto S, Hanioka N, Narimatsu S, Ishikawa T, Ogura K, Watabe T, Nishimura M, Ueda N, Naito S. Involvement of SULT1A3 in elevated sulfation of 4-hydroxypropranolol in Hep G2 cells pretreated with beta-naphthoflavone. Biochem. Pharmacol. 2005;69:941–950. doi: 10.1016/j.bcp.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Mulder GM, Jakoby WB. Sulfation. In: Mulder GJ, editor. Conjugation Reactions in Drug Metabolism. London: Taylor and Francis; 1990. pp. 107–161. [Google Scholar]
- Orman JS, Keating GM. Buprenorphine/naloxone: a review of its use in the treatment of opioid dependence. Drugs. 2009;69:577–607. doi: 10.2165/00003495-200969050-00006. [DOI] [PubMed] [Google Scholar]
- Pai TG, Sugahara T, Suiko M, Sakakibara Y, Xu F, Liu M-C. Differential xenoestrogen-sulfating activities of the human cytosolic sulfotransferases: molecular cloning, expression, and purification of human SULT2B1a and SULT2B1b sulfotransferases. Biochim. Biophys. Acta. 2002;1573:165–170. doi: 10.1016/s0304-4165(02)00416-6. [DOI] [PubMed] [Google Scholar]
- Pacifici GM. Sulfation of drugs and hormones in mid-gestation human fetus. Early Hum. Dev. 2005;81:573–581. doi: 10.1016/j.earlhumdev.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Pasternak GW. Pharmacological mechanisms of opioid analgesics. Clin. Neuropharmacol. 1993;16:1–18. doi: 10.1097/00002826-199302000-00001. [DOI] [PubMed] [Google Scholar]
- Riches Z, Stanley EL, Bloomer JC, Coughtrie MW. Quantitative evaluation of the expression and activity of five major sulfotransferases (SULTs) in human tissues: the SULT 'pie'. Drug Metab. Dispos. 2009;37:2255–2261. doi: 10.1124/dmd.109.028399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runge-Morris M, Kocarek TA. Regulation of Sulfotransferase and UDP-Glucuronosyltransferase Gene Expression by the PPARs. PPAR Res. 2009 doi: 10.1155/2009/728941. ID 728941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara Y, Suiko M, Liu M-C. De novo sulfation of L-tyrosine in HepG2 human hepatoma cells and its possible functional implication. Eur. J. Biochem. 1994;226:293–301. doi: 10.1111/j.1432-1033.1994.tb20053.x. [DOI] [PubMed] [Google Scholar]
- Sakakibara Y, Takami Y, Nakayama T, Suiko M, Liu M-C. Localization and functional analysis of the substrate specificity/catalytic domains of human M-form and P-form phenol sulfotransferases. J. Biol. Chem. 1998;273:6242–6247. doi: 10.1074/jbc.273.11.6242. [DOI] [PubMed] [Google Scholar]
- Sarhill N, Walsh D, Nelson KA. Hydromorphone: pharmacology and clinical applications in cancer patients. Support. Care Cancer. 2001;9:84–96. doi: 10.1007/s005200000183. [DOI] [PubMed] [Google Scholar]
- Shwed JA, Walle UK, Walle T. Hep G2 cell line as a human model for sulphate conjugation of drugs. Xenobiotica. 1992;22:973–82. doi: 10.3109/00498259209049903. [DOI] [PubMed] [Google Scholar]
- Skarke C, Lötsch J. Morphine metabolites: clinical implications. Semin. Anesth. Perioperat. Med. Pain. 2002;21:258–264. [Google Scholar]
- Sloan P, Slatkin N, Ahdieh H. Effectiveness and safety of oral extended-release oxymorphone for the treatment of cancer pain: a pilot study. Support. Care Cancer. 2005;13:57–65. doi: 10.1007/s00520-004-0731-1. [DOI] [PubMed] [Google Scholar]
- Smith DS, Peterson RE, Fujimoto JM. Species differences in the biliary excretion of morphine, morphine-3-glucuronide and morphine-3-ethereal sulfate in the cat and rat. Biochem. Pharmacol. 1973;22:485–492. doi: 10.1016/0006-2952(73)90290-6. [DOI] [PubMed] [Google Scholar]
- Smith HS. Opioid metabolism. Mayo Clin. Proc. 2009;84:613–624. doi: 10.1016/S0025-6196(11)60750-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith HS. Morphine sulfate and naltrexone hydrochloride extended release capsules for the management of chronic, moderate-to-severe pain, while reducing morphine-induced subjective effects upon tampering by crushing. Expert Opin. Pharmacother. 2011;12:1111–1125. doi: 10.1517/14656566.2011.571205. [DOI] [PubMed] [Google Scholar]
- Sugahara T, Pai TG, Suiko M, Sakakibara Y, Liu M-C. Differential roles of human monoamine (M)-form and simple phenol (P)-form phenol sulfotransferases in drug metabolism. J. Biochem. 2003;133:259–262. doi: 10.1093/jb/mvg033. [DOI] [PubMed] [Google Scholar]
- Suiko M, Sakakibara Y, Liu M-C. Sulfation of environmental estrogen-like chemicals by human cytosolic sulfotransferases. Biochem. Biophys. Res. Commun. 2000;267:80–84. doi: 10.1006/bbrc.1999.1935. [DOI] [PubMed] [Google Scholar]
- Tunblad K, Jonsson EN, Hammarlund-Udenaes M. Morphine blood-brain barrier transport is influenced by probenecid co-administration. Pharm. Res. 2003;20:618–623. doi: 10.1023/a:1023250900462. [DOI] [PubMed] [Google Scholar]
- Teubner W, Meinl W, Florian S, Kretzschmar M, Glatt H. Identification and localization of soluble sulfotransferases in the human gastrointestinal tract. Biochem J. 2007;404:207–215. doi: 10.1042/BJ20061431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo R, Barkin RL, Wang VC. Pharmacology of opioids in the treatment of chronic pain syndromes. Pain Physician. 2011;14:343–360. [PubMed] [Google Scholar]
- Weinshilboum RM, Otterness DM. Sulfotransferase enzymes. In: Kaufman FC, editor. Conjugation-Deconjugation Reactions in Drug Metabolism and Toxicity. Berlin: Springer-Verlag; 1994. pp. 45–78. [Google Scholar]
- Westerink WM, Schoonen WG. Phase II enzyme levels in HepG2 cells and cryopreserved primary human hepatocytes and their induction in HepG2 cells. Toxicol. In Vitro. 2007;21:1592–1602. doi: 10.1016/j.tiv.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Xie R, Hammarlund-Udenaes M. Blood-brain barrier equilibration of codeine in rats studied with microdialysis. Pharm. Res. 1998;15:570–575. doi: 10.1023/a:1011929910782. [DOI] [PubMed] [Google Scholar]
- Yanagisawa K, Sakakibara Y, Suiko M, Takami Y, Nakayama T, Nakajima H, Takayanagi K, Natori Y, Liu M-C. cDNA cloning, expression, and characterization of the human bifunctional ATP sulfurylase/adenosine 5’-phosphosulfate kinase enzyme. Biosci. Biotechnol. Biochem. 1998;62:1037–1040. doi: 10.1271/bbb.62.1037. [DOI] [PubMed] [Google Scholar]
- Yeh SY, Woods LA. Isolation and characterization of urinary metabolites of nalorphine in dogs and cats. J. Pharm. Sci. 1971;60:148–150. doi: 10.1002/jps.2600600137. [DOI] [PubMed] [Google Scholar]
- Yeh SY, Gorodetzky CW, Krebs HA. Isolation and identification of morphine 3- and 6-glucuronides, morphine 3,6-diglucuronide, morphine 3-ethereal sulfate, normorphine, and normorphine 6-glucuronide as morphine metabolites in humans. J. Pharm. Sci. 1977;66:1288–1293. doi: 10.1002/jps.2600660921. [DOI] [PubMed] [Google Scholar]
- Zheng M, McErlane KM, Ong MC. Hydromorphone metabolites: isolation and identification from pooled urine samples of a cancer patient. Xenobiotica. 2002;32:427–439. doi: 10.1080/00498250110119090. [DOI] [PubMed] [Google Scholar]
- Zuckerman A, Bolan E, de Paulis T, Schmidt D, Spector S, Pasternak GW. Pharmacological characterization of morphine-6-sulfate and codeine-6-sulfate. Brain Res. 1999;842:1–5. doi: 10.1016/s0006-8993(99)01766-7. [DOI] [PubMed] [Google Scholar]