Abstract
The circadian timing system influences a vast array of behavioral responses. Substantial evidence indicates a role for the circadian system in regulating reward processing. Here we explore time of day effects on drug anticipation, locomotor activity, and voluntary methamphetamine (MA) and food intake in animals with ad libitum food access. We compared responses to drug versus a palatable treat during their normal sleep times in early day (zeitgeber time (ZT) 0400) or late day (ZT 1000). In the first study, using a between-subjects design, mice were given daily 1-h access to either peanut butter (PB-Alone) or to a low or high concentration of MA mixed in PB (MA + PB). In study 2, we repeated the experiment using a within-subjects design in which mice could choose between PB-Alone and MA + PB at either ZT 0400 or 1000. In study 3, the effects of MA-alone were investigated by evaluating anticipatory activity preceding exposure to nebulized MA at ZT 0400 vs. ZT 1000. Time of day effects were observed for both drug and palatable treat, such that in the between groups design, animals showed greater intake, anticipatory activity, and post-ingestional activity in the early day. Furthermore, there were differences among mice in the amount of MA ingested but individuals were self-consistent in their daily intake. The results for the within-subjects experiment also revealed robust individual differences in preference for MA + PB or PB-Alone. Interestingly, time of day effects on intake were observed only for the preferred substance. Anticipatory activity preceding administration of MA by nebulization was also greater at ZT 0400 than ZT 1000. Finally, pharmacokinetic response to MA administered intraperitoneally did not vary as a function of time of administration. The results indicate that time of day is an important variable mediating the voluntary intake and behavioral effects of reinforcers.
Keywords: Methamphetamine, Circadian, Anticipatory behaviors, Motor activity, Time of day, Time of day
1. Introduction
The circadian timing system has a pervasive influence in that it modulates numerous behavioral and physiological responses, including the response to natural and drug reinforcers (Hasler et al., 2012). Indeed, for several types of reinforcers the pharmacological, physiological, and behavioral effects vary as a function of time of administration or availability over a 24-h cycle (Falcon and McClung, 2009; Webb et al., 2009a). These rhythms persist under constant conditions (Terman and Terman, 1975; Kosobud et al., 1998), suggesting that they are under endogenous circadian control by the brain clock located in the suprachiasmatic nucleus of the hypothalamus. Although there have been studies investigating the influence of time of day on the behavioral responses to drugs of abuse, surprisingly methamphetamine (MA) has received limited experimental attention. In humans, time-of-day effects may influence acute subjective, cognitive, and adverse effects of MA.
Data from participants in a prior experiment in our laboratory suggest that time of day influences of the euphoric effects of MA. When participants received MA at 0115 h, ratings of “good drug effects” were similar across low and moderate doses (5 versus 10 mg) (Hart et al., 2003). In contrast, unpublished data from this experiment reveal that when the same participants received 5 mg MA at 0915, their ratings of “good drug effects” were indistinguishable from ratings for placebo [Fig. S1.a (data) and b (study design)], (Supplementary material; Hart et al., 2003). While this experiment was not designed to examine the influence of time of day, the results do raise a question about how such an effect might influence drug self-administration: a question optimally addressed in studies of laboratory animals.
Although there have been few studies of time-of day effects of MA in humans (Shappell et al., 1996), diurnal variations in response to amphetamines have been reported in laboratory animals using a variety of procedures including operant avoidance, sensitization, tolerance, general activity, conditioned place preference (CPP), and stereotypic behavior (Arvanitogiannis et al., 2000; Evans et al., 1973; Gaytan et al., 1998a,b; Gaytan et al., 1999; Kuribara and Tadokoro, 1982, 1984; Martin-Iverson and Iversen, 1989; Uchihashi et al., 1994; Urba-Holmgren et al., 1977; Webb et al., 2009b). Overall, these data show greater drug effects around dawn compared to dusk. However, to our knowledge, there has been no prior attempt to explore the impact of diurnal variations on self-administration of amphetamines.
The goal of the present experiment was to examine time of day effects on reinforcer intake, associated behaviors, and pharmacokinetics. A second goal was to compare time of day effects of two different reinforcers, specifically a palatable treat, peanut butter (PB-Alone), versus drug, methamphetamine (MA) mixed in peanut butter (MA + PB). We also investigated dose–response relationships and individual differences in these behaviors. Finally, we sought to explore changes in these behaviors over time. To investigate these questions, we used a paradigm involving voluntary intake, thereby allowing for simultaneous measurement of anticipatory behaviors, self-administered voluntary intake of drug and/or palatable treat, and locomotor activity. This paradigm is analogous to voluntary human drug use, and does not require surgical implantation of an indwelling catheter for acquisition of self-administration data. Further, because the mice are provided with food ad libitum they have very low activity levels during the day, allowing assessment of responses to reinforcers against low baselines (Mistlberger, 1994; Escobar et al., 2011). In Protocol 1, we used a between-subjects design to compare the behavioral responses to drug and/or palatable treat in the early versus late day. In that work, we noted that marked individual differences in MA intake with self-consistent responses over the course of the experiment. Protocol 2 used a within-subjects design, thereby permitting more detailed measurement of individual differences in intake and time of day effects. To isolate the effects of MA from PB, in Protocol 3 we investigated time of day effects on anticipatory activity associated with nebulized MA. Finally, to assess time of day effects on pharmacokinetic factors, in Protocol 4 MA was injected at several times of day and serum measurement of the drug were taken for the subsequent 4 h.
2. Methods and materials
2.1. Animals and housing
Adult male C57BL/6 N mice 6 weeks of age, weighing an average of 22 g (range 16°26 g) at the beginning of each experiment were subjects (Charles River, Wilmington, MA). Mice were housed individually in transparent polycarbonate cages (32 × 14 × 13 cm), equipped with a running wheel (diameter, 11 cm) placed in sound attenuating, ventilated chambers (Phenome Technologies Inc. Lincolnshire, IL). The room was maintained at 23 ± 2 °C and 72% humidity. Standard mouse chow(Purina, St. Louis, MO) and water were available ad libitum except as noted. The experimenter changed cages every two weeks, and the data from the 24 h following a cage change were not included in the analyses. For Protocols 1 and 2, body weight was taken on the first and last day of each experiment. For Protocol 3, to measure anticipation to nebulized MA, a skeleton photoperiod with lights on ZT 0000–0030 and ZT 1130–1200 was used to avoid the masking effects of light on activity. Nebulization was performed under dim red light (1 lx) illumination at ZT 0400–0415 or ZT 1000–1015. For all experiments, animals were adapted to a 12:12 light:dark cycle (200 lx), with lights off at zeitgeber time 1200 (ZT 1200) and on at ZT 0000 for 14–16 days before the start of the experiment. Animals were cared for in accordance with the Columbia University Institutional Animal Care and Use Committee and Animal Welfare regulations.
2.2. Preparation of drugs
For Protocols 1 and 2, stock solutions of MA hydrochloride (Sigma-Aldrich Inc., St. Louis, MO) were prepared at two concentrations as follows: MA (34 or 68 mg) was added to distilled water (30 ml) to create 1.13 mg/ml and 2.26 mg/ml. PB was commercially available (Jif® Brand, Creamy Peanut Butter). Each animal was assigned its own Petri dish (BD Falcon, 35 × 10 mm tissue culture dish) for the duration of the study. For use, 1.00 ± 0.01 g PB was placed in the center the dish.MA stock solution or water (40 µl of 0, 1.13, or 2.26 mg/ml MA) was mixed thoroughly in the PB to create 45 or 90 µg/g MA + PB or PB-Alone. Ingestion of the entire mixture yields a dose of 2 mg/kg and 4 mg/kg, based on the mean initial body weight of 22 g/mouse. For Protocol 3 the concentration of MA was 0.4 and 1.0 mg/ml. For Protocol 4, body weight was measured immediately prior to injection of 2 mg/kg MA.
2.3. Protocol 1: Study design
2.3.1. Experimental groups
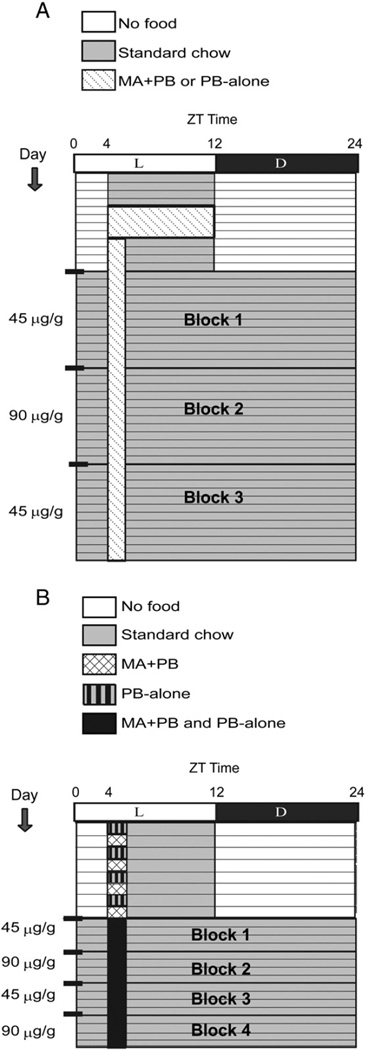
Animals were placed in one of four groups (N = 10/grp) differentiated by the time, either early (ZT 0400) or late day (ZT 1000) at which they were given access to either PB-Alone or MA + PB. The training and testing intervals are shown schematically for the ZT 0400 group in Fig. 1A. During an initial 4 day training period, animals were acclimated to food restriction conditions by giving them access to standard chow for 8-h/day from either ZT 0400–1200 or ZT 1000–1800. On days 5–8, animals were provided MA + PB (3 g PB mixed with 45 µg MA) or PB-Alone (3 g PB) for the same 8-h intervals. On days 9–12, they received 1-h access to 1 g of either 45 µg/g MA + PB or PB-Alone, followed by 7-h access to standard chow (ZT 0400–1200 or ZT 1000– 1800). On days 13–23 (Block 1), mice had ad-libitum access to chow, and daily 1-h access to 1 g of 45 µg/g MA + PB or PB-Alone at ZT 0400–0500 or ZT 1000–1100 continued. On days 24–34 (Block 2), the concentration of MA was doubled to 90 µg/g MA + PB, and subsequently returned on days 35–45 (Block 3) to 45 µg/g MA + PB.
Fig. 1.
Schematics depict the design for the Protocol 1 (A, between-subjects design) and Protocol 2 (B, within-subjects design). Each horizontal line represents one day. The black and white bar across the top of the schematic shows the light:dark cycle. The legend indicates times of no food availability (white), standard chow (gray) and PB-Alone and/or MA + PB availability (pattern). The concentration of MA + PB available during each block of the study is depicted on the left. As shown, in Protocol 1 there were three 11-day blocks of 1-h daily access to either PB-Alone or MA + PB, while in Protocol 2 there were four 4-day blocks of 1-h daily access to both PB-Alone and MA + PB.
2.3.2. Measures
The behavioral measures assessed were amount eaten, MA intake, anticipatory activity, activity after ingestion, and total daily activity. For determination of amount eaten, the experimenter was in the room for 20 min for placement and removal of Petri dishes. MA intake is reported in mg/kg body weight, adjusted for interpolated daily individual weight gain. Body weight on the last day of the study (day 45) averaged 28 g (range 24–31 g).Wheel running was monitored continuously using a computer-based data acquisition system, VitalView (Minimitter, Bend, OR, USA) and was quantified in 10 min bins across the 24 h day using Actiview(MiniMitter) and Excel (Microsoft). Activity was normalized within each animal to control for wheel resistance and was calculated by dividing the sum of activity counts in each activity measure by the number of wheel revolutions per bin averaged over 24 h. Anticipatory activity was defined as the average number of wheel revolutions in the 2-h prior to reinforcer access (ZT 0200–0400 or ZT 0800–1000). Activity after ingestion was the average wheel revolutions in the 2 h after the start of food access (ZT 0400–0600 or ZT 1000–1200).
2.3.3. Data analysis
Data was analyzed using a linear mixed model with both fixed and random effects in SAS (SAS Institute Inc., Cary, N.C., USA). One animal died during the first four days of training and data for this animal was not used. After removing outliers (or 2 s.d. from mean), observations from the last 10 days of each 11-day block were averaged for each animal (Total observations = 117). Independent variables included time of day (ZT 0400, ZT 1000), treatment group (PB-Alone,MA + PB), concentration (45, 90 µg/g MA + PB), and block (1, 2, and 3). Animal identity was analyzed as a random effect. Analyses were conducted in two ways: first by time of day, concentration, and block, and second by time of day, treatment group and block. To assess the possibility that the presence of the experimenter influenced the activity of the animals, activity after ingestion was analyzed including and excluding the first 20 min of access (ZT 420–600 and 1020 to 1200). No differences were found. We also examined the relationship between MA intake and activity after ingestion with a correlation using data from all 33 days of the experiment for each animal that received MA + PB (Total observations = 660). Finally, we examined the relationship between time of day and amount of activity after ingestion by analyzing all cases in which animals ingested comparable amounts of MA in early and late time points (0.55–0.75 mg/kg MA; ZT 4, N = 57 and ZT 10, N = 68; independent t test).
2.4. Protocol 2: Study design
In a pilot study, we conducted a taste aversion study to see whether animals would eat MA + PB when PB-Alone was also available. Here, drug-naïve animals that had access to food for 6 h/day were presented with both PB-Alone and MA + PB for 15 min. Mice ate from both dishes, indicating that the taste of MA in PB was not aversive.
2.4.1. Experimental groups
Because substantial individual differences among mice were found in the foregoing between subjects study, we next examined individual differences in a within subjects design. Here, mice received 8 days of food restriction training and 16 days of 1-h access to MA + PB and PB-Alone during ad libitum food availability, for a total of 24 days of the experiment. Animals were placed into two groups (N = 10/grp) differentiated by the time of day (ZT 0400 or ZT 1000) at which they were given a choice between one Petri dish containing MA + PB and another containing PB-Alone (Fig. 1B, shows the ZT 0400 group). During the training period, either MA + PB or PB-Alone was presented on alternate days for 1-h daily followed by 7-h access to standard chow. Here, on 1 day, a dish of MA + PB was placed one side of the home cage and on the next day, a dish of PB-Alone was placed on the opposite side, and so on. In order to create distinct environments within the home cage, vertical and horizontal striped paper was taped to the outside of the cage near the placement of each dish with the pattern held constant for each animal and counter-balanced across animals. For the experimental period, animals were given ad libitum access to standard chow and 1-h access to both dishes for 16 days with stripe patterns present, in four 4-day blocks of alternating concentrations of 45 or 90 µg/g MA + PB.
2.4.2. Measures
Some of the behavioral measures were identical to the first experiment, as follows: amount eaten (amount of MA + PB and of PB-Alone), MA intake, anticipatory activity, activity after ingestion, and total daily activity. In addition, MA preference and first approach were assessed. MA preference was defined as the percent MA + PB eaten out of the overall amount eaten each day [(MA + PB / (MA + PB + PB-Alone) * 100)]. First approach was defined as the dish eaten from first. The calculation of MA intake was adjusted for individual weight gain. On the last day of the study (day 24), average body weight of the mice was 25 g (range 22 to 27 g).
2.4.3. Data analysis
Data was analyzed using a linear mixed model in SAS with observations from the last 3 days of each 4-day block averaged for each animal, as in the first study. During Block 1 intake was 50% higher than in other blocks, likely due to the preceding period of food restriction, and this interval was omitted from further analysis (Total observations = 45).One animal died during the study and this data was not used. Independent variables included time of day (ZT 0400, ZT 1000), concentration (45, 90 µg/g MA + PB), and block (2, 3, and 4).
To categorize animals into MA- or PB-preferring groups, we calculated the MA + PB eaten/total amount eaten, for the last 12 days of the study (Blocks 2–4). The data were applied a one sample t-test to compare individuals' average preference to that expected by chance (no-preference or 50%). This yielded four animals with no preference, 8 preferred MA + PB and 7 preferred PB-Alone. Using these categories, we evaluated intake and activity of the MA + PB and PB-Alone preferring groups at each time of day for blocks in which at least 60% of the intake was from one dish. Finally, to explore whether time of day influenced amount of activity after ingestion, we analyzed cases in which meal size was kept within a narrow range — cases in which animals ingested 0.6–0.8 mg/kg MA (ZT 4, N = 20 and ZT 10, N = 13; independent t test). We also examined the relationship between MA intake and activity after ingestion by correlating these measures using data from all 16 days of the experiment for each animal (Total observations = 240).
2.5. Protocol 3: Study design
In a pilot study, we confirmed that nebulized MA increased wheel running activity – measured as in Protocol 1 – (F(2, 13) = 5.846, p = .017: water = 1.94 ± 1.27; 0.4 mg/ml MA = 9.46 ± 3.50; 1 mg/ml MA: 25.98 ± 5.99). A dose of 1 mg/ml nebulized MA was selected as it elicited a similar amount of activity as IP injection of 4 mg/kg MA (1 mg/ml nebulized MA: 25.98 ± 5.99; 4 mg/kg MA (IP): 19.77 ± 4.42).
2.5.1. Experimental groups
To measure anticipation of MA, animals were placed in one of four groups (MA: N = 6/grp; Control: N = 3/grp) differentiated by the time of day (ZT 0400, 1000) at which they were administered 1 mg/ml nebulized MA for 15 min at 10:00–10:15 AM for 16 consecutive days. Animals were adapted to the nebulization chamber (chambers: Brain Tree Scientific, Braintree, MA, catalog # MPC-3 AERO; Nebulizer: Briggs Medical Service Company, Waukegan, IL, catalog# 40-370-000) for 4 days at ZT 0400 for 15 min while nebulized with water. Then they were undisturbed for 2 days, received 0.4 mg/ml nebulized MA for 2 days, undisturbed for 2 days, received 1.0 mg/ml MA for 2 days.
2.5.2. Measures
Wheel running activity was collected and normalized as described in Protocol 1. Anticipatory activity was defined as the average number of wheel revolutions in the 2-h prior to nebulization (ZT 0200–0400 or ZT 0800–1000).
2.5.3. Data analysis
To test the effect of time of day on anticipatory activity in response to nebulized MA, data was analyzed using a two way ANOVA.
2.6. Protocol 4: Study design
2.6.1. Experimental groups
Animals were placed in one of four groups (N = 6/grp) differentiated by the time of day (ZT 0200, 0800, 1400, and 2000) at which they were administered a single injection of 2 mg/kg methamphetamine (IP). Six blood samples were taken: baseline, 15 min, 30 min, 1-h, 2-h, and 4-h post-injection.
2.6.2. Measures
Blood samples were collected via the lateral saphenous vein. For each time point, samples were collected into Microvette capillary tubes (Sarstedt, Numbrecht, DE) and allowed to clot. They were then centrifuged (Eppendorf centrifuge 5417r) at 14,000 rpm for 15 min at room temperature. Serum was removed from the centrifuged sample and frozen at −80 °C until analysis. MA levels were quantified in duplicate with sensitivity at 1 ng/ml by commercially available competitive enzyme linked immunosorbent assay (ELISA/EIA) kits from BioQuant, Inc. (San Diego, CA), following manufacturer's instructions. A 1 mg/ml MA standard purchased from Cerilliant (Round Rock, TX) was serially diluted to create standards (ranging from 2 µg/ml to 5 ng/ml) which were used for all analyses. Test samples were balanced across assay plates so that each plate had the same number of animals from each time of administration. Samples within each animal were kept together on a single assay plate.
2.6.3. Data analysis
To assess effects of ZT administration on serum levels of MA, a mixed-factor repeated measures analysis of variance (RMANOVA) was conducted with ZT time of administration as the between-subjects factor and blood sample time as the repeated measure. This analysis was conducted on raw serum [MA] and normalized values for each time-point (percent maximum blood conc.), with no difference in the results. For pharmacokinetic estimates, half-life values were calculated in GraphPad Prism software (San Diego, CA) by fitting the equation for one-phase exponential decay to the data. Area under the curve (AUC), a measure of gross drug effect, was also calculated in GraphPad Prism software using the trapezoid method. To assess whether ZT time of administration affected primary pharmacokinetic measures (half-life, AUC, time to peak serum concentration (Tmax), and peak concentration (Cmax)), data was analyzed using a one-way ANOVA.
3. Results
3.1. Protocol 1
3.1.1. Amount eaten
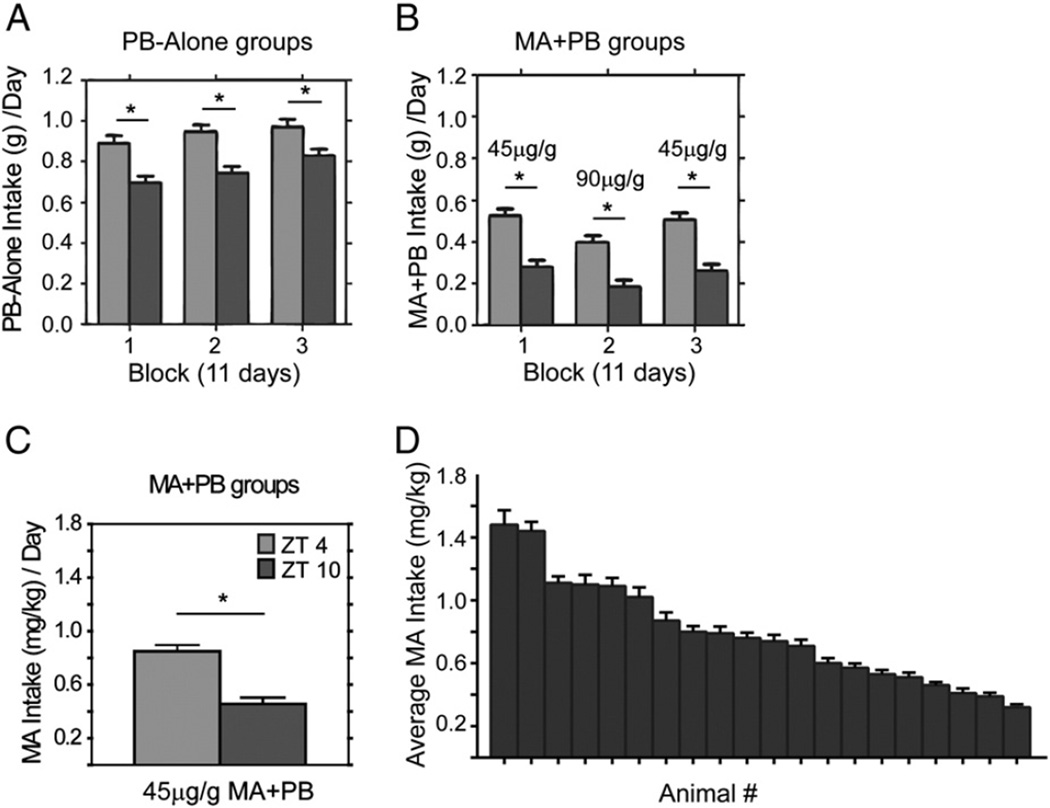
There was a substantial effect of time of day, treatment, MA concentration, and block on the amount of PB and MA + PB eaten (Fig. 2A,B; smallest main effect F2,70 = 11.91, p < 0.001). It is noteworthy that in all cases, animals ate more at ZT 0400 than ZT 1000 in both PB-Alone and MA + PB groups (~30 and 50% difference, respectively). The MA + PB groups ate less of the mixture each day than did the PB-Alone groups. In addition, the MA + PB groups ate less of the mixture when the MA concentration was increased and this returned to prior levels when the lower MA concentration was reinstated (Fig. 2B). In contrast, the PB-Alone groups steadily increased their consumption over blocks of trials (Fig. 2A). Interestingly, animals in both groups were self-consistent in amount eaten, with 27% of the variability in the data ascribed to individual differences (t38 = 7.12, p < .0001, estimate = 27.29 ± 3.8).
Fig. 2.
Upper panels: Bar histograms show amount of PB eaten for the PB-Alone (A) and MA + PB (B) groups over serial blocks of time in Protocol 1. Lower panel: The bar histogram shows average MA intake at the low concentration of MA (C) and MA intake averaged over both concentrations for individuals, with animals ordered from highest to lowest average intake. (D). * indicates significant differences between ZT 0400 and ZT 1000. All post hoc t-tests are p < .01.
3.1.2. MA intake
The amount of MA ingested was significantly influenced by time of day and drug concentration (smallest F value of F2,70 = 4.44, p = 0.015). Animals ingested more MA at ZT 0400 than ZT 1000 (Fig. 2C, 45 µg/g corresponding to 2 mg/kg, t70 = 5.79, p < 0.001; 90 µg/g corresponding to 4 mg/kg; t70 = 9.55 p < 0.001, data not shown) and when the higher concentration was available (ZT 0400; t70 = 16.07, p < .0001; ZT 1000; t70 = 5.87, p < .0001, data not shown). There was a significant interaction between time of day and concentration (F2,70 = 28.01, p < 0.001), with a greater increase in MA intake at ZT 0400 when the higher concentration was introduced. Interestingly, as was the case for PB intake, there were marked individual differences among animals, but mice were self-consistent in MA intake (Fig. 2D) and this was true for each concentration (4 mg/kg; t18 = 6.0 p < 0.001; 2 mg/kg t18 = 5.82, p < 0.001). Individual differences accounted for 62% of the variability in the data (t38 = 12.29, p < .0001, estimate = 62.43 ± 5).
3.1.3. Activity
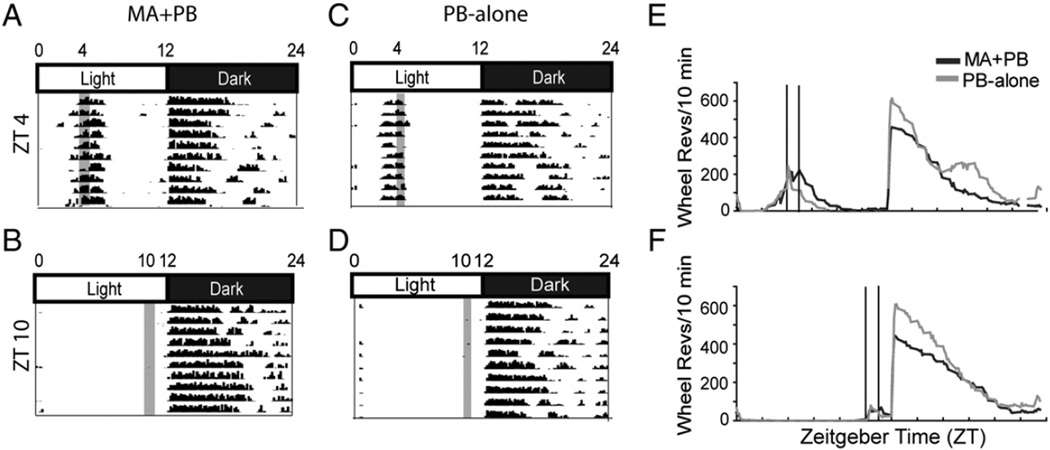
Several aspects of activity were tracked, including overall activity of each group, anticipatory activity prior to reinforcer availability, and activity following reinforcer access. Fig. 3 shows wheel-running activity for representative MA + PB and PB-Alone animals from ZT 0400 and 1000 groups (Fig. 3A–D) and for these groups as a whole (Fig. 3E, F). It is evident in these records that anticipatory activity occurred at ZT 0400 but not at ZT 1000 (Fig. 3E versus F). Furthermore, there was more activity after ingestion at ZT 0400 than ZT 1000 (Fig. 3E versus F).
Fig. 3.
For representative individual animals, actograms depict locomotor activity of each of the four experimental groups: MA + PB at ZT 0400 (A) and ZT 1000 (B), and PB-Alone at ZT 0400 (C) and ZT 1000 (D) during the first block of the study, while group averages are shown on the right (E, F). For the actograms, time of day and light:dark cycles are shown across the top. Data are plotted in 10 min time bins on the X-axis and the height of the black mark reflects the accumulated number of wheel revolutions for consecutive bins. Consecutive days are shown on the Y axis. The time of reinforcer availability is given by the gray shaded bar (A–D) or the vertical line (E, F).
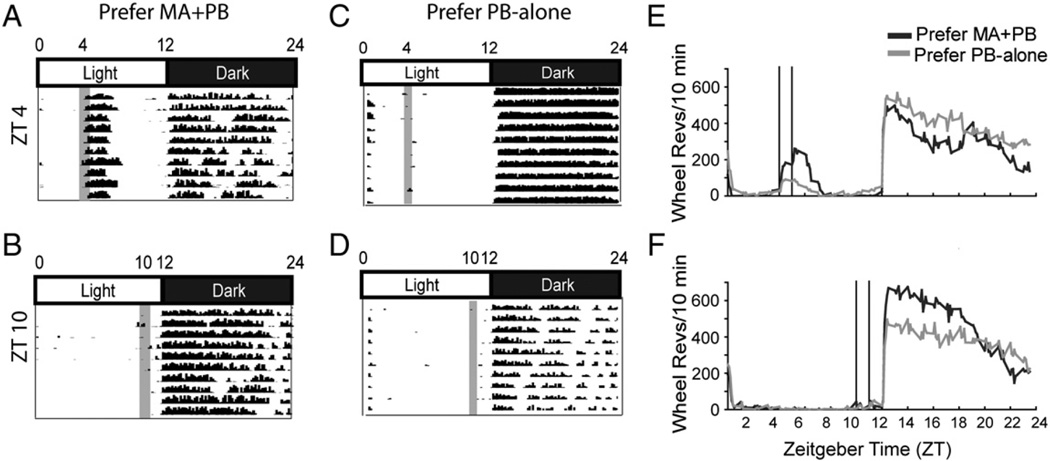
Fig. 6.
For representative individual animals, actograms depict locomotor activity of each of the groups: MA preferring at ZT 0400 (A) and ZT 1000 (B), and PB preferring at ZT 0400 (C) and ZT 1000 (D) during the third block of the study, while group averages are shown on the right (E, F). For the actograms, time of day and light:dark cycles are shown across the top. Data are plotted in 10 min time bins on the X-axis and the height of the black mark reflects the accumulated number of wheel revolutions for consecutive bins. Consecutive days are shown on the Y axis. The time of reinforcer availability is given by the gray shaded bar (A–D) or the vertical line (E, F).
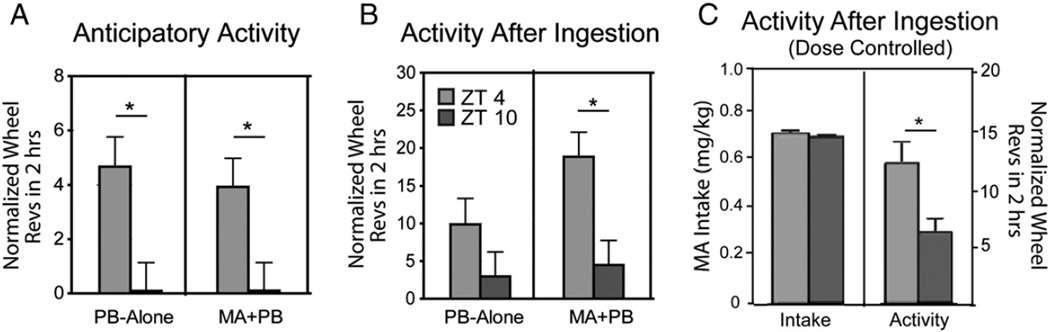
There were no significant main effects of time of day, treatment, or concentration on total daily activity (data not shown). On the other hand, anticipatory activity was substantially influenced by time of day and block (Fig. 4A; smallest F value, F2,70 = 3.26, p = 0.04). More specifically, six animals from each group at ZT 0400 exhibited anticipatory activity, while none did so at ZT 1000 (PB-Alone group; t70 = 3.02, p = .003; MA + PB group; t70 = 2.6, p = .01).
Fig. 4.
Bar histograms show anticipatory activity (A), activity after ingestion (B) and activity after ingestion at comparable intake of MA (C). * indicates significant differences between ZT 0400 and ZT 1000 within each frame. All post hoc t-tests are p < .05.
Activity after ingestion was significantly affected by time of day with more activity at ZT 0400 than at ZT 1000 (Fig. 4B; F1,70 = 11.43, p = 0.001). However, this difference only reached significance in the MA + PB group (t70 = 3.11, p = .002). There was a significant correlation between MA intake and activity after ingestion at each time of day (R660 = 0.362, p < .0001), though at ZT 1000, the animals showed relatively little activity after MA treatment. To assess whether this was due to differences in intake, we examined data for days where comparable amounts of MA were ingested at ZT 0400 and 1000. Even when equivalent amounts of MA were ingested, there was more post ingestion activity at ZT 0400 versus 1000 (Fig. 4C right panel; t123 = 2.84, p = .005).
3.2. Protocol 2
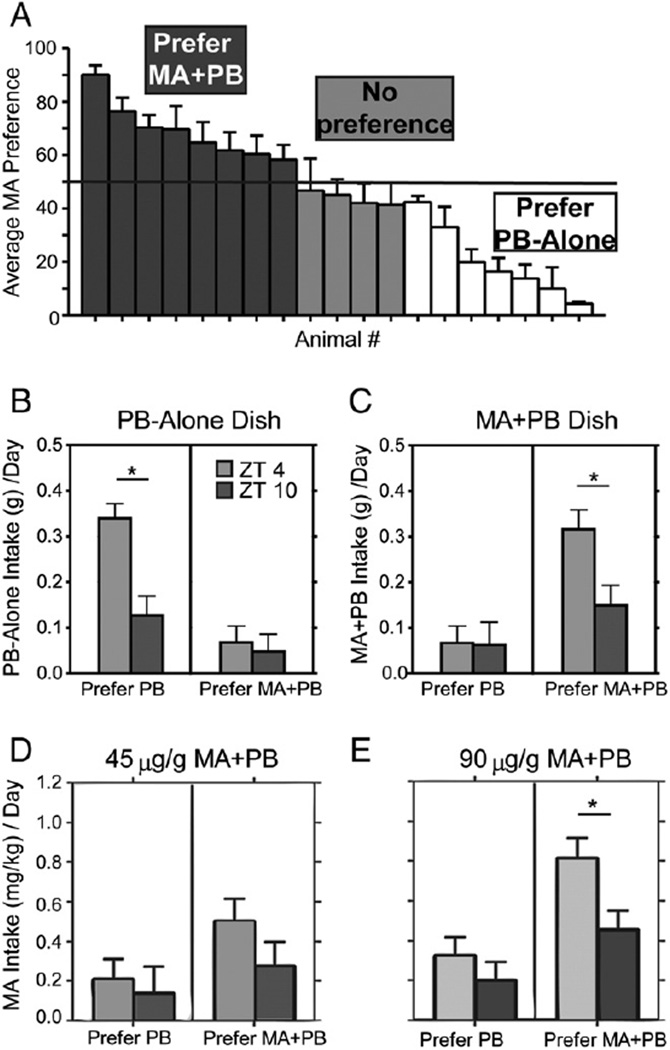
3.2.1. Preference
As in Protocol 1, at each concentration individuals were consistent in their preference for either MA + PB or PB-Alone, with the random effect of subjects accounting for 48% of the variability in preference scores (Fig. 5A; t18 = 3.68, p = 0.0019, estimate = 48.2 ± 13.1). Assessment of preferred reinforcer eaten/total eaten indicates that both MA + PB and PB-Alone preferring animals ate a greater percentage of the preferred substance at ZT 0400 than at 1000 (preference by time of day interaction, F1,16 = 6.76, p = 0.019). This difference reached significance only for the MA + PB group at the higher concentration (t16 = 2.14, p = .05, data not shown).
Fig. 5.
Top panel: Bar histograms show MA preference for individual animals, ordered from highest to lowest (A). Middle panels: Bar histograms show group averages for PB-Alone intake (g) (B), and MA + PB intake (g) (C) at the low concentration of MA. Bottom panels: The bar histograms show MA intake (mg/kg) at the low (D) and high (E) concentration of MA. * indicates significant differences between ZT 0400 and ZT 1000 within each frame. All post hoc t-tests are p < .05.
3.2.2. Amount eaten
While there was no effect of MA concentration, there were main effects of time of day, preference, and preference by time of day interactions for both intake of PB-Alone or MA + PB (Fig. 5B; smallest F value, F1,16 = 8.64, p < 0.01 and Fig. 5C; smallest F value, F1,16 = 4.37, p = 0.05 respectively). The time of day effect on amount eaten was observed only for the preferred substance; PB-Alone intake was greater at ZT 0400 in the PB-preferring group but not in the MA + PB preferring group (left versus right panel of Fig. 5B; data shown for 2 mg/kg: t16 = 3.98, p = .0007; 4 mg/kg: t16 = 4.02, p = .0007). MA + PB intake was also greater at ZT 0400 only in the MA + PB preferring animals (left versus right panel of Fig. 5C; data shown for 2 mg/kg: t16 = 2.71, p = .014; 4 mg/kg: t16 = 2.43, p = .024).
3.2.3. MA intake
There was a main effect of preference group on MA intake (Fig. 5D, E; F1,16 = 14.63, p = 0.001). Animals also ingested larger amounts of MA at ZT 0400 than ZT 1000, and MA intake increased with concentration, but these main effects did not reach significance (time of day, F1,16 = 3.85, p = 0.067; concentration, F1,16 = 4.22, p = 0.057). MA intake was significantly higher at ZT 0400 in the MA + PB preferring animals when the higher concentration was available (right panel of Fig. 5E; t16 = 2.57, p = .018).
3.2.4. First approach
Animals approached their preferred substance first (F1,16 = 61.57, p < 0.0001).
3.2.5. Activity
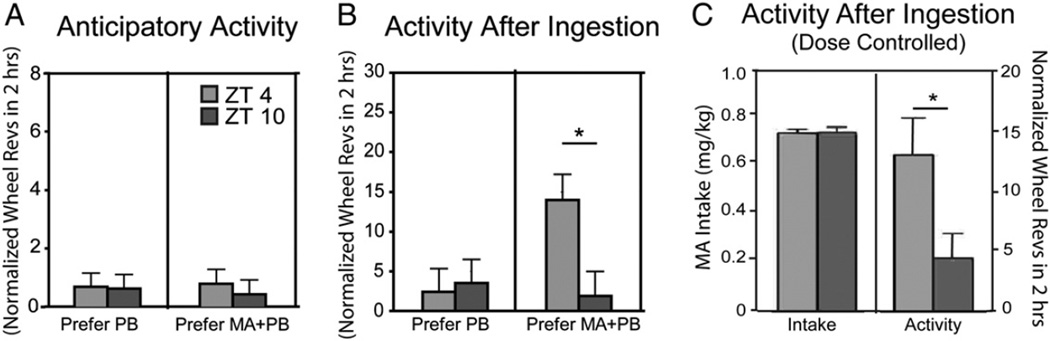
As evident from the actograms for individual animals (Fig. 6A–D) and in the group data (Fig. 6E, F), anticipatory activity was not seen at either ZT 0400 or 1000. The amount of activity following ingestion was greater at ZT 0400 as in Protocol 1.
As in Protocol 1 total daily activity was not affected by time of day or preference group (data not shown), but in contrast to the first study, animals did not show anticipatory activity (Fig. 7A).
Fig. 7.
Bar histograms show anticipatory activity (A), activity after ingestion (B) and activity after ingestion at comparable intake of MA (C). * indicates significant differences between ZT 0400 and ZT 1000 within each frame. All post hoc t-tests are p < .05.
As in Protocol 1, in activity after ingestion there were main effects of preference group and preference × time of day; (Fig. 7B; smallest F value was F1,16 = 11.86, p = 0.003). Time of day effects on activity were observed in MA-preferring animals (right panel, Fig. 7B; t16 = 2.7, p = .014), and there was a correlation between amount of MA ingested and amount of activity after ingestion (R240 = 0.264, p < 0.001). Greater activity at ZT 0400 cannot be attributed to greater MA intake as time of day effects were significant when MA intake (0.6 to 0.8 mg/kg) at ZT 0400 and 1000 was taken into account (Fig. 7C; t31 = 2.06, p = .048).
3.3. Protocol 3
3.3.1. Anticipatory activity
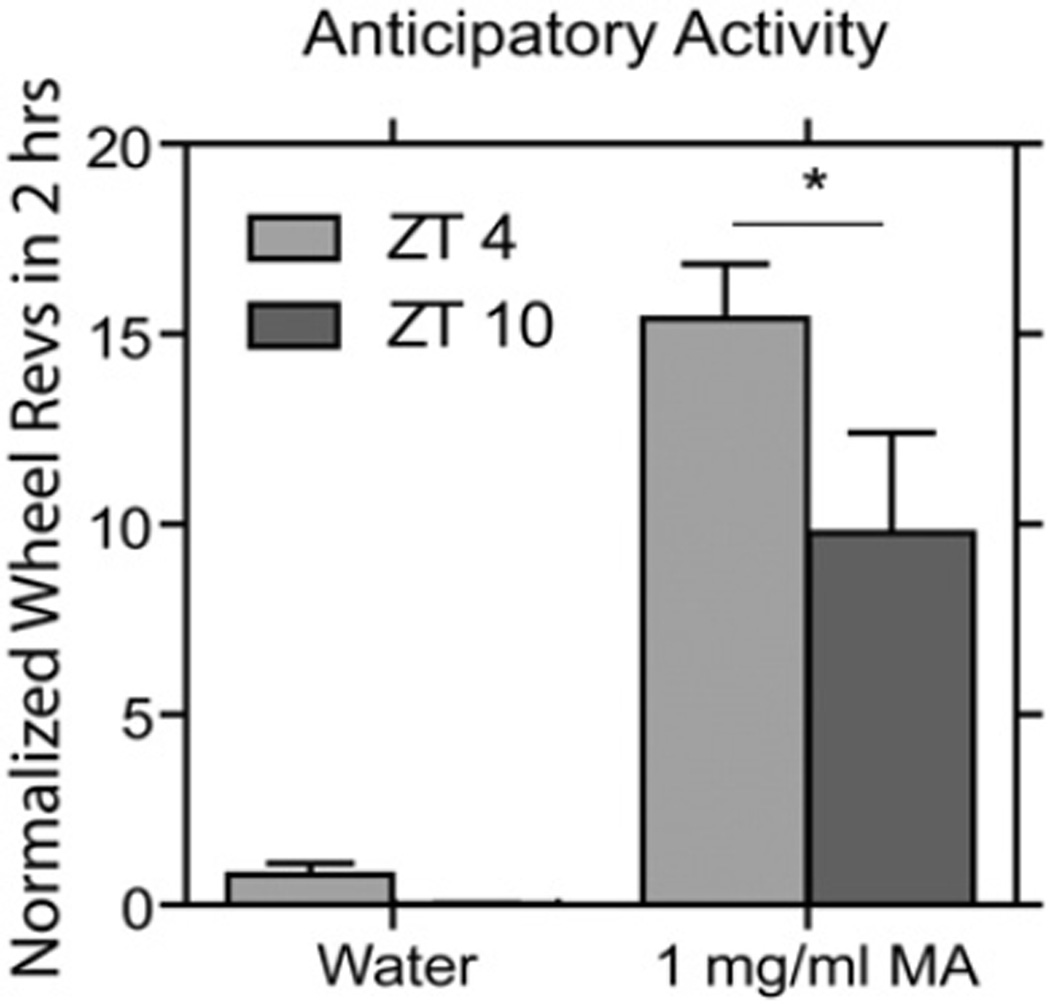
Animals showed anticipatory wheel running before nebulized MA but not water administration (F(1, 17) = 30.418, p < .001). Anticipatory activity was significantly higher at ZT 0400 than at ZT 1000 (Fig. 8; t10 = 1.869, p = .045).
Fig. 8.
Bar histograms show mean (+SEM) anticipatory activity levels of all animals nebulized at ZT 0400 or ZT 1000 with water or 1 mg/ml MA (*p < 0.05 vs. water; #p < 0.05 vs. MA ZT 1000).
3.4. Protocol 4
3.4.1. Serum MA concentration
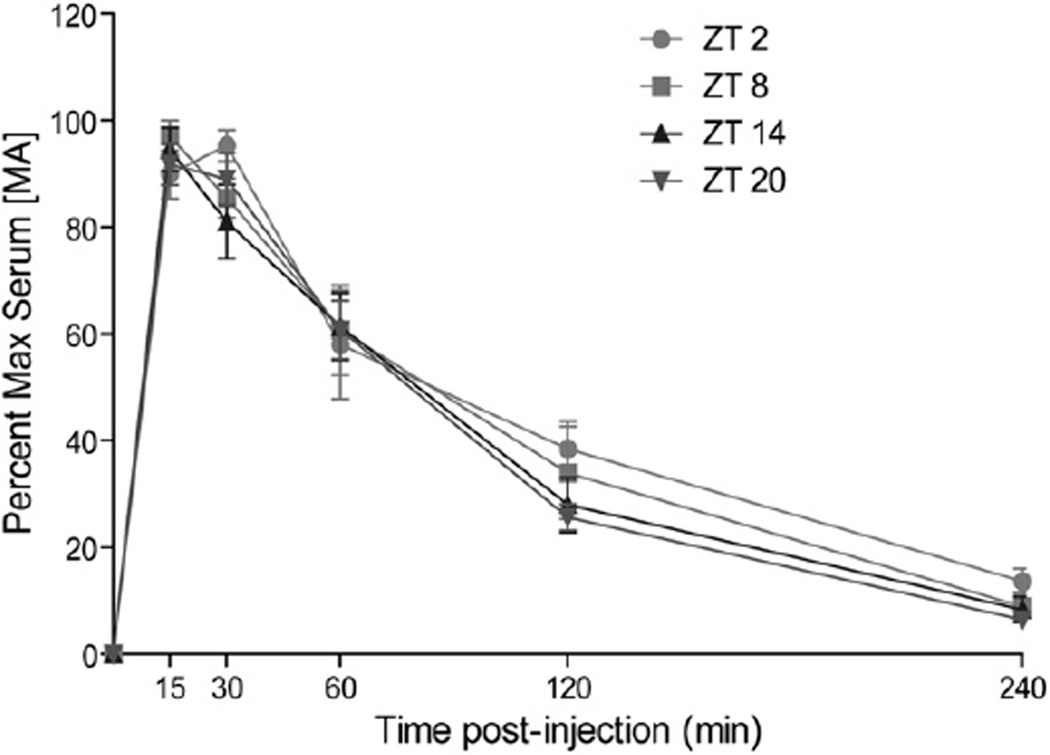
Fig. 9 displays normalized serum [MA] up to 4 h after 2 mg/kg MA administration. A significant, time-dependent decline in [MA] was observed for all groups (F(4, 40) = 22.185, p = 0.000). There was no significant main effect of ZT time-of-day (F(3, 116) = 0.879, p = 0.47) or interaction between ZT and blood sample time.
Fig. 9.
Line graph shows serum [MA] up to 4 h after 2 mg/kg MA administration (IP) at ZT 0200, 0800, 1400, and 2000. There were no significant effects of ZT time of administration on serum [MA].
3.4.2. Pharmacokinetic parameters
There were no significant main effects of ZT time of administration on half-life, AUC, Cmax, or Tmax (largest F value, F(3, 116) = 1.440, p = 0.257).
4. Discussion
A challenge in the development of animal models of drug use is to assess intake in a way that models human drug use as closely as possible. Some studies of diurnal variations in drug intake and effects involve access to drugs throughout the light/dark cycle (Deneau et al., 1969; Fitch and Roberts, 1993; Hollingsworth and Mueller, 1988; Lynch and Roberts, 2004; Lynch and Taylor, 2004; Martin-Iverson and Iversen, 1989). Restricted access paradigms, such as the one used here, more closely mimics drug availability for recreational human drug users. Furthermore, the present paradigm permits the experimenter to quantify a variety of measures that depend on free movement, and can be used in either a between- or a within-subjects design.
The present results demonstrate that under controlled conditions, time of day significantly affects reinforcer-related responses, both to palatable food and drug (Protocols 1 and 2) and to drug-alone (Protocols 1, 2, and 3). Further, these differences cannot be attributed to diurnal variations in pharmacokinetics (Protocol 4). Some effects were consistently seen in both the between and within-subjects design. Animals were more responsive to reinforcers in early day (ZT 0400) compared to late day (ZT 1000). Specifically, there were time of day differences in intake, anticipatory activity, and activity after ingestion in both the between (Protocol 1) and the within subjects study (Protocol 2). In the latter, time of day effects in intake were strongest for the preferred substance. Furthermore, robust, self consistent individual differences were observed in both experiments in MA intake and preference.
4.1. Time of day effects on drug intake
Diurnal variations in self-administration have been reported previously for another stimulant, cocaine, in restricted access conditions using indwelling catheters. Rats trained to self-administer cocaine show a diurnal variation in bar presses at low (but not at high) concentrations with more responses in mid-day versus mid-night (Baird and Gauvin, 2000). Furthermore, there is a leftward shift in the dose–response curve when access is restricted to mid-day, with peak rates of responding at lower doses. Rhesus monkeys also self-administered more low doses of cocaine in mid-day compared to the other times tested, namely, the transitions from light:dark and dark:light (Negus et al., 1995). The current results, indicating greater methamphetamine intake in the early versus late day, are consistent with these findings on cocaine. Furthermore, animals ingested low to moderate doses of MA in the present experiment (avg dose, Protocol 1: 0.8 mg/kg, Protocol 2: 0.55 mg/kg). Although it is difficult to extrapolate the reinforcing (or other) effects produced by this dosing range in mice to effects produced in humans, it is noteworthy that 0.2–0.8 mg/kg of methamphetamine has been shown to serve as a reinforcer in humans (Hart et al., 2001; Kirkpatrick et al., 2012). Taken together, these studies indicate that time of day modulates drug intake and may be particularly salient at lower doses of stimulants. While the present experimental design counterbalanced visual cues and drug-paired side across animals, it is possible that the mice had a visual pattern or side bias — factors that should be controlled in future studies.
4.2. TOD effects on MA-induced activity
There is a growing body of evidence supporting circadian and time of day effects following administration of stimulants in a variety of activity measures. Daily methamphetamine injections or chronic consumption in water induces marked changes in activity rhythms that occur independently of the central circadian pacemaker in the suprachiasmatic nucleus (Honma and Honma, 2009). However, experiments that utilized temporally restricted administration of lower doses of amphetamines indicate variations in drug effects over the course of the light/dark cycle, though with differences among reports in peak times of drug effects (Evans et al., 1973; Gaytan et al., 1998a, 1999; Kuribara and Tadokoro, 1982; Urba-Holmgren et al., 1977; Webb et al., 2009b), possibly due to differences in drug and dosing regimen, housing conditions, species and behaviors measured. However, overall, these previous publications show greater drug effects around dawn compared to dusk. The present results indicate that in the early day, animals showed MA + PB-induced activity, but in late day, there were no differences between MA + PB versus PB-Alone activity (Figs. 4B and 7B). This is further consistent with prior reports of amphetamine-induced CPP showing a peak in early day and no difference between saline and amphetamine in late day (Webb et al., 2009b).
4.3. TOD effects on anticipation
In addition to voluntary intake and activity after ingestion, the present self-administration method allowed for measurement of anticipatory activity, an appetitive behavior. We found a robust effect of time of day on the development of anticipatory activity. Specifically, most animals in the early day MA + PB and PB-Alone groups exhibited anticipatory activity while none of the animals in the late day groups displayed anticipatory activity. This effect was significant even when we controlled for amount of intake of PB-Alone and MA + PB. This time of day effect was also seen when MA was administered through nebulization. The absence of anticipatory responses in the within subjects study was unexpected. It is not likely due to change in reinforcer intake, as anticipatory activity was not correlated with intake in Protocol 1 and there were no main effects of treatment (PB-Alone versus MA + PB) or self-administered dose of MA in this measure. The absence of anticipation may be due to the shorter training duration in the within subjects study. While food anticipatory activity can take 3–14 days to develop in food-restricted animals (Mistlberger, 1994), reinforcers such as drugs and palatable treats in freely-fed rodents produce relatively weak anticipatory response that develop slowly over several weeks or not at all (Abe and Rusak, 1992; Angeles-Castellanos et al., 2008; Hsu et al., 2010; Mendoza et al., 2005; Mistlberger and Rusak, 1987; Pecoraro et al., 2002; Verwey et al., 2007; Waddington-Lamont et al., 2007; Webb et al., 2009a).
Anticipatory activity in response to non-nutritive reinforcers is mediated by an SCN-independent oscillator that is theorized to be specific to rewarding stimuli (Webb et al., 2009a). We speculate that this anticipatory activity may be related to anthropomorphic concepts of “craving,” specifically, motivational processes associated with positive affective states of craving (for review of craving, see Littleton, 2000; Siegel, 1999). In this view, the current data suggests that craving and/or motivation for reinforcers may be greater in the early day. However, interpretations of anticipatory activity as a measure of craving must be circumscribed, as appetitive measures in both laboratory animals and humans may have limited predictive for actual drug intake (Drummond et al., 2000; Littleton, 2000). In keeping with these findings, anticipatory activity was not correlated with drug intake in the current experiment. As anticipatory activity is a learned response, these time of day effects may be associated with diurnal variation in reward-related learning. This hypothesis is supported by data from preference measures. Early day groups exhibited significantly more extreme preferences than late day groups, possibly because they were better able to distinguish between the two dishes.
4.4. TOD effects on reinforcer type
Another goal of the experiment was to compare time of day effects in different types of reinforcers. It has been suggested that all rewards engage the mesolimbic dopamine pathway (Wanat et al., 2009). In fact, chronic drug use is sometimes referred to as “hijacking” reward pathways meant to promote evolutionarily adaptive natural rewards. Furthermore, recent data suggests that chronic engagement of natural reinforcers such as highly caloric/palatable foods or sex can cause changes in brain circuitry in reward-related areas and corresponding addiction-like behaviors (Avena et al., 2008; Blum et al., 2000; Johnson and Kenny, 2010; Stice et al., 2010; Trinko et al., 2007). Our results contribute to this literature by indicating that food and drug display similar time of day effects in intake and associated behaviors. Furthermore, within individuals, the early day increase in intake occurred only in the preferred substance, supporting the idea that time of day differences are specifically related to reinforcer value.
A possible caveat in the experimental design used here is that the MA was provided in a PB mixture possibly masking the distinct effects of these reinforcers. The results indicate that animals responded differently to MA + PB compared to PB-Alone (Protocol 1). The amount of PB eaten increased over days of the study (Fig. 2A), while the amount of MA + PB ingested was stable for each concentration (Fig. 2B). That is, animals reduced the amount of PB eaten when MA was present, and they also regulated consumption according to MA concentration. The amount of MA + PB eaten may have been modulated by the anorexigenic effects of MA, by self-regulation of amount of MA intake, and/or by taste aversion. The present results are consistent with both anorexogenic and/or self-regulatory mechanisms. While MA is known to have a bitter taste, the pilot study negated this notion (Methods, Section 2.4.1), and this was confirmed in experimental data indicating that animals with ad-libitum access to food voluntarily ate MA + PB, even when PB-Alone was also available (Fig. 6C).
4.5. Individual differences in inbred mice
Interestingly, although these experiments were conducted in a well documented inbred mouse strain (Zurita et al., 2011; personal communication with Charles Parady, Senior Specialist in Technical Services, Charles River), marked individual differences in preferences were seen. There are a number of possible explanations for robust individual differences including epigenetics, polymorphisms/genetic drift, or gene–environment interactions. Individual differences have been previously reported in inbred mouse strains and this has sparked controversy over possible mechanisms (Blizard et al., 2004, 2005; Griffin et al., 2007; Wahlsten et al., 2006). The mechanism underlying individual differences in inbred mouse strains, particularly with respect to drugs of abuse is a fertile line of study. A caveat in the ingestion study is that non-pharmacological factors such as position bias may account for some of the observed results.
5. Overall conclusions
Taken together, the results indicate that there are robust time of day effects in the voluntary intake and behavioral response to food and drug. In Protocols 1 and 2, the greatest intake occurred in the early light phase, regardless of reinforcer type. In parallel, there was more activity in the early day in both protocols. In Protocol 1, both reinforcers elicited an anticipatory response only in the early light phase, and the effect on activity held up when we controlled for meal size. Furthermore, this time of day effect was replicated when MA was administered through nebulization. MA-induced activity was also greater in the early day in both Protocols 1 and 2. When we controlled for MA dose, activity remained greater in the early day indicating greater potency. Finally, pharmacokinetic data indicated that time-of-day effects in behavior cannot be attributed to diurnal variations in the metabolism of MA.
The effects of the two reinforcers differed in several aspects. There was more post-ingestion activity and less total PB intake following MA ingestion compared to PB-Alone. The amount of PB eaten remained stable at the two different MA concentrations, while the PB intake increased over time in the PB-Alone group. Further, when given a choice, most animals developed a consistent preference for either MA + PB or PB-Alone. Taken together, we conclude that MA + PB intake was regulated differently from PB intake.
Overall, the present design, allowing the study of temporally restricted, voluntary intake in animals during the inactive phase enables a reasonable parallel to this aspect of human drug use where access to drugs is restricted to the night, or the inactive phase for humans. This has long been considered due to matters of convenience and privacy; however, the present results indicate that drug effects vary by time of day, and, this may importantly modulate drug-taking behaviors and drug effects.
Acknowledgments
We thank Aabir Das and Dr. Matthew Butler for technical assistance and helpful discussions. This work was supported primarily by the Psychology Department of Columbia University (to CLH and RS) and secondarily by NIH grants 37919 and MH075045 (to RS) and by an Undergraduate Summer Research Fellowship from The American Physiological Society (MRR) and from Barnard College.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.pbb.2013.05.011.
Disclosure of financial interests and potential conflicts of interest
None of the authors have biomedical financial interests or potential conflicts of interest.
References
- Abe H, Rusak B. Anticipatory activity and entrainment of circadian rhythms in Syrian hamsters exposed to restricted palatable diets. Am J Physiol. 1992;263:R116–R124. doi: 10.1152/ajpregu.1992.263.1.R116. [DOI] [PubMed] [Google Scholar]
- Angeles-Castellanos M, Salgado-Delgado R, Rodriguez K, Buijs RM, Escobar C. Expectancy for food or expectancy for chocolate reveals timing systems for metabolism and reward. Neuroscience. 2008;155:297–307. doi: 10.1016/j.neuroscience.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Arvanitogiannis A, Sullivan J, Amir S. Time acts as a conditioned stimulus to control behavioral sensitization to amphetamine in rats. Neuroscience. 2000;101:1–3. doi: 10.1016/s0306-4522(00)00401-2. [DOI] [PubMed] [Google Scholar]
- Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008;32:20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird TJ, Gauvin D. Characterization of cocaine self-administration and pharmacokinetics as a function of time of day in the rat. Pharmacol Biochem Behav. 2000;65:289–299. doi: 10.1016/s0091-3057(99)00207-5. [DOI] [PubMed] [Google Scholar]
- Blizard DA, Vandenbergh DJ, Jefferson AL, Chatlos CD, Vogler GP, McClearn GE. Effects of periadolescent ethanol exposure on alcohol preference in two BALB substrains. Alcohol. 2004;34:177–185. doi: 10.1016/j.alcohol.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Blizard DA, Wada Y, Onuki Y, Kato K, Mori T, Taniuchi T, et al. Use of a standard strain for external calibration in behavioral phenotyping. Behav Genet. 2005;35:323–332. doi: 10.1007/s10519-005-3224-1. [DOI] [PubMed] [Google Scholar]
- Blum K, Braverman ER, Holder JM, Lubar JF, Monastra VJ, Miller D, et al. Reward deficiency syndrome: a biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. J Psychoactive Drugs. 2000;32(Suppl. i–iv):1–112. doi: 10.1080/02791072.2000.10736099. [DOI] [PubMed] [Google Scholar]
- Deneau G, Yanagita T, Seevers MH. Self-administration of psychoactive substances by the monkey. Psychopharmacologia. 1969;16(1):30–48. doi: 10.1007/BF00405254. [DOI] [PubMed] [Google Scholar]
- Drummond DC, Litten RZ, Lowman C, Hunt WA. Craving research: future directions. Addiction. 2000;95(Suppl. 2):S247–S255. doi: 10.1080/09652140050111816. [DOI] [PubMed] [Google Scholar]
- Escobar C, Salgado R, Rodriguez K, Blancas Vazquez AS, Angeles-Castellanos M, Buijs RM. Scheduled meals and scheduled palatable snacks synchronize circadian rhythms: consequences for ingestive behavior. Physiol Behav. 2011;104(4):555–561. doi: 10.1016/j.physbeh.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Evans HL, Ghiselli WB, Patton RA. Diurnal rhythm in behavioral effects of methamphetamine, p-chloramethamphetamine and scopolamine. J Pharmacol Exp Ther. 1973;186:10–17. [PubMed] [Google Scholar]
- Falcon E, McClung CA. A role for the circadian genes in drug addiction. Neuropharmacology. 2009;56(Suppl. 1):91–96. doi: 10.1016/j.neuropharm.2008.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch TE, Roberts DCS. The effects of dose and access restrictions on the periodicity of cocaine self-administration in the rat. Drug Alcohol Depend. 1993;33(2):119–128. doi: 10.1016/0376-8716(93)90053-s. [DOI] [PubMed] [Google Scholar]
- Gaytan O, Swann A, Dafny N. Diurnal differences in rat's motor response to amphetamine. Eur J Pharmacol. 1998a;345:119–128. doi: 10.1016/s0014-2999(97)01558-6. [DOI] [PubMed] [Google Scholar]
- Gaytan O, Swann A, Dafny N. Time-dependent differences in the rat's motor response to amphetamine. Pharmacol Biochem Behav. 1998b;59:459–467. doi: 10.1016/s0091-3057(97)00438-3. [DOI] [PubMed] [Google Scholar]
- Gaytan O, Lewis C, Swann A, Dafny N. Diurnal differences in amphetamine sensitization. Eur J Pharmacol. 1999;374:1–9. doi: 10.1016/s0014-2999(99)00243-5. [DOI] [PubMed] [Google Scholar]
- Griffin WC, III, Randall PK, Middaugh LD. Intravenous cocaine self-administration: individual differences in male and female C57BL/6J mice. Pharmacol Biochem Behav. 2007;87:267–279. doi: 10.1016/j.pbb.2007.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CL, Ward AS, Haney M, Foltin RW, Fischman MW. Methamphetamine self-administration by humans. Psychopharmacology. 2001;157:75–81. doi: 10.1007/s002130100738. [DOI] [PubMed] [Google Scholar]
- Hart CL, Ward AS, Haney M, Nasser J, Foltin RW. Methamphetamine attenuates disruptions in performance and mood during simulated night-shift work. Psychopharmacology (Berl) 2003;169:42–51. doi: 10.1007/s00213-003-1464-4. [DOI] [PubMed] [Google Scholar]
- Hasler BP, Smith LJ, Cousins JC, Bootzin RR. Circadian rhythms, sleep, and substance abuse. Sleep Med Rev. 2012;16:67–81. doi: 10.1016/j.smrv.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth EM, Mueller K. Patterns of locomotor and stereotypic behavior during continuous amphetamine administration in rats. Pharmacol Biochem Behav. 1988;30(2):535–537. doi: 10.1016/0091-3057(88)90493-5. [DOI] [PubMed] [Google Scholar]
- Honma K, Honma S. The SCN-independent clocks, methamphetamine and food restriction. Eur J Neurosci. 2009;30:1707–1717. doi: 10.1111/j.1460-9568.2009.06976.x. [DOI] [PubMed] [Google Scholar]
- Hsu CT, Patton DF, Mistlberger RE, Steele AD. Palatable meal anticipation in mice. PLoS One. 2010;5:e12093. doi: 10.1371/journal.pone.0012903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13:635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick MG, Gunderson EW, Johanson CE, Levin FR, Foltin RW, Hart CL. Comparison of intranasal methamphetamine and d-amphetamine self-administration by humans. Addiction. 2012;107:783–791. doi: 10.1111/j.1360-0443.2011.03706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosobud AE, Pecoraro NC, Rebec GV, Timberlake W. Circadian activity precedes daily methamphetamine injections in the rat. Neurosci Lett. 1998;250:99–102. doi: 10.1016/s0304-3940(98)00439-x. [DOI] [PubMed] [Google Scholar]
- Kuribara H, Tadokoro S. Circadian variation in methamphetamine- and apomorphineinduced increase in ambulatory activity in mice. Pharmacol Biochem Behav. 1982;17:1251–1256. doi: 10.1016/0091-3057(82)90129-0. [DOI] [PubMed] [Google Scholar]
- Kuribara H, Tadokoro S. Circadian variation in susceptibility to methamphetamine after repeated administration in mice. Pharmacol Biochem Behav. 1984;20:247–250. doi: 10.1016/0091-3057(84)90250-8. [DOI] [PubMed] [Google Scholar]
- Littleton J. Can craving be modeled in animals? The relapse prevention perspective. Addiction. 2000;95(Suppl. 2):S83–S90. doi: 10.1080/09652140050111672. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roberts DC. Effects of cocaine self-administration on food-reinforced responding using a discrete trial procedure in rats. Neuropsychopharmacology. 2004;29(4):669–575. doi: 10.1038/sj.npp.1300363. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Taylor JR. Sex differences in the behavioral effects of 24-h/day access to cocaine under a discrete trial procedure. Neuropsychopharmacology. 2004;29(5):943–951. doi: 10.1038/sj.npp.1300389. [DOI] [PubMed] [Google Scholar]
- Martin-Iverson MT, Iversen SD. Day and night locomotor activity effects during administration of (+)-amphetamine. Pharmacol Biochem Behav. 1989;34:465–471. doi: 10.1016/0091-3057(89)90542-x. [DOI] [PubMed] [Google Scholar]
- Mendoza J, Angeles-Castellanos M, Escobar C. Entrainment by a palatable meal induces food-anticipatory activity and c-Fos expression in reward-related areas of the brain. Neuroscience. 2005;133:293–303. doi: 10.1016/j.neuroscience.2005.01.064. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE. Circadian food-anticipatory activity: formal models and physiological mechanisms. Neurosci Biobehav Rev. 1994;18:171–195. doi: 10.1016/0149-7634(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Mistlberger R, Rusak B. Palatable daily meals entrain anticipatory activity rhythms in free-feeding rats: dependence on meal size and nutrient content. Physiol Behav. 1987;41:219–226. doi: 10.1016/0031-9384(87)90356-8. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Lukas SE, Mendelson JH. Diurnal patterns of cocaine and heroin self-administration in rhesus monkeys responding under a schedule of multiple daily sessions. Behav Pharmacol. 1995;6:763–775. [PubMed] [Google Scholar]
- Pecoraro N, Gomez F, Laugero K, Dallman MF. Brief access to sucrose engages food-entrainable rhythms in food-deprived rats. Behav Neurosci. 2002;116:757–776. [PubMed] [Google Scholar]
- Shappell SA, Kearns GL, Valentine JL, Neri DF, DeJohn CA. Chronopharmacokinetics and chronopharmacodynamics of dextromethamphetamine in man. J Clin Pharmacol. 1996;36:1051–1063. doi: 10.1177/009127009603601109. [DOI] [PubMed] [Google Scholar]
- Siegel S. Drug anticipation and drug addiction. The 1998 H. David Archibald Lecture. Addiction. 1999;94:1113–1124. doi: 10.1046/j.1360-0443.1999.94811132.x. [DOI] [PubMed] [Google Scholar]
- Stice E, Yokum S, Blum K, Bohon C. Weight gain is associated with reduced striatal response to palatable food. J Neurosci. 2010;30:13105–13109. doi: 10.1523/JNEUROSCI.2105-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terman M, Terman JS. Control of the rat's circadian self-stimulation by light–dark cycles. Physiol Behav. 1975;14:781–789. doi: 10.1016/0031-9384(75)90070-0. [DOI] [PubMed] [Google Scholar]
- Trinko R, Sears RM, Guarnieri DJ, DiLeone RJ. Neural mechanisms underlying obesity and drug addiction. Physiol Behav. 2007;91:499–505. doi: 10.1016/j.physbeh.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Uchihashi Y, Kuribara H, Yasuda H, Umezu T, Tadokoro S. Long-continuous observation of the effects of methamphetamine on wheel-running and drinking in mice. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18:397–407. doi: 10.1016/0278-5846(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Urba-Holmgren R, Holmgren B, Aguiar M. Circadian variation in an amphetamine induced motor response. Pharmacol Biochem Behav. 1977;7:571–572. doi: 10.1016/0091-3057(77)90257-x. [DOI] [PubMed] [Google Scholar]
- Verwey M, Khoja Z, Stewart J, Amir S. Differential regulation of the expression of Period2 protein in the limbic forebrain and dorsomedial hypothalamus by daily limited access to highly palatable food in food-deprived and free-fed rats. Neuroscience. 2007;147:277–285. doi: 10.1016/j.neuroscience.2007.04.044. [DOI] [PubMed] [Google Scholar]
- Waddington-Lamont E, Harbour VL, Barry-Shaw J, Renteria Diaz L, Robinson B, Stewart J, et al. Restricted access to food, but not sucrose, saccharine, or salt, synchronizes the expression of Period2 protein in the limbic forebrain. Neuroscience. 2007;144:402–411. doi: 10.1016/j.neuroscience.2006.09.027. [DOI] [PubMed] [Google Scholar]
- Wahlsten D, Bachmanov A, Finn DA, Crabbe JC. Stability of inbred mouse strain differences in behavior and brain size between laboratories and across decades. Proc Natl Acad Sci U S A. 2006;103:16364–16369. doi: 10.1073/pnas.0605342103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanat KA, Anadkat MJ, Klekotka PA. Seasonal variation of Stevens–Johnson syndrome and toxic epidermal necrolysis associated with trimethoprim–sulfamethoxazole. J Am Acad Dermatol. 2009;60:589–594. doi: 10.1016/j.jaad.2008.11.884. [DOI] [PubMed] [Google Scholar]
- Webb IC, Baltazar RM, Lehman MN, Coolen LM. Bidirectional interactions between the circadian and reward systems: is restricted food access a unique zeitgeber? Eur J Neurosci. 2009a;30:1739–1748. doi: 10.1111/j.1460-9568.2009.06966.x. [DOI] [PubMed] [Google Scholar]
- Webb IC, Baltazar RM, Wang X, Pitchers KK, Coolen LM, Lehman MN. Diurnal variations in natural and drug reward, mesolimbic tyrosine hydroxylase, and clock gene expression in the male rat. J Biol Rhythms. 2009b;24:465–476. doi: 10.1177/0748730409346657. [DOI] [PubMed] [Google Scholar]
- Zurita E, Chagoyen M, Cantero M, Alonso R, Gonzalez-Neira A, Lopez-Jimenez A, et al. Genetic polymorphisms among C57BL/6 mouse inbred strains. Transgenic Res. 2011;20:481–489. doi: 10.1007/s11248-010-9403-8. [DOI] [PubMed] [Google Scholar]