Abstract
Late stent thrombosis (LST) and very LST (VLST) are infrequent complications after drug-eluting stent (DES) implantation, but they carry a significant risk for patients. Delayed healing, which may be represented by incomplete stent coverage, has been observed in necropsy vessel specimens treated with DES. As a result, in vivo assessment of stent coverage, as well as stent apposition using optical coherence tomography (OCT), have been recently used as surrogate safety endpoints in clinical trials testing DES platforms. By adopting strut coverage assessed by OCT, one can assess the safety profile of the new generation of DES in preregistration studies. This article focuses on stent strut coverage as a central predictor of late DES thrombosis from the histopathological point of view, discusses the limitations of the current imaging modalities and presents the technical characteristics of OCT for the detection of neointimal coverage after stent implantation. We also review the preclinical and clinical investigations using this novel imaging modality.
Keywords: Strut coverage, Optical coherence tomography, Drug-eluting stent
Introduction
Drug-eluting stents (DES), originally conceived to locally deliver antiproliferative agents to the vascular wall in a controlled manner [1, 2], have met their primary objectives of reducing the excessive formation of neointimal tissue and reducing restenosis and repeat revascularization rates that are major limitations of bare-metal stents (BMS) in a wide variety of clinical and angiographic scenarios [3–7].
Nevertheless, these impressive results have been tempered by recent observations of late stent thrombosis (LST) and very LST (VLST); an infrequent (up to 0.6% per year) but potentially life-threatening complication [8–10]. Late DES thrombosis is thought to be multifactorial in origin. Although patient-, lesion- and procedure-related factors may impact its occurrence by various degrees, pathological studies have suggested that the absence of stent strut coverage due to delayed vascular healing by DES and the persistence of fibrin may be the most important underlying substrates [11–18].
Until recently, the only available method to accurately evaluate the vascular healing of post-stent implantations was histopathology, which carried some limitations, including small number of cases, selection bias, tissue preparation and the inability of assessing the stent coverage in vivo and longitudinally over several time-points [19].
Intravascular ultrasound (IVUS), formerly the gold standard for the assessment of stent strut apposition and the vascular responses to stents, lacks the axial and lateral resolution (150–250 μm) of the ultrasound waves for the identification of small amounts of neointimal tissue [20].
Intravascular optical coherence tomography (OCT) is a novel catheter-based invasive imaging modality, which uses infrared light rather than ultrasound. By producing high-resolution images (~20 μm), it provides unique insights into the interaction between the stent and the vessel wall and has allowed a more precise characterization of atherosclerotic plaques [19–21]. For this reason, OCT has been considered a promising endovascular imaging technique for the evaluation of neointimal formation after DES implantation. Being able to accurately identify in vivo the so-called ‘‘vulnerable stents’’ may shed light on a better understanding of the LST and VLST phenomena, its mechanisms and the temporal patterns of occurrence.
In addition, given the rarity of stent thrombosis, several thousand patients would be required in a clinical trial to assess the safety of a new device, making such a trial unrealistic due to excessive cost and duration. Near-histology range strut-level analysis by OCT may also serve as a surrogate end-point for clinical trials assessing the safety of new devices and providing a measurable variable for the comparison between different technologies. In the present review, we highlight the importance of stent strut coverage as a central predictor of late DES thrombosis from the histopathological point of view, briefly discuss the limitations of the current imaging modalities and present the technical characteristics of OCT that make it a promising imaging tool for evaluation of healing after stent implantation. We also present preclinical validation studies and current clinical trials using this novel imaging modality in the identification of new surrogate end-points.
Pathological findings after DES implantation
Traditionally, histological studies have been the most effective form of evaluating vascular healing after stent implantation [22]. In the DES era, the initial preclinical studies on sirolimus-eluting stents (SES) have presented conflicting results, with different degrees of biological effects regarding arterial healing, inflammation and neointimal growth. In the study by Klugherz et al. [23], polymer-based SES implanted in rabbit iliac arteries demonstrated a dose-dependent inhibition of neointimal formation, with little evidence of increased inflammation and delayed endo-thelialization at 28 days in both the high- (196 μg/ stent) and low-dose (64 μg/stent) stents used. Suzuki et al. [24] reported on higher amounts of accumulated fibrin with SES (180 μg/stent) in comparison to BMS in porcine coronary arteries, although the degree of endothelialization was similar between both stents. More recently, Carter et al. [25] studied SES implanted in porcine coronary arteries and arterial inflammation, characterized by giant cells, gradually increased from 90 to 180 days, with a corresponding increase in neointimal formation.
Farb et al. [11], investigating the response of various doses of polymer-based paclitaxel-eluting stents, (PES) deployed in the iliac arteries of rabbits, demonstrated at the end of 28 days a dose-dependent reduction of mean neointimal thickness, with incomplete healing in the higher dose PES, consisting of persistent intimal fibrin deposition, intraintimal hemorrhage, and increased intimal and adventitial inflammation, compared to the low-dose PES, the stent coated with polymer but without a drug, and the bare stent.
The first reports on the pathological observations of DES implanted in humans came from patients who died from causes not related to the DES previously implanted. Histological examinations demonstrated few inflammatory cells and fibrin deposits, along with approximately 80% coverage of a SES implanted 16 months previously [12] and nearly 95% coverage in another stent implanted 4 years earlier [13].
Virmani et al. [14] described the first pathological report of a patient who suffered a fatal acute myocardial infarction and cardiac rupture as a result of late thrombosis 18 months after the implantation of two overlapping SESs in the left circumflex artery. At autopsy, they found a localized hypersensitivity reaction, with an extensive inflammatory infiltrate involving the intima, media and adventitia, consisting of lymphocytes, plasma cells, macrophages and eosinophils. Luminal fibrin-rich thrombus, aneurysmal arterial remodeling and focal malapposition areas were additional findings. The extent of stent endothelial coverage was not mentioned.
Subsequently, Joner et al. [15] compared 32 DES (SES or PES) from 23 patients who died >30 days after implantation, with 36 matched BMS implanted in 25 patients. Of the 23 patients with DES, 14 (61%) had evidence of LST. SES and PES showed greater delayed healing characterized by persistent fibrin deposition and poorer endothelialization (55.8 ± 26.5%) compared with BMS (89.8 ± 20.9%, P = 0.0001). In addition, DES with LST showed a higher fibrin score and more delayed healing compared to patent DES (27.1 ± 25.9% vs. 66.1 ± 25.4%, P = 0.001). Delayed arterial healing was found to be a cardinal risk factor in all 14 patients with LST. In three cases (21%), delayed healing was the only risk factor encountered. In the other 11 patients (79%), additional procedural and pathological factors for LST were: (1) local hypersensitivity reactions; (2) ostial and/or bifurcation stenting; (3) malapposition/incomplete stent apposition; (4) restenosis and (5) strut penetration into the necrotic core.
In another publication from the same group, an expansion of the above-mentioned necropsy database was performed. From 62 lesions in 46 patients, which had a DES (SES or PES) implanted >30 days, Finn et al. [17] identified 28 lesions (in 23 patients) with thrombi compared to 34 lesions without thrombi from another 23 patients. By using the multiple logistic generalized estimating equations model, they demonstrated that endothelialization was the most powerful histological predictor of stent thrombosis, and that the morphometric parameter that best correlated with endothelialization was the ratio of uncovered to total stent struts per section. The odds ratio for thrombus in a stent with a ratio of uncovered to total stent struts per section>30% is 9.0 (95% CI: 3.5–22), demonstrating a marked increase in the risk for LST as the number of uncovered struts increased. It is unclear how these 30% rates were calculated, and the study might have been too ambitious given the limited data, lack of longitudinal follow-up, heterogeneity of the study sample and intrinsic methodological limitations of histology, which does not allow determination of the cause-effect relationship. Nevertheless, accurate in vivo identification of stent coverage has been postulated as the central surrogate endpoint in prognosticating the risk of future thrombotic complications.
In vivo determination of stent coverage status: limitations of the current imaging modalities and feasibility of optical coherence tomography
Intravascular ultrasound
IVUS has established itself as the standard intravascular imaging modality for the assessment of vascular responses after stent implantation [26]. Studies in patients who suffered late DES thrombosis have identified a high rate of late acquired incomplete stent apposition, suggesting that this phenomenon could constitute an important risk factor for LST [27–30]. DES under-expansion and residual reference segment stenosis have also been associated with DES thrombosis [31, 32]. Nevertheless, the limited axial resolution of IVUS (100–150 μm) and the frequent presence of artifacts around stent struts (side-lobes and shadowing) preclude an accurate assessment of vascular microstructures, such as thin layers of neointimal tissue covering DES struts [20, 33, 34]. In contrast to BMS that develop circumferential coverage with an average neointimal thickness of 500 μm or more, DES significantly inhibit the hyperplastic response, with an average late lumen loss for SES or PES that can be lower than 100 μm and unlikely to be detected by IVUS [35].
In a study by Matsumoto et al. [36], 57 SES in 34 patients were evaluated by IVUS and OCT at 6-month follow-up. The presence of neointima (mean neointimal thickness 52.5 μm) undetectable by IVUS was 64%. In addition, the average rate of neointima-covered struts in an individual SES was 89%. Only 16% of SES showed full coverage by neointima, whereas the remaining stents had partially uncovered struts. While optimal stent sizing using IVUS can help reduce the rate of uncovered struts [37], tiny level strut coverage is beyond the capacity of IVUS.
Intracoronary angioscopy
Intracoronary angioscopy is another optical imaging modality, which provides unique insights into the visualization of stent coverage that is able to identify even tiny layers of tissue covering stent struts. Its elevated sensitivity in detecting intravascular thrombus has allowed the demonstration of subclinical thrombus formation associated with delayed neointimal coverage over DES struts [38]. Recently, Higo et al. [39] have demonstrated that SES promoted the formation of atherosclerotic yellow neointima after a 10-month follow-up. In addition, they showed that thrombus was detected more frequently on the yellow area than on the white area and was never identified in the sites where the stent struts were buried under neointima. In another study, Awata et al. [40] performed serial angioscopic examinations 3.6 ± 1.1 months, 10.5 ± 1.6 months and 21.2 ± 2.2 months after SES (n = 17) and BMS (n = 11). Neointimal coverage was completed by 3–6 months in BMS. In contrast, 94% of SES had incomplete neointimal coverage after 2 years, along with the presence of thrombi and yellow plaques. No yellow plaques were evident in BMS at the second and third follow-ups. Moreover, in the entire cohort of 74 angioscopic observations, a significant relationship existed between neointimal coverage grades and the presence of thrombi (P = 0.002) and yellow plaques (P < 0.0001).
Nevertheless, there are also significant limitations in angioscopic evaluations. Imaging is restricted to the surface of the coronary lumen, thus limiting quantitative assessment of the neointima. Assessment of the quality and functionality of the tissue covering the stent struts is also not possible by angioscopic evaluation. Moreover, angiographic complexity, such as tortuous and angulated vessels, may also limit a complete circumferential view of the vessel. A large profile of angioscopy is also not suitable for easy use.
Optical coherence tomography
Intravascular OCT generates ultra-high resolution cross-sectional images of tissue layers using back-reflected light with a band-width in the near-infrared spectrum. The image is formed by the backscattering of light from the vessel wall, or the time it takes for emitted light to travel between the target tissue and back to the lens, producing an ‘‘echo time delay’’ with a measurable signal intensity or ‘‘magnitude’’ [21].
Current OCT systems use a central wavelength of approximately 1,300 nm, which makes tissue penetration limited to 1–3 mm as compared to 4–8 mm achieved by IVUS [21]. On the other hand, the axial resolution, which is also determined by the light wavelength, ranges from 15 to 10 μm as compared to 200 to 250 μm achieved with IVUS [19–21]. Lateral resolution is typically 20–90 μm against 150–300 μm from IVUS [21]. These high resolution properties have permitted OCT to provide a near histology-level capacity for detection and quantification of even small layers of neointimal tissue over DES struts.
In an era when DES safety is the current challenge of interventional cardiology, the so-called ‘‘strut-level analysis’’, with accurate assessment and quan-tification of neointimal coverage over stents, has been adopted as a surrogate end point in numerous clinical trials of DES and as an important parameter for the approval of new DES technologies by regulatory agencies. In this regard, intravascular OCT has emerged as a promising in vivo imaging modality for the evaluation of vascular healing post-DES implantation.
Validation of stent coverage measurement by optical coherence tomography: from preclinical studies
Preclinical testing in animal models is an important part of the regulatory process used for determining the safety and efficacy of a new device before human use. Accuracy of strut coverage evaluated by OCT has been validated in pre clinical studies.
Prati et al. [41] investigated the capacity of OCT to accurately monitor the occurrence of arterial healing after stenting. Thirty-two cross-sections from eight BMS implanted in the right common carotid arteries of rabbits, with 384 stent struts, were analyzed. OCT was able to detect all stent struts in 30 of 32 cross-sections (93.7%), and correctly identify the presence/ absence of tissue for every strut. Histological and OCT measurements of mean neointimal thickness were similar and closely related (r = 0.85, P < 0.001) with an excellent intra- and interobserver reproducibility of OCT measurements (R2 = 0.90 and 0.88, respectively).
More recently, Murata et al. [42] investigated the accuracy of time domain OCT in analyzing the neointimal response to several DES types, comparing OCT images acquired in vivo with corresponding histological specimens. A total of 84 stents were implanted (22 BMS, 22 everolimus-eluting stents, 20 zotarolimus-eluting stents, and 20 PES) in normal porcine coronary arteries and were harvested at 28 (n = 42) and 90 (n = 42) days. The luminal and stent areas were consistently larger in OCT compared with histology. Mean neointimal thickness was also very similar between the techniques (3.27% variation). There was a high correlation between OCT and histology for the evaluation of neointimal area (R2 = 0.804), luminal area (R2 = 0.825), and neointimal thickness (R2 = 0.789). These results were independent of stent type. Correlation for total stent area was poor (R2 = 0.352), maybe as a result of damaging of the stent during histological sectioning. When strut coverage measurements were stratified according to thickness and a strut-by-strut analysis was performed, OCT and histology detected a similar proportion of uncovered stents (1.16% OCT vs. 1.84% histology). However, in vivo OCT showed some variability in the quantification of neointimal thickness ranging from 20 to 80 μm, differing significantly from histology. Particularly, this difference was marked between the 20-and 60-μm range. This variation for neointimal thickness converged between 80 and 100 μm. Finally, OCT determined a greater proportion of struts covered by >100 μm than histology (84.9% vs. 71.95%). OCT has also shown a higher accuracy for detecting small degrees of neointimal tissue (occupying\30% of the stent area measured by histology) than IVUS. In the study by Suzuki et al. [43], OCT and IVUS imaging were performed in six pigs across 33 stents 1 month after implantation. Eleven stents (33%) had a small degree of neointimal tissue (average area: 1.26 ± 0.46 mm2 and percent area obstruction: 21.4 ± 5.2%). Compared with histology, the diagnostic accuracy of OCT (area under the receiver operating characteristic curve [AUC]: 0.967, 95% CI: 0.914–1.019) was higher than that of IVUS (AUC: 0.781, 95% CI: 0.612–0.838).
Taken together, this data validates OCT as a reliable and highly reproducible method for the evaluation of neointimal formation and strut coverage after DES implantation.
Clinical trials for stent coverage assessment
OCT clinical trials that evaluated strut level neointimal coverage as one endpoint are listed in Table 1. Since time-domain OCT was first approved in European countries in 2007, more than 30 clinical OCT studies have been published or presented at scientific conferences. Each trial can be characterized by variables related to study design and OCT analysis methodology, which are critical for the interpretation of the results (Table 2).
Table 1.
Optical coherence tomography clinical trials
| Author | Study type | Blind analysis |
Population/lesion typea | Number of patient |
OCT follow-up time (months) |
Statistical level of strut level analysis |
OCT analysis interval (mm) |
Adding the OCT axial resolution to malapposition values |
Follow-up OCT image assessment results
|
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total analyzed frame |
Total strut number |
Stent type
|
|||||||||||
| S1 | S2 | S3 | |||||||||||
| Guagliumi et al. [44] | Randomized controlled | Yes (corelab) | Long lesion (non-CTO) | 77 | 6 | Patient, segment | 0.3 | No | 6,968 | 53,047 | SES | PES | ZES |
| Matsumoto et al. [36] | Prospective observational | Yes | Angina (71%) OMI (29%), [De novo 82%, restenosis 18%] [CTO 18%] | 34 | 6 | Strut | 1 | Yes (20 μm) | N/A | 6,770 | SES | ||
| Guagliumi et al. [45] | Randomized controlled | Yes (corelab) | AMI | 118 | 13 | Lesion | 0.3 | No | N/A | 45,996 | PES (ExpressTM) | BMS | |
| Guagliumi et al. [46] | Randomized controlled | Yes (corelab) | AMI | 42 | 6 | Patient | 0.06 | No | 17,088 | 153,452 | ZES | BMS | |
| Guagliumi et al. [47] | Randomized controlled | Yes (corelab) | UA (25%), SA (75%) | 60 | 6 | Patient | 0.06 | No | 17,698 | 252,243 | JACTAX HD | JACTAX LD | PES (LibertéTM) |
| Kim et al. [54] | Cross-sectional prospective observation | No | ACS (56%), SA (44%) | 68 | 9 | Strut | 1 | No | 1,473 | 16,563 | ZES | SES | |
| Katoh et al. [57] | Observational | Yes | Angina (69%), OMI (31%), [Denovo 77%, restenosis 15%] [CTO 8%] | 13 | 6/12 | Strut | 1 | Yes (20 μm) | N/A | 4,606 | SES | ||
| Kubo et al. [52] | Observational | Yes | UA (43%), SA (57%) | 55 | Pre/0/9 | Patient | 1 | No | 911 | 7,532 | SES (UA) | SES (SA) | |
| Barlis et al. [48] | Randomized controlled | Yes (corelab) | SA (61%), ACS (39%) | 46 | 9 | Strut | N/A | No | N/A | 11,068 | BES | SES | |
| Serruys et al. [58] | Prospective open-label | Yes (corelab) | SA (70%), UA (27%), Silent ischemia (3%) | 7 | 0/6/24 | Strut | N/A | No | N/A | 1,035 | BVS | ||
| Ormiston et al. [59] | Prospective open-label | Yes (corelab) | SA (70%), UA (27%), Silent ischemia (3%) | 13 | 0/6 | Strut | N/A | No | N/A | 1,409 | BVS | ||
| Tamburino et al. [60] | Prospective open-label | Yes (corelab) | SA (24%), UA (63%), Silent ischemia (2%), recent MI (11%) | 15 | 6 | Strut | 0.5 | No | 1,904 | 19,028 | Catania stent | ||
| Takano et al. [61] | Prospective observational | No | ACS (43%) | 21 | 3 | Patient | 1 | No | 567 | 4,516 | SES | ||
| Yao et al. [68] | Observational | Yes | N/A | 36 | 6 or 12 | Strut | 1 | No | 903 | 6,561 | SES (6 M) | SES (12 M) | |
| Ozaki et al. [53] | Prospective observational | Yes | STEMI (28%), UA (17%), SA (55%) | 32 | 0/10 | Strut | 1 | Yes (20 μm) | 616 | 4,320 | SES | ||
| Ishigami et al. [66] | Observational | No | ACS (17%) | 60 | 5–8 or 9–24 or 25–43 | Strut | 1 | Yes (30 μm) | N/A | 8,475 | SES (5–8 M) | SES (9–24 M) | SES (25–43 M) |
| Kim et al. [49] | Prospective observational | Yes | ACS (77%) | 244 | SES (thrombus: 9.1, non: 9.7), PES (thrombus: 9.3, non: 9.4), ZES (thrombus: 3.0, non: 8.4) | Patient | 1 | No | 6,097 | 61,907 | SES | PES | ZES |
| Murakami et al. [50] | Prospective observational | No | ACS (35%), SA | 40 | PES (5.9 ± 0.3), SES (5.8 ± 2.6) | Patient | 1 | No | 983 | 8,434 | PES | SES | |
| Chen et al. [55] | Prospective observational | Yes | N/A | 23 | SES (8.6), BMS1 (7.2), BMS2 (44.5) | Strut | 1 | N/A | N/A | 5,132 | SES | BMS1 | BMS2 |
| Xie et al. [56] | Prospective observational | No | ACS (52%) | 40 | 3 | Strut | 1 | No | 911 | 7,951 | SES | BMS | |
| Fan et al. [51] | Prospective observational | No | AMI | 46 | 9 | Patient | 1 | No | N/A | 11,514 | SES | PES | ZES |
| Kim et al. [62] | Prospective observational | Yes | ACS (52%), SA | 31 | 0/3 | Patient | 0.5 | No | N/A | N/A | ZES | ||
| Author | Follow-up OCT image assessment results
|
Uncoverage rate comparison | Comments | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Stent type |
% Uncovered strut
|
% Malapposed strut
|
|||||||||
| S1 | S2 | S3 | S4 | S1 | S2 | S3 | S4 | ||||
| Guagliumi et al. [44] | BMS | Total: 8.1 ± 11.2 | Total: 4.0 ± 10.3 | Total: 0.06 ± 0.24 | Total: 0.86 ± 2.89 | Total: 2.3 ± 5.3 | Total: 2.3 ± 9.3 | Total: 0.01 ± 0.12 | Total: 0.12 ± 0.70 | DES > BMS (P = 0.08) | First OCT randomized trial performed |
| OLP: 6.7 ± 9.6 | OLP: 6.7 ± 16.5 | OLP: 0.10 ± 0.37 | OLP: 1.52 ± 3.39 | OLP 2.9 ± 6.9 | OLP 5.5 ± 15.6 | OLP 0.04 ± 0.18 | OLP 0.001 ± 0.001 | SES (OLP =NonOLP) | |||
| NonOLP: 8.6 ± 12.0 | NonOLP: 2.7 ± 4.5 | NonOLP: 0.02 ± 0.11 | NonOLP: 0.56 ± 2.66 | NonOLP 1.9 ± 4.2 | NonOLP 0.73 ± 2.1 | NonOLP 0.001 ± 0.001 | NonOLP 0.17 ± 0.85 | PES (OLP > NonOLP) ZES (OLP =NonOLP) |
|||
| Matsumoto et al. [36] | 8 | 1 | |||||||||
| Guagliumi et al. [45] | 5.68 ± 6.96 | 1.1 ± 2.5 | 0.90 ± 2.08 | 0.07 ± 0.21 | PES > BMS | Single-center OCT sub-trial of multicenter HORIZONS-AMI | |||||
| Guagliumi et al. [46] | 1.39 ± 2.55 | 6.68 ± 10.35 | 0.74 ± 1.93 | 3.54 ± 5.74 | ZES = BMS | ||||||
| Guagliumi et al. [47] | 7.0 ± 12.2 | 4.6 ± 7.3 | 5.3 ± 14.7 | 0.80 ± 1.89 | 1.12 ± 2.77 | 1.35 ± 4.38 | JACTAX HD = JACTAX LD = PES |
||||
| Kim et al. [54] | 0.3 | 12.3 | 0.08 | 2.6 | SES > ZES | ||||||
| Katoh et al. [57] | 6 M:10.4 12 M:5.7 |
6 M:1.7 12 M: 0.2 | 6 M > 12 M | Serial observation of neointimal coverage in same population | |||||||
| Kubo et al. [52] | 14 | 4 | 2.4 | 1.2 | UA > SA | ||||||
| Barlis et al. [48] | 0.6 (95% CI: 0.2–1.6) | 2.1 (95% CI: 0.9–4.4) | 0.2 (95% CI: 0.1–0.5) | 0.4 (95% CI: 0.2–1.0) | SES > BES | This study was performed as OCT substudy of LEADERS trial | |||||
| Serruys et al. [58] | 24 M: 0 | 24 M: 0 | This study was performed as OCT substudy of ABSORB trial | ||||||||
| Ormiston et al. [59] | 1 | 6 | This study was performed as OCT substudy of ABSORB trial | ||||||||
| Tamburino et al. [60] | 0.5 | 0.15 | This study was performed as OCT substudy of ATLANTA trial | ||||||||
| Takano et al. [61] | 15 | 15 | |||||||||
| Yao et al. [68] | 9.2 | 6.6 | 1.3 | 1.4 | 6 M > 1 2 M | ||||||
| Ozaki et al. [53] | 12.26b | 5.04 | Main goal of this study was to evaluate stent apposition and coverage at follow-up based on post-procedural strut apposition | ||||||||
| Ishigami et al. [66] | 14.8 | 11.7 | 4.1 | 1.4 | 0.5 | 0.3 | S1 > S2 > S3 | ||||
| Kim et al. [49] | 10 ± 12 | 4 ± 6 | 0.3 ± 0.9 | 1.3 ± 0.9 | 0.9 ± 3.3 | 0.3 ± 1.9 | SES > PES > ZES | % of uncovered struts were higher in thrombus PES group than non thrombus PES group (P = 0.01) | |||
| Murakami et al. [50] | 5 ± 5 | 15 ± 6 | 6 ± 4 | 15 ± 5 | SES > PES | ||||||
| Chen et al. [55] | 15.2 | 0.34 | 0.32 | 1.8 | 0.0 | 0.0 | SES > BMS | ||||
| Xie et al. [56] | 15 | 0.1 | 15 | 1.1 | SES > BMS | ||||||
| Fan et al. [51] | 16.2 ± 17.8 | 4.7 ±7.4 | 0.6 ± 1.5 | 4.0 ± 8.2 | 2.1 ± 4.5 | 0 ± 0 | SES > PES > ZES | ||||
| Kim et al. [62] | 0.1 | 0.2 ± 1.1 | |||||||||
OCT Optical coherence tomography, CTO chronic total occlusion, OMI old myocardial infarction, ACS acute coronary syndrome, UA unstable angina, SA stable angina, MI myocardial infarction, SES sirolimus-eluting stent, PES paclitaxel-eluting stent, ZES zotarolimus-eluting stent, BMS bare metal stent, OLP overlap, NonOLP non-overlap, CI confidence interval
Uncovered strut with malapposition was not included
Table 2.
Significant parameters for interpretation of OCT clinical trial results
| Study design | OCT methodology |
|---|---|
| Randomization | Blind analysis |
| Sites (multi or single) | Analysis interval |
| Sample size (statistical power) | Definition of variables (uncoverage, malapposition) |
| Included population | |
| OCT endpoints | |
| Time points of OCT procedure (single or serial) | |
| Multiple modalities | |
| Statistical methods (statistical level, model) |
1. Study design
Randomization, number of sites (single-center vs. multi-center), sample size, statistical methods (statistical level), OCT endpoints, target population/lesion, follow-up time points (single vs. serial), use of multiple modalities, blind assessment by independent core laboratory.
Randomization is an ideal allocation method to eliminate selection bias. Among 31 trials, randomization was performed only in 5 trials [44–48]. Two of them are single-center OCT sub-studies from large multi-center clinical trials [45, 48]; a strategy expected to increase in the future. All but one study [48] have been performed at a single-center, a trend which is expected to change in the near future as the new OCT systems become available worldwide.
In considering statistical power, sufficient populations should be included based on the level of analysis, although data points of OCT strut level analysis are large. For randomization trials using more than 2 types of stents, sample sizes can simply be calculated depending on the trial design, such as superiority or non-inferiority, using published data (or estimated data in case no previous data exists, such as for novel stents) on the rates of uncovered or malapposed struts with standard deviations and alpha and beta errors. Too small of a sample size might result in inconclusive findings.
The statistical level of strut coverage is diverse between trials and is categorized into 4 groups; per-patient, per-lesion, per-segment, and per-strut. Patient level or lesion level analysis is stricter, and generates a single value for each patient/lesion. On the contrary, for data presented at strut level or segment level, multiple data points are being generated and combined, and cluster phenomenon must be addressed with a complex samples general linear model [44] or Bayesian hierarchical random-effects model [48].
Lesion/patient level assessment of strut coverage was used in 9 trials, 6 of which displayed the degree of neointimal coverage as mean ± SD of % total uncovered struts [44, 45, 47, 49–51]. In a single study [46], median and interquartiles were used. Besides the total percentage of uncoverage, the various arbitrary cut-off values used for the degree of uncoverage were also associated with LST [44–48] at the discretion of the center investigating each trial. In the OCTAMI trial [46], the incidence of >10% uncovered struts was used as an arbitrary variable. In the LEADERS trial [48], any, >5 and >10% uncovered struts were used. As of yet, no clinically-relevant cut-off value of % uncovered struts or malapposed struts associated with stent thrombosis exists. Thus, a larger OCT study is warranted to evaluate the reliability of the degree of incomplete coverage for identifying patients at a clinically-relevant increased risk of LST.
For most OCT clinical trials, the primary OCT endpoint was strut coverage, except in 3 trials, which evaluated lesion morphology [52], the course of baseline malapposition [53], and intracoronary thrombus [49].
Many OCT studies were performed for the evaluation of strut coverage between different stent types at the same follow-up time point [44–51, 54–56]. Single-arm studies with one stent type were also performed [36, 53, 57–62]. Using the same stent type, assessment of coverage patterns between different target populations is also one of the strategies that provide useful information. Kubo et al. [52] evaluated long-term strut coverage of SES for both stable angina and unstable angina populations. This study revealed strut coverage was significantly delayed in unstable angina population. The impact of different stenting techniques on coverage could also evaluated in OCT clinical trials. Pathological findings from preclinical studies [63] drove clinicians to evaluate specific stent segments, such as overlap segments, which were exclusively susceptible to uncovered and malapposed struts in paclitaxel-eluting stents (PES) compared to non-overlap segments [44, 64]. Furthermore, pathological findings from autopsy studies [18] triggered 3 clinical trials targeting AMI populations for DES strut coverage [45, 46, 51].
The majority of trials have a single follow-up time point. The timing of the follow-up OCT procedure depends on the main goals of each trial: targeting long-term safety or early completion of coverage and drug-eluting kinetics of the target stent. Owing to the ability of detecting small changes of neointimal tissue by OCT, serial evaluation at different time points seems to be feasible. Katoh et al. [57] evaluated SES strut coverage at different time points in the same population. In their study, although almost 90% of struts were covered within 6 months, tissue coverage increased for both uncovered struts and those already covered from 6 to 12 months. Serial OCT evaluation is also useful in assessing novel biodegradable stents with which temporal absorption and local vessel responses are even more puzzling than with current DES. In the ABSORB trial for the BVS stent, both strut coverage and absorbing processes were sequentially evaluated in a small number of the population [58, 59].
There is an on-going trial (OCTAXUS, PI: Guagliumi G, MD, Bergamo, Italy), which is a single-center, prospective trial to evaluate serial strut coverage and vessel wall response of PES implanted at 2 sequences of staged percutaneous coronary intervention (PCI) procedure with a 3 month interval. OCT evaluation at 3 time points, including post-procedure (0, 3, and 9 M), will give us more information on the heterogeneity of neointimal growth regulation by time and the impact of post-procedural OCT lumen/wall interface appearance on strut coverage.
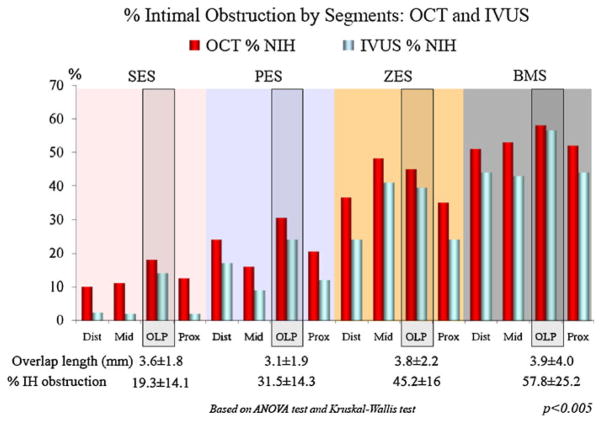
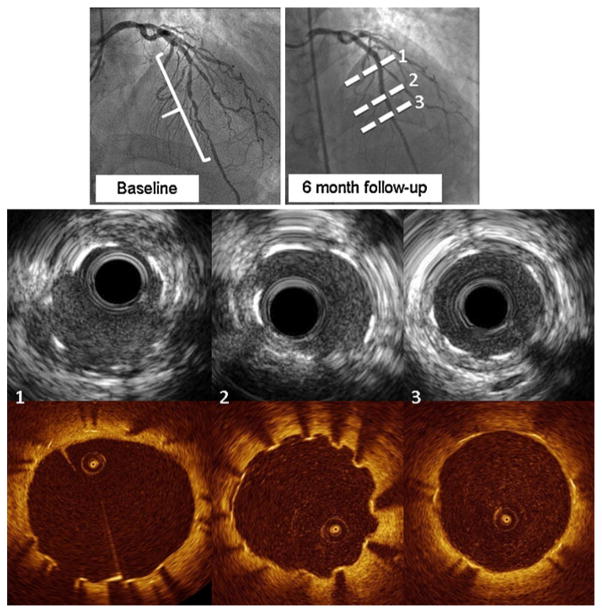
While OCT strut level analysis is a unique method among intracoronary modalities, it is still a relatively new technology and has not accumulated clinical data. Thus, a combined usage of other modalities, such as IVUS, may provide an opportunity to learn from clinical findings already validated by other modalities, but not yet by OCT. In the ODESSA trial [44], OCT was more sensitive to a small amount of neointimal hyperplasia compared to IVUS (Fig. 2). From this trial, we assessed the ability of OCT to detect and measure the magnitude of neointimal hyperplasia (NIH) at 6 months after DES and BMS implantation compared to IVUS [65]. Although no NIH was detected by IVUS, OCT measured an average of 0.72 ± 0.63 mm2 of NIH. Actually, 92.7 ± 15% of struts were covered by tissue, 5 ± 8.4% of struts were uncovered and 2.3 ± 3% of struts were malapposed. The heterogeneity of minimal tissue coverage after DES was obvious by OCT when compared with IVUS, which showed a similar ‘‘no NIH’’ (Fig. 1).
Fig. 2.
% neointimal hyperplasia evaluated by both OCT and IVUS: from ODESSA trial. OCT is more sensitive for small amounts of neointimal reaction observed in sirolimus-eluting stents (SES) and paclitaxel-eluting stents (PES) compared to zotarolimus-eluting stents (ZES) and bare metal stents (BMS), although both modalities are similarly sensitive for high % of neointimal hyperplasia (figure is courtesy of Dr Guagliumi) [44]
Fig. 1.
Heterogeneity of strut coverage of sirolimus-eluting stents. Corresponding IVUS and OCT images for each line presented in angiography were presented, respectively. All images were selected from ODESSA trial population
2. Methodology of OCT image acquisition and analysis
OCT image acquisition method, blind analysis, definitions of coverage/malapposition, analysis interval.
OCT images were acquired using a commercially available TD-OCT system. Therefore, all trials, except one trial [48], which used the flushing technique, used balloon occlusion for blood clearance.
OCT provides unprecedented details of the vessel wall-stent-lumen interface but must be used judiciously. OCT image interpretation must be performed in a blind fashion, without the knowledge of the type of stent or clinical scenario, to avoid potential bias and misinterpretation of the various facts and artifacts that one can visualize.
The analysis performed at an independent core laboratory is considered to be most reliable. Actually, 6 out of 31 trials adopted core laboratory analysis [44–48, 58, 59].
Definition of variables that are evaluated by OCT strut level analysis may influence the results and interestingly, some of the variables are similarly defined between studies, but some are not.
Not all studies described the definition of ‘‘coverage’’ in the manuscript, but the definition of ‘‘uncoverage’’ was similar between all studies, based on the OCT image figures presented in studies. On the contrary, a lack of tissue coverage on the strut blooming was considered to be the universal consensus of ‘‘uncoverage’’.
Except for one study [64] without a clear description of malapposition criteria, malapposition was determined when the distance between the stent inner surface reflection (blooming) and the vessel wall was identified as clearly separated. For this determination, the strut thickness, with the polymer thickness in cases of polymer-coating DES, according to each stent manufacturer’s specification was supposed to be used for calculation. Nevertheless, the value, even for the same stent, is diverse among studies. 15 trials, for which SES were assigned, used the malapposition cut-off value range from 150 to 200 μm. Regarding 4 trials [44–47] that our core laboratory performed blind analysis of, the cut-off values for malapposition were larger than the ones used in other studies, since our methodology measured the distance from the inner surface of strut blooming to the vessel lumen surface and added 20 μm to correct for half-strut blooming artifacts [21]. This discrepancy is related to what distance was actually measured. However, in 4 studies, OCT axial resolution (20–30 μm) was added as a limit of error to the actual strut thickness [36, 53, 57, 66], which might underestimate the incidence of malapposition.
Classification of malapposition is another issue with some discrepancy between trials. Some trials separately assessed malapposition with coverage and without coverage. However, in our core laboratory analysis, we treated malapposition as a single population regardless of tissue coverage. There are several reasons for this. The existence of tissue coverage behind struts cannot be evaluated due to strut shadowing, particularly when tissue coverage is minimal without apparent continuity from the lateral tissues. In such cases, we can just assess the existence of tissue on the inner surface of struts and are not sure of the entire coverage of malapposed struts. Furthermore, while OCT provides micron-level resolution, it does not yet offer functional tissue differentiation. Therefore, so far, we cannot characterize the types of tissue covering the strut surfaces and distinguish between benign or ‘‘malignant’’ tissue coverage, which is represented in autopsy studies, such as fibrin deposition [18]. We also believe that a malapposed segment may disturb the flow, independent of the presence of coverage. Considering the widespread use of OCT and possible future multi-center large OCT trials, a universal consensus on the definition of ‘‘malapposition’’ by OCT is mandatory, and we strongly suggest that malapposed struts with any tissue coverage should not be treated as totally benign phenomena, unless functional tissue characterization can be available.
The analysis interval is also an important variable for the accuracy of incidence of uncoverage. Various intervals, from 0.06 mm (every 1 frame) to 1.0 mm (every 15.6–20 frames) were used in these studies. We evaluated the impact of analysis intervals using our OCT database, including 293 patients, 139,507 cross-sections, and 878,486 struts [67]. The ideal interval for cross-sectional data was highly dependent on the variable of interest. Lumen and stent area by OCT showed low (<10%) variability independent of analysis intervals from 0.3 to 2.4 mm. However, the variability for the percentage of uncovered struts was high (11.7 ± 25.1%) even at every 2 frames (0.12 mm) and reached an unacceptably high variability (51.9 ± 132.5%) at a 15.6 frames (1 mm) analysis interval. From these results, we conclude that a shorter interval is ideally recommended for an accurate quantification of strut coverage/malapposition at a strut level analysis.
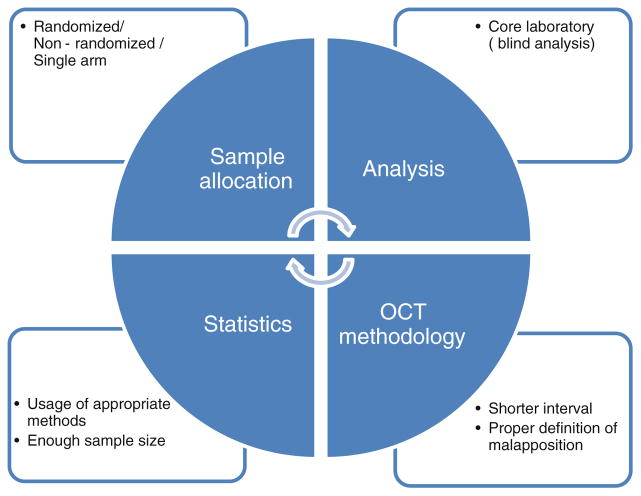
When interpreting the results of OCT clinical studies, 4 important elements should be paid attention to: whether randomization was performed, whether a large enough sample population was included, whether proper methodology of OCT strut level analysis was selected, and whether the statistical method was appropriate (Fig. 3). As an ideal model of OCT clinical trials for comparison of different stent types, we suggest a randomized trial with blind analysis, particularly at a core laboratory to minimize possible study-related biases.
Fig. 3.
Ideal approach for OCT clinical trial design
Guagliumi et al. systematically applied OCT in dedicated prospective, randomized, controlled studies with core laboratory analysis dealing with high risk patient ‘‘off-label indication’’ cohorts, such as long lesions requiring overlapping stents [44], DES for STEMI population [45, 46], and novel bioabsorbable-coating stents [47]. ODESSA is the first OCT randomized trial enrolling 77 consecutive patients with long lesions requiring multiple overlapping stents to assess thickness of coverage and strut apposition of 3 different overlapping long DES, such as SES, PES, zotarolimus-eluting stent (ZES) and BMS at 6 months by OCT and IVUS [44], which showed a trend towards higher incidences of uncovered struts at the overlap site of DES compared to BMS (P = 0.08) and different degrees of strut coverage and NIH among different DES platforms. The percentage of uncovered struts of PES at overlap segments was significantly higher than at non-overlap segments. OCTAMI [46] evaluated neointimal responses in 44 AMI patients between ZES and BMS at a 3:1 ratio, and there were no differences in the percentage of uncovered struts and the percentage of malapposed struts. In the study, they also presented the maximum length of the uncovered segment, which is the longest continuous segment of frames with any uncovered strut, as well as the malapposed segment, which is the longest continuous segment of frames with any malapposed strut, as an arbitrary variable for strut coverage. OCTDESI [47] evaluated the proportion of uncovered struts in PES coated with durable vs. ultrathin biodegradable abluminal polymers (JACTAX-HD, JACTAX-LD), which resulted in similar 6 month rates of uncovered struts per patient. HORIZONS-OCT is an OCT large sub-trial (n = 118) of a multicenter trial (Harmonizing Outcomes with RevascularIZatiON and Stents in Acute Myocardial Infarction) prospectively randomized with 3,602 worldwide patients with STEMI receiving in a 3:1 ratio PES vs. BMS to evaluate long-term (13 months) strut coverage and vessel wall response at an independent core laboratory [45]. Strut coverage rates were lower in PES compared to BMS, although the amount of neointimal thickening was smaller in PES. The LEADERS trial was a multicenter, randomized trial to compare the biolimus-eluting stent (BES) with a biodegradable polymer with SES using a durable polymer. They applied a random-effect model for data analysis of per-strut assessment, and the % of uncoverage was significantly lower in BES compared to SES (P = 0.04), which was similar after adjustment for pre-procedure lesion length, reference vessel diameter, stent number per lesion and the presence of overlap segments.
Future clinical OCT trial
An OCT clinical trial that aims to evaluate the major adverse cardiac event (MACE), especially LST, as a primary endpoint has yet to be conducted. Since the incidence of LST is very low in clinical practice, the inclusion of a large number of populations is needed to identify the characteristics of coronary arteries that are associated with LST. The proposed Massachusetts General Hospital OCT registry plans to include 3,000 patients, starting in June 2010, with follow-ups up to 5 years and is expected to possibly give further insights. The correlation between plaque characteristics and long-term cardiac events, such as myocardial infarctions, would be also evaluated. However, even such a large registry may be underpowered to define predictors of LST and the inherent limitations of registry designs, such as the variability of the study population, lack of control groups, lack of strict data monitoring and follow-up, and unclear endpoints of the study are potential limitations.
SPUM-ACS is another large on-going trial (n = 2,000, PI: Luscher TF, MD, Zurich, Switzer-land) to evaluate MACE, as well as the correlation of vulnerable plaque in an ACS population and clinical outcomes and biomarkers. As a large OCT trial evaluating the safety of one particular stent, the multicenter ABSORB EXTEND Clinical Investigation is including 1,000 patients to continue the assessment of the safety and performance of BVS in 1,000 patients, including performing OCT for all participants.
A number of new OCT trials that evaluate strut coverage of novel stents are ongoing. The TEST-6-OCT trial (n = 45, PI: Mehilli J, MD, PhD, Muenchen, Germany), which assesses the superiority of the biodegradable polymer based limus-eluting stents vs. the permanent polymer based everolimus-eluting stent (EES) in terms of uncoverage at 6–8 months, BASE-OCT (n = 40, PI: Karjalainen PP, MD, PhD, Pori, Finland), which evaluates the completeness of strut coverage and vessel wall response to bio-active-stent (BAS) vs. EES at 6–8 months in an ACS population, the STACCATO trial (n = 60, PI: Adria-enssens T, MD, Leuven, Belgium) that compares strut coverage between EES and BES at 9 months, the COVER OCT (n = 40) and COVER OCT-II (n = 40, PI: Kim JS, MD, PhD, Seoul, Korea) that compare ZES (Endeavor Resolute®) and EES at 9 months and at 3 months, respectively, the PONTI-NA trial (n = 30, PI: Sgueglia GA, Goretti, Italy) that assesses serial neointimal coverage of BES at 6 and 7 months after implantation, the OISTER trial (n = 40, PI: Sangiorgi G, MD, Modena, Italy) that compares the neointimal coverage of a novel trapidil eluting stent vs. PES at 3 months in NSTACS, have just been completed or are on-going trials, the OCT-EVEREST trial (n = 42, PI: Guagliumi G, MD, Bergamo, Italy) which assesses the neointimal coverage of EES at 6 months with post-procedural OCT assessment.
Several OCT trials for evaluating drug (paclit-axel)-eluting balloons (DEB) are also on-going. The OCTOPAS trial (n = 80, PI: Poerner TC, MD, PhD, Jena, Germany) assesses neointimal coverage between EES and BMS predilated with DEB. The SEDUCE trial (n = 50, PI: Adriaenssens T, MD, Leuven, Belgium) will evaluate different healing responses after stenting (BMS and DES) vs. DEB at 9 month follow-up. Although the inclusion number is small, the IN-PACT CORO trial (n = 30, PI: Burzotta F, MD, PhD, Rome, Italy) will compare the neointimal hyperplasia at 6 months in patients with BMS implantation alone compared to those receiving additional DEB use and will assess whether the technique of DEB use (pre or after BMS) may affect the degree of neointimal hyperplasia.
Conclusion
OCT has quickly established itself as a standard tool to evaluate strut coverage for next generation devices. While OCT is providing the information of ‘‘completeness’’ of strut coverage as a surrogate safety endpoint, it is still unknown whether OCT can predict real clinical outcomes, such as restenosis and late stent thrombosis. Upcoming larger clinical OCT trials are expected to answer such questions.
Abbreviations
- OCT
Optical coherence tomography
- DES
Drug-eluting stent
- IVUS
Intravascular ultrasound
- SES
Sirolimus-eluting stent
- PES
Paclitaxel-eluting stent
- ZES
Zotarolimus-eluting stent
- UA
unstable angina
- SA
Stable angina
- CTO
Chronic total occlusion
- MACE
Major cardiovascular event
- MI
Myocardial infarction
- AMI
Acute myocardial infarction
- LST
Late stent thrombosis
- OLP
Overlap
- TLR
Target lesion revascularization
- AUC
Area under the receiver operating characteristic curve
- PCI
Percutaneous coronary intervention
- NIH
Neointimal hyperplasia
- TD-OCT
Time-domain optical coherence tomography
- FD-OCT
Fourier-domain optical coherence tomography
- TVR
Target vessel revascularization
- NSTACS
Non-ST elevation acute coronary syndrome
- CAD
Coronary artery disease
- CI
Confidence interval
- BMS
Bare metal stent
- PI
Principle investigator
- EES
Everolimus-eluting stent
- BAS
Bio-active stent
- DEB
Drug-eluting balloon
- BES
Biolimus-eluting stent
Footnotes
Conflict of interest
None.
Contributor Information
Satoko Tahara, Cardiovascular Research Core Laboratory, University Hospitals Case Medical Center, Cleveland, OH, USA.
Daniel Chamié, Cardiovascular Research Core Laboratory, University Hospitals Case Medical Center, Cleveland, OH, USA.
Motaz Baibars, Cardiovascular Research Core Laboratory, University Hospitals Case Medical Center, Cleveland, OH, USA.
Chadi Alraies, Cardiovascular Research Core Laboratory, University Hospitals Case Medical Center, Cleveland, OH, USA.
Marco Costa, Email: Marco.Costa@Uhhospitals.org, Harrington McLaughlin Heart and Vascular Institute, University Hospitals Case Medical Center, 11100 Euclid Avenue, Cleveland, OH 44106-5038, USA.
References
- 1.Morice MC, Serruys PW, Sousa JE, et al. Randomized study with the sirolimus-coated Bx velocity balloon-expandable stent in the treatment of patients with de novo native coronary artery lesions. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. RAVEL study group. N Engl J Med. 2002;346:1773–1780. doi: 10.1056/NEJMoa012843. [DOI] [PubMed] [Google Scholar]
- 2.Stone GW, Ellis SG, Cox DA, et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. TAXUS-IV investigators. N Engl J Med. 2004;350:221–231. doi: 10.1056/NEJMoa032441. [DOI] [PubMed] [Google Scholar]
- 3.Sousa JE, Costa MA, Abizaid A, et al. Sirolimus-eluting stent for the treatment of in-stent restenosis: a quantitative angiography and three-dimensional intravascular ultrasound study. Circulation. 2003;107:24–27. doi: 10.1161/01.cir.0000047063.22006.41. [DOI] [PubMed] [Google Scholar]
- 4.Stone GW, Ellis SG, Cannon L, Mann JT, Greenberg JD, Spriggs D, et al. Comparison of a polymer-based paclitaxel-eluting stent with a bare metal stent in patients with complex coronary artery disease. A randomized controlled trial. JAMA. 2005;294:1215–1223. doi: 10.1001/jama.294.10.1215. [DOI] [PubMed] [Google Scholar]
- 5.Thuesen L, Kelbaek H, Kløvgaard L, et al. Comparison of sirolimus-eluting and bare metal stents in coronary bifurcation lesions: subgroup analysis of the stenting coronary arteries in non-stress/Benestent disease trial (SCANDSTENT) Am Heart J. 2006;152:1140–1145. doi: 10.1016/j.ahj.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 6.Pasceri V, Patti G, Speciale G, et al. Meta-analysis of clinical trials on use of drug-eluting stents for treatment of acute myocardial infarction. Am Heart J. 2007;153:749–754. doi: 10.1016/j.ahj.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Baumgart D, Klauss V, Baer F, et al. One-year results of the SCORPIUS study. A german multicenter investigation on the effectiveness of sirolimus-eluting stents in diabetic patients. J Am Coll Cardiol. 2007;50:1627–1634. doi: 10.1016/j.jacc.2007.07.035. [DOI] [PubMed] [Google Scholar]
- 8.Camenzind E, Steg PG, Wijns W. Stent thrombosis late after implantation of first-generation drug-eluting stents: a cause for concern. Circulation. 2007;115:1440–1455. doi: 10.1161/CIRCULATIONAHA.106.666800. [DOI] [PubMed] [Google Scholar]
- 9.Daemen J, Wenawesser P, Tsuchida K, Abrecht L, Vaina S, Morger C, et al. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet. 2007;369:667–678. doi: 10.1016/S0140-6736(07)60314-6. [DOI] [PubMed] [Google Scholar]
- 10.Lagerqvist B, James SK, Stenestrand U, Lindbäck J, Nilsson T, Wallentin L SCAAR Study Group. Long-term outcomes with drug-eluting stents versus bare-metal stents in Sweden. N Engl J Med. 2007;356:1009–1019. doi: 10.1056/NEJMoa067722. [DOI] [PubMed] [Google Scholar]
- 11.Farb A, Heller PF, Shroff S, et al. Pathological analysis of local delivery of paclitaxel via a polymer-coated stent. Circulation. 2001;104:473–479. doi: 10.1161/hc3001.092037. [DOI] [PubMed] [Google Scholar]
- 12.Guagliumi G, Farb A, Musumeci G, et al. Images in cardiovascular medicine: sirolimus-eluting stent implanted in human coronary artery for 16 months: pathological findings. Circulation. 2003;107:1340–1341. doi: 10.1161/01.cir.0000062700.42060.6f. [DOI] [PubMed] [Google Scholar]
- 13.Sousa JE, Costa MA, Farb A, et al. Images in cardiovascular medicine: vascular healing 4 years after the implantation of sirolimus-eluting stent in humans: a his-tophatological examination. Circulation. 2004;110:e5–e6. doi: 10.1161/01.CIR.0000134307.00204.B3. [DOI] [PubMed] [Google Scholar]
- 14.Virmani R, Guagliumi G, Farb A, et al. Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent: should we be cautious? Circulation. 2004;109:701–705. doi: 10.1161/01.CIR.0000116202.41966.D4. [DOI] [PubMed] [Google Scholar]
- 15.Joner M, Finn AV, Farb A, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48:193–202. doi: 10.1016/j.jacc.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 16.Luscher TF, Steffel J, Eberli FR, et al. Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications. Circulation. 2007;115:1051–1058. doi: 10.1161/CIRCULATIONAHA.106.675934. [DOI] [PubMed] [Google Scholar]
- 17.Finn AV, Joner M, Nakazawa G, et al. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation. 2007;115:2435–2441. doi: 10.1161/CIRCULATIONAHA.107.693739. [DOI] [PubMed] [Google Scholar]
- 18.Nakazawa G, Finn AV, Joner M, et al. Delayed arterial healing and increased late stent thrombosis at culprit sites after drug-eluting stent placement for acute myocardial infarction patients: an autopsy study. Circulation. 2008;118:1138–1145. doi: 10.1161/CIRCULATIONAHA.107.762047. [DOI] [PubMed] [Google Scholar]
- 19.Guagliumi G, Sirbu V. Optical coherence tomography: high resolution intravascular imaging to evaluate vascular healing after coronary stenting. Catheter Cardiovasc Interv. 2008;72:237–247. doi: 10.1002/ccd.21606. [DOI] [PubMed] [Google Scholar]
- 20.Tanigawa J, Barlis P, Di Mario C, et al. Intravascular optical coherence tomography: optimization of image acquisition and quantitative assessment of stent strut apposition. EuroIntervention. 2007;3:128–136. [PubMed] [Google Scholar]
- 21.Bezerra HG, Costa MA, Guagliumi G, et al. Intra-coronary optical coherence tomography: a comprehensive review: clinical and research applications. JACC Cardiovasc Interv. 2009;2:1035–1046. doi: 10.1016/j.jcin.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joner M, Nakazawa G, Finn AV, et al. Endothelial cell recovery between comparator polymer-based drug-eluting stents. J Am Coll Cardiol. 2008;52:333–342. doi: 10.1016/j.jacc.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 23.Klugherz BD, Llanos G, Lieuallen W, et al. Twenty-eight-day efficacy and pharmacokinetics of the sirolimus-eluting stent. Coron Artery Dis. 2002;13:183–188. doi: 10.1097/00019501-200205000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki T, Kopia G, Hayashi S, et al. Stent-based delivery of sirolimus reduces neointimal formation in a porcine coronary model. Circulation. 2001;104:1188–1193. doi: 10.1161/hc3601.093987. [DOI] [PubMed] [Google Scholar]
- 25.Carter AJ, Aggarwal M, Kopia GA, et al. Long-term effects of polymer-based, slow-release, sirolimus-eluting stents in porcine coronary model. Cardiovasc Res. 2004;63:617–624. doi: 10.1016/j.cardiores.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 26.Regar E, Werner F, Siebert U, et al. Reproducibility of neointima quantification with motorized intravascular ultrasound pullback in stented coronary arteries. Am Heart J. 2000;139:632–637. doi: 10.1016/s0002-8703(00)90040-1. [DOI] [PubMed] [Google Scholar]
- 27.Cook S, Wenaweser P, Togni M, et al. Incomplete stent apposition and very late stent thrombosis after drug-eluting stent implantation. Circulation. 2007;115:2426–2434. doi: 10.1161/CIRCULATIONAHA.106.658237. [DOI] [PubMed] [Google Scholar]
- 28.Alfonso F, Suárez A, Pérez-Vizcayno MJ, et al. Intravascular ultrasound findings during episodes of drug-eluting stent thrombosis. J Am Coll Cardiol. 2007;50:2095–2097. doi: 10.1016/j.jacc.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 29.Lee CW, Kang SJ, Park DW, et al. Intravascular ultrasound findings in patients with very late stent thrombosis after either drug-eluting or bare-metal stent implantation. J Am Coll Cardiol. 2010;55:1936–1942. doi: 10.1016/j.jacc.2009.10.077. [DOI] [PubMed] [Google Scholar]
- 30.Hassan AK, Bergheanu SC, Stijnen T, et al. Late stent malapposition risk is higher after drug-eluting stent compared with bare-metal stent implantation and associates with late stent thrombosis. Eur Heart J. 2010;31:1172–1180. doi: 10.1093/eurheartj/ehn553. [DOI] [PubMed] [Google Scholar]
- 31.Fujii K, Carlier SG, Mintz GS, et al. Stent under-expansion and residual reference segment stenosis are related to stent thrombosis after drug-eluting stent implantation. An intravascular ultrasound study. J Am Coll Cardiol. 2005;45:995–998. doi: 10.1016/j.jacc.2004.12.066. [DOI] [PubMed] [Google Scholar]
- 32.Okabe T, Mintz GS, Buch AN, Roy P, et al. Intra-vascular ultrasound parameters associated with stent thrombosis after drug-eluting stent deployment. Am J Cardiol. 2007;100:615–620. doi: 10.1016/j.amjcard.2007.03.072. [DOI] [PubMed] [Google Scholar]
- 33.Lermusiaux P, Martinez R, Donadey A, et al. Intravascular ultrasound: limitations and prospects. J Mal Vasc. 2000;25:229–236. [PubMed] [Google Scholar]
- 34.Di Mario C, Gorge G, Peters R, et al. Clinical application and image interpretation in intracoronary ultrasound. Study group on intracoronary imaging of the working group of coronary circulation and of the subgroup on intravascular ultrasound of the working group of echocardiography of the european society of cardiology. Eur Heart J. 1998;19:207–229. doi: 10.1053/euhj.1996.0433. [DOI] [PubMed] [Google Scholar]
- 35.Di Mario C, Barlis P. Optical coherence tomography: a new tool to detect tissue coverage in drug-eluting stents. JACC Cardiovasc Interv. 2008;1:174–175. doi: 10.1016/j.jcin.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 36.Matsumoto D, Shite J, Shinke T, et al. Neointimal coverage of sirolimus-eluting stents at 6-month follow-up: evaluated by optical coherence tomography. Eur Heart J. 2007;28:961–967. doi: 10.1093/eurheartj/ehl413. [DOI] [PubMed] [Google Scholar]
- 37.Sera F, Awata M, Uematsu M, et al. Optimal stent-sizing with intravascular ultrasound contributes to complete neointimal coverage after sirolimus-eluting stent implantation assessed by angioscopy. JACC Cardiovasc Interv. 2009;2:989–994. doi: 10.1016/j.jcin.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 38.Kotani J, Awata M, Nanto S, et al. Incomplete ne-ointimal coverage of sirolimus-eluting stents: angioscopic findings. J Am Coll Cardiol. 2006;47:2108–2111. doi: 10.1016/j.jacc.2005.11.092. [DOI] [PubMed] [Google Scholar]
- 39.Higo T, Ueda Y, Oyabu J, et al. Atherosclerotic and thrombogenic neointima formed over sirolimus drug-eluting stent: an angioscopic study. JACC Cardiovasc Imaging. 2009;2:616–624. doi: 10.1016/j.jcmg.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 40.Awata M, Kotani J, Uematsu M, et al. Serial angioscopic evidence of incomplete neointimal coverage after sirolimus-eluting stent implantation: comparison with bare-metal stents. Circulation. 2007;116:910–916. doi: 10.1161/CIRCULATIONAHA.105.609057. [DOI] [PubMed] [Google Scholar]
- 41.Prati F, Zimarino M, Stabile D, et al. Does optical coherence tomography identify arterial healing after stenting? An in vivo comparison with histology on a rabbit carotid model. Heart. 2008;94:217–294. doi: 10.1136/hrt.2006.112482. [DOI] [PubMed] [Google Scholar]
- 42.Murata A, Wallace-Bradley D, Tellez A, et al. Accuracy of optical coherence tomography in the evaluation of neointimal coverage after stent implantation. JACC Cardiovasc Imaging. 2010;3:76–84. doi: 10.1016/j.jcmg.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki Y, Ikeno F, Koizumi T, et al. In vivo comparison between optical coherence tomography and intra-vascular ultrasound for detecting small degrees of in-stent neointima after stent implantation. JACC Cardiovasc Interv. 2008;1:168–173. doi: 10.1016/j.jcin.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 44.Guagliumi G, Musumeci G, Sirbu V, Bezerra HG, Suzuki N, Fiocca L, Matiashvili A, Lortkipanidze N, Trivisonno A, Valsecchi O, Biondi-Zoccai G, Costa MA. Optical coherence tomography assessment of in vivo vascular response following implantation of overlapping bare-metal and drug-eluting stents. JACC Cardivasc Interv. 2010;3:531–539. doi: 10.1016/j.jcin.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 45.Guagliumi G, Sirbu V, Costa MA, Musumeci G, Trivisonno A, Matiashvili A, Lortkipanidze N, Mihalcsik L, Valsecchi O, Bezerra HG, Suzuki N, Coletta J, Mintz G, Maehara A, Parise H, Lansky A, Cristea E, Mehran R, Stone G. Long-term strut coverage of paclitaxel eluting stents compared to bare-metal stents implanted during primary PCI in acute myocardial infarction: HORIZONS-OCT. Circulation. 2011;123:274–281. doi: 10.1161/CIRCULATIONAHA.110.963181. [DOI] [PubMed] [Google Scholar]
- 46.Guagliumi G, Sirbu V, Bezerra HG, Zoccai GB, Fiocca L, Musumeci G, Matiashvili A, Lortkipanidze N, Tahara S, Valsecchi O, Costa MA. Strut coverage and vessel wall response to zotarolimus-eluting and bare metal stents implanted in patients with ST-elevation myocardial infarction: the OCTAMI (optical coherence tomography in acute myocardial infarction) study. JACC Cardiovasc Interv. 2010;3:680–687. doi: 10.1016/j.jcin.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 47.Guagliumi G, Sirbu V, Musumeci G, Bezerra HG, Aprile A, Kyono H, Fiocca L, Mathiasvili A, Lortkipanidze N, Vassileva A, Popma JJ, Allocco DJ, Dawkins KD, Valsecchi O, Costa MA. Strut coverage and vessel wall response to a new generation paclitaxel-eluting stent with an ultrathin biodegradable abluminal polymer: optical coherence tomography drug eluting stent investigation (OCTDESI) Circ Cardiovasc Interv. 2010;3:367–375. doi: 10.1161/CIRCINTERVENTIONS.110.950154. [DOI] [PubMed] [Google Scholar]
- 48.Barlis P, Regar E, Serruys PW, Dimopoulos K, van der Giessen WJ, van Geuns RJ, Ferrante G, Wandel S, Windecker S, van Es GA, Eerdmans P, Jüni P, di Mario C. An optical coherence tomography study of a biodegradable vs. durable polymer-coated limus-eluting stent: a LEADERS trial sub-study. Eur Heart J. 2010;31:165–176. doi: 10.1093/eurheartj/ehp480. [DOI] [PubMed] [Google Scholar]
- 49.Kim JS, Hong MK, Fan C, Kim TH, Shim JM, Park SM, Ko YG, Choi D, Jang Y. Intracoronary thrombus formation after drug-eluting stents implantation: optical coherence tomographic study. Am Heart J. 2010;159:278–283. doi: 10.1016/j.ahj.2009.11.029. [DOI] [PubMed] [Google Scholar]
- 50.Murakami D, Takano M, Yamamoto M, Inami S, Ohba T, Seino Y, Mizuno K. Advanced neointimal growth is not associated with a low risk of instent thrombus. Optical coherence tomographic findings after first-generation drug-eluting stent implantation. Circ J. 2009;73:1627–1634. doi: 10.1253/circj.cj-08-1166. [DOI] [PubMed] [Google Scholar]
- 51.Fan C, Kim JS, Lee JM, Kim TH, Park SM, Wi J, Paik SI, Ko YG, Choi D, Hong MK, Jang Y. Different vascular healing patterns with various drug-eluting stents in primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: optical coherence tomographic findings. Am J Cardiol. 2010;105:972–976. doi: 10.1016/j.amjcard.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 52.Kubo T, Imanishi T, Kitabata H, Kuroi A, Ueno S, Yamano T, Tanimoto T, Matsuo Y, Masho T, Takarada S, Tanaka A, Nakamura N, Mizukoshi M, Tomobuchi Y, Akasaka T. Comparison of vascular response after sirolimus-eluting stent implantation between patients with unstable and stable angina pectoris: a serial optical coherence tomography study. JACC Cardiovasc Imaging. 2008;1:475–484. doi: 10.1016/j.jcmg.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 53.Ozaki Y, Okumura M, Ismail TF, Naruse H, Hattori K, Kan S, Ishikawa M, Kawai T, Takagi Y, Ishii J, Prati F, Serruys PW. The fate of incomplete stent apposition with drug-eluting stents: an optical coherence tomography-based natural history study. Eur Heart J. 2010;31:1470–1476. doi: 10.1093/eurheartj/ehq066. [DOI] [PubMed] [Google Scholar]
- 54.Kim JS, Jang IK, Kim JS, Kim TH, Takano M, Kume T, Hur NW, Ko YG, Choi D, Hong MK, Jang Y. Optical coherence tomography evaluation of zotarolimus-eluting stents at 9-month follow-up: comparison with sirolimus-eluting stents. Heart. 2009;95:1907–1912. doi: 10.1136/hrt.2009.167759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen BX, Ma FY, Luo W, Ruan JH, Xie WL, Zhao XZ, Sun SH, Guo XM, Wang F, Tian T, Chu XW. Neointimal coverage of bare-metal and sirolimus-eluting stents evaluated with optical coherence tomography. Heart. 2008;94:566–570. doi: 10.1136/hrt.2007.118679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xie Y, Takano M, Murakami D, Yamamoto M, Okamatsu K, Inami S, Seimiya K, Ohba T, Seino Y, Mizuno K. Comparison of neointimal coverage by optical coherence tomography of a sirolimus-eluting stent versus a bare-metal stent three months after implantation. Am J Cardiol. 2008;102:27–31. doi: 10.1016/j.amjcard.2008.02.091. [DOI] [PubMed] [Google Scholar]
- 57.Katoh H, Shite J, Shinke T, Matsumoto D, Tanino Y, Ogasawara D, Sawada T, Miyoshi N, Kawamori H, Yoshino N, Hirata K. Delayed neointimalization on sirolimus-eluting stents: 6-month and 12-month follow up by optical coherence tomography. Circ J. 2009;73:1033–1037. doi: 10.1253/circj.cj-08-0746. [DOI] [PubMed] [Google Scholar]
- 58.Serruys PW, Ormiston JA, Onuma Y, Regar E, Gonzalo N, Garcia-Garcia HM, Nieman K, Bruining N, Dorange C, Miquel-Hébert K, Veldhof S, Webster M, Thuesen L, Dudek D. A bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2-year outcomes and results from multiple imaging methods. Lancet. 2009;373:897–910. doi: 10.1016/S0140-6736(09)60325-1. [DOI] [PubMed] [Google Scholar]
- 59.Ormiston JA, Serruys PW, Regar E, Dudek D, Thuesen L, Webster MW, Onuma Y, Garcia-Garcia HM, McGreevy R, Veldhof S. A bioabsorbable everolimus-eluting coronary stent system for patients with single de novo coronary artery lesions (ABSORB): a prospective open-label trial. Lancet. 2008;371:899–907. doi: 10.1016/S0140-6736(08)60415-8. [DOI] [PubMed] [Google Scholar]
- 60.Tamburino C, La Manna A, Di Salvo ME, Sacchetta G, Capodanno D, Mehran R, Dangas G, Corcos T, Prati F. First-in-man 1-year clinical outcomes of the catania coronary stent system with nanothin polyzene-F in de novo native coronary artery lesions: the ATLANTA (assessment of the latest non-thrombogenic angioplasty stent) trial. JACC Cardiovasc Interv. 2009;2:197–204. doi: 10.1016/j.jcin.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 61.Takano M, Inami S, Jang IK, Yamamoto M, Murakami D, Seimiya K, Ohba T, Mizuno K. Evaluation by optical coherence tomography of neointimal coverage of sirolimus-eluting stent three months after implantation. Am J Cardiol. 2007;99:1033–1038. doi: 10.1016/j.amjcard.2006.11.068. [DOI] [PubMed] [Google Scholar]
- 62.Kim JS, Jang IK, Fan C, Kim TH, Kim JS, Park SM, Choi EY, Lee SH, Ko YG, Choi D, Hong MK, Jang Y. Evaluation in 3 months duration of neointimal coverage after zotarolimus-eluting stent implantation by optical coherence tomography: the ENDEAVOR OCT trial. JACC Cardiovasc Interv. 2009;2:1240–1247. doi: 10.1016/j.jcin.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 63.Finn AV, Nakazawa G, Joner M, Kolodgie FD, Mont EK, Gold HK, Virmani R. Vascular responses to drug eluting stents: importance of delayed healing. Arterioscler Thromb Vasc Biol. 2007;27:1500–1510. doi: 10.1161/ATVBAHA.107.144220. [DOI] [PubMed] [Google Scholar]
- 64.Tian F, Chen YD, Sun ZJ, Chen L, Yuan F, Song XT, Lü SZ. Evaluation of neointimal coverage of overlapping sirolimus-eluting stents by optical coherence tomography. Chin Med J. 2009;20(122):670–674. [PubMed] [Google Scholar]
- 65.Bezerra HG, Guagliumi G, Valescchi O, Lortkipanidze N, Rosenthal N, Tahara S, Kyono H, Simon DI, Costa MA. Unraveling the lack of neointimal hyperplasia detected by IVUS using OCT: lack of spatial resolution or a true biological effect? The ODESSA sub-study. J Am Coll Cardiol. 2009;53:2523. [Google Scholar]
- 66.Ishigami K, Uemura S, Morikawa Y, Soeda T, Okayama S, Nishida T, Takemoto Y, Onoue K, Somekawa S, Takeda Y, Kawata H, Horii M, Saito Y. Long-term follow-up of neointimal coverage of sirolimus-eluting stents—evaluation with optical coherence tomography. Circ J. 2009;73:2300–2307. doi: 10.1253/circj.cj-08-1116. [DOI] [PubMed] [Google Scholar]
- 67.Bezerra HG, Guagliumi G, Kyono H, Tahara S, Rosenthal N, Musumeci G, Sirbu V, Valescchi O, Aprile A, Simon DI, Costa MA. Determining the optimal cross-sectional analysis interval for OCT assessment of coronary stenting. Circulation. 2009;120:S1000. [Google Scholar]
- 68.Yao ZH, Matsubara T, Inada T, Suzuki Y, Suzuki T. Neointimal coverage of sirolimus-eluting stents 6 months and 12 months after implantation: evaluation by optical coherence tomography. Chin Med J. 2008;121:503–507. [PubMed] [Google Scholar]