Highlights
-
•
Striga spp. resistance in NERICA cultivars, observed in vitro, was confirmed in situ.
-
•
NERICA-2, -10 (S. asiatica) and -10, -17 (S. hermonthica) are most resistant.
-
•
NERICA-1, -17, -9 (S. asiatica) and -1, -17, -10 (S. hermonthica) show tolerance.
-
•
Some NERICA cultivars yielded 1.5–2 t ha−1 despite Striga spp. infestation.
Keywords: Parasitic weeds, Tolerance, Upland rice, Oryza sativa, Oryza glaberrima, Africa
Abstract
The parasitic weeds Striga asiatica and Striga hermonthica cause high yield losses in rain-fed upland rice in Africa. Two resistance classes (pre- and post-attachment) and several resistant genotypes have been identified among NERICA (New Rice for Africa) cultivars under laboratory conditions (in vitro) previously. However, little is known about expression of this resistance under field conditions. Here we investigated (1) whether resistance exhibited under controlled conditions would express under representative Striga-infested field conditions, and (2) whether NERICA cultivars would achieve relatively good grain yields under Striga-infested conditions. Twenty-five rice cultivars, including all 18 upland NERICA cultivars, were screened in S. asiatica-infested (in Tanzania) and S. hermonthica-infested (in Kenya) fields during two seasons. Additionally, a selection of cultivars was tested in vitro, in mini-rhizotron systems. For the first time, resistance observed under controlled conditions was confirmed in the field for NERICA-2, -5, -10 and -17 (against S. asiatica) and NERICA-1 to -5, -10, -12, -13 and -17 (against S. hermonthica). Despite high Striga-infestation levels, yields of around 1.8 t ha−1 were obtained with NERICA-1, -9 and -10 (in the S. asiatica-infested field) and around 1.4 t ha−1 with NERICA-3, -4, -8, -12 and -13 (in the S. hermonthica-infested field). In addition, potential levels of tolerance were identified in vitro, in NERICA-1, -17 and -9 (S. asiatica) and in NERICA-1, -17 and -10 (S. hermonthica). These findings are highly relevant to rice agronomists and breeders and molecular geneticists working on Striga resistance. In addition, cultivars combining broad-spectrum resistance with good grain yields in Striga-infested fields can be recommended to rice farmers in Striga-prone areas.
1. Introduction
In sub-Saharan Africa (SSA), rice is an increasingly important cereal crop (Seck et al., 2012) in rain-fed agro-ecosystems. Of the total area under rice in SSA, 32% can be characterized as rain-fed upland, with average estimated yields of around 1.2 t ha−1 (Diagne et al., 2013). The extremely low productivity in these rain-fed upland environments is caused by a myriad of bio-physical and socio-economic constraints (e.g. Balasubramanian et al., 2007). Major production constraints for smallholder farms in rain-fed agro-ecosystems in Africa are drought, poor soil fertility and weeds (Waddington et al., 2010). Weed species that are frequently observed on these poorly fertile and drought-prone soils are those of the parasitic Orobanchaceae family (e.g. Parker, 2009, Rodenburg and Johnson, 2009). Striga spp., in particular Striga hermonthica (Del.) Benth. and Striga asiatica (L.) Kuntze, are the most wide-spread and economically important species of parasitic weeds in cereal cropping systems (e.g. Mohamed et al., 2001, Rodenburg et al., 2010, Spallek et al., 2013).
Striga species negatively affect the growth and yield of the crops they infect (e.g. Webb and Smith, 1996, Frost et al., 1997, Gurney et al., 1999). However, the extent of these negative effects is a function of the environment and the genetic make-up of the host and parasite. Host plant genotypes may show various levels, mechanisms and combinations of resistance and tolerance to Striga species, where resistance (antonym: susceptibility) reduces the Striga infection levels and tolerance (antonym: sensitivity) alleviates the effects of infection (e.g. Yoder and Scholes, 2010, Rodenburg and Bastiaans, 2011). Some rice cultivars (e.g. Oryza sativa cultivars IR47255-B-B-5-4, IR49255-B-B-5-2, Nipponbare and IR64 and O. glaberrima cultivars ACC102196, Makassa, CG14 and IG10) exhibit good resistance against some ecotypes of S. hermonthica whilst other cultivars (e.g. IAC165 and Koshihikari) are susceptible (Harahap et al., 1993, Johnson et al., 1997, Gurney et al., 2006, Kaewchumnong and Price, 2008, Swarbrick et al., 2009). High genetic variability has also been observed among different species and ecotypes (i.e. genetically distinct populations within a species) of the parasite (Botanga et al., 2002, Huang et al., 2012), making Striga management complex as the resistance found in some cultivars may be overcome by a small subset of Striga individuals within the seed bank leading to the development of a virulent population over time (e.g. Rodenburg and Bastiaans, 2011, Kountche et al., 2013).
Nevertheless, the use of Striga resistant cultivars is widely considered as one of the most suitable and effective control options for resource-poor farmers (Haussmann et al., 2000). However, very few rice cultivars are known that combine resistance to Striga species and/or ecotypes, with adaptability to African upland rice growing environments (Rodenburg et al., 2010). This gap can potentially be filled by a group of inter-specific, NERICA (‘New Rice for Africa’) rice cultivars which are widely distributed and adopted across Africa (Diagne, 2006, Kijima et al., 2006, Wopereis et al., 2008). The NERICA cultivars are the progeny of crosses between the African rice species Oryza glaberrima (Steud.) and the Asian rice species Oryza sativa (L.). They were generated to combine the weed competitiveness and resilience to abiotic and biotic stresses of the African rice species with the high yield and grain quality of the Asian rice species (Jones et al., 1997a, Jones et al., 1997b).
Recently, Jamil et al. (2011a) and Cissoko et al. (2011) evaluated the 18 upland NERICA cultivars released so far (and their parental genotypes) for pre- and post-attachment resistance respectively, against different Striga species and ecotypes under controlled environment conditions. Pre-attachment resistance entails all mechanisms that hamper the development of the parasite before attachment to the host root. Post-attachment resistance are all mechanisms that prevent or hamper the attached parasite to establish the necessary xylem–xylem connection with the host root. Some of the NERICA cultivars displayed an excellent degree of post-attachment resistance against ecotypes of S. hermonthica and S. asiatica (Cissoko et al., 2011). Also, variation in the quantity and type of strigolactone production, and consequently the ability to germinate S. hermonthica seeds was found among this group of rice cultivars. Those that produced low amounts of strigolactones showed good levels of pre-attachment resistance (Jamil et al., 2011a, Jamil et al., 2011b). Some cultivars, e.g. NERICA-1, -3, -4, -12 and -17, showed excellent combinations of pre- and post-attachment resistance which may increase the durability of resistance under field conditions. However, whilst we now know which of the NERICA cultivars show resistance to Striga spp. and ecotypes under highly controlled growth conditions, we know much less about the impact of environment on the expression of resistance, hence whether the resistance exhibited by some cultivars in vitro (laboratory) will be effective in situ (field). In addition, apart from a study by Atera et al. (2012) where a selection of four NERICA cultivars was grown under S. hermonthica-infested conditions, there is no published information about the adaptability and yield performance of different NERICA cultivars under Striga-infested field conditions.
The objectives of this study were therefore to determine (1) whether the resistance of the NERICA cultivars identified under controlled environment conditions (i.e. pre- and/or post-attachment resistance) is exhibited as reduced above-ground parasite numbers in S. hermonthica and S. asiatica-infested fields in Africa, and (2) whether cultivars that exhibit good resistance also have good rice grain yields. To achieve this, two seasons of field screening trials were conducted with all 18 upland NERICA cultivars, their parents, and known susceptible, resistant and local check cultivars, in two different locations, at Kyela, Tanzania (where fields are infested with S. asiatica) and at Mbita, Kenya (under S. hermonthica infestation). In addition, the resistance of selected cultivars was investigated under controlled environment conditions following infection with the Striga ecotypes obtained from the field sites.
2. Materials and methods
2.1. Plant materials
All 18 interspecific upland NERICA rice cultivars, NERICA-1 to -18, their O. glaberrima parent, CG14, and O. sativa ssp. japonica parents, WAB56-104, WAB56-50 and WAB181-18, were grown in Striga-infested plots at Kyela, Tanzania under S. asiatica infestation, and at Mbita, Kenya under S. hermonthica infestation. In addition to these 22 cultivars, in Kyela, two traditional and locally popular cultivars, Supa India (synonym: Kilombero; included as locally adapted but Striga-susceptible check) and Mwangulu (included as locally adapted and Striga-resistant check), and an international cultivar originally from Brazil, IAC165 (Oryza sativa ssp. Japonica; included as Striga-susceptible check), were selected, making a total of 25 cultivars. For the trials in Mbita, Mwangulu was replaced by the cultivar IR49255-B-B-5-2 (O. sativa ssp. Indica; included as resistant check) (identified by Harahap et al., 1993, Johnson et al., 1997). Seeds of all rice cultivars were obtained from the Africa Rice Center (AfricaRice), Cotonou, Benin except for Supa India and Mwangulu, which were supplied by the Agricultural District Office of Kyela. Seeds of S. asiatica and S. hermonthica were collected in the previous season from plants parasitizing rice at Kyela, Tanzania (Sa-Kyela) and maize at Mbita, Kenya (Sh-Mbita) in farmer’ fields surrounding the experimental field sites. These seeds were used to supplement the soil seed bank in the field trials as well as for the controlled environment studies.
2.2. Experimental sites
The S. asiatica field screening trials were conducted during the rainy seasons (February–July) of 2011 and 2012 in Mbako (9°35′ S–33°48′ E; 525 m a.s.l.), a village approximately 15 km from Kyela, in Kyela district, Mbeya region in southern Tanzania (Table 1). The district is part of the Southern Highlands and located in the west arm of the African Rift Valley on the shores of Lake Malawi. Kyela district is a S. asiatica-infested upland rice-growing area. Since no experimental station in Africa exists where screening work in S. asiatica-infested fields can be conducted, we opted to execute this work in two already infested farmers’ fields in this S. asiatica endemic area. Cumulative rainfall measured in the field during the trials was 2474 mm in 2011 and 2499 mm in 2012 (Table 1). In 2012 two field trials were conducted. The first was carried out in the same field as the 2011 trial and is referred to as Kyela 2012-1. This field trial was duplicated in a second field in the same village, approximately 1 km from the first field and is referred to as Kyela 2012-2.
Table 1.
Overview of experimental conditions of the field trials conducted at Kyela, Tanzania (2011 and 2012), and at Mbita, Kenya (2010 and 2011).
| Location | Kyela–Tanzania (S. asiatica) | Mbita–Kenya (S. hermonthica) | |||
|---|---|---|---|---|---|
| (9°37′30′′ S–33°52′30′′E) | (0°42′82′′ S–34°20′53′′ E) | ||||
| Altitude (m a.s.l.) | 525 | 1141 | |||
| Year | 2011 | 2012-1 | 2012-2 | 2010 | 2011 |
| Season/Period | Single rain/Feb–Jun | Short rain/Sep–Jan | Long rain/Mar–Aug | ||
| Cumulative rainfall (mm) | 2474 | 2499 | 281a | 615 | |
| Sowing dates | 09/02/211 | 22/02/2012 | 29/02/2012 | 17/09/2010 | 17/03/2011 |
| Cultivars | 24 + Mwangulu | 24 + IR49255-B-B-5-2 | |||
| Net plot size (m2) | 117.25 m2 | 86 m2 | |||
| Net sub-plot size (m2) | 4.69 m2 | 3.44 m2 | |||
| Fertilizer application | 100 kg ha−1 N-P-K: 20-10-10 | 50 kg ha−1 N-P-K: 17-17-17 | |||
| Striga infestation density (m−2) | 0.91 g (243,000 seedsb) | 0.60 g (85,000 seeds) | |||
| Soil parameters | |||||
| Sand:silt:clay | 63:14:23 | 63:14:23 | 63:13:25 | – | – |
| pH | 5.21 | 4.80 | 4.75 | 5.70 | 5.95 |
| N (%) | 0.10 | 0.11 | 0.11 | 0.60 | 1.46 |
| P (ppm) | 4.11 | 6.8 | 5.9 | – | – |
| K (ppm) | 230 | 218 | 229 | – | – |
Supplementary irrigation was provided.
Seed weights according to Parker and Riches (1993).
The S. hermonthica field screening trials were conducted during the short rainy season of 2010 (September to January) and the long rainy season of 2011 (March to August) at the farm of the International Center of Insect Physiology and Ecology (ICIPE) at Mbita (0°42′ S–34°20′ E; around 1141 m a.s.l.), located on a peninsula in Lake Victoria, Suba District, western Kenya (Table 1). The trial was laid out on a heavily Striga-infested field at the west-side of the peninsula that was formerly under sorghum – cassava rotation. Cumulative rainfall was 281 mm in 2010 (short rain) and 615 mm in 2011 (long rain) (Table 1). Rainfall data were obtained from ICIPE's meteorological station 500 m from the field. Supplementary irrigation (by sprinkler) was applied when rainfall was insufficient (in Mbita only).
2.3. Experimental design, plot sizes and field preparation
All field trials were laid out in a 5 × 5 lattice design with six replicates. At Kyela each plot, representing an individual cultivar, measured 1.25 m × 3.75 m (4.69 m2) and contained 5 rows of 15 hills with a plant distance of 0.25 m × 0.25 m (Table 1). At Mbita each plot measured 1.25 m × 2.75 m (3.44 m2) with 5 rows of 11 rice planting hills with the same plant distance as in Kyela (Table 1). Plots were separated by one open row of 0.25 m to avoid neighbor effects and to allow easy access. Each replicate was separated by a 1.25 m alley.
Each plot received supplementary Striga seeds mixed with white sand. An amount of 4.25 g of S. asiatica seed (germination rate: 55–65%) mixed with 450 g sand at Kyela and 2.07 g of S. hermonthica seed (germination rate: 75–80%) in 450 g sand at Mbita were used, resulting in an infestation density of 0.9 g seed m−2 at Kyela (approx. 146,000 viable S. asiatica seeds) and 0.6 g seeds m−2 at Mbita (approx. 66,000 viable S. hermonthica seeds). The mixture was broadcast and incorporated into the upper 5–10 cm of soil using short-handled-hoes, prior to rice sowing. Implications of additional Striga infestation in the selected farmers’ fields in Kyela were carefully explained to the farmers owning the land, during discussions prior to the experimental seasons. Measures to restore the original conditions were presented and our technical and financial assistance to achieve this was guaranteed.
In all trials, rice was directly sown at approximately 6 seeds per hill, and thinned to 2–3 plants per hill 25 days after sowing (DAS). To arrive at the desired plant density, in some cases gap filling was carried out by using supplemental plants from a rice nursery planted at the edge of the field on the same sowing date. From sowing onwards, each trial was regularly hand weeded (at least every 2–3 weeks) to remove all weeds other than Striga. At both sites fertilizer was applied at 35 DAS. In Kyela N-P-K (20-10-10) was applied at an equivalent rate of 100 kg ha−1, while at Mbita, with relatively nutrient-rich soils, N-P-K (17:17:17) was applied at a rate of 50 kg ha−1 (Table 1).
2.4. Experimental measurements
The number of above-ground Striga plants in each plot, emerged within the central area comprising 27 rice hills, was counted weekly in the Mbita trials, and in Kyela at 57, 85 and 114 DAS (2011), 49, 68, 102 and 118 DAS (2012-1) and 47, 95 and 113 DAS (2012-2). These data enabled the assessment of the maximum number of above-ground Striga plants (NSmax), which is a reliable measure for Striga resistance in the field, following Rodenburg et al. (2005). At harvest emerged Striga plants within each observation area of 27 hills in each plot were collected, dried and weighted for the assessment of Striga biomass, as an additional resistance measure. At harvest, rice panicles were harvested from the same central 27 hills of each plot. Rice panicles were air-dried for 2 weeks after which rice grains were separated from the panicles and weighed. Grain moisture content was assessed, using a digital grain moisture meter of SATAKE (Model SS-7), to correct rice grain dry weights to 14% moisture.
2.5. Phenotyping of Striga resistance levels under controlled environment conditions
To determine the impact of the field environment on the resistance ranking of the NERICA cultivars, a subset of the cultivars was phenotyped for post-attachment resistance under controlled environment conditions at the University of Sheffield using the same ecotypes of S. hermonthica (Sh-Mbita) and S. asiatica (Sh-Kyela) present at the field sites. In addition, the tolerance of these cultivars was assessed as described by Cissoko et al. (2011). Six-day-old single rice seedlings were transferred to rhizotrons, which consist of 25 cm × 25 cm × 2 cm perspex containers packed with vermiculite covered by a 100 μm polyester mesh, with openings at the top and bottom to allow shoot growth and drainage. Ten days later the rice plants were infected with 12.5 mg of germinated S. hermonthica seeds or 20 mg of germinated S. asiatica seeds (Cissoko et al., 2011). Uninfected control plants were treated in a similar manner but without the Striga seeds. Four replicates were evaluated for each cultivar × Striga sp. combination. The cultivars tested were NERICA-1, -7, -9, -10 and -17, CG14, WAB56-104, WAB56-50, WAB181-18, IAC165 and Supa India. Quantification of post-attachment resistance levels was based on mean parasite dry biomass per host root system for the different cultivars. Host tolerance was assessed by plotting the relative host plant biomass of infected plants (i.e. the biomass of parasite infected plants as percentage of the biomass of parasite-free control plants) against the Striga infection level, expressed as the total biomass of the parasitizing plants collected from the host roots.
2.6. Statistical analyses
Prior to analyses, data were checked for homoscedasticity and normality following Sokal and Rohlf (1995). Following these tests, field data on rice grain and Striga dry weights were analyzed using a Linear Mixed Model. We tested whether there was a significant Trial × Cultivar interaction effect for both locations (Kyela and Mbita). We first performed a log-likelihood ratio test for the homogeneity of variance and when the variance was not constant, we combined the data taking into account the heterogeneity of the variances. When the Trial × Cultivar interaction effect was significant (P < 0.05), we fitted a model for each trial separately, where Cultivar was considered as fixed effect and Block, nested into Replicate, and Replicate as random effects. For analyses of maximum above-ground Striga numbers (NSmax) a Generalized Linear Mixed Model (McCullagh and Nelder, 1989) was used under the assumption of a Poisson distribution. Standard Errors of Differences of Means (SED), LS means and associated standard errors were computed. A Squared Euclidian Distance matrix was computed based on LS means and Ward's clustering procedure (Ward 1963), in which incremental sums of squares as fusion criteria, were applied using a hierarchical agglomerative clustering (Kettenring, 2006). Three measures were used for the validation of the results of the cluster analysis (1) Connectivity (Handl et al., 2005), (2) Dunn Index (Dunn, 1974), and (3) Silhouette Width (Rousseeuw, 1987). Each of these measures evaluates the hierarchical clustering while varying the number of clusters. The optimum number of clusters, provided by at least two of these measures, was presented here. This facilitated clustering of the cultivars in statistically distinct groups, based on Striga field resistance and rice grain yield under Striga-infested conditions. Spearman rank correlations were calculated between LS means of NSmax and Striga dry weights and between LS means of NSmax and rice yields. The rhizotron data were analyzed following checks for homoscedasticity and normality. ANOVAs were conducted followed by a comparison of means using Tukey's honest significant difference test. All field data were analyzed using the statistical package Genstat (v. 11), the cluster analysis was performed using the clValid package (Brock et al., 2008) of the R software version 3.1.1 (R-Core-Team, 2014) and the rhizotron data were analyzed using Minitab (v. 15).
3. Results
3.1. How resistant are the NERICA cultivars to S. hermonthica and S. asiatica?
For both locations (Kyela and Mbita) the Trial × Cultivar interaction effects on maximum above-ground Striga numbers were highly significant (P < 0.0001), and therefore data were analyzed separately for each field trial. Rice cultivar had a highly significant effect (P < 0.001) on the maximum number of emerged S. asiatica and S. hermonthica (NSmax) in all screening trials (Table 2). Rice cultivar also significantly affected Striga dry weights at harvest (except in the Kyela 2012-1 trial).
Table 2.
Variance components analysis (F-stat. and F-prob.) and standard errors of differences of means (SED) of cultivar effects on rice grain dry weights (rice grain DW), maximum above-ground Striga numbers (NSmax) and above-ground Striga biomass (dry weights) (Striga DW) at harvest, obtained from S. asiatica (Kyela) and S. hermonthica (Mbita) infested fields during two seasons per location.
| Striga sp.a | Trial | df | Rice grain DW |
NSmaxb |
Striga DW |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| F-stat. | F-prob. | SED | F-stat. | F-prob. | SED | F-stat. | F-prob. | SED | |||
| Sa | Kyela 2011 | 24 | 2.982 | <0.001 | 0.030 | 9.477 | <0.001 | 0.490 | 2.922 | <0.001 | 0.077 |
| Kyela 2012-1 | 24 | 4.953 | <0.001 | 0.025 | 4.172 | <0.001 | 0.496 | 1.255 | 0.217 | 0.096 | |
| Kyela 2012-2 | 24 | 3.058 | <0.001 | 0.026 | 75.408 | <0.001 | 0.304 | 2.017 | 0.009 | 2.430 | |
| Sh | Mbita 2010 | 24 (23)c | 1.881 | 0.019 | 0.048 | 11.115 | <0.001 | 1.060 | 1.946 | 0.012 | 5.008 |
| Mbita 2011 | 24 (23)c | 1.256 | 0.221 | 0.043 | 55.181 | <0.001 | 0.627 | 5.548 | <0.001 | 11.100 | |
Sa = S. asiatica; Sh = S. hermonthica.
Based on a generalized linear model with Poisson distribution.
Grain DW of Mbita trials have 23 degrees of freedom, as Supa India did not reach flowering due to photoperiodicity.
In all the field trials, mean maximum above-ground Striga numbers (NSmax) per cultivar correlated positively and highly significantly (P < 0.001) with the mean Striga dry weights at harvest per cultivar (Spearman correlation coefficients for S. asiatica were r2011 = 0.77, r2012-1 = 0.73, and r2012-2 = 0.76; for S. hermonthica correlation coefficients were r2010 = 0.88 and r2011 = 0.89).
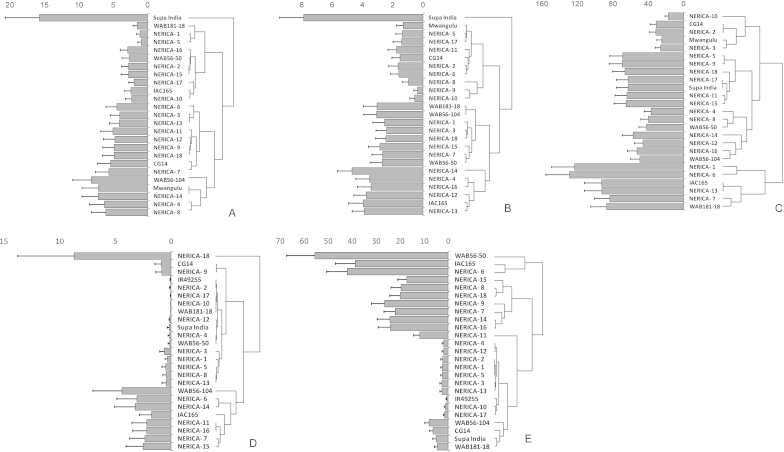
Based on maximum above-ground S. asiatica numbers (NSmax) observed in the field, and using the hierarchical cluster analysis and evaluation measures outlined above, rice cultivars were clustered into three groups, representing different resistance levels in each field trial in Kyela (Fig. 1A–C). In the 2011 field trial, the cluster with the most resistant cultivars, comprised NERICA-1, -5, -2, -10, -15, -16 and -17, WAB181-18, WAB56-50 and IAC165 (Fig. 1A). For the Kyela 2012 -1 field trial (the same field as the 2011 trial), the most resistant cultivars were NERICA-8, -9, -10, -2, -5, -6, -11 and -17 and CG14 and Mwangulu (Fig. 1B). The cultivars at the second field site (Kyela 2012-2) had much higher Striga infestation levels compared to those at the Kyela 2012-1 field site (Fig. 1B and C). The most resistant cultivars in the Kyela 2012-2 trial were NERICA-2, -3 -10, -4, -8, -12, -14 and -16 and CG14, Mwangulu, WAB56-50 and WAB56-104 (Fig. 1C). Table 3, summarizing the groupings based on NSmax using cluster analysis, shows that the cultivars expressing consistently high levels of field resistance against S. asiatica across the two seasons and sites were NERICA-2 -10, -5 and -17.
Fig. 1.
Maximum number of emerged Striga plants m−2 per cultivar for Kyela 2011 (A), 2012-1 (B) and 2012-2 (C) – S. asiatica – and for Mbita 2010 (D) and 2011 (E) – S. hermonthica. Left side: means and standard errors of means; right side: cluster analyses.
Table 3.
Summary of hierarchical cluster analysesa based on maximum above-ground Striga numbers (NSmax), a measure for resistance in the field, observed in the S. asiatica-infested field in Kyela, Tanzania (2011, 2012-1 and 2012-2) and the S. hermonthica-infested field in Mbita, Kenya (2010 and 2011); cluster 1 groups together the most resistant cultivars of a particular screening trial, cluster 2 groups cultivars of intermediate resistance/susceptibility and cluster 3 represents cultivars with susceptibility to a particular Striga species. For each cluster, the mean NSmax (in number of plants m−2) is shown. Underlined are names of cultivars showing consistent good resistance against Striga sp.
| Cluster |
S. asiatica |
S. hermonthica |
|||
|---|---|---|---|---|---|
| 2011 | 2012-1 | 2012–2 | 2010 | 2011 | |
| Resistant | |||||
| 1 | N1b, N5, N2, N10, N15, N16, N17, WAB181-18, WAB56-50, IAC165 | N8, N9, N10, N2, N5, N6, N11, N17, CG14, Mwangulu | N2, N3, N10, N4, N8, N12, N14, N16, N5, N9, N11, N15, N17, N18, CG14, Mwangulu, WAB56-50, WAB56-104, Supa India | N2, N4, N10,N12, N17, N1, N3, N5, N8, N13, N9, WAB181-18, WAB56-50, Supa India, IR49255-B-B-5-2, CG14 | N1, N2, N3, N4, N5, N10, N12, N13, N17, N11, IR49255-B-B-5-2, WAB181-18, WAB56-104, CG14, Supa India |
| Mean | 2.1 | 1.3 | 48.0 | 0.3 | 3.6 |
| Intermediate | |||||
| 2 | N3, N6, N7, N9, N11, N12, N13, N18, N8, N4, N14, CG14, Mwangulu, WAB56-104 | N1, N3, N7, N15, N18, N4, N12, N13, N14, N16, WAB56-50, WAB56-104, WAB181-18, IAC165 | N7, N13, IAC165, WAB181-18 | N6, N7, N11, N14, N15, N16, WAB56-104, IAC165 | N7, N8, N9, N14, N15, N16, N18 |
| Mean | 5.6 | 3.2 | 88.9 | 2.7 | 22.0 |
| Susceptible | |||||
| 3 | Supa India | Supa India | N1, N6 | N18 | N6, WAB56-50, IAC165 |
| Mean | 15.7 | 7.9 | 126.1 | 8.7 | 45.4 |
The Connectivity (Handl et al., 2005), Dunn Index (Dunn, 1974), and Silhouette Width (Rousseeuw, 1987) measures are used to optimize the number of clusters (see Section 2.6).
NERICA cultivars are abbreviated by ‘N’ following the specific number.
Based on maximum above-ground numbers of S. hermonthica (NSmax), and using the hierarchical cluster analysis and evaluation measures outlined above, rice cultivars were clustered again into three groups in both field trials carried out in Mbita (Fig. 1D and E). In 2010, the most resistant cultivars comprised NERICA-2, -4, -10, -12, -17, -1, -3, -5, -8 and -13 and WAB181-18, WAB56-50, Supa India and IR49255-B-B-5-2 (Fig. 1D). In the second season (2011) the Striga infection levels were much higher than in the first season. Here the most resistant cultivars were NERICA-1, -2, -3, -4, -5, -10, -12, -13, -17 and -11, and IR49255-B-B-5-2, WAB181-18, WAB56-50, CG14 and Supa India (Fig. 1E). Following the cluster analysis, the cultivars expressing consistently high levels of field resistance against S. hermonthica were NERICA-1, -2, -3, -4, -5, -10, -12, -13 and -17, and IR49255-B-B-5-2, CG14 and Supa India (Table 3).
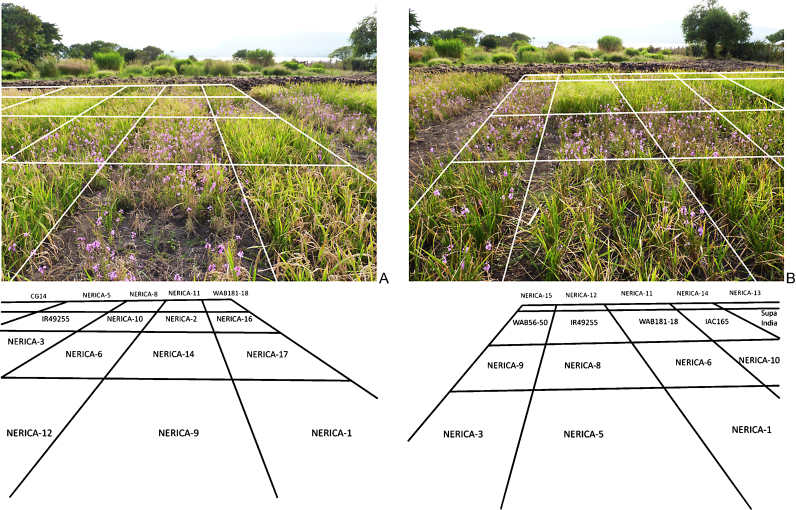
The variation in resistance to S. hermonthica among cultivars is illustrated in Fig. 2, which shows plots within two adjacent replicates of the Mbita 2011 trial. NERICA-1, -10 and -17 have little emerged Striga whereas NERICA-14 and -9 are very susceptible and highly infected by S. hermonthica. Most of the NERICA-9 rice plants in this replicate have died because of the high infection levels (Fig. 2A). The number of Striga plants parasitizing susceptible cultivars WAB56-50 and NERICA-8 and -6 contrasts with the good resistance of NERICA-1, -3 and -5 and the cultivar IR49255-B-B-5-2 (Fig. 2B).
Fig. 2.
Contrasting Striga infection levels in the S. hermonthica screening trial at Mbita, Kenya (July 2011) replicate 6 (A) and replicate 3 (B); Sub-plots, representing cultivars are delimited by white lines.
3.2. Rice grain yields under S. hermonthica and S. asiatica infestation
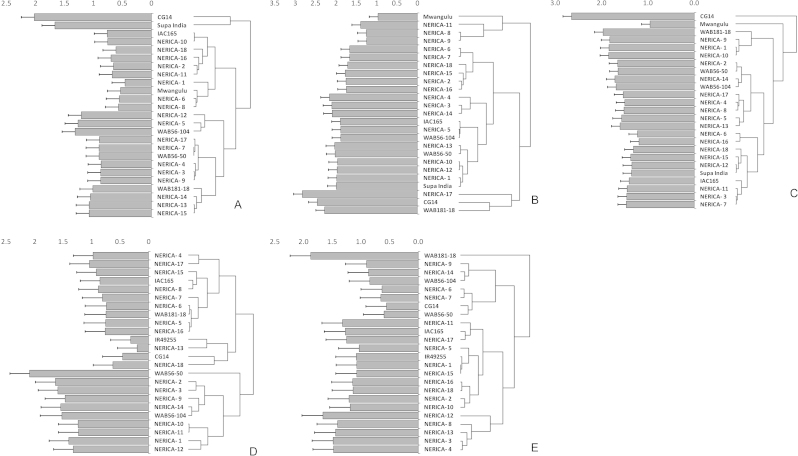
For both locations (Kyela and Mbita) the Trial × Cultivar interaction effects on rice grain dry weights were significant (Kyela: P = 0.0013; Mbita: P = 0.0207), and therefore data were analyzed separately for each field trial. With a few exceptions the extrapolated rice grain yields obtained in both the S. asiatica and the S. hermonthica-infested fields did not exceed 2 t ha−1 (Fig. 3). In the S. asiatica infested fields, average yield per cultivar ranged from 0.4 to 2.0 t ha−1 (average: 0.9 t ha−1) in 2011, and from 0.9 to 2.8 t ha−1 (average: 1.9 t ha−1) and 1.0 to 2.6 t ha−1 (average: 1.6 t ha−1) in 2012 (fields 1 and 2 respectively). Based on rice grain weights obtained under S. asiatica infested conditions, and using the hierarchical cluster analysis and evaluation measures outlined above, cultivars were clustered in three groups (Fig. 3A–C). In 2011, the highest yielding cultivars were CG14 and Supa India (Fig. 3A). The second-highest yielding group consisted of NERICA-5, -12, -3, -4, -7, -9, -13, -14, -15 and -17 and WAB56-104, WAB56-50 and WAB181-18. Cultivars in the remaining clusters yielded well below 1 t ha−1. The cluster with the highest yielding cultivars in the 2012-1 trial contained NERICA-17, CG14 and WAB181-18 (Fig. 3B). The third cluster included NERICA-8, -9 and -11 and Mwangulu and the second cluster included all other cultivars. In the second S. asiatica screening trial conducted in 2012, the highest yielding cultivar was again CG14, with well over 2 t ha−1 (Fig. 3C). The lowest yielding cultivar, in cluster 3, was Mwangulu. The second cluster included all other cultivars, with an average yield of 1.5 t ha−1. The only cultivar expressing consistent high levels of grain yield under S. asiatica infested conditions was CG14 (Table 4).
Fig. 3.
Rice grain dry weights (t ha−1) per cultivar for Kyela 2011 (A), 2012-1 (B) and 2012-2 (C) – S. asiatica – and for Mbita 2010 (D) and 2011 (E) – S. hermonthica. Left side: means and standard errors of means; right side: cluster analyses.
Table 4.
Summary of hierarchical cluster analysesa based on rice grain yields (at 14% grain moisture content), measured in the S. asiatica-infested field in Kyela, Tanzania (2011, 2012-1 and 2012-2) and the S. hermonthica-infested field in Mbita, Kenya (2010 and 2011); cluster 1 groups together the highest yielding cultivars of a particular screening trial, cluster 2 groups cultivars with intermediate high yields, cluster 3 represents cultivars of intermediate low yields and clusters 4 and 5 groups low yielding cultivars. Underlined are names of cultivars showing consistent good yields under Striga sp. infestation. For each cluster, the mean extrapolated rice grain yield (in t ha−1) is shown.
| Cluster |
S. asiatica |
S. hermonthica |
|||
|---|---|---|---|---|---|
| 2011 | 2012-1 | 2012-2 | 2010 | 2011 | |
| High yielding | |||||
| 1 | Supa India, CG14 | N17b, CG14, WAB181-18 | CG14 | WAB56-50 | WAB181-18 |
| Mean | 1.8 | 2.5 | 2.7 | 2.1 | 1.9 |
| Intermediate-high yielding | |||||
| 2 | N5, N12, N3, N4, N7, N9, N13, N14, N15, N17, WAB56-104, WAB56-50, WAB181-18 | N3, N4, N14, N1, N5, N10, N12, N13, N2, N6, N7, N15, N16, N18, WAB56-50, WAB56-104, IAC165, Supa India | N1, N9, N10, WAB181-18, N2, N4, N5, N8, N13, N14, N17, N3, N6, N7, N11, N12, N15, N16, N18, WAB56-50, WAB56-104, Supa India, IAC165 | N1, N2, N3, N9, N10, N11, N12, N14, WAB56-104 | N3, N4, N8, N12, N13 |
| Mean | 1.0 | 1.9 | 1.5 | 1.4 | 1.5 |
| Intermediate-low yielding | |||||
| 3 | N2, N10, N11, N16, N18, IAC165, N1, N6, N8, Mwangulu | N8, N9, N11, Mwangulu | Mwangulu | N4, N5, N6, N7, N8, N15, N16- N17, N13, N18, WAB181-18, IAC165, CG14, IR49255-B-B-5-2 | N1, N2, N5, N10, N11, N15, N16, N17, N18, IAC165, IR49255-B-B-5-2 |
| Mean | 0.6 | 1.2 | 1.0 | 0.7 | 1.2 |
| Low yielding | |||||
| 4 | N9, N14, WAB56-104 | ||||
| Mean | 0.9 | ||||
| 5 | N6, N7, WAB56-50, CG14 | ||||
| Mean | 0.6 | ||||
The Connectivity (Handl et al., 2005), Dunn Index (Dunn, 1974), and Silhouette Width (Rousseeuw, 1987) measures are used to optimize the number of clusters (see Section 2.6).
NERICA cultivars are abbreviated by ‘N’ following the specific number.
In the S. hermonthica-infested fields, in Mbita, based on grain yields and using the hierarchical cluster analysis and evaluation measures outlined above, cultivars were clustered into three (2010) and five (2011) groups (Fig. 3D and E). Averaged across the cultivars, the yield in 2011 (1.1 t ha−1) was similar to that in 2010 (1.0 t ha−1), but the variability in average yield among cultivars was higher in 2010 (ranging from 0.2 to 2.1 t ha−1) than in 2011 (ranging from 0.5 to 1.9 t ha−1). The cluster with best yielding cultivars in 2010 contained only WAB56-50. This was followed by a cluster of NERICA-1 to -3, NERICA-9 to -12 and NERICA-14 and WAB56-104. In 2011, the highest yielding cultivar was WAB181-18. The cluster with second-highest yielding cultivars included NERICA-3, -4, -8, -12 and -13. It was closely followed by a third cluster containing NERICA-1, -2, -5, -10, -11, and NERICA-15 to -18, IAC165 and IR49255-B-B-5-2. The cultivars expressing consistent high levels of grain yield under S. hermonthica infested conditions were NERICA-3 and -12, (Table 4).
3.3. Is there a relationship between rice grain yield and resistance to Striga?
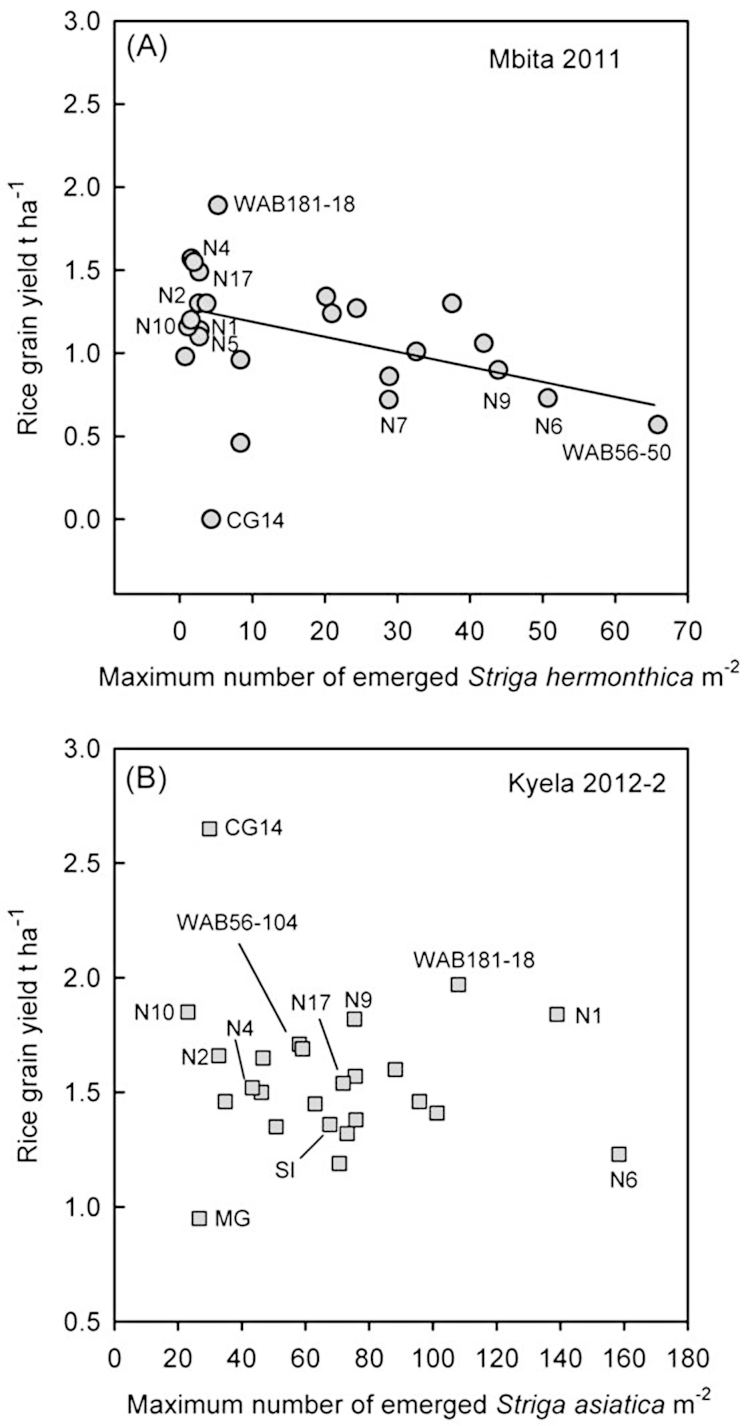
In the 2011 field trial at Mbita (when S. hermonthica infection levels were high) there was a moderate but significant correlation between the resistance ranking of the cultivars, based on NSmax, and grain yield (r = −0.45; P = 0.027) with the more resistant cultivars showing the greatest grain yields (Fig. 4A). In 2010, when infection levels were low, no such relationship was seen. In the field trials at Kyela there was no consistent pattern between the level of resistance to S. asiatica and grain yield as illustrated by data from Kyela 2012-2, the trial with the highest S. asiatica infection levels (Fig. 4B). In some cases cultivars with good resistance had some of the highest yields whereas others had yields that were similar to more susceptible cultivars (Fig. 1, Fig. 3, Fig. 4). Interestingly, in Kyela, CG14 showed very good resistance to S. asiatica under both low (2011 and 2012-1) and high (2012-2) infestation levels and achieved the highest yield (greater than 2 t ha−1) each year. However, although CG14 also showed good resistance in both trials at Mbita, it yielded poorly in that site (Fig. 1, Fig. 3, Fig. 4).
Fig. 4.
The relationship between resistance of the cultivars (maximum number of emerged Striga m−2) and rice grain yield (t ha−1). (A) Mbita field trial 2011 (R2 = −0.28); (B) Kyela field trial 2012-2. NERICA cultivars are abbreviated by ‘N’ following the specific number, Mwangulu is abbreviated as ‘MG’ and Supa India as ‘SI’.
3.4. How resistant are the NERICA cultivars and their parental genotypes to the S. hermonthica (Sh-Mbita) and S. asiatica (Sa-Kyela) ecotypes under controlled environment conditions?
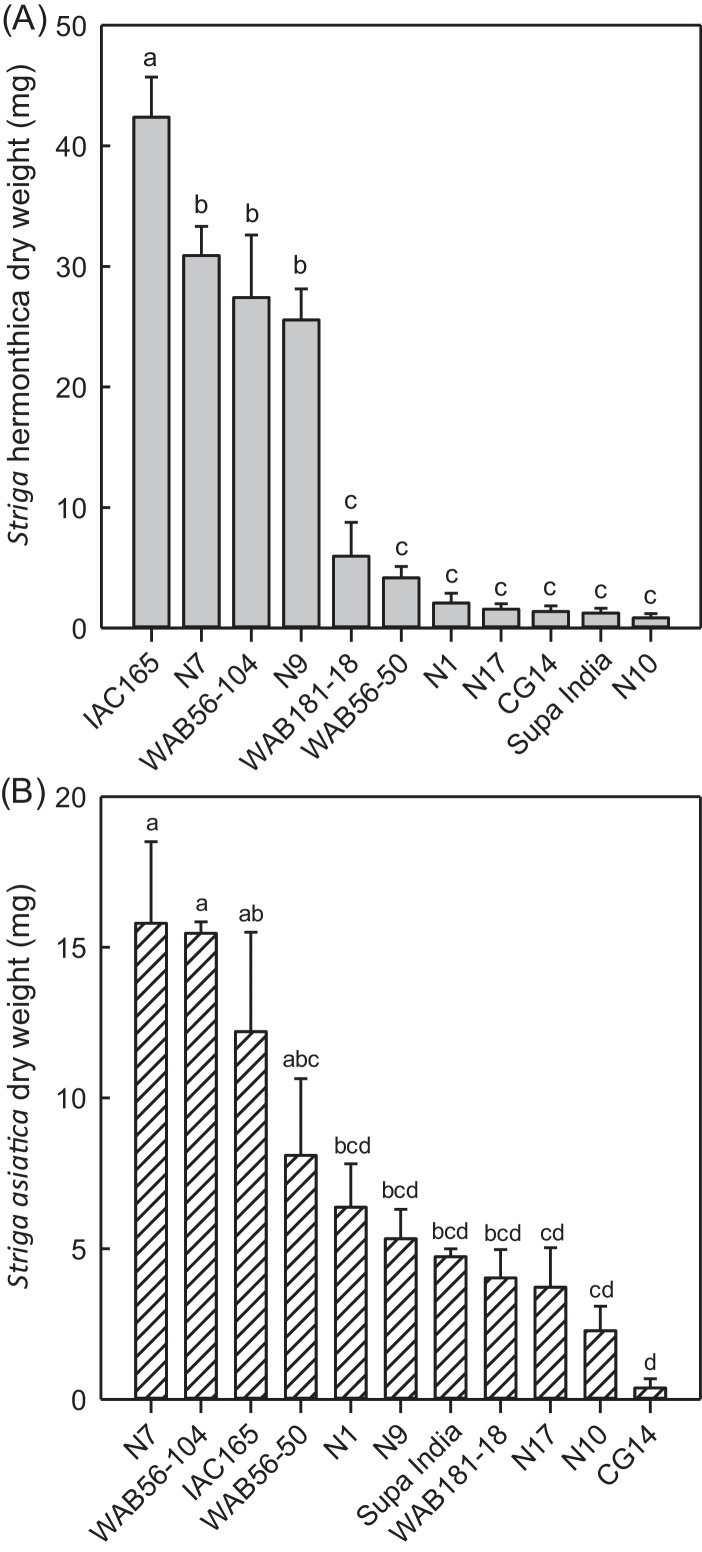
A significant cultivar effect on S. hermonthica (Sh-Mbita) infection levels (F = 57.1, df = 10, P = 0.001) was observed under controlled environment conditions (in rhizotrons) with WAB181-18, WAB56-50, CG14, Supa India and NERICA-1, -10 and -17 exhibiting good resistance (Fig. 5A). The most resistant cultivars had few successful attachments resulting in low parasite biomass on the roots. IAC165, WAB56-104 and NERICA-7 and -9 were very susceptible with a large number of attachments and high parasite biomass. A significant cultivar effect on S. asiatica (Sa-Kyela) infection levels (F = 11.0, df = 10, P = 0.001) was also observed. The most resistant cultivars were CG14, NERICA-10 and -17 supporting few attachments and low Striga biomass (Fig. 5B) whilst the most susceptible were NERICA-7, WAB56-104, WAB56-50 and IAC165, supporting the largest number and biomass of parasites on their roots.
Fig. 5.
Post attachment resistance of selected NERICA rice cultivars (N1, N7, N9, N10 and N17) and their parents to (A) Striga hermonthica (Sh-Mbita) and (B) S. asiatica (Sa-Kyela) ecotypes collected from the field sites at Mbita Point, Kenya and Kyela, Tanzania respectively. Striga dry weight was assessed at 21 days after infection. Data are means of four replicates ± SE. Means with the same letter do not differ significantly from each other (Tukey multiple comparison test, P > 0.05).
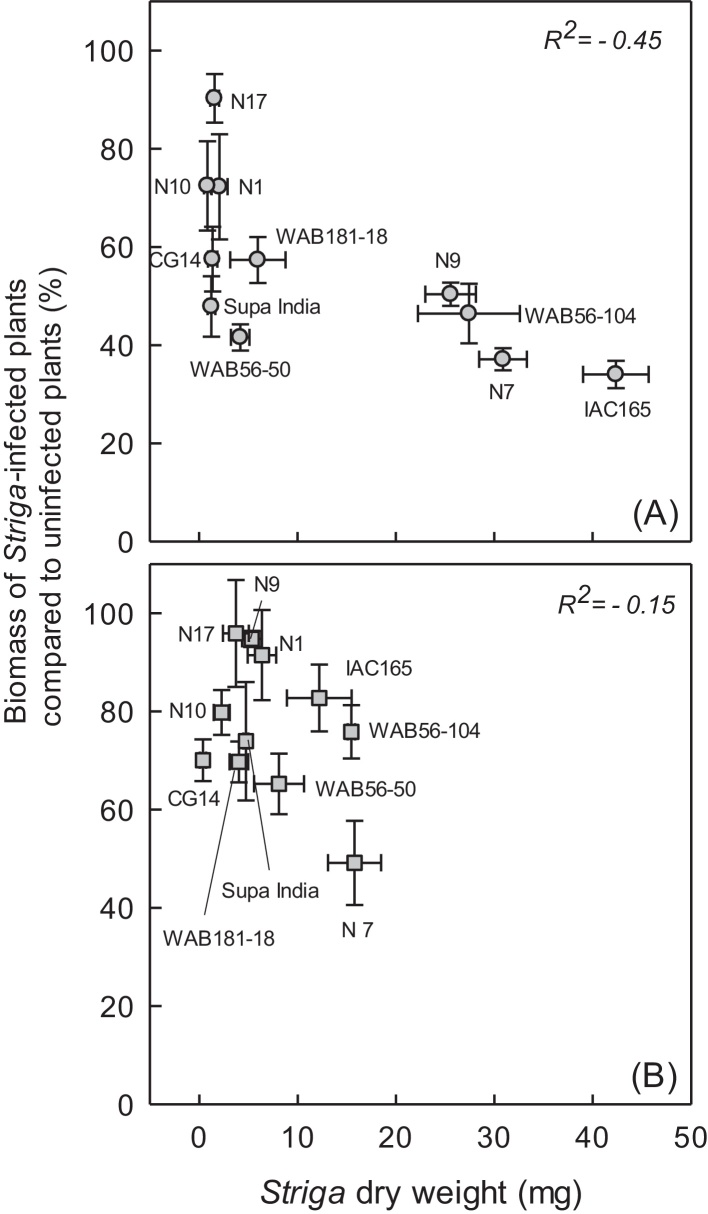
With both S. hermonthica and S. asiatica, there was a negative relation between the parasitic biomass on the roots and the percentage biomass of infected host-plants compared to uninfected control plants (Fig. 6). The most resistant cultivars (NERICA-17, -10 and -1) only showed a small (10–25%) reduction in biomass compared with the uninfected controls. This contrasted with the most susceptible cultivars, NERICA-9, -7, WAB56-104 and IAC165, which all lost 50–65% of their biomass compared to their respective control plants, when infected with either S. hermonthica or S. asiatica (Fig. 6). There was also a difference in growth performance (tolerance) between cultivars subjected to the same amount of Striga infection. For example, NERICA-17 showed 10% reduction in biomass when infected by S. hermonthica or S. asiatica, while Supa India showed 40 and 50% at similar infection levels of S. hermonthica and S. asiatica respectively.
Fig. 6.
Relationship between the biomass of Striga-infected plants compared to uninfected plants (%) and the dry weight of (A) Striga hermonthica (Sh-Mbita) and (B) S. asiatica (Sa-Kyela), attached to the roots of NERICA rice cultivars (N1, N7, N9, N10 and N17), parental lines and checks, 21 days after infection. Data are presented as means ± SE of four replicates.
4. Discussion
The interspecific NERICA cultivars have been widely adopted by farmers in rain-fed upland rice growing areas in sub-Saharan Africa (Wopereis et al., 2008). Recently however, mixed levels of the resistance of different NERICA cultivars have been reported (J. Rodenburg, personal observation). In 2011 Jamil et al., and Cissoko et al., analyzed pre-and post-attachment resistance levels of the 18 upland NERICA cultivars and their parental genotypes to different Striga species and ecotypes under controlled environment growth conditions. They found that some NERICA cultivars showed good pre- and/or post-attachment resistance against different Striga species and ecotypes whereas others only showed resistance against a specific species or ecotype and some were susceptible (to varying degrees) to all Striga ecotypes. However, the impact of the environment on the expression of these host resistance mechanisms (i.e. pre- and post-attachment) and the adaptability and yield of different cultivars under Striga-infested field conditions is largely unknown. The only previously published study, conducted by Atera et al. (2012) with a small selection of NERICA cultivars, showed that NERICA-1 and NERICA-10 yielded anywhere between 1.7 and 2.5 t ha−1 under S. hermonthica-infested field conditions in Kenya. Atera et al. (2012), did however no provide any information on Striga infection levels hence no inference could be drawn on the Striga resistance or tolerance levels of the rice cultivars under review. Information on resistance and yield levels under field conditions is of paramount importance to farmers when selecting cultivars for different agro-ecological zones.
4.1. How resistant are the NERICA rice cultivars to Striga spp. in the field? Is there a correlation between resistance rankings obtained under controlled environment conditions and in the field?
Among the set of 25 rice cultivars screened under field conditions in Tanzania and Kenya, significant differences were found in their levels of resistance against S. asiatica and S. hermonthica as summarized in Table 5. Nine of the 18 NERICA cultivars (NERICA 1-5, -10, -12, -13 and -17), one of the three O. sativa parents (WAB181-18) and the O. glaberrima parent (CG14) showed good or excellent resistance to the S. hermonthica ecotype from Mbita in the field. The same nine NERICA cultivars were also ranked as the most resistant (post-attachment resistance) to the S. hermonthica ecotype from Kibos, western Kenya (Sh-Kibos) in a previous rhizotron (controlled environment) study by Cissoko et al. (2011). For the selected cultivars we tested in the current study in a rhizotron, with the same S. hermonthica ecotype as the one present in the field (Sh-Mbita), resistance found in the field was confirmed. Many of these same cultivars (i.e. NERICA-1, -3, -4, -12 and -17, CG14 and WAB181-18) also had good pre-attachment resistance to an ecotype of S. hermonthica from Medani (Sudan) (see: Jamil et al., 2011b).
Table 5.
Summary of the resistance levels of rice cultivars to S. hermonthica and S. asiatica ecotypes in field and controlled environments (pre and post-attachment resistance). Cultivars are ranked Resistant (R), Susceptible (S) or Intermediate (I); based on NSmax (field) or the average number of attached Striga plants (controlled environment).
| Cultivar/Striga ecotype | Field |
Controlled environment | |||||
|---|---|---|---|---|---|---|---|
| Post-attachmentb |
Pre-attachment resistanceb |
||||||
| Sh-Mba | Sa-Ky | Sh-Mb | Sa-Ky | Sh-Ki | Sa-US | Sh-Me | |
| NERICA-1 | R | I/S | R | I | R | R | R |
| NERICA-2 | R | R | – | – | R | R | I |
| NERICA-3 | R | R | – | – | R | R | R |
| NERICA-4 | R | I/S | – | – | R | R | R |
| NERICA-5 | R | R | – | – | R | R | I |
| NERICA-6 | S | I/S | – | – | S | S | I |
| NERICA-7 | S | S | S | S | S | S | S |
| NERICA-8 | I | I/R | – | – | S | S | S |
| NERICA-9 | I/S | I/S | S | I | S | I | I |
| NERICA-10 | R | R | R | R | R | R | I |
| NERICA-11 | I/S | I/S | – | – | S | S | S |
| NERICA-12 | R | I | – | – | R | R | R |
| NERICA-13 | R | S | – | – | I | R | I |
| NERICA-14 | S | S | – | – | S | S | S |
| NERICA-15 | S | I/S | – | – | S | S | I |
| NERICA-16 | S | I/S | – | – | S | S | R |
| NERICA-17 | R | R | R | R | R | R | R |
| NERICA-18 | S | I/S | – | – | S | S | I |
| CG14 | R | R | R | R | R | R | R |
| WAB56-104 | I/S | I | S | S | I | I | R |
| WAB56-50 | I/S | I | R | I | I | R | S |
| WAB181-18 | R | I | R | R | I | R | R |
| IAC165 | S | S | S | S | S | S | – |
| Supa India | R | S | R | I | – | – | – |
Striga ecotypes: Sh-Mb = S. hermonthica from Mbita (Kenya); Sh-Ki = S. hermonthica from Kibos (Kenya); Sh-Me = S. hermonthica from Medani (Sudan); Sa-Ky = S. asiatica from Kyela (Tanzania); Sa-US = S. asiatica from USA. IR49255-B-B-2 and Mwangulu are not shown as they were only tested in one field site and not in controlled environments.
Information on post-attachment resistance is derived from Cissoko et al. (2011) and on pre-attachment resistance from Jamil et al. (2011b).
This suggests that these NERICA cultivars have broad-spectrum resistance to at least several S. hermonthica ecotypes. IR49255-B-B-5-2 which was used as resistant ‘check cultivar’ in this study also exhibited a good level of resistance to Sh-Mbita confirming previous field and pot studies where this cultivar was highly resistant to other S. hermonthica ecotypes (Harahap et al., 1993, Johnson et al., 1997).
The classification of S. asiatica resistance in the field seems to be highly dependent on the Striga infection levels, as NERICA-5 and -17, for instance, showed relatively high field resistance against S. asiatica under moderate to low infection levels (2011 and 2012-1 trials) but were more susceptible under the high infection levels of the 2012-2 field. Only three NERICA cultivars (NERICA-2, -3 and -10) and the O. glaberrima parent CG14 showed very good field resistance to Sa-Kyela under high infestation levels (in the 2012-2 trial) although several others e.g. NERICA-4, -8, -12, -14 and -16, as well as NERICA-5, -9, -11, -15 -17 and -18 showed intermediate resistance. Of the above mentioned cultivars NERICA-10 and -17 and CG14 were assessed against Sa-Kyela, for post-attachment resistance, under controlled environment conditions where they also exhibited good resistance (Table 5). Field resistance of NERICA-2, -3, -4, -10, -12 and -17 and CG14 confirmed the post-attachment resistance ranks based on a previous rhizotron study by Cissoko et al. (2011), with a S. asiatica ecotype from the USA (Table 5).
Supa India was very susceptible to Sa-Kyela but resistant to Sh-Mbita. This cultivar has been grown for many years by the farmers at Kyela and it is likely that the virulence levels of the local parasite population against this cultivar have increased in time. Supa India had not been grown at Mbita prior to this study and showed good resistance to S. hermonthica. Based on insights presented by Huang et al. (2012), this would suggest that the S. hermonthica population in this field did not have the virulence loci to overcome resistance in this cultivar. It is also interesting to note that many of the NERICA cultivars exhibited different resistance levels (in both field and controlled environment studies) when infected with Sh-Mbita compared to Sa-Kyela. For example NERICA-14 and -16 were very susceptible to Sh-Mbita but showed intermediate resistance under high infestation levels of Sa-Kyela and NERICA-1 and -13 showed good resistance to Sh-Mbita but were susceptible to Sa-Kyela. These differences in host-parasite specificity again suggest that the ecotypes of these two species of Striga have very different suites of virulence loci.
Although the correspondence between the resistance levels of the cultivars when screened in the field and under controlled environment conditions was remarkably good, they were not always exact. For example, WAB56-50 proved susceptible against S. hermonthica in the field, but resistant against the same ecotype in the rhizotron (i.e. in the post-attachment stage). NERICA-8, -14 and -16 showed intermediate resistance against S. asiatica in the field, but proved susceptible to Sa-USA in the rhizotron study by Cissoko et al. (2011). WAB56-104 was susceptible against S. asiatica in the rhizotron but had intermediate resistance in the field, while the reverse situation was observed with WAB181-18. Such differences confirm earlier findings that resistance observed under controlled environments do not always express in exactly the same way in the field (e.g. Omanya et al., 2004). There are a number of reasons for this including, differences in Striga infestation level and non-homogenous distribution of seeds in the soil (Haussmann et al., 2000), variability in soil fertility (particularly P and N), which may affect the production of strigolactones by the host roots and hence the germination of Striga seeds (Yoneyama et al., 2007, Jamil et al., 2011a, Umehara, 2011) and the soil moisture, flora and fauna of infested fields. All these factors create a different screening environment compared to the fully controlled situations in the laboratory (e.g. Haussmann et al., 2000). In addition, the characteristics of the host root system play a role in the responses of cultivars to Striga infection in the field. Cultivars with simpler, less branched roots can avoid or escape Striga parasitism in the field (Arnaud et al., 1999, Delft et al., 1996) and thus have fewer attached parasites. In the rhizotron study, Striga seeds were aligned along the host roots even if apparent differences are observed in root morphology or architecture of rice cultivars tested.
4.2. Do rice cultivars that exhibit good resistance responses in the field also produce good yields under Striga infested conditions?
Rice cultivars with good resistance are suitable for rice production in sub-Saharan Africa where S. hermonthica and S. asiatica are prevalent, provided that they are adapted to the prevailing growing environments (Rodenburg et al., 2010). Environmental adaptation is reflected in growth and reproduction parameters, such as biomass and yield. Rice grain weights (at 14% moisture content) showed a significant negative correlation with maximum above-ground Striga numbers (NSmax), as a measure for susceptibility (i.e. in general the most resistant cultivars produced the highest yields), only in the 2011 trial in Mbita, under high S. hermonthica pressure. This is in line with earlier field screening results with sorghum cultivars, where only under high S. hermonthica infestation the negative correlation between Striga numbers and yield under Striga infestation appears significant (Rodenburg et al., 2005). This result would imply that Striga resistance only provides a yield advantage under high infection levels.
Under Striga-infested conditions the best performing rice cultivars yielded an equivalent of 1.5–2.5 t ha−1 at Mbita (e.g. WAB56-50, NERICA-2 and -3 in 2010; WAB181-18, NERICA-3, -4 and -12 in 2011) and at Kyela (e.g. CG14 and Supa India in 2011; CG14, WAB181-18 and NERICA-17, -3, -4 and -14 in 2012-1; CG14, WAB181-18 and NERICA-1, -9 and -10 in 2012-2). These yields were similar to experimentally obtained upland rice yields of sub-optimally weeded plots (e.g. Ekeleme et al., 2009, Toure et al., 2011) or in sub-optimally fertilized plots (e.g. Saito et al., 2012) elsewhere in SSA. Yields of the best performing NERICA cultivars were mostly higher than the overall average estimated yield of upland rice (i.e. 1–1.25 t ha−1) obtained by farmers in Eastern Africa (e.g. Mghase et al., 2010, Sekiya et al., 2013) as well as the wider region (e.g. Seck et al., 2012, Diagne et al., 2013).
Cultivar performance in the two field trials is probably not only limited by Striga parasitism. The two field sites (particularly Kyela) are characterized by poor soil fertility, caused by continuous crop production without nutrient replenishment by appropriate fertilizer applications. Confirming our own soil fertility assessments, soils in Kyela are characterized by 0.16% N and around 5 ppm of available P (Mghase et al., 2010) while soils in Mbita have been reported to have 0.09–0.12% N and 6.3–13.3 ppm available P (Weisskopf et al., 2009). This relative poor soil fertility has certainly negatively affected crop performance, in particular in Kyela. The optimization of crop performance, including that of NERICA rice cultivars, in these nutrient-limited soils will require a good management and application of fertilizers (e.g. Saito and Futakuchi, 2009). Improving soil fertility will also improve performance of rice in Striga-infested fields as shown by Adagba et al. (2002) where an application of 90–120 kg N ha−1 helped to reduce the number of emerged Striga plants and boost rice yields.
Under Striga-infested field conditions, Striga resistance may have an important contribution to satisfactory yields, but final crop yields will depend on a suite of other genetic and non-genetic factors and interactions. The highly resistant rice cultivars IR49255-B-B-5-2 and CG14, for instance, showed lower grain yields at Mbita than some of the resistant NERICA cultivars (e.g. -3, -4, -12, and -13) despite similar infection levels. This may be caused by differences in Striga tolerance, a general lower level of genetically determined yield potential, or differences in environmental adaptation. The interspecific NERICA cultivars are known to combine relatively high yields with overall good adaptability to rain-fed upland environments (e.g. Saito et al., 2012). While for IR49255-B-B-5-2, only tested at one field site, causes for the poor yields cannot be conclusively established based on data presented here, the poor performance of CG14 under S. hermonthica infestation in Mbita must be a result to the lack of adaptability to the prevailing growing conditions at that site; CG14 was the highest yielding cultivar under S. asiatica infestation in Kyela and the rhizotron study with the two ecotypes of these Striga species did not reveal any differences in tolerance of CG14 to any of these species.
4.3. Can rice cultivars be differentiated based on variation in tolerance against Striga spp.?
The difference in yield between equally resistant or equally susceptible cultivars observed in the field may be due to inherent genetic differences in levels of Striga tolerance, the physiological capacity of the host plant to alleviate parasitism effects, as previously shown in sorghum (e.g. Rodenburg et al., 2005, Rodenburg et al., 2006, Rodenburg et al., 2008). Revealing such traits requires a combination of Striga-free and Striga-infested plots in the experimental design. While in our study, no Striga-free control plants were grown under the same field conditions, both uninfected and infected plants were grown in the rhizotrons, which allowed us to compare tolerance of different cultivars, provided that they had similar infection levels. At similar S. hermonthica infection levels, NERICA-17, was markedly less affected by parasitism than NERICA-10, which in turn performed better than CG14 and Supa India. The same was observed when these cultivars were infected by S. asiatica. At higher infection levels, WAB56-104 performed better than NERICA-7. Such variation in tolerance levels in rice cultivars, reported before by Cissoko et al. (2011), should be further explored and exploited for breeding purposes. If tolerance can be introgressed into adapted, high yielding (with desirable grain quality) Striga-resistant cultivars, this trait will provide an additional safety net for farmers coping with Striga infested soils (Rodenburg and Bastiaans, 2011).
5. Conclusion
This study showed that some NERICA cultivars displayed good levels of resistance and tolerance to the two most important Striga species occurring in rain-fed cereal cropping systems. A number of NERICA cultivars, notably NERICA-2, -10, -5 and -17 for S. asiatica and NERICA-1, -2, -3, -4, -5, -10, -12, -13 and -17 for S. hermonthica, possessed superior resistance in the field. In addition, NERICA-1, -17 and -10 have been identified, in vitro, as cultivars with potentially good levels of S. hermonthica tolerance. Potential tolerance to S. asiatica has been observed in NERICA-1, -17 and -9, at low infection levels, and with WAB56-104, at high infection levels. These cultivars suffered less Striga-inflicted total plant biomass reduction compared to some other cultivars when subjected to similar Striga infection (biomass) levels. Yields obtained under Striga-infested conditions in the field show a high variability among cultivars, years and Striga species. However, under high parasite pressure reasonable yields were obtained by a number of NERICA cultivars, i.e. NERICA-1, -9 and -10 (under S. asiatica infestation) and NERICA-3, -4, -8, -12 and -13 (under S. hermonthica infestation).
This study showed that the use of in vitro methods to identify resistance based on single mechanisms (i.e. either pre-attachment or post-attachment) are useful for the identification of superior breeding material in particular when such methods are used in succession to identify material with resistance based on multiple mechanisms (i.e. pre- and post-attachment). The resistant cultivars identified in this study, could be used in breeding programs aiming at the development and improvement of Striga resistance in adapted and high yielding rice cultivars. Cultivars that combine such broad-based resistance with the ability to maintain satisfactory yield levels in the field (i.e. NERICA-10 for S. asiatica-infested fields and NERICA-3, -4, -13 and -12 for S. hermonthica infested fields) are also suitable for inclusion in an integrated Striga control program.
These findings are highly relevant to rice breeders and molecular geneticist working on Striga defence mechanisms, as well as to resource-poor rice farmers typically working in the poorly fertile, drought-prone and Striga infested upland ecosystems commonly found in sub-Saharan Africa.
Acknowledgements
The authors would like to thank the Biotechnology and Biological Sciences Research Council (BBSRC) and the Department of International Development (DfID) for funding this work through the Sustainable Agriculture for International Development (SARID) programme (Grant number: BB/F004303/1) and the BBSRC, DfID and (through a grant awarded to BBSRC) the Bill & Melinda Gates Foundation through the Sustainable Crop Production Research for International Development (SCPRID) programme (Grant number: BB/J011703/1). The second author was supported by a BBSRC studentship, under the SARID grant.
References
- Adagba M.A., Lagoke S.T.O., Imolehin E.D. Nitrogen effect on the incidence of Striga hermonthica (Del.) Benth in upland rice. Acta Agron. Hung. 2002;50:145–150. [Google Scholar]
- Arnaud M.C., Véronési C., Thalouarn P. Physiology and histology of resistance to Striga hermonthica in Sorghum bicolor var. Framida. Australian Journal of Plant Physiology. 1999;26:63–70. [Google Scholar]
- Atera E.A., Itoh K., Azuma T., Ishii T. Response of NERICA rice to Striga hermonthica infections in western Kenya. Int. J. Agric. Biol. 2012;14:271–275. [Google Scholar]
- Balasubramanian V., Sie M., Hijmans R.J., Otsuka K. Increasing rice production in sub-Saharan Africa: challenges and opportunities. Adv. Agron. 2007;94:55–133. [Google Scholar]
- Botanga C.J., Kling J.G., Berner D.K., Timko M.P. Genetic variability of Striga asiatica (L.) Kuntz based on AFLP analysis and host-parasite interaction. Euphytica. 2002;128:375–388. [Google Scholar]
- Brock G., Pihur V., Datta S., Datta S. clValid: an R package for cluster validation. J. Stat. Softw. 2008;25:1–22. [Google Scholar]
- Cissoko M., Boisnard A., Rodenburg J., Press M.C., Scholes J.D. New rice for Africa (NERICA) cultivars exhibit different levels of post-attachment resistance against the parasitic weeds Striga hermonthica and Striga asiatica. New Phytol. 2011;192:952–963. doi: 10.1111/j.1469-8137.2011.03846.x. [DOI] [PubMed] [Google Scholar]
- Delft G.J., Graves J.D., Fitter A.H. Sorghum root system architecture in relation to Striga parasitism. In: Moreno M.T., Cubero J.I., Berner D., Joel D., Musselman L.J., Parker C., editors. Advances in Parasitic Plant Research. Proceedings of the Sixth International Parasitic Weed Symposium; Cordoba, Spain; 1996. pp. 777–786. [Google Scholar]
- Diagne A. Diffusion and adoption of NERICA rice varieties in Cote d’Ivoire. Dev. Econ. 2006;44:208–231. [Google Scholar]
- Diagne A., Amovin-Assagba E., Futakuchi K., Wopereis M.C.S. In: Realizing Africa's Rice Promise. Wopereis M.C.S., Johnson D.E., Ahmadi N., Tollens E., Jalloh A., editors. CABI, Wallingford; Oxfordshire, UK: 2013. Estimation of cultivated area, number of farming households and yield for major rice-growing environments in Africa; pp. 35–45. [Google Scholar]
- Dunn J.C. Well separated clusters and fuzzy partitions. J. Cybernet. 1974;4:95–104. [Google Scholar]
- Ekeleme F., Kamara A.Y., Oikeh S.O., Omoigui L.O., Amaza P., Abdoulaye T., Chikoye D. Response of upland rice cultivars to weed competition in the savannas of West Africa. Crop Prot. 2009;28:90–96. [Google Scholar]
- Frost D.L., Gurney A.L., Press M.C., Scholes J.D. Striga hermonthica reduces photosynthesis in sorghum: the importance of stomatal limitations and a potential role for ABA. Plant Cell Environ. 1997;20:483–492. [Google Scholar]
- Gurney A.L., Press M.C., Scholes J.D. Infection time and density influence the response of sorghum to the parasitic angiosperm Striga hermonthica. New Phytol. 1999;143:573–580. doi: 10.1046/j.1469-8137.1999.00467.x. [DOI] [PubMed] [Google Scholar]
- Gurney A.L., Slate J., Press M.C., Scholes J.D. A novel form of resistance in rice to the angiosperm parasite Striga hermonthica. New Phytol. 2006;169:199–208. doi: 10.1111/j.1469-8137.2005.01560.x. [DOI] [PubMed] [Google Scholar]
- Handl J., Knowles J., Kell D.B. Computational cluster validation in post-genomic data analysis. Bioinformatics. 2005;21:3201–3212. doi: 10.1093/bioinformatics/bti517. [DOI] [PubMed] [Google Scholar]
- Harahap Z., Ampong Nyarko K., Olela J.C. Striga hermonthica resistance in upland rice. Crop Prot. 1993;12:229–231. [Google Scholar]
- Haussmann B.I.G., Hess D.E., Welz H.G., Geiger H.H. Improved methodologies for breeding Striga-resistant sorghums. Field Crop. Res. 2000;66:195–211. [Google Scholar]
- Huang K., Whitlock R., Press M.C., Scholes J.D. Variation for host range within and among populations of the parasitic plant Striga hermonthica. Heredity. 2012;108:96–104. doi: 10.1038/hdy.2011.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamil M., Charnikhova T., Cardoso C., Jamil T., Ueno K., Verstappen F., Asami T., Bouwmeester H.J. Quantification of the relationship between strigolactones and Striga hermonthica infection in rice under varying levels of nitrogen and phosphorus. Weed Res. 2011;51:373–385. [Google Scholar]
- Jamil M., Rodenburg J., Charnikhova T., Bouwmeester H.J. Pre-attachment Striga hermonthica resistance of new rice for Africa (NERICA) cultivars based on low strigolactone production. New Phytol. 2011;192:964–975. doi: 10.1111/j.1469-8137.2011.03850.x. [DOI] [PubMed] [Google Scholar]
- Johnson D.E., Riches C.R., Diallo R., Jones M.J. Striga on rice in West Africa; crop host range and the potential of host resistance. Crop Prot. 1997;16:153–157. [Google Scholar]
- Jones M.P., Dingkuhn M., Aluko G.K., Semon M. Interspecific Oryza sativa L. × O. glaberrima Steud. progenies in upland rice improvement. Euphytica. 1997;94:237–246. [Google Scholar]
- Jones M.P., Mande S., Aluko K. Diversity and potential of Oryza glaberrima Steud in upland rice breeding. Breed. Sci. 1997;47:395–398. [Google Scholar]
- Kaewchumnong K., Price A.H. A study on the susceptibility of rice cultivars to Striga hermonthica and mapping of Striga tolerance quantitative trait loci in rice. New Phytol. 2008;180:206–216. doi: 10.1111/j.1469-8137.2008.02568.x. [DOI] [PubMed] [Google Scholar]
- Kettenring J.R. The practice of cluster analysis. J. Classif. 2006;23:3–30. [Google Scholar]
- Kijima Y., Sserunkuuma D., Otsuka K. How revolutionary is the NERICA revolution? Evidence from Uganda. Dev. Econ. 2006;44:252–267. [Google Scholar]
- Kountche B.A., Hash C.T., Dodo H., Laoualy O., Sanogo M.D., Timbeli A., Vigouroux Y., This D., Nijkamp R., Haussmann B.I.G. Development of a pearl millet Striga-resistant genepool: response to five cycles of recurrent selection under Striga-infested field conditions in West Africa. Field Crop. Res. 2013;154:82–90. [Google Scholar]
- McCullagh P., Nelder J.A. Chapman & Hall; London: 1989. Generalized Linear Models. [Google Scholar]
- Mghase J.J., Shiwachi H., Nakasone K., Takahashi H. Agronomic and socio-economic constraints to high yield of upland rice in Tanzania. African J. Agr. Res. 2010;5:150–158. [Google Scholar]
- Mohamed K.I., Musselman L.J., Riches C.R. The genus Striga (Scrophulariaceae) in Africa. Ann. Mo. Bot. Gard. 2001;88:60–103. [Google Scholar]
- Omanya G.O., Haussmann B.I.G., Hess D.E., Reddy B.V.S., Kayentao M., Welz H.G., Geiger H.H. Utility of indirect and direct selection traits for improving Striga resistance in two sorghum recombinant inbred populations. Field Crop. Res. 2004;89:237–252. [Google Scholar]
- Parker C. Observations on the current status of Orobanche and Striga problems worldwide. Pest Manag. Sci. 2009;65:453–459. doi: 10.1002/ps.1713. [DOI] [PubMed] [Google Scholar]
- Parker C., Riches C.R. CABI, Wallingford; Oxon, England: 1993. Parasitic Weeds of the World: Biology and Control. [Google Scholar]
- R-Core-Team . R Foundation for Statistical Computing; Vienna, Austria: 2014. R. A language and environment for statistical computing. http://www.R-project.org/ [Google Scholar]
- Rodenburg J., Bastiaans L. Host-plant defence against Striga spp.: reconsidering the role of tolerance. Weed Res. 2011;51:438–441. [Google Scholar]
- Rodenburg J., Johnson D.E. Weed management in rice-based cropping systems in Africa. Adv. Agron. 2009;103:149–218. [Google Scholar]
- Rodenburg J., Bastiaans L., Weltzien E., Hess D.E. How can field selection for Striga resistance and tolerance in sorghum be improved? Field Crop Res. 2005;93:34–50. [Google Scholar]
- Rodenburg J., Bastiaans L., Kropff M.J. Characterization of host tolerance to Striga hermonthica. Euphytica. 2006;147:353–365. [Google Scholar]
- Rodenburg J., Bastiaans L., Schapendonk A.H.C.M., van der Putten P.E.L., van Ast A., Dingemanse N.J., Haussmann B.I.G. CO2-assimilation and chlorophyll fluorescence as indirect selection criteria for host tolerance against Striga. Euphytica. 2008;160:75–87. [Google Scholar]
- Rodenburg J., Riches C.R., Kayeke J.M. Addressing current and future problems of parasitic weeds in rice. Crop Prot. 2010;29:210–221. [Google Scholar]
- Rousseeuw P.J. Silhouettes: a graphical aid to the interpretation and validation of cluster analysis. J. Comput. Appl. Math. 1987;20:53–65. [Google Scholar]
- Saito K., Futakuchi K. Performance of diverse upland rice cultivars in low and high soil fertility conditions in West Africa. Field Crop Res. 2009;111:243–250. [Google Scholar]
- Saito K., Sokei Y., Wopereis M.C.S. Enhancing rice productivity in West Africa through genetic improvement. Crop Sci. 2012;52:484–493. [Google Scholar]
- Seck P.A., Diagne A., Mohanty S., Wopereis M.C.S. Crops that feed the world 7: Rice. Food Secur. 2012;4:7–24. [Google Scholar]
- Sekiya N., Khatib K.J., Makame S.M., Tomitaka M., Oizumi N., Araki H. Performance of a number of NERICA cultivars in Zanzibar, Tanzania: yield, yield components and grain quality. Plant Prod. Sci. 2013;16:141–153. [Google Scholar]
- Sokal R.R., Rohlf F.J. W.H. Freeman and Company; New York: 1995. Biometry. [Google Scholar]
- Spallek T., Mutuku M., Shirasu K. The genus Striga: a witch profile. Mol. Plant Pathol. 2013;14:861–869. doi: 10.1111/mpp.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarbrick P.J., Scholes J.D., Press M.C., Slate J. A major QTL for resistance of rice to the parasitic plant Striga hermonthica is not dependent on genetic background. Pest Manag. Sci. 2009;65:528–532. doi: 10.1002/ps.1719. [DOI] [PubMed] [Google Scholar]
- Toure A., Rodenburg J., Saito K., Oikeh S., Futakuchi K., Gumedzoe D., Huat J. Cultivar and weeding effects on weeds and rice yields in a degraded upland environment of the Coastal Savanna. Weed Technol. 2011;25:322–329. [Google Scholar]
- Umehara M. Strigolactone, a key regulator of nutrient allocation in plants. Plant Biotechnol. 2011;28:429–437. [Google Scholar]
- Waddington S.R., Li X.Y., Dixon J., Hyman G., de Vicente M.C. Getting the focus right: production constraints for six major food crops in Asian and African farming systems. Food Secur. 2010;2:27–48. [Google Scholar]
- Weisskopf L., Akello P., Milleret R., Khan Z.R., Schulthess F., Gobat J.M., Le Bayon R.C. White lupin leads to increased maize yield through a soil fertility-independent mechanism: a new candidate for fighting Striga hermonthica infestation? Plant Soil. 2009;319:101–114. [Google Scholar]
- Webb M., Smith M.C. Biology of Striga hermonthica (Scrophulariaceae) in Sahelian Mali: effects on pearl millet yield and prospects of control. Weed Res. 1996;36:203–211. [Google Scholar]
- Wopereis M.C.S., Diagne A., Rodenburg J., Sié M., Somado E.A. Why NERICA is a successful innovation for African farmers: a response to Orr et al. from the Africa Rice Center. Outlook Agr. 2008;37:169–176. [Google Scholar]
- Yoder J.I., Scholes J.D. Host plant resistance to parasitic weeds; recent progress and bottlenecks. Curr. Opin. Plant Biol. 2010;13:478–484. doi: 10.1016/j.pbi.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Yoneyama K., Xie X.N., Kusumoto D., Sekimoto H., Sugimoto Y., Takeuchi Y. Nitrogen deficiency as well as phosphorus deficiency in sorghum promotes the production and exudation of 5-deoxystrigol, the host recognition signal for arbuscular mycorrhizal fungi and root parasites. Planta. 2007;227:125–132. doi: 10.1007/s00425-007-0600-5. [DOI] [PubMed] [Google Scholar]