Abstract
Antibodies capable of decarboxylating orotate were sought by immunization with a hapten designed to elicit antibodies with combining sites that resemble the orotate-binding and catalytic portion of the active site of the enzyme orotidine 5'-monophosphate (OMP) decarboxylase (orotidine-5'-monophosphate carboxy-lyase, EC 4.1.1.23). Active recombinant antibody fragments (Fabs) were selected from a combinatorial cDNA library by complementation of a pyrF strain of Escherichia coli and growth of the library-expressing cells on pyrimidine-free medium. In this biological screen, a sufficiently active antibody from the library would decarboxylate orotate to produce uracil, a pyrimidine source for the auxotroph, and would provide the cells with a growth advantage compared to cells without an active antibody. Six recombinant Fabs yielded identifiable colonies in a screen of 16,000 transformants. To enhance its stability and expression level, one of the six positive fragments was converted into single-chain form. In this form, the antibody fragment conferred a definite growth advantage to the auxotroph that was eliminated when the hapten was included in the medium. The purified single-chain antibody displayed orotate decarboxylase activity in vitro, as determined by a 14CO2 displacement assay. The specific activity of the antibody is approximately 10(-7) times that of naturally occurring OMP decarboxylase, but this antibody-catalyzed rate is estimated to be 10(8) times the background rate. The results offer the potential to use these methods to obtain catalytic antibodies for other biosynthetic reactions as well as to assess the effectiveness of the hapten transition state or active site analog in eliciting antibody catalysts.
Full text
PDF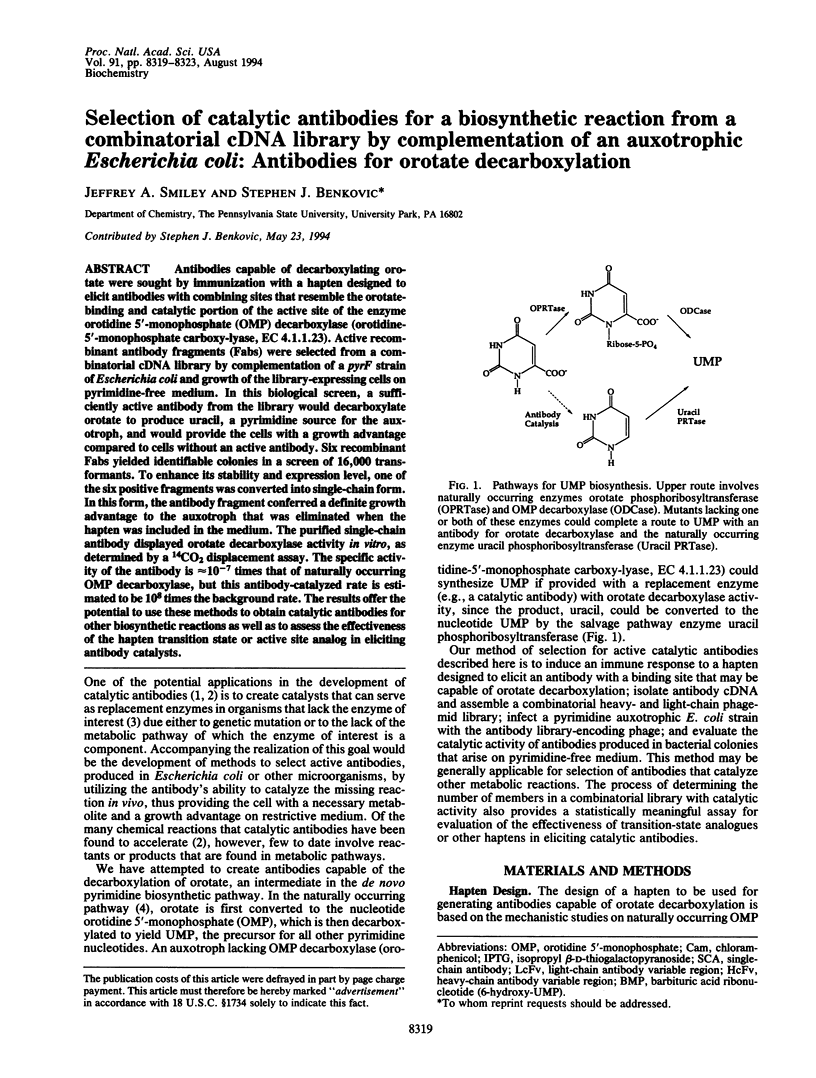



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbas C. F., 3rd, Kang A. S., Lerner R. A., Benkovic S. J. Assembly of combinatorial antibody libraries on phage surfaces: the gene III site. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):7978–7982. doi: 10.1073/pnas.88.18.7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beak P., Siegel B. Mechanism of decarboxylation of 1,3-dimethylorotic acid. A model for orotidine 5'-phosphate decarboxylase. J Am Chem Soc. 1976 Jun 9;98(12):3601–3606. doi: 10.1021/ja00428a035. [DOI] [PubMed] [Google Scholar]
- Bell J. B., Jones M. E. Purification and characterization of yeast orotidine 5'-monophosphate decarboxylase overexpressed from plasmid PGU2. J Biol Chem. 1991 Jul 5;266(19):12662–12667. [PubMed] [Google Scholar]
- Benkovic S. J. Catalytic antibodies. Annu Rev Biochem. 1992;61:29–54. doi: 10.1146/annurev.bi.61.070192.000333. [DOI] [PubMed] [Google Scholar]
- Chaudhary V. K., Batra J. K., Gallo M. G., Willingham M. C., FitzGerald D. J., Pastan I. A rapid method of cloning functional variable-region antibody genes in Escherichia coli as single-chain immunotoxins. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1066–1070. doi: 10.1073/pnas.87.3.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer P., Leu S. J., Yang Y. Y., Chen P. P. Rapid simultaneous screening for DNA integrity and antigen specificity of clones selected by phage display. Biotechniques. 1994 May;16(5):828–830. [PubMed] [Google Scholar]
- Gibbs R. A., Posner B. A., Filpula D. R., Dodd S. W., Finkelman M. A., Lee T. K., Wroble M., Whitlow M., Benkovic S. J. Construction and characterization of a single-chain catalytic antibody. Proc Natl Acad Sci U S A. 1991 May 1;88(9):4001–4004. doi: 10.1073/pnas.88.9.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huse W. D., Sastry L., Iverson S. A., Kang A. S., Alting-Mees M., Burton D. R., Benkovic S. J., Lerner R. A. Generation of a large combinatorial library of the immunoglobulin repertoire in phage lambda. Science. 1989 Dec 8;246(4935):1275–1281. doi: 10.1126/science.2531466. [DOI] [PubMed] [Google Scholar]
- Jones M. E. Pyrimidine nucleotide biosynthesis in animals: genes, enzymes, and regulation of UMP biosynthesis. Annu Rev Biochem. 1980;49:253–279. doi: 10.1146/annurev.bi.49.070180.001345. [DOI] [PubMed] [Google Scholar]
- Lerner R. A., Benkovic S. J. Principles of antibody catalysis. Bioessays. 1988 Oct;9(4):107–112. doi: 10.1002/bies.950090402. [DOI] [PubMed] [Google Scholar]
- Lerner R. A., Benkovic S. J., Schultz P. G. At the crossroads of chemistry and immunology: catalytic antibodies. Science. 1991 May 3;252(5006):659–667. doi: 10.1126/science.2024118. [DOI] [PubMed] [Google Scholar]
- Lesley S. A., Patten P. A., Schultz P. G. A genetic approach to the generation of antibodies with enhanced catalytic activities. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1160–1165. doi: 10.1073/pnas.90.4.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine H. L., Brody R. S., Westheimer F. H. Inhibition of orotidine-5'-phosphate decarboxylase by 1-(5'-phospho-beta-d-ribofuranosyl)barbituric acid, 6-azauridine 5'-phosphate, and uridine 5'-phosphate. Biochemistry. 1980 Oct 28;19(22):4993–4999. doi: 10.1021/bi00563a010. [DOI] [PubMed] [Google Scholar]
- Lewis C., Krämer T., Robinson S., Hilvert D. Medium effects in antibody-catalyzed reactions. Science. 1991 Aug 30;253(5023):1019–1022. doi: 10.1126/science.1887215. [DOI] [PubMed] [Google Scholar]
- McCafferty J., Griffiths A. D., Winter G., Chiswell D. J. Phage antibodies: filamentous phage displaying antibody variable domains. Nature. 1990 Dec 6;348(6301):552–554. doi: 10.1038/348552a0. [DOI] [PubMed] [Google Scholar]
- Ohmstede C. A., Langdon S. D., Chae C. B., Jones M. E. Expression and sequence analysis of a cDNA encoding the orotidine-5'-monophosphate decarboxylase domain from Ehrlich ascites uridylate synthase. J Biol Chem. 1986 Mar 25;261(9):4276–4282. [PubMed] [Google Scholar]
- Prabhakararao K., Jones M. E. Radioassay of orotic acid phosphoribosyltransferase and orotidylate decarboxylase utilizing a high-voltage paper electrophoresis technique or an improved 14CO2- release method. Anal Biochem. 1975 Dec;69(2):451–457. doi: 10.1016/0003-2697(75)90147-5. [DOI] [PubMed] [Google Scholar]
- Sastry L., Alting-Mees M., Huse W. D., Short J. M., Sorge J. A., Hay B. N., Janda K. D., Benkovic S. J., Lerner R. A. Cloning of the immunological repertoire in Escherichia coli for generation of monoclonal catalytic antibodies: construction of a heavy chain variable region-specific cDNA library. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5728–5732. doi: 10.1073/pnas.86.15.5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokat K. M., Leumann C. J., Sugasawara R., Schultz P. G. A new strategy for the generation of catalytic antibodies. Nature. 1989 Mar 16;338(6212):269–271. doi: 10.1038/338269a0. [DOI] [PubMed] [Google Scholar]
- Smiley J. A., Jones M. E. A unique catalytic and inhibitor-binding role for Lys93 of yeast orotidylate decarboxylase. Biochemistry. 1992 Dec 8;31(48):12162–12168. doi: 10.1021/bi00163a027. [DOI] [PubMed] [Google Scholar]
- Smiley J. A., Paneth P., O'Leary M. H., Bell J. B., Jones M. E. Investigation of the enzymatic mechanism of yeast orotidine-5'-monophosphate decarboxylase using 13C kinetic isotope effects. Biochemistry. 1991 Jun 25;30(25):6216–6223. doi: 10.1021/bi00239a020. [DOI] [PubMed] [Google Scholar]
- Stewart J. D., Roberts V. A., Thomas N. R., Getzoff E. D., Benkovic S. J. Site-directed mutagenesis of a catalytic antibody: an arginine and a histidine residue play key roles. Biochemistry. 1994 Mar 1;33(8):1994–2003. doi: 10.1021/bi00174a004. [DOI] [PubMed] [Google Scholar]
- Struhl K., Cameron J. R., Davis R. W. Functional genetic expression of eukaryotic DNA in Escherichia coli. Proc Natl Acad Sci U S A. 1976 May;73(5):1471–1475. doi: 10.1073/pnas.73.5.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Hicks J. B., Hilvert D. In vivo catalysis of a metabolically essential reaction by an antibody. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8784–8786. doi: 10.1073/pnas.88.19.8784. [DOI] [PMC free article] [PubMed] [Google Scholar]