The synthesis of proteins with a fully native sequence is an ongoing challenge in protein chemistry. Native chemical ligation (NCL) approaches have proven to be generally applicable where cysteine (Cys) residues are appropriately positioned,[1,2] however, the synthesis of many proteins often require ligation at non-Cys sites in the polypeptide sequence.[3-7] Previously, we introduced a reductive strategy for ligation at Ala sites[7] based on global desulfurization of Cys[8] that has found widespread utility for the synthesis of complex proteins by NCL.[9,10] Selective desulfurization can be affected by both Rainey Ni and Pd/C/H2[7] and, more recently, by the radical initiator VA-044 in combination with the water soluble phosphine TCEP.[11] However, since these conditions result in global desulfurization of all thiols in the protein, the method requires protection and deprotection of all other Cys residues in the native sequence.[6a,12] These additional steps complicate the synthesis of larger polypeptides and limit the use of natural Cys residues for ligation.[13]
Selenocysteine (Sec, U) has been shown to expand the NCL method to Xaa-Sec site, allowing the synthesis of selenoproteins.[14-16] Additionally, the resulting selenopeptides can be deselenized under similar conditions to that used for Cys containing peptides to yield the corresponding Ala peptide sequences.[11,17] We reasoned that the high propensity of selenols to form radicals[18] could be harnessed for selective reduction of selenols in the presence of thiols (Scheme 1), thus avoiding the need for protection/deprotection steps. This approach was inspired by our observation that synthetic analogs of glutaredoxin 3 (Grx3) containing Sec were incompatible with reduction by the water soluble reducing agent TCEP,[19] leading to the generation of significant levels of a deselenized side products. By contrast, the wt-Grx3 was found to be stable to TCEP. Indeed, the sensitivity of Sec in peptides and proteins to reduction by TCEP has been previously noted in the development of selenocysteine ligation methods and in the context of selenoproteins.[15,16a,20] Importantly, TCEP[21] is widely used to reduce disulfides to thiols in peptides and proteins without reduction of the C-S bond.[22-24]
Scheme 1.
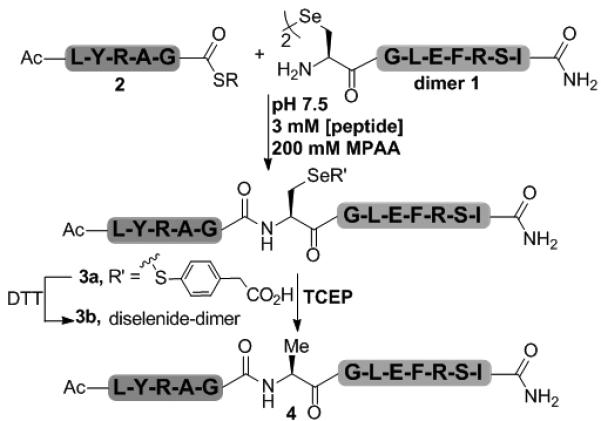
Native Chemical Ligation of N-terminal Sec-peptide 1 with C-terminal thioester-peptide 2 gives the ligated product, 3a. Following purification, 3a was deselenized to the alanyl peptide 4, using excess TCEP. R = 3-mercaptopropionyl-Leu.
Accordingly, we ligated the N-terminal Sec-peptide 1 (UGLEFRSI-amide, isolated in the form of a diselenide dimer) to the thioester peptide 2 (Ac-LYRAG-SR) (Scheme 1 and Figure S1), to produce 3a.[14-16] The ligation conditions of 6 M GdmCl, 200 mM Na2HPO4, pH 7.5, saturated with ~200 mM 4-mercaptophenylacetic acid, (MPAA),[25] gave the best results. The aromatic thiol acted as both a catalyst to activate the alkyl thioester and as a mild reducing agent to generate a small pool of free selenol to facilitate the ligation reaction. In addition, under these conditions the product was spontaneously converted to selenylsulfide 3a, with MPAA, which was beneficial for the HPLC purification.[26]
The purified selenylsulfide peptide 3a was treated with 50-fold excess TCEP at pH 5.5 to produce the deselenized alanyl-peptide 4 (Scheme 1, Figure S2 and S3). Under these conditions, the reaction proceeded to completion yet was slower than anticipated (17 h). We reasoned that the presence of the aromatic thiol, MPAA (1 equiv) could interfere with the deselenization reaction[27,28] by acting as a radical scavenger, thus competing with the deselenization of the Sec residue.[27-29] To remove the aromatic thiol, compound 3a was reduced with 50-fold excess DTT to produce the free selenol, which subsequently reoxidized to give the dimeric diselenide peptide, 3b. This reaction sequence was accomplished in one pot due to the high reactivity of the selenol and low redox potential of diselenides.[19] Deselenization of the diselenide dimer 3b with excess TCEP proceeded rapidly, generating the Ala peptide 4 in less than 4 h, a time comparable to previously reported deselenization reactions.
TCEP is a common reagent in protein chemistry that is selective for disulfide reduction.[21-24] The chemoselectivity of the deselenization conditions was further evaluated with peptide 5a, H-Ala-Sec-Gly-Ser-Cys-Lys-Trp-Thr-Met-Ala-NH2 which contains the potentially sensitive amino acids Cys, Met and Trp. Peptide 5a oxidized under ambient conditions to generate the cyclic selenylsulfide peptide 5b (Scheme 2). Treatment of 5b with only TCEP (40-fold excess) at pH 5.5 resulted in rapid deselenization of the Sec to yield peptide 6. Surprisingly, analysis of the minor side product identified peptide 7, H-Ala-Ala-Gly-Ser-Ala-Lys-Trp-Thr-Met-Ala-NH2, consistent with a slow additional desulfurization of the Cys residue.[30,31] However, when the reaction was run (200 mM NaH2PO4, pH 5.1) with 9.4 equiv DTT to reduce the selenylsulfide, addition of just 2.2 equiv TCEP led exclusively to deselenization. Mono-reduced deselenized product 6, H-Ala-Ala-Gly-Cys-Lys-Trp-Thr-Met-Ala-NH2, was obtained cleanly in 2 h with only traces of the deselenized-desulfurized peptide 7 detected (Scheme 2, Figure 1 and Figure S4).
Scheme 2.
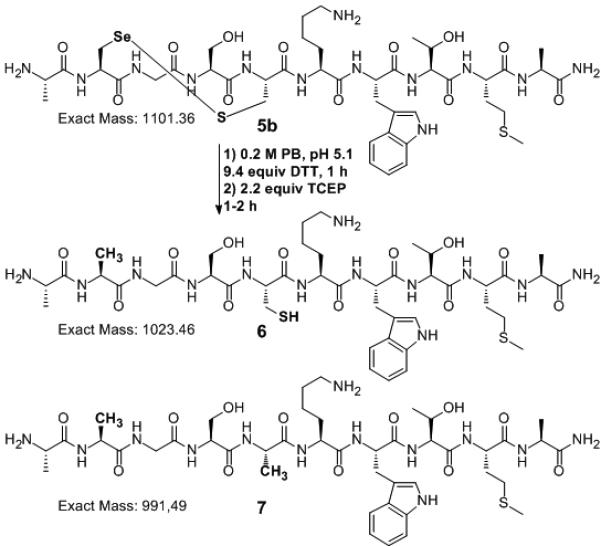
Selective deselenization of peptide 5b. The deselenized peptide 6 was the major product with only traces of side product, 7.
Figure 1.
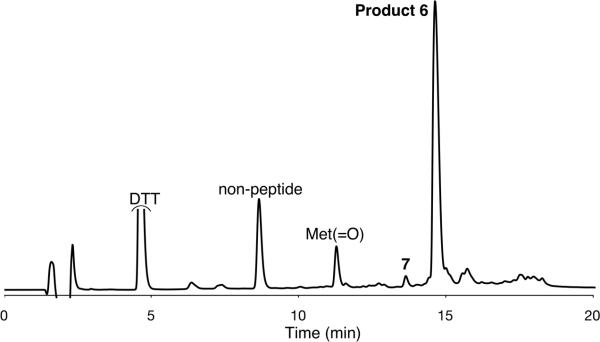
HPLC chromatogram for the deselenization reaction mixture of peptide 5b, which was carried out with 9.4 equiv DTT for 1 h, and 2.2 equiv TCEP for 2 h to give major product, 6, with traces of the deselenized-desulfurized product, 7. Met(=O) corresponds to deselenized product with oxidized Met present in the starting material.
We propose that this deselenization of Sec to Ala occurs through a radical-mediated mechanism (Scheme 3), similar to the desulfurization of thiols by trialkyl phosphines (and phosphites under elevated temperatures or UV light) proposed first by Walling et al[27] and recently by Wan and Danishefsky (in the presence of a radical initiator such as VA-044).[11,32]
Scheme 3.
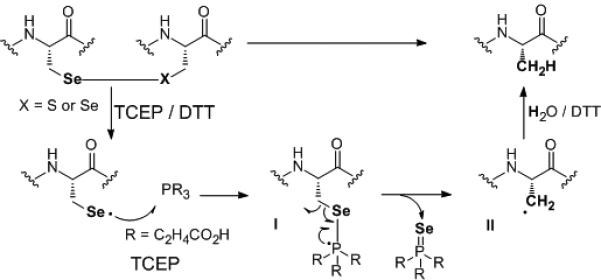
Proposed deselenization mechanism of Sec-containing peptides with TCEP in aqueous solution.
In principle, loss of selenium could occur through an elimination mechanism to generate dehydroalanine.[33] To rule out this possibility, we carried out two experiments. First, the diselenide 1 was treated with either hydrogen peroxide[34] or TCEP (Scheme 4). Under hydrogen peroxide the selenium atom was converted to the corresponding selenoxide with subsequent β-syn elimination to produce the modified peptide 8 where the Sec has been converted to dehydroalanine (Scheme 4, bottom).[34] Peptide 8 was rapidly hydrolyzed under the reaction conditions to the corresponding pyruvoyl peptide 9a, which was observed together with its hydrate, 9b (Figure S6B). In contrast, when diselenide dimer 1 was reacted with TCEP under the deselenization conditions, the corresponding Ala peptide, 10, was obtained without any traces of either 9a or 9b (Scheme 4 top, Figure S6C).[33] Second, we carried out the deselenization reaction of peptide 5b (Scheme 2) under the regular conditions using D2O instead of H2O. The reaction mixture was worked up and analyzed in H2O to reverse any deuterium incorporation at acidic sites. The resulting deselenization product was found to be consistent with 6-d, having a mass of M+1 in comparison to the product from H2O, 6 (Scheme 5, Figure S5). Furthermore, the chromatographically distinct minor side-product (additional desulfurization) was found to be consistent with 7-d2 having a mass of M+2 in comparison with the above-described 7. Any mechanism involving dehydroalanine formation would lead to an additional deuterium being incorporated at the α-carbon (Scheme 5, Figure S5).[31]
Scheme 4.
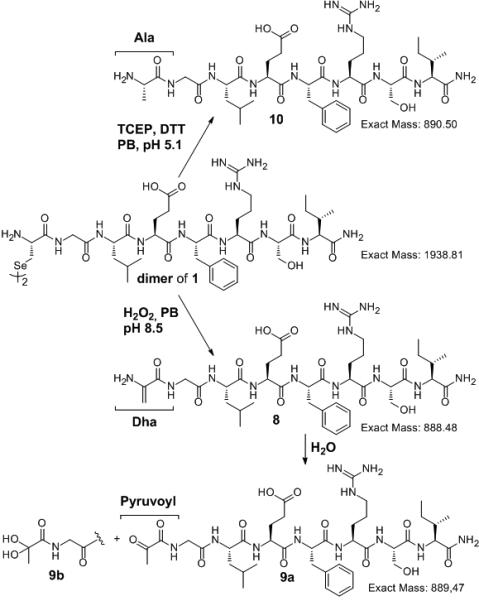
Reaction of dimer peptide 1 with H2O2 produced the dehydroalanine analog, 8, which was rapidly hydrolyzed under the reaction conditions to the corresponding pyruvoyl peptide 9a (in equilibrium with its hydrate 9b). In contrast, reaction of peptide 1 with TCEP gave only the deselenized product, 10, with an N-terminal Ala.
Scheme 5.
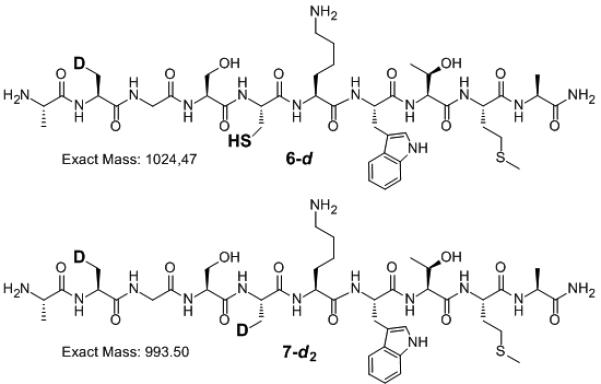
Deselenization of peptide 5b in phosphate buffered D2O by TCEP forms two products; the deselenized mono-deuterated product, 6-d as the major product and the doubly-deuterated deselenized-desulfurized minor product, 7-d2.
Epimerization is always a concern in the development of new methods. While the desulfurization of Cys residues by Raney Ni has been experimentally shown to be racemization-free,[7,35] the radical-mediated mechanism could result in epimerization.[11] The proposed deselenization mechanism proceeds through a radical on the β-carbon, which is expected to be the major intermediate.[11,32] However, radical migration from β- to the α-carbon could lead to undesired epimerization at the Sec residue. Furthermore, it has been experimentally confirmed that thiyl radicals can abstract hydrogen from α-carbons in peptides and proteins (as well as acidic protons in lipids and DNA).[36,37] To test for epimerization, the pentapeptide H-Phe-Lys-Sec-Ser-Asp-NH2 (FKUSD.amide, isolated in the form of a diselenide dimer, 11) was synthesized together with H-Phe-Lys-(l)Ala-Ser-Asp-NH2 (FK(l)ASD.amide, 12) and H-Phe-Lys-(d)Ala-Ser-Asp-NH2 (FK(d)ASD.amide, 13, which by HPLC elutes at 8.8 min, 1 min earlier than the l-enantiomer). Deselenization of 11 with TCEP was complete within 2 h, producing only the l-enantiomer product FK(l)ASD, which co-eluted with the synthetic peptide, and has an identical mass. (Scheme 6, Figures S7 and S8).
Scheme 6.
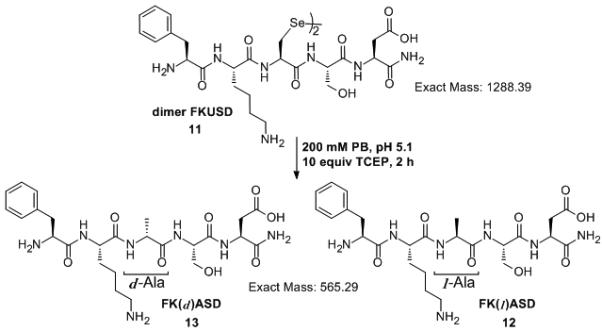
The diselenide dimer of FKUSD, 11, was deselenized with 10 equiv TCEP in 200 mM phosphate buffer, pH 5.1 to give only FK(l)ASD product, 12, no traces of the of 13 were observed.
Taken together, these results support the formation of a radical intermediate at the β-carbon of Sec, without migration to the α-carbon, indicating a chemoselective process with complete retention of stereoselectivity.
Finally, to evaluate selective deselenization of a complex polypeptide following ligation, we prepared the 38-mer peptide Grx3(1-38)(Cys11Sec-Cys14Sec-Ala38Cys) containing two Sec and one Cys residues by NCL (Scheme S1), together with the all-Cys control, Grx3(1-38)(Ala38Cys). Following removal of thiophenol by HPLC, deselenization of Grx3(1-38)(Cys11Sec-Cys14Sec-Ala38Cys) by TCEP gave a doubly-deselenized product Grx3(1-38)(Cys11Ala-Cys14Ala-Ala38Cys) as the major product with a minor Cys reduction side-product (Figure S9 to S12).[31] In contrast the Cys-containing analog Grx3(1-38)(Ala38Cys) was fully stable to the treatment with TCEP.
In conclusion, we have developed a straightforward method for the selective reduction of selenocysteine to alanine in the presence of unprotected cysteine. The deselenization reaction by TCEP is highly chemo- and enantioselective. In effect, this strategy will allow ligation at both Ala and Cys sites in the same protein sequence and maintain native Cys residues without the need for side chain protection. This general approach has the potential to be extended to other amino acids with the appropriate β- or γ- selenol group.[7,9] Furthermore, despite the widespread use of TCEP in protein chemistry, we recommend its use with caution, particularly in the production of selenoproteins.[38]
Supplementary Material
Footnotes
This study was supported by the Israel-US Binational Science Foundation, the German-Israeli Project Cooperation (DIP) (EK), NIH GM059380 (PED), the Israeli Higher Education Planning and Budgeting Committee and Israel Ministry of Science (NM), and the Skaggs Institute for Chemical Biology. EK is the incumbent of the Benno Gitter & Ilana Ben-Ami Chair of Biotechnology, Technion.
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.((Please delete if not appropriate))
References
- 1.Dawson PE, Muir TW, Clark-Lewis I, Kent SBH. Science. 1994;266:776–779. doi: 10.1126/science.7973629. [DOI] [PubMed] [Google Scholar]
- 2.Dawson PE, Kent SBH. Annu. Rev. Biochem. 2000;69:923–960. doi: 10.1146/annurev.biochem.69.1.923. [DOI] [PubMed] [Google Scholar]
- 3.a Dirksen A, Dawson PE. Curr. Opin. Chem. Biol. 2008;12:760–766. doi: 10.1016/j.cbpa.2008.10.009. [DOI] [PubMed] [Google Scholar]; b Hackenberger CPR, Schwarzer D. Angew. Chem. 2008;120:10182–10228. [Google Scholar]; Angew. Chem. Int. Ed. 2008;47:10030–10074. doi: 10.1002/anie.200801313. [DOI] [PubMed] [Google Scholar]
- 4.a Nilsson BL, Kiessling LL, Raines RT. Org. Lett. 2000;2:1939–1941. doi: 10.1021/ol0060174. [DOI] [PubMed] [Google Scholar]; b Saxon E, Armstrong JI, Bertozzi CR. Org. Lett. 2000;2:1941–1943. doi: 10.1021/ol006054v. [DOI] [PubMed] [Google Scholar]; c Bode JW, Fox RM, Baucom KD. Angew. Chem. Int. Ed. 2006;45:1248–1252. doi: 10.1002/anie.200503991. [DOI] [PubMed] [Google Scholar]; d Okamoto R, Souma S, Kajihara Y. J. Org. Chem. 2009;74:2494–2501. doi: 10.1021/jo8026164. [DOI] [PubMed] [Google Scholar]
- 5.a Low DW, Hill MG, Carrasco MR, Kent SBH, Botti P. Proc. Natl. Acad. Sci. U.S.A. 2001;98:6554–6559. doi: 10.1073/pnas.121178598. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Marinzi C, Offer J, Longhi R, Dawson PE. Bioorg. Med. Chem. 2004;12:2749–2757. doi: 10.1016/j.bmc.2004.02.039. [DOI] [PubMed] [Google Scholar]; c Offer J, Dawson PE. Org. Lett. 2000;1:23–26. doi: 10.1021/ol991184t. [DOI] [PubMed] [Google Scholar]; d Botti P, Carrasco MR, Kent SBH. Tet. Lett. 2001;42:1831–1833. [Google Scholar]; e Offer J, Boddy CNC, Dawson PE. J. Am. Chem. Soc. 2002;124:4642–4646. doi: 10.1021/ja016731w. [DOI] [PubMed] [Google Scholar]; f Kawamaki T, Aimoto S. Tetrahedron Lett. 2003;44:6059–6061. [Google Scholar]
- 6.a Yang YY, Ficht S, Brik A, Wong C-H. J. Am. Chem. Soc. 2007;129:7690–7701. doi: 10.1021/ja0708971. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Lutsky MY, Nepomniaschiy N, Brik A. Chem. Commun. 2008;10:1229–1231. doi: 10.1039/b718945a. [DOI] [PubMed] [Google Scholar]
- 7.Yan LZ, Dawson PE. J. Am. Chem. Soc. 2001;123:526–533. doi: 10.1021/ja003265m. [DOI] [PubMed] [Google Scholar]
- 8.Turner RA, Pierce JG, du Vigneaud V. J. Biol. Chem. 1951;193:359–361. [PubMed] [Google Scholar]
- 9.a Crich D, Banerjee A. J. Am. Chem. Soc. 2007;129:10064–10065. doi: 10.1021/ja072804l. The extension of desulfurization to other amino acids through β or γ thiol substitution was originally proposed in ref. 7 and has been shown for Abu [7], Phe and Val. [DOI] [PubMed] [Google Scholar]; b Haase C, Rohde H, Seitz O. Angew. Chem. 2008;120:6912–6915. doi: 10.1002/anie.200801590. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2008;47:6807–6810. doi: 10.1002/anie.200801590. [DOI] [PubMed] [Google Scholar]; c Chen J, Wan Q, Yuan Y, Zhu J, Danishefsky SJ. Angew. Chem. 2008;120:8649–8652. [Google Scholar]; Angew. Chem. Int. Ed. 2008;47:8521–8524. doi: 10.1002/anie.200803523. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Xuechen L, Lam HY, Zhang Y, Chan CK. Org. Lett. 2010;12:1724–1727. doi: 10.1021/ol1003109. and Thr. [DOI] [PubMed] [Google Scholar]; e Chen J, Wang P, Zhu J, Wan Q, Danishefsky SJ. Tetrahedron. 2010;66:2277–2283. doi: 10.1016/j.tet.2010.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.a Yang R, Pasunooti KK, Li F, Liu X-W, Liu C-F. J. Am. Chem. Soc. 2009;131:13592–13593. doi: 10.1021/ja905491p. [DOI] [PubMed] [Google Scholar]; b Ajish Kumar KS, Haj-Yahya M, Olschewski D, Lashuel HA, Brik A. Angew. Chem. Int. Ed. 2009;48:8090–8094. doi: 10.1002/anie.200902936. [DOI] [PubMed] [Google Scholar]; c Harpaz Z, Siman P, Ajish Kumar KS, Brik A. ChemBioChem. 2010;11:1232–1235. doi: 10.1002/cbic.201000168. [DOI] [PubMed] [Google Scholar]
- 11.Wan Q, Danishefsky SJ. Angew. Chem. 2007;119:9408–9412. [Google Scholar]; Angew. Chem. Int. Ed. 2007;46:9248–9252. doi: 10.1002/anie.200704195. [DOI] [PubMed] [Google Scholar]
- 12.Pentelute BL, Kent SBH. Org. Lett. 2007;9:687–690. doi: 10.1021/ol0630144. [DOI] [PubMed] [Google Scholar]
- 13.Durek T, Torbeev VY, Kent SBH. Proc. Natl. Acad. Sci. U.S.A. 2007;104:4846–4851. doi: 10.1073/pnas.0610630104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hondal RJ, Nilsson BL, Raines RT. J. Am. Chem. Soc. 2001;123:5140–5141. doi: 10.1021/ja005885t. [DOI] [PubMed] [Google Scholar]
- 15.a Gieselman MD, Xie L, van der Donk WA. Org. Lett. 2001;3:1331–1334. doi: 10.1021/ol015712o. [DOI] [PubMed] [Google Scholar]; b Ralle M, Berry SM, Nilges MJ, Gieselman MD, van der Donk WA, Lu Y, Blackburn NJ. J. Am. Chem. Soc. 2004;126:7244–7256. doi: 10.1021/ja031821h. [DOI] [PubMed] [Google Scholar]
- 16.a Quaderer R, Sewing A, Hilvert D. Helv. Chim. Acta. 2001;84:1197–1206. [Google Scholar]; b Casi G, Roelfes G, Hilvert D. ChemBioChem. 2008;9:1623–1631. doi: 10.1002/cbic.200700745. [DOI] [PubMed] [Google Scholar]
- 17.Quaderer R, Hilvert D. Chem. Commun. 2002;22:2620–2621. doi: 10.1039/b208288h. [DOI] [PubMed] [Google Scholar]
- 18.Nauser T, Dockheer S, Kissner R, Koppenol WH. Biochemistry. 2006;45:6038–6043. doi: 10.1021/bi0602260. [DOI] [PubMed] [Google Scholar]
- 19.Metanis N, Keinan E, Dawson PE. J. Am. Chem. Soc. 2006;128:16684–16691. doi: 10.1021/ja0661414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorlatov SN, Stadtman TC. Proc. Natl. Acad. Sci. U.S.A. 1998;95:8520–8525. doi: 10.1073/pnas.95.15.8520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burns JA, Butler JC, Moran J, Whitesides GM. J. Org. Chem. 1991;56:2648–2650. [Google Scholar]
- 22.Nallamsetty S, Waugh DS. Nat. Protocols. 2007;2:383–391. doi: 10.1038/nprot.2007.50. [DOI] [PubMed] [Google Scholar]
- 23.Kirsch T, Boehm M, Schuckert O, Metzger AU, Willbold D, Frank RW, Rosch P. Prot. Exp. and Purification. 1996;8:75–84. doi: 10.1006/prep.1996.0076. [DOI] [PubMed] [Google Scholar]
- 24.Valcu C-M, Schlink K. Proteomics. 2006;6:1599–1605. doi: 10.1002/pmic.200500314. [DOI] [PubMed] [Google Scholar]
- 25.Johnson ECB, Kent SBH. J. Am. Chem. Soc. 2006;128:6640–6646. doi: 10.1021/ja058344i. [DOI] [PubMed] [Google Scholar]
- 26. Reduced Sec peptides elute poorly from HPLC columns.
- 27.a Walling C, Rabinowitz R. J. Am. Chem. Soc. 1957;79:5326. The solvent free desulfurization of thiols by trialkylphosphites (and later phosphines) is inhibited by the presence of thiophenol. [Google Scholar]; b Walling C, Basedow OH, Savas ES. J. Am. Chem. Soc. 1960;82:2181–2184. [Google Scholar]
- 28. Deselenization of Sec during native chemical ligation has been observed ref. [15a]
- 29. A minor side product of Sec to Ser conversion was observed, consistent with the Cys to Ser conversion observed in the free-radical-based desulfurization of Cys-Crotogossamide ref.. [11] (Figure 54, page S36 in the SI). This side product is not observed under our optimized conditions with less TCEP.
- 30.Wang Z, Rejtar T, Zhou ZS, Karger BL. Rapid Commun. Mass Spectrom. 2010;24:267–275. doi: 10.1002/rcm.4383. The desulfurization of cysteines residues in proteins at elevated temperatures (up to 60 °C) with TCEP was observed by mass spectrometry, and is consistent with a thiyl radical-based mechanism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. The desulfurized minor-product is likely to result from transfer of the selanyl radical to the Cys thiol, and subsequent desulfurization with TCEP as suggested by Danishefsky ref. [11]
- 32. The mechanism is thought to occur through attack of a selanyl radical with the phosphine TCEP to yield a phosphoranyl radical with an expanded valence shell. The homolytic cleavage of the C-Se bond forms an alkyl radical at the β-carbon, which abstracts hydrogen to form alanine. The driving force of the process is the formation of the strong Se=P bond, ref. [27]
- 33.Bernardes GJL, Grayson EJ, Thompson S, Chalker JM, Errey JC, El Oualid F, Claridge TDW, Davis BG. Angew. Chem. Int. Ed. 2008;47:2244–2247. doi: 10.1002/anie.200704381. Treatment of disulfides with electron rich phosphines can result in β-elimination to form dehydroalanine, subsequent Michael addition leads to epimerization of the Cys residue. [DOI] [PubMed] [Google Scholar]
- 34.Levengood MR, van der Donk WA. Nat. Protoc. 2006;1:3001–3010. doi: 10.1038/nprot.2006.470. [DOI] [PubMed] [Google Scholar]
- 35.Bang D, Makhatadze GI, Tereshko V, Kossiakoff AA, Kent SBH. Angew. Chem. Int. Ed. 2005;44:3852–3856. doi: 10.1002/anie.200463040. [DOI] [PubMed] [Google Scholar]
- 36.a Nauser T, Schöneich C. J. Am. Chem. Soc. 2003;125:2042–2043. doi: 10.1021/ja0293599. [DOI] [PubMed] [Google Scholar]; b Nauser T, Schöneich C. Chem. Res. Toxicol. 2004;17:1323–1328. doi: 10.1021/tx049856y. [DOI] [PubMed] [Google Scholar]
- 37.a Chatgilialoglu C, Altieri A, Fischer H. J. Am. Chem. Soc. 2002;124:12816–12823. doi: 10.1021/ja027428d. [DOI] [PubMed] [Google Scholar]; b Nauser T, Schöneich C. Chem. Res. Toxicol. 2003;16:1056–1061. doi: 10.1021/tx034094c. [DOI] [PubMed] [Google Scholar]; c Pogocki D, Schöneich C. Free Radical Biol. Med. 2001;31:98–107. doi: 10.1016/s0891-5849(01)00559-7. [DOI] [PubMed] [Google Scholar]
- 38.Muttenthaler M, Nevin ST, Grishin AA, Ngo ST, Choy PT, Daly NL, Hu S-H, Armishaw CJ, Wang C-IA, Lewis RJ, Martin JL, Noakes PG, Craik DJ, Adams DJ, Alewood PF. J. Am. Chem. Soc. 2010;132:3514–3522. doi: 10.1021/ja910602h. Recent attempts to reduce a seleno-α-conotoxin in the presence of excess reducing agents TCEP led to significant deselenization. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


