Abstract
The autonomic nervous system is a key regulator of cardiovascular system. In this review we focus on how sex and aging influence autonomic regulation of blood pressure in humans in an effort to understand general issues related to how the autonomic nervous system regulates blood pressure, and the cardiovascular system as a whole. Younger women generally have lower blood pressure and sympathetic activity than younger men. However, both sexes show marked inter-individual variability across age groups with significant overlap seen. Additionally, while men across the lifespan show a clear relationship between markers of whole body sympathetic activity and vascular resistance, such a relationship is not seen in young women. In this context, the ability of the sympathetic nerves to evoke vasoconstriction is lower in young women likely as a result of concurrent β2 mediated vasodilation that offsets α-adrenergic vasoconstriction. These differences reflect both central sympatho-inhibitory effects of estrogen and also its influence on peripheral vasodilation at the level of the vascular smooth muscle and endothelium. By contrast post-menopausal women show a clear relationship between markers of whole body sympathetic traffic and vascular resistance, and sympathetic activity rises progressively in both sexes with aging. These central findings in humans are discussed in the context of differences in population-based trends in blood pressure and orthostatic intolerance. The many areas where there is little sex-specific data on how the autonomic nervous system participates in the regulation of the human cardiovascular system are highlighted.
Keywords: sympathetic activity, vasoconstriction, estrogen, blood pressure
1. INTRODUCTION AND OVERVIEW
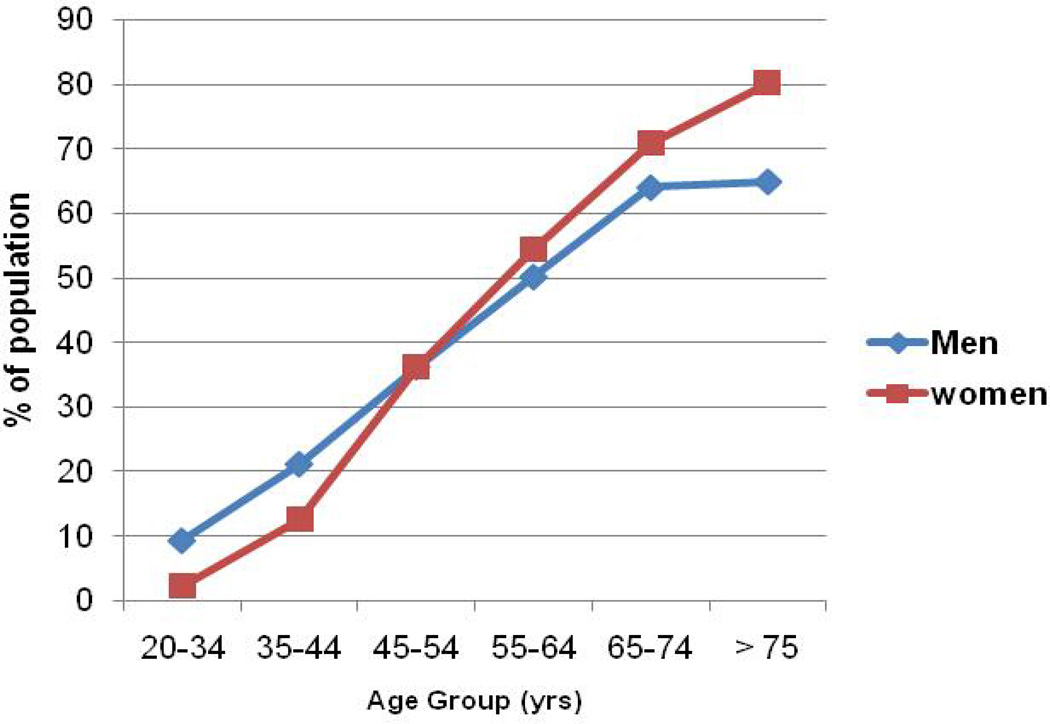
After heart rate, arterial blood pressure is perhaps the most commonly measured vital sign in humans. The case can also be made that blood pressure is the fundamental regulated variable in the cardiovascular system and that by considering it, insights into the larger question of neural control of the circulation can be gained (86). Additionally, when blood pressure is chronically elevated it is a marker of cardiovascular disease risk that can be measured noninvasively. Conversely, when blood pressure is low, the risk of fainting and falls is increased. On a population basis, in the developed world, blood pressure is generally lower in young women than men and rises slowly with age until menopause (73). After menopause, blood pressure increases more rapidly in women than men in similar age groups and by age 65 or 70 years more women are hypertensive than men. These trends are shown in Figure 1. By contrast fainting and orthostatic intolerance as shown in Figure 2 are much more common in young women than young men (3, 39, 42). These observations indicate that, in general, there are important sex differences in human blood pressure regulation consistent with differences in the function of the autonomic nervous system function.
Figure 1.
Prevalence of hypertension from 2003–2006 by sex and age in the United States. These data shows that blood pressure increases with age in wealthy, industrialized countries with low levels of physical activity, food abundance, and the social stresses of urbanization. It also shows that blood pressure is generally lower in young women than young men but that the rate of change in blood pressure is higher for women especially in the perimenopausal period (Figure adapted from ref NCHS, (73).
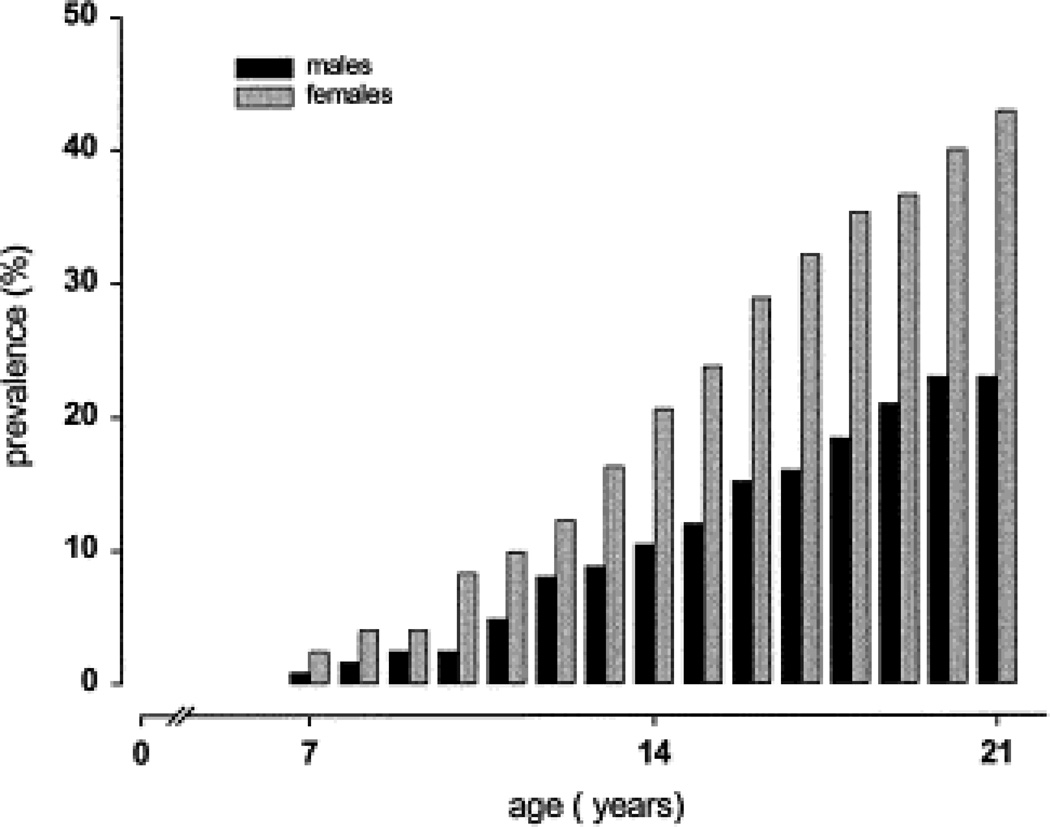
Figure 2.
The prevalence of syncope in young women (grey bars) is higher than young men (black bars) between the ages of 7–21 years. Figure adapted from (42).
Unique Issues for Human Blood Pressure Regulation
Humans are bipedal and thus spend the majority of time in the upright position. With the head above the heart, it can be argued that blood pressure regulation in humans has an additional challenge compared to commonly studied rodents and other quadruped models (86). This difference is perhaps amplified because our species can go rapidly from the supine to upright position. Additionally, unlike many species, adult human females are normally fertile year round until menopause, and life expectancy after menopause for many women can be on the order of 40 or more years which is approximately 50% of lifespan. The population trends in blood pressure after menopause mentioned above are also consistent with the general idea that sex steroid hormones influence blood pressure in women. In this context, the widespread medicinal use of these exogenous hormones for birth control or to modulate the symptoms of menopause might also important implications for blood pressure control (52). Because of this unique collection of features germane to blood pressure regulation in women, and because of our expertise in human studies, we will focus on primary data in humans to the greatest possible extent and rely on limited data from animal models when relevant data are not available in humans.
Scope of this Review
With the above introductory comments as a background, we want to detail four other elements of our approach prospectively. First, the boundaries of issues related to neural control of the circulation are potentially expansive, especially if there is a major focus on epidemiology and pathophysiology. Thus we emphasize the role of sex differences and neural control of the circulation in the context of blood pressure regulation (see Figure 3). Our focus will be on healthy normotensive young and older adults with caveats from studies that focus on hypertension and other clinical conditions. Second, almost by definition the term “neural control” implies a dominant role for the autonomic nervous system. For example during common non-resting conditions like mental stress or exercise, multiple neural factors can influence blood pressure and these influences are exerted via the autonomic nervous system (62). Third, we will not review changes in blood pressure during pregnancy and the associated hypertensive conditions in great detail. What is known about autonomic control in these conditions has been reviewed by Fu (38, 40). Fourth, we also avoid an extended discussion of the renal mechanisms that contribute to blood pressure regulation beyond those areas that clearly intersect with the autonomic nervous system. We have previously challenged what we view as the overly renal-centric view of blood pressure regulation and hypertension and these arguments have been made in detail elsewhere (17, 54, 55). Thus, the scope of this review will be narrow by design. Within this scope, we seek to address seven fundamental questions:
What clues or questions do temporal trends in population-based blood pressure data provide about sex differences and neural control of the circulation?
Can these trends be explained in part by sex related differences in the activity of the autonomic nervous system?
Does aging affect sex related differences in how the autonomic nervous system contributes blood pressure regulation at rest?
Do differences in central autonomic outflow contribute to sex related differences in how the autonomic nervous system contributes blood pressure regulation?
Do differences in baroreflex responses to posture (orthostatic stress) contribute to sex related differences in how the autonomic nervous system contributes to blood pressure regulation?
Do the blood pressure responses to somatic afferent stimulation or mental stress demonstrate sex related differences in how the autonomic nervous system contributes blood pressure regulation?
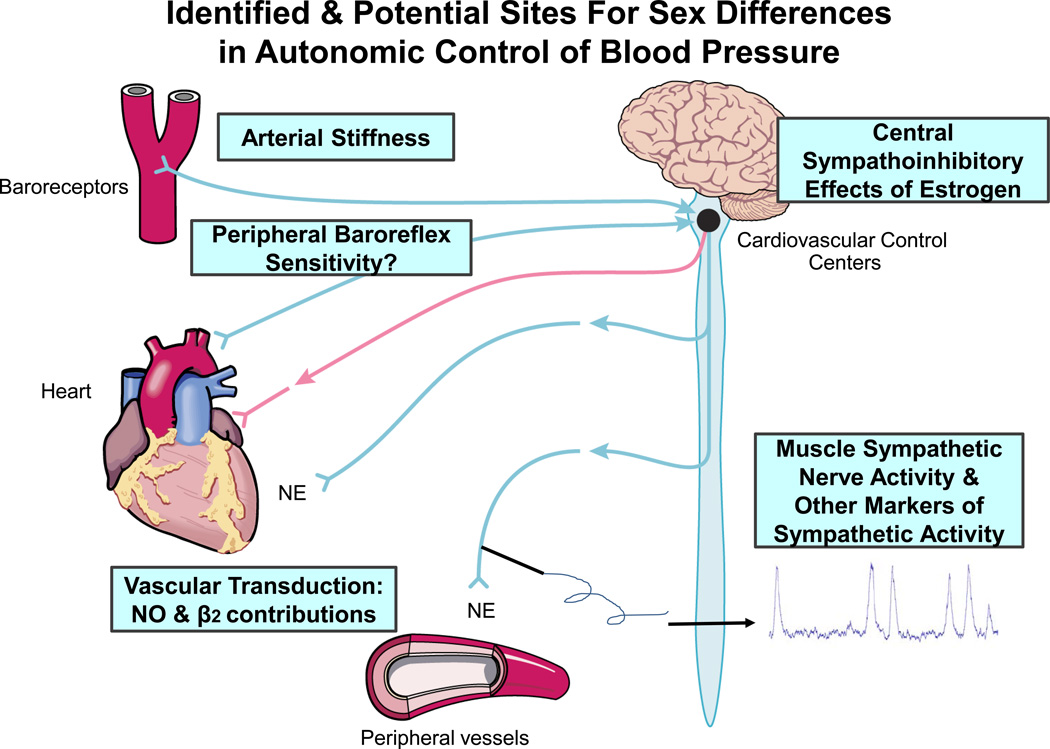
Figure 3.
This illustrates the sites for sex differences in autonomic control of blood pressure discussed in this review. NE=norepinephrine NO=nitric oxide; β2= β2-adrenergic receptor.
Do changes in sympathetic vasoconstrictor nerve activity evoke similar vasoconstrictor responses in both sexes and are these responses altered by age in a sex specific manner? Can any differences in vasoconstrictor responses be explained on the basis of adrenergic receptor sensitivity?
The fundamental role of sex steroid hormones in these differences will be addressed for each question. Before addressing these questions, we will first discuss how to assess the contribution of the autonomic nervous system to blood pressure regulation in humans. We will also discuss our fundamental observations about interindividual variability in how the various determinants of blood pressure interact in healthy humans to generate a relatively narrow range of blood pressure values at rest. Finally, we will try to identify areas that are relatively unexplored and where data are generally lacking.
AUTONOMIC REGULATION OF BLOOD PRESSURE
Because we are focusing on blood pressure to frame our discussion, we start with the classic physiologic approach (based on Darcy’s Law) that mean arterial pressure equals cardiac output multiplied by total peripheral resistance (11). Thus cardiac output (as determined by heart rate and stroke volume) and vascular tone (as described by total peripheral resistance) operate together as determinants of blood pressure, and the equation below is essentially Darcy’s law for fluid applied to the cardiovascular system.
MAP = CO X TPR
Of note, only blood pressure and cardiac output can be measured. Total peripheral resistance is always calculated from the measurement of these variables. Blood pressure is easy to measure both invasively and non-invasively but it is important to remember that measurements of peripheral blood pressure in the limb differ from central blood pressure in the aorta and large central blood vessels (87). Blood pressure is also highly variable over 24 hours and can change dramatically during periods of mental and physical stress. For example, a standard cold pressor test can evoke >20 mmHg increases in both systolic and diastolic blood pressure in normotensive subjects at risk for future hypertension (67). In this context, some subjects show 50% or greater increases in mean arterial pressure in response to mental stress, handgrip, or a cold pressor test (62). By contrast, during sleep most normotensive young people have marked falls in blood pressure. In some individuals the reductions in blood pressure seen during sleep might be interpreted as potentially “low” during wakefulness (68). During periods of physical stress like weight lifting, there can be marked but temporary increases in blood pressure (>250/150 or higher) that many clinicians would find alarming when considered out of context (64).
Cardiac output is more challenging to measure and “gold standard” techniques are invasive and typically require access to mixed venous blood from the pulmonary artery and arterial blood samples. Other noninvasive techniques include the uptake of foreign gases and rebreathing techniques to measure pulmonary blood flow as a surrogate for total cardiac output (50). These are well validated but usually require specialized equipment such as a customized mass spectrometer to measure breath-by-breath gas uptake, and may not be feasible for all laboratories. Simpler, non-invasive ways to estimate cardiac output include the arterial waveform analysis and transthoracic impedance (9, 61, 82, 113). These need to be used with care and are frequently best applied to evaluate changes within a subject during acute interventions rather than absolute values.
In general, both the parasympathetic (vagal) and sympathetic arms of the autonomic nervous system regulate heart rate and vascular resistance. Vagal tone predominates at rest and with a combination of vagal withdrawal and increases in cardiac sympathetic activity causing heart rate to increase during activities like exercise (66). By contrast, vascular resistance is under the predominant control of the sympathetic nervous system along with adrenergic neurotransmitters and receptors. With the exception of human sweating, there is little evidence for sympathetic cholinergic nerves in humans (56).
Implicit in the above discussion is the idea that a given blood pressure might be generated with a relatively high cardiac output and low total peripheral resistance, or a relatively low cardiac output and a high peripheral resistance. Table 1 represents idealized values and ranges at rest based on data from hundreds of younger normotensive subjects we have studied over the past decade. These subjects have been primarily Caucasian, non-obese (BMI less than 30), non-smokers, physically active but not involved in intensive competitive athletic training. They have been rigorously screened for other health problems and are not on any chronic medications except for oral contraceptives in some of the women. We have also included values for muscle sympathetic nerve activity (MSNA), which is a direct measurement of efferent sympathetic activity to skeletal muscles in humans (see below).
Table 1.
Representative normative values for healthy, 25-yr old adults
| Men | Women | |
|---|---|---|
| Height, m | 1.8 | 1.7 |
| Weight, kg | 80 | 65 |
| BMI, kg/m2 | 25 | 23 |
| Heart rate, bpm | 60 | 60 |
| MAP, mmHg | 90 | 90 |
| Stroke volume, mL | 100 | 85 |
| Cardiac output, L | 6 | 5 |
| TPR, mmHg/L/min | 15 | 18 |
| MSNA burst frequency, bursts/min | 24 | 18 |
| MSNA burst incidence, bursts/100 hb | 38 | 31 |
The key points in the table include that the women are shorter and lighter than the men with slightly lower resting blood pressure and similar heart rates. Consistent with the differences in body size, the women also have lower cardiac outputs and smaller stroke volumes. Total peripheral resistance is higher in the women, again due to body size, but MSNA also tends to be lower. There is also considerable interindividual variability in both sexes, especially for cardiac output, total peripheral resistance and MSNA. As this review progresses, the sources of this variability and the physiological ramifications of it will be discussed in more detail, but it is important to remember that broad based claims about sex differences in blood pressure and blood pressure regulation need to be viewed with caution based on how variable the determinants of blood pressure in different individuals of either sex can be. This variability is also amplified by a number of other physiological and anthropomorphic sex differences.
Body Size & Sex Differences
One of the most obvious sex differences is that on average women are smaller than men. This raises issues about how best to scale the determinants of blood pressure. Arguments can be made to support scaling per kg of body weight, on the basis of body surface area, and perhaps on the basis of body weight to the .67–.75 power (49). There are pros and cons to each of these approaches, and scaling typically has its greatest utility when there are vast differences in body size, similar to that observed across the animal kingdom in so-called mice to elephant comparisons.
Further issues related to blood pressure and body size that should be mentioned include the general observation that blood volume is higher in men than women, but hematocrit is typically lower in women (78). Another important consideration when comparing total peripheral resistance between men and women are differences in body composition and resting metabolic rate. While metabolic rate is typically similar per kg of lean tissue in men and women, women typically have more body fat (6). Blood flow to adipose tissue is very low, so the lower lean tissue mass (especially skeletal muscle) typically seen in women means that there is less peripheral demand for oxygen and thus less need for oxygen delivery to peripheral tissues. Again it should be emphasized that these concepts are based on data from the developed world. In this context, much of the world is anemic by Western standards, meaning that for any given blood pressure individuals with chronically low hematocrits are likely to have higher cardiac outputs and lower total peripheral resistance in comparison to developed world standards (30, 65, 109) .
Summary of Section 2
The physiological determinants of blood pressure are described by relationships between standard physical and physiological variables. However, cardiac output can be difficult to measure accurately, and total peripheral resistance is a calculated variable. Comparisons between men and women are also confounded by differences in body size, body composition and other variables. There is no generally accepted or universal approach to scaling these differences among men and women and assumptions about normal values typically reflect measurements made at rest in more economically developed parts of the world. However it is important to remember that blood pressure is usually the main focus of most studies, both in terms of mechanisms of control and in terms of clinical relevance. Although scaling can be useful in some contexts, it is the absolute values (e.g., cardiac output) which contribute directly to blood pressure, not the scaled versions. Thus, we will generally avoid scaling and will discuss potentially confounding issues related to body size, composition and related factors when appropriate.
2. AGE-RELATED TRENDS IN BLOOD PRESSURE
Changes in Blood Pressure with Age and Sex
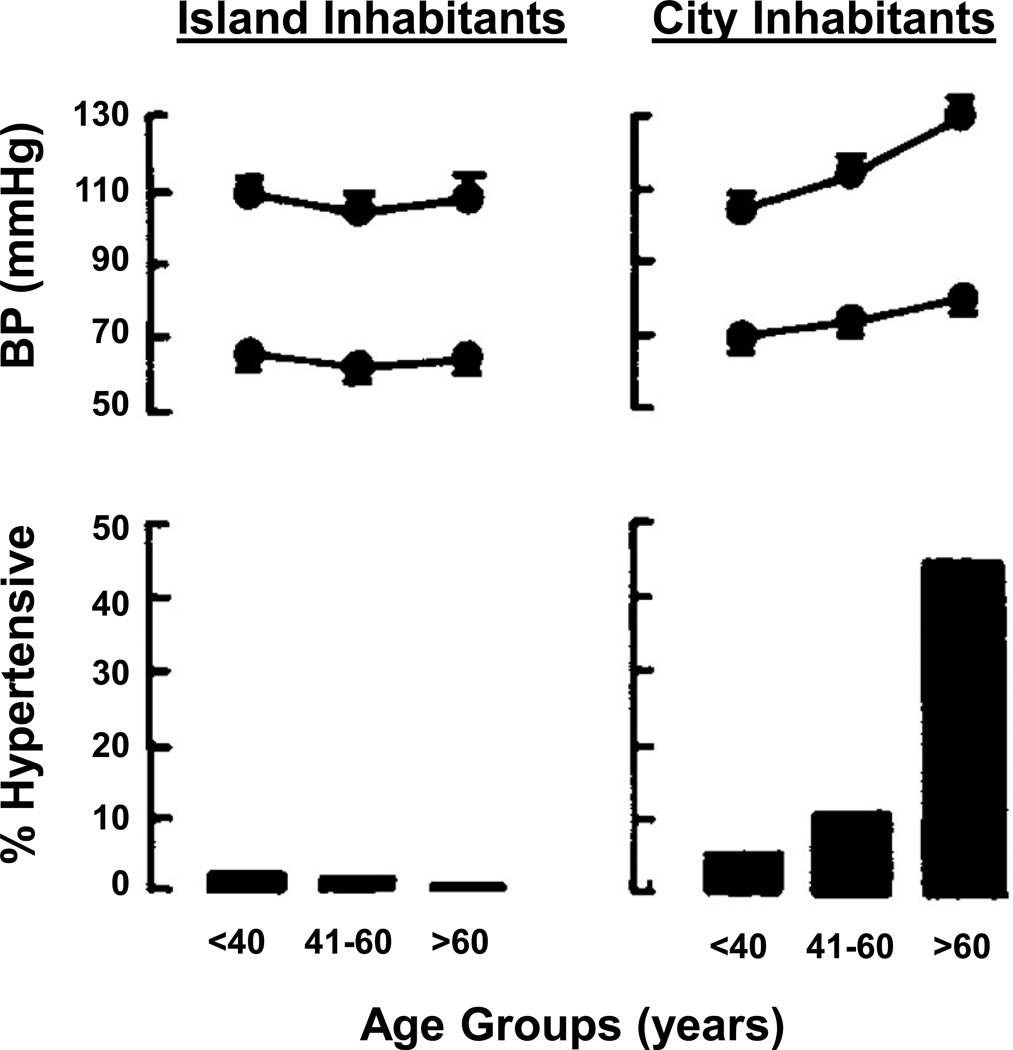
Figure 1 shows age related trends in blood pressure for the U.S (73). This figure is typical for a developed country where physical inactivity, obesity, and high salt diets are common. As mentioned at the outset, a key point in this figure is that blood pressure tends to rise steadily in men over the course of adulthood. By contrast, in women, blood pressure remains lower until middle age and then rises more rapidly after menopause. While the upward trends in blood pressure seen in both sexes in Figure 1 are typical of developed countries, they are not obligatory. Figure 4 shows that in cultures that might be described as less developed from a western perspective, where physical activity is high, processed food consumption and obesity rates are low, and dietary sodium is also low; blood pressure does not rise as dramatically with age (48). Additionally, in individuals and populations that follow what might be described as physical activity and dietary health guidelines the changes in blood pressure commonly seen in Figure 1 can be blunted (7).
Figure 4.
Age and sex-related trends in blood pressure vary by cultural and environmental factors. The top panel shows blood pressure (BP) with age among isolated island Kuna Indian inhabitants and Kuna residing in the urban environment of Panama City. The bottom panel shows the prevalence of hypertension amount these communities. Note that the blood pressure trends typically observed in industrialized areas is not present in rural island dweller, indicating that age-related changes in blood pressure are not an obligatory feature of human biology. Figure adapted from ref (48).
Arterial Stiffness
While the increased cardiovascular risk associated with hypertension is well known, recent research has also focused on the related problem of vascular (arterial) stiffness in large conducting arteries. Hypertension and increased arterial stiffness almost always coexist, and increased arterial stiffness precedes the onset of hypertension (57). Furthermore, many of the problems associated with hypertension are likely due to stiffening of the arteries. The sex differences in blood pressure regulation, and how they are altered by aging, may further be complicated by the increases in arterial stiffness.
With advancing age, the increase in arterial stiffness is thought to be caused by several factors (36). There is a progressive increase in the collagen protein located in the large conduit arteries, and a decrease in the elastin protein. The medial smooth muscle cell layer of the artery may hypertrophy and further contribute to an increase in arterial stiffness. Additionally, a greater deposition of advanced glycation end-products, causing protein cross-linking within each layer of the arterial wall also contributes to the age-associated increase in arterial stiffness. Such structural changes create a stronger, more rigid vessel and may be viewed as adaptive to prevent large fluctuations in blood pressure from rupturing the artery. However, this rigidity of the aorta in particular also creates resistance that the left side of the heart must overcome to open the aortic valve to expel blood into the arterial tree, thereby increasing systolic blood pressure.
Tone in the conduit arteries per se does not seem to be affected by acute changes in sympathetic nerve activity (98). However, acute elevations in sympathetic nerve activity will increase total peripheral resistance. Depending on the method of estimating arterial stiffness, an acute increase in sympathetic nerve activity may result in augmented measures of systemic arterial stiffness, providing an additional mechanism affecting blood pressure regulation. Chronically elevated sympathetic nerve activity often coincides with greater arterial stiffness (e.g. advancing age, diabetes, hypertension, etc.) so it is difficult to determine each variable’s contribution to blood pressure. We speculate that chronically elevated sympathetic nerve activity may independently induce resistance vessel hypertrophy and therefore increase arterial stiffness. Along these lines, Dinenno, et al. has shown an association between conduit artery wall thickness in the femoral artery and sympathetic nerve activity in healthy men (27). This suggests an additional mechanism by which elevated sympathetic nerve activity may influence arterial stiffness and blood pressure regulation with advancing age.
The age-associated increase in arterial stiffness appears to occur earlier in men, compared with women (1, 2, 51, 97), similar to the pattern of change in blood pressure. However, other studies have suggested that there are no differences in arterial stiffness (79). Furthermore, it has also been suggested that women, when matched with men for mean arterial pressure, have a greater age-associated increase in arterial stiffness (103). The idea that elderly women have greater arterial stiffness compared to men has been supported by several recent studies (23, 76, 88). Such discrepancies may be due to the variety of methods to measure arterial stiffness, including the assessment of regional stiffness and wall motion using ultrasonography or systemic arterial stiffness using pulse wave analysis to determine the augmentation of the waveform, or calculating the velocity between arterial pulse sites.
Age-related changes in arterial stiffness in women and men will be affected by differences in sex hormones. Although estrogen is thought to exert protective effects on the vasculature, some studies suggest otherwise. For example, age-related arterial stiffening was not accelerated by the menopausal transition (77) and remained unchanged with hormone therapy (24), suggesting a limited effect of estrogen. The specific effects of sex hormones on the vasculature and total peripheral resistance are discussed in greater detail in section 6.
Potential sex differences in arterial stiffness should also be cautiously interpreted because women usually have smaller arterial diameters due to differences in body size. Therefore, sex differences may be augmented when assessing regional arterial structure and function. Additionally, normative data for pulse wave velocity (PWV) or augmentation index (AI) are often separated by sex, because women have higher PWV and AI values, compared to age-matched men (16, 92, 96, 118). In this context, these differences in estimates of arterial stiffness are thought to be a function of the method of measurement, and not differences in estimated cardiovascular risk.
Summary of Section 3
In the developed world, blood pressure rises with age. The patterns of increase differ between men and women. Data from other cultures or subsets of Westerners who follow health guidelines related to diet and exercise indicate that this increase is not obligatory. Of note is that many of the health consequences of hypertension may be related arterial stiffness; this has become an increasingly important topic for blood pressure research in recent years (69, 80).
3. ASSESSMENT OF AUTONOMIC CONTRIBUTIONS TO BLOOD PRESSURE REGUATION
In addition to simple non-invasive measurements of HR and BP, there are a number of ways to assess the contributions of the sympathetic and parasympathetic nervous systems to blood pressure regulation. These include measurements of blood borne hormones, tracer techniques to determine norepinephrine spillover, direct neural recordings of sympathetic activity, and pharmacological agents to block or activate key autonomic neural pathways or effector responses. Analysis of heart rate variability is also useful to assess autonomic (especially vagal) control of heart rate.
Sympathetic Activity
Plasma Norepinephrine
There are three widely used approaches to assess the activity of the sympathetic nervous system in humans; the first is to simply measure the concentration of norepinephrine in venous blood. During activities associated with marked sympathetic activation like graded exercise there can be large increases in NE. NE concentrations also increase during less stressful activities like changes in posture (movement from supine to upright position). In resting humans, plasma norepinephrine is often correlated with directly measured sympathetic nerve activity (53, 91). However, because values measured in venous blood reflect the balance of NE release, reuptake and metabolism, it is not possible to state with certainty that the circulating values are directly related to changes in efferent sympathetic nervous system activity over a range of activities or conditions. An additional issue is that values obtained from a peripheral vein (e.g. the antecubital vein) might also be overly influenced by regional sympathetic outflow (to the forearm for example) vs. reflecting more global changes in whole body sympathetic activity.
Norepinephrine Spillover
To calculate norepinephrine spillover, radioactive tracers of norepinephrine are infused at a known rate and specific activity. If the arterial concentration of both labeled and unlabeled NE is known, then by obtaining either a whole body (mixed venous) or regional venous blood sample along with an estimate of blood flow, it is possible to estimate either whole body or regional norepinephrine release. This technique also accounts for norepinephrine re-uptake and metabolism, which can vary under different physiological conditions (33, 34). An advantage of NE spillover is that it can provide insight about sympathetic activity to regions of the body where microneurography is impossible. It is also possible to use the technique during physiological stressors like exercise. However, the technique is invasive and technically demanding and thus is only used in a limited number of specialized laboratories. An additional limitation is that NE spillover has a relatively limited temporal resolution in comparison to MSNA.
Muscle Sympathetic Nerve Activity (MSNA)
MSNA measured using microneurography is a direct measurement of the electrical activity of peripheral sympathetic nerves in humans. In this technique, a small tungsten microelectrode is placed in a peripheral (usually the peroneal) nerve and it is possible to record from efferent sympathetic vasoconstrictor nerve fibers. One advantage of MSNA is its rapid time resolution allowing beat to beat changes in sympathetic activity to be measured. Additionally, under resting conditions, changes in MSNA to one region typically reflect changes to most or all of the skeletal muscles (85, 104). This means that MSNA reflects sympathetic activity to the largest vascular bed in the body. A key question is the extent to which MSNA is an indicator of systemic sympathetic activity, Wallin, Esler and colleagues showed that MSNA is directly proportional to renal and cardiac sympathetic activity (in men), as measured using norepinephrine spillover techniques, and to whole body norepinephrine spillover (105, 107).
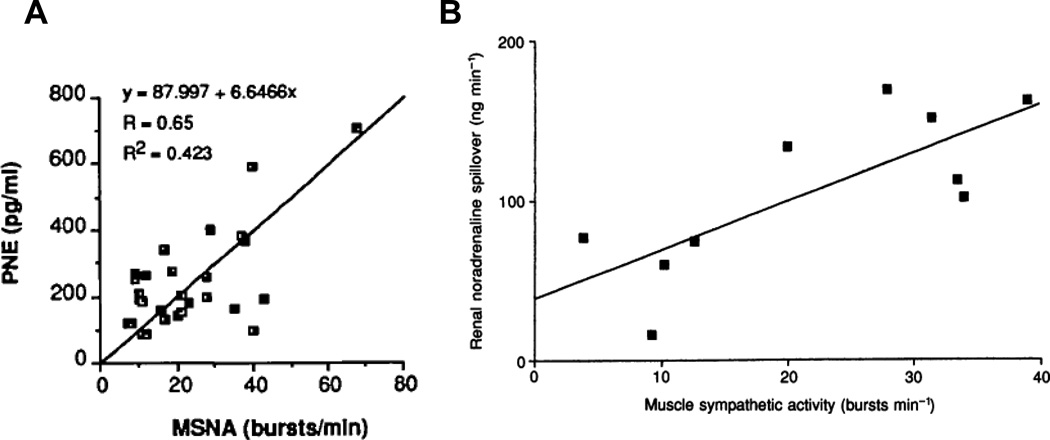
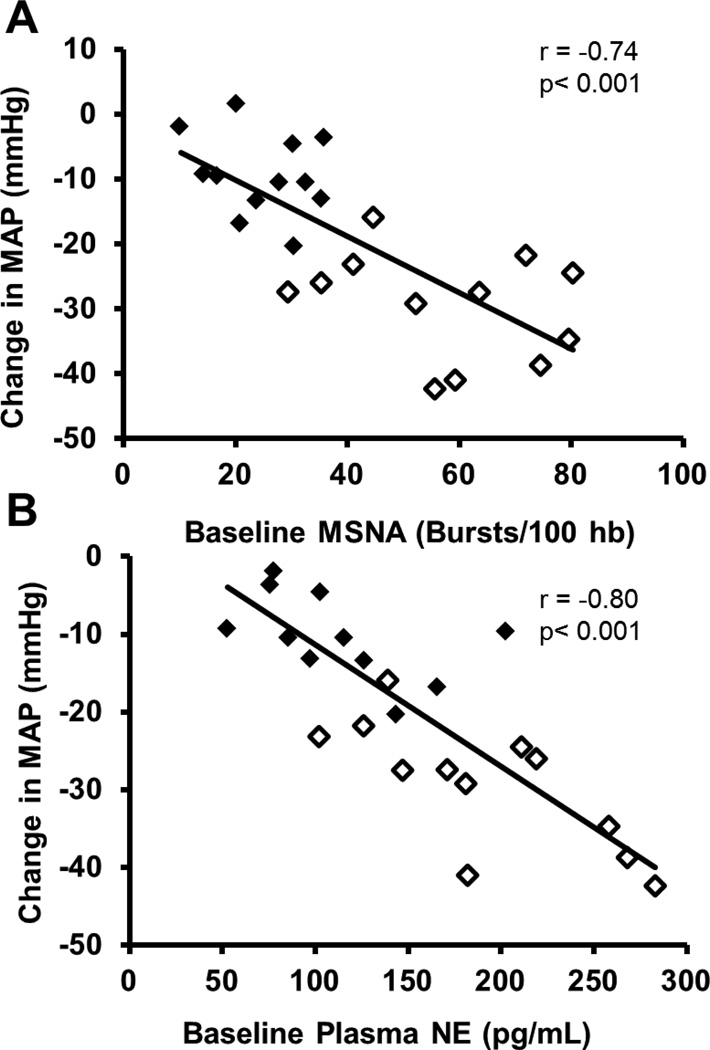
In general, MSNA correlates with simple measurements of plasma norepinephrine, more detailed tracer based measurements of whole body norepinephrine spillover, and many organ specific measurements of norepinephrine spillover. MSNA is more difficult to measure in non-resting situations that require significant movement by the subject. Figure 5 shows two examples of correlations between plasma NE, MSNA and NE spillover.
Figure 5.
The relationship between baseline muscle sympathetic nerve activity (MSNA) and plasma norepinephrine levels (PNE) is shown in panel A (Figure adapted from) (74). Panel B shows the relationship between MSNA and renal norepinephrine (noradrenaline) spillover. Figure adapted from (1097). These data highlight the general agreement between several commonly used indices of sympathetic activity in humans. In these studies only a limited number or no women were included.
Pharmacological Tools
Autonomic agonists and antagonists can also be used to “pharmacodissect” how the autonomic nervous system controls the circulation. These drugs can be given systemically or via a peripheral artery across specific vascular beds. With systemic doses, a major potential confounding factor is that any changes in blood pressure caused by administration of one class of drugs might cause reflex adjustments in neural activity. Under these circumstances, effector responses not targeted by the drug being used could occur due to the reflex adjustments, thereby potentially confounding data interpretation. For example, high dose α-adrenergic blocking drugs could be given to estimate the contribution of α-adrenergic tone to blood pressure regulation. However, a drop in blood pressure (due to peripheral vasodilation) might evoke reflex-mediated increases in heart rate that would keep blood pressure relatively normal and also alter either sympathetic or parasympathetic tone to the heart. At first glance, one (erroneous) conclusion might be that α-adrenergic receptors on the myocardium play a key role regulating heart rate. In this context, care must be taking to ensure the potential reflex and other systemic effects of the drugs are fully understood so that the data generated using systemic administration of these drugs can be interpreted in a coherent fashion. Another issue with the use of whole body adrenergic antagonists is that the extent of receptor blockade (partial vs. complete blockade) will alter both direct and reflex responses.
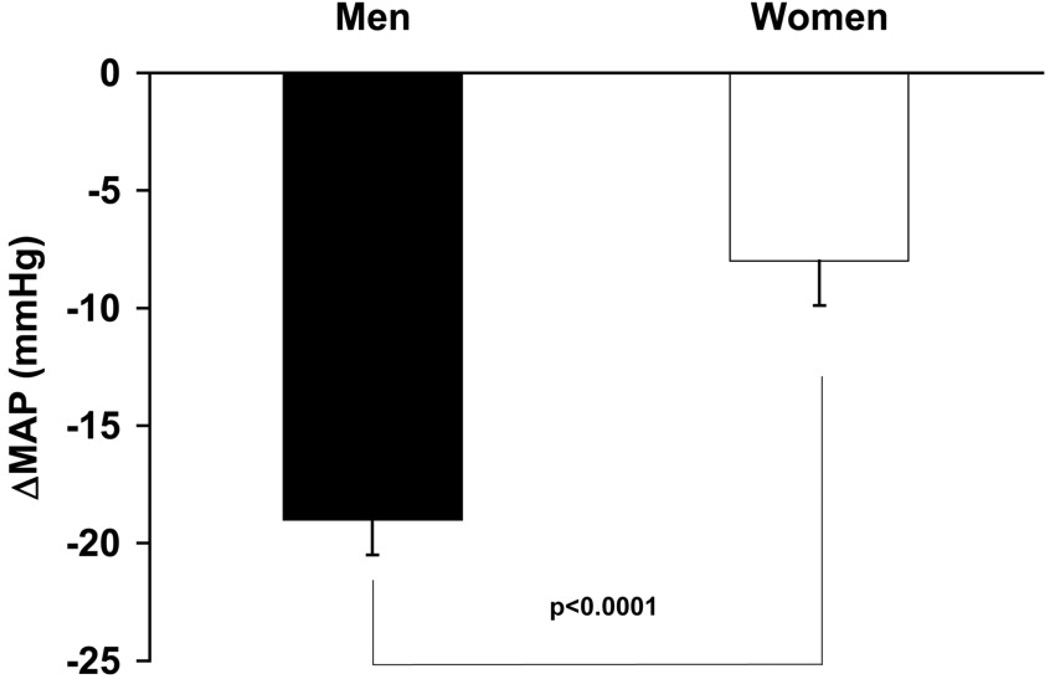
One strategy to understand the contribution of the autonomic nervous system to blood pressure regulation is to give either several classes of drugs (e.g. α and β blockers) at the same time or use ganglionic blockade with drugs like trimethaphan camsylate to assess “autonomic support” of blood pressure (53, 60). In this technique, changes in blood pressure in the absence of any influence of the autonomic nervous system on blood pressure are used to determine “autonomic support” of blood pressure. If blood pressure falls more in one subject group vs. another, then the group with the largest fall in blood pressure is said to have greater autonomic support of blood pressure. We and others have interpreted the reductions in blood pressure during ganglionic (or multi-modal pharmacologic blockade) of the ANS as primary evidence that the autonomic nervous system plays a major role in long term blood pressure regulation (4, 20, 53) . Figure 6 shows the difference in the decrease in blood pressure, or autonomic support between young men and young women.
Figure 6.
The autonomic support of blood pressure is estimated by the blood pressure response after ganglionic blockade, where both sympathetic and parasympathetic nerve activity is abolished. The change in mean arterial pressure (MAP) in response to ganglionic blockade is greater in young men compared with young women, indicating greater autonomic support of blood pressure in young men. Figure adapted from (20).
An additional problem with the use of whole body pharmacologic strategies including autonomic blockade is that changes in blood pressure and heart rate, while interesting, provide an incomplete picture of vascular resistance unless cardiac output is also measured. This is analogous to the discussion above regarding compensatory reflex adjustments evoked with various autonomic agonists and antagonists. However, when this approach is used in conjunction with measures of cardiac output even more insight is possible into the role of the autonomic nervous system in blood pressure regulation. This additional insight is possible because with knowledge of cardiac output, the changes in vascular resistance can be calculated giving a more complete picture of overall autonomic regulation of the circulation.
Finally, there are a limited number of compounds available for use in humans and existing ideas about the specificity of the drugs and the idealized role of specific receptor subtypes in various physiological responses are not always accurate. For example, it is generally taught that the β1 adrenergic receptor subtype is specific to cardiac control (changes in heart rate and contractility). However, at least some of the increase in heart rate during sympathetic stimulation is due to β2 adrenergic receptors and there is also substantial post-junctional α2 adrenergic vasoconstrictor tone (10, 26, 63). Observations such as these conflict with standard ideas and teaching about the specificity and physiological roles of adrenergic receptor subtypes.
Autonomic agonists and antagonists can also be used across a specific vascular bed to gain insight into the contribution of sympathetic activity and adrenergic receptor subtypes to vascular tone. Over many years, this approach has been applied to the human forearm. This permits large regional doses of drugs to be given to a small volume of tissue so that detailed pharmacologic studies can be performed in humans with little or no systemic effects of the drugs. Thus the problem of engaging cardiovascular reflexes is avoided. This technique can also be used on conjunction with other techniques. For example, microneurography can be used to measure sympathetic outflow to the limbs and plethysmography can be used to measure limb blood flow. Autonomic blocking drugs can then be used to understand the extent to which any changes in sympathetic outflow evoke changes in limb blood flow. While these approaches are extremely useful, extrapolation of changes observed across the forearm to the whole body is an obvious limitation. For example, in men non-selective alpha adrenergic blockade of the forearm leads to larger increases in forearm blood flow and reductions in forearm vascular resistance in young men compared to older men. By contrast the effects on femoral blood flow are similar between young and older men (28). These findings demonstrate less vasoconstrictor tone for a given level of sympathetic activity in older men and regional differences in the two age groups. For older and younger women there is essentially no comparable data. For both sexes across age groups, essentially no data is available concerning these issues in visceral vascular beds. The obvious accessibility of the limbs for study is the obvious explanation for this lack of data.
Parasympathetic Tone
Assessment of parasympathetic tone, especially to the heart, is challenging. It is not possible to make direct measurements of vagal nerve activity to the heart in conscious humans. Additionally, acetylcholine, the main neurotransmitter released by the parasympathetic nerves that influence heart rate, is short-lived and it is not possible to gain insight into changes in parasympathetic activity via measurements of plasma acetylcholine levels. In general, the most commonly used methods to assess vagal control of heart rate rely on the measurement of heart rate variability or muscarinic blocking drugs like atropine. In this context, high frequency heart rate variability is typically thought to reflect vagal influences on the heart (46). The extent to which other indices of heart rate variability (low frequency, low-to-high frequency ratio, etc) reflect changes sympathetic activity to the heart is controversial (8, 29). Additionally the mechanisms responsible for the changes in vagal nerve activity that cause high frequency HRV are uncertain.
The general idea is that vagally-mediated changes in heart rate can occur very rapidly (with high frequency) because of two main factors: first, the vagus nerve is large and myelinated (unlike postganglionic sympathetic nerves, which are small and unmyelinated), and therefore neurotransmission is rapid. Second, the existence of acetylcholinesterase at the neuro-effector junction means that “turning off” the vagal influence can occur very rapidly as well. There is evidence suggesting that the variability reflects centrally driven vagal outflow, changes in vagal outflow evoked by afferents in the lungs and thorax, and also baroreflex mediated changes in HR caused by subtle breathing related changes in central blood volume.
Parasympathetic control of heart rate can also be evaluated using muscarinic antagonists like atropine. In general, the change in heart rate from rest to the “blocked” condition is taken as an index of parasympathetic tone to the heart, but the many caveats noted above for whole body drug administration apply to this technique as well.
Summary of Section 4
In this section, we have reviewed the fundamental techniques used to assess the influence of the autonomic nervous system on blood pressure and related variables in humans. These techniques can be used at rest or to measure the responses of the variables of interest to physiological stressors like exercise, orthostatic challenge, or experimentally evoked changes in blood pressure. There are positives and negatives associated with each technique including issues of cost, complexity, and temporal resolution. Additionally, when many of the approaches are used in isolation, they can provide an incomplete picture of the role the autonomic nervous system in blood pressure regulation as reflex adjustments in other elements of the system occur which mask the contribution of the system under study. Thus, a thorough understanding of the strengths and limitations of each approach along with how they can be used in combination with hemodynamic measurements and other interventions is critical for their optimal application to a specific experimental question.
INTER-INDIVIDUAL DIFFERENCES IN SYMPATHETIC NEURAL AND CARDIOVASCULAR VARIABLES
Hagbarth, Vallbo, Wallin and colleagues originally discovered, in the late 1960s, that peripheral sympathetic nerve activity could be directly recorded in awake human subjects (101). As clinical neurophysiologists, these pioneers had a goal of identifying a range of normal values, above which, for example, an individual might be at increased risk of cardiovascular disease such as hypertension (100, 101). Unfortunately, from the perspective of clinical predictive value, MSNA exhibits a very wide inter-individual variation among otherwise similar, healthy, normotensive young men. Indeed, Sundlöf and Wallin (and many subsequent investigators) reported inter-individual variation of as much as 7 to 10-fold (19, 43–45, 72, 74, 100) . Figures 7, 8, 9, and 10 shows this inter-individual variability and also group differences for a large number of young men and women and also middle aged and older subjects. A key point is that while the range in high in both sexes, the average values are clearly lower in young women in comparison to young men.
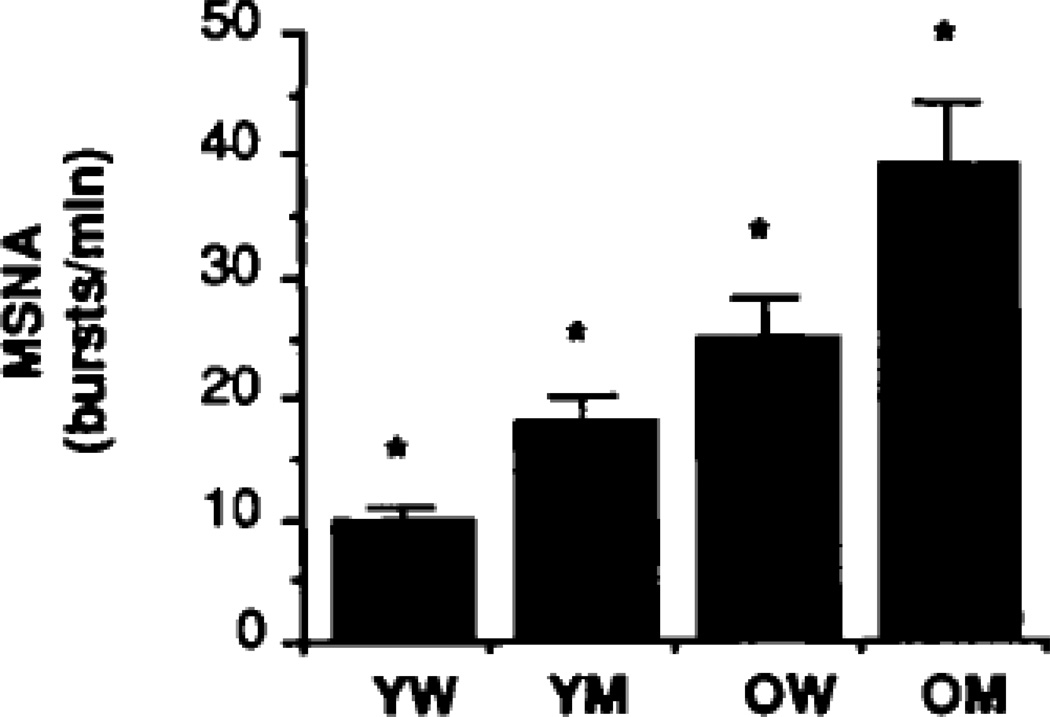
Figure 7.
Mean levels of muscle sympathetic nerve activity (MSNA) are lower in young women (YW) compared with young men (YM) but rise similarly with age. Note the sex differences remain between older women (OW) and older men (OM). *p<0.05 for pairwise comparisons. Figure adapted from (74).
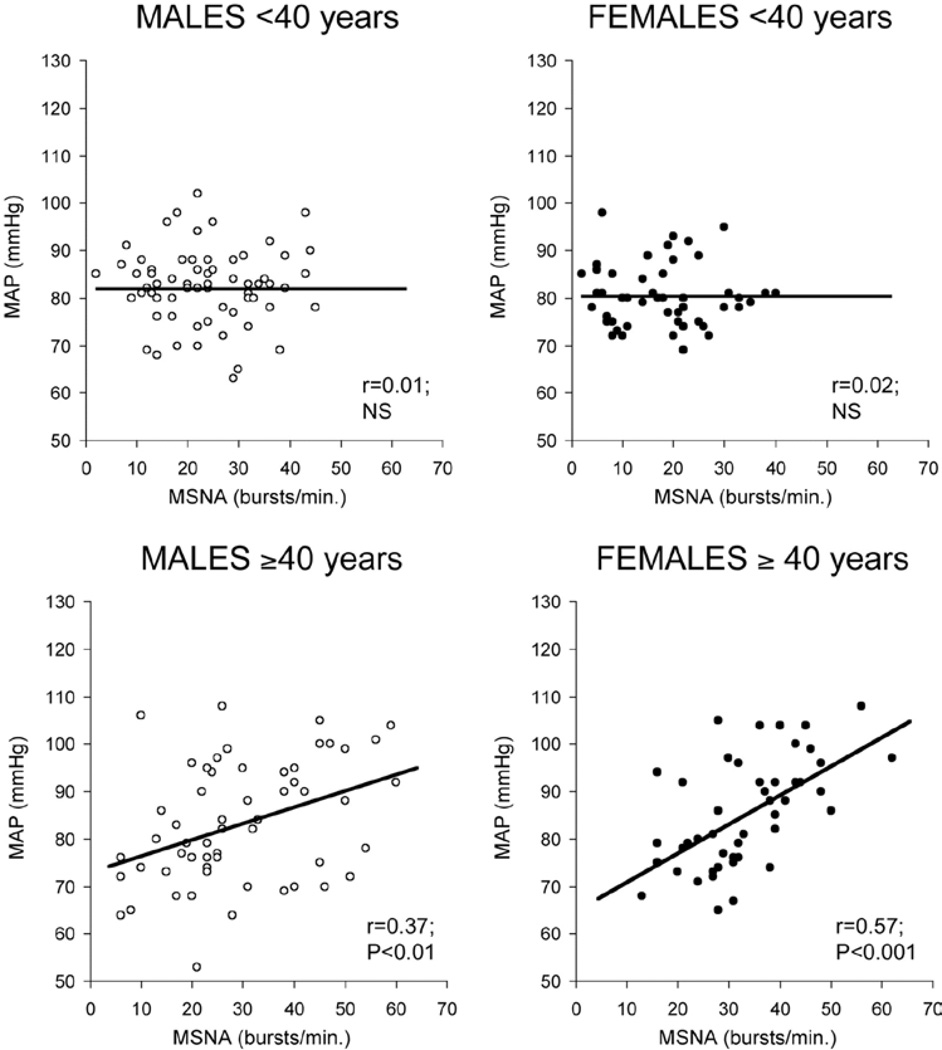
Figure 8.
The relationship between muscle sympathetic nerve activity (MSNA) and mean arterial blood pressure (MAP) in large cohort of healthy humans is shown. All four panels show the marked variability in MSNA seen in normal humans. The top panels show that for young men and women there is no relationship between MSNA and MAP. The bottom panels show that a weak relationship emerges in men over 40 and that this relationship is stronger in women. Data from (72).
Figure 9.
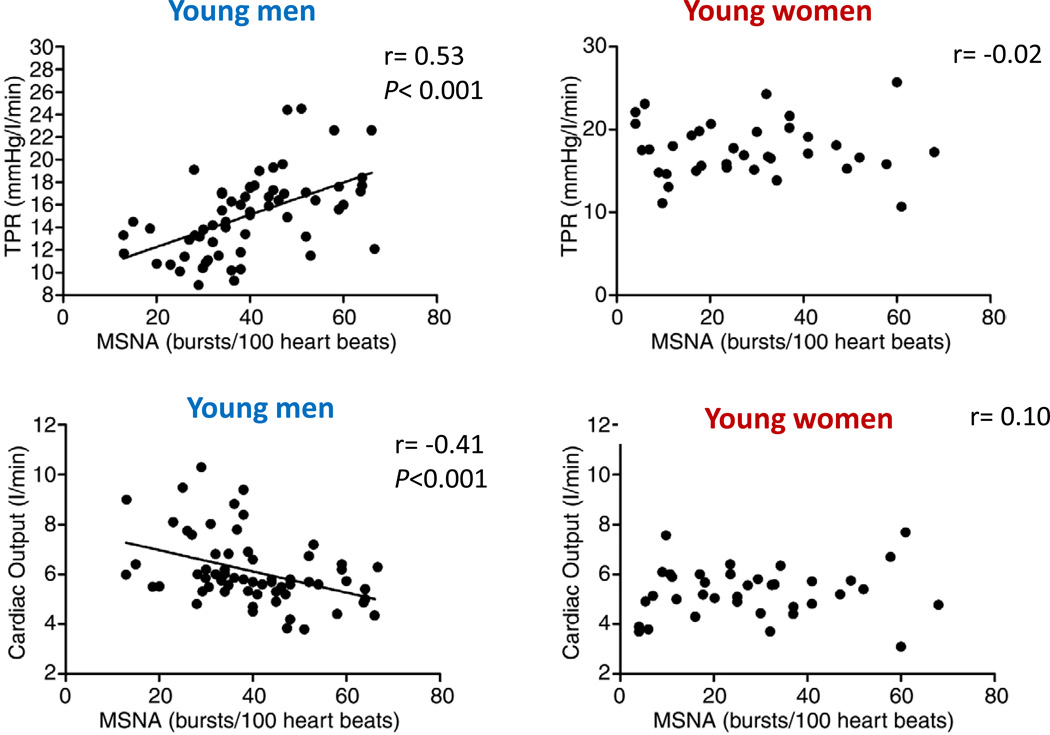
Mean arterial pressure is determined by total peripheral resistance (TPR) and cardiac output (CO). The relationship between muscle sympathetic nerve activity (MSNA) and TPR or CO is only apparent in young men (left panels). The positive correlation between MSNA and TPR, and the inverse correlation between MSNA and CO in young men balances the effect of MSNA so that higher MSNA does not translate to higher MAP. However, these relationships are not present in young women which suggest that young women regulate blood pressure differently than young men. In addition, it suggests that the transduction of sympathetic activity to vasoconstrictor responses differs between young men and women. Data compiled from several studies from our laboratory (19, 43–45).
Figure 10.
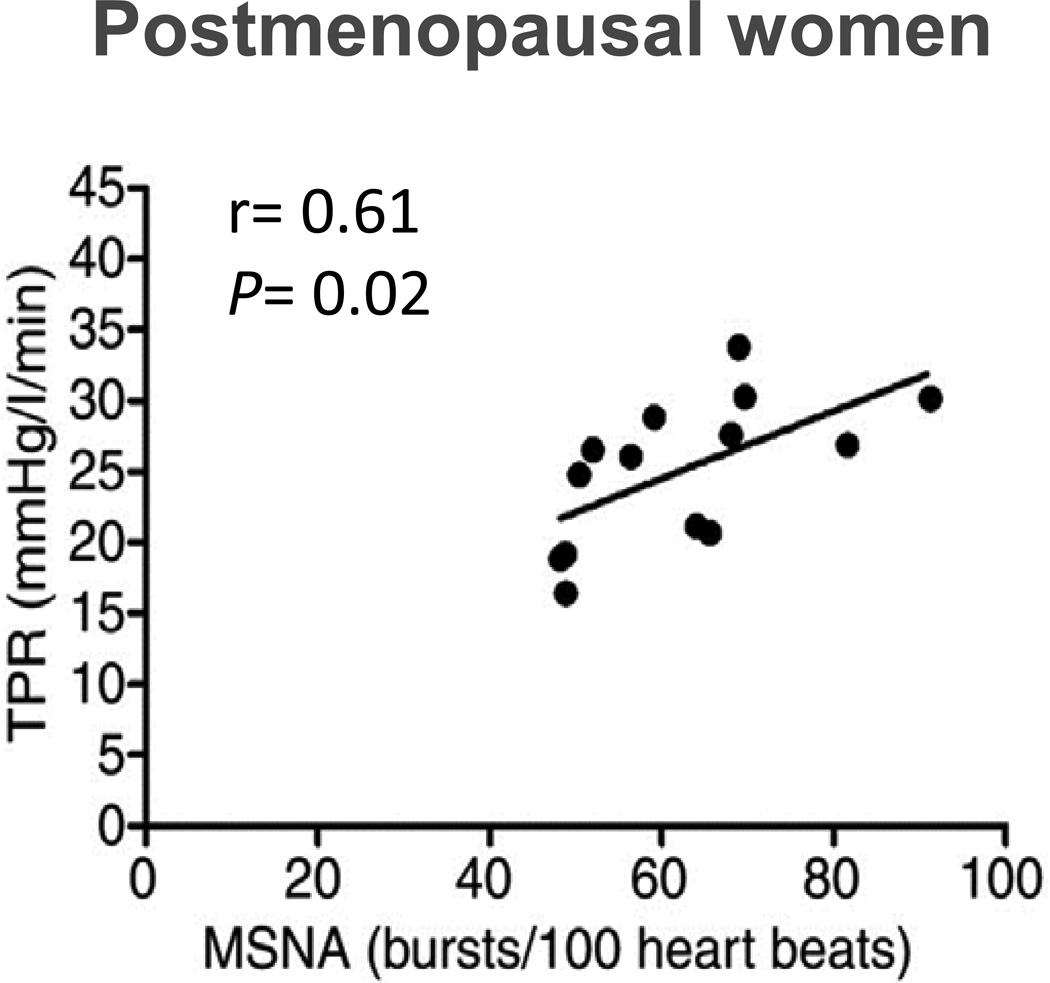
The relationship between muscle sympathetic nerve activity (MSNA) and total peripheral resistance (TPR) also exists in healthy postmenopausal women. This pattern is similar between young men (Figure 9) and postmenopausal women, but not young women suggests a role for sex hormones in blood pressure regulation. Data from (43).
Even more surprising was the observation that this variability in MSNA was not related to resting blood pressure in young people. Sympathetic nerves are primarily vasoconstrictor in nature, and maneuvers which increase MSNA tend to increase blood pressure acutely. Therefore, it was initially thought that individuals with higher MSNA would have higher blood pressure chronically, and that MSNA that was “too high” could be predictive for hypertension. However, among young healthy men and women, it has repeatedly been shown that people with higher MSNA did not necessarily have higher blood pressure than people with MSNA several fold lower (19, 43–45, 94, 100). As Figure 8 shows, there is some relationship between MSNA and blood pressure in older subjects but it is not particularly impressive. While mean values for MSNA are generally higher in groups of hypertensive subjects, the range of values seen in patients has significant overlap with the range seen in normotensive controls. These findings also demonstrate the challenges of directly linking sympathetic activity or changes in it to changes in vascular resistance in all circumstances across different groups of subject. In addition to the need to consider cardiac output when considering blood pressure, the vasoconstrictor effects of sympathetic activity may also dependent on the amount of NE released and sensitivity of targeted blood vessels to it (25). Maneuvers that increase sympathetic activity like hypoxia might also have direct vasodilator effects on blood vessels further confounding what is a conceptually straight-forward relationship (108). This relationship might be further confounded by local autoregulation via factors like the myogenic response (81). However, these responses are difficult to study in intact humans and the influences of sex and age on these responses are virtually unknown.
Following the initial observations of wide inter-individual variation in MSNA and lack of relationship with blood pressure, several decades passed in which the apparent paradox remained unexplained. One interpretation was that this was evidence against a major role of the sympathetic nervous system in human blood pressure regulation, and/or that MSNA might not be a good indicator of systemic sympathetic nerve activity. Another idea was that if SNA was high to one vascular bed it might be lower to another bed. For example if cardiac SNA was high and MSNA was low then perhaps the balance between the two resulted in no net effect on blood pressure. However, as noted above, most indices of sympathetic activity in different vascular beds track each other in resting healthy humans (105, 107).
Sympathetic Activity vs. TPR
More recent studies, including our own, have provided evidence of an integrated balance of several key neural and hemodynamic factors, which together contribute to the maintenance of normal blood pressure over a range of MSNA values in young healthy people. Notably for the purpose of this review, the balance of factors appears to be different for each of the sexes (19, 43–45). We reported in young men that MSNA at rest showed an inverse correlation with resting cardiac output, and a positive correlation with total peripheral resistance (TPR) (19). Thus, MSNA is an important contributor to resting TPR, and individuals with higher MSNA (and higher TPR) have lower cardiac output. Going back to the basic cardiovascular/ hydraulic equivalent of Ohm’s law (MAP = CO x TPR), these relationships clearly explain the lack of relationship between MSNA and MAP in healthy young men.
Subsequently, MSNA was shown to be inversely related to vascular adrenergic responsiveness in a group of young healthy (mostly male) subjects (18). Individuals with higher MSNA showed less forearm vasoconstriction during brachial artery infusions of both norepinephrine and tyramine (which elicits endogenous norepinephrine release). This relationship is also an important aspect of the multifactorial neural-hemodynamic balance maintaining normal blood pressures in healthy individuals over a range of MSNA values at rest.
As discussed in subsequent sections, the inter-individual variability in MSNA, and the balance of neural with hemodynamic factors, show important differences between men and women, and between women before and after menopause.
Basis of Inter-Individual Differences in MSNA
Currently the factors responsible for the large differences in MSNA seen in relatively homogenous young healthy subjects remain obscure. Candidates include differences in central sympathetic outflow and alterations in peripheral baroreflex function. That genetic factors contribute to differences among people is suggested by a study showing that identical twins have similar levels of MSNA (106). Another idea is that vasodilator tone may differ among subjects and individuals with relatively more dilator tone would require increased sympathetic activity to offset this tone so that blood pressure could be maintained in the normal range. In this context, Skarphedinsson and colleagues found that MSNA was inversely related to markers of whole body nitric oxide (NO) synthesis in a group of healthy young men, consistent with the interpretation that individuals with robust NO-mediated vasodilation at rest require higher levels of sympathetic activity to maintain a normal blood pressure (94). In a study designed follow up on those observations, we found that individuals with high levels of baseline MSNA experienced more marked increases in blood pressure when large systemic doses of the nitric oxide synthase inhibitor L-NMMA were administered (18). However, interpretation of these latter data were complicated by the fact that the blood pressure increases were due to differences in cardiac output (baseline cardiac output being lower in people with higher MSNA), and that changes in peripheral vascular resistance (TPR) with LNMMA were similar between groups.
Sex Differences in Inter-Individual Variability
We have conducted similar studies on relationships between blood pressure, cardiac output, and muscle sympathetic nerve activity in young women. The overall amount of variability appears to be similar between young men and women, although as noted above, average values tend to be lower in women. We originally hypothesized that the basic relationships seen in men would be observed in women, but would be shifted to lower cardiac output/ MSNA values (i.e., shifted “down and to the left”). However, we found that the positive relationship between MSNA and TPR seen in young men was absent in young women as seen in Figure 9. We also found that inverse relationship between MSNA and cardiac output was absent in young women (19, 43–45).
Effects of Aging
While the relationships seen between blood pressure, cardiac output, MSNA, and TPR are not as robust, they are generally seen in middle-aged and older men as well as younger men. One reason for these age-related differences in men may be due to the fact that aging, even in healthy subjects, is associated with reduced endothelial function, and this reduction in endothelial function can be quite variable clouding the relationship between TPR and MSNA. Additionally, older men are less responsive to α-adrenergic vasoconstriction (25, 45, 95).
Once again, when women are considered, important and unexpected sex differences were observed. The relationships seen in younger women (or lack thereof) are reversed and older women show a pattern of relationships between blood pressure, cardiac output, and MSNA/TPR similar to those seen in younger men. The correlation between MSNA and TPR is shown in Figure 10, and are similar to the young men shown in Figure 9.
Other Evidence that there are Sex Differences in Neural Control of the Circulation
In general, our findings related to the relationships between mean arterial pressure, cardiac output, MSNA, and TPR are supported by studies that have given autonomic blocking drugs to younger and older members of both sexes. Results from these studies indicate that the effects of ganglionic blockade on blood pressure are less in young women than in young men (53) (see Figure 6). This is also true when other pharmacological strategies are used to disrupt the relationship between sympathetic outflow and vascular resistance in young men and women. This general observation is consistent with the idea that MSNA and TPR are related in young men, but unrelated in young women (90).
In older subjects of both sexes, the reductions in blood pressure during ganglionic blockade are marked and there is a positive relationship between the fall in blood pressure during ganglionic blockade and the baseline level of MSNA. One potential confound in comparing younger and older subjects during autonomic blockade is that in addition to eliminating sympathetic vasoconstrictor activity, ganglionic blockade also eliminates parasympathetic activity. Thus, young subjects show a larger increase in heart rate during ganglionic blockade than older subjects because aging is associated with a reduction in intrinsic heart rate. However, the results of ganglionic blocking studies outlined above, in conjunction with other whole body pharmacologic blockade studies, are consistent with the idea that there are important sex differences in the relationship between inherent sympathetic activity and total peripheral resistance in young men and women, and that these relationships shift dramatically in post-menopausal older women.
Interim Summary Sections 1–5
We have now outlined a number of technical issues related to studying neural control of the circulation in humans. We have also reviewed data showing that autonomic support of blood pressure is higher in young men than young women and that it increases in both sexes with aging. We have discussed the importance of inter-individual variability in neural and hemodynamic variables in men and women, and the relationships among these variables that contribute to normal blood pressure regulation in healthy people. Importantly, the positive relationship seen between MSNA and TPR along with the inverse relationship seen between MSNA and cardiac output in young men are absent in young women. By contrast the relationships seen in young men are seen in older women. With these fundamental observations, we have answered the first two questions laid out at the beginning of this review on how sex and age related population trends in blood pressure might reflect differences in the activity of the autonomic nervous system. We will now explore the physiological mechanisms behind the differences in the autonomic nervous system and blood pressure regulation by addressing the remaining questions outlined at the outset of this review. To put these issues in context, we will first discuss what known about the effects of sex steroids on the variables we have discussed up to this point.
4. INFLUENCE OF SEX STEROIDS
Effects of Estrogen on Sympathetic Activity
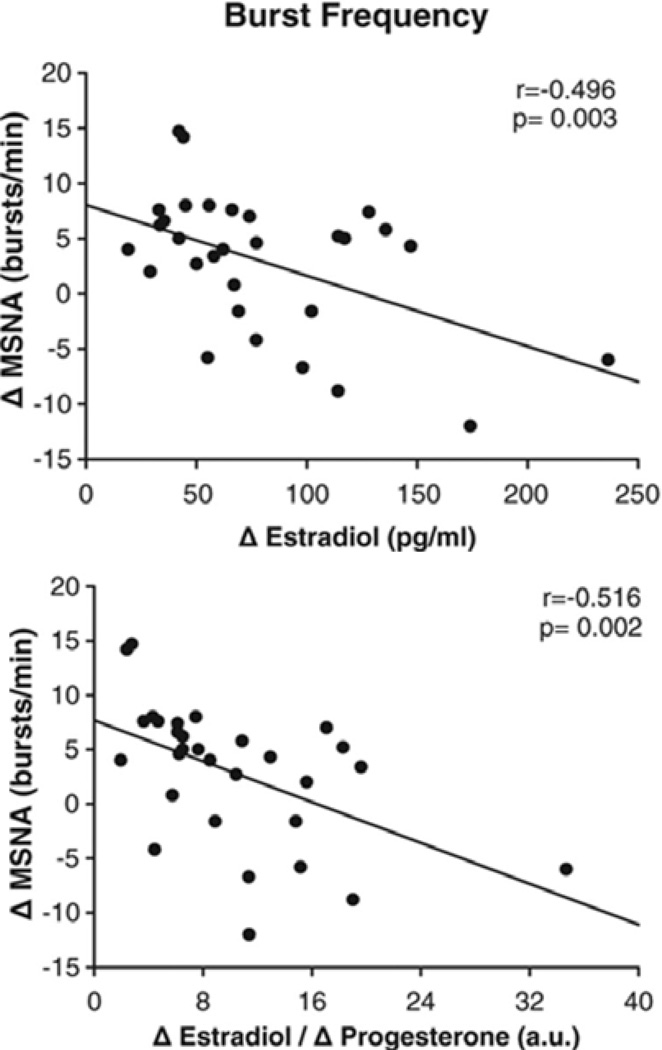
The obvious question raised by the observations outlined showing sex differences between young men and women and changes as women age, is to what extent are these differences due to sex steroid hormones? In this context, there are several lines of evidence favoring the view that estrogen is sympatho-inhibitory. First, in an analysis from several laboratories of 30 young women with normal ovarian cycles, Figure 11 shows that MSNA was inversely correlated with estrogen levels (14). Second, as shown in Figure 12, several interventional trials of hormone replacement therapy have been shown to reduce MSNA (99, 102, 110). Taken together, these observations are consistent with the idea that estrogen is sympatho-inhibitory. The influence of progesterone is less clear. In the analysis noted above, MSNA at rest was also inversely related to the E2/P4 ratio, suggesting a potential sympatho-excitatory role for the hormone. However, the specifics of the influence of progesterone remain to be clearly elucidated.
Figure 11.
Sex hormones vary throughout the ovarian cycle in young women. The change between mid-luteal and early follicular phases in estradiol (top panel) and in the ratio estradiol/progesterone (bottom panel) was inversely associated with the change in muscle sympathetic nerve activity (MSNA). This suggests that higher estrogen in mid luteal phases may be sympatho-inhibitory. Data adapted from (13).
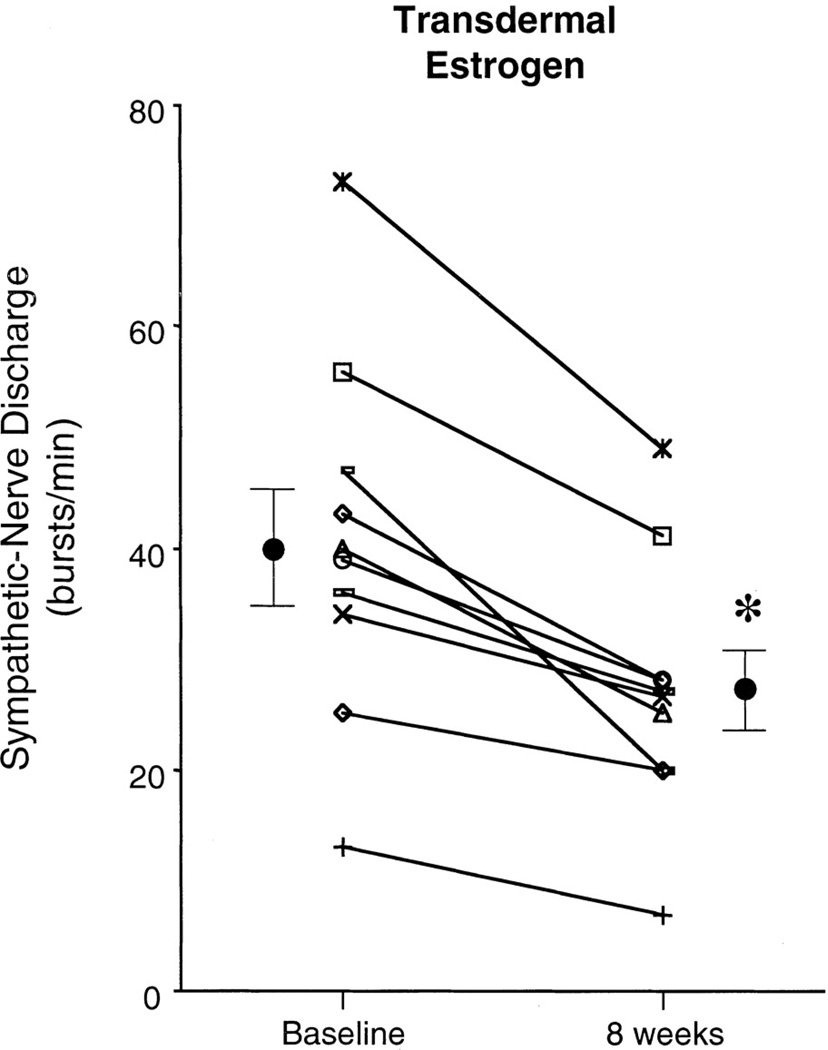
Figure 12.
Sex hormones and specifically estrogen has been shown to affect neural outflow. Administration of transdermal estrogen for 8 weeks in postmenopausal women reduces muscle sympathetic nerve activity. *p<0.05. Data adapted from (102).
There is also accumulating evidence that menstrual cycle phase and levels of sex hormones might play a role in determining baseline levels of MSNA in young women. For convenience, (and to facilitate comparisons between normally menstruating women and those on oral contraceptives) most studies are made at the onset of menstruation when hormones are low. However, recent evidence points to elevated MSNA when sex hormone levels are high (13). These cycle dependent fluctuations in MSNA are an obvious sex difference in younger humans.
A key question about the effects of estrogen on sympathetic activity is the site of the inhibitory effects. In subsequent sections several potential mechanisms that can be evaluated in humans will be discussed. However, there are data from rodent models indicating that estrogen acts in the central nervous system to reduce sympathetic activity by enhancing NO mediated sympatho-inhibitory pathways and blunting the central sympatho-excitatory effects of activating the renin angiotensin aldosterone system (21, 114 115). Estrogen also appears to augment central sympatho-inhibitory responses to baroreflex stimulation.
Peripheral Adrenergic Sensitivity and Vascular Transduction
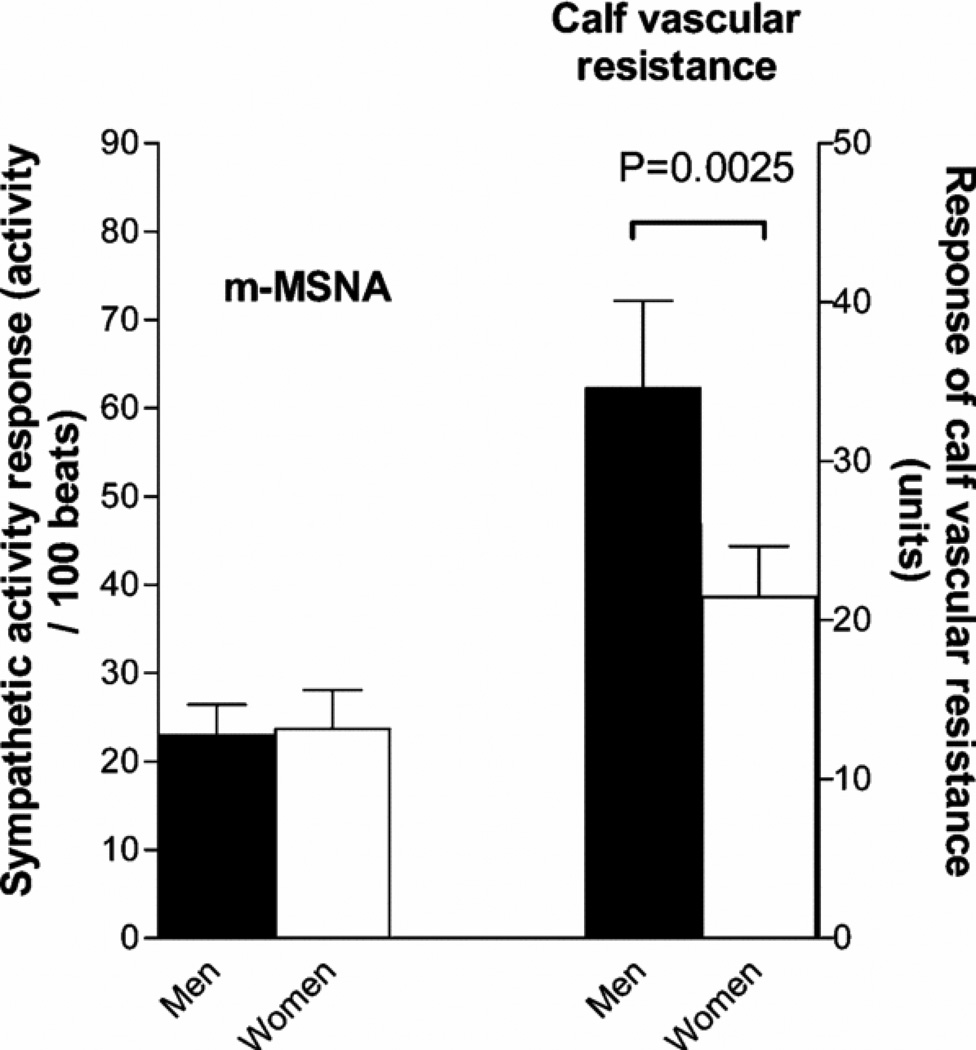
Our observations that MSNA is related to TPR in men and older women, but not younger women, suggests that there are sex differences (that interact with aging effects) in how sympathetic activity is transduced into changes in vascular resistance. For example, use of lower body negative pressure to evoke increases in sympathetic activity typically causes more robust vasoconstrictor responses in young men than young women (112). However, there are limited or no data on this topic in older women. Along these lines, Hogarth et al (47) showed that despite similar increases in MSNA, HR and blood pressure in response to a cold pressor test in women, increases in calf vascular resistance in women were blunted vs. men. This also suggests that how sympathetic nerve activity is transferred in vasoconstrictor tone is different in women. (Figure 13).
Figure 13.
The left panel shows that the rise in MSNA is similar in young men and women during a cold pressor test. The right panel shows that the rise in calf vascular resistance is lower in the women. These data are consistent with the idea that the transduction of sympathetic activity to vascular tone is lower in young women than men. Data from (48).
Mechanisms for these differences appear to include differences in the sub-populations of adrenergic receptors responsible for the transduction of sympathetic activity (norepinephrine) into vasoconstriction. For example, Figure 14 shows brachial artery infusions of norepinephrine cause more vasoconstriction for a given dose in young men and postmenopausal women than young women (43, 59). However, when β-adrenergic blocking drugs are co-administered, vasoconstrictor responses are similar in both sexes. This observation suggests that a main cause of the lack of relationship between MSNA and TPR in young women is that vasodilating β-adrenergic receptors are activated at the same time that vasoconstricting α-adrenergic receptors are activated, thus blunting or eliminating vasoconstriction caused by activation of the sympathetic nerves. In addition to the lack of TPR MSNA relationships seen at rest in young women, concurrent β-adrenergic vasodilation might also limit sympathetic vasoconstriction in young women during periods of sympathetic activation.
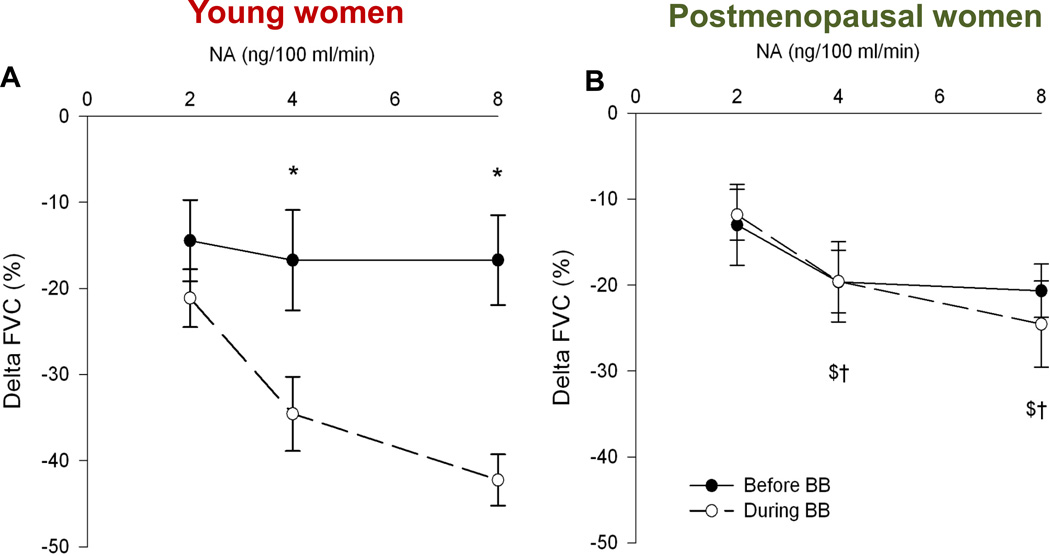
Figure 14.
Effects of brachial artery administration of norepinephrine (noradrenaline, NA) on forearm vascular conductance before and after local administration of the non-selective β-blocker propranolol (BB). In young women (panel A) increasing doses of NA did not evoke marked vasoconstriction at rest. However, marked vasoconstriction is seen after administration of propranolol. In postmenopausal women propranolol had no effect and the constriction caused by administration of NA caused more marked vasoconstriction. In men, β-blockade has little effect on these responses (not shown). The responses indicate that concurrent β-adrenergic vasodilation limits α-adrenergic vasoconstriction in young women. This sex difference might explain many of the findings highlighted in Figures 7, 8, and 9. Data from (43).
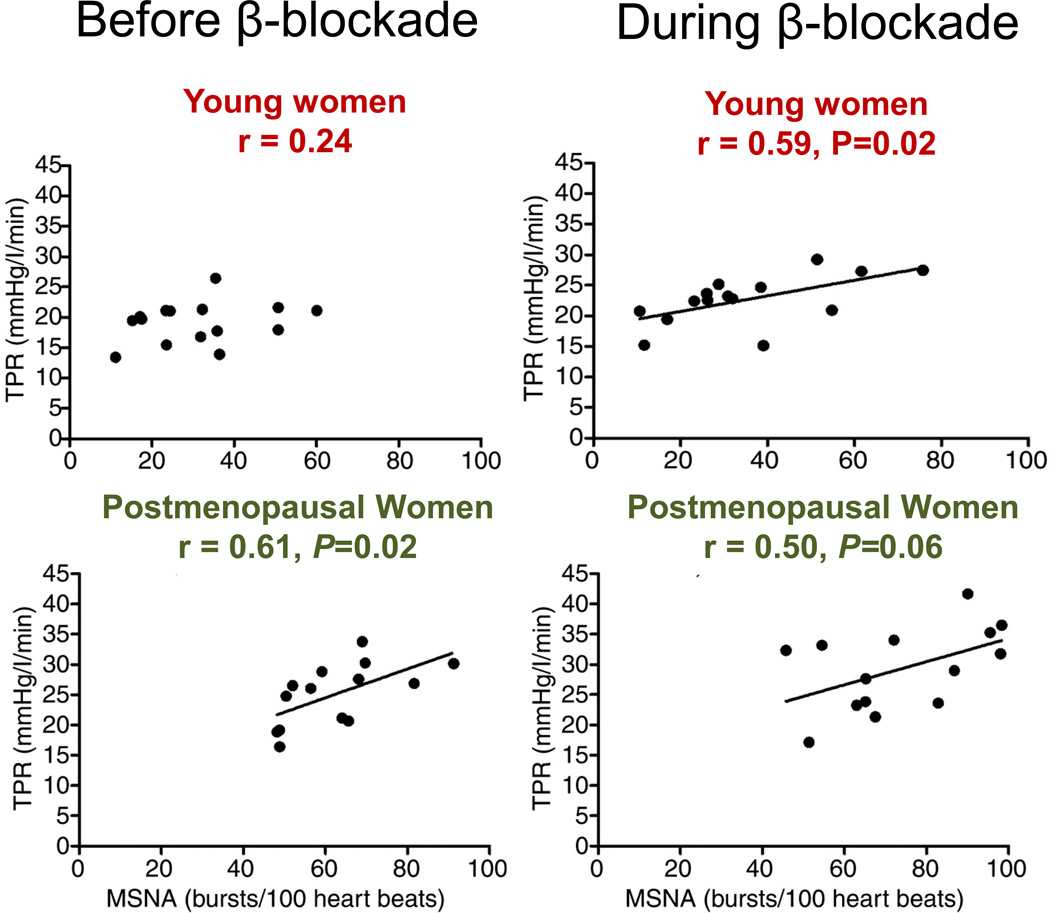
Along similar lines, when young women are given high doses of systemic β-adrenergic blocking drugs, a positive relationship between MSNA and TPR emerges. Essentially, the responses seen in young women with β-blockade mirror those seen in older women (without β-blockade) (43) (see Figure 15). Thus, a major sex difference between men and women is a limitation in the ability of vasoconstrictor sympathetic activity to cause increases in vascular tone in the young women as a result of concurrent competing β-adrenergic receptor-mediated vasodilation. Additionally, data from animals support this interpretation (70).
Figure 15.
The is no association between muscle sympathetic nerve activity (MSNA) and total peripheral resistance (TPR) in young women, however, there is a significant positive association in young men (not shown) and in postmenopausal women (left panels). After systemic β-blockade, where β-adrenergic vasodilation is attenuated, a positive association between MSNA and TPR emerges in young women (right panel). This suggests that β-adrenergic vasodilation blunts vascular transduction of the sympathetic nerves. Data from (43).
At least some β-adrenergic vasodilation is nitric oxide-dependent (31). In this context estrogen augments endothelial function in humans and also limits the potentially negative effects of dietary salt consumption on the NO mediated vasodilator component of endothelial function (32). Thus, estrogen could operate to either increase the number of vascular β-adrenergic receptors and/or the potency of the dilation that they cause when activated by having a more general effect on NO-mediated endothelial function (35). Both of these vascular responses would tend lower blood pressure.
Summary of Section 6
In humans several lines of evidence suggest that estrogen reduces sympathetic activity. These include the observation that on average MSNA and other indices of baseline sympathetic activity are lower in young women than young men. The effects of hormone replacement therapy to reduce MSNA or NE spillover in older women are consistent with this conclusion. Based on observations in animals at least some of these sex differences are due to the central sympatho-inhibitory effects of estrogen. The effects of the estrogen mediated changes in sympathetic activity on blood pressure are likely amplified by estrogen mediated improvements in endothelial function and enhanced β2 mediated vasodilation. These vascular changes limit the ability of sympathetic neural activity to evoke vasoconstriction in young women and their loss during or after menopause contributes to the rise in baseline blood pressure seen as women age. However, as is the case for all of the responses being discussed in this review, the magnitude of interindividual variability for a give response within a given group of subjects is typically greater than either the mean sex or age related differences that are observed.
5. ACUTE NEURAL-HEMODYNAMIC RESPONSES
At the outset of this review, we pointed out that blood pressure is highly variable in humans over a 24-hour period. In this context, the awake supine resting state is relatively unusual (i.e., healthy people don’t commonly spend a lot of their time lying down, resting and awake). Thus, there could be important sex differences in how humans respond to other forms of sympatho-excitatory stimulation.
Acute blood pressure reactivity (the increase in blood pressure in response to a transient sympatho-excitatory stimulus) may be predictive of risk for future chronic hypertension, even in people who are currently normotensive. Because young women tend to have lower blood pressure and lower risk of hypertension compared to young men, it has been of interest to evaluate if the lower risk of hypertension in women may be related to a difference between the sexes in neural and hemodynamic responses to acute stressors. These can be divided into two general categories: 1) baroreflex responses to actual or simulated orthostasis, and 2) non-baroreflex responses (mental stress and responses to somatic afferent activation).
Orthostatic Stress
With regard to orthostatic responses, there is evidence both for (58, 93) and against (41) attenuated MSNA responses to orthostatic stress in women compared to men. Fu and colleagues showed that blood volume and stroke volume were important factors in determining the MSNA responses to acute head up tilt (HUT) (41). They acutely administered a diuretic (furosemide) to groups of healthy young men and women and compared MSNA responses to HUT with and without the diuretic and between groups. They found that both the diuretic and the HUT increased MSNA acutely, but that these increases were not different between groups. Kimmerly and Shoemaker reported smaller increases in MSNA during postural stress in women, which appeared to be primarily due to smaller increases in burst size (58). The reasons for differences among studies are not clear, but may be related to (1) differences in methodology related to MSNA quantification (e.g., total activity vs. burst counts); and/or (2) inherent biological variability in MSNA, as discussed above.
To further investigate potential mechanisms for sex differences in sympathetic responses to orthostatic stress, Yang et al used frequency domain analysis to evaluate if the coherence between diastolic pressure and MSNA was different between men and women during progressive LBNP (116). They showed that DBP-MSNA coherence was greater in men at baseline, and that coherence increased in men (but not in women) with progressive LBNP. These investigators also demonstrated that women had a smaller percentage of consecutive MSNA bursts (at least two consecutive cardiac cycles with a burst) at baseline and throughout LBNP, compared to men. These findings give further insight into differences between men and women in the mechanisms by which sympathetic nerves participate in blood pressure regulation during orthostatic stress.
While most of the focus on the autonomic responses to orthostatic challenges blood pressure has centered on cardiopulmonary and arterial baroreflexes, the vestibular system can also modulate sympathetic activity in a way that would increase MSNA during some forms of orthostatic challenge. In young subjects there is no evidence for a major sex difference in the responsiveness of the vestibulosympathetic reflex. However, there is evidence that it is less responsive in aging and might contribute to age related reductions in blood pressure with postural change (15, 83, 84).
The potential sex differences in MSNA responses to tilt aside, young women also generally have lower LBNP tolerance (e.g the maximum negative pressure achieved before presyncope or syncope (22, 37, 111). In addition to potential sex differences in sympathetic and vascular responses noted above, sex differences in blood volume, venous compliance and muscle mass might influence the responses to orthostatic stress. However, only sex differences in muscle mass are clearly established. Additionally, there is very little comparative data that would allow coherent conclusions to be drawn on sex differences and orthostatic tolerance between older men and women. Finally, it is interesting to note that the prevalence of fainting outside the laboratory, as shown in Figure 2, is much more common in young women than in young men (42).
Arterial Baroreflexes
Potential mechanisms for sex differences in acute baroreflex responses might include physical differences in blood pressure signal transduction due to differences in arterial stiffness between the sexes. At this time there is no clear cut evidence that young women have more compliant carotid arteries than young men and this is further confounded by differences in carotid artery diameter. However, it is tempting to speculate that if there were more distension of the barosensitive areas in the aorta and carotid sinus in young women, then a given level of blood pressure would generate more sympatho-inhibitory afferent signals to the brain might be responsible for greater reflex inhibition of sympathetic outflow. This is an idea worthy of exploration that could explain the tonic differences in MSNA at rest. Likewise, altered peripheral baroreceptor function could also lead to greater reflex reductions in heart rate and more vagal tone, as is the case for baroreceptor control of MSNA little is known about potential sex differences between young men and young women (5). Importantly, any differences in peripheral baroreceptor function on sympathetic activity might also be augmented in young women if the central inhibitory effects of estrogen on baroreflex function seen in rodents are also present in humans (89).
The ideas about arterial stiffness outlined above may also contribute to the changes in MSNA associated with aging. If arterial stiffness is altering baroreflex sensitivity, this may explain at least some of the increase in MSNA seen in older humans (71). Furthermore, if the net increase in arterial stiffness as women age is greater (as some studies suggest), it could account for some of the temporal patterns in blood pressure and autonomic nervous system regulation that have been outlined previously and are exemplified in Figures 1, 8, and 10 (76). There are data in older men showing that about 50% of the decline in baroreflex function (at least baroreflex control of heart rate) with aging is due to reduced distensibility of the carotid arteries and about 50% is likely due to altered central integration of afferent signals from the baroreceptors (71). We have shown that sex differences exist in the relationship between surrogate measures of arterial stiffness (augmentation index) and sympathetic activity (Figure 16) (16). However, as is the case for so many of the potential sex differences discussed in this review, there are little or no conclusive data on this topic in older women.
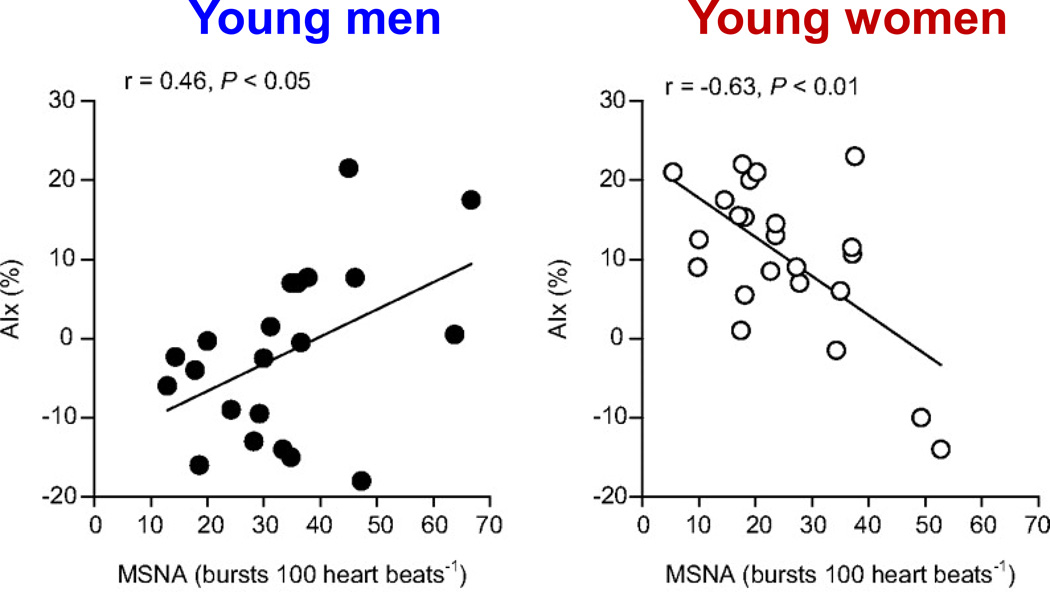
Figure 16.
Augmentation index (AIx%) is a pulse wave characteristic that is affected by increases in central arterial stiffness. Individuals with greater arterial stiffness typically demonstrate higher values of AIx. In young men, higher muscle sympathetic nerve activity (MSNA) is associated with greater AIx and presumably, higher arterial stiffness (left panel). However, this association is inverse in young women, highlighting the important differences in neurovascular regulation. Data from (16).
Other Acute Sympatho-excitatory Responses
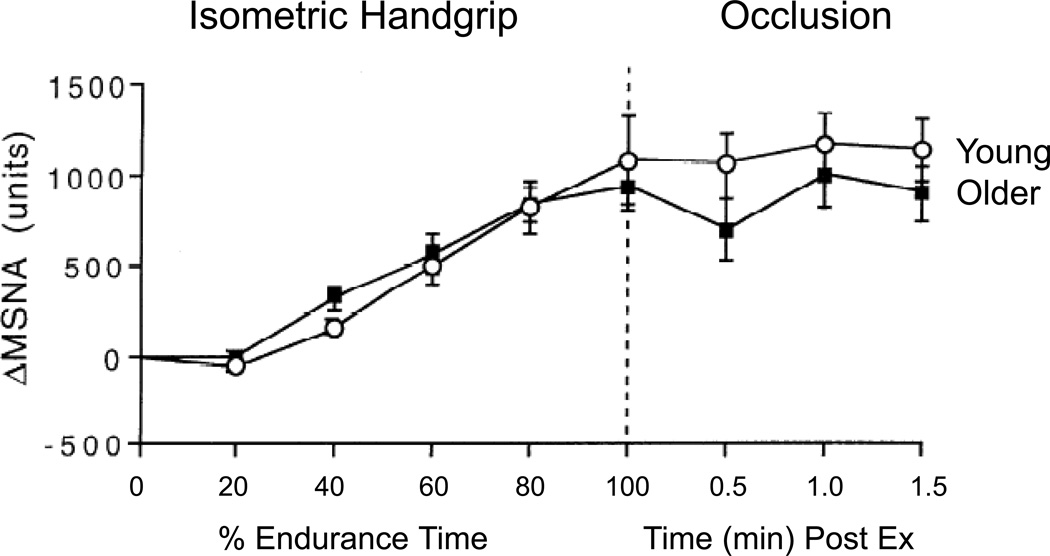
MSNA and blood pressure responses to non-orthostatic stimuli (e.g., isometric handgrip exercise, mental/emotional stress and the cold pressor test) may be predictive of risk for chronic hypertension later in life. Potential sex differences in sympathetic and vascular responses to mental stress have proven challenging to study due to a large amount of inter-individual variability in both sexes. Hogarth and colleagues reported that although women had lower MSNA at rest, MSNA responses to cold pressor and isometric handgrip tests were similar in men and women (47). Importantly, however, calf vascular resistance increased more in the men. This is consistent with the discussion above regarding attenuated vasoconstrictor responses in women due to the increased presence of vasodilating β-adrenergic receptors in this group, and may be an additional factor contributing to the lower risk of hypertension in young women. Additionally, while baseline differences in MSNA are seen in older and younger humans the absolute increases in MSNA in response to many stressors are similar (see Figure 17) (74, 75).
In terms of sympathetic neural and hemodynamic measurements, there are often subgroups of “responders” and “non-responders” to mental stress. In groups of mental stress responders, Carter and Ray demonstrated that men and women showed similar MSNA responses to mental arithmetic, but that the men had greater? blood pressure responses, consistent with the vascular transduction ideas outlined above (14). Yang and colleagues further evaluated sex differences in MSNA, forearm and calf blood flow responses before and during mental arithmetic (12, 117). They reported that men and women exhibited similar MSNA responses to mental stress, but that women exhibited significantly greater vasodilation in the calf, whereas men did not (and some men exhibited vasoconstriction). In their study, MSNA in the men was inversely related to calf vascular conductance, but no such relationship was observed in women. They conclude that sex differences in transduction of sympathetic activity to vascular responses contribute to their results, which are also consistent with the idea that women have a greater tendency for vasodilation.
Summary of Section 7
While it is clear that healthy young women generally have lower orthostatic tolerance than young men, the reasons are multifactorial with differences in vascular transduction likely playing a primary role. These differences in vascular transduction are consistent many observations about the relationship or lack thereof between sympathetic activity and vascular resistance in young women made throughout this review. For other sympatho-excitatory stressors the changes in MSNA in younger and older men and women are generally similar and it is unclear if there are any major sex differences in baroreflex control of either sympathetic activity or heart rate. The lack of data in healthy older women is particularly noticeable.
6. SUMMARY, UNANSWERED QUESTIONS AND CONCLUDING COMMENTS
Blood Pressure is “The” Regulated Variable
In this review we have taken the position espoused by Loring Rowell that it is “as if blood pressure is the regulated variable” (86). Thus, by examining potential sex differences in how the autonomic nervous system contributes to blood pressure regulation, broader insights into sex differences and the autonomic nervous system might be gained. Using blood pressure as a starting point, lower blood pressure values often observed in healthy non-obese young women suggest that primary sex differences exist. This conclusion is reinforced by the way blood pressure changes with age, especially the increases seen in peri- and post-menopausal women.
Sex Differences in Sympathetic Activity
While these group differences in blood pressure are clear cut, we have also emphasized that the values for key autonomic determinants of blood pressure in normotensive humans show marked interindividual variability. For example, MSNA is ∼20–25% lower on average in healthy young women in comparison to healthy young men. However, the range of values in each group can vary 5–10 fold, so substantial overlap is seen. Group differences in cardiac output and TPR also exist, but less profound within-group variability is seen. Therefore, a key question going forward is, should future research focus on individual patterns of regulation or differences in groups? The answer to this question likely depends of the nature of the problem being addressed, for the needs of epidemiology, public health and personalized medicine in the 21st century will certainly be different.
Estrogen and Sympathetic Activity
In the context of the two points above, is it possible to reconcile the group and individual differences into a coherent story about sex differences? Based on the changes in blood pressure around the time of menopause, it appears that estrogen has powerful sympatho-inhibitory and blood pressure lowering effects. This view is supported by: 1) studies in young women showing an inverse relationship between plasma estrogen levels and MSNA, and 2) data showing that hormone replacement therapy can lower MSNA and NE spillover. Based on animal models it seems reasonable to suggest that key sites for estrogen mediated sympatho-inhibition lie within the brainstem.
However, estrogen also has powerful effects on the blood vessels themselves. If these influences result in increased distensibility of the baroreceptors, a given blood pressure might evoke more reflex sympatho-inhibition in women than in men. At this time, there is little or no comprehensive and mechanistic data on this topic in younger and older women. Thus, it is not possible to infer if the effects of estrogen on sympathetic activity in women are due primarily to central sympathetic inhibition, altered peripheral baroreflex function, or both. This conclusion also highlights a general lack of information on sex differences, the autonomic nervous system and blood pressure in humans.
Sex Differences in Vascular Transduction
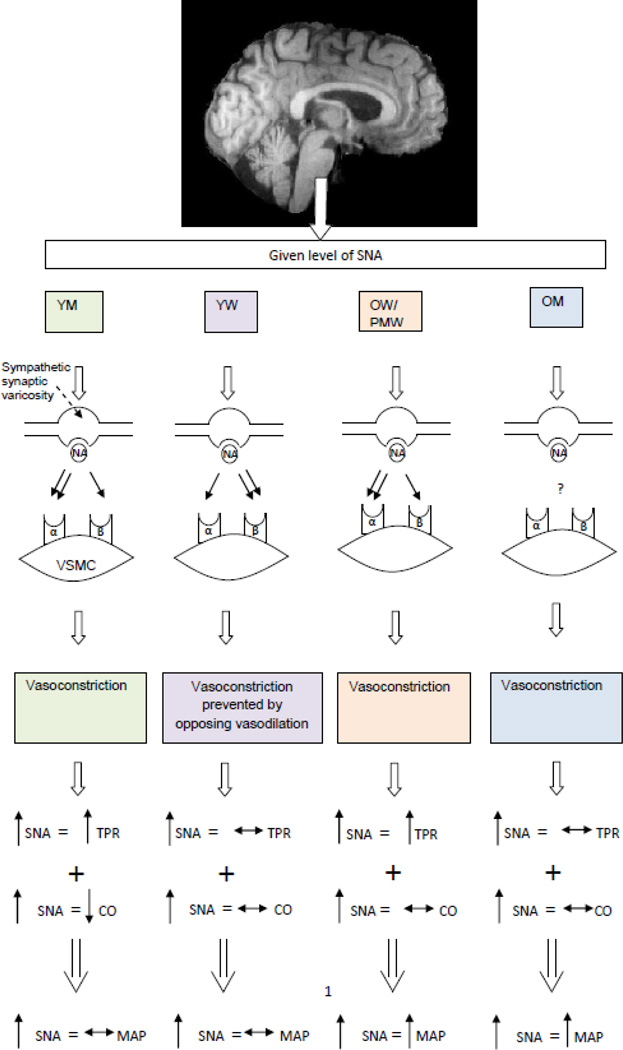
Perhaps the most striking sex difference in autonomic function is in how the relationship between MSNA and TPR differs by sex and with age across a wide range of MSNA values at rest. In healthy men from approximately 20 to 70 years of age, there is a clear positive relationship between MSNA and TPR. In young women, this relationship is absent, but re-emerges in post-menopausal women. At this time the best possible explanation for these observations centers on β2 mediated vasodilation, which appears to offset α-adrenergic vasoconstriction in young women but not young men. Because estrogen can have profound effects of peripheral vascular function, a key role for estrogen in these differences is likely. Loss of the estrogen-mediated effect on β-receptors and vascular smooth muscle also explains why the relationship between MSNA and TPR becomes positive in post-menopausal women. Parenthetically, individuals with high baseline MSNA might also be at risk for hypertension as they age or accumulate risk factors that result in endothelial dysfunction permitting unopposed vasoconstriction to drive blood pressure higher (Figure 18).
Figure 18.
Summary figure illustrating vascular transduction and how this influences sex differences in blood pressure. Specifically the effect on the vasculature appears to be dependent on estrogen, as young women are different than young men and older women. α=α-adrenergic receptors; β=β –adrenergic receptors; CO=cardiac output; MAP=mean arterial pressure; NA=noradrenaline; OM=older men; OW=older women; PMW:postmenopausal women; SNA=sympathetic nerve activity; TPR=total peripheral resistance; VSMC=vascular smooth muscle YM=young men; YW=young women.
The differences and physiological mechanisms behind the MSNA-TPR relationship in young men and women also offers important clues regarding sex differences in acute blood pressure regulation and the responsiveness to acute sympatho-excitatory maneuvers. It appears that young women and young men typically show similar increases in sympathetic activity during orthostatic challenge and maneuvers like handgripping, but the changes in MSNA typically cause either less vasoconstriction and smaller increases in blood pressure in the young women than in the young men. This blunted transduction of sympathetic activity into vasoconstriction is likely an important contributor to the reduced orthostatic tolerance and increased fainting seen in young women. At this time, there is little or no comprehensive and mechanistic data on the topic of vascular transduction during sympatho-excitation in older women. However, both older men and women do show robust increases in MSNA during sympatho-excitatory stressors like handgripping.
Autonomic Support of Blood Pressure
Consistent with the way sex and age affect MSNA, TPR and vascular transduction, there are differing effects of ganglionic and other forms of autonomic blockade on blood pressure. There are smaller reductions in blood pressure during ganglionic blockade in young women compared to young men (20); older women show reductions that are similar to younger men (4, 53). In addition, the magnitude of the drop in blood pressure is related to sympathetic nerve activity in men (53) and women (4) as shown in Figure 19.
Figure 19.
In women, both muscle sympathetic nerve activity (MSNA) and plasma norepinephrine (NE) is associated with the change in mean arterial pressure (MAP) after ganglionic blockade. Young women are shown in black squares and older women are shown in white squares. Note that older women demonstrate a greater reduction in MAP after ganglionic blockade. Figure adapted from (4).
Context Specific Conclusions and Questions?
Several places in this review, we have emphasized that most of the data we are synthesizing come from subjects who are likely relatively affluent and educated subjects of European descent, living in industrialized countries. Figure 3 shows that the age related trends in BP do not hold in all societies. This leads us to wonder if our conclusions would be similar if more observations were made in humans who lived in high physical activity low social stress environments that included what might be described as “healthy” diets? How generalizable are our conclusions and are the effects of sex and aging, even in healthy “Western” subjects, obligatory? Do they represent primary biological destiny or complex interactions among biology, behavior and the cultural environment that surrounds, and sometimes engulfs us?
Figure 17.
An example of age differences in acute sympatho-excitatory maneuvers. In this study, isometric handgrip was performed and then the forearm was occluded. Note the absolute change in MSNA during these maneuvers was similar in both age groups. Data from. (75).
ACKNOWLEDGEMENTS
The authors would like to thank the many subjects who have volunteered for their studies over the years. The nursing support of Shelly Roberts, Jean Knutson, and Sarah Wolhart along with the technical support of Christopher Johnson, Branton Walker, and Jessica Sawyer has been superb throughout these studies. Drs. Johns Eisenach and Timothy Curry have also been excellent collaborators and offered both intellectual and medical support. In addition, we thank Maja Johnson for her assistance in editing the manuscript and organizing the references. Finally, our primary findings have been supported by the NIH (Grant HL-83947 to MJJ, BGW, and NC) as well as NIH grant AG 38067 (JNB) and American Heart Association grant 2170087 (ECH).
This publication was made possible by CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Footnotes
The views, opinions, and/or findings contained in this article are those of the authors and should not be construed as an official Department of the Army position, or decision, unless so designated by other official documentation. Approved for public release; distribution unlimited.
REFERENCES
- 1.Alecu C, Gueguen R, Aubry C, Salvi P, Perret-Guillaume C, Ducrocq X, Vespignani H, Benetos A. Determinants of arterial stiffness in an apparently healthy population over 60 years. J Hum Hypertens. 2006;20:749–756. doi: 10.1038/sj.jhh.1002072. [DOI] [PubMed] [Google Scholar]
- 2.Alghatrif M, Strait JB, Morrell CH, Canepa M, Wright J, Elango P, Scuteri A, Najjar SS, Ferrucci L, Lakatta EG. Longitudinal trajectories of arterial stiffness and the role of blood pressure: the Baltimore Longitudinal Study of Aging. Hypertension. 2013;62:934–941. doi: 10.1161/HYPERTENSIONAHA.113.01445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ali YS, Daamen N, Jacob G, Jordan J, Shannon JR, Biaggioni I, Robertson D. Orthostatic intolerance: a disorder of young women. Obstet Gynecol Surv. 2000;55:251–259. doi: 10.1097/00006254-200004000-00025. [DOI] [PubMed] [Google Scholar]
- 4.Barnes JN, Hart EC, Curry TB, Nicholson WT, Eisenach JH, Wallin BG, Charkoudian N, Joyner MJ. Aging enhances autonomic support of blood pressure in women. Hypertension. 2014;63:303–308. doi: 10.1161/HYPERTENSIONAHA.113.02393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes JN, Matzek LJ, Charkoudian N, Joyner MJ, Curry TB, Hart EC. Association of cardiac baroreflex sensitivity with blood pressure transients: influence of sex and menopausal status. Frontiers Physiol. 2012;3:187. doi: 10.3389/fphys.2012.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behnke ARWJH. Evaluation and Regulation of Body Build and Composition. Prentice-Hall, Inc: Englewood Cliffs, New Jersey; 1974. [Google Scholar]
- 7.Beilin LJ, Burke V, Cox KL, Hodgson JM, Mori TA, Puddey IB. Non pharmacologic therapy and lifestyle factors in hypertension. Blood Pressure. 2001;10:352–365. doi: 10.1080/080370501753400656. [DOI] [PubMed] [Google Scholar]
- 8.Berntson GG, Bigger JT, Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M, Nagaraja HN, Porges SW, Saul JP, Stone PH, van der Molen MW. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- 9.Bogert LW, van Lieshout JJ. Non-invasive pulsatile arterial pressure and stroke volume changes from the human finger. Exp Physiol. 2005;90:437–446. doi: 10.1113/expphysiol.2005.030262. [DOI] [PubMed] [Google Scholar]
- 10.Brodde OE. Beta 1- and beta 2-adrenoceptors in the human heart: properties, function, and alterations in chronic heart failure. Pharmacol Rev. 1991;43:203–242. [PubMed] [Google Scholar]
- 11.Brown GO. Henry Darcy and the making of a law. Water Resour Res. 2002;38 [Google Scholar]
- 12.Butt C, Pathmadeva C, Spencer C. Forearm and calf blood flow in response to cortical arousal in normal male and female subjects. Clin Auton Res. 1999;9:103–107. doi: 10.1007/BF02311767. [DOI] [PubMed] [Google Scholar]
- 13.Carter JR, Fu Q, Minson CT, Joyner MJ. Ovarian cycle and sympathoexcitation in premenopausal women. Hypertension. 2013;61:395–399. doi: 10.1161/HYPERTENSIONAHA.112.202598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carter JR, Ray CA. Sympathetic neural responses to mental stress: responders, nonresponders and sex differences. Am J Physiol: Heart Circ Physiol. 2009;296:H847–H853. doi: 10.1152/ajpheart.01234.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carter JR, Ray CA. Sympathetic responses to vestibular activation in humans. Am J Physiol Regul Integr Comp Physiol. 2008;294:R681–R688. doi: 10.1152/ajpregu.00896.2007. [DOI] [PubMed] [Google Scholar]
- 16.Casey DP, Curry TB, Joyner MJ, Charkoudian N, Hart EC. Relationship between muscle sympathetic nerve activity and aortic wave reflection characteristics in young men and women. Hypertension. 2011;57:421–427. doi: 10.1161/HYPERTENSIONAHA.110.164517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charkoudian N, Hart EC, Barnes JN, Joyner MJ. Comments on Point:Counterpoint: The dominant contributor to systemic hypertension: Chronic activation of the sympathetic nervous system vs. The role of the sympathetic nervous system--influences of sex and aging. J Appl Physiol (1985) 2010;109:2005. doi: 10.1152/japplphysiol.01160.2010. [DOI] [PubMed] [Google Scholar]
- 18.Charkoudian N, Joyner MJ, Barnes SA, Johnson CP, Eisenach JH, Dietz NM, Wallin BG. Relationship between muscle sympathetic nerve activity and systemic hemodynamics during nitric oxide synthase inhibition in humans. Am J Physiol : Heart Circ Physiol. 2006;291:H1378–H1383. doi: 10.1152/ajpheart.00234.2006. [DOI] [PubMed] [Google Scholar]
- 19.Charkoudian N, Joyner MJ, Johnson CP, Eisenach JH, Dietz NM, Wallin BG. Balance between cardiac output and sympathetic nerve activity in resting humans: role in arterial pressure regulation. J Physiol. 2005;568:315–321. doi: 10.1113/jphysiol.2005.090076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christou DD, Jones PP, Jordan J, Diedrich A, Robertson D, Seals DR. Women have lower tonic autonomic support of arterial blood pressure and less effective baroreflex buffering than men. Circulation. 2005;111:494–498. doi: 10.1161/01.CIR.0000153864.24034.A6. [DOI] [PubMed] [Google Scholar]
- 21.Ciriello J, Roder S. 17beta-Estradiol alters the response of subfornical organ neurons that project to supraoptic nucleus to plasma angiotensin II and hypernatremia. Brain Res. 2013;1526:54–64. doi: 10.1016/j.brainres.2013.06.038. [DOI] [PubMed] [Google Scholar]
- 22.Convertino VA. Gender differences in autonomic functions associated with blood pressure regulation. Am J Physiol. 1998;275:R1909–R1920. doi: 10.1152/ajpregu.1998.275.6.R1909. [DOI] [PubMed] [Google Scholar]
- 23.Coutinho T, Borlaug BA, Pellikka PA, Turner ST, Kullo IJ. Sex differences in arterial stiffness and ventricular-arterial interactions. J Amer Coll Cardiol. 2013;61:96–103. doi: 10.1016/j.jacc.2012.08.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dias AR, Jr, de Mello NR, Eluf Gebara OC, Nussbacher A, Wajngarten M, Petti DA. Conjugated equine estrogen, raloxifene and arterial stiffness in postmenopausal women. Climacteric. 2008;11:390–396. doi: 10.1080/13697130802325635. [DOI] [PubMed] [Google Scholar]
- 25.Dinenno FA, Dietz NM, Joyner MJ. Aging and forearm postjunctional alpha-adrenergic vasoconstriction in healthy men. Circulation. 2002;106:1349–1354. doi: 10.1161/01.cir.0000028819.64790.be. [DOI] [PubMed] [Google Scholar]
- 26.Dinenno FA, Eisenach JH, Dietz NM, Joyner MJ. Post-junctional alpha-adrenoceptors and basal limb vascular tone in healthy men. J Physiol. 2002;540:1103–1110. doi: 10.1113/jphysiol.2001.015297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dinenno FA, Jones PP, Seals DR, Tanaka H. Age-associated arterial wall thickening is related to elevations in sympathetic activity in healthy humans. Am J Physiol Heart Circ Physiol. 2000;278:H1205–H1210. doi: 10.1152/ajpheart.2000.278.4.H1205. [DOI] [PubMed] [Google Scholar]
- 28.Dinenno FA, Joyner MJ. Alpha-adrenergic control of skeletal muscle circulation at rest and during exercise in aging humans. Microcirculation. 2006;13:329–341. doi: 10.1080/10739680600618843. [DOI] [PubMed] [Google Scholar]
- 29.Eckberg DL. Sympathovagal balance: a critical appraisal. Circulation. 1997;96:3224–3232. doi: 10.1161/01.cir.96.9.3224. [DOI] [PubMed] [Google Scholar]
- 30.Edgerton VR, Gardner GW, Ohira Y, Gunawardena KA, Senewiratne B. Iron-deficiency anaemia and its effect on worker productivity and activity patterns. Br Med J. 1979;2:1546–1549. doi: 10.1136/bmj.2.6204.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eisenach JH, Clark ES, Charkoudian N, Dinenno FA, Atkinson JL, Fealey RD, Dietz NM, Joyner MJ. Effects of chronic sympathectomy on vascular function in the human forearm. J Appl Physiol (1985) 2002;92:2019–2025. doi: 10.1152/japplphysiol.01025.2001. [DOI] [PubMed] [Google Scholar]
- 32.Eisenach JH, Gullixson LR, Kost SL, Joyner MJ, Turner ST, Nicholson WT. Sex differences in salt sensitivity to nitric oxide dependent vasodilation in healthy young adults. J Appl Physiol (1985) 2012;112:1049–1053. doi: 10.1152/japplphysiol.01197.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Esler M. Clinical application of noradrenaline spillover methodology: delineation of regional human sympathetic nervous responses. Pharmacol Toxicol. 1993;73:243–253. doi: 10.1111/j.1600-0773.1993.tb00579.x. [DOI] [PubMed] [Google Scholar]
- 34.Esler M, Jennings G, Korner P, Blombery P, Sacharias N, Leonard P. Measurement of total and organ-specific norepinephrine kinetics in humans. Am J Physiol. 1984;247:E21–E28. doi: 10.1152/ajpendo.1984.247.1.E21. [DOI] [PubMed] [Google Scholar]
- 35.Ferrer M, Meyer M, Osol G. Estrogen replacement increases beta-adrenoceptor-mediated relaxation of rat mesenteric arteries. J Vasc Res. 1996;33:124–131. doi: 10.1159/000159140. [DOI] [PubMed] [Google Scholar]
- 36.Fleenor BS. Large elastic artery stiffness with aging: novel translational mechanisms and interventions. Aging Dis. 2013;4:76–83. [PMC free article] [PubMed] [Google Scholar]
- 37.Franke WD, Johnson CP, Steinkamp JA, Wang R, Halliwill JR. Cardiovascular and autonomic responses to lower body negative pressure: do not explain gender differences in orthostatic tolerance. Clin Auton Res. 2003;13:36–44. doi: 10.1007/s10286-003-0066-x. [DOI] [PubMed] [Google Scholar]
- 38.Fu Q. Microneurographic research in women. Frontiers Physiol. 2012;3:278. doi: 10.3389/fphys.2012.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fu Q, Arbab-Zadeh A, Perhonen MA, Zhang R, Zuckerman JH, Levine BD. Hemodynamics of orthostatic intolerance: implications for gender differences. Am J Physiol: Heart Circ Physiol. 2004;286:H449–H457. doi: 10.1152/ajpheart.00735.2002. [DOI] [PubMed] [Google Scholar]
- 40.Fu Q, Levine BD. Autonomic circulatory control during pregnancy in humans. Seminars in reproductive medicine. 2009;27:330–337. doi: 10.1055/s-0029-1225261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fu Q, Witkowski S, Okazaki K, Levine BD. Effects of gender and hypovolemia on sympathetic neural responses to orthostatic stress. Am J Physiol: Reg Integ Comp Physiol. 2005;289:R109–R116. doi: 10.1152/ajpregu.00013.2005. [DOI] [PubMed] [Google Scholar]
- 42.Ganzeboom KS, Colman N, Reitsma JB, Shen WK, Wieling W. Prevalence and triggers of syncope in medical students. Am J Cardiol. 2003;91:1006–1008. doi: 10.1016/s0002-9149(03)00127-9. A1008. [DOI] [PubMed] [Google Scholar]
- 43.Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach J, Joyner MJ. Sex and ageing differences in resting arterial pressure regulation: the role of the beta-adrenergic receptors. J Physiol. 2011;589:5285–5297. doi: 10.1113/jphysiol.2011.212753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hart EC, Joyner MJ, Wallin BG, Charkoudian N. Sex, ageing and resting blood pressure: gaining insights from the integrated balance of neural and haemodynamic factors. J Physiol. 2012;590:2069–2079. doi: 10.1113/jphysiol.2011.224642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hart EC, Joyner MJ, Wallin BG, Johnson CP, Curry TB, Eisenach JH, Charkoudian N. Age-related differences in the sympathetic-hemodynamic balance in men. Hypertension. 2009;54:127–133. doi: 10.1161/HYPERTENSIONAHA.109.131417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hedman AE, Hartikainen JE, Tahvanainen KU, Hakumaki MO. The high frequency component of heart rate variability reflects cardiac parasympathetic modulation rather than parasympathetic 'tone'. Acta Physiol Scand. 1995;155:267–273. doi: 10.1111/j.1748-1716.1995.tb09973.x. [DOI] [PubMed] [Google Scholar]
- 47.Hogarth AJ, Mackintosh AF, Mary DA. Gender-related differences in the sympathetic vasoconstrictor drive of normal subjects. Clin Sci. 2007;112:353–361. doi: 10.1042/CS20060288. [DOI] [PubMed] [Google Scholar]
- 48.Hollenberg NK, Martinez G, McCullough M, Meinking T, Passan D, Preston M, Rivera A, Taplin D, Vicaria-Clement M. Aging, acculturation, salt intake, and hypertension in the Kuna of Panama. Hypertension. 1997;29:171–176. doi: 10.1161/01.hyp.29.1.171. [DOI] [PubMed] [Google Scholar]
- 49.Jensen K, Johansen L, Secher NH. Influence of body mass on maximal oxygen uptake: effect of sample size. Eur J Appl Physiol. 2001;84:201–205. doi: 10.1007/s004210170005. [DOI] [PubMed] [Google Scholar]
- 50.Johnson BD, Beck KC, Proctor DN, Miller J, Dietz NM, Joyner MJ. Cardiac output during exercise by the open circuit acetylene washin method: comparison with direct Fick. J Appl Physiol (1985) 2000;88:1650–1658. doi: 10.1152/jappl.2000.88.5.1650. [DOI] [PubMed] [Google Scholar]
- 51.Jonason T, Henrikssen E, Kangro T, Nilsson H, Vessby B, Ringqvist I. Stiffness of the common carotid artery in healthy 50-year-old subjects. Clin Physiol. 1997;17:569–577. doi: 10.1046/j.1365-2281.1997.00062.x. [DOI] [PubMed] [Google Scholar]
- 52.Jones JMW, Daniels K. National Health Statistics Report. Hyattsville, MD: United States Department of Health and Human Services; 2012. Current Contraceptive Use in the United States, 2006–2010, and Changes in Patterns of Use Since 1995; p. 26. [PubMed] [Google Scholar]
- 53.Jones PP, Shapiro LF, Keisling GA, Jordan J, Shannon JR, Quaife RA, Seals DR. Altered autonomic support of arterial blood pressure with age in healthy men. Circulation. 2001;104:2424–2429. doi: 10.1161/hc4501.099308. [DOI] [PubMed] [Google Scholar]
- 54.Joyner MJ, Charkoudian N, Wallin BG. Sympathetic nervous system and blood pressure in humans: individualized patterns of regulation and their implications. Hypertension. 2010;56:10–16. doi: 10.1161/HYPERTENSIONAHA.109.140186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Joyner MJ, Charkoudian N, Wallin BG. A sympathetic view of the sympathetic nervous system and human blood pressure regulation. Exp Physiol. 2008;93:715–724. doi: 10.1113/expphysiol.2007.039545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Joyner MJ, Dietz NM. Sympathetic vasodilation in human muscle. Acta Physiol Scand. 2003;177:329–336. doi: 10.1046/j.1365-201X.2003.01090.x. [DOI] [PubMed] [Google Scholar]
- 57.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308:875–881. doi: 10.1001/2012.jama.10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kimmerly DS, Wong S, Menon R, Shoemaker JK. Forebrain neural patterns associated with sex differences in autonomic and cardiovascular function during baroreceptor unloading. Am J Physiol: Reg Integ Comp Physiol. 2007;292:R715–R722. doi: 10.1152/ajpregu.00366.2006. [DOI] [PubMed] [Google Scholar]
- 59.Kneale BJ, Chowienczyk PJ, Brett SE, Coltart DJ, Ritter JM. Gender differences in sensitivity to adrenergic agonists of forearm resistance vasculature. J Am Coll Cardiol. 2000;36:1233–1238. doi: 10.1016/s0735-1097(00)00849-4. [DOI] [PubMed] [Google Scholar]
- 60.Korner PI, Shaw J, Uther JB, West MJ, McRitchie RJ, Richards JG. Autonomic and non-autonomic circulatory components in essential hypertension in man. Circulation. 1973;48:107–117. doi: 10.1161/01.cir.48.1.107. [DOI] [PubMed] [Google Scholar]
- 61.Leonetti P, Audat F, Girard A, Laude D, Lefrere F, Elghozi JL. Stroke volume monitored by modeling flow from finger arterial pressure waves mirrors blood volume withdrawn by phlebotomy. Clin Auton Res. 2004;14:176–181. doi: 10.1007/s10286-004-0191-1. [DOI] [PubMed] [Google Scholar]
- 62.Liu Z, Hesse C, Curry TB, Pike TL, Issa A, Bernal M, Charkoudian N, Joyner MJ, Eisenach JH. Ambulatory arterial stiffness index is not correlated with the pressor response to laboratory stressors in normotensive humans. J Hypertens. 2009;27:763–768. doi: 10.1097/HJH.0b013e328324eb27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lohmeier TE, Hildebrandt DA, Dwyer TM, Iliescu R, Irwin ED, Cates AW, Rossing MA. Prolonged activation of the baroreflex decreases arterial pressure even during chronic adrenergic blockade. Hypertension. 2009;53:833–838. doi: 10.1161/HYPERTENSIONAHA.109.128884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.MacDougall JD, Tuxen D, Sale DG, Moroz JR, Sutton JR. Arterial blood pressure response to heavy resistance exercise. J Appl Physiol (1985) 1985;58:785–790. doi: 10.1152/jappl.1985.58.3.785. [DOI] [PubMed] [Google Scholar]
- 65.Mackerras D, Singh G. The prevalence of anaemia depends on the definition: an example from the Aboriginal Birth Cohort Study. Eur J Clin Nutrit. 2007;61:135–139. doi: 10.1038/sj.ejcn.1602483. [DOI] [PubMed] [Google Scholar]
- 66.Matsukawa K. Central command: control of cardiac sympathetic and vagal efferent nerve activity and the arterial baroreflex during spontaneous motor behaviour in animals. Exp Physiol. 2012;97:20–28. doi: 10.1113/expphysiol.2011.057661. [DOI] [PubMed] [Google Scholar]
- 67.Matthews KA, Katholi CR, McCreath H, Whooley MA, Williams DR, Zhu S, Markovitz JH. Blood pressure reactivity to psychological stress predicts hypertension in the CARDIA study. Circulation. 2004;110:74–78. doi: 10.1161/01.CIR.0000133415.37578.E4. [DOI] [PubMed] [Google Scholar]
- 68.Millar-Craig MW, Bishop CN, Raftery EB. Circadian variation of blood-pressure. Lancet. 1978;1:795–797. doi: 10.1016/s0140-6736(78)92998-7. [DOI] [PubMed] [Google Scholar]
- 69.Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol (1985) 2008;105:1652–1660. doi: 10.1152/japplphysiol.90549.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Molinari C, Battaglia A, Grossini E, Mary DA, Surico N, Vacca G. The role of beta 2-adrenergic vascular receptors in the peripheral vasodilation caused by 17 beta-estradiol in anesthetized pigs. Life Sci. 1999;65:1545–1552. doi: 10.1016/s0024-3205(99)00399-9. [DOI] [PubMed] [Google Scholar]
- 71.Monahan KD, Dinenno FA, Seals DR, Clevenger CM, Desouza CA, Tanaka H. Age-associated changes in cardiovagal baroreflex sensitivity are related to central arterial compliance. Am J Physiol: Heart Circ Physiol. 2001;281:H284–H289. doi: 10.1152/ajpheart.2001.281.1.H284. [DOI] [PubMed] [Google Scholar]
- 72.Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L, Somers VK. Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension. 2005;45:522–525. doi: 10.1161/01.HYP.0000160318.46725.46. [DOI] [PubMed] [Google Scholar]
- 73.NCHS. Health, United States, 2009: With Special Feature on Medical Technology. Hyattsville (MD): 2010. [PubMed] [Google Scholar]
- 74.Ng AV, Callister R, Johnson DG, Seals DR. Age and gender influence muscle sympathetic nerve activity at rest in healthy humans. Hypertension. 1993;21:498–503. doi: 10.1161/01.hyp.21.4.498. [DOI] [PubMed] [Google Scholar]
- 75.Ng AV, Callister R, Johnson DG, Seals DR. Sympathetic neural reactivity to stress does not increase with age in healthy humans. Am J Physiol. 1994;267:H344–H353. doi: 10.1152/ajpheart.1994.267.1.H344. [DOI] [PubMed] [Google Scholar]
- 76.Okada Y, Galbreath MM, Shibata S, Jarvis SS, VanGundy TB, Meier RL, Vongpatanasin W, Levine BD, Fu Q. Relationship between sympathetic baroreflex sensitivity and arterial stiffness in elderly men and women. Hypertension. 2012;59:98–104. doi: 10.1161/HYPERTENSIONAHA.111.176560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.O'Neill SM, Liu J, O'Rourke MF, Khoo SK. The menopausal transition does not appear to accelerate age-related increases in arterial stiffness. Climacteric. 2013;16:62–69. doi: 10.3109/13697137.2012.739220. [DOI] [PubMed] [Google Scholar]
- 78.Pate RR, Sparling PB, Wilson GE, Cureton KJ, Miller BJ. Cardiorespiratory and metabolic responses to submaximal and maximal exercise in elite women distance runners. Int J Sports Med. 1987;8(Suppl 2):91–95. doi: 10.1055/s-2008-1025712. [DOI] [PubMed] [Google Scholar]
- 79.Pearson AC, Guo R, Orsinelli DA, Binkley PF, Pasierski TJ. Transesophageal echocardiographic assessment of the effects of age, gender, and hypertension on thoracic aortic wall size, thickness, and stiffness. Am Heart J. 1994;128:344–351. doi: 10.1016/0002-8703(94)90488-x. [DOI] [PubMed] [Google Scholar]
- 80.Pettersen KH, Bugenhagen SM, Nauman J, Beard DA, Omholt SW. Arterial stiffening provides sufficient explanation for primary hypertension. PLoS Comput Biol. 2014;10:e1003634. doi: 10.1371/journal.pcbi.1003634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ping P, Johnson PC. Role of myogenic response in enhancing autoregulation of flow during sympathetic nerve stimulation. Am J Physiol. 1992;263:H1177–H1184. doi: 10.1152/ajpheart.1992.263.4.H1177. [DOI] [PubMed] [Google Scholar]
- 82.Raaijmakers E, Faes TJ, Scholten RJ, Goovaerts HG, Heethaar RM. A meta-analysis of published studies concerning the validity of thoracic impedance cardiography. Ann NY Acad Sci. 1999;873:121–127. doi: 10.1111/j.1749-6632.1999.tb09458.x. [DOI] [PubMed] [Google Scholar]
- 83.Ray CA. Effect of gender on vestibular sympathoexcitation. Am J Physiol Regul Integ Comp Physiol. 2000;279:R1330–R1333. doi: 10.1152/ajpregu.2000.279.4.R1330. [DOI] [PubMed] [Google Scholar]
- 84.Ray CA, Monahan KD. Aging attenuates the vestibulosympathetic reflex in humans. Circulation. 105:956–961. doi: 10.1161/hc0802.104289. [DOI] [PubMed] [Google Scholar]
- 85.Rea RF, Wallin BG. Sympathetic nerve activity in arm and leg muscles during lower body negative pressure in humans. J Appl Physiol (1985) 1989;66:2778–2781. doi: 10.1152/jappl.1989.66.6.2778. [DOI] [PubMed] [Google Scholar]
- 86.Rowell LB. Human Circulation Regulation During Physical Stress. New York, NY: Oxford University Press, Inc; 1986. [Google Scholar]
- 87.Rowell LB, Brengelmann GL, Blackmon JR, Bruce RA, Murray JA. Disparities between aortic and peripheral pulse pressures induced by upright exercise and vasomotor changes in man. Circulation. 1968;37:954–964. doi: 10.1161/01.cir.37.6.954. [DOI] [PubMed] [Google Scholar]
- 88.Russo C, Jin Z, Palmieri V, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Arterial stiffness and wave reflection: sex differences and relationship with left ventricular diastolic function. Hypertension. 2012;60:362–368. doi: 10.1161/HYPERTENSIONAHA.112.191148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Saleh MC, Connell BJ, Saleh TM. Autonomic and cardiovascular reflex responses to central estrogen injection in ovariectomized female rats. Brain Res. 2000;879:105–114. doi: 10.1016/s0006-8993(00)02757-8. [DOI] [PubMed] [Google Scholar]
- 90.Schmitt JA, Joyner MJ, Charkoudian N, Wallin BG, Hart EC. Sex differences in alpha-adrenergic support of blood pressure. Clin Auton Res. 2010;20:271–275. doi: 10.1007/s10286-010-0061-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Seals DR, Victor RG, Mark AL. Plasma norepinephrine and muscle sympathetic discharge during rhythmic exercise in humans. J Appl Physiol (1985) 1988;65:940–944. doi: 10.1152/jappl.1988.65.2.940. [DOI] [PubMed] [Google Scholar]
- 92.Segers P, Rietzschel ER, De Buyzere ML, Vermeersch SJ, De Bacquer D, Van Bortel LM, De Backer G, Gillebert TC, Verdonck PR. Noninvasive (input) impedance, pulse wave velocity, and wave reflection in healthy middle-aged men and women. Hypertension. 2007;49:1248–1255. doi: 10.1161/HYPERTENSIONAHA.106.085480. [DOI] [PubMed] [Google Scholar]
- 93.Shoemaker JK, Hogeman CS, Khan M, Kimmerly DS, Sinoway LI. Gender affects sympathetic and hemodynamic response to postural stress. Am J Physiol: Heart Circ Physiol. 2001;281:H2028–H2035. doi: 10.1152/ajpheart.2001.281.5.H2028. [DOI] [PubMed] [Google Scholar]
- 94.Skarphedinsson JO, Elam M, Jungersten L, Wallin BG. Sympathetic nerve traffic correlates with the release of nitric oxide in humans: implications for blood pressure control. J Physiol. 1997;501(Pt 3):671–675. doi: 10.1111/j.1469-7793.1997.671bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Smith EG, Voyles WF, Kirby BS, Markwald RR, Dinenno FA. Ageing and leg postjunctional alpha-adrenergic vasoconstrictor responsiveness in healthy men. J Physiol. 2007;582:63–71. doi: 10.1113/jphysiol.2007.130591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Smulyan H, Asmar RG, Rudnicki A, London GM, Safar ME. Comparative effects of aging in men and women on the properties of the arterial tree. J Am Coll Cardiol. 2001;37:1374–1380. doi: 10.1016/s0735-1097(01)01166-4. [DOI] [PubMed] [Google Scholar]
- 97.Sonesson B, Lanne T, Vernersson E, Hansen F. Sex difference in the mechanical properties of the abdominal aorta in human beings. J Vasc Surg. 1994;20:959–969. doi: 10.1016/0741-5214(94)90234-8. [DOI] [PubMed] [Google Scholar]
- 98.Sonesson B, Vernersson E, Hansen F, Lanne T. Influence of sympathetic stimulation on the mechanical properties of the aorta in humans. Acta Physiol Scand. 1997;159:139–145. doi: 10.1046/j.1365-201X.1997.581343000.x. [DOI] [PubMed] [Google Scholar]
- 99.Sudhir K, Elser MD, Jennings GL, Komesaroff PA. Estrogen supplementation decreases norepinephrine-induced vasoconstriction and total body norepinephrine spillover in perimenopausal women. Hypertension. 1997;30:1538–1543. doi: 10.1161/01.hyp.30.6.1538. [DOI] [PubMed] [Google Scholar]
- 100.Sundlof G, Wallin BG. The variability of muscle nerve sympathetic activity in resting recumbent man. J Physiol. 1977;272:383–397. doi: 10.1113/jphysiol.1977.sp012050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vallbo AB, Hagbarth KE, Wallin BG. Microneurography: how the technique developed and its role in the investigation of the sympathetic nervous system. J Appl Physiol (1985) 2004;96:1262–1269. doi: 10.1152/japplphysiol.00470.2003. [DOI] [PubMed] [Google Scholar]
- 102.Vongpatanasin W, Tuncel M, Mansour Y, Arbique D, Victor RG. Transdermal estrogen replacement therapy decreases sympathetic activity in postmenopausal women. Circulation. 2001;103:2903–2908. doi: 10.1161/01.cir.103.24.2903. [DOI] [PubMed] [Google Scholar]
- 103.Waddell TK, Dart AM, Gatzka CD, Cameron JD, Kingwell BA. Women exhibit a greater age-related increase in proximal aortic stiffness than men. J Hypertens. 2001;19:2205–2212. doi: 10.1097/00004872-200112000-00014. [DOI] [PubMed] [Google Scholar]
- 104.Wallin BG, Charkoudian N. Sympathetic neural control of integrated cardiovascular function: insights from measurement of human sympathetic nerve activity. Muscle Nerve. 2007;36:595–614. doi: 10.1002/mus.20831. [DOI] [PubMed] [Google Scholar]
- 105.Wallin BG, Esler M, Dorward P, Eisenhofer G, Ferrier C, Westerman R, Jennings G. Simultaneous measurements of cardiac noradrenaline spillover and sympathetic outflow to skeletal muscle in humans. J Physiol. 1992;453:45–58. doi: 10.1113/jphysiol.1992.sp019217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wallin BG, Kunimoto MM, Sellgren J. Possible genetic influence on the strength of human muscle nerve sympathetic activity at rest. Hypertension. 1993;22:282–284. doi: 10.1161/01.hyp.22.3.282. [DOI] [PubMed] [Google Scholar]
- 107.Wallin BG, Thompson JM, Jennings GL, Esler MD. Renal noradrenaline spillover correlates with muscle sympathetic activity in humans. J Physiol. 1996;491(Pt 3):881–887. doi: 10.1113/jphysiol.1996.sp021265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Weisbrod CJ, Minson CT, Joyner MJ, Halliwill JR. Effects of regional phentolamine on hypoxic vasodilatation in healthy humans. J Physiol. 2001;537:613–621. doi: 10.1111/j.1469-7793.2001.00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Weiskopf RB, Viele MK, Feiner J, Kelley S, Lieberman J, Noorani M, Leung JM, Fisher DM, Murray WR, Toy P, Moore MA. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA. 1998;279:217–221. doi: 10.1001/jama.279.3.217. [DOI] [PubMed] [Google Scholar]
- 110.Weitz G, Elam M, Born J, Fehm HL, Dodt C. Postmenopausal estrogen administration suppresses muscle sympathetic nerve activity. J Clin Endocrinol Metab. 2001;86:344–348. doi: 10.1210/jcem.86.1.7138. [DOI] [PubMed] [Google Scholar]
- 111.Wenner MM, Haddadin AS, Taylor HS, Stachenfeld NS. Mechanisms contributing to low orthostatic tolerance in women: the influence of oestradiol. J Physiol. 2013;591:2345–2355. doi: 10.1113/jphysiol.2012.247882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wenner MM, Stachenfeld NS. Blood pressure and water regulation: understanding sex hormone effects within and between men and women. J Physiol. 2012;590:5949–5961. doi: 10.1113/jphysiol.2012.236752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wesseling KH, Jansen JR, Settels JJ, Schreuder JJ. Computation of aortic flow from pressure in humans using a nonlinear, three-element model. J Appl Physiol (1985) 1993;74:2566–2573. doi: 10.1152/jappl.1993.74.5.2566. [DOI] [PubMed] [Google Scholar]
- 114.Xue B, Johnson AK, Hay M. Sex differences in angiotensin II- and aldosterone-induced hypertension: the central protective effects of estrogen. Am J Physiol: Reg Integ Com Physiol. 2013;305:R459–R463. doi: 10.1152/ajpregu.00222.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xue B, Singh M, Guo F, Hay M, Johnson AK. Protective actions of estrogen on angiotensin II-induced hypertension: role of central nitric oxide. Am J Physiol: Heart Circ Physiol. 2009;297:H1638–H1646. doi: 10.1152/ajpheart.00502.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang H, Cooke WH, Reed KS, Carter JR. Sex differences in hemodynamic and sympathetic neural firing patterns during orthostatic challenge in humans. J Appl Physiol (1985) 2012;112:1744–1751. doi: 10.1152/japplphysiol.01407.2011. [DOI] [PubMed] [Google Scholar]
- 117.Yang H, Drummer TD, Carter JR. Sex differences in sympathetic neural and limb vascular reactivity to mental stress in humans. Am J Physiol: Heart Circ Physiol. 2013;304:H436–H443. doi: 10.1152/ajpheart.00688.2012. [DOI] [PubMed] [Google Scholar]
- 118.Yasmin, Brown MJ. Similarities and differences between augmentation index and pulse wave velocity in the assessment of arterial stiffness. QJM. 1999;92:595–600. doi: 10.1093/qjmed/92.10.595. [DOI] [PubMed] [Google Scholar]