Abstract
Background
Noninvasive brain stimulation (NIBS) techniques such as transcranial magnetic stimulation (TMS) and transcranial current stimulation (tCS) have the potential to mitigate a variety of symptoms associated with neurological and psychiatric conditions, including stroke, cerebral palsy, autism, depression, and Tourette syndrome. While the safety of these modalities has been established in adults, there is a paucity of research assessing the safety of NIBS among children.
Objective
To examine the existing literature regarding the safety of NIBS techniques in children and adolescents with neurologic and neuropsychiatric disorders.
Methods
An electronic search was performed on online databases for studies using NIBS in individuals less than 18 years of age. Non-English publications, diagnostic studies, electroconvulsive therapy, single/dual pulse TMS studies, and reviews were excluded. Adverse events reported in the studies were carefully examined and synthesized to understand the safety and tolerability of NIBS among children and adolescents.
Results
The data from 48 studies involving more than 513 children/adolescents (2.5–17.8 years of age) indicate that the side-effects of NIBS were, in general, mild and transient [TMS: headache (11.5%), scalp discomfort (2.5%), twitching (1.2%), mood changes (1.2%), fatigue (0.9%), tinnitus (0.6%); tCS: tingling (11.5%), itching (5.8%), redness (4.7%), scalp discomfort (3.1%)] with relatively few serious adverse events.
Conclusion
Our findings indicate that both repetitive TMS and tCS are safe modalities in children and adolescents with various neurological conditions, especially when safety guidelines are followed. The incidence of adverse events appears to be similar to that observed in adults; however, further studies with longer treatment and follow-up periods are needed to better understand the benefits and tolerance of long-term use of NIBS in children.
Keywords: TMS, tDCS, theta burst, safety, tolerability, guidelines
Introduction
Noninvasive brain stimulation (NIBS) refers to a group of modalities that are used to induce electric currents to and within the brain for diagnostic or therapeutic purposes [1–4]. A growing body of evidence suggests that NIBS techniques may have a promising role in the diagnosis, monitoring, and treatment of a variety of neurological and psychiatric conditions [5–9]. The therapeutic potential of NIBS stems from the capacity to evoke immediate and sustained modulation of neural network activity through alterations in neuronal excitation. The induced neuromodulation can be either excitatory or inhibitory, depending on the polarity, frequency, and duration of the stimulation [2, 10]. Moreover, the ability to induce directional modulation further enhances the therapeutic possibilities of NIBS, as the necessary direction of the brain excitability for recovery varies with different disease conditions [10, 11].
Two major types of NIBS techniques are currently in use on humans for clinical and research applications: Transcranial Magnetic Stimulation (TMS) and Transcranial Current Stimulation (tCS) [12]. TMS uses a varying magnetic field to induce weak electric currents in the brain. It can be delivered as a single pulse or as a train of pulses. Single-pulse TMS is typically used to study brain physiology and plasticity [3, 13–16], whereas repetitive-pulse TMS (rTMS) is commonly used to elicit neuromodulation and neuroplasticity, and can result in prolonged excitability changes that outlast the stimulation period [6, 15]. Typically, the direction of neuromodulation is driven by the frequency at which the stimulation is performed, such that high-frequency rTMS increases cortical excitability and low-frequency rTMS decreases cortical excitability [17]. However, theta burst stimulation (a variation of high frequency rTMS) can induce either depression or facilitation of cortical excitability, depending on burst-train duration, such that intermittent theta burst stimulation increases cortical excitability and continuous theta burst stimulation decreases cortical excitability [18].
tCS refers to the application of direct or alternating current on a specific region of the brain, transmitted via electrodes attached to the scalp. A wide range of tCS modalities exists, but only a few have been well-studied. Transcranial direct current stimulation (tDCS), (or “Transcranial Micropolarization”), is the most commonly used type of tCS [2, 19–25]. It employs a battery-driven stimulator to deliver weak direct currents (0.5–2.0 mA) through contact electrodes over the scalp. The current flow modulates neuronal excitability by altering the resting membrane potential of the neurons and produces aftereffects (i.e., prolonged changes in neuronal excitability) that are thought to be driven by Glutamatergic and GABAergic synapsic plasticity [26]. tDCS can be used to elicit an excitatory (anodal) or inhibitory (cathodal) effect, depending on the polarity of stimulation. Specifically, anodal stimulation has a depolarizing effect, which increases neuronal excitability; whereas, cathodal stimulation has a hyperpolarizing effect, which decreases neuronal excitability [1, 19, 27, 28].
During the past two decades, a large number of studies have evaluated the therapeutic benefits of NIBS modalities in a wide range of patient populations, including children with neurological disorders. These studies have demonstrated that NIBS modalities may provide therapeutic benefits for a wide variety of disease-specific symptoms, such as aphasia [29–32], dystonia [33–35], depression [36–41], epilepsy [10, 42–47], migraine [48, 49], motor dysfunction [11, 50–53], neurocognitive impairments [54], and pain [55–57], and are generally safe when the safety guidelines are observed [3, 20, 58–61]. The majority of the NIBS safety studies have been conducted in adults, and there is a paucity of research specifically devoted to examining the safety of NIBS in children. The few studies that have reviewed the safety of NIBS in children were limited to reporting on single-pulse and paired-pulse TMS protocols [59, 62]. A 2010 review on the safety of rTMS for children and adolescents indicated that rTMS was a feasible technique to facilitate recovery in adolescents with neurological and neuropsychiatric conditions with no major adverse events reported [63]. However, that review did not include any studies that examined children less than seven years old, and only three subjects were less than 16 years of age. In light of the growing interest of research directives and clinical applications of NIBS for children and adolescents, it is imperative to better understand the safety of these techniques for this population.
Therefore, the purpose of this review was to collect evidence related to the safety of NIBS application in children and adolescents, and to expand upon the D’agati study [63] by including younger subjects, subsequent studies published after 2009, as well as studies pertaining to the use of tCS in these populations.
Methods
We performed an electronic search of MEDLINE, EMBASE, PubMed, Web of Science, SPORTDiscus™, Evidence Based Medicine Reviews, and Multifile (EBMR) databases from their inception to September, 2014. Permutations of the text keyword combinations for topic or study interventions included the following: “transcranial direct current stimulation”, “transcranial current stimulation”, “micropolarization”, “transcranial magnetic stimulation”, “rapid transcranial magnetic stimulation”, “repetitive transcranial magnetic brain stimulation”, “deep transcranial magnetic stimulation”, and their respective abbreviations along with search terms “children”, “pediatric”, and “adolescent”. These terms were then combined with CNS diseases or disorders, such as cerebral palsy, stroke, autism, Tourette syndrome, epilepsy, depression, and delayed neuropsychological development. The references of the papers retrieved through this electronic search were manually inspected to find other potential studies that fit our inclusion criteria.
Inclusion criteria were limited to studies on children and adolescents less than 18 years of age that incorporated NIBS. Non-English language publications, diagnostic studies, retrospective studies, electroconvulsive therapy, single/dual pulse TMS studies, and reviews were excluded. Due to the limited number of papers regarding the application of NIBS in pediatric populations, we included both single-session and intervention studies. We did not impose an inclusion criteria based on quality of the study (i.e., study design), as this would have resulted in the exclusion of case reports, case series, and letters to the editors. Since serious adverse events were often communicated through such studies or reports, the use of qualitative assessment and subsequent exclusion of these studies would artificially lower the apparent incidence of such side effects, and thus lead to a misrepresentation of the safety of NIBS in children.
Studies were examined by the authors to determine the eligibility criteria. When there was insufficient information from the abstract, we read the full-length paper to ensure that each potential study was eligible to be included in our review. A data extraction sheet was developed to summarize the following variables: (1) sample size, (2) age, (3) diagnosis, (4) adverse effects, (5) treatment parameters, and (6) stimulation parameters. When there was insufficient information on subject demographics, treatment parameters, and/or adverse events, the corresponding authors of studies were contacted. We also cross-referenced the ClinicalTrials.gov website to retrieve additional information related to adverse events, when the reports were not clearly described in the manuscript and/or in the event that corresponding authors failed to reply. Extracted data were coded and evaluated using descriptive statistics. Adverse effects data were pooled among studies to calculate the incidence (i.e., frequency) of each event. This was performed by dividing the total number of subjects that had reported an adverse event (n), by the total sample size of the pooled data (N), and expressing it as a percentage . There were several studies that included both children and adults. In those instances, and when the exact number of children versus adults reporting an adverse event couldn’t be determined (i.e., from the study or by contacting the authors), we made an assumption that all the adverse events occurred in children/adolescents. This was done in order to obtain a more conservative estimate of the incidence of an adverse event.
Results
Our search retrieved 51 studies that met inclusion criteria (Table 1). Of these, eight published manuscripts were case reports [44, 64–70]. Three publications, one on tDCS [23] and two on TMS [66, 71], did not report the adverse effects of NIBS modalities, nor was the information available from the corresponding authors or ClinicalTrials.gov. The remaining 48 studies addressed the adverse effects of tCS and TMS in more than 513 children and adolescents between 2.5–17.8 years of age (Table 1). A total of 23 studies reported the absence of side effects and/or tolerability of TMS/tCS. The longest follow-up period was 1.5 years [72]. Among the variety of diagnoses reported, autism, cerebral palsy, epilepsy, and depression were the most common. There were nine single-session studies (3 rTMS and 6 tCS) [32, 34, 35, 43, 47, 56, 73–75]. As a therapeutic tool, TMS and tCS were applied as many times as 2–3 sessions/day [45, 46, 72, 76]. Twenty-two publications reported conducting the NIBS sessions on consecutive weekdays [31, 38, 39, 45, 46, 51, 65–67, 69, 70, 72, 76–85], and five reported weekly sessions [24, 55, 57, 86, 87].
Table 1.
Description of subject demographics, clinical characteristics, and adverse effects reported for transcranial current stimulation (tCS) and repetitive transcranial magnetic stimulation.
| References | N (n<18) | Age (y) | Diagnosis | Adverse events** |
|---|---|---|---|---|
| Bogdanov et al., 1994 | 21* | 6–18 | Infantile cerebral palsy | Not reported |
| Alon et al., 1998 | 7 | 2.5–7.5 | Cerebral palsy | Well tolerated No reports of seizures, episodes of nausea or vomiting or sleep disruption |
| Shelyakin et al., 2001 | 18 | 4–8 | Infatile cerebral palsy and organic CNS lesions | No negative adverse effects |
| Ilyukhina et al., 2005 | 30 | 4–6 | Delayed neuropsychological development | No adverse effects |
| San-Juan et al., 2011 | 2 (1 < 18) | 17 and 31 | Rasmussen’s encephalitis | No complications or skin injuries |
| Schneider et al., 2011 | 10 | 6–21 | Autism (language disorder) | No adverse effects |
| Mattai A et al., 2011 | 13 | 10.5–17.6 | Schizophrenia | Redness, tingling, itching, fatigue |
| Varga et al., 2011 | 5 | 6.1–11 | Epilepsy | No adverse effects |
| Yook et al., 2011 | 1 | 11 | Seizure | No adverse effects |
| Andrade et al., 2013 | 14 | 5–12 | Language disorders | Tingling, burning sensation, scalp pain, local redness, headache, sleepiness, trouble concentrating, mood changes, irritability |
| Auvichayapat et al., 2013 | 36 | 6–15 | Epilepsy | Well tolerated Erythematous rash with no pain or pruritus (one patient) |
| Young et al., 2013† | 11 (8 < 18) | 7–18 | Dystonia | Uncomfortable at the stimulation electrodes |
| Grecco et al., 2014 | 12 | 5–10 | Cerebral Palsy | No adverse effects |
| Duarte et al., 2014 | 12 | Mean (SD): 7.8(2.0) | Cerebral Palsy | Tingling, redness in the supra-orbital region No other behavioral changes, headache, or discomfort |
| Prehn-Kristensen et al., 2014 | 12 | 10–14 | ADHD | No effect on mood, alertness, or memory |
| Young et al., 2014 | 14 (12 < 18) | 7–19 | Dystonia | Skin discomfort |
| Brasil-Neto et al., 2004 | 5 (1 < 18) | 6–50 | Frontal focal epilepsy | No untoward effects |
| Graff-Guerrero et al., 2004§ | 2 | 11 and 7 | Epilepsia Partialis Continua | No major side effects |
| Morales et al., 2005 | 2 | 8 and 16 | Epilepsy | Case A: Well tolerated; no adverse effects Case B: Leg pain and mild headache (resolved after stimulation) |
| Kinoshita et al., 2005 | 7 (1 < 18) | 16–33 | Extratemporal lobe epilepsy | No adverse effects |
| Fregni et al., 2006 | 27* | Mean (SD): 21.6(7.4)y/20.8(5.2) | Epilepsy/healthy | Not reported |
| Loo et al., 2006 | 2 | 16 | Depression | No adverse effects |
| Valle et al., 2007 | 17 | Mean (SD): 9·1(3.2) | Cerebral palsy | No adverse effects |
| Bloch et al., 2008 | 9 (5 < 18) | 16–18 | Depression | Mild headache |
| Kirton et al., 2008‡ | 5 (4 < 18) | 10–16.7 | Stroke | Neurocardiogenic syncope, mild headache, nausea, neck stiffness |
| Rotenberg et al., 2008 | 1 | 14 | Rasmussen’s encephalitis | Well tolerated with no adverse effects |
| Jardri et al., 2009 | 1 | 11 | Schizophrenia | Well tolerated |
| Mylius et al., 2009 | 1 | 6 | Pantothenate kinase-associated neurodegenerative disease (PKAN) | Not reported |
| Sokhadze et al., 2009§ | 8 (5 <18) | 12–27 | Autism | No adverse effects |
| Baruth et al., 2010§ | 16 (14 < 18) | 9–26 | Autism spectrum disorder | Itching, mild headache |
| Kirton et al., 2010‡ | 5 (4<18) | 10.3–16.7 | Stroke | Neurocardiogenic syncope |
| Sokhadze et al., 2010§ | 13 (11 <18) | 8–27 | Autism spectrum disorder | No adverse effects |
| Hu et al., 2011 | 1 | 15 | Depression | Seizure, hypomania |
| Kwon et al., 2011 | 10 | 9–14 | Tourette’s syndrome | Well tolerated No adverse effects or worsening of symptoms |
| Sun et al., 2011 | 17 (8 < 18) | 3–32 | Epilepsy | Well tolerated; no adverse effects |
| Wall et al., 2011 | 8 | 14.6–17.8 | Depression | Well tolerated |
| Casanova et al., 2012 | 25 | Mean (SD): 12.9(3.1) | Autism | No adverse effects |
| Croarkin et al., 2012† | 8 | 14–17 | Depression | Scalp discomfort |
| Helfrich et al., 2012 | 25 | 8–14 | ADHD | Mild headache; well tolerated |
| Jardri et al., 2012§ | 10 | Mean (SD): 15.5(2.3) | Childhood-onset schizophrenia | Scalp discomfort |
| Sokhadze et al., 2012§ | 20 (18 < 18) | 9–21 | Autism | No adverse effects |
| Sun et al., 2012 | 60* | Group 1: Mean (SD): 21.3 (7.5) Group 2: Mean (SD): 19.7 (6.4) |
Epilepsy | Mild or moderate headache, tinnitus; well tolerated |
| Weaver et al., 2012 | 9 (4 < 18) | 14–21 | ADHD | Mild headache, scalp discomfort |
| Wu et al., 2012 | 40 | 8–17 | Tourette’s syndrome/healthy | Mild headache, neck stiffness, finger twitching; no reports of seizures |
| Chiramberro et al., 2013 | 1 | 16 | Depression | Seizure |
| Le et al., 2013 | 25 | 7–16 | Tourette Syndrome | Mild sleepness |
| Wall et al., 2013 | 14 | 13.9–17.8 | Depression | Scalp discomfort, no adverse neurocognitive effect |
| Gillick et al., 2014§ | 10 | 8–15 | Congenital hemiparesis | Self-limiting headache, dizziness, mood changes, fatigue, abnormal muscle contractions |
| Gomez et al., 2014 | 10 | 7–12 | ADHD | Slight headache, neck pain, slight brief dizziness |
| Oberman et al., 2014 | 19 (17 < 18) | 9–18 | Autism | Mild headache, mild fatigue; no serious adverse effects |
| Sokhadze et al., 2014§ | 27 (22 < 18) | Mean (SD): 14.8(3.2) | Autism spectrum disorder | Mild headache, jaw twitches, itching |
N: number of subjects that underwent stimulation. ADHD: Attention Deficit Hyperactivity Disorder.
Unknown number of subjects under 18 years old.
Adverse effects as described by the authors.
Each of these studies reported dropout of one subject from the study due to discomfort and did not include the subject in their analysis.
Sample consists of same subjects.
Adverse effects were gathered by contacting the authors through email. Note that the total number of subjects reported in the manuscript (n > 513) does not include the studies that did not report adverse effects, those with unknown number of subjects under 18 years old, and was adjusted for samples representing the same subjects.
rTMS was the most frequently examined NIBS modality, with 35 publications addressing the application of TMS on children and adolescents (3.0 to 17.8 years old) [38–40, 44–46, 52, 55–57, 64–67, 70, 71, 73, 75–81, 83, 85–94]. The duration of the interventions ranged from one day to 18 weeks, and the stimulation provided was administered in frequencies ranging from 0.3Hz to 10Hz; two studies reported priming the subject with a frequency of 6Hz before the procedure [46, 52]. The intensity of stimulation ranged from 55% active motor threshold to 128% resting motor threshold, with one study using 20% resting motor threshold as a control condition [80] (Table 2).
Table 2.
Transcranial magnetic stimulation (TMS) parameters used in the studies included in the review.
| Authors | Intensity | Frequency (Hz) |
Train
|
Inter-train interval (s) |
Total number of pulses/session |
Sessions | Time (minutes) |
Coil | Treatment site | |
|---|---|---|---|---|---|---|---|---|---|---|
| Number | Duration(s) | |||||||||
| Brasil-Neto et al., 2004 | 5% below MT | 0.3 | 5 | 60 | 60 | 100 | 2 session/week 3 months |
9 | Circular | Vertex (Cz) |
|
| ||||||||||
| Graff-Guerrero et al., 2004 | Case (A) 50% MSO Case (B) 128%RMT |
20 | 15 | 2 | 58 | 600 | 1 session | 15 | Fig.-8 | Left frontal cortex Epileptogenic focus |
|
| ||||||||||
| Morales et al., 2005 | Case (A) 100% MSO Case (B) 90–100%MT |
1 (1st session) 6Hz-priming &1Hz (2nd session) 1 (day 2) 1 (1st session) 6Hz-priming & 1Hz (2nd session) |
Continuous | 4 × 600 | ||||||
| 10 | 5 | 25 | 300 | 3 sessions/2 days | 16–40 | Circular | Left motor cortex | |||
| Continuous | 600 | |||||||||
| Continuous | 4 × 600 | |||||||||
| Continuous | 300 | |||||||||
| 10 | 50 | 2 session/day | 2 × 15 | Fig.-8 | Seizure focus | |||||
| Continuous | 5 | 25 | 900 | |||||||
|
| ||||||||||
| Kinoshita et al., 2005 | 90%RMT or 100%AMT | 0.9 | Not reported | 2 sessions/day 5 days/week 1 week |
15 | Circular | FCz or PCz | |||
|
| ||||||||||
| Fregni et al., 2006 | 90%RMT | 1 | Continuous | 900 | 2 sessions 1 session/week |
15 | Fig.-8 | Left Primary Motor Cortex | ||
|
| ||||||||||
| Loo et al., 2006 | Case (A) 110%RMT |
10 | 40 | 5 | 25 | 2000 | 29 sessions | 20 | No specified | Not specified |
| Case (B) 110%RMT |
10 | 40 | 5 | 25 | 2000 | 38 sessions | 20 | |||
|
| ||||||||||
| Valle et al., 2007 | Group (A) 90%RMT |
1 | Continuous | 1500 | 5 consecutive days | 25 | Fig.-8 | Motor cortex | ||
| Group (B) 90%RMT |
5 | 5 | 60 | 120 | 1500 | 5 consecutive days | 15 | Fig.-8 | Motor cortex | |
|
| ||||||||||
| Bloch et al., 2008 | 80%MT | 10 | 20 | 2 | 58 | 14 working days | 20 | Circular | Left DLPFC | |
|
| ||||||||||
| Kirton et al., 2008** | 100%RMT | 1 | Continuous | 1200 | 8 days | 20 | Fig.-8 | Motor cortex | ||
|
| ||||||||||
| Rotenberg et al., 2008 | 100%MT | 1 | Continuous | 1800 | 9 consecutive days | 30 | Fig.-8 | Epilepitogenic focus | ||
|
| ||||||||||
| Jardri et al., 2009 | 100% MT | 1 | Continuous | 1000 | 10 consecutive working days | 17 | Fig.-8 | R and L Temporo-parietal junction | ||
|
| ||||||||||
| Mylius et al., 2009 | 95%RMT | 1 | Continuous | 1200 | 5 consecutive days | 20 | Fig.-8 | Left premotor cortex | ||
|
| ||||||||||
| Sokhadze et al., 2009 | 90%MT | 0.5 | 15 | 10 | 20–30 | 150 | 2 sessions/week 3 weeks |
7.5–10 | Fig.-8 | DLPFC |
|
| ||||||||||
| Baruth et al., 2010 | 90%MT | 1 | 15 | 10 | 20–30 | 150 | 1 session/week 12 weeks |
7.5–10 | Fig.-8 | Left/Right DLFPC |
|
| ||||||||||
| Kirton et al., 2010** | 100%RMT | 1 | Continuous | 1200 | 8 days | 20 | Fig.-8 | Motor cortex | ||
|
| ||||||||||
| Sokhadze et al., 2010 | 90%MT | 0.5 | 15 | 10 | 20–30 | 150 | 2 sessions/week 3 weeks |
7.5–10 | Fig.-8 | Left DLPFC |
|
| ||||||||||
| Hu et al., 2011 | 80%RMT | 10 | 20 | 4 | 26 | 800 | Daily | Fig.-8 | Left prefrontal lobe | |
|
| ||||||||||
| Kwon et al., 2011 | 100%RMT | 1 | 4 | 300 | 120 | 1200 | 10 sessions | 26 | Fig.-8 | Supplementary motor area |
|
| ||||||||||
| Sun et al., 2011 | 90%RMT | 0.5 | Continuous | 3 × 500 | 1 session/day Daily 2 weeks |
70 | Fig.-8 | Epileptogenic zone | ||
|
| ||||||||||
| Wall et al., 2011 | 120%RMT | 10 | 75 | 4 | 26 | 3000 | 30 sessions 5 days/week 6–8 weeks |
37.5 | Not specified | Left DLPFC |
|
| ||||||||||
| Casanova et al., 2012 | 90%MT | 1 | 15 | 10 | 20–30 | 150 | 1 session/week 12 weeks |
7.5–10 | Fig.-8 | Left DLPFC Right DLPFC |
|
| ||||||||||
| Croarkin et al., 2012 | 120%RMT | 10 | 75 | 4 | 26 | 3000 | 30 sessions 5 days/week 6–8 weeks |
37.5 | Not specified | Left prefrontal cortex |
|
| ||||||||||
| Helfrich et al., 2012 | 80%RMT or 100%MSO | 1 | Continuous | 900 | 1 session | 15 | Fig.-8 | Left primary motor cortex | ||
|
| ||||||||||
| Jardri et al., 2012 | 90%MT | 1 | Continuous | 1200 | 2 sessions/day 5 consecutive days |
20 | Not specified | Left temporoparietal junction | ||
|
| ||||||||||
| Sokhadze et al., 2012 | 90%RMT | 1 | 15 | 10 | 20–30 | 150 | 1 session/week 12 weeks |
7.5–10 | Fig.-8 | DLPFC and opposite side of DLPFC |
|
| ||||||||||
| Sun et al., 2012 | Group (A) 90%RMT Group (B) 20%RMT |
0.5 | Continuous | 3 × 500 | 2 weeks Daily |
70 | Fig.-8 | Epileptogenic focus | ||
|
| ||||||||||
| Weaver et al., 2012 | 100%MT | 10 | 50 | 4 | 26 | 2000 | 10 sessions 5 sessions/week |
25 | Fig.-8 | Prefrontal cortex |
|
| ||||||||||
| Wu et al., 2012* | 55–80% AMT | 30–50 | Continuous Intermitent Θ-burst |
8 | 600 600 |
43 sessions | 0.667 3.167 |
Fig. 8 | Motor cortex | |
|
| ||||||||||
| Chiramberro et al., 2013 | 100%RMT | 10 | 60 | 5 | 25 | 3000 | 5 days/week 4 weeks |
30 | Fig.-8 | Left DLPFC |
|
| ||||||||||
| Le et al., 2013 | 110%RMT | 1 | Continuous | 1200 | 5 days/week 4 weeks |
20 | Fig.-8 | Supplementary motor area | ||
|
| ||||||||||
| Wall et al., 2013 | 120%RMT | 10 | 75 | 4 | 26 | 3000 | 30 sessions 6–8 weeks |
37.5 | Not specified | Left DLPFC |
|
| ||||||||||
| Gillick et al., 2014 | 90%RMT 90%RMT |
6Hz-priming & 1Hz | 20 Continuous |
5 | 25 0 |
600 600 |
5 alternative weekdays | 10 10 |
Fig.-8 | Contralesional primary motor cortex |
|
| ||||||||||
| Gomez et al., 2014 | 90%RMT | 1 | Continous | 1500 | 5 consecutive days | 25 | Butterfly | Left DLFPC | ||
|
| ||||||||||
| Oberman et al., 2014* | 80%AMT | 50 | Continuous Θ-burst | 600 | 1 session | 0.667 | Fig.-8 | Left motor cortex | ||
|
| ||||||||||
| Sokhadze et al., 2014 | 90%MT | 1Hz | 9 | 20 | 20–30 | 180 | 18 sessions 1 session/week 18 weeks |
5.7–7 | Fig.-8 | Left/Right/Bilateral DLPFC |
R: right; L: left; MSO: maximum stimulator output; MT: Motor Threshold; RMT: Resting Motor Threshold; AMT: Active Motor Threshold; DLPFC: Dorsolateral Prefrontal Cortex
Theta-burst stimulation (TBS),
Sample consists of same subjects
Of the more than 322 enrolled children and adolescents that underwent TMS, only four (1.2%) encountered major negative side effects. Of these, two subjects experienced a seizure (0.62%) [64, 70] and two others had syncope (0.62%) [89, 91]. TMS studies reported several minor negative side effects, the most common being headache (11.5%) and scalp discomfort (2.5%) (Figure 1 and Figure 2A). The headaches (and scalp discomfort) reported were almost always transient and resolved spontaneously without any medical intervention. Only two studies reported that subjects needed a single-dose of acetaminophen/ibuprofen for the treatment of headache [75]. The other TMS-related minor adverse effects (e.g., neck stiffness, twitching, fatigue, etc.) were also temporary and often resolved within 24 hours after the stimulation. Table 1 summarizes population data and adverse effects.
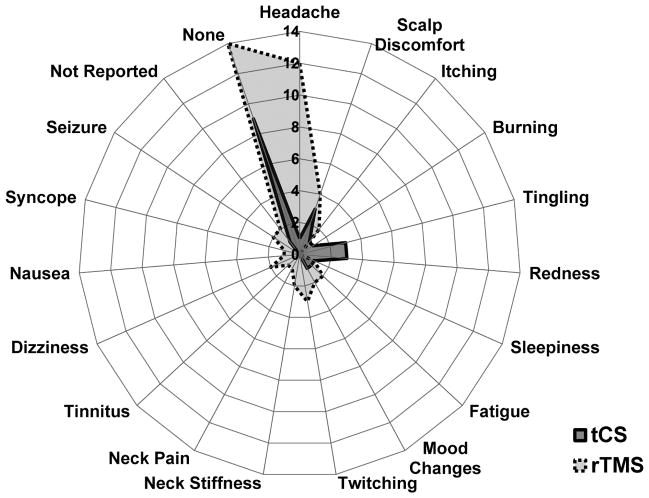
Figure 1.
Radial plot depicting the reported adverse effects of repetitive transcranial magnetic stimulation (rTMS) and transcranial current stimulation (tCS) in children and adolescents from 51 studies (35 rTMS and 16 tCS). The angular data represent the reported adverse effects and the circular data represent the total number of studies reporting a particular adverse effect. 23 studies (14 rTMS and 9 tCS) reported no adverse effects. Scalp discomfort and minor headaches were the most commonly reported adverse effect of rTMS. Tingling, redness, and scalp discomfort were the most commonly reported adverse effect of tCS. Note that a study was counted for an adverse effect even if one subject was reported to have an adverse effect.
Figure 2.
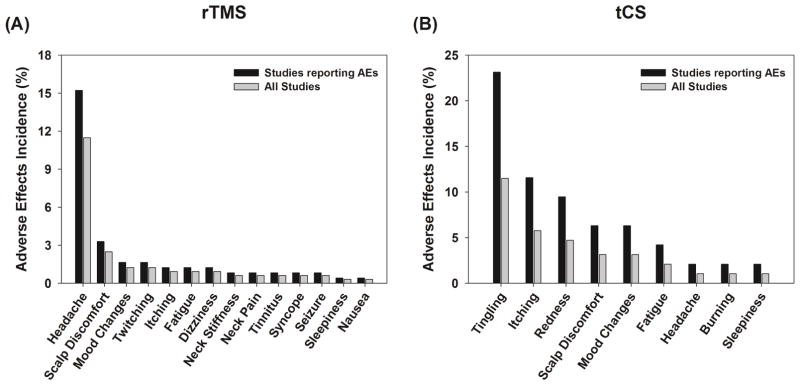
Bar graphs representing the incidence (i.e., frequency) of various adverse effects reported for (A) rTMS and (B) tCS in children/adolescents. The gray bars indicate the percentage of each adverse event calculated based on the total number of subjects (N = 322 for rTMS, N= 191 for tCS) and the black bars indicate a conservative estimate of the percentage of each adverse event calculated based on the total number of subjects on the studies that reported an adverse event (N = 243 for rTMS and N = 95 for tCS). The conservative estimate assumed that only those studies that reported an adverse event attempted to capture the incidence of adverse effects using appropriate follow-up or questionnaires.
Our search also resulted in 16 publications in which greater than 191 children and adolescents (2.5–17.6 years old) underwent tCS (Table 1 and 3) [23–25, 31, 32, 34, 35, 43, 47, 51, 68, 69, 72, 74, 82, 84]. There were 14 studies that used tDCS and two studies that used transcranial alternating current stimulation (tACS) (Table 3). There were 15 studies that addressed the adverse effects of tCS (13 tDCS and 2 tACS). The intensity of current used ranged from 0.03 to 2.0 mA, and stimulation was applied from 18 to 50 minutes per session, with total treatment ranging from 1 to 102 sessions. Detailed information on current parameters can be found in Table 3.
Table 3.
Transcranial current stimulation (tCS) parameters used in the studies included in the review.
| Articles | Electrode size (cm2) | Current density (mA/cm2) / intensity (mA) | Electrode site | Time (Minutes) | Sessions |
|---|---|---|---|---|---|
| Bogdanov et al., 1994 | 6.0 | I: 0.2–0.8 D: 0.04–0.16 |
A: frontal region C: sensorimotor cortex/mastoid process |
Up to 20 (1st 3 sessions) 45–50 (subsequent) |
7–15 sessions 3–4 sessions/week |
| Alon et al., 1998** | 11.34 | I: 0.5 peak D: 0.044 peak |
Right and left temporal area | 10 | Twice a day 7 days/week 8-weeks |
| Shelyakin et al., 2001 | 5.0 | I: 0.3–0.7 D: 0.06–0.14 |
A: projection of the posterior temporal area C: projection of the parietal area |
20–40 | Every day or every two day Up to 15 sessions |
| Ilyukhina et al., 2005 | Not reported | I: 0.03–0.08 D: Unknown |
A: left frontal and parietal cortex C: not reported |
15–20 | Up to 6 sessions 1–2 weeks interval between sessions |
| Mattai et al., 2011* | 25.0 | I: 2.0 D: 0.08 |
A: DLPFC or non-dominat forearm C: STG or non-dominat forearm |
20 | 1 session/day 5 days/week 2 weeks |
| San-Juan et al., 2011 | 0.15 | Case (A): I: 1.0 / D:6.6 Case (B): I: 2.0 / D: 13.2 |
Case A A: contralateral supraorbital area C: scalp (C3) Case B A: scalp (F2) C: scalp (F8) |
60 | 4 (0, 7, 30 and 60 days) |
| Schneider et al., 2011 | 25.0 | I: 2.0 D: 0.08 |
A: DLPFC (F3) C: Right supraorbital area |
30 | 1 session |
| Varga et al., 2011 | 25.0 | I: 1.0 D: 0.04 |
A: epileptogenic focus (Peak −ve) C: epileptogenic focus (Peak +ve) |
20 | 1 session |
| Yook, et al., 2011 | 25.0 | I: 2.0 D: 0.08 |
A: left supraorbital C: right temporo-parietal area |
20 | 1 session/day 5 days/week 2 weeks |
| Andrade et al., 2013* | 35.0 | I: 1.0 (1st min) 2.0 (30 min) D: 0.057 |
A: broca area C: right supraorbital |
1 30 |
1 session/day 5 consecutive days |
| Auvichayapat et al., 2013* | 35.0 | I: 1.0 D: 0.029 |
A: contralateral shoulder area C: epileptogenic focus |
20 | 1 session |
| Young et al., 2013* | 35.0 | I: 1.0 D: 0.029 |
A: contralateral supraorbital C: motor cortex |
9 + 20 (pause) + 9 |
1 session |
| Grecco et al., 2014 | 25.0 | I: 1.0 D: 0.04 |
A: motor cortex C: contralateral supraorbital |
20 | 1 session/day 5 days/week 2 weeks |
| Duarte et al., 2014* | 25.0 | I: 1.0 D: 0.04 |
A: motor cortex C: contralateral supraorbital |
20 | 1 session/day 5 days/week 2 weeks |
| Prehn-Kristensen et al., 2014** | 0.503 | I: 0.25 peak D: 0.497 peak |
A: bilateral frontolateral (F3, F4) C: bilateral mastoid (M1, M2) |
5 × 5 1 min rest between blocks |
1 session |
| Young et al., 2014* | 35.0 | I:1.0 D: 0.029 |
A: contralateral supraorbital C: motor cortex |
9 + 20 (pause) + 9 |
1 session |
A: anode; C: cathode; I: intensity; D: density; STG: superior temporal gyrus; DLPFC: dorsolateral prefrontal cortex
Studies that reported mild adverse effects.
Studies that used transcranial alternating current stimulation.
tCS was well-tolerated and no studies reported any serious adverse effects; however, subjects commonly reported minor side effects such as tingling (n = 22), itching (n = 11), and redness (n = 9). Pooled analysis indicated that the frequency of the adverse effects (i.e., incidence) ranged between 1% and 11.5% (Figure 2B). Tingling and itching sensations were reported to be brief and transient, and typically subsided after the completion of stimulation. Redness associated with the stimulation resolved within 1–2 hours after the end of the treatment [43, 51]. Six subjects complained of minor scalp discomfort [34, 35], four continued the study following a reduction in current intensity [34, 35], while another withdrew from the study [35]. None of the adverse effects required any medical intervention.
Discussion
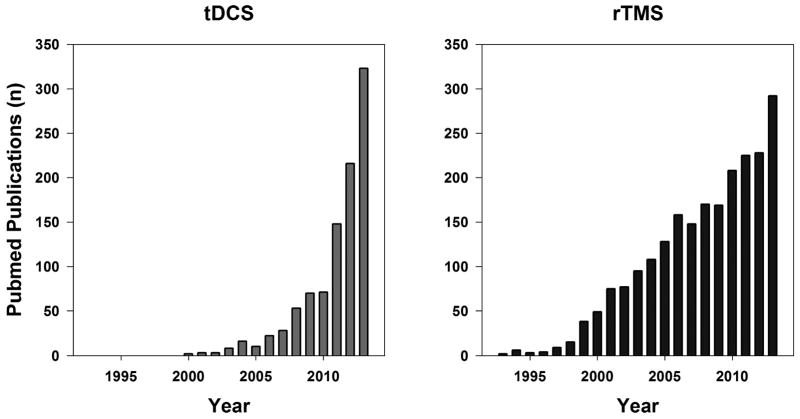
NIBS modalities are considered to be promising methods to enhance brain plasticity and neuromotor function among individuals with neurological disorders. As a result, it is not surprising to see a tremendous increase in both short-term and long-term studies evaluating the efficacy and effectiveness of NIBS for various disease conditions (Figure 3). While rTMS is the most extensively studied NIBS technique, there is a growing interest in the use of tDCS for facilitating neuroplasticity (Figure 3). This is because tDCS is simple and relatively inexpensive, and can be administered in conjunction with cognitive and motor training protocols [95, 96]. However, there are very few studies that address the safety of these techniques, especially in children. In this review, we sought to examine the safety of NIBS (i.e., specifically rTMS and tDCS) among children and adolescents. Our analyses of the existing published literature, to date, revealed a very low incidence of serious adverse events (< 1%), and suggests that these NIBS modalities are safe in children and adolescents.
Figure 3.
Bar charts representing number of published papers per year on tDCS (left panel) and rTMS (right panel) until 2013. The search was conducted on the PubMed database using the search terms “transcranial direct current stimulation” and “repetitive transcranial magnetic stimulation”.
Safety of rTMS
The present review of the literature confirms that rTMS poses little risk to children and adolescents, especially when the specific safety guidelines for the application of rTMS are followed. However, several major and minor adverse effects were reported that are worth describing in further detail. The most severe side effects were seizures and neurocardiogenic syncope, and minor side effects included headache, scalp discomfort, twitching, fatigue, and tinnitus. Specifically, one report described a case in which rTMS led to seizure and hypomania in a 15 year old girl who was diagnosed with adolescent-onset depressive disorder [64]. Another case report described rTMS induced seizures in an adolescent with major depression [70]. Although previous reviews of TMS on children and adolescents have not reported seizure as an adverse event [60, 63], studies on adults have reported seizure as a side effect of rTMS [3]. Epileptogenic medications (i.e., medications that lower seizure threshold), alcohol consumption, and prior seizure history may play a significant role in triggering seizure during rTMS [58, 97]. The subjects in the case reports, which we present in this review, had no history of seizure, cerebral trauma, or any other conditions that could induce seizure, and the researchers were following the safety parameters recommended in the guidelines [3]. However, the subjects were taking medications (Sertraline 100–150 mg/day [64, 70], olanzapine 75 mg/day [70]) that have a potential risk for seizure induction during the application of rTMS [3]. Moreover, one of the subjects had consumed high levels of alcohol (reported levels: 0.20% of blood alcohol ½ hour after seizure), despite being a “minor”, prior to rTMS application [70]. For these reasons, researchers should carefully screen and remove potentially eligible subjects from inclusion if they report recent intake of alcohol or if they are taking epileptogenic medications, especially when there is not a significant benefit-to-risk ratio [3].
In another study included in this review, authors discussed the occurrence of neurocardiogenic syncope in 2 patients under 18 years of age, following rTMS [89]. Both patients, who were participants of an rTMS randomized control trial, had a prior history of syncope (or presyncope) [98]. Even though the authors reported syncope as an adverse event, it is important to note that both cases of syncope occurred during subjects’ initial exposure to TMS (i.e., single-pulse TMS), and not during rTMS. Vasovagal response has not been reported as an adverse effect of rTMS among children (as noted in a previous review) [60, 63]; however, syncope has been reported as an adverse effect of rTMS in an elderly adult subject with depression [99]. This particular event was not thought to be related to the rTMS, as syncope occurred 6 hours after cessation of the stimulation period [99]. In both children and adults, reported TMS-induced syncope lasted only a few minutes and subjects recovered spontaneously after cessation of stimulation, without any additional treatment [98, 99]. Further, these studies reported that patients respond favorably to further rTMS without any subsequent syncopal events, suggesting that, unlike seizures, syncopal attacks may not require exclusion of the subject from the study. For safety purposes, we recommend screening subjects to identify possible history of syncope and/or predisposition to autonomic disorders, and employing suitable measures to minimize the incidence of syncope in these subjects (e.g., adequate hydration, recent food intake, gradual increase in TMS intensity, etc.) [98].
Several studies included in this review reported mild side effects after the application of rTMS [38, 39, 46, 52, 80, 81, 83, 94]. The most commonly reported mild side effect was headache, with ~35% of the studies citing at least one subject with this event. While the exact cause of TMS-related headache is not entirely clear, it is thought to be caused by the activation of muscles and nerves near the stimulation coil, which results in contraction/twitches of the scalp and upper face muscles in some patients [3, 58]. In all cases, headaches attributed to rTMS were mild and brief and were also observed in the sham TMS group [100]; a phenomenon that is consistent with the adult literature [101]. Mild headaches were also reported in conjunction with rTMS among children, in a previous review [63], and as a side effect among adults in several previous studies [101, 102]. Machii and others [101] reported that frontal area and low frequency rTMS were associated with higher rates of headaches in adults; however, our review did not corroborate a link between treatment area, or TMS intensity/frequency, and localized headache.
An additional minor side effect attributed to rTMS is tinnitus. Our review found two reported cases of tinnitus (0.6% incidence) [80]. While previous reviews on children/adolescents did not report the occurrence of tinnitus [59, 60, 63], tinnitus has been reported as a side-effect of rTMS in adults [101]. While the exact reason for the occurrence of tinnitus is not clear, it appears that intensity, frequency, and site of treatment may play a role. In both cases reported in this review, the subjects underwent high intensity stimulation (90% RMT). These are consistent with Machii and colleagues [101], who reported 3 cases of tinnitus in adult subjects that had no history of the illness, and 2 complaints of tinnitus exacerbation in patients with a history of tinnitus after high intensity stimulation. In all 5 cases reported by Machii et al., patients were also subjected to high frequency stimulation; however, both subjects highlighted in this review underwent low frequency stimulation. It is important to note, though, that the study that had reported tinnitus included both adults and children, and it was not clear from the manuscript whether tinnitus occurred in children or in adults. We made several attempts to contact the corresponding author to retrieve this information, but did not receive a response from them. As a result, we assumed that both cases occurred in children, although it is to be recognized that this may not be the case.
The effect of rTMS on neuropsychological functions, such as attention, learning, and memory has been well studied in adults [103–105]. These studies report no adverse effects of TMS on memory or cognition. Unfortunately, we were only able to retrieve a single study that specifically examined this issue in adolescents with depression [40]. The authors of this study reported no negative impact of rTMS on neurocognitive functioning, such as learning, memory, and executive function. Further, the authors of one of the rTMS studies included in this review [38] followed eight of the nine subjects three years after their initial treatment for depression and reported no evidence of cognitive deterioration in any of their patients [106]. These data further support the long-term safety of rTMS in adolescents.
Safety of tCS
Our findings support the safety of tCS in children and adolescents with neurologic and neuropsychiatric disorders. The adverse effects reported were rare, mild, and transient; with “redness”, “slight tingling”, “itching”, and “burning sensation” as the most commonly reported events. It is important to note that while the literature regarding the use of TMS is comprehensive, there is a paucity of studies (especially long-term intervention) on the application of tCS among children and adolescents.
Safety of tDCS application in adults has been well tested and established, and the few adverse side effects that have been reported were mild and transient [19, 107, 108]. Poreisz and colleagues [61] provided a detailed account of the adverse events associated with tDCS among healthy subjects as well as patients, and stated that mild tingling was the most common adverse effect. Itching and tingling under the electrodes, followed by headache and burning sensation, were also the most common adverse effects reported by several authors during and/or after direct current application [19, 61, 109, 110]. Our findings are in accordance with previous studies, in that the most common side effects were redness, tingling, and itching (Tables 1 and 3; Figure 1 and 2). These discomforts are mostly related to the electrode site (especially on the electrode over the supraorbital region), and are associated with the onset of the bout of stimulation. Our review also highlights that the mild adverse events reported in conjunction with tCS usually disappeared within a few minutes after the beginning of the stimulation. None of adverse events lasted longer than two hours after cessation of the stimulation, and no studies reported delayed onset side effects [35, 43, 82]. Approximately 60% of the studies reported that tCS was well tolerated, and no adverse events were noted. Given the physics and physiology of tCS, minor nuisance sensations such as tingling, itching, or slight burning are virtually unavoidable, and thus in all likelihood, these studies did not specifically consider reporting transient minor side-effects. Therefore, to fully document all adverse effects related to tCS, we support the use of the questionnaire proposed by Brunoni and colleagues, which systematically characterizes the severity of each of the possible adverse effects of tCS, along with a risk attribution scale [110].
There are various factors that can influence a subject’s perception of comfort during the application of tDCS. It is well known that intensity of current, as well as density and electrode size can modulate the tingling and itch perception during transcranial stimulation. A recent study by Dundas and colleagues [111] has also demonstrated a relationship between the solution concentration for electrode placement and the perception of comfort, such that higher concentrations (220 mM) are perceived as less comfortable than lower concentrations of NaCl solution (15 mM). Although current density and electrode sizes have been well-described by the published papers in this review, no papers reported electrolytic concentration of saline solution used during stimulation. A limitation of this review was an inherent assumption that most studies used normal saline (i.e., 0.9% NaCl solution), due to its common availability. The 0.9% saline solution contains 154 mM of NaCl per liter, which is slightly above the recommended concentration for optimal subject comfort [111]. Hence, it may be beneficial to reduce the concentration (e.g., < 100 mM) of the saline solution in the event that a subject reports unusual discomfort during tDCS application. We suggest that all future studies report the concentration of the electrolyte solution along with the related observed adverse effects, as this would enable researchers to better understand the relationship between electrolyte concentration and perceived comfort during stimulation.
Recently, a few reports have indicated the occurrence of skin lesions after repeated tDCS application in adults with no history of skin disease or injury [51, 112, 113]. There is also a report of delayed onset contact dermatitis after a single bout of tDCS in a single adult subject [114]. Interestingly, none of the studies in our review among children and adolescents reported any of these same adverse effects that have been noted in adults. The reported skin lesions after repeated tDCS application in adults have been attributed to the use of water soaked sponge electrodes, instead of the conventional saline soaked sponge electrodes [113, 115]. Hence, it is possible that skin related adverse effects were not observed in our review, as all studies used saline soaked sponge electrodes. It is also plausible that skin lesions did not occur because very few studies occurred over a long duration. Regardless, and based on the currently-available evidence, we recommend that researchers carefully and regularly monitor the skin under the electrode site, before each tDCS session, and only use saline soaked sponges to minimize risk of skin lesions.
It is important to note that only seven [23, 25, 69, 72, 82] of the 16 tCS studies had interventions beyond 10 sessions, and were prospective in nature. While these studies demonstrated adequate safety of tCS in children and adolescents, future research is needed to better understand the benefits and tolerance of long-term use of tCS in children. Although several studies have demonstrated no brain tissue damage on animal models and humans after direct stimulation [19, 22, 116, 117], there is a well-accepted threshold of tCS current density (<142.9A/m2 or 14.29mA/cm2) [118], above which has been theorized to be harmful to the brain structures. The current density commonly used for tCS studies, including those presented in this review (0.029mA/cm2 – 13.2mA/cm2), is typically lower than the aforementioned threshold. Moreover, unlike rTMS, tCS does not elicit action potentials in the cortical neurons, which is thought to minimize the risk of serious adverse events, such as seizures. It is thus reasonable to posit that tCS could be as safe as rTMS for long-term interventions in children.
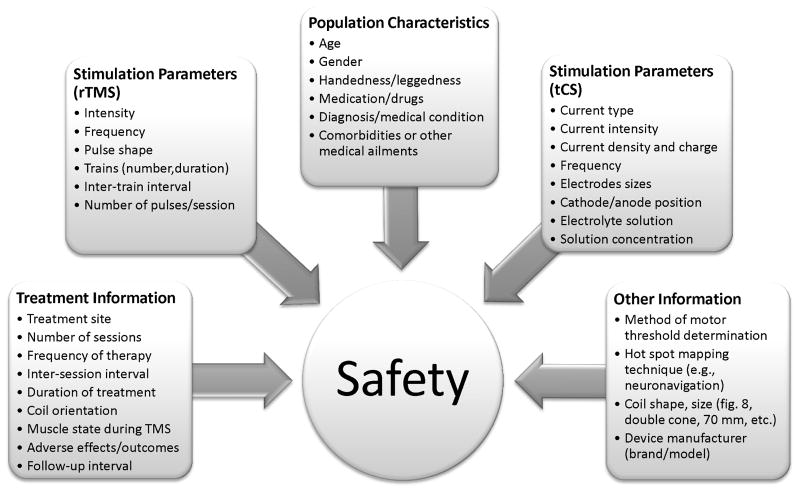
Recommendations for NIBS Reporting Standards
Despite the growing number of publications pertaining to NIBS modalities in children, adolescents, and adults, there are no definitive standards for reporting NIBS data, especially for tCS. Further, very few studies systematically quantify adverse events by reporting the frequency of a side-effect. As a result and at present, it was impossible to perform an appropriate meta-analysis on this topic, or to understand the granular associations between every potential combination of NIBS parameter and potential safety outcomes. In order to improve the critical appraisal of published investigations for device safety and efficacy, we suggest that authors include detailed information regarding various factors that could influence safety and efficacy (e.g., stimulation parameters, technical factors, patient demographics, etc.) and clearly report the frequency of all adverse effects observed in their population when constructing manuscripts for publication (Figure 4 and Supplementary materials 1 & 2).
Figure 4.
Reporting guidelines for noninvasive brain stimulation (NIBS) research. We suggest that authors consider including detailed information regarding various factors that could influence safety and efficacy when constructing manuscripts for publication. A checklist for reporting standards is also included in the supplementary material, which could serve as a guideline for writing NIBS manuscripts.
Potential Limitations
There are some limitations to this review that are worth discussing. While our pooled sample size exceeds 500 subjects, it is still relatively small to detect rare adverse events. Further, as mentioned, very few studies have evaluated the long-term effects of NIBS in children/adolescents, which makes it difficult to understand the effects of long-term use of NIBS in this population. Moreover, unlike for TMS use, there are no clear dosage and safety guidelines for tCS use (other than the density criteria), which restrict our ability to evaluate whether tCS studies utilized parameters that are physiologically safe. Finally, many of the studies included in the review were not designed as a safety study, per se, and perhaps did not quantify adverse effects through appropriate questionnaires or follow-ups. As a result, it is likely that the frequency of adverse events reported may not reflect the true incidence of an adverse event in this population. It is to be noted that while there are a few studies in adults that have included physiological data (e.g., MRI-based structural or physiological alterations), or blood samples (e.g., serum markers of neuron specific enolase or protein S-100) to quantify alterations at the molecular levels [116, 119, 120], such studies are lacking in the youth population. Therefore, we recommend that readers consider these limitations while interpreting the study results.
Conclusion
This is the first attempt to evaluate and provide a comprehensive review on safety of both TMS and tCS in children and adolescents. Our review identified 51 studies involving children less than 18 years of age of which 48 discussed adverse events. In over 322 subjects, rTMS caused seizure in two subjects and neurocardiogenic syncope in two subjects. In all four cases, the subjects had potential risk factors for seizure or neurocardiogenic syncope prior to involvement. In more than 191 children that underwent tCS, no subject experienced any major side effects. These data support the feasibility, tolerability, and safety of NIBS, and especially tCS, for children and adolescents. When conducting NIBS studies on human subjects, researchers should be aware of the most common adverse effects attributed to the application of NIBS. All risks should be openly discussed with patients, parents, and/or caregivers so that they can make informed treatment decisions and consent/assent. A priori screening of all subjects, based on the established guidelines, would substantially help to better identify high risk patients and minimize adverse events. We recommend that future studies systematically record and report all adverse events so that all potential side effects of NIBS may be adequately understood (e.g., supplementary material 1). We also suggest providing adequate detail on the TMS/tCS parameters and dosage so that potential mediating links between these factors and safety outcomes can be discerned (e.g., supplementary material 2). There is a need to conduct more controlled clinical trials, with longer follow-up periods, in order to evaluate the long-term effects of these technologies in a young population, as well as to develop optimum parameters for generating the desired therapeutic effects.
Supplementary Material
Highlights.
The safety of noninvasive brain stimulation techniques in children and adolescents was examined through a review of existing literature.
Adverse effects reported by the studies retrieved from the online search were systematically examined and reviewed.
The data from 48 studies involving more than 500 children/adolescents indicate that the side-effects of noninvasive brain stimulation were, in general, mild and transient.
Noninvasive brain stimulation appears to be safe in children and adolescents with various neurological conditions, especially when safety guidelines are followed; however, further studies with longer treatment and follow-up periods are needed.
Acknowledgments
We thank the authors of previously published manuscripts (especially Dr. Estate Sokhadze) who provided the requested information for this review through their prompt email responses. We also thank three anonymous reviewers for their insightful comments on an earlier version of the manuscript. The authors were supported for their time and effort on this project through the University of Michigan Department of Physical Medicine & Rehabilitation and through grants NIH R01 EB019834 and NIH K01 HD074706. There are no other funding sources involved in this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Guleyupoglu B, Schestatsky P, Edwards D, Fregni F, Bikson M. Classification of methods in transcranial electrical stimulation (tES) and evolving strategy from historical approaches to contemporary innovations. J Neurosci Methods. 2013;219(2):297–311. doi: 10.1016/j.jneumeth.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527(Pt 3):633–9. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rossi S, Hallett M, Rossini PM, Pascual-Leone A Safety of TMSCG. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120(12):2008–39. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003;2(3):145–56. doi: 10.1016/s1474-4422(03)00321-1. [DOI] [PubMed] [Google Scholar]
- 5.Eldaief MC, Press DZ, Pascual-Leone A. Transcranial magnetic stimulation in neurology: A review of established and prospective applications. Neurol Clin Pract. 2013;3(6):519–26. doi: 10.1212/01.CPJ.0000436213.11132.8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paulus W, Peterchev AV, Ridding M. Transcranial electric and magnetic stimulation: technique and paradigms. Handb Clin Neurol. 2013;116:329–42. doi: 10.1016/B978-0-444-53497-2.00027-9. [DOI] [PubMed] [Google Scholar]
- 7.Webster BR, Celnik PA, Cohen LG. Noninvasive brain stimulation in stroke rehabilitation. NeuroRx. 2006;3(4):474–81. doi: 10.1016/j.nurx.2006.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Radhu N, de Jesus DR, Ravindran LN, Zanjani A, Fitzgerald PB, Daskalakis ZJ. A meta-analysis of cortical inhibition and excitability using transcranial magnetic stimulation in psychiatric disorders. Clin Neurophysiol. 2013;124(7):1309–20. doi: 10.1016/j.clinph.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 9.Edwards MJ, Talelli P, Rothwell JC. Clinical applications of transcranial magnetic stimulation in patients with movement disorders. Lancet Neurol. 2008;7(9):827–40. doi: 10.1016/S1474-4422(08)70190-X. [DOI] [PubMed] [Google Scholar]
- 10.Nitsche MA, Paulus W. Noninvasive brain stimulation protocols in the treatment of epilepsy: current state and perspectives. Neurotherapeutics. 2009;6(2):244–50. doi: 10.1016/j.nurt.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boggio PS, Nunes A, Rigonatti SP, Nitsche MA, Pascual-Leone A, Fregni F. Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients. Restor Neurol Neurosci. 2007;25(2):123–9. [PubMed] [Google Scholar]
- 12.Davis NJ, van Koningsbruggen MG. “Non-invasive” brain stimulation is not non-invasive. Front Syst Neurosci. 2013;7:76. doi: 10.3389/fnsys.2013.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krishnan C, Dhaher Y. Corticospinal responses of quadriceps are abnormally coupled with hip adductors in chronic stroke survivors. Exp Neurol. 2012;233(1):400–7. doi: 10.1016/j.expneurol.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krishnan C, Ranganathan R, Kantak SS, Dhaher YY, Rymer WZ. Active robotic training improves locomotor function in a stroke survivor. J Neuroeng Rehabil. 2012;9:57. doi: 10.1186/1743-0003-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pascual-Leone A, Tormos JM, Keenan J, Tarazona F, Canete C, Catala MD. Study and modulation of human cortical excitability with transcranial magnetic stimulation. J Clin Neurophysiol. 1998;15(4):333–43. doi: 10.1097/00004691-199807000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Madhavan S, Krishnan C, Jayaraman A, Rymer WZ, Stinear JW. Corticospinal tract integrity correlates with knee extensor weakness in chronic stroke survivors. Clin Neurophysiol. 2011;122(8):1588–94. doi: 10.1016/j.clinph.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peterchev AV, Wagner TA, Miranda PC, Nitsche MA, Paulus W, Lisanby SH, et al. Fundamentals of transcranial electric and magnetic stimulation dose: definition, selection, and reporting practices. Brain Stimul. 2012;5(4):435–53. doi: 10.1016/j.brs.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mix A, Benali A, Eysel UT, Funke K. Continuous and intermittent transcranial magnetic theta burst stimulation modify tactile learning performance and cortical protein expression in the rat differently. Eur J Neurosci. 2010;32(9):1575–86. doi: 10.1111/j.1460-9568.2010.07425.x. [DOI] [PubMed] [Google Scholar]
- 19.Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008;1(3):206–23. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Nitsche MA, Liebetanz D, Lang N, Antal A, Tergau F, Paulus W. Safety criteria for transcranial direct current stimulation (tDCS) in humans. Clin Neurophysiol. 2003;114(11):2220–2. doi: 10.1016/s1388-2457(03)00235-9. author reply 2–3. [DOI] [PubMed] [Google Scholar]
- 21.Nitsche MA, Nitsche MS, Klein CC, Tergau F, Rothwell JC, Paulus W. Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Clin Neurophysiol. 2003;114(4):600–4. doi: 10.1016/s1388-2457(02)00412-1. [DOI] [PubMed] [Google Scholar]
- 22.Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57(10):1899–901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- 23.Bogdanov OV, Pinchuk D, Pisar’kova EV, Shelyakin AM, Sirbiladze KT. The use of the method of transcranial micropolarization to decrease the severity hyperkineses in patients with infantile cerebral palsy. Neurosci Behav Physiol. 1994;24(5):442–5. doi: 10.1007/BF02359800. [DOI] [PubMed] [Google Scholar]
- 24.Ilyukhina VA, Kozhushko NY, Matveev YK, Ponomareva EA, Chernysheva EM, Shaptilei MA. Transcranial micropolarization in the combined therapy of speech and general psychomotor retardation in children of late preschool age. Neurosci Behav Physiol. 2005;35(9):969–76. doi: 10.1007/s11055-005-0153-7. [DOI] [PubMed] [Google Scholar]
- 25.Shelyakin AM, Preobrazhenskaya IG, Kassil MV, Bogdanov OV. The effects of transcranial micropolarization on the severity of convulsive fits in children. Neurosci Behav Physiol. 2001;31(5):555–60. doi: 10.1023/a:1010487201282. [DOI] [PubMed] [Google Scholar]
- 26.Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. Neuroscientist. 2011;17(1):37–53. doi: 10.1177/1073858410386614. [DOI] [PubMed] [Google Scholar]
- 27.Arul-Anandam AP, Loo C, Sachdev P. Transcranial direct current stimulation - what is the evidence for its efficacy and safety? F1000 Med Rep. 2009;1 doi: 10.3410/M1-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krishnan C, Ranganathan R, Kantak SS, Dhaher YY, Rymer WZ. Anodal transcranial direct current stimulation alters elbow flexor muscle recruitment strategies. Brain Stimul. 2014;7(3):443–50. doi: 10.1016/j.brs.2014.01.057. [DOI] [PubMed] [Google Scholar]
- 29.Cotelli M, Manenti R, Petesi M, Brambilla M, Cosseddu M, Zanetti O, et al. Treatment of primary progressive aphasias by transcranial direct current stimulation combined with language training. J Alzheimers Dis. 2014;39(4):799–808. doi: 10.3233/JAD-131427. [DOI] [PubMed] [Google Scholar]
- 30.Berthier ML, Pulvermuller F. Neuroscience insights improve neurorehabilitation of poststroke aphasia. Nat Rev Neurol. 2011;7(2):86–97. doi: 10.1038/nrneurol.2010.201. [DOI] [PubMed] [Google Scholar]
- 31.Andrade AC, Magnavita GM, Allegro JV, Neto CE, Lucena RD, Fregni F. Feasibility of Transcranial Direct Current Stimulation Use in Children Aged 5 to 12 Years. J Child Neurol. 2013 doi: 10.1177/0883073813503710. [DOI] [PubMed] [Google Scholar]
- 32.Schneider HD, Hopp JP. The use of the Bilingual Aphasia Test for assessment and transcranial direct current stimulation to modulate language acquisition in minimally verbal children with autism. Clin Linguist Phon. 2011;25(6–7):640–54. doi: 10.3109/02699206.2011.570852. [DOI] [PubMed] [Google Scholar]
- 33.Wu AD, Fregni F, Simon DK, Deblieck C, Pascual-Leone A. Noninvasive brain stimulation for Parkinson’s disease and dystonia. Neurotherapeutics. 2008;5(2):345–61. doi: 10.1016/j.nurt.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Young SJ, Bertucco M, Sanger TD. Cathodal transcranial direct current stimulation in children with dystonia: a sham-controlled study. J Child Neurol. 2014;29(2):232–9. doi: 10.1177/0883073813492385. [DOI] [PubMed] [Google Scholar]
- 35.Young SJ, Bertucco M, Sheehan-Stross R, Sanger TD. Cathodal transcranial direct current stimulation in children with dystonia: a pilot open-label trial. J Child Neurol. 2013;28(10):1238–44. doi: 10.1177/0883073812460092. [DOI] [PubMed] [Google Scholar]
- 36.Iyer MB, Schleper N, Wassermann EM. Priming stimulation enhances the depressant effect of low-frequency repetitive transcranial magnetic stimulation. J Neurosci. 2003;23(34):10867–72. doi: 10.1523/JNEUROSCI.23-34-10867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pascual-Leone A, Rubio B, Pallardo F, Catala MD. Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet. 1996;348(9022):233–7. doi: 10.1016/s0140-6736(96)01219-6. [DOI] [PubMed] [Google Scholar]
- 38.Bloch Y, Grisaru N, Harel EV, Beitler G, Faivel N, Ratzoni G, et al. Repetitive transcranial magnetic stimulation in the treatment of depression in adolescents: an open-label study. J ECT. 2008;24(2):156–9. doi: 10.1097/YCT.0b013e318156aa49. [DOI] [PubMed] [Google Scholar]
- 39.Croarkin PE, Wall CA, Nakonezny PA, Buyukdura JS, Husain MM, Sampson SM, et al. Increased cortical excitability with prefrontal high-frequency repetitive transcranial magnetic stimulation in adolescents with treatment-resistant major depressive disorder. J Child Adolesc Psychopharmacol. 2012;22(1):56–64. doi: 10.1089/cap.2011.0054. [DOI] [PubMed] [Google Scholar]
- 40.Wall CA, Croarkin PE, McClintock SM, Murphy LL, Bandel LA, Sim LA, et al. Neurocognitive effects of repetitive transcranial magnetic stimulation in adolescents with major depressive disorder. Front Psychiatry. 2013;4:165. doi: 10.3389/fpsyt.2013.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berlim MT, Van den Eynde F, Daskalakis ZJ. Clinical utility of transcranial direct current stimulation (tDCS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. J Psychiatr Res. 2013;47(1):1–7. doi: 10.1016/j.jpsychires.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 42.Tergau F, Naumann U, Paulus W, Steinhoff BJ. Low-frequency repetitive transcranial magnetic stimulation improves intractable epilepsy. Lancet. 1999;353(9171):2209. doi: 10.1016/S0140-6736(99)01301-X. [DOI] [PubMed] [Google Scholar]
- 43.Auvichayapat N, Rotenberg A, Gersner R, Ngodklang S, Tiamkao S, Tassaneeyakul W, et al. Transcranial direct current stimulation for treatment of refractory childhood focal epilepsy. Brain Stimul. 2013;6(4):696–700. doi: 10.1016/j.brs.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 44.Brasil-Neto JP, de Araujo DP, Teixeira WA, Araujo VP, Boechat-Barros R. Experimental therapy of epilepsy with transcranial magnetic stimulation: lack of additional benefit with prolonged treatment. Arq Neuropsiquiatr. 2004;62(1):21–5. doi: 10.1590/s0004-282x2004000100004. [DOI] [PubMed] [Google Scholar]
- 45.Kinoshita M, Ikeda A, Begum T, Yamamoto J, Hitomi T, Shibasaki H. Low-frequency repetitive transcranial magnetic stimulation for seizure suppression in patients with extratemporal lobe epilepsy-a pilot study. Seizure. 2005;14(6):387–92. doi: 10.1016/j.seizure.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 46.Morales OG, Henry ME, Nobler MS, Wassermann EM, Lisanby SH. Electroconvulsive therapy and repetitive transcranial magnetic stimulation in children and adolescents: a review and report of two cases of epilepsia partialis continua. Child Adolesc Psychiatr Clin N Am. 2005;14(1):193–210. viii–ix. doi: 10.1016/j.chc.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 47.Varga ET, Terney D, Atkins MD, Nikanorova M, Jeppesen DS, Uldall P, et al. Transcranial direct current stimulation in refractory continuous spikes and waves during slow sleep: a controlled study. Epilepsy Res. 2011;97(1–2):142–5. doi: 10.1016/j.eplepsyres.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 48.Lipton RB, Dodick DW, Silberstein SD, Saper JR, Aurora SK, Pearlman SH, et al. Single-pulse transcranial magnetic stimulation for acute treatment of migraine with aura: a randomised, double-blind, parallel-group, sham-controlled trial. Lancet Neurol. 2010;9(4):373–80. doi: 10.1016/S1474-4422(10)70054-5. [DOI] [PubMed] [Google Scholar]
- 49.Lo YL. Headache: migraine, magnetic stimulation and cortical excitability. Nat Rev Neurol. 2010;6(8):425–7. doi: 10.1038/nrneurol.2010.109. [DOI] [PubMed] [Google Scholar]
- 50.Hummel FC, Cohen LG. Non-invasive brain stimulation: a new strategy to improve neurorehabilitation after stroke? Lancet Neurol. 2006;5(8):708–12. doi: 10.1016/S1474-4422(06)70525-7. [DOI] [PubMed] [Google Scholar]
- 51.deDuarte NA, Grecco LA, Galli M, Fregni F, Oliveira CS. Effect of transcranial direct-current stimulation combined with treadmill training on balance and functional performance in children with cerebral palsy: a double-blind randomized controlled trial. PLoS One. 2014;9(8):e105777. doi: 10.1371/journal.pone.0105777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gillick BT, Krach LE, Feyma T, Rich TL, Moberg K, Thomas W, et al. Primed low-frequency repetitive transcranial magnetic stimulation and constraint-induced movement therapy in pediatric hemiparesis: a randomized controlled trial. Dev Med Child Neurol. 2014;56(1):44–52. doi: 10.1111/dmcn.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grecco LA, deDuarte NA, de Mendonca ME, Pasini H, Lima VL, Franco RC, et al. Effect of transcranial direct current stimulation combined with gait and mobility training on functionality in children with cerebral palsy: study protocol for a double-blind randomized controlled clinical trial. BMC Pediatr. 2013;13:168. doi: 10.1186/1471-2431-13-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mondino M, Bennabi D, Poulet E, Galvao F, Brunelin J, Haffen E. Can transcranial direct current stimulation (tDCS) alleviate symptoms and improve cognition in psychiatric disorders? World J Biol Psychiatry. 2014;15(4):261–75. doi: 10.3109/15622975.2013.876514. [DOI] [PubMed] [Google Scholar]
- 55.Sokhadze EM, El-Baz AS, Sears LL, Opris I, Casanova MF. rTMS neuromodulation improves electrocortical functional measures of information processing and behavioral responses in autism. Front Syst Neurosci. 2014;8:134. doi: 10.3389/fnsys.2014.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Graff-Guerrero A, Gonzales-Olvera J, Ruiz-Garcia M, Avila-Ordonez U, Vaugier V, Garcia-Reyna JC. rTMS reduces focal brain hyperperfusion in two patients with EPC. Acta Neurol Scand. 2004;109(4):290–6. doi: 10.1046/j.1600-0404.2003.00222.x. [DOI] [PubMed] [Google Scholar]
- 57.Baruth JM, Casanova MF, El-Baz A, Horrell T, Mathai G, Sears L, et al. Low-Frequency Repetitive Transcranial Magnetic Stimulation (rTMS) Modulates Evoked-Gamma Frequency Oscillations in Autism Spectrum Disorder (ASD) J Neurother. 2010;14(3):179–94. doi: 10.1080/10874208.2010.501500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108(1):1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- 59.Quintana H. Transcranial magnetic stimulation in persons younger than the age of 18. J ECT. 2005;21(2):88–95. doi: 10.1097/01.yct.0000162556.02720.58. [DOI] [PubMed] [Google Scholar]
- 60.Vicario CM, Nitsche MA. Non-invasive brain stimulation for the treatment of brain diseases in childhood and adolescence: state of the art, current limits and future challenges. Front Syst Neurosci. 2013;7:94. doi: 10.3389/fnsys.2013.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Poreisz C, Boros K, Antal A, Paulus W. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res Bull. 2007;72(4–6):208–14. doi: 10.1016/j.brainresbull.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 62.Gilbert DL, Garvey MA, Bansal AS, Lipps T, Zhang J, Wassermann EM. Should transcranial magnetic stimulation research in children be considered minimal risk? Clin Neurophysiol. 2004;115(8):1730–9. doi: 10.1016/j.clinph.2003.10.037. [DOI] [PubMed] [Google Scholar]
- 63.D’Agati D, Bloch Y, Levkovitz Y, Reti I. rTMS for adolescents: Safety and efficacy considerations. Psychiatry Res. 2010;177(3):280–5. doi: 10.1016/j.psychres.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 64.Hu SH, Wang SS, Zhang MM, Wang JW, Hu JB, Huang ML, et al. Repetitive transcranial magnetic stimulation-induced seizure of a patient with adolescent-onset depression: a case report and literature review. J Int Med Res. 2011;39(5):2039–44. doi: 10.1177/147323001103900552. [DOI] [PubMed] [Google Scholar]
- 65.Jardri R, Delevoye-Turrell Y, Lucas B, Pins D, Bulot V, Delmaire C, et al. Clinical practice of rTMS reveals a functional dissociation between agency and hallucinations in schizophrenia. Neuropsychologia. 2009;47(1):132–8. doi: 10.1016/j.neuropsychologia.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 66.Mylius V, Gerstner A, Peters M, Prokisch H, Leonhardt A, Hellwig D, et al. Low-frequency rTMS of the premotor cortex reduces complex movement patterns in a patient with pantothenate kinase-associated neurodegenerative disease (PKAN) Neurophysiol Clin. 2009;39(1):27–30. doi: 10.1016/j.neucli.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 67.Rotenberg A, Depositario-Cabacar D, Bae EH, Harini C, Pascual-Leone A, Takeoka M. Transient suppression of seizures by repetitive transcranial magnetic stimulation in a case of Rasmussen’s encephalitis. Epilepsy Behav. 2008;13(1):260–2. doi: 10.1016/j.yebeh.2007.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.San-Juan D, de Calcaneo JD, Gonzalez-Aragon MF, Bermudez Maldonado L, Avellan AM, Argumosa EV, et al. Transcranial direct current stimulation in adolescent and adult Rasmussen’s encephalitis. Epilepsy Behav. 2011;20(1):126–31. doi: 10.1016/j.yebeh.2010.10.031. [DOI] [PubMed] [Google Scholar]
- 69.Yook SW, Park SH, Seo JH, Kim SJ, Ko MH. Suppression of seizure by cathodal transcranial direct current stimulation in an epileptic patient - a case report. Ann Rehabil Med. 2011;35(4):579–82. doi: 10.5535/arm.2011.35.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chiramberro M, Lindberg N, Isometsa E, Kahkonen S, Appelberg B. Repetitive transcranial magnetic stimulation induced seizures in an adolescent patient with major depression: a case report. Brain Stimul. 2013;6(5):830–1. doi: 10.1016/j.brs.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 71.Fregni F, Boggio PS, Valle AC, Otachi P, Thut G, Rigonatti SP, et al. Homeostatic effects of plasma valproate levels on corticospinal excitability changes induced by 1Hz rTMS in patients with juvenile myoclonic epilepsy. Clin Neurophysiol. 2006;117(6):1217–27. doi: 10.1016/j.clinph.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 72.Alon G, Syron SC, Smith GV. Is transcranial electrical stimulation (TCES) a safe intervention for children with cerebral palsy? Neurorehabil Neural Repair. 1998;12:65–72. [Google Scholar]
- 73.Helfrich C, Pierau SS, Freitag CM, Roeper J, Ziemann U, Bender S. Monitoring cortical excitability during repetitive transcranial magnetic stimulation in children with ADHD: a single-blind, sham-controlled TMS-EEG study. PLoS One. 2012;7(11):e50073. doi: 10.1371/journal.pone.0050073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Prehn-Kristensen A, Munz M, Goder R, Wilhelm I, Korr K, Vahl W, et al. Transcranial Oscillatory Direct Current Stimulation During Sleep Improves Declarative Memory Consolidation in Children With Attention-deficit/hyperactivity Disorder to a Level Comparable to Healthy Controls. Brain Stimul. 2014 doi: 10.1016/j.brs.2014.07.036. [DOI] [PubMed] [Google Scholar]
- 75.Oberman LM, Pascual-Leone A, Rotenberg A. Modulation of corticospinal excitability by transcranial magnetic stimulation in children and adolescents with autism spectrum disorder. Front Hum Neurosci. 2014;8:627. doi: 10.3389/fnhum.2014.00627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jardri R, Bubrovszky M, Demeulemeester M, Poulet E, Januel D, Cohen D, et al. Repetitive transcranial magnetic stimulation to treat early-onset auditory hallucinations. J Am Acad Child Adolesc Psychiatry. 2012;51(9):947–9. doi: 10.1016/j.jaac.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 77.Valle AC, Dionisio K, Pitskel NB, Pascual-Leone A, Orsati F, Ferreira MJ, et al. Low and high frequency repetitive transcranial magnetic stimulation for the treatment of spasticity. Dev Med Child Neurol. 2007;49(7):534–8. doi: 10.1111/j.1469-8749.2007.00534.x. [DOI] [PubMed] [Google Scholar]
- 78.Sun W, Fu W, Mao W, Wang D, Wang Y. Low-frequency repetitive transcranial magnetic stimulation for the treatment of refractory partial epilepsy. Clin EEG Neurosci. 2011;42(1):40–4. doi: 10.1177/155005941104200109. [DOI] [PubMed] [Google Scholar]
- 79.Wall CA, Croarkin PE, Sim LA, Husain MM, Janicak PG, Kozel FA, et al. Adjunctive use of repetitive transcranial magnetic stimulation in depressed adolescents: a prospective, open pilot study. J Clin Psychiatry. 2011;72(9):1263–9. doi: 10.4088/JCP.11m07003. [DOI] [PubMed] [Google Scholar]
- 80.Sun W, Mao W, Meng X, Wang D, Qiao L, Tao W, et al. Low-frequency repetitive transcranial magnetic stimulation for the treatment of refractory partial epilepsy: a controlled clinical study. Epilepsia. 2012;53(10):1782–9. doi: 10.1111/j.1528-1167.2012.03626.x. [DOI] [PubMed] [Google Scholar]
- 81.Weaver L, Rostain AL, Mace W, Akhtar U, Moss E, O’Reardon JP. Transcranial magnetic stimulation (TMS) in the treatment of attention-deficit/hyperactivity disorder in adolescents and young adults: a pilot study. J ECT. 2012;28(2):98–103. doi: 10.1097/YCT.0b013e31824532c8. [DOI] [PubMed] [Google Scholar]
- 82.Mattai A, Miller R, Weisinger B, Greenstein D, Bakalar J, Tossell J, et al. Tolerability of transcranial direct current stimulation in childhood-onset schizophrenia. Brain Stimul. 2011;4(4):275–80. doi: 10.1016/j.brs.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Le K, Liu L, Sun M, Hu L, Xiao N. Transcranial magnetic stimulation at 1 Hertz improves clinical symptoms in children with Tourette syndrome for at least 6 months. J Clin Neurosci. 2013;20(2):257–62. doi: 10.1016/j.jocn.2012.01.049. [DOI] [PubMed] [Google Scholar]
- 84.Grecco LA, Duarte ND, Mendonca ME, Cimolin V, Galli M, Fregni F, et al. Transcranial direct current stimulation during treadmill training in children with cerebral palsy: A randomized controlled double-blind clinical trial. Res Dev Disabil. 2014;35(11):2840–8. doi: 10.1016/j.ridd.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 85.Gomez L, Vidal B, Morales L, Baez M, Maragoto C, Galvizu R, et al. Low frequency repetitive transcranial magnetic stimulation in children with attention deficit/hyperactivity disorder. Preliminary results. Brain Stimul. 2014;7(5):760–2. doi: 10.1016/j.brs.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 86.Casanova MF, Baruth JM, El-Baz A, Tasman A, Sears L, Sokhadze E. Repetitive Transcranial Magnetic Stimulation (rTMS) Modulates Event-Related Potential (ERP) Indices of Attention in Autism. Transl Neurosci. 2012;3(2):170–80. doi: 10.2478/s13380-012-0022-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sokhadze EM, Baruth JM, Sears L, Sokhadze GE, El-Baz AS, Casanova MF. Prefrontal neuromodulation using rTMS improves error monitoring and correction function in autism. Appl Psychophysiol Biofeedback. 2012;37(2):91–102. doi: 10.1007/s10484-012-9182-5. [DOI] [PubMed] [Google Scholar]
- 88.Loo C, McFarquhar T, Walter G. Transcranial magnetic stimulation in adolescent depression. Australas Psychiatry. 2006;14(1):81–5. doi: 10.1080/j.1440-1665.2006.02251.x. [DOI] [PubMed] [Google Scholar]
- 89.Kirton A, Chen R, Friefeld S, Gunraj C, Pontigon AM, Deveber G. Contralesional repetitive transcranial magnetic stimulation for chronic hemiparesis in subcortical paediatric stroke: a randomised trial. Lancet Neurol. 2008;7(6):507–13. doi: 10.1016/S1474-4422(08)70096-6. [DOI] [PubMed] [Google Scholar]
- 90.Sokhadze EM, El-Baz A, Baruth J, Mathai G, Sears L, Casanova MF. Effects of low frequency repetitive transcranial magnetic stimulation (rTMS) on gamma frequency oscillations and event-related potentials during processing of illusory figures in autism. J Autism Dev Disord. 2009;39(4):619–34. doi: 10.1007/s10803-008-0662-7. [DOI] [PubMed] [Google Scholar]
- 91.Kirton A, Deveber G, Gunraj C, Chen R. Cortical excitability and interhemispheric inhibition after subcortical pediatric stroke: plastic organization and effects of rTMS. Clin Neurophysiol. 2010;121(11):1922–9. doi: 10.1016/j.clinph.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 92.Sokhadze E, Baruth J, Tasman A, Mansoor M, Ramaswamy R, Sears L, et al. Low-frequency repetitive transcranial magnetic stimulation (rTMS) affects event-related potential measures of novelty processing in autism. Appl Psychophysiol Biofeedback. 2010;35(2):147–61. doi: 10.1007/s10484-009-9121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kwon HJ, Lim WS, Lim MH, Lee SJ, Hyun JK, Chae JH, et al. 1-Hz low frequency repetitive transcranial magnetic stimulation in children with Tourette’s syndrome. Neurosci Lett. 2011;492(1):1–4. doi: 10.1016/j.neulet.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 94.Wu SW, Shahana N, Huddleston DA, Lewis AN, Gilbert DL. Safety and tolerability of theta-burst transcranial magnetic stimulation in children. Dev Med Child Neurol. 2012;54(7):636–9. doi: 10.1111/j.1469-8749.2012.04300.x. [DOI] [PubMed] [Google Scholar]
- 95.Segrave RA, Arnold S, Hoy K, Fitzgerald PB. Concurrent cognitive control training augments the antidepressant efficacy of tDCS: a pilot study. Brain Stimul. 2014;7(2):325–31. doi: 10.1016/j.brs.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 96.Reis J, Fritsch B. Modulation of motor performance and motor learning by transcranial direct current stimulation. Curr Opin Neurol. 2011;24(6):590–6. doi: 10.1097/WCO.0b013e32834c3db0. [DOI] [PubMed] [Google Scholar]
- 97.Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Screening questionnaire before TMS: an update. Clin Neurophysiol. 2011;122(8):1686. doi: 10.1016/j.clinph.2010.12.037. [DOI] [PubMed] [Google Scholar]
- 98.Kirton A, Deveber G, Gunraj C, Chen R. Neurocardiogenic syncope complicating pediatric transcranial magnetic stimulation. Pediatr Neurol. 2008;39(3):196–7. doi: 10.1016/j.pediatrneurol.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 99.Figiel GS, Epstein C, McDonald WM, Amazon-Leece J, Figiel L, Saldivia A, et al. The use of rapid-rate transcranial magnetic stimulation (rTMS) in refractory depressed patients. J Neuropsychiatry Clin Neurosci. 1998;10(1):20–5. doi: 10.1176/jnp.10.1.20. [DOI] [PubMed] [Google Scholar]
- 100.Gillick BT, Krach LE, Feyma T, Rich TL, Moberg K, Menk J, et al. Safety of primed repetitive transcranial magnetic stimulation and modified constraint-induced movement therapy in a randomized controlled trial in pediatric hemiparesis. Arch Phys Med Rehabil. 2014 doi: 10.1016/j.apmr.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Machii K, Cohen D, Ramos-Estebanez C, Pascual-Leone A. Safety of rTMS to non-motor cortical areas in healthy participants and patients. Clin Neurophysiol. 2006;117(2):455–71. doi: 10.1016/j.clinph.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 102.Janicak PG, O’Reardon JP, Sampson SM, Husain MM, Lisanby SH, Rado JT, et al. Transcranial magnetic stimulation in the treatment of major depressive disorder: a comprehensive summary of safety experience from acute exposure, extended exposure, and during reintroduction treatment. J Clin Psychiatry. 2008;69(2):222–32. doi: 10.4088/jcp.v69n0208. [DOI] [PubMed] [Google Scholar]
- 103.Loo CK, Mitchell PB, McFarquhar TF, Malhi GS, Sachdev PS. A sham-controlled trial of the efficacy and safety of twice-daily rTMS in major depression. Psychol Med. 2007;37(3):341–9. doi: 10.1017/S0033291706009597. [DOI] [PubMed] [Google Scholar]
- 104.O’Connor M, Brenninkmeyer C, Morgan A, Bloomingdale K, Thall M, Vasile R, et al. Relative effects of repetitive transcranial magnetic stimulation and electroconvulsive therapy on mood and memory: a neurocognitive risk-benefit analysis. Cogn Behav Neurol. 2003;16(2):118–27. doi: 10.1097/00146965-200306000-00005. [DOI] [PubMed] [Google Scholar]
- 105.Vanderhasselt MA, De Raedt R, Leyman L, Baeken C. Acute effects of repetitive transcranial magnetic stimulation on attentional control are related to antidepressant outcomes. J Psychiatry Neurosci. 2009;34(2):119–26. [PMC free article] [PubMed] [Google Scholar]
- 106.Mayer G, Aviram S, Walter G, Levkovitz Y, Bloch Y. Long-term follow-up of adolescents with resistant depression treated with repetitive transcranial magnetic stimulation. J ECT. 2012;28(2):84–6. doi: 10.1097/YCT.0b013e318238f01a. [DOI] [PubMed] [Google Scholar]
- 107.Iyer MB, Mattu U, Grafman J, Lomarev M, Sato S, Wassermann EM. Safety and cognitive effect of frontal DC brain polarization in healthy individuals. Neurology. 2005;64(5):872–5. doi: 10.1212/01.WNL.0000152986.07469.E9. [DOI] [PubMed] [Google Scholar]
- 108.Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006;117(4):845–50. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 109.Kessler SK, Turkeltaub PE, Benson JG, Hamilton RH. Differences in the experience of active and sham transcranial direct current stimulation. Brain Stimul. 2012;5(2):155–62. doi: 10.1016/j.brs.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brunoni AR, Amadera J, Berbel B, Volz MS, Rizzerio BG, Fregni F. A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. Int J Neuropsychopharmacol. 2011;14(8):1133–45. doi: 10.1017/S1461145710001690. [DOI] [PubMed] [Google Scholar]
- 111.Dundas JE, Thickbroom GW, Mastaglia FL. Perception of comfort during transcranial DC stimulation: effect of NaCl solution concentration applied to sponge electrodes. Clin Neurophysiol. 2007;118(5):1166–70. doi: 10.1016/j.clinph.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 112.Palm U, Keeser D, Schiller C, Fintescu Z, Nitsche M, Reisinger E, et al. Skin lesions after treatment with transcranial direct current stimulation (tDCS) Brain Stimul. 2008;1(4):386–7. doi: 10.1016/j.brs.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 113.Frank E, Wilfurth S, Landgrebe M, Eichhammer P, Hajak G, Langguth B. Anodal skin lesions after treatment with transcranial direct current stimulation. Brain Stimul. 2010;3(1):58–9. doi: 10.1016/j.brs.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 114.Riedel P, Kabisch S, Ragert P, von Kriegstein K. Contact dermatitis after transcranial direct current stimulation. Brain Stimul. 2012;5(3):432–4. doi: 10.1016/j.brs.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 115.Palm U, Feichtner KB, Hasan A, Gauglitz G, Langguth B, Nitsche MA, et al. The Role of Contact Media at the Skin-electrode Interface During Transcranial Direct Current Stimulation (tDCS) Brain Stimul. 2014;7(5):762–4. doi: 10.1016/j.brs.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 116.Nitsche MA, Niehaus L, Hoffmann KT, Hengst S, Liebetanz D, Paulus W, et al. MRI study of human brain exposed to weak direct current stimulation of the frontal cortex. Clin Neurophysiol. 2004;115(10):2419–23. doi: 10.1016/j.clinph.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 117.Vandermeeren Y, Jamart J, Ossemann M. Effect of tDCS with an extracephalic reference electrode on cardio-respiratory and autonomic functions. BMC Neurosci. 2010;11:38. doi: 10.1186/1471-2202-11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liebetanz D, Koch R, Mayenfels S, Konig F, Paulus W, Nitsche MA. Safety limits of cathodal transcranial direct current stimulation in rats. Clin Neurophysiol. 2009;120(6):1161–7. doi: 10.1016/j.clinph.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 119.Chaieb L, Antal A, Pisoni A, Saiote C, Opitz A, Ambrus GG, et al. Safety of 5 kHz tACS. Brain Stimul. 2014;7(1):92–6. doi: 10.1016/j.brs.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 120.Ullrich H, Kranaster L, Sigges E, Andrich J, Sartorius A. Neuron specific enolase and serum remain unaffected by ultra high frequency left prefrontal transcranial magnetic stimulation in patients with depression: a preliminary study. J Neural Transm. 2013;120(12):1733–6. doi: 10.1007/s00702-013-1050-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.