Over the last decade, widespread adoption of solid-phase assays by histocompatibility laboratories has led to increased sensitivity to detect anti-HLA antibody and greater accuracy in determining anti-HLA antibody specificity [1]. The influence of these changes on listing practices in terms of requirements for a negative prospective crossmatch is unknown. This is important because the clinical significance of very low strength anti-HLA donor specific antibodies detected by highly sensitive methods is unclear [2], yet listing with a requirement for a prospective crossmatch is associated with higher waitlist mortality [3].
We analyzed national registry data of all pediatric listings (age <18 years) from the Organ Procurement and Transplantation Network (OPTN) from 1996 to 2009. The dataset and exclusion criteria have been previously described [3]. Patients were classified on the basis of their earliest listing as requiring a traditional prospective crossmatch, a virtual prospective crossmatch, both, or neither. The requirement for a virtual crossmatch is indicated in the dataset by the specification of ≥1 unacceptable antigen(s). We chose January 1996 as the start date for this analysis because that was the first full calendar year in which data on traditional crossmatch requirement and unacceptable antigens were collected. We hypothesized that the proportion of patients listed with a requirement for a traditional and/or virtual prospective crossmatch has increased over time.
The requirement for a prospective crossmatch was analyzed using the Chi-square test for trend with two-sided alpha of 0.05 using STATA v10.1 (StataCorp LP, College Station, TX). The study was conducted with approval of the University of Pittsburgh Institutional Review Board and OPTN.
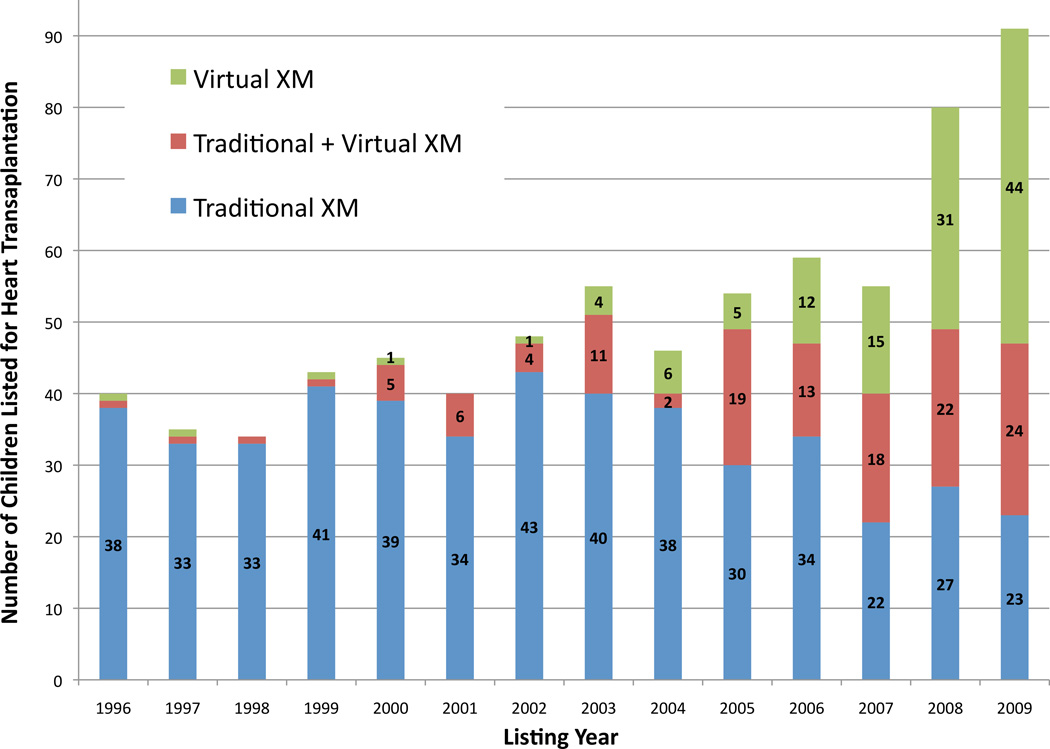
Of the 5,990 children in the study cohort, 725 (12%) had a requirement for a prospective crossmatch during listing. Of these, 475 (8%) were listed with the requirement for a traditional prospective crossmatch, 122 (2%) with the requirement for a virtual prospective crossmatch, and 128 (2%) with both. The requirement for a prospective crossmatch increased over time, from 10% of listings in 1996 to 18% of listings in 2009 (p<0.0001).
As shown in figure 1, the increased prevalence of the prospective crossmatch requirement over time is directly attributed to the growing utilization of the virtual crossmatch. Since 2006, the requirement for a virtual prospective crossmatch has comprised the majority of pediatric listings in which a prospective crossmatch was required. These changes in listing requirements correspond to the increased utilization of highly sensitive alloantibody detection methods during this same time [4].
Figure 1.
Change in composition of the prospective crossmatch requirement from 1996 to 2009. XM, crossmatch.
While it may seem obvious that there would have been an increase in the requirement for a prospective crossmatch over time, we felt that it was important to investigate this topic because there is uncertainty about the clinical relevance of low titer alloantibodies detected using high sensitivity assays and there are no formal policies that mandate listing of patients with respect to a requirement for a prospective crossmatch. Thus, even the increases shown here in the utilization of the virtual prospective crossmatch may underestimate the true prevalence if centers chose to not specify this information to the OPTN but rather to perform the virtual crossmatch internally, in real-time, with each organ offer. The advantage of this approach is that listing centers could weigh the relative risks and benefits of transplantation on a donor-by-donor basis, rather than be restricted to a subset of donor organs, and thereby potentially diminish the longer wait-list duration and increased risks of mortality associated with listing with the requirement for a prospective crossmatch [3].
It is clear that data about individual centers’ listing practices with regard to the requirement for a prospective crossmatch would have been valuable in this analysis. In particular, it is unknown whether antibody titers/MFIs were used as criteria for listing with the requirement for virtual crossmatch and how these criteria may have changed over time. Other data that were not available but would have been valuable include laboratory methods used to detect HLA antibodies and panel reactive antibody data of patients listed with a requirement for a prospective crossmatch. In the OPTN dataset, PRA data are only collected in recipients, at the time of transplantation.
Of note, 17% of patients with a requirement for a prospective crossmatch during listing had a requirement for both a prospective traditional crossmatch and a virtual crossmatch. While some of these may have resulted from changes in the crossmatch requirement during listing (i.e. initial requirement for traditional crossmatch that was subsequently ‘relaxed’ to a virtual crossmatch), the rationale for specifying both the traditional and virtual requirements at listing (93 patients, 12.5% of patients listed with a requirement for prospective crossmatch) is unclear. Because there is no advantage to listing with both requirements simultaneously, such listings may reflect misinterpretation of the requirements by listing centers. Further education about the meaning of prospective crossmatch requirements may be warranted.
In summary, we found an increase in the requirement for a prospective crossmatch from 1996 to 2009 due to an increase in use of the virtual crossmatch.
Acknowledgments
This project was supported by the National Institutes of Health (KL2RR024154, KL2TR000146). Content is solely the responsibility of the authors and does not necessarily represent the views of the National Institutes of Health or OPTN.
Footnotes
DISCLOSURES
No author has a financial interest or other potential conflict of interest related to subject matter or materials mentioned in the manuscript.
REFERENCES
- 1.Zeevi A, Girnita A, Duquesnoy R. HLA antibody analysis: sensitivity, specificity, and clinical significance in solid organ transplantation. Immunol Res. 2006;36:255–264. doi: 10.1385/IR:36:1:255. [DOI] [PubMed] [Google Scholar]
- 2.Vaidya S. Antigen-Specific antibody concentration in performing calculated panel reactive antibody and virtual crossmatches. Transplantation. 2008;85:1046–1050. doi: 10.1097/TP.0b013e318168fdb5. [DOI] [PubMed] [Google Scholar]
- 3.Feingold B, Comer DM, Park SL, Moore CG, Webber SA, Bryce CL. Outcomes on the basis of a prospective XM in pediatric heart transplantation. J Heart Lung Transplant. 2012;31:S208–S209. doi: 10.1016/j.healun.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cecka JM. Calculated PRA (CPRA): The New Measure of Sensitization for Transplant Candidates. Am J Transplant. 2010;10:26–29. doi: 10.1111/j.1600-6143.2009.02927.x. [DOI] [PubMed] [Google Scholar]