Abstract
The signal transduction modulator Rgs9-2 (Regulator of G protein signaling 9-2) plays a key role in dopaminergic and opioidergic transmission in the striatum. Rgs9-2 is a potent modulator of opiate reward and analgesia, but its role in chronic pain remains unknown. Here, we use the spared nerve injury model (SNI), to evaluate the influence of Rgs9-2 in sensory symptoms, as well as in anxiety and depression-like behaviors observed under neuropathic pain conditions. Our data demonstrate that knockout of the Rgs9 gene reduces the intensity of thermal hyperalgesia and mechanical allodynia the first few days after nerve injury. This small, but significant effect is only observed at early time points after nerve injury, whereas after the first week of SNI, Rgs9 knockout (Rgs9KO) and Rgs9 wildtype (Rgs9WT) mice show similar levels of mechanical allodynia and thermal hyperalgesia. Furthermore, Rgs9-2 deletion exacerbates anxiety and depression like behaviors several weeks after the emergence of the neuropathic pain symptoms. Our findings also reveal a temporal and regional regulation of Rgs9-2 protein expression by neuropathic pain, as Rgs9-2 levels are reduced in the spinal cord a few days after nerve injury, whereas decreased Rgs9-2 levels in the Nucleus Accumbens (NAc) are only observed several weeks after nerve injury. Thus, adaptations in Rgs9-2 activity in the spinal cord and in the NAc may contribute to sensory and affective components of neuropathic pain.
Keywords: spared nerve injury, forced swim, chronic pain, thermal hyperalgesia, mechanical allodynia, signal transduction, nociception
INTRODUCTION
Regulator of G protein signaling 9–2 (Rgs9-2) is a striatal enriched modulator of GPCR signal transduction (Hollinger & Hepler 2002). Rgs9-2 controls the signaling amplitude and duration of several GPCRs in the dorsal and ventral striatum, including dopamine and mu opioid receptors (Kimple et al 2011, Traynor et al 2009). Rgs9-2 protein exerts its modulatory actions by binding to activated Gα subunits and accelerating their GTPase activity. Rgs9-2 also prevents Gα effector activation (Kimple et al 2011). The protein is expressed at very high levels in the striatum and at moderate to low abundance in the dorsal horn of the spinal cord. Rgs9-2 immunoreactivity is not detected in the dorsal root ganglia (Gold et al 1997, Psigfogeorgou et al 2011, Rahman et al 2003). Studies from various groups reveal a potent modulatory role of Rgs9-2 in drug addiction, opiate dependence and analgesia (Traynor et al., 2009). In addition, Rgs9-2 regulates the actions of antipsychotic and anti-Parkinsonian agents (Gold et al 2007, Traynor et al 2009). The mechanism of Rgs9-2 action involves the formation of complexes with several signal transduction and adaptor molecules. Interactions with these partner proteins determine the localization and stability of Rgs9-2. In particular, Rgs9-2 interacts with the adaptor molecule R7BP (Anderson, Semeov, Song and Martemyanov, 2007, Martemyanov, Yoo, Skiba and Arshavsky, 2005) which targets the protein near the cell membrane, whereas Rgs9-2 association with the Gβ5 subunit confers stability (Kimple et al 2011, Masuho, Wakasugi-Masuho, Posokhva, Patton and Martemyanov. 2011). Recent studies from our group demonstrated that Rgs9-2 plays either a positive or a negative modulatory role in mu opioid receptor (MOR) signaling, that is agonist dependent (Psifogeorgou et al 2007). Our more recent work also demonstrated that increased Rgs9-2 activity in the nucleus accumbens modulates opiate reward as well as opiate analgesia and tolerance (Gaspari et al., 2014).
Rgs9-2 expression in CNS regions associated with mood disorders (NAc) and nociceptive transmission (spinal cord), led us to hypothesize that this protein modulates chronic pain responses, as well as anxiety/depression-like behaviors observed under chronic pain conditions. Using the spared nerve injury (SNI) paradigm of neuropathic pain (Shields, Eckert, Lii and Basbaum, 2003), we show that knockout of the Rgs9 gene leads to a small delay in the development of maximal mechanical allodynia and thermal hyperalgesia, but does not have a marked effect on the intensity of these symptoms lasting beyond the first week after nerve injury. Neuropathic pain also predisposes humans to depression (Bair, Wu, Damush, Sutherlans and Kroeke 2008), and induces anxiety/depression-like behaviors in mice (Yalcin et al., 2011). Our findings from Rgs9KO and Rgs9WT mice in the open field and forced swim tests suggest that Rgs9-2 plays a protective role in anxiety and depression-like behaviors observed several weeks after nerve injury.
METHODS
Animals and treatments
Mice were kept on a 12h light/dark cycle, were group-housed (4 per cage) with food and water available ad libitum. Homozygous female Rgs9KO and Rgs9WT mice were used in all behavioral studies. For western blot analysis we used adult C57Bl/6 female mice (Jackson Laboratories). Animal handling and experiments were in accordance to the guidelines of the Institutional Animal Care and Use Committees of the University of Crete and Icahn Medical School at Mount Sinai.
Spared nerve injury model
The spared nerve injury (SNI) model of neuropathic pain was performed under Avertine (2,2,2-tribromoethanol, Aldrich) general anesthesia, as previously described (Shields et al 2003, Stratinaki et al 2013). Skin and muscle incision of the left hind-limp at mid-thigh level revealed the sciatic nerve and its three branches, with the help of a stereomicroscope. The common peroneal and the sural nerves were carefully ligated with 6.0 silk suture (Ethicon, Johnson & Johnson Intl.), transected and 1–2mm sections of these nerves were removed, while the tibial nerve was left intact. Skin was then closed with silk 4.0 sutures (Ethicon, Johnson & Johnson Intl). In sham-operated mice the same procedure was followed but the nerves were left intact.
Von Frey Response
The mechanical threshold for hindpaw withdrawal was determined using von Frey hairs with ascending forces expressed in grams (0.1 – 3.6gr, Electronic von Frey Anesthesiometer, IITC). Mice were single-placed in Plexiglas boxes on an elevated mesh screen. Each von Frey hair was applied on the plantar surface of mice hind-paws, 5 times per paw, and the mechanical threshold was defined as 3 or more withdrawals out of 5 trials (Benbouzid et al 2008, Stratinaki et al 2013).
Hargreaves Response
Thermal nociception was measured using the hind-paw withdrawal test (Plantar Analgesia Meter IITC, CA). Mice were single-placed in Plexiglas boxes atop a glass surface. A high-intensity heat lamp (40% intensity setting) was applied on the mid-plantar hindpaw of the injured limb and two measurements were obtained with a 2 min interval (Patwardhan, Scotland, Akopian, and Hargreaves 2009). The average of the two measurements was defined as the thermal nociceptive threshold. The cut-off time of 20 sec was used to avoid tissue damage.
Western blot analysis
Lumbar spinal cord from the ipsilateral side to the SNI and bilateral punches from NAc were rapidly dissected from sham or SNI-operated C57Bl/6 mice as previously described (Zachariou et al 2003). Samples were sonicated in 1% SDS buffer containing a protease inhibitor cocktail (Sigma) and phosphatase inhibitors (sodium orthovanadate, aprotinin-Sigma) 20–30 μl of protein per sample were loaded and separated on 8% or10% SDS-PAGE. We used a rabbit protein A-purified anti-RGS9 antibody (1:10,000, (Psifogeorgou et al 2007) a mouse G beta 5 antibody (1:20,000 W. Simonds, National Institute of Diabetes, Digestive and Kidney Diseases, Bethesda, MS) and a mouse anti-beta-tubulin antibody (1:40,000 Sigma). The specificity of the Rgs9-2 antiserum has been demonstrated by western blot analysis studies using striatal tissue from Rgs9WT and Rgs9KO mice (Psifogeorgou et al 2011).
Open Field
Mice were tested during 5 min in an open field arena (44 × 44 cm). A video tracking system (Ethovision 3.0, Noldus) was used to measure the time spent in the center (34 x 34 cm) middle and periphery of the test arena (Chaudhury et al 2013).
Forced swim test
For the forced swim test (FST), mice were transferred to the testing room one by one, just before the experiment. Mice were placed in individual glass cylinders (46cm height x 18cm diameter) containing water at room temperature (25°C) at a depth of 15cm. The session was videotaped for 6 minutes for offline analysis. Behavioral measures were taken by a blind experimenter of the “latency” (the time elapsed before the mouse is still for the first time for at least 5 sec) and the total “immobility” time (the state in which mice make only movements necessary for remaining afloat). Mice that were unable to keep their head above water were excluded (Stratinaki et al 2013).
Statistical Analysis
For all behavioral experiments we used two-way ANOVAs followed by Bonferroni post hoc test or t-test, as indicated in the Figure legends. For western blot analysis we used one-way ANOVAs followed by Dunnett’s post hoc test, or t-test, as indicated in Figure legends.
RESULTS
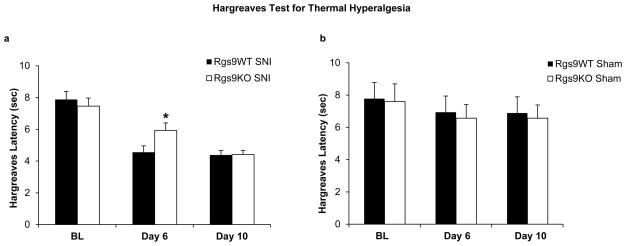
Our earlier work had shown that Rgs9-2 does not affect nociceptive behaviors associated with inflammatory pain, as formalin injection to the hindpaw elicited indistinguishable responses between Rgs9WT and Rgs9KO mice (Papachatzaki et al 2011). Here, we used the mouse SNI model to test the role of Rgs9-2 in symptoms of neuropathic pain. We first monitored thermal hyperalgesia and mechanical allodynia in different cohorts of Rgs9WT and Rgs9KO mice which had SNI surgery in the left hindlimb. Thermal hyperaglasia was monitored using the Hargreaves assay (Hargreaves, Dumner, Brown, Flores and Koris 1988). As shown in Fig. 1a, Rgs9KO mice reach maximal thermal hyperalgesia a few days later than their wildtype controls (day 6 for Rgs9WT mice versus day 10 for Rgs9KO mice), but both genotypes show similar Hargreaves responses by day 10 post SNI (baseline Hargreaves response Rgs9WT mice 7.88±0,5, baseline Hargeaves response Rgs9KO mice=7.44±0.5, day 6 Rgs9WT=4.56±0.4, Rgs9KO=5.93±0.4, day 10, Rgs9WT=4.38±0.3, Rgs9KO= 4.41±0.3). The onset of hyperalgesia is not affected by knockout of the Rgs9 gene. Sham operated controls from both genotypes show similar Hargreaves responses (Fig. 1b).
Figure 1. Thermal hyperalgesia in SNI and Sham operated Rgs9WT and Rgs9KO mice.
Rgs9KO mice exhibited a statistically significant delay in the development of maximal hyperalgesia behavior: Rgs9WT animals show maximal hyperalgesia by day 6, while Rgs9KO mice reach maximal hyperalgesia by day 10 (Fig. 1a). Figure 1b shows Hargreaves responses of control, sham-operated Rgs9WT and Rgs9KO mice. No differences in Hargreaves responses between genotypes were observed in the sham group. Data are expressed as means ± SEM, n=20–22 per genotype in SNI groups, n=7–8 per genotype in Sham groups *p<0.05 between genotypes for day 6.
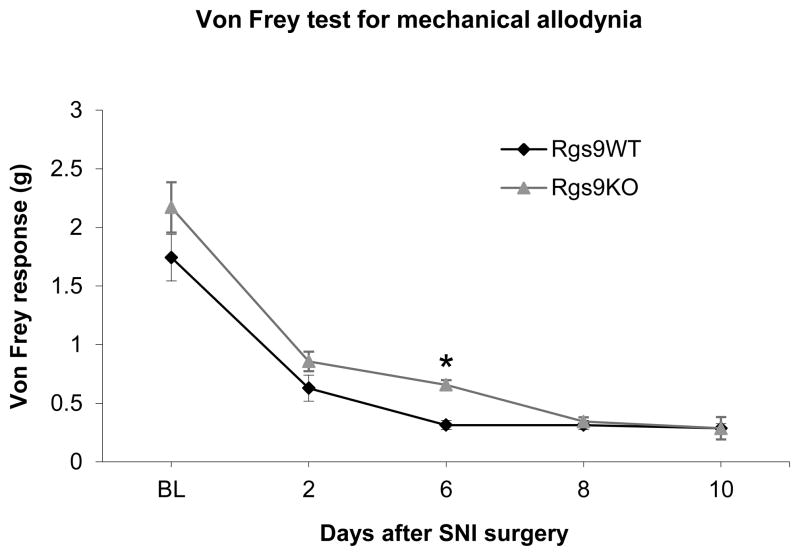
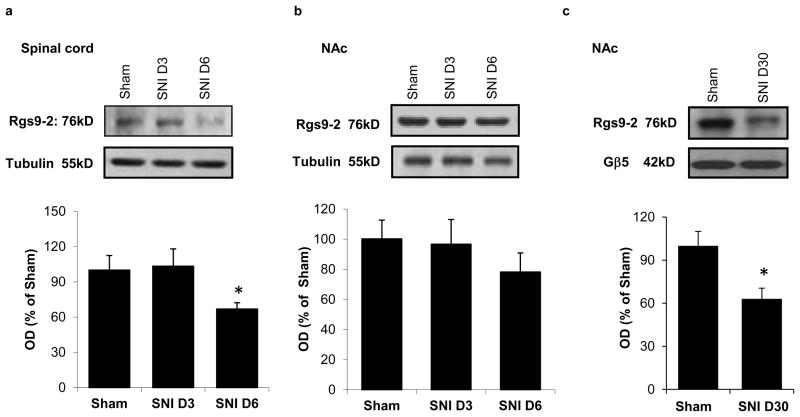
A similar delay was observed in the development of mechanical allodynia, as assessed by the Von Frey assay. As shown in Fig. 2, Rgs9WT mice develop maximal allodynia 6 days post SNI whereas Rgs9KO mice develop maximal allodynia 8 days post SNI. By day 10 both groups show similar Von Frey responses and this level of allodynia is maintained for several weeks (Von Frey response: day 6 Rgs9WT mice=0.31±0.04, day 6 Rgs9KO mice=0.65±0.09, day 10 Rgs9WT mice=0.28±0.04, day 10 Rgs9KO mice=0.28±0.05; day 30 Rgs9WT mice=0.33±0.04, day 30 Rgs9KO mice=0.28±0.05). No differences between genotypes are observed in the Von Frey assay between sham operated animals (Von Frey response on baseline: Rgs9WT mice=1.65±0.10, Rgs9KO mice=1.86±0,14, day 6: Rgs9WT mice =1.64±.19, Rg9KO mice=1.7±.21, Day 10: Rgs9WT mice=1.64±0.19, Rgs9KO mice=1.74±0.19). We next used western blot analysis to determine if neuropathic pain modulates Rgs9-2 expression in the spinal cord and the NAc. Our earlier western blot analysis studies demonstrated Rgs9-2 immunoreactivity levels that were very abundant in the striatum with low to moderate abundances in spinal cord (Terzi, Cao, Agrimaki, Martemyanov and Zachariou 2012). This pattern of expression was also observed using in situ hybridization analysis (Zachariou et al 2003). We found that 6 days post SNI, Rgs9-2 expression in the spinal cord is significantly reduced (OD for day 6=58.6±5% of control, Fig. 3a), with no change in Rgs9-2 expression in the NAc at this time point (Fig. 3b). In contrast, at the longer 30 day-post SNI time point, Rgs9-levels in the NAc are significantly decreased (63±7,5% of control, Fig. 3c).
Figure 2. Mechanical allodynia in SNI and Sham operated Rgs9WT and Rgs9KO mice.
Using the Von Frey assay we demonstrate that SNI induced mechanical allodynia in Rgs9WT and Rgs9KO mice. Rgs9KO mice exhibited a statistically significant delay in the development of maximal allodynia, as Rgs9WT mice reach maximal allodynia by day 6 and Rgs9KO mice reach maximal allodynia by day8 (n=7 per genotype, * p<0.05 between genotypes for day 6 of SNI).
Figure 3. Regulation of Rgs9-2 levels in the spinal cord and NAc by neuropathic pain.
Western blot analysis studies show that Rgs9-2 expression in the mouse ipsilateral lumbar spinal cord is significantly reduced six days after SNI surgery (Fig 3a). SNI has no significant effect on Rgs9-2 expression in the NAc 3 or 6 days post SNI (Fig 3b, n=3–5 per group, * p<0.05, *** p<0.001). One way ANOVA followed by Dunnett’s test). As shown in Fig. 3c Rgs9-2 levels are significantly reduced in the NAc at 30 days after SNI surgery (Data are expressed as means ± SEM, n=5–6 per group, *p<0.05, t-test).
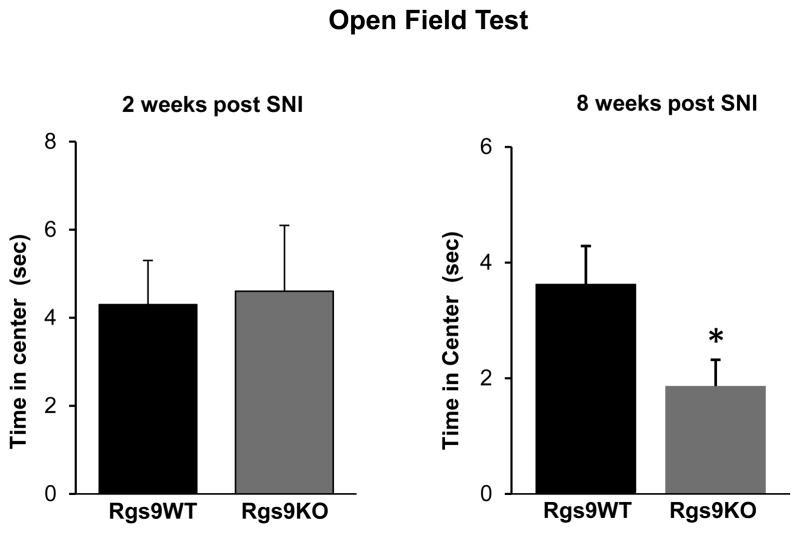
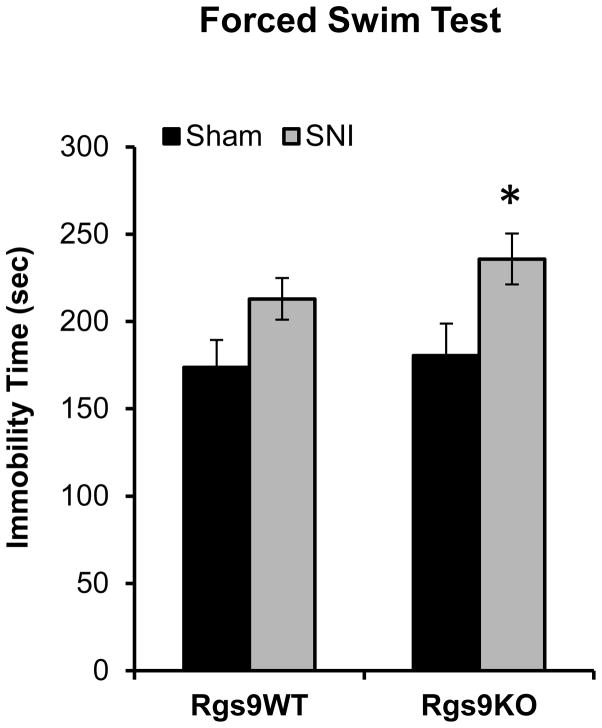
In the second series of experiments we again used the SNI model to test the role of the Rgs9 gene in anxiety and depression related behaviors that emerge from neuropathic pain. Specifically, we monitored responses of different cohorts of Rgs9WT and Rgs9KO mice, in the open field and forced swim tests at 2 and 8 weeks after SNI. As shown in Fig. 4, there is no significant difference between genotypes in the open field test 2 weeks after SNI. However, 8 weeks post-SNI, Rgs9KO mice show more anxiety-like behavior, with less time spent in the center of the open field arena, relative to their wildtype controls (Rgs9WT: time in periphery 254±4.6 sec, time in middle=41.6±4.2 sec, time in center=3.63±0.6 sec Rgs9KO mice: time in periphery 266±6 sec, time in middle 31.6±5.6, time in center=1.86±0.45). Based on the above findings on a role of Rgs9-2 in anxiety-like behaviors, we explored the role of Rgs9-2 in modulating despair-like behaviors that emerge several weeks after SNI surgery. We used the FST and monitored responses of Sham and SNI operated Rgs9WT and Rgs9KO mice, 8 weeks post SNI. Indeed, genetic ablation of the Rgs9 gene exacerbates depression-like behaviors, as mutant mice show increased immobility time in the Forced Swim test (immobility time (sec): Rgs9WT Sham=173±15.8, Rgs9WT SNI =213±11.9, Rgs9KO Sham =180±18, Rgs9KO SNI=235±14.6, Fig. 5).
Figure 4. Rgs9KO mice show more anxiety than their wild type controls 8 weeks after SNI.
We evaluated anxiety like behaviors of Rgs9WT and Rgs9KO mice using the open field test. Time spent in center of the open arena was monitored 2 and 8 weeks post SNI surgery. Rgs9KO mice behave similar to their Rgs9WT controls at 2 weeks after SNI surgery, but they show increased anxiety-like behavior at the 8 week time-point, spending less time in the center of the arena compared to their Rgs9WT controls. Data are expressed as means ± SEM. n=8–9 per genotype. *p<0.05, t-test
Figure 5. Rgs9KO mice show despair like behaviors at 8 weeks post SNI.
Sham and SNI operated Rgs9WT and Rgs9KO mice were tested in the Forced Swim assay 8 weeks post-surgery. While there is a trend for the wildtype SNI animals to show a higher immobility time compared to their sham operated controls, only Rgs9KO SNI mice show a significant increase in immobility compared to their sham operated controls. Data are expressed as means ± SEM, n=5–6 per genotype, *p<0.05 for Rgs9KO Sham versus Rgs9KO SNI, two way ANOVA followed by Bonferroni posthoc test.
DISCUSSION
Our findings suggest that Rgs9-2 exerts a transient and negative modulation of sensory components of neuropathic pain in the SNI model. Rgs9-2 does not appear to impact long term thermal hyperalgesia and mechanical allodynia responses. Our findings also indicate that Rgs9-2 modulates anxiety- and depression-like behaviors observed several weeks after induction of neuropathic pain.
Our previous work indicated that Rgs9KO mice show no deficits in nociceptive responses in acute thermal noxious stimuli (hot plate test, tail flick test, Zachariou et al 2003). More recent studies from our group indicate that Rgs9-2 does not affect nociceptive behaviors in the early (peripherally mediated) or late (centrally mediated) phases of the formalin model of inflammatory pain (Papachatzaki et al 2011). In this study we assessed the role of Rgs9-2 in symptoms of neuropathic pain deriving from injury of the sciatic nerve. SNI leads to thermal hyperalgesia, which is observed within a few days post-surgery. While both Rgs9WT and Rgs9KO mice develop similar hyperalgesia responses to radiant heat in the Hargreaves assay, the time course of hyperalgesia development in Rgs9 mutant mice is delayed: wildtype mice show maximal hyperalgesia by day 6, and mutant mice by day 10. These data suggest that Rgs9-2 actions promote intracellular adaptations associated with nerve injury, and contribute to the development of sensitized behaviors, such as thermal hyperalgesia. However, in our study the intensity but not the onset time of hyperalgesia is affected by Rgs9 knockout, and both genotypes develop similar maximal hyperalgesia responses at 10 days post SNI operation. This negative modulation of hyperalgesic responses might be a direct effect of Rgs9-2 on mu opioid receptor function, or an effect on the function of monoamine receptors or other GPCRs involved in nociceptive transmission, central sensitization and endogenous analgesia.
Our data also indicate that prevention of Rgs9-2 actions delay the development of maximal mechanical allodynia by 2–3 days. Thus, while mechanical allodynia is developed in both genotypes Rgs9KO mice show a lower degree of allodynia compared to Rgs9WT mice at 6 days post SNI. However by 8 days post SNI, both genotypes reach maximal allodynic responses in the Von Frey test. Our findings suggest that Rgs9-2 modulates sensory components of neuropathic pain at early time points post-SNI, but at later post-SNI time points this protein does not affect the severity of allodynia or thermal hyperalgesia. Rgs9-2 is expressed in high amounts in the striatum and in low to medium amounts in the spinal cord (Gold et al 1997, Zachariou et al 2003), and its expression levels have been shown to be dynamically modulated by opioid and psychoactive substances (Terzi, Stergiou, King, Zacahriou, 2009). Here, we use western blot analysis to test whether Rgs9-2 levels in the NAc and spinal cord are regulated by neuropathic pain-like conditions. Our data show that while Rgs9-2 levels are not altered in the mouse NAc at 3 or 6 days post SNI, there is a reduction in Rgs9-2 expression in the spinal cord at 6 days post SNI. Based on the behavioral phenotypes of the Rgs9KO mice, the spinal cord adaptations coincide with the time point of maximal hyperalgesia. Thus we speculate that Rgs9-2 positively modulates nociceptive information at early stages of neuropathic pain. In contrast, the delayed adaptations in Rgs9-2 expression in the NAc at later time points are more likely to be related to the mood and motivation-like behaviors observed several weeks post SNI.
Notably, a growing amount of literature links chronic pain conditions to adaptations in the mesolimbic pathway. For example, neuropathic pain induces changes in dopamine and norepinephrine release in the ventral striatum of mice (Taylor, Murphy, Evans and Cahill, 2014) and local electrical stimulation of the NAc or application of dopamine D2 receptor agonists reduce nociceptive responses (Sotres-Bayon, Torres-Lopez, Lopez-Avila, del Angel and Lellicer 2001, Taylor et al., 2014). Thus, it is possible that modulation of monoaminergic transmission in the NAc by Rgs9-2 (or other Rgs proteins) affect nociceptive responses. However, since our findings on a role of Rgs9-2 in the intensity of SNI induced tactile allodynia and thermal hyperalgesia relied in constitutive Rgs9KO mice, it is not possible to determine the exact contribution of NAc Rgs9-2 in these phenotypes. The regulation pattern suggests that Rgs9-2 actions in the spinal cord are more likely to be involved in the modulation of sensory symptoms, but further experiments are needed to determine the actions of Rgs9-2 in the NAc and the spinal cord under neuropathic pain conditions. Future work will use advanced genetic models to specifically target Rgs9-2 in the NAc or in the spinal cord, to understand the actions of this molecule in neuropathic pain symptoms.
The last part of this study examined the role of Rgs9-2 in affective symptoms of neuropathic pain. As indicated by numerous clinical studies, depression is frequently observed in patients suffering from neuropathic pain (Bair et al., 2008, Maletic & Raison 2009, Reid et al., 2011). In preclinical models, several studies have demonstrated the development of anxiety- and depression-like behaviors in mice within a few weeks after nerve injury (Narita et al,, 2006, Yalcin et al., 2011). Several brain structures, including the NAc, have been implicated in the affective components of chronic pain. In the striatum, chronic pain conditions are associated with decreased dopamine and norepinephrine release (Taylor et al., 2014). Conversely, pain relief has been correlated with increased dopamine release in the NAc (Xie et al., 2014). Until now, the temporal adaptations in signal transduction that occur in neurons of the mesolimbic pathway under chronic pain have not been characterized. Our findings show that at early stages of neuropathic pain, where both thermal hyperalgesia and mechanical allodynia are observed, Rgs9-2 protein expression in the NAc remains unaffected. However, Rgs9-2 levels in the NAc are significantly reduced at later time points which suggests that this decrease in Rgs9-2 activity is related to the development of anxiety and depression-like behaviors. Our behavioral findings from groups of Rgs9WT and Rgs9KO mice further support the hypothesis that inhibition of Rgs9-2 activity exacerbates anxiety- and depression-like behaviors several weeks after the induction of neuropathic pain. Future behavioral studies will use neuroanatomically targeted overexpression or inactivation methods, to evaluate the role of Rgs9-2 actions in anxiety, anhedonia and despair-like behaviors in naïve as well as in neuropathic pain suffering mice. Furthermore, future work will characterize the protein-protein interactions mediating Rgs9-2 actions in the NAc under neuropathic pain conditions.
In summary, our work demonstrates that Rgs9-2 modulates the intensity of thermal hyperalgesia and mechanical allodynia at early time points after nerve injury, and anxiety/depression like behaviors at several weeks after nerve injury. Neuropathic pain promotes changes in Rgs9-2 protein levels in the spinal cord and the NAc, suggesting that this molecule plays a physiological role in adaptive responses to nerve injury. Together, these findings provide important new information on the cellular mechanisms underlying symptoms of chronic neuropathic pain.
Acknowledgments
Supported by the Greek Secretariat of Research and Technology and the 7th EU Framework (Aristia 2012).
References
- Anderson GR, Semenov A, Song JH, Martemyanov KA. The membrane anchor R7BP controls the proteolytic stability of the striatal specific RGS protein, RGS9-2. The Journal of biological chemistry. 2007;282:4772–81. doi: 10.1074/jbc.M610518200. [DOI] [PubMed] [Google Scholar]
- Bair MJ, Wu J, Damush TM, Sutherland JM, Kroenke K. Association of Depression and Anxiety Alone and in Combination With Chronic Musculoskeletal Pain in Primary Care Patients. Psychosom Med. 2008;70:890–97. doi: 10.1097/PSY.0b013e318185c510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbouzid M, Pallage V, Rajalu M, Waltisperger E, Doridot S, et al. Sciatic nerve cuffing in mice: A model of sustained neuropathic pain. European Journal of Pain. 2008;12:591–99. doi: 10.1016/j.ejpain.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2013;493:532–6. doi: 10.1038/nature11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspari S, Papachatzaki MM, Koo JW, Carr FB, Tsimpanouli ME, et al. Nucleus Accumbens-Specific Interventions in RGS9-2 Activity Modulate Responses to Morphine. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold SJ, Hoang CV, Potts BW, Porras G, Pioli E, et al. RGS9-2 negatively modulates L-3,4-dihydroxyphenylalanine-induced dyskinesia in experimental Parkinson’s disease. J Neurosci. 2007;27:14338–48. doi: 10.1523/JNEUROSCI.4223-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold SJ, Ni YG, Dohlman HG, Nestler EJ. Regulators of G-Protein Signaling (RGS) Proteins: Region-Specific Expression of Nine Subtypes in Rat Brain. The Journal of Neuroscience. 1997;17:8024–37. doi: 10.1523/JNEUROSCI.17-20-08024.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hollinger S, Hepler JR. Cellular regulation of RGS proteins: modulators and integrators of G protein signaling. Pharmacol Rev. 2002;54:527–59. doi: 10.1124/pr.54.3.527. [DOI] [PubMed] [Google Scholar]
- Kimple AJ, Bosch DE, Giguère PM, Siderovski DP. Regulators of G-Protein Signaling and Their Gα Substrates: Promises and Challenges in Their Use as Drug Discovery Targets. Pharmacological Reviews. 2011;63:728–49. doi: 10.1124/pr.110.003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maletic V, Raison CL. Neurobiology of depression, fibromyalgia and neuropathic pain. Frontiers in bioscience. 2009;14:5291–338. doi: 10.2741/3598. [DOI] [PubMed] [Google Scholar]
- Martemyanov KA, Yoo PJ, Skiba NP, Arshavsky VY. R7BP, a Novel Neuronal Protein Interacting with RGS Proteins of the R7 Family. Journal of Biological Chemistry. 2005;280:5133–36. doi: 10.1074/jbc.C400596200. [DOI] [PubMed] [Google Scholar]
- Masuho I, Wakasugi-Masuho H, Posokhova EN, Patton JR, Martemyanov KA. Type 5 G protein beta subunit (Gbeta5) controls the interaction of regulator of G protein signaling 9 (RGS9) with membrane anchors. The Journal of biological chemistry. 2011;286:21806–13. doi: 10.1074/jbc.M111.241513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Kaneko C, Miyoshi K, Nagumo Y, Kuzumaki N, et al. Chronic pain induces anxiety with concomitant changes in opioidergic function in the amygdala. Neuropsychopharmacology. 2006;31:739–50. doi: 10.1038/sj.npp.1300858. [DOI] [PubMed] [Google Scholar]
- Papachatzaki MM, Antal Z, Terzi D, Szucs P, Zachariou V, Antal M. RGS9-2 modulates nociceptive behaviour and opioid-mediated synaptic transmission in the spinal dorsal horn. Neurosci Lett. 2011;501:31–4. doi: 10.1016/j.neulet.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwardhan AM, Scotland PE, Akopian AN, Hargreaves KM. Activation of TRPV1 in the spinal cord by oxidized linoleic acid metabolites contributes to inflammatory hyperalgesia. Proc Natl Acad Sci U S A. 2009;106:18820–4. doi: 10.1073/pnas.0905415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psifogeorgou K, Papakosta P, Russo SJ, Neve RL, Kardassis D, et al. RGS9-2 is a negative modulator of μ-opioid receptor function. Journal of Neurochemistry. 2007;103:617–25. doi: 10.1111/j.1471-4159.2007.04812.x. [DOI] [PubMed] [Google Scholar]
- Psifogeorgou K, Terzi D, Papachatzaki MM, Varidaki A, Ferguson D, et al. A unique role of RGS9-2 in the striatum as a positive or negative regulator of opiate analgesia. J Neurosci. 2011;31:5617–24. doi: 10.1523/JNEUROSCI.4146-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psigfogeorgou K, Terzi D, Papachatzaki MM, Varidaki A, Ferguson D, et al. A Unique Role of RGS9-2 in the Striatum as a Positive or Negative Regulator of Opiate Analgesia. The Journal of Neuroscience. 2011;31:5617–24. doi: 10.1523/JNEUROSCI.4146-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman Z, Schwarz J, Gold SJ, Zachariou V, Wein MN, et al. RGS9 Modulates Dopamine Signaling in the Basal Ganglia. Neuron. 2003;38:941–52. doi: 10.1016/s0896-6273(03)00321-0. [DOI] [PubMed] [Google Scholar]
- Reid KJ, Harker J, Bala MM, Truyers C, Kellen E, et al. Epidemiology of chronic non-cancer pain in Europe: narrative review of prevalence, pain treatments and pain impact. Curr Med Res Opin. 2011;27:449–62. doi: 10.1185/03007995.2010.545813. [DOI] [PubMed] [Google Scholar]
- Shields SD, Eckert WA, III, Basbaum AI. Spared nerve injury model of neuropathic pain in the mouse: a behavioral and anatomic analysis. The Journal of Pain. 2003;4:465–70. doi: 10.1067/s1526-5900(03)00781-8. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Torres-Lopez E, Lopez-Avila A, del Angel R, Pellicer F. Lesion and electrical stimulation of the ventral tegmental area modify persistent nociceptive behavior in the rat. Brain Res. 2001;898:342–9. doi: 10.1016/s0006-8993(01)02213-2. [DOI] [PubMed] [Google Scholar]
- Stratinaki M, Varidaki A, Mitsi V, Ghose S, Magida J, et al. Regulator of G protein signaling 4 is a crucial modulator of antidepressant drug action in depression and neuropathic pain models. Proceedings of the National Academy of Sciences. 2013;110:8254–59. doi: 10.1073/pnas.1214696110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AM, Murphy NP, Evans CJ, Cahill CM. Correlation Between Ventral Striatal Catecholamine Content and Nociceptive Thresholds in Neuropathic Mice. The journal of pain: official journal of the American Pain Society. 2014 doi: 10.1016/j.jpain.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzi D, Cao Y, Agrimaki I, Martemyanov KA, Zachariou V. R7BP Modulates Opiate Analgesia and Tolerance but not Withdrawal. Neuropsychopharmacology. 2012;37:1005–12. doi: 10.1038/npp.2011.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzi D, Stergiou E, King SL, Zachariou V. Regulators of G protein signaling in neuropsychiatric disorders. Progress in molecular biology and translational science. 2009;86:299–333. doi: 10.1016/S1877-1173(09)86010-9. [DOI] [PubMed] [Google Scholar]
- Traynor JR, Terzi D, Caldarone BJ, Zachariou V. RGS9-2: probing an intracellular modulator of behavior as a drug target. Trends in Pharmacological Sciences. 2009;30:105–11. doi: 10.1016/j.tips.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie JY, Qu C, Patwardhan A, Ossipov MH, Navratilova E, et al. Activation of mesocorticolimbic reward circuits for assessment of relief of ongoing pain: A potential biomarker of efficacy. Pain. 2014;155:1659–66. doi: 10.1016/j.pain.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalcin I, Bohren Y, Waltisperger E, Sage-Ciocca D, Yin JC, et al. A Time-Dependent History of Mood Disorders in a Murine Model of Neuropathic Pain. Biological Psychiatry. 2011;70:946–53. doi: 10.1016/j.biopsych.2011.07.017. [DOI] [PubMed] [Google Scholar]
- Zachariou V, Georgescu D, Sanchez N, Rahman Z, DiLeone R, et al. Essential role for RGS9 in opiate action. Proceedings of the National Academy of Sciences. 2003;100:13656–61. doi: 10.1073/pnas.2232594100. [DOI] [PMC free article] [PubMed] [Google Scholar]