Abstract
Follicular helper T cells (TFH cells) compose a heterogeneous subset of CD4+ T cells that induce the differentiation of B cells into plasma cells and memory cells. They are found within and in proximity to germinal centers in secondary lymphoid organs, and their memory compartment also circulates in the blood. Our knowledge on the biology of TFH cells has increased significantly during the past decade, largely as a result of mouse studies. However, recent studies on human TFH cells isolated from lymphoid organ and blood samples and recent observations on the developmental mechanism of human TFH cells have revealed both similarities and differences between human and mouse TFH cells. Here we present the similarities and differences between mouse and human lymphoid organ–resident TFH cells and discuss the role of TFH cells in response to vaccines and in disease pathogenesis.
A number of seminal discoveries made in mice and humans led to the description of B follicular helper T (TFH) cells in the early 2000s. The requirement of T cell help for the development of antibody responses was first described in the 1960s (ref. 1). CD4+ helper T cells (TH cells) were then found to be necessary for the development of germinal centers, discrete structures in secondary lymphoid organs where the selection of high-affinity B cells and the development of B cell memory occur2–4. In vitro studies in the 1980s, mostly involving CD4+ T cell clones and recombinant cytokines, showed that TH2 cells are the major TH subset engaged in helping B cells by secreting interleukin 4 (IL-4) and IL-10 (refs. 5,6). In mouse, TH1 cells also contribute to the regulation of antibody responses by inducing B cell class switching toward IgG2a. However, for almost two decades it was unclear how the TH1 and TH2 cells engaged in B cell help in lymphoid organs were biologically and developmentally distinct from those that exit lymphoid organs and migrate into peripheral tissues. The chemokine receptor CXCR5 was discovered in 1993 as a G protein–coupled receptor expressed primarily by B cells7, and in 1996 it was shown to be critical for the migration of B cells into follicles in lymphoid organs in mice8. In 1999, CD4+ T cells activated in lymphoid organs of immunized mice were found to express CXCR5, which was required for the cells’ migration into follicles9. In the early 2000s, studies on CD4+ T cells in human tonsils showed that cells expressing CXCR5 have a superior capacity to induce immunoglobulin production in B cells in vitro relative to CD4+ T cells lacking CXCR5 expression. On the basis of their localization and functions, tonsillar CXCR5+ CD4+ T cells were designated as TFH cells10–12. A similar CD4+ T cell subset was found in mouse lymph nodes13. Profiling of cytokine production and gene expression in human and mouse TFH cells showed that these cells are distinct from TH1 and TH2 cells14–16 and help B cells mainly by delivering activating signals with the TNF family molecule CD40L and the cytokine IL-21 (refs. 14,17–20). In 2009, the transcription repressor Bcl-6 was discovered to be an essential factor for TFH cell generation in vivo in mice21–23, and since then TFH cells have been recognized as an independent TH subset distinct from TH1, TH2 and TH17 cells.
Our knowledge of the biology of TFH cells has increased significantly during the past decade (reviewed in refs. 24,25). Like in other fields of immunology, important biological features of TFH cells have been learned of from studies in mouse models, whereas studies of the ontogeny and function of TFH cells in humans have remained relatively limited, mainly because of difficulties in investigating and manipulating TFH cells from human secondary lymphoid organs. Furthermore, there are only two main sources of human TFH cells for research: tonsils from children who have experienced recurrent throat infections but are otherwise healthy, and spleens, generally from cadaveric organ donors. This poses a challenge in investigations of human TFH cells’ association with human diseases such as cancer and autoimmunity. Over 60 million years of independent evolution have introduced significant differences in the immune systems of humans and mice. Thus, it is important to address whether conclusions drawn in mouse TFH studies also hold true for human TFH cells. Recent progress in our understanding of the biology of blood-circulating TFH cells in humans has provided clues on how to determine whether alteration of TFH responses contributes to human diseases. Furthermore, analyses of blood memory TFH cells (and also lymph node cells in some instances) from patients with primary or acquired immunodeficiencies have also provided important insights regarding the development and/or maintenance of TFH cells in humans. Together with in vitro studies aiming at determining the developmental mechanisms of human TFH cells, these studies have started revealing similarities as well as differences between humans and mice.
In this review, we first summarize our current knowledge of the biology of lymphoid organ TFH cells and discuss the similarities and differences between these cells in mice and humans. Then we discuss recent findings on the circulating memory compartment of TFH cells in human blood (hereinafter called blood memory TFH cells). Last, we summarize recent insights into the role of TFH cells in disease pathogenesis and discuss how TFH cells participate in or contribute to both beneficial and aberrant immune responses observed in various human diseases.
TFH subsets and dynamics in mice
Recent studies in mice and humans show that TFH lineage cells in lymphoid organs are composed of subsets that differ in their localization, phenotype and function. The circulating memory compartment of TFH cells in human blood also contains subsets that differ in phenotype and function.
Studies in mice have shown that after interaction with dendritic cells (DCs) in the T cell zones of secondary lymphoid organs, a fraction of activated CD4+ T cells migrate toward B cell follicles by upregulating the chemokine receptor CXCR5 (refs. 9,26) (mediated by increased expression of the transcription factors Bcl-6 (refs. 27,28) and Ascl2 (ref. 29)) while downregulating the chemokine receptor CCR7 (ref. 30) and the cell adhesion molecule PSGL-1 (ref. 31). These CXCR5+Bcl-6+CD4+ T cells (hereinafter called TFH precursors) interact with antigen-presenting B cells at the border of the B cell follicle and T cell zone9,32, a required process for the generation of germinal center (GC)-resident TFH cells (GC TFH cells) and the differentiation of primed B cells along both GC and extrafollicular pathways33. Bcl-6 expression in CD4+ T cells is a prerequisite for GC formation21–23. The origin of TFH cells is not restricted to naive cells, and there is some evidence suggesting that other TH subsets including TH1, TH2, TH17 and regulatory T (Treg) cells may become TFH cells in GCs (reviewed in ref. 25). This is consistent with the heterogeneity in cytokine expression patterns among GC TFH cells developed under different immunization protocols and by different types of infectious agents (reviewed in ref. 34).
In mice, GC TFH cells and TFH precursors have been defined largely on the basis of differences in cell surface markers, particularly the expression of CXCR5 and PD-1, which are more highly expressed in GC TFH cells than in their precursors outside GCs. During the acute or early phase following immunization, IL-21 is expressed almost exclusively by CXCR5hiPD-1hi GC TFH cells in lymphoid organs35. Investigation of TFH cells is, however, no longer restricted to their identification on the basis of phenotypic markers and cytokine secretion; it is now possible to visualize and track GC TFH cells directly with intravital imaging. Such imaging studies have revealed that GC TFH cells display unique cell dynamics: GC TFH cells continuously immigrate and redistribute to other follicles and neighboring GCs36, in a manner dependent on high expression of CXCR5 and the trafficking molecule sphingosine-1-phosphate receptor 2 (S1PR2)37. Signals derived from interacting B cells induce GC TFH cells to increase intracellular Ca2+ and their expression of IL-4 and IL-21, which enhance their capacity to promote the growth and differentiation of B cells38. A dynamic exchange of GC TFH cells among multiple GCs and bidirectional signals between GC TFH cells and B cells during their cognate interactions likely represent important mechanisms associated with the efficient selection and expansion of high-affinity B cells during GC responses. GC TFH cells exiting GCs rarely remain in the T cell zone or enter the bloodstream (at least while GC responses are actively in progress)36, suggesting that GC TFH cells and their precursors display different cell dynamics.
Mouse studies also identified cell subsets important for the suppression of GC response34. These suppressors contain Foxp3+ T follicular regulatory (TFR) cells39–41. Current evidence shows that, at least in mice, TFR cells are differentiated from thymus-derived Foxp3+ regulatory T cells. TFR cells dampen GC responses by limiting the numbers of both TFH and B cells in GCs39–41. Given that mice lacking functional TFR cells favor the accumulation of non-antigen-specific B cells40, TFR cells might be specialized in repressing self-reactive B cells in GCs. TFR cells are also likely to be responsible for terminating the GC response39. In either case, the balance between TFH and TFR cells in the GC environment likely represents a key factor in the generation of both high-affinity protective antibodies and pathogenic autoantibodies.
TFH subsets in human tonsils
Studies in the early 1980s showed that CD4+ T cells in human tonsillar GCs express CD57 (ref. 42) and that these CD57+ T cells display a limited ability to express IL-2 (ref. 43). Although early studies suggested that CD57 is a marker for functionally mature GC TFH cells in humans, CD57 is expressed by only approximately 30% of GC TFH cells, and it is also expressed by a fraction (~10%) of CD4+ T cells localized outside GCs15,18. TFH cells in human GCs are currently defined by their high expression of CXCR5, inducible costimulator (ICOS) and T cell inhibitory receptor PD-1 (refs. 15,18,44). Of note, ICOS is not a useful marker for defining GC TFH cells in mice, as ICOS expression is largely similar between GC TFH cells and TFH precursors36. In human tonsils, Foxp3+ TFR cells within GCs are much rarer than in mice (unpublished observations), and current knowledge on human TFR cells is very limited.
While Bcl-6 is well recognized as a transcription factor defining the TFH lineage, mouse studies show that GC TFH cells contain cells coexpressing Bcl-6 and T-bet (the transcription factor typically expressed by TH1 cells)35,45. In humans, GC TFH cells in tonsils also contain a subset coexpressing Bcl-6 and RORγt (the transcription factor typically expressed by TH17 cells), in addition to a subset coexpressing Bcl-6 and T-bet46. It remains to be established whether these GC TFH cell subsets and GC TFH cells lacking expression of T-bet or RORγt have distinct functions; nevertheless, this observation suggests that other TH subsets might be able to differentiate into TFH cells in humans, similar to cells in mice. An alternative explanation is that there are developmental paths that are shared between TH1 and TFH cells and between TH17 and TFH cells in humans, as also proposed in mice47,48.
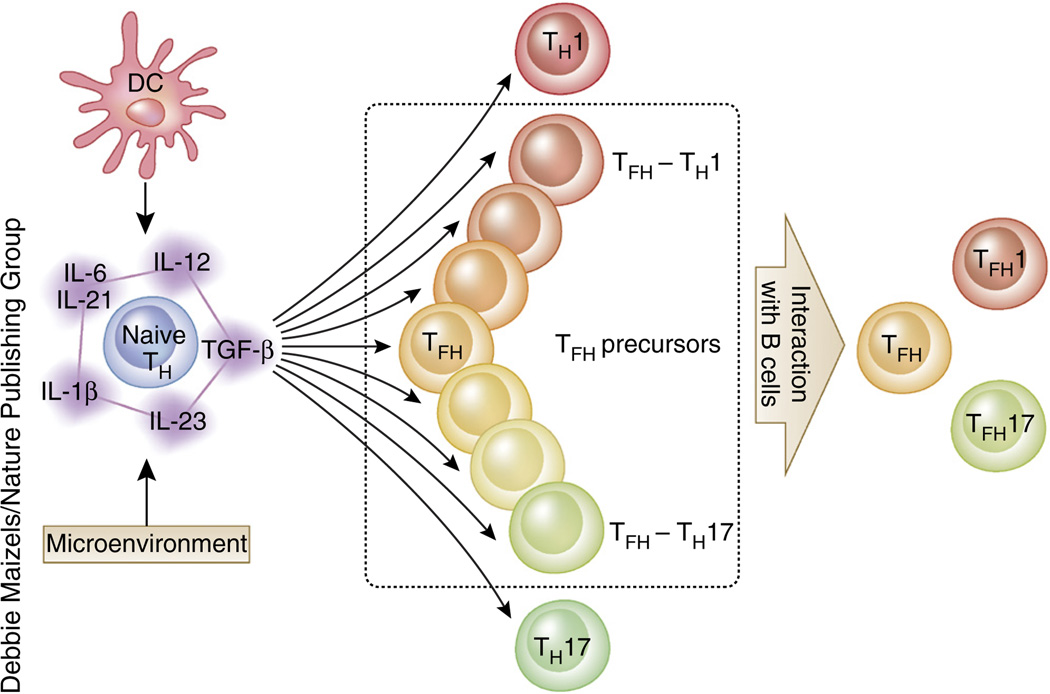
Recent data point to the possibility that the development of TFH cells might differ between mice and humans. In humans, the cytokine TGF-β acts with IL-12 and IL-23 to promote the expression of multiple TFH molecules, including CXCR5, IL-21 and Bcl-6 (ref. 46), on activated naive CD4+ T cells (Fig. 1). Furthermore, human CD4+ T cells cultured under conditions used to generate TH17 cells (for example, a combination of TGF-β, IL-23, IL-6 and IL-1β) coexpress TFH molecules and TH17 molecules46. This is in stark contrast to mouse CD4+ T cells, in which TGF-β signals suppress the expression of TFH molecules including IL-21, ICOS and Bcl-6 (refs. 21,46,49,50).
Figure 1.
Potential mechanism in the generation of human TFH subsets. As with other TH subsets, signals derived from antigen-presenting cells (including DCs) and the microenvironment instruct naive CD4+ T cells to differentiate into the TFH lineage. The major cytokines driving the early TFH differentiation process in humans are IL-12 and IL-23, and TGF-β synergizes with these cytokines. Other STAT3-activating cytokines, including IL-6, IL-21 and IL-1β, also support this process in the presence of IL-12, IL-23 and TGF-β. The differentiation of human naive CD4+ T cells is regulated by the balance of signals derived from these cytokines, and activated CD4+ T cells differentiate into precursors of variable TH subsets such as TH1, TH17 and TFH cells. Some TFH precursors share properties of TH1 and TH17 cells (dotted rectangle in middle panel); interactions with B cells promote their differentiation into mature TFH cells, including TFH1 and TFH17 cells. The mechanism associated with the generation of TFH2 cells is currently unknown.
In human tonsils, CD4+ T cells expressing low amounts of CXCR5 and ICOS (CXCR5loICOSlo cells) are exclusively localized outside GCs18. CXCR5loICOSloCD4+ T cells in tonsils appear to be TFH precursors (or extrafollicular helper T cells), as these cells express multiple TFH molecules such as CD40L, IL-21 and CXCL13 but lack the expression of Bcl-6 protein18. Functionally, isolated CXCR5loICOSloCD4+ T cells are more effective than TFH cells in vitro at helping naive B cells to become immunoglobulin-producing cells, possibly because they produce large amounts of IL-21. In contrast, GC TFH cells but not CXCR5loICOSloCD4+ T cells provide help to GC B cells and promote their survival, proliferation and differentiation into immunoglobulin-producing cells in vitro. The inability of CXCR5loICOSloCD4+ T cells to help GC B cells is due to their expression of Fas ligand, which induces the death of Fas-expressing GC B cells18. Thus, TFH cells at different maturation stages differ in their location and biological functions in human tonsils18. The observation that human TFH precursors help naive B cells is consistent with the presence of mouse TFH precursors with low expression of PD-1 and Bcl-6 at the T-B border shortly after T cell priming and before the induction of GCs33. These T cells likely induce the differentiation of B cells that have recently engaged their antigen receptors.
Memory TFH subsets in human blood
CD4+ T cells expressing the chemokine receptor CXCR5 were first described in human blood in 1994 (ref. 51) and were considered to represent recently activated T cells10,12,52. More recent studies indicate that blood CXCR5+CD4+ T cells contain long-lived memory cells that share functional properties with TFH cells53. Accordingly, blood CXCR5+CD4+ T cells are currently termed blood (or peripheral) memory TFH cells. Unlike GC TFH cells, blood memory TFH cells—even those expressing ICOS and the proliferation marker Ki67—do not express the Bcl-6 protein54–58, indicating that Bcl-6 is dispensable for their maintenance. The molecular mechanisms by which blood memory TFH cells maintain their TFH characteristics remain largely unknown and thus represent an important research topic.
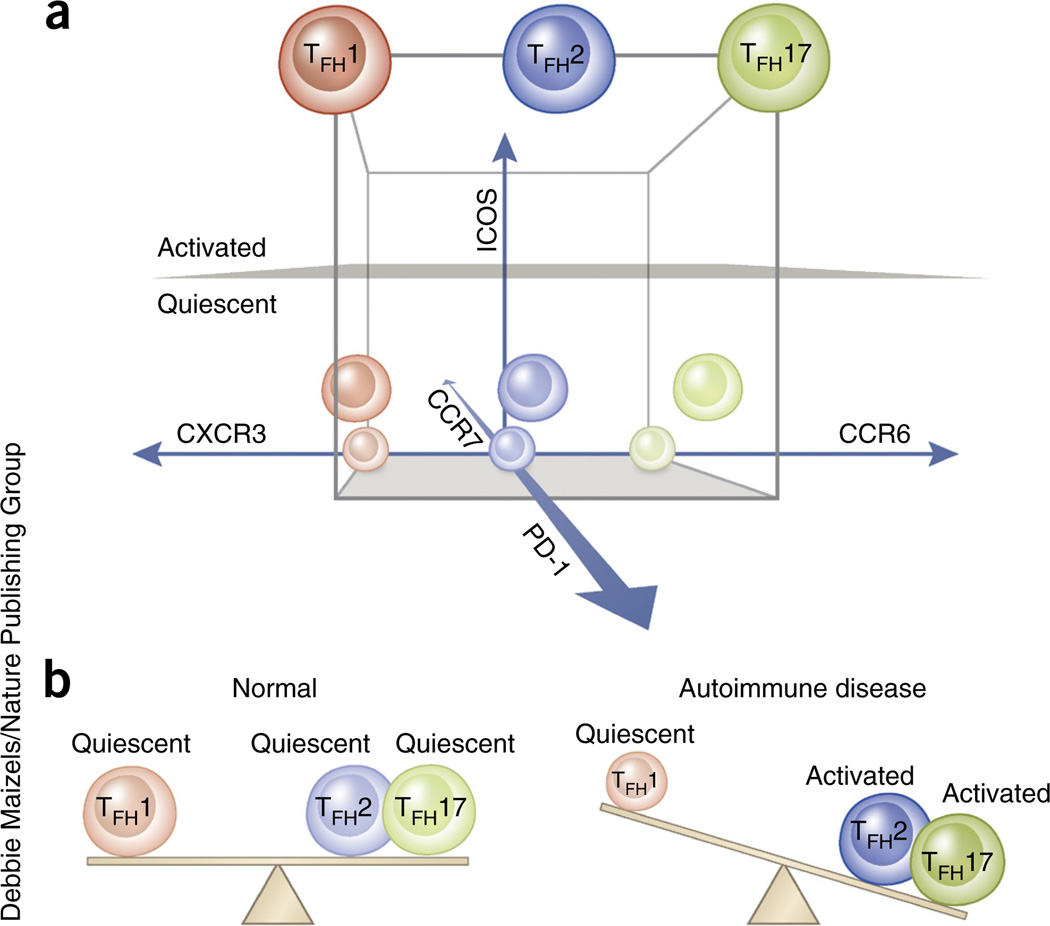
Human blood memory TFH cells actually include several populations with unique phenotypes and functions (see ref. 53 for a detailed review). Although staining strategies and markers selected to define blood TFH subsets differ among laboratories, we propose that blood memory TFH cells can be assessed on the basis of the following three sets of parameters: the presence of the chemokine receptors CXCR3 and CCR6; of the immunoregulatory molecule PD-1 and the chemokine receptor CCR7; and of the costimulatory molecule ICOS (Fig. 2a).
Figure 2.
Alteration of blood memory TFH subsets in human autoimmune diseases. (a) Three parameters—CXCR3 versus CCR6, PD-1 and CCR7, and ICOS—can subdivide human blood memory TFH cells (CD4+CD45RA−CXCR5+) into at least three TFH1, TFH2 and TFH17 subsets (nine TFH subsets in total). PD-1 and CCR7 define two quiescent subpopulations, PD-1−CCR7hi and PD-1+CCR7int, and ICOS defines the ICOS+PD-12+ activated population within the blood memory TFH1, TFH2 and TFH17 subsets. The nine blood memory TFH subsets are indicated in a three-dimensional scale. (b) TFH2 and TFH17 subsets represent efficient helpers among blood memory TFH subsets. In the blood of patients with active autoimmune disease (such as SLE, juvenile dermatomyositis, Sjögren’s syndrome or multiple sclerosis), the frequency of active (ICOS+PD-12+) TFH2 and TFH17 subsets increases, whereas the frequency of TFH1 subsets decreases. This alteration likely reflects the increase of functional TFH cells in lymphoid organs and/or inflamed tissues.
The first set of parameters defines three major subsets: CXCR3+CCR6− cells that share properties with TH1 cells (hereinafter called blood memory TFH1 cells), CXCR3−CCR6− cells resembling TH2 cells (hereinafter called blood memory TFH2 cells) and CXCR3−CCR6+ cells resembling TH17 cells (hereinafter called blood memory TFH17 cells)55. Experiments in which isolated T cell subsets were cultured with B cells in the presence of superantigen (such as staphylococcus enterotoxin B to induce cognate interactions between T and B cells) indicated that blood memory TFH2 and TFH17 cells can induce naive B cells to produce immunoglobulins and to switch isotypes through IL-21 secretion. In contrast, blood memory TFH1 cells lack the capacity to help naive B cells55,59,60. Furthermore, whereas blood memory TFH2 cells promote IgG and IgE secretion, blood memory TFH17 cells induce IgG and IgA secretion55. Thus, TFH2 and TFH17 cells represent efficient B cell helper cells with a distinct capacity to regulate immunoglobulin isotype switching.
The second (PD-1 and CCR7) and third (ICOS) sets of parameters define three other subsets: one activated subset (ICOS+PD-12+CCR7lo) and two quiescent subsets (ICOS−PD-1+CCR7int and ICOS−PD-1−CCR7hi). In healthy subjects ICOS expression is limited to a small population of blood TFH cells; this population substantially increases after vaccination, and thus defines activated memory TFH cells. In mice these cells appear before the formation of GC TFH cells, because deletion of the Sh2d1a gene, encoding the SAP, an adaptor protein essential for the functions of SLAM family receptors, does not affect the formation of this circulating subset but prevents the appearance of terminally differentiated GC TFH cells58. ICOS− cells are further divided into PD-1+ cells (~30% of blood memory TFH cells) and PD-1− cells, both of which lack the expression of Ki67 and thus are in a quiescent state58,59. CCR7 expression by blood memory TFH cells negatively correlates with that of PD-1 (refs. 52,53).
The matrix combination of the three parameters defines nine blood memory TFH subsets (Fig. 2a). The activated ICOS+PD-12+CCR7lo populations within blood memory TFH2 and TFH17 cells might represent the most efficient helpers. Among quiescent TFH2 and TFH17 subsets, the ICOS−PD-1+CCR7int population provides help to memory B cells more promptly than the ICOS−PD-1−CCR7hi population59,60, but the ICOS−PD-1+CCR7int and ICOS−PD-1−CCR7hi TFH2 and TFH17 subsets are equally capable of helping naive B cells.
In contrast, blood memory TFH1 cells lack the capacity to help naive or memory B cells54,55,59,60. Therefore, whether blood memory TFH1 cells represent a subset of memory TFH cells remains controversial. First, upon T cell receptor stimulation, blood memory TFH1 cells produce CXCL13, a chemokine that is highly expressed by GC TFH cells and whose receptor, CXCR5, is expressed at high levels by B cells18,61. Second, blood memory TFH1 cells contain antigen-specific memory cells59. Third, upon polyclonal stimulation with the phorbol ester PMA and ionomycin, blood memory TFH1 cells produce IL-21 in amounts equivalent to those of blood memory TFH2 and TFH17 cells59. Finally, influenza vaccination transiently induces ICOS expression exclusively on blood memory TFH1 cells54. The increase in ICOS+ TFH1 cells in blood is positively correlated with the generation of protective antibody responses54. Mechanistically, ICOS+ blood memory TFH1 cells help memory, but not naive, B cell differentiation into plasma cells via secretion of IL-21 and IL-1054. This observation suggests that blood memory TFH1 cells also contribute to antibody responses, but only when they become ICOS+PD-12+CCR7lo activated cells. Collectively, blood memory TFH cells can be subdivided into nonefficient helpers (TFH1) and efficient helpers (TFH2 and TFH17). The differential expression of ICOS, PD-1 and CCR7 further defines developmentally and functionally distinct subpopulations within the subsets.
TFH cells in primary immunodeficiencies
Primary immunodeficiencies are caused by defects in the expression of molecules involved in immune system development and/or function. Analyses of lymphoid tissue and blood samples from patients with primary immunodeficiencies have helped clarify the requirements for the development of normal immune responses in humans, including the generation of TFH cells and GCs. With the success of whole exome sequencing in identifying gene variants responsible for primary immunodeficiencies (at least 34 new gene defects were identified in the past 4 years62), investigation of immune responses in affected patients is likely to accelerate understanding of human immunity in the near future.
Patients with ICOS deficiency suffer from common variable immunodeficiency and show severely impaired GC formation in lymphoid tissues and severely decreased blood memory TFH cells, accompanied by a severe deficiency of memory B cells63. This demonstrates that ICOS is essential for the generation of TFH and GC responses in humans, as shown in mice64.
Multiple mouse models show that B cells play a fundamental role in the generation of GC TFH cells by interacting with TFH precursors25. This is also the case in humans, as patients with X-linked agammaglobulinemia, who lack mature B cells because of a deficiency of the tyrosine kinase Btk, and those with CVID, who have a significantly reduced number of B cells (<2% in lymphocytes), display significantly reduced frequencies of blood memory TFH cells65. Patients with hyper IgM syndrome caused by a deficiency of functional CD40L also show severely impaired GC formation, as well as reduced blood memory TFH cells63 and memory B cells66, which indicates the importance of CD40-CD40L interactions for GC and TFH development in humans, as shown in mice67.
SAP-deficient mice show profoundly altered GC reactions and generation of TFH cells68, as SAP is required for durable interactions between helper T cells and B cells, a process essential for the maturation of TFH cells69. Consistently, patients with X-linked lymphoproliferative disease caused by SAP deficiency show impaired formation of GCs and impaired development of memory B cells70. Interestingly, both SAP-deficient mice and human patients with X-linked lymphoproliferative disease show normal frequencies of blood memory TFH cells58, suggesting that at least a subset of circulating blood TFH cells develop before GC TFH formation. Furthermore, migration of GC TFH cells into the circulation is limited during the acute phase of the GC response36,38. It remains possible that GC TFH cells and their descendent memory TFH cells in lymphoid organs39,40 might start circulating during the termination of the GC response.
In vitro studies have suggested that IL-12 promotes antibody responses in humans via two mechanisms. IL-12 produced by DCs (CD14+ dermal DCs in human skin71) can induce the in vitro differentiation of CD40-activated naive B cells into IgM-producing plasma cells72,73 and of naive CD4+ T cells into TFH-like cells74,75. Consistently, children deficient for the IL-12 receptor β1 (IL-12Rβ1) chain, which is shared by the IL-12 and IL-23 receptors, have reduced TFH and GC responses74. Thus, in addition to the recent in vitro observation that TGF-β acts as a critical co-factor of IL-12 and IL-23 for the early TFH cell differentiation process in humans46, impaired TFH response in IL-12Rβ1 deficiency in vivo provides evidence that IL-12 and IL-23 contribute to the development of TFH responses in humans. Although the frequency of blood memory TFH cells is significantly reduced in subjects with IL-12Rβ1 deficiency during childhood (less than 10 years old), it gradually increases with age and becomes normal in adults. This indicates that the development and/or maintenance of the TFH cell response can be compensated through other pathways and/or cytokines, possibly IL-6, IL-21 and IL-27.
Mutations in the intracellular signaling molecule STAT3 cause most cases of hyper IgE syndrome. People with this syndrome are susceptible to a narrow spectrum of infections linked to defective TH17 responses and show altered antibody responses with reduced blood memory TFH cells76,77, indicating that STAT3 is required for optimal TFH cell generation in humans. Given that B cell responses to IL-10 and IL-21, cytokines delivering activation signals via STAT3, are severely altered in STAT3-deficient subjects78, the reduced number of blood memory TFH cells in STAT3-mutant subjects might be dependent on both B cell–intrinsic and T cell–intrinsic defects. Notably, upon exposure to IL-12, human STAT3 mutant naive CD4+ T cells are induced to express high amounts of several TFH molecules, such as CXCR5, ICOS and Bcl-6, despite lower expression of IL-21 (ref. 76). Thus, other molecules such as STAT4 might functionally compensate for the lack of STAT3. This concept is supported by the finding that STAT3 and STAT4 play largely redundant roles in the expression of TFH molecules by human naive CD4+ T cells exposed to TGF-β46.
TFH cells and autoimmunity
The generation of autoantibodies is a hallmark of autoimmune disease. Autoantibodies target a broad range of self-antigens, including nuclear components (such as double-stranded DNA), organ-specific antigens and soluble factors. These autoantibodies can profoundly dysregulate the function of multiple organs or systems through a variety of mechanisms. Autoantibodies result from a breakdown of tolerance mechanisms during B cell development79, from T cell–dependent or –independent B cell activation, or as a consequence of somatic mutation and rogue selection within GCs80,81. Yet GCs can in some cases redeem self-reactive B cells82. There is now compelling evidence in mice and in humans that aberrant generation and/or activation of TFH cells and extrafollicular helper T cells contributes to the pathogenesis of autoimmune diseases. Here we first describe evidence of aberrant TFH responses in mouse and human autoimmunity, and then discuss how genetic factors are potentially associated with aberrant TFH responses in human autoimmune diseases.
TFH cells in autoimmune mouse models
The first evidence linking aberrant TFH responses and autoimmunity came from studies of the sanroque mouse model, which carries a single-amino-acid mutation known as ‘san’ in the RNA-binding protein Roquin-1 (ref. 16). Roquinsan/san (or Rc3h1san/san) mice spontaneously develop lupus-like clinical symptoms and, in a genetically susceptible background, type 1 diabetes (T1D). The development of these diseases is accompanied by high amounts of antinuclear and anti-islet antibodies16,83. Mechanistically, the mutated Roquin in Roquinsan/san mice displays an impaired ability to repress the expression of Icos16 and Ifng84 in activated helper T cells and promotes the generation of TFH cells. Ox40 (also known as Tnfrsf4), a co-stimulatory molecule highly expressed by TFH cells25, and Tnf are additional targets of Roquin and its paralog Roquin-2 (refs. 85–87). TFH cells and myeloid cells accumulate and are overactive in Roquinsan/san mice and in mice doubly deficient in Roquin and Roquin-2 (refs. 86,87). Genetic ablation of Sh2d1a (encoding SAP) in Roquinsan/san mice prevents lupus pathology, whereas transfer of sanroque TFH cells into wild-type mice promotes spontaneous GC formation, supporting a causal role for TFH cell accumulation in the lupus-like disease88. These findings suggest that excessive TFH cells in GCs corrupt positive selection, such as diminished competition for T cell help, and a lower threshold for selection allows the emergence of self-reactive clones. Excessive expression of ICOS89 and, more prominently, of IFN-γ84 was found to be an important contributor to Roquinsan-mediated TFH cell accumulation. Of note, IFN-γ signaling blockade has been found not only to alleviate TFH and GC B cell accumulation but also to virtually prevent all clinical manifestations associated with Roquinsan-mediated disease, unlike SAP deficiency, which did not correct the splenomegaly or hypergammaglobulinemia88, or ICOS deficiency, which did not eliminate autoantibody formation84. Other mouse models have also revealed additional mechanisms that cause aberrant TFH responses in autoimmune disease. In the BXSB-Yaa mouse model, which displays a duplication of the Tlr7 gene, aberrant TFH responses and the development of glomerulonephritis are completely dependent on IL-21 signals90, in contrast to what occurs in Roquinsan/san mice, in which IL-21 signals are not essential for pathology91. The generation of autoantibodies in the MRL/lpr lupus mouse model, which is characterized by deficiency of the proapoptotic molecule Fas, is dependent on the T and B cell interactions at extrafollicular sites31. Extrafollicular TH cells that develop in MRL/lpr mice share features with GC TFH cells: their development is dependent on ICOS and Bcl-6, and their helper function is dependent on IL-21 and CD40L31. These studies demonstrate that inhibiting the generation of pathogenic TFH and extrafollicular helper T cells by blocking ICOS, CD40L, IFN-γ or IL-21 ameliorates the generation of autoantibodies and/or the development of glomerulonephritis, thereby providing a rationale for targeting these molecules for therapeutics.
Although T1D has long been thought to be a T cell–driven and organ-specific autoimmune disease, there is now evidence for a pathogenic role for both B cells and antibodies, as well as for TFH cell dysregulation, in T1D etiopathogenesis. B cells are required for diabetes development in the nonobese diabetic (NOD) mouse model of T1D92–94 and in the Roquinsan/san model83. Direct evidence for a pathogenic role of autoantibodies comes from experiments demonstrating protection from diabetes in NOD mice made deficient for activating Fcγ receptors95,96 and, conversely, diabetes induction upon passive transfer of antibodies against islet-expressed neo-self-antigens83,96. In line with these observations, it is not surprising that correlations are being described between TFH cells and autoimmune diabetes. In the Roquinsan/san model, aberrant TFH response was directly linked with the development of high anti-islet autoantibody titers and T1D83. TFH cells were increased in the pancreatic draining lymph nodes of mice that developed autoimmune diabetes83, and cells sharing characteristics of TFH cells, but expressing CCR9 instead of CXCR5, have been found in the pancreas of diabetic-prone NOD mice97.
TFH cells in human autoimmune diseases
Increased GC response in patients with systemic lupus erythematosus (SLE) was suggested in early studies by an increased frequency of CD27+CD38hi somatically mutated antibody-producing plasmablasts in peripheral blood98,99. Because SLE patients display higher frequencies of self-reactive mature naive B cells than healthy controls owing to a defect in the early checkpoint of B cell repertoires100,101, SLE patients seem predisposed to the development of a broader range of autoantibodies than healthy subjects. Whether and how TFH cells contribute to the pathogenesis of human autoimmunity has been unclear, but recent progress in understanding the biology of blood memory TFH cells has rendered the analysis of human TFH responses in the context of autoimmunity feasible.
Since the description of an association between the frequency of CXCR5+ICOS+ and/or CXCR5+PD1+ circulating TFH cells and the severity of SLE and Sjögren’s syndrome57, multiple studies have confirmed102 or refined these findings, providing improved phenotypic characterization (for example, a reproducible association with the CCR7loPD-1+/hi subset58), and extended them to additional autoimmune diseases such as myasthenia gravis103, rheumatoid arthritis104,105, autoimmune thyroid diseases106 and T1D107. An increase of ICOS+ blood memory TFH cells showed a positive correlation with serum autoantibody titers and disease activity and/or severity in these diseases57,58,104,106–108. These observations suggest that patients with active autoimmune disease display aberrant TFH responses; this can be monitored through assessment of the increase of activated blood memory TFH cells.
Accumulating evidence also indicates that patients with autoimmune disease display an alteration in the balance of blood memory TFH1, TFH2 and TFH17 cells. In patients with juvenile dermatomyositis55, adult SLE109 and Sjögren’s syndrome102, TFH1 cells are underrepresented among blood memory TFH cells, whereas TFH2 and/or TFH17 cells are overrepresented. Such alterations were found to correlate with disease activity, serum autoantibody titers and/or the frequency of blood plasmablasts55,102,109. Furthermore, although multiple sclerosis (MS) is not generally considered an autoantibody-mediated autoimmune disease, patients with MS also show the same alteration (low TFH1 and high TFH17) in the composition of blood memory TFH subsets110. Two additional lines of evidence also support a pathogenic role of TFH cells in MS. First, B cells are now thought to play a major pathogenic role in MS, as depletion of B cells with anti-CD20 significantly reduces the number of brain inflammatory lesions and halts the development of new lesions111. Second, ectopic B cell follicles are formed in the brain lesions of more than 40% of patients with secondary progressive MS, and the development of these structures correlates with disease severity112.
Collectively, although definitive evidence is yet to be found, these observations suggest that an increase in activated TFH2 and/or TFH17 cell subsets and a decrease of TFH1 cells within blood memory TFH cells might be common across multiple autoimmune diseases (Fig. 2b). Such alterations in blood memory TFH cells might reflect an overall increase of efficient helpers that promote the generation of antibodies in lymphoid organs and/or inflammatory sites in patients with autoimmune diseases. However, to date, studies linking human blood memory TFH cells and TFH cells in lymphoid organs and/or ectopic GCs in inflammatory tissues are lacking. TFH cell responses in ectopic GCs might be of considerable importance, as discussed below.
TFH cells in inflamed tissues
Inflammatory sites in autoimmune diseases often develop lymphoid cell aggregations including helper T cells and B cells, which leads to the formation of well-structured GCs (hereinafter called ectopic GCs). The mechanisms that control the initial development, cellular composition and functional maintenance of ectopic GCs seem to be largely shared with GCs in lymphoid organs113. For example, in lupus tubulointerstitial nephritis lesions, TH cells found in lymphoid T and B cell aggregates are phenotypically similar to TFH cells in lymphoid organs114. Although the precise mechanisms by which TFH cells accumulate in inflammatory sites in humans remain largely unknown, studies in mouse models suggest the involvement of TH17 cells113. In the experimental autoimmune encephalomyelitis mouse model, TH17 cells induce the formation of ectopic lymphoid follicles in inflammatory brain lesions via IL-17 and the cell surface molecule podoplanin and show features of TFH cells in these tertiary lymphoid structures115. Given that human patients with autoimmune diseases have increased activated memory TFH17 cells in blood (as discussed earlier), that early developmental pathways for TFH and TH17 cells in inflammatory environments are shared in humans46, and that in mice TH17-derived IL-17 directly promotes B cell differentiation into GC B cells, which are thought to be the source of pathogenic autoantibodies116, it is tempting to speculate that TFH17 cells might be involved in the formation of ectopic GCs in human autoimmune diseases.
Among patients with autoimmune disease, those with ectopic GCs in inflammatory lesions often have higher disease activity and are refractory to treatment. Yet the formation of ectopic GCs does not simply reflect the extent or the duration of inflammation in the lesions, as only 20% to 40% of patients with chronic inflammation develop ectopic GCs113. The formation of ectopic GCs is preceded by aggregates of T and B cells in inflammatory sites. Therefore, at variance with B cell follicles in lymphoid tissues, where autoreactive B cells are excluded because of their reduced expression of CXCR5 (ref. 117), lymphoid aggregates in inflammatory tissues are likely to be permissive to the entry of autoreactive B cells. Encounters with TFH-like cells at these sites114 might induce their cell growth and/or differentiation into antibody-producing cells. This possibility is directly supported by the observation that B cells in lymphoid aggregates and/or ectopic GCs produce autoantibodies113. Moreover, organ-specific self-antigens are easily accessible by antigen-presenting cells, including B cells, because of the abundance of damaged and/or apoptotic cells caused by the inflammatory process. Therefore, ectopic GCs might represent a major site of autoantibody production in autoimmune diseases, and thus a potentially good target for therapeutics. The nature of TFH cells in inflamed tissues requires further studies. Questions regarding subjects such as the developmental mechanism of TFH cells at inflammatory lesions and how lymphoid aggregates develop into ectopic GCs can be addressed in mouse models, but an effort to determine whether the observations apply to humans will be required. Another important question is whether activated blood memory TFH cells present in active autoimmune disease patients originate from inflammatory lesions. It will be critical to answer these questions in order to directly link the information obtained from the analysis of blood TFH subsets with autoimmune disease pathogenesis.
Genetics of altered TFH cell response
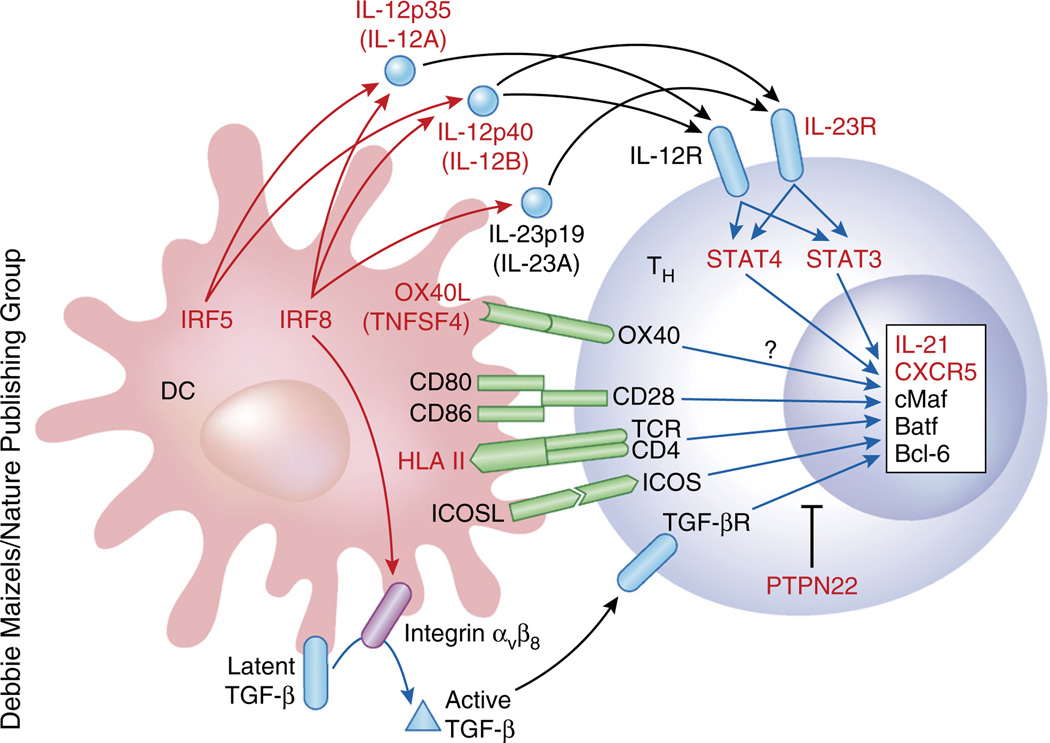
Autoimmune diseases result from complex interplay between genetic and environmental factors. Genome-wide association studies (GWAS) on multiple autoimmune diseases have yielded risk-associated loci118 (summary at http://www.genome.gov/gwastudies). Variants in HLA class II alleles are at the top of the list of susceptibility loci in most autoantibody-mediated autoimmune diseases, supporting a requirement of CD4+ helper T cells in disease pathogenesis. Importantly, multiple risk-loci identified in autoimmune diseases are potentially associated with the regulation of the development and/or the function of human TFH cells (Fig. 3). The list includes genes associated with IL-12 and IL-23, as well as cytokines implicated in human TFH cell differentiation44,46,74,75, such as IL12A, IL12B (both in multiple sclerosis), IL23R (rheumatoid arthritis), STAT3 (multiple sclerosis), STAT4 (SLE, rheumatoid arthritis, Sjögren’s syndrome), IRF5 (SLE, rheumatoid arthritis) and IRF8 (SLE, rheumatoid arthritis, multiple sclerosis). The transcription factors IRF5 and IRF8 positively regulate the production of IL-12 and IL-23 by macrophages and DCs119,120. IRF8 is essential for IL-12 production in humans. IRF8-deficient human subjects experience disseminated infection caused by bacille Calmette-Guérin vaccines, a clinical manifestation also seen in IL-12 receptor deficiency, due to a profound reduction of IL-12 production by monocytes119. IRF8 also contributes to signaling in TGF-β, another cytokine important for the generation of human TFH cells46, by promoting the expression of TGF-β-activating integrin αvβ8 on the surface of DCs121. Studies in SLE and Sjögren’s syndrome suggest that the OX40 ligand (OX40L)-coding TNFSF4 might be involved in the generation and/or homeostasis of TFH cells: overexpression of OX40L causes the accumulation of T cells in B cell follicles122, and OX40 receptor signals induce CD4+ T cells to express CXCR5 in both mice26,123,124 and humans (unpublished observations). Furthermore, GWAS identified multiple risk-loci encoding molecules expressed by TFH cells, including IL21 (SLE, rheumatoid arthritis) and CXCR5 (multiple sclerosis, Sjögren’s syndrome). A recent study showed that in addition to Blimp-1-encoding PRDM1 (ref. 22), PTPN22, a risk locus identified in GWAS of SLE, rheumatoid arthritis, myasthenia gravis and T1D, negatively regulates the generation of TFH and GC responses in mice125. However, whether and how most of these gene variants contribute to aberrant TFH responses in autoimmune diseases remains unknown. The majority of risk loci consist of single-nucleotide polymorphisms within noncoding, putatively regulatory DNA in the proximity of genes126, suggesting either that there is a disease-causing coding mutation in linkage disequilibrium with the GWAS-identified single-nucleotide polymorphism, or that alteration in pre- and/or post-transcriptional regulation is central to dysregulated immune responses in subjects with autoimmune disease traits. Recent studies aimed at mapping genetic variation contributing to transcriptional variation (termed expression quantitative trait locus mapping studies) through the use of purified immune cell subsets have started to reveal how gene variants regulate immune responses in different cell types126–128. The integration of expression quantitative trait locus mapping with blood memory TFH cells and/or TFH cells within inflammatory tissues and antigen-presenting cells obtained from patients with autoimmune disease might reveal how gene variants identified in GWAS contribute to the aberrant TFH and GC responses.
Figure 3.
Risk loci of human autoimmune diseases associated with the TFH developmental pathway. Multiple risk loci identified in GWAS in autoimmune diseases (indicated in red) are potentially associated with the regulation of the development and/or the function of human TFH cells. At least seven risk loci—IL12A, IL12B, IL23R, STAT3, STAT4, IRF5 and IRF8—are associated with IL-12 and IL-23. IRF8 also might contribute to TGF-β signaling by promoting the expression of TGF-β-activating integrin αvβ8 on the surface of DCs. Risk loci contain genes encoding TFH-specific molecules (such as IL21 and CXCR5), as well as genes associated with the inhibition of TFH cell development (such as PRDM1 and PTPN22). Whether and how these gene variants are associated with aberrant TFH responses in autoimmune diseases remains to be established.
TFH cells in cancer
Given the demonstrated mutual dependence of T and B cells for growth and survival, it is not surprising that TFH cells play important roles in supporting the growth and survival of follicular B cell tumors. Furthermore, TFH cells themselves can give rise to a peripheral T cell tumor known as angioimmunoblastic T cell lymphoma (AITL). Other peripheral T cell tumors also present with phenotypic features and genetic abnormalities that suggest a TFH origin. Recently, TFH cells were found to infiltrate solid-organ tumors, where they might play both protective and pathogenic roles.
AITL, which accounts for ~20% of peripheral T cell lymphomas (PTCLs), is an aggressive tumor associated with a poor survival rate (33% 5-year survival)129. Most patients present with systemic disease associated with lymphadenopathy, hepatosplenomegaly, anemia and hypergammaglobulinemia and suffer from systemic illness. The neoplastic T cells account for only a small fraction of the lymphoid infiltrate and are mixed with a large number of reactive immune cell types, including small lymphocytes, eosinophils and plasma cells, and expansion of follicular dendritic cell networks. Genetic profiling of AITL and immunohistochemical analysis of neoplastic T cells have provided strong evidence that TFH cells are the normal cellular counterpart of the neoplastic cells in AITL, which typically express BCL6, CD10 and other TFH markers, including CXCL13 and PD-1 (ref. 130). In mice, heterozygosity for a Roquin-1 mutation that causes T cell–autonomous TFH expansion also leads to an AITL-like disease131. These mutations have not been found in humans, but mutations in TET2, IDH2, DNMT3A and RHOA are commonly found in AITL132–134. Although the first three genes are known to play a role in DNA methylation and epigenetic modification of gene expression, how these mutations selectively affect TFH cells remains to be understood.
Up to 40% of other PTCLs (PTCLs not otherwise specified (NOS)) also express TFH markers, and some share genetic mutations typically found in AITL, including in TET2, IDH2 and RHOA. For this reason, these tumors are now being referred to as TFH-like PTCL-NOS135. The neoplastic T cells of various types of primary cutaneous cell lymphomas also express various TFH cell markers, with the exception of CD10, and in some cases also form rosettes with B cells, which typically occurs in AITL136–139.
Evidence for a pathogenic role of TFH cells in B cell lymphoma first came from studies demonstrating that the number of T cells infiltrating B cell tumors was an important predictor of outcome140. Follicular lymphoma, the most frequent indolent non-Hodgkin lymphoma, is thought to originate from GC B cells141. The gene expression profiles of tumor-infiltrating T cells and myeloid cells, rather than that of malignant B cells, determine the prognosis for follicular lymphoma142. Furthermore, that prognosis worsens if T cells are localized within neoplastic follicles143. Tumor-infiltrating T cells have the phenotypic features of TFH cells and overexpress IL-4 (ref. 144), TNF, IFN-γ and LT-α145. In particular, IL-4 produced by tumor-infiltrating TFH-like cells causes follicular lymphoma tumor cells to secrete the chemokines CCL17 and CCL22, which attract the migration of Treg cells and TH2 cells145. Foxp3+ T cells that resemble TFR cells described in mice are also present in neoplastic follicles and are expanded during lymphomagenesis146, although their significance is still unclear. While overall numbers of tumor-infiltrating Foxp3+ T cells may be associated with improved survival147, their follicular localization may be associated with worse survival and increased risk of transformation148, perhaps through the dampening of the cytotoxic T lymphocyte–driven anti-tumor response149. Recent reports have described increased circulating TFH-like cells in patients with chronic lymphocytic leukemia150,151, particularly during the more advanced stages. These expanded TFH cells may be of pathological relevance, given that the combination of IL-21 and CD40L induces robust chronic lymphocytic leukemia cell proliferation150,152.
In nonlymphoid malignancies, TFH cells are a component of the inflammatory infiltrate of breast cancer, and their presence, as assessed by an eight–TFH gene signature, was associated with increased survival153. Moreover, a single TFH-associated gene, CXCL13, conferred the dominant prognostic value. Extensively infiltrated tumors were found to contain tertiary lymphoid structures with visible GCs and CXCL13-producing TFH cells. These findings suggest that TFH cells, by virtue of their ability to secrete CXCL13 and organize ectopic lymphoid filtrates, may be important in coordinating the recruitment of immune cells that mediate the anti-tumor response.
In other tumor types, a high proportion and altered distribution of TFH cells has been reported in colorectal cancer154 and non–small cell lung cancer155. TFH cells infiltrate thymomas, correlating with the severity of myasthenia gravis156. A recent comprehensive gene analysis study on various immune cells isolated from human colorectal tumors revealed that the infiltration of B cells correlates with the infiltration of TFH cells at tumor sites157 and that the infiltration of B cells and TFH cells, which is associated with the expression of CXCL13 and IL21, positively correlates with patient survival. Whether and how TFH cells might promote the development of colorectal tumors remains to be established.
TFH cells in HIV and vaccine design
In the past couple of years an intriguing relationship was revealed between TFH cells and both human and simian immunodeficiency virus (HIV and SIV, respectively). HIV eliminates the circulating memory CD4+ T cells it infects, but several groups showed that TFH cells accumulate in lymph nodes during chronic SIV158,159 and HIV160–163 infection. Furthermore, TFH cells expand, despite old164–166 and new158,160 evidence that HIV infects TFH cells, in which viral replication occurs. Reports from the late 1980s demonstrated that follicular dendritic cell networks in GCs represent a large reservoir of HIV virions167, retained as immune complexes163. Thus, TFH cells are constantly exposed to the virus during chronic infection. In contrast to the TFH cell accumulation seen in lymph nodes, blood memory PD-1+ TFH17 cells capable of providing help to B cells are decreased in chronic HIV infection, but they recover after antiretroviral therapy60.
The relationships among viral load, chronicity and numbers of TFH cells are complex. Although most studies have failed to identify a correlation between lymph node TFH cell numbers and viral load158,159,161, there are possible explanations for the reduction in TFH cells after antiretroviral therapy160,162: the antigen load might control the total number of TFH cells, the compartmentalization of TFH cells between secondary lymphoid tissues and the circulation, or the survival of the blood memory TFH cells. Further work is clearly needed to explain the mechanisms behind these findings.
Chronic HIV infection is associated with an increased frequency of TFH cells in lymph nodes together with increased expression of Bcl-6 (ref. 158). The expression of IL-6 receptor on TFH cells is increased, which might mediate increased responsiveness to IL-6 (ref. 158). The importance of IL-6 signals for the maintenance of the TFH response in chronic infection is also highlighted in a mouse model of chronic viral infection with LCMV clone 13 (ref. 168). Increased TFH generation during chronic HIV infection may in turn affect the host’s immune response not only against HIV, but also against other, unrelated viral and bacterial infections169. High TFH numbers in HIV-infected individuals correlate with B cell dysregulation, including hypergammaglobulinemia, loss of memory B cells and, occasionally, production of autoantibodies and development of autoimmunity170. It is possible that the lowered threshold of B cell selection in GCs due to an excess of TFH cells171 might lead to the generation of antibodies with low affinities. Alternatively, TFH cell function might be dampened by either T cell–intrinsic factors (e.g., increased sensitivity to IL-6 signaling)158 or the microenvironment (e.g., increased PD-L1 expression by GC B cells that dampens IL-21 production by TFH cells162).
Designing potent HIV vaccines remains a major challenge. It is now established that antibodies can be protective and prevent infection, as passive transfer of broadly neutralizing antibodies confers protection to HIV challenge in humanized mice infected with HIV172,173. However, most vaccine trials have failed to protect vaccinated individuals to any significant degree. A number of factors associated with HIV make the creation of a decently protective antibody-based vaccine difficult, such as fast mutation rates, structural properties of the envelope complex that make conserved epitopes relatively inaccessible to antibodies, and the need for B cells to undergo extensive somatic mutation to generate broadly neutralizing antibodies. The recent HIV vaccine RV144 “Thai” trial showed that binding of IgG antibodies to envelope proteins is associated with protection, whereas binding of IgA antibodies to envelope proteins correlates directly with the rate of infection174. These data suggest a need to better understand the nature and isotype of the antibody response to be elicited by vaccines for protection. There is encouraging evidence that numbers of blood ICOS−PD1+ TFH2 and TFH17 cells (but not TFH1 cells) may serve as a useful biomarker for patients producing broadly neutralizing antibodies to HIV175. However, the significant overlap in measurements between high- and low-affinity groups calls for additional studies to further delineate the best correlates of protection. Although numerous studies have concluded that a potent TFH response correlates with antibody titers and protective responses54,58,59, there is also evidence that an excessive response might be deleterious. Thus, limiting of TFH cells is required for optimal affinity maturation176, and an excessive number of TFH cells lowers the threshold for positive selection, allowing survival of low-affinity and self-reactive clones88. Furthermore, the balance between TFH and TFR cells is also likely to be important for the overall duration and quality control of GC responses34. So far, very little is known about how different adjuvants and prime-boost regime strategies influence the magnitude, longevity and quality of antibody responses. This knowledge will be important for the improved and rational design of protective vaccines.
Conclusions
In the past 5 years considerable progress has been made in the understanding of TFH cells, particularly in humans. We predict that this progress will lead to improved vaccine designs, better management of autoimmune diseases and novel prognostic biomarkers for lymphoid and solid tumors.
ACKNOWLEDGMENTS
Supported by the US National Institutes of Health (U19-AI057234, U19-AI082715 and U19-AI089987), the Alliance for Lupus Research, the Baylor Health Care System (H.U.) and the Australian National Health and Medical Research Council (C.G.V.).
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Miller JF, Mitchell GF. Cell to cell interaction in the immune response. I. Hemolysin-forming cells in neonatally thymectomized mice reconstituted with thymus or thoracic duct lymphocytes. J. Exp. Med. 1968;128:801–820. doi: 10.1084/jem.128.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MacLennan IC. Germinal centers. Annu. Rev. Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 3.Allen CD, Okada T, Cyster JG. Germinal-center organization and cellular dynamics. Immunity. 2007;27:190–202. doi: 10.1016/j.immuni.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Victora GD, Nussenzweig MC. Germinal centers. Annu. Rev. Immunol. 2012;30:429–457. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau J, et al. The CD40 antigen and its ligand. Annu. Rev. Immunol. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- 6.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 7.Dobner T, Wolf I, Emrich T, Lipp M. Differentiation-specific expression of a novel G protein-coupled receptor from Burkitt’s lymphoma. Eur. J. Immunol. 1992;22:2795–2799. doi: 10.1002/eji.1830221107. [DOI] [PubMed] [Google Scholar]
- 8.Förster R, et al. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell. 1996;87:1037–1047. doi: 10.1016/s0092-8674(00)81798-5. [DOI] [PubMed] [Google Scholar]
- 9.Ansel KM, McHeyzer-Williams LJ, Ngo VN, McHeyzer-Williams MG, Cyster JG. In vivo-activated CD4 T cells upregulate CXC chemokine receptor 5 and reprogram their response to lymphoid chemokines. J. Exp. Med. 1999;190:1123–1134. doi: 10.1084/jem.190.8.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breitfeld D, et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J. Exp. Med. 2000;192:1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schaerli P, et al. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J. Exp. Med. 2000;192:1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim CH, et al. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. J. Exp. Med. 2001;193:1373–1381. doi: 10.1084/jem.193.12.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campbell DJ, Kim CH, Butcher EC. Separable effector T cell populations specialized for B cell help or tissue inflammation. Nat. Immunol. 2001;2:876–881. doi: 10.1038/ni0901-876. [DOI] [PubMed] [Google Scholar]
- 14.Chtanova T, et al. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J. Immunol. 2004;173:68–78. doi: 10.4049/jimmunol.173.1.68. [DOI] [PubMed] [Google Scholar]
- 15.Rasheed AU, Rahn HP, Sallusto F, Lipp M, Muller G. Follicular B helper T cell activity is confined to CXCR5(hi)ICOS(hi) CD4 T cells and is independent of CD57 expression. Eur. J. Immunol. 2006;36:1892–1903. doi: 10.1002/eji.200636136. [DOI] [PubMed] [Google Scholar]
- 16.Vinuesa CG, et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 2005;435:452–458. doi: 10.1038/nature03555. [DOI] [PubMed] [Google Scholar]
- 17.Bryant VL, et al. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J. Immunol. 2007;179:8180–8190. doi: 10.4049/jimmunol.179.12.8180. [DOI] [PubMed] [Google Scholar]
- 18.Bentebibel SE, Schmitt N, Banchereau J, Ueno H. Human tonsil B-cell lymphoma 6 (BCL6)-expressing CD4+ T-cell subset specialized for B-cell help outside germinal centers. Proc. Natl. Acad. Sci. USA. 2011;108:E488–E497. doi: 10.1073/pnas.1100898108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han S, et al. Cellular interaction in germinal centers. Roles of CD40 ligand and B7-2 in established germinal centers. J. Immunol. 1995;155:556–567. [PubMed] [Google Scholar]
- 20.Casamayor-Palleja M, Khan M, MacLennan IC. A subset of CD4+ memory T cells contains preformed CD40 ligand that is rapidly but transiently expressed on their surface after activation through the T cell receptor complex. J. Exp. Med. 1995;181:1293–1301. doi: 10.1084/jem.181.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nurieva RI, et al. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnston RJ, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu D, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31:457–468. doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Tangye SG, Ma CS, Brink R, Deenick EK. The good, the bad and the ugly—TFH cells in human health and disease. Nat. Rev. Immunol. 2013;13:412–426. doi: 10.1038/nri3447. [DOI] [PubMed] [Google Scholar]
- 25.Crotty S. Follicular helper CD4 T cells (TFH) Annu. Rev. Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 26.Flynn S, Toellner KM, Raykundalia C, Goodall M, Lane P. CD4 T cell cytokine differentiation: the B cell activation molecule, OX40 ligand, instructs CD4 T cells to express interleukin 4 and upregulates expression of the chemokine receptor, Blr-1. J. Exp. Med. 1998;188:297–304. doi: 10.1084/jem.188.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kerfoot SM, et al. Germinal center B cell and T follicular helper cell development initiates in the interfollicular zone. Immunity. 2011;34:947–960. doi: 10.1016/j.immuni.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kitano M, et al. Bcl6 protein expression shapes pre-germinal center B cell dynamics and follicular helper T cell heterogeneity. Immunity. 2011;34:961–972. doi: 10.1016/j.immuni.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 29.Liu X, et al. Transcription factor achaete-scute homologue 2 initiates follicular T-helper-cell development. Nature. 2014;507:513–518. doi: 10.1038/nature12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haynes NM, et al. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. J. Immunol. 2007;179:5099–5108. doi: 10.4049/jimmunol.179.8.5099. [DOI] [PubMed] [Google Scholar]
- 31.Odegard JM, et al. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. J. Exp. Med. 2008;205:2873–2886. doi: 10.1084/jem.20080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garside P, et al. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 1998;281:96–99. doi: 10.1126/science.281.5373.96. [DOI] [PubMed] [Google Scholar]
- 33.Lee SK, et al. B cell priming for extrafollicular antibody responses requires Bcl-6 expression by T cells. J. Exp. Med. 2011;208:1377–1388. doi: 10.1084/jem.20102065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramiscal RR, Vinuesa CG. T-cell subsets in the germinal center. Immunol. Rev. 2013;252:146–155. doi: 10.1111/imr.12031. [DOI] [PubMed] [Google Scholar]
- 35.Lüthje K, et al. The development and fate of follicular helper T cells defined by an IL-21 reporter mouse. Nat. Immunol. 2012;13:491–498. doi: 10.1038/ni.2261. [DOI] [PubMed] [Google Scholar]
- 36.Shulman Z, et al. T follicular helper cell dynamics in germinal centers. Science. 2013;341:673–677. doi: 10.1126/science.1241680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moriyama S, et al. Sphingosine-1-phosphate receptor 2 is critical for follicular helper T cell retention in germinal centers. J. Exp. Med. 2014;211:1297–1305. doi: 10.1084/jem.20131666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shulman Z, et al. Dynamic signaling by T follicular helper cells during germinal center B cell selection. Science. 2014;345:1058–1062. doi: 10.1126/science.1257861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wollenberg I, et al. Regulation of the germinal center reaction by Foxp3+ follicular regulatory T cells. J. Immunol. 2011;187:4553–4560. doi: 10.4049/jimmunol.1101328. [DOI] [PubMed] [Google Scholar]
- 40.Linterman MA, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat. Med. 2011;17:975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chung Y, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat. Med. 2011;17:983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Porwit-Ksiazek A, Ksiazek T, Biberfeld P. Leu 7+ (HNK-1+) cells. I. Selective compartmentalization of Leu 7+ cells with different immunophenotypes in lymphatic tissues and blood. Scand. J. Immunol. 1983;18:485–493. doi: 10.1111/j.1365-3083.1983.tb00882.x. [DOI] [PubMed] [Google Scholar]
- 43.Velardi A, Mingari MC, Moretta L, Grossi CE. Functional analysis of cloned germinal center CD4+ cells with natural killer cell-related features. Divergence from typical T helper cells. J. Immunol. 1986;137:2808–2813. [PubMed] [Google Scholar]
- 44.Ma CS, et al. Early commitment of naive human CD4(+) T cells to the T follicular helper (T(FH)) cell lineage is induced by IL-12. Immunol. Cell Biol. 2009;87:590–600. doi: 10.1038/icb.2009.64. [DOI] [PubMed] [Google Scholar]
- 45.Fazilleau N, McHeyzer-Williams LJ, Rosen H, McHeyzer-Williams MG. The function of follicular helper T cells is regulated by the strength of T cell antigen receptor binding. Nat. Immunol. 2009;10:375–384. doi: 10.1038/ni.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmitt N, et al. The cytokine TGF-β co-opts signaling via STAT3-STAT4 to promote the differentiation of human TFH cells. Nat. Immunol. 2014;15:856–865. doi: 10.1038/ni.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakayamada S, et al. Early Th1 cell differentiation is marked by a Tfh cell-like transition. Immunity. 2011;35:919–931. doi: 10.1016/j.immuni.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bauquet AT, et al. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat. Immunol. 2009;10:167–175. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nurieva RI, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suto A, et al. Development and characterization of IL-21-producing CD4+ T cells. J. Exp. Med. 2008;205:1369–1379. doi: 10.1084/jem.20072057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Förster R, Emrich T, Kremmer E, Lipp M. Expression of the G-protein–coupled receptor BLR1 defines mature, recirculating B cells and a subset of T-helper memory cells. Blood. 1994;84:830–840. [PubMed] [Google Scholar]
- 52.Schaerli P, Loetscher P, Moser B. Cutting edge: induction of follicular homing precedes effector Th cell development. J. Immunol. 2001;167:6082–6086. doi: 10.4049/jimmunol.167.11.6082. [DOI] [PubMed] [Google Scholar]
- 53.Schmitt N, Bentebibel SE, Ueno H. Phenotype and functions of memory Tfh cells in human blood. Trends Immunol. 2014;35:436–442. doi: 10.1016/j.it.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bentebibel SE, et al. Induction of ICOS+CXCR3+CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Sci. Transl. Med. 2013;5:176ra132. doi: 10.1126/scitranslmed.3005191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morita R, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34:108–121. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chevalier N, et al. CXCR5 expressing human central memory CD4 T cells and their relevance for humoral immune responses. J. Immunol. 2011;186:5556–5568. doi: 10.4049/jimmunol.1002828. [DOI] [PubMed] [Google Scholar]
- 57.Simpson N, et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 2010;62:234–244. doi: 10.1002/art.25032. [DOI] [PubMed] [Google Scholar]
- 58.He J, et al. Circulating precursor CCR7(lo)PD-1(hi) CXCR5(+) CD4(+) T cells indicate Tfh cell activity and promote antibody responses upon antigen reexposure. Immunity. 2013;39:770–781. doi: 10.1016/j.immuni.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 59.Locci M, et al. Human circulating PD-1(+)CXCR3(−)CXCR5(+) memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity. 2013;39:758–769. doi: 10.1016/j.immuni.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boswell KL, et al. Loss of circulating CD4 T cells with B cell helper function during chronic HIV infection. PLoS Pathog. 2014;10:e1003853. doi: 10.1371/journal.ppat.1003853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim CH, et al. Unique gene expression program of human germinal center T helper cells. Blood. 2004;104:1952–1960. doi: 10.1182/blood-2004-03-1206. [DOI] [PubMed] [Google Scholar]
- 62.Conley ME, Casanova JL. Discovery of single-gene inborn errors of immunity by next generation sequencing. Curr. Opin. Immunol. 2014;30:17–23. doi: 10.1016/j.coi.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bossaller L, et al. ICOS deficiency is associated with a severe reduction of CXCR5+CD4 germinal center Th cells. J. Immunol. 2006;177:4927–4932. doi: 10.4049/jimmunol.177.7.4927. [DOI] [PubMed] [Google Scholar]
- 64.Akiba H, et al. The role of ICOS in the CXCR5+ follicular B helper T cell maintenance in vivo. J. Immunol. 2005;175:2340–2348. doi: 10.4049/jimmunol.175.4.2340. [DOI] [PubMed] [Google Scholar]
- 65.Martini H, et al. Importance of B cell co-stimulation in CD4(+) T cell differentiation: X-linked agammaglobulinaemia, a human model. Clin. Exp. Immunol. 2011;164:381–387. doi: 10.1111/j.1365-2249.2011.04377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lougaris V, Badolato R, Ferrari S, Plebani A. Hyper immunoglobulin M syndrome due to CD40 deficiency: clinical, molecular, and immunological features. Immunol. Rev. 2005;203:48–66. doi: 10.1111/j.0105-2896.2005.00229.x. [DOI] [PubMed] [Google Scholar]
- 67.Kawabe T, et al. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1994;1:167–178. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 68.Crotty S, Kersh EN, Cannons J, Schwartzberg PL, Ahmed R. SAP is required for generating long-term humoral immunity. Nature. 2003;421:282–287. doi: 10.1038/nature01318. [DOI] [PubMed] [Google Scholar]
- 69.Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature. 2008;455:764–769. doi: 10.1038/nature07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cannons JL, Tangye SG, Schwartzberg PL. SLAM family receptors and SAP adaptors in immunity. Annu. Rev. Immunol. 2011;29:665–705. doi: 10.1146/annurev-immunol-030409-101302. [DOI] [PubMed] [Google Scholar]
- 71.Klechevsky E, et al. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29:497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Caux C, et al. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to granulocyte-macrophage colony-stimulating factor plus tumor necrosis factor alpha: II. Functional analysis. Blood. 1997;90:1458–1470. [PubMed] [Google Scholar]
- 73.Dubois B, et al. Critical role of IL-12 in dendritic cell-induced differentiation of naive B lymphocytes. J. Immunol. 1998;161:2223–2231. [PubMed] [Google Scholar]
- 74.Schmitt N, et al. IL-12 receptor beta1 deficiency alters in vivo T follicular helper cell response in humans. Blood. 2013;121:3375–3385. doi: 10.1182/blood-2012-08-448902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schmitt N, et al. Human dendritic cells induce the differentiation of interleukin-21-producing T follicular helper-like cells through interleukin-12. Immunity. 2009;31:158–169. doi: 10.1016/j.immuni.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ma CS, et al. Functional STAT3 deficiency compromises the generation of human T follicular helper cells. Blood. 2012;119:3997–4008. doi: 10.1182/blood-2011-11-392985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mazerolles F, Picard C, Kracker S, Fischer A, Durandy A. Blood CD4+CD45RO+CXCR5+ T cells are decreased but partially functional in signal transducer and activator of transcription 3 deficiency. J. Allergy Clin. Immunol. 2013;131:1146–1156. e1141–e1145. doi: 10.1016/j.jaci.2012.12.1519. [DOI] [PubMed] [Google Scholar]
- 78.Avery DT, et al. B cell-intrinsic signaling through IL-21 receptor and STAT3 is required for establishing long-lived antibody responses in humans. J. Exp. Med. 2010;207:155–171. S151–S155. doi: 10.1084/jem.20091706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yurasov S, Nussenzweig MC. Regulation of autoreactive antibodies. Curr. Opin. Rheumatol. 2007;19:421–426. doi: 10.1097/BOR.0b013e328277ef3b. [DOI] [PubMed] [Google Scholar]
- 80.Di Zenzo G, et al. Pemphigus autoantibodies generated through somatic mutations target the desmoglein-3 cis-interface. J. Clin. Invest. 2012;122:3781–3790. doi: 10.1172/JCI64413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vinuesa CG, Sanz I, Cook MC. Dysregulation of germinal centres in autoimmune disease. Nat. Rev. Immunol. 2009;9:845–857. doi: 10.1038/nri2637. [DOI] [PubMed] [Google Scholar]
- 82.Sabouri Z, et al. Redemption of autoantibodies on anergic B cells by variable-region glycosylation and mutation away from self-reactivity. Proc. Natl. Acad. Sci. USA. 2014;111:E2567–E2575. doi: 10.1073/pnas.1406974111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Silva DG, et al. Anti-islet autoantibodies trigger autoimmune diabetes in the presence of an increased frequency of islet-reactive CD4 T cells. Diabetes. 2011;60:2102–2111. doi: 10.2337/db10-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee SK, et al. Interferon-gamma excess leads to pathogenic accumulation of follicular helper T cells and germinal centers. Immunity. 2012;37:880–892. doi: 10.1016/j.immuni.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 85.Leppek K, et al. Roquin promotes constitutive mRNA decay via a conserved class of stem-loop recognition motifs. Cell. 2013;153:869–881. doi: 10.1016/j.cell.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 86.Pratama A, et al. Roquin-2 shares functions with its paralog Roquin-1 in the repression of mRNAs controlling T follicular helper cells and systemic inflammation. Immunity. 2013;38:669–680. doi: 10.1016/j.immuni.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 87.Vogel KU, et al. Roquin paralogs 1 and 2 redundantly repress the Icos and Ox40 costimulator mRNAs and control follicular helper T cell differentiation. Immunity. 2013;38:655–668. doi: 10.1016/j.immuni.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 88.Linterman MA, et al. Follicular helper T cells are required for systemic autoimmunity. J. Exp. Med. 2009;206:561–576. doi: 10.1084/jem.20081886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yu D, et al. Roquin represses autoimmunity by limiting inducible T-cell co-stimulator messenger RNA. Nature. 2007;450:299–303. doi: 10.1038/nature06253. [DOI] [PubMed] [Google Scholar]
- 90.Bubier JA, et al. A critical role for IL-21 receptor signaling in the pathogenesis of systemic lupus erythematosus in BXSB-Yaa mice. Proc. Natl. Acad. Sci. USA. 2009;106:1518–1523. doi: 10.1073/pnas.0807309106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Linterman MA, et al. Roquin differentiates the specialized functions of duplicated T cell costimulatory receptor genes CD28 and ICOS. Immunity. 2009;30:228–241. doi: 10.1016/j.immuni.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 92.Serreze DV, et al. B lymphocytes are essential for the initiation of T cell-mediated autoimmune diabetes: analysis of a new “speed congenic” stock of NOD.Ig mu null mice. J. Exp. Med. 1996;184:2049–2053. doi: 10.1084/jem.184.5.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Akashi T, et al. Direct evidence for the contribution of B cells to the progression of insulitis and the development of diabetes in non-obese diabetic mice. Int. Immunol. 1997;9:1159–1164. doi: 10.1093/intimm/9.8.1159. [DOI] [PubMed] [Google Scholar]
- 94.Noorchashm H, et al. B-cells are required for the initiation of insulitis and sialitis in nonobese diabetic mice. Diabetes. 1997;46:941–946. doi: 10.2337/diab.46.6.941. [DOI] [PubMed] [Google Scholar]
- 95.Inoue Y, et al. Activating Fc gamma receptors participate in the development of autoimmune diabetes in NOD mice. J. Immunol. 2007;179:764–774. doi: 10.4049/jimmunol.179.2.764. [DOI] [PubMed] [Google Scholar]
- 96.Harbers SO, et al. Antibody-enhanced cross-presentation of self antigen breaks T cell tolerance. J. Clin. Invest. 2007;117:1361–1369. doi: 10.1172/JCI29470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McGuire HM, et al. A subset of interleukin-21+ chemokine receptor CCR9+ T helper cells target accessory organs of the digestive system in autoimmunity. Immunity. 2011;34:602–615. doi: 10.1016/j.immuni.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 98.Arce E, et al. Increased frequency of pre-germinal center b cells and plasma cell precursors in the blood of children with systemic lupus erythematosus. J. Immunol. 2001;167:2361–2369. doi: 10.4049/jimmunol.167.4.2361. [DOI] [PubMed] [Google Scholar]
- 99.Jacobi AM, et al. Correlation between circulating CD27high plasma cells and disease activity in patients with systemic lupus erythematosus. Arthritis Rheum. 2003;48:1332–1342. doi: 10.1002/art.10949. [DOI] [PubMed] [Google Scholar]
- 100.Yurasov S, et al. Persistent expression of autoantibodies in SLE patients in remission. J. Exp. Med. 2006;203:2255–2261. doi: 10.1084/jem.20061446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yurasov S, et al. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J. Exp. Med. 2005;201:703–711. doi: 10.1084/jem.20042251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li XY, et al. Role of the frequency of blood CD4(+) CXCR5(+) CCR6(+) T cells in autoimmunity in patients with Sjogren’s syndrome. Biochem. Biophys. Res. Commun. 2012;422:238–244. doi: 10.1016/j.bbrc.2012.04.133. [DOI] [PubMed] [Google Scholar]
- 103.Luo C, et al. Expansion of circulating counterparts of follicular helper T cells in patients with myasthenia gravis. J. Neuroimmunol. 2013;256:55–61. doi: 10.1016/j.jneuroim.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 104.Wang J, et al. High frequencies of activated B cells and T follicular helper cells are correlated with disease activity in patients with new-onset rheumatoid arthritis. Clin. Exp. Immunol. 2013;174:212–220. doi: 10.1111/cei.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu R, et al. A regulatory effect of IL-21 on T follicular helper-like cell and B cell in rheumatoid arthritis. Arthritis Res. Ther. 2012;14:R255. doi: 10.1186/ar4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhu C, et al. Increased frequency of follicular helper T cells in patients with autoimmune thyroid disease. J. Clin. Endocrinol. Metab. 2012;97:943–950. doi: 10.1210/jc.2011-2003. [DOI] [PubMed] [Google Scholar]
- 107.Xu X, et al. Inhibition of increased circulating Tfh cell by anti-CD20 monoclonal antibody in patients with type 1 diabetes. PLoS ONE. 2013;8:e79858. doi: 10.1371/journal.pone.0079858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Luo C, et al. Expansion of circulating counterparts of follicular helper T cells in patients with myasthenia gravis. J. Neuroimmunol. 2013;256:55–61. doi: 10.1016/j.jneuroim.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 109.Le Coz C, et al. Circulating TFH subset distribution is strongly affected in lupus patients with an active disease. PLoS ONE. 2013;8:e75319. doi: 10.1371/journal.pone.0075319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Romme Christensen J, et al. Systemic inflammation in progressive multiple sclerosis involves follicular T-helper, Th17- and activated B-cells and correlates with progression. PLoS ONE. 2013;8:e57820. doi: 10.1371/journal.pone.0057820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hauser SL, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N. Engl. J. Med. 2008;358:676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 112.Magliozzi R, et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain. 2007;130:1089–1104. doi: 10.1093/brain/awm038. [DOI] [PubMed] [Google Scholar]
- 113.Pitzalis C, Jones GW, Bombardieri M, Jones SA. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat. Rev. Immunol. 2014;14:447–462. doi: 10.1038/nri3700. [DOI] [PubMed] [Google Scholar]
- 114.Liarski VM, et al. Cell distance mapping identifies functional T follicular helper cells in inflamed human renal tissue. Sci. Transl. Med. 2014;6:230ra246. doi: 10.1126/scitranslmed.3008146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Peters A, et al. Th17 cells induce ectopic lymphoid follicles in central nervous system tissue inflammation. Immunity. 2011;35:986–996. doi: 10.1016/j.immuni.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wu HJ, et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ekland EH, Forster R, Lipp M, Cyster JG. Requirements for follicular exclusion and competitive elimination of autoantigen-binding B cells. J. Immunol. 2004;172:4700–4708. doi: 10.4049/jimmunol.172.8.4700. [DOI] [PubMed] [Google Scholar]
- 118.Stranger BE, De Jager PL. Coordinating GWAS results with gene expression in a systems immunologic paradigm in autoimmunity. Curr. Opin. Immunol. 2012;24:544–551. doi: 10.1016/j.coi.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hambleton S, et al. IRF8 mutations and human dendritic-cell immunodeficiency. N. Engl. J. Med. 2011;365:127–138. doi: 10.1056/NEJMoa1100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Krausgruber T, et al. IRF5 promotes inflammatory macrophage polarization and TH1–TH17 responses. Nat. Immunol. 2011;12:231–238. doi: 10.1038/ni.1990. [DOI] [PubMed] [Google Scholar]
- 121.Yoshida Y, et al. The transcription factor IRF8 activates integrin-mediated TGF-beta signaling and promotes neuroinflammation. Immunity. 2014;40:187–198. doi: 10.1016/j.immuni.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Brocker T, et al. CD4 T cell traffic control: in vivo evidence that ligation of OX40 on CD4 T cells by OX40-ligand expressed on dendritic cells leads to the accumulation of CD4 T cells in B follicles. Eur. J. Immunol. 1999;29:1610–1616. doi: 10.1002/(SICI)1521-4141(199905)29:05<1610::AID-IMMU1610>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 123.Walker LS, et al. Compromised OX40 function in CD28-deficient mice is linked with failure to develop CXC chemokine receptor 5-positive CD4 cells and germinal centers. J. Exp. Med. 1999;190:1115–1122. doi: 10.1084/jem.190.8.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kim MY, et al. CD4(+)CD3(−) accessory cells costimulate primed CD4 T cells through OX40 and CD30 at sites where T cells collaborate with B cells. Immunity. 2003;18:643–654. doi: 10.1016/s1074-7613(03)00110-9. [DOI] [PubMed] [Google Scholar]
- 125.Maine CJ, Marquardt K, Cheung J, Sherman LA. PTPN22 controls the germinal center by influencing the numbers and activity of T follicular helper cells. J. Immunol. 2014;192:1415–1424. doi: 10.4049/jimmunol.1302418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Okada Y, et al. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature. 2014;506:376–381. doi: 10.1038/nature12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fairfax BP, et al. Innate immune activity conditions the effect of regulatory variants upon monocyte gene expression. Science. 2014;343:1246949. doi: 10.1126/science.1246949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Raj T, et al. Polarization of the effects of autoimmune and neurodegenerative risk alleles in leukocytes. Science. 2014;344:519–523. doi: 10.1126/science.1249547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Federico M, et al. Clinicopathologic characteristics of angioimmunoblastic T-cell lymphoma: analysis of the international peripheral T-cell lymphoma project. J. Clin. Oncol. 2013;31:240–246. doi: 10.1200/JCO.2011.37.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.de Leval L, Gisselbrecht C, Gaulard P. Advances in the understanding and management of angioimmunoblastic T-cell lymphoma. Br. J. Haematol. 2010;148:673–689. doi: 10.1111/j.1365-2141.2009.08003.x. [DOI] [PubMed] [Google Scholar]
- 131.Ellyard JI, et al. Heterozygosity for Roquinsan leads to angioimmunoblastic T-cell lymphoma-like tumors in mice. Blood. 2012;120:812–821. doi: 10.1182/blood-2011-07-365130. [DOI] [PubMed] [Google Scholar]
- 132.Sakata-Yanagimoto M, et al. Somatic RHOA mutation in angioimmunoblastic T cell lymphoma. Nat. Genet. 2014;46:171–175. doi: 10.1038/ng.2872. [DOI] [PubMed] [Google Scholar]
- 133.Odejide O, et al. A targeted mutational landscape of angioimmunoblastic T-cell lymphoma. Blood. 2014;123:1293–1296. doi: 10.1182/blood-2013-10-531509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yoo HY, et al. A recurrent inactivating mutation in RHOA GTPase in angioimmunoblastic T cell lymphoma. Nat. Genet. 2014;46:371–375. doi: 10.1038/ng.2916. [DOI] [PubMed] [Google Scholar]
- 135.Lemonnier F, et al. Recurrent TET2 mutations in peripheral T-cell lymphomas correlate with TFH-like features and adverse clinical parameters. Blood. 2012;120:1466–1469. doi: 10.1182/blood-2012-02-408542. [DOI] [PubMed] [Google Scholar]
- 136.Cetinözman F, Jansen PM, Willemze R. Expression of programmed death-1 in primary cutaneous CD4-positive small/medium-sized pleomorphic T-cell lymphoma, cutaneous pseudo-T-cell lymphoma, and other types of cutaneous T-cell lymphoma. Am. J. Surg. Pathol. 2012;36:109–116. doi: 10.1097/PAS.0b013e318230df87. [DOI] [PubMed] [Google Scholar]
- 137.Meyerson HJ, et al. Follicular center helper T-cell (TFH) marker positive mycosis fungoides/Sezary syndrome. Mod. Pathol. 2013;26:32–43. doi: 10.1038/modpathol.2012.124. [DOI] [PubMed] [Google Scholar]
- 138.Rodríguez Pinilla SM, et al. Primary cutaneous CD4+ small/medium-sized pleomorphic T-cell lymphoma expresses follicular T-cell markers. Am. J. Surg. Pathol. 2009;33:81–90. doi: 10.1097/PAS.0b013e31818e52fe. [DOI] [PubMed] [Google Scholar]
- 139.Park JH, Han JH, Kang HY, Lee ES, Kim YC. Expression of follicular helper T-cell markers in primary cutaneous T-cell lymphoma. Am. J. Dermatopathol. 2014;36:465–470. doi: 10.1097/DAD.0b013e3182a72f8c. [DOI] [PubMed] [Google Scholar]
- 140.Lee AM, et al. Number of CD4+ cells and location of forkhead box protein P3-positive cells in diagnostic follicular lymphoma tissue microarrays correlates with outcome. J. Clin. Oncol. 2006;24:5052–5059. doi: 10.1200/JCO.2006.06.4642. [DOI] [PubMed] [Google Scholar]
- 141.Shaffer AL, Rosenwald A, Staudt LM. Lymphoid malignancies: the dark side of B-cell differentiation. Nat. Rev. Immunol. 2002;2:920–932. doi: 10.1038/nri953. [DOI] [PubMed] [Google Scholar]
- 142.Dave SS, et al. Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. N. Engl. J. Med. 2004;351:2159–2169. doi: 10.1056/NEJMoa041869. [DOI] [PubMed] [Google Scholar]
- 143.Glas AM, et al. Gene-expression and immunohistochemical study of specific T-cell subsets and accessory cell types in the transformation and prognosis of follicular lymphoma. J. Clin. Oncol. 2007;25:390–398. doi: 10.1200/JCO.2006.06.1648. [DOI] [PubMed] [Google Scholar]
- 144.Pangault C, et al. Follicular lymphoma cell niche: identification of a preeminent IL-4-dependent T(FH)-B cell axis. Leukemia. 2010;24:2080–2089. doi: 10.1038/leu.2010.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rawal S, et al. Cross talk between follicular Th cells and tumor cells in human follicular lymphoma promotes immune evasion in the tumor microenvironment. J. Immunol. 2013;190:6681–6693. doi: 10.4049/jimmunol.1201363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Amé-Thomas P, et al. Characterization of intratumoral follicular helper T cells in follicular lymphoma: role in the survival of malignant B cells. Leukemia. 2012;26:1053–1063. doi: 10.1038/leu.2011.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Carreras J, et al. High numbers of tumor-infiltrating FOXP3-positive regulatory T cells are associated with improved overall survival in follicular lymphoma. Blood. 2006;108:2957–2964. doi: 10.1182/blood-2006-04-018218. [DOI] [PubMed] [Google Scholar]
- 148.Farinha P, et al. The architectural pattern of FOXP3-positive T cells in follicular lymphoma is an independent predictor of survival and histologic transformation. Blood. 2010;115:289–295. doi: 10.1182/blood-2009-07-235598. [DOI] [PubMed] [Google Scholar]
- 149.Yang ZZ, Novak AJ, Ziesmer SC, Witzig TE, Ansell SM. Attenuation of CD8(+) T-cell function by CD4(+)CD25(+) regulatory T cells in B-cell non-Hodgkin’s lymphoma. Cancer Res. 2006;66:10145–10152. doi: 10.1158/0008-5472.CAN-06-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ahearne MJ, et al. Enhancement of CD154/IL4 proliferation by the T follicular helper (Tfh) cytokine, IL21 and increased numbers of circulating cells resembling Tfh cells in chronic lymphocytic leukaemia. Br. J. Haematol. 2013;162:360–370. doi: 10.1111/bjh.12401. [DOI] [PubMed] [Google Scholar]
- 151.Cha Z, et al. Association of peripheral CD4+ CXCR5+ T cells with chronic lymphocytic leukemia. Tumour Biol. 2013;34:3579–3585. doi: 10.1007/s13277-013-0937-2. [DOI] [PubMed] [Google Scholar]
- 152.Pascutti MF, et al. IL-21 and CD40L signals from autologous T cells can induce antigen-independent proliferation of CLL cells. Blood. 2013;122:3010–3019. doi: 10.1182/blood-2012-11-467670. [DOI] [PubMed] [Google Scholar]
- 153.Gu-Trantien C, et al. CD4(+) follicular helper T cell infiltration predicts breast cancer survival. J. Clin. Invest. 2013;123:2873–2892. doi: 10.1172/JCI67428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang Z, et al. Circulating follicular helper T cells in Crohn’s disease (CD) and CD-associated colorectal cancer. Tumour Biol. 2014;35:9355–9359. doi: 10.1007/s13277-014-2208-2. [DOI] [PubMed] [Google Scholar]
- 155.Shi W, et al. Dysregulation of circulating follicular helper T cells in nonsmall cell lung cancer. DNA Cell Biol. 2014;33:355–360. doi: 10.1089/dna.2013.2332. [DOI] [PubMed] [Google Scholar]
- 156.Zhang M, et al. Thymic TFH cells involved in the pathogenesis of myasthenia gravis with thymoma. Exp. Neurol. 2014;254:200–205. doi: 10.1016/j.expneurol.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 157.Bindea G, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39:782–795. doi: 10.1016/j.immuni.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 158.Petrovas C, et al. CD4 T follicular helper cell dynamics during SIV infection. J. Clin. Invest. 2012;122:3281–3294. doi: 10.1172/JCI63039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hong JJ, Amancha PK, Rogers K, Ansari AA, Villinger F. Spatial alterations between CD4(+) T follicular helper, B, CD8(+) T cells during simian immunodeficiency virus infection: T/B cell homeostasis, activation, and potential mechanism for viral escape. J. Immunol. 2012;188:3247–3256. doi: 10.4049/jimmunol.1103138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Perreau M, et al. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J. Exp. Med. 2013;210:143–156. doi: 10.1084/jem.20121932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lindqvist M, et al. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. J. Clin. Invest. 2012;122:3271–3280. doi: 10.1172/JCI64314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cubas RA, et al. Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nat. Med. 2013;19:494–499. doi: 10.1038/nm.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Keele BF, et al. Characterization of the follicular dendritic cell reservoir of human immunodeficiency virus type 1. J. Virol. 2008;82:5548–5561. doi: 10.1128/JVI.00124-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tenner-Racz K, von Stemm AM, Guhlk B, Schmitz J, Racz P. Are follicular dendritic cells, macrophages and interdigitating cells of the lymphoid tissue productively infected by HIV? Res. Virol. 1994;145:177–182. doi: 10.1016/s0923-2516(07)80020-3. [DOI] [PubMed] [Google Scholar]
- 165.Haase AT. Population biology of HIV-1 infection: viral and CD4+ T cell demographics and dynamics in lymphatic tissues. Annu. Rev. Immunol. 1999;17:625–656. doi: 10.1146/annurev.immunol.17.1.625. [DOI] [PubMed] [Google Scholar]
- 166.Gratton S, Cheynier R, Dumaurier MJ, Oksenhendler E, Wain-Hobson S. Highly restricted spread of HIV-1 and multiply infected cells within splenic germinal centers. Proc. Natl. Acad. Sci. USA. 2000;97:14566–14571. doi: 10.1073/pnas.97.26.14566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ringler DJ, et al. Cellular localization of simian immunodeficiency virus in lymphoid tissues. I. Immunohistochemistry and electron microscopy. Am. J. Pathol. 1989;134:373–383. [PMC free article] [PubMed] [Google Scholar]
- 168.Harker JA, Lewis GM, Mack L, Zuniga EI. Late interleukin-6 escalates T follicular helper cell responses and controls a chronic viral infection. Science. 2011;334:825–829. doi: 10.1126/science.1208421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Vinuesa CG. HIV and T follicular helper cells: a dangerous relationship. J. Clin. Invest. 2012;122:3059–3062. doi: 10.1172/JCI65175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Moir S, Fauci AS. B cells in HIV infection and disease. Nat. Rev. Immunol. 2009;9:235–245. doi: 10.1038/nri2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Linterman MA, et al. Follicular helper T cells are required for systemic autoimmunity. J. Exp. Med. 2009;206:561–576. doi: 10.1084/jem.20081886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.West AP, Jr, et al. Structural insights on the role of antibodies in HIV-1 vaccine and therapy. Cell. 2014;156:633–648. doi: 10.1016/j.cell.2014.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ahlers JD. All eyes on the next generation of HIV vaccines: strategies for inducing a broadly neutralizing antibody response. Discov. Med. 2014;17:187–199. [PubMed] [Google Scholar]
- 174.Haynes BF, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N. Engl. J. Med. 2012;366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Locci M, et al. Human circulating PD-(+)1CXCR3(−)CXCR5(+) memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity. 2013;39:758–769. doi: 10.1016/j.immuni.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Victora GD, et al. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. 2010;143:592–605. doi: 10.1016/j.cell.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]