Abstract
Objective:
The purpose of this longitudinal study was to examine the prognostic value of subjective memory complaints in 156 cognitively intact community-dwelling older adults with a mean age of 83 years.
Methods:
Participants were assessed for subjective memory complaints, cognitive performance, functional status, and mood at annual evaluations with a mean follow-up of 4.5 years.
Results:
Subjective memory complaint at entry (n = 24) was not associated with impaired memory performance and did not predict memory decline or progression to incipient dementia. Memory complaints were inconsistent across examinations for 62% of participants who reported memory problems.
Conclusion:
Memory complaints by older adults are inconsistent over time. Memory complaints’ value as a research criterion for selecting people at risk of dementia is weak among community-dwelling older adults. Age, length of follow-up, and other population characteristics may affect the implication of self-reported memory problems.
Keywords: Subjective memory complaints, memory complaint, mild cognitive impairment, Alzheimer’s disease, preclinical dementia, cognitive aging, dementia
Introduction
Interest in subjective memory complaints as a possible indicator of impending dementia has escalated in recent years as research focus has shifted toward identifying at the earliest possible stage people who will develop Alzheimer’s disease (AD). The possibility that these complaints will prove a valuable clinical complement to other early detection methods derives from the finding that progressive episodic memory impairment is one of the earliest cognitive changes associated with most cases of AD.1 The attempt to identify impending dementia at its earliest clinical stage has led researchers to focus on patients with mild cognitive impairment (MCI), often a precursor to AD. Subjective memory complaint has been described as a stage prior to MCI in the eventual development of AD dementia.2,3 Many older adults who are aware of their own memory changes are worried about developing AD.
Recent studies of “pre-MCI” have focused on community samples because of the advantage of documenting the onset of the earliest cognitive decline compared to clinic patients who present with complaints but are often more cognitively impaired. The prevalence of subjective memory complaints in community-based studies of participants aged 65 years and older varies from approximately 25% to over 50%.4
The accuracy and reliability of older adults in reporting their memory abilities compared to age-appropriate expectations is unclear because published reports are mixed.4–6 It may be difficult to judge what is normal age-related decline as opposed to abnormal deterioration, even for health care professionals. A variety of factors can affect daily memory. Memory complaints may be influenced by psychological factors such as depression and anxiety,7 and cognitive variables such as information processing speed,8,9 attention and working memory,10 and executive functions.11 In addition, MCI patients, like Alzheimer patients, may have impaired awareness of cognitive deficits,12 which can result in under reporting of memory problems by some individuals.
The diagnostic criteria for MCI often include a subjective memory complaint;13–15 hence, establishing the predictive value of memory complaints has important research and clinical value. It could assist in targeting individuals most appropriate for Alzheimer prevention research and help older adults and their clinicians make more informed decisions about treatments or other management strategies.
When older adults with complaints about failing memory are recruited for research, what is the likelihood that this information suggests an impending dementia process? The purpose of this longitudinal study is to assess the significance and stability of subjective memory complaints over time in community volunteers 69 years and older who are cognitively intact at baseline. Based on previously published mixed results of the prognostic significance of memory complaints, we tested the hypothesis that during follow-up, participants with memory complaints at entry into the longitudinal study, compared to those without, will have more impaired memory on examination and will have an increased risk for MCI. We also examined whether subjective memory complaints are stable over time.
Methods
Participants
All participants were volunteers in the Intelligent Systems for Assessing Aging Changes (ISAAC) study and were community-dwelling seniors who agreed to participate in research related to the use of in-home technologies.16 Written consent for the study was obtained from each participant in accordance with requirements of the Institutional Review Board at the Oregon Health & Science University. Volunteers resided in retirement communities or free-standing, single-family homes and were functionally independent. Inclusion criteria included 69 years or older, English as a primary language, and average health for age, which included well-controlled, chronic medical conditions common with advanced age such as hypertension and coronary artery disease. The current sample is a subset of this cohort comprising 156 participants who were cognitively intact. None qualified for a diagnosis of MCI as described below.
Examinations
Annual examinations consisted of physical and neurological examinations, a battery of cognitive tests, and questionnaires and rating forms, including the Oregon Brain Aging Memory Questionnaire.17 Cognitive screening during the neurological examination consisted of the Mini-Mental State Examination (MMSE).18 The Hollingshead four-factor index was used to rate socioeconomic status (SES).19 Depression was accessed with the 15-item abbreviated Geriatric Depression Scale (GDS).20 Functional status also was rated by asking each participant’s informant, usually a family member, to answer the Functional Activity Questionnaire (FAQ).21 Health status was reported using the Cumulative Illness Rating Scale (CIRS).22,23 Higher scores represent more symptoms/problems for the GDS, FAQ, and CIRS. Apolipoprotein E (APOE)-epsilon4 allele presence or absence was measured via blood samples. Participants were unaware of their APOE status. They were followed for at least 3 years and up to 6 years.
Classification criteria
The diagnosis of MCI was based on comparison of participants’ performances on neuropsychological tests with normative data from healthy (control) older adults in our Layton Alzheimer’s Disease Center. Based on the model by Jak et al.,24 MCI was defined as scores ⩾1 standard deviation (SD) below age-appropriate normative data on two out of three tests within one or more of five cognitive domains: (1) memory: Wechsler Memory Scale–Revised (WMS-R) Logical Memory II Story A, WMS-R Visual Reproduction II, and Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Word-List Recall; (2) executive function: category fluency (animals), Trail Making Test–Part B, and Stroop color-word conflict; (3) processing speed: Wechsler Adult Intelligence Scale–Revised (WAIS-R) Digit Symbol, Trail Making Test–Part A, and Stroop color naming; (4) working memory: WMS-R Digits Backward, WAIS-III Letter-Number Sequencing or WAIS-IV Digit Sequencing, and MMSE item WORLD backward; and (5) visual perception/construction: WAIS-R Block Design, WAIS-R Picture Completion, and WMS-R Visual Reproduction I. Although memory complaint is often included as requirement for the diagnosis of MCI, it was not included in the diagnostic criteria for these analyses because it is the main predictor variable. Incipient dementia was defined as diagnosis of MCI on the participant’s last two annual evaluations.
Methods for assessing subjective memory complaints have varied across studies from a single question to more lengthy questionnaires or interviews. In the current study, memory complaint was determined by asking participants two questions. A positive complaint was defined as the subject endorsing both “My memory is fair, poor, or very poor” (as opposed to “good” or “excellent”) and “My memory has gotten worse in the past year,” criteria consistent with memory complaint criteria of the U.S. National Institute of on Aging and Alzheimer’s Association workgroup,13 the European Consortium on Alzheimer’s disease,14 and the International Working Group on Mild Cognitive Impairment.15 The participant’s informant was asked, “Has the individual’s memory gotten worse in the past year? Yes/No.” This information appears in Table 1, although it was not used for classification of a memory complaint.
Table 1.
Baseline demographic and clinical characteristics for participants with and without memory complaints expressed as means (standard deviations) or percentages.
| Baseline characteristics | No memory complaint, n = 132 | Memory complaint, n = 24 | p value |
|---|---|---|---|
| Age, years | 83.1 (5.5); [69–96] | 84.5 (4.5); [74–92] | 0.29 |
| Sex, % female | 81% | 67% | 0.11 |
| Race, % non-White | 16% | 13% | 0.67 |
| Education, years | 15.8 (2.3); [10–20] | 16.1 (2.7); [12–20] | 0.58 |
| SES | 51.7 (9.1); [26–66] | 51.8 (9.8); [34–66] | 0.90 |
| CIRS | 20.8 (3.0); [15–34] | 21.7 (3.0); [18–32] | 0.16 |
| APOE-epsilon4, % presencea | 21% | 43% | 0.02* |
| MMSE | 29.0 (1.2); [24–30] | 28.0 (1.7); [25–30] | 0.0047** |
| FAQ | 0.3 (1.2); [0–10] | 1.4 (2.2); [0–7] | 0.0031** |
| GDS | 0.8 (1.1) | 1.4 (1.6) | 0.0073** |
| Years of follow-up | 4.5 (0.9); [2–5] | 4.4 (1.0); [2–5] | 0.90 |
| Informant memory complaint | 19% | 35% | 0.10 |
| Progression to incipient dementia % | 13% | 21% | 0.34 |
CIRS: Cumulative Illness Rating Scale; APOE-epsilon4: apolipoprotein E-epsilon4; SES: socioeconomic status; MMSE: Mini-Mental State Examination; FAQ: Functional Activity Questionnaire; GDS: Geriatric Depression Scale.
Ranges are presented in brackets.
APOE-epsilon4 available for 23 with memory complaints and 123 with no complaint.
p < 0.05; **p < 0.01.
Statistical analysis
Demographic and cognitive performances at entry between groups with and without memory complaints were assessed using t-test or Wilcoxon rank-sum test for continuous variables and Pearson’s chi-square test or Fisher’s exact test for categorical variables as appropriate. Because of multiple comparisons a statistical level of p <.01 was adopted to protect against type 1 error. Longitudinal mixed-effects models were used to examine changes in domain-specific cognitive z-scores over time between groups (complainers and non-complainers at baseline) and the group × time interactions. Cox proportional hazard models were built to examine the likelihood of progression to incipient dementia according to baseline memory complaint. Differences between groups in time to progression were examined using Kaplan–Meier survival curves. All analyses were performed using SAS 9.2 software (SAS Institute, Inc., Cary, NC). Frequency data were used to assess the stability of cognitive status and memory complaints over time and to determine when memory complaints develop in relation to the onset of MCI classification.
Results
Participants
The mean age of the 156 cognitively intact participants at baseline was 83.3 years (SD = 5.4 years), and mean education was 15.8 years (SD = 2.4 years). The majority were women and Caucasian. Although all were cognitive intact at entry based on neuropsychological criteria, 24 (15%) had a memory complaint as defined above. With the exception of one person’s memory self-rating of “poor,” participants were classified as having a memory complaint based on rating their memory both as less than good and having gotten worse in the past year. We did not expect participants to report more than minor memory problems at baseline because they were selected from a larger research cohort to be those with age-expected memory performance on cognitive tests and daily functioning.
Group comparison
Baseline demographic and clinical characteristics for those with and without memory complaints are presented in Table 1. The groups did not differ in age, education, sex, race, education, SES, or in health measured with the CIRS. A higher percentage of the memory complaint group members were APOE-epsilon4+. Informants reported more problems with daily functioning via FAQ among participants with a memory complaint but not worsening memory over the previous year. Even though there are small group differences, scores on the MMSE, GDS, and FAQ were within normative non-impaired ranges for both groups. In our study, informant memory complaints were similar for both groups. On average, participants had 4.5 years of follow-up.
The groups were compared for their performance on neuropsychological tests at baseline (Table 2). Verbal memory differences between those with and without a memory complaint did not reach statistical significance. Slight group differences on measures of executive function were seen. No differences were observed on tests of processing speed and visual perception/construction. The only statistical difference between groups occurred on spelling “world” backward.
Table 2.
Baseline neuropsychological test scores for participants with and without memory complaint expressed as means (standard deviations).
| Test scores by cognitive domain | No memory complaint, n = 132 | Memory complaint, n = 24 | p valuea |
|---|---|---|---|
| Memory | |||
| Logical Memory Delayed Recall | 12.8 (3.7) | 11.3 (3.2) | 0.06 |
| Visual Reproduction IIb | 22.9 (9.6) | 18.5 (10.0) | 0.047* |
| CERAD Delayed Recall | 7.2 (1.8) | 6.5 (1.7) | 0.07 |
| Processing speed | |||
| Digit Symbol Test | 41.3 (9.1) | 39.8 (8.9) | 0.35 |
| Trail Making Test–Part A | 37.6 (12.6) | 39.3 (9.7) | 0.31 |
| Stroop Color Namingb | 61.4 (13.4) | 56.0 (15.3) | 0.08 |
| Working memory | |||
| Digit Span Backward | 4.7 (1.1) | 4.7 (1.0) | 0.93 |
| Letter-Number Sequencing | 8.8 (2.3) | 8.4 (1.8) | 0.27 |
| World Backward (MMSE item) | 4.9 (0.6) | 4.4 (1.2) | 0.0018** |
| Executive function | |||
| Trail Making Test–Part B | 104 (44) | 121 (42) | 0.04* |
| Stroop Color–Word Conflict | 30.9 (7.7) | 26.6 (8.0) | 0.04* |
| Category Fluency: Animals | 19.1 (4.9) | 16.6 (4.4) | 0.03* |
| Visual perception/construction | |||
| Block Design | 22.9 (7.3) | 22.5 (6.8) | 0.70 |
| Picture Completion | 13.6 (3.1) | 13.9 (2.0) | 0.80 |
| Visual Reproduction I | 30.2 (6.1) | 29.0 (5.8) | 0.29 |
CERAD: Consortium to Establish a Registry for Alzheimer’s Disease; MMSE: Mini-Mental State Examination.
Wilcoxon rank-sum non-parametric test unless noted otherwise.
Student’s t-test
p < 0.05; **p < 0.01.
There was no statistical group difference in the percentage of participants progressing to incipient dementia (see Table 1). For those who developed incipient dementia, the mean time from baseline to this classification was 2.0 years (SD = 1.3 years). Using the non-complainers as the reference group, the memory complainers’ hazard ratio of progression to incipient dementia was 1.8 (95% confidence interval (CI): 0.6–4.8, p = 0.27).
Each participant’s memory performance was calculated by taking an average of their z-scores on the three tests within the memory domain. A longitudinal mixed-effects model adjusted for age, sex, and education examined whether decline in memory domain z-scores over time differed between the groups with and without memory complaints at baseline. Although the baseline memory performance between groups (complainers vs not) was slightly different, the change in memory scores over time was not significantly different (see Table 3). Memory scores significantly declined in both groups over time.
Table 3.
Longitudinal mixed-effects model for participants with and without memory complaints at baseline with changes in the average of each person’s three memory domain z-scores as the dependent variable.
| Effect | Estimate | Standard error | Degrees freedom | t value | p value |
|---|---|---|---|---|---|
| Intercept | 3.56560 | 1.03950 | 152 | 3.43 | 0.0008 |
| Follow-up, years | −0.00025 | 0.00003 | 671 | −8.29 | <.0001 |
| Memory complaint | −0.34530 | 0.17130 | 671 | −2.02 | 0.0442 |
| Years from baseline × complaint | −0.00012 | 0.00008 | 671 | −1.53 | 0.1259 |
| Baseline age | −0.05502 | 0.01064 | 671 | −5.17 | <.0001 |
| Female | 0.32870 | 0.14680 | 671 | 2.24 | 0.0255 |
| Education, years | 0.04208 | 0.02497 | 671 | 1.68 | 0.0925 |
Stability of memory complaints
The presence or absence of subjective memory complaints at annual examinations was tallied for all but five subjects who had missing memory complaint data on at least one examination. A subjective memory complaint occurred on at least one examination for 72 (48%) participants (see Figure 1). Of those with memory complaints, the prevalence of complaints at annual visits increased over time. The percentage was lowest at baseline, as expected, and it nearly doubled by the fourth year (see Figure 2). Of these participants with memory complaints, roughly half (34% or 47%) had an operationally defined MCI on any follow-up examination. By contrast, only 18 (23%) subjects who never had a memory complaint had at least one MCI classification. For the 34 participants with both memory complaints and a classification of MCI, the memory complaint preceded the classification of MCI for 21 (62%) participants.
Figure 1.
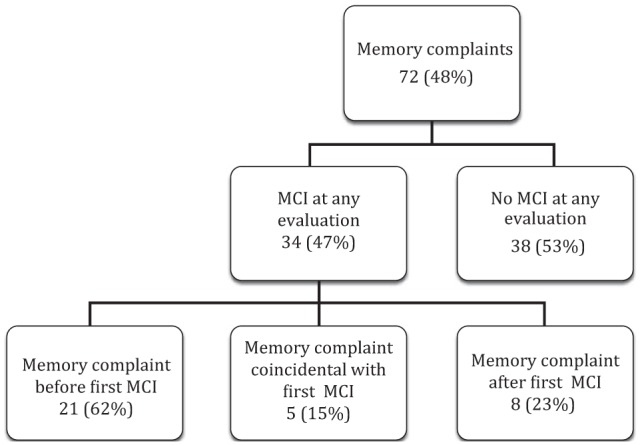
Percent of subjects with memory complaints on at least one evaluation during follow-up and whether they also had a MCI diagnosis.
Figure 2.
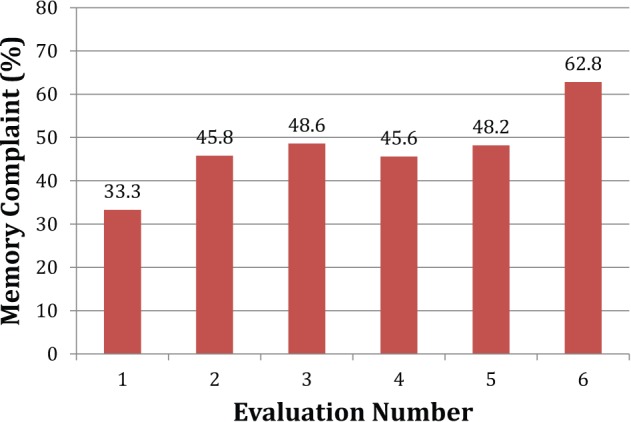
Prevalence of memory complaints over time by participants with memory complaints on at least one annual examination.
Memory complaints were inconsistent across annual visits for many of these community-dwelling participants. That is, the evaluation in which the participant endorsed the two memory complaint questions was followed by at least one subsequent annual evaluation in which the person did not endorse both of these questions. Of the 52 subjects, 20 (38%) who were classified as MCI on any evaluation inconsistently reported memory problems over time, while 24% of the 99 subjects who remained cognitively intact had memory reporting inconsistencies. MCI was inconsistent over time in 35% of participants with MCI.
Discussion
Our baseline results are similar to previous studies of community-dwelling older adults showing a weak, if any, relationship between subjective memory complaints and impaired memory performance.25 In our study, differences on memory tests between groups with and without memory complaints were short of statistical significances, and decline in memory scores during follow-up did not differ between groups. The relatively low prevalence of memory complaints at baseline in our sample likely is related to the low level of depression of participants. Numerous studies have shown a relationship between subjective memory complaints and psychological factors, particularly depression and anxiety 4,5,7,11,26,27 and poor sleep.28 Also, high level of education4 and possible selection bias associated with volunteering for an in-home technology study also may have contributed to the low baseline rate.
It has been established that informant-based memory complaints often are more accurate than self-reports,7,29 although in one large study, they did not occur as early as self reports.30 We did not find informants’ reports more predictive than self-reports.
In this study, the percent of people progressing to incipient dementia was higher, but not statistically different, in the memory complaint group than in the no-complaint group. Four subjects have progressed to dementia after the formal study period, three with no memory complaint at baseline. Previous research has found mixed results for the prognostic significance of subjective memory complaints (for reviews, see Iliffe and Pealing31 and Jonker et al.4). For example, the conclusion that memory complaint is a strong indicator of developing dementia was reached in a very large sample of cognitively intact elders who were followed an average of 3.2 years.32 These researchers found an association between baseline memory complaints and incident AD for participants who were cognitively intact at baseline defined as an MMSE score >25. Given the relatively short interval between “normal” cognition and incident AD, it is possible that some participants had cognitive impairment at baseline that was not detected by the MMSE. By contrast, in another large study, memory complaints at baseline were associated with slower information processing speed and delayed word-list recall but were not associated with cognitive decline at a 6-year follow-up.8
The current longitudinal study sheds light on the reasons for conflicting results of the prognostic significance of subjective memory complaints. To our knowledge, this is the first time memory complaint stability over several years has been reported.
Existing literature shows that the diagnosis of MCI is unstable,24,33–35 and this was true in our study. MCI was defined as having a score ⩾1 SD below age-appropriate normative data on at least two cognitive tests in the same domain. This criterion provides a balance between sensitivity and specificity,24 although this definition is likely to include some individuals with age-appropriate cognition.36 However, all participants who acquired a diagnosis of MCI in our study showed evidence of cognitive decline because they did not meet criteria for MCI at baseline.
A limitation of the current study was the small sample of community-dwelling participants with memory complaints at baseline. The prevalence of memory complaints may differ in clinical samples. Most participants were Caucasian, and there was a preponderance of women. Also, the sample consisted of participants selected because of their interest in in-home technology who were older and better educated than in many studies. To clarify the significance and stability of subjective memory complaints over time, memory complaints should be assessed in a sample larger than used in the present study. Also, the best questions to ask to elicit self-evaluation of memory merits further study. Methods have varied from asking a single question37 to more complex interviews.28 In a study of types of memory complaints by patients in a memory clinic, recalling conversations, books, and movies were judged by the patients to be more difficult than other types of memories.38
We conclude that subjective memory complaints of community-dwelling older adults as presently studied are weakly associated with future cognitive decline and have limited value as a research criterion for selecting older adults at risk of MCI, at least among older adults. This conclusion is consistent with a large meta-analysis of the relationship between subjective memory complaints and objective memory performance in older adults.39 These authors strongly cautioned against relying on subjective complaints as a proxy for objective memory performance. Memory complaints may be more reliable in patients who are referred to memory disorder or dementia clinics when they are likely to have more cognitive impairment. It may be that subjective memory complaints by younger adults also are more predictive. Wang et al.40 found that age modified the association between subjective memory complaints and future dementia, with a hazard ratio of 6.0 at age 70 and dropping to 1.6 at age 80.
We agree with Lenehan et al.41 that the MCI diagnostic requirement of subjective memory complaint elevates the rate of false positive. We also agree with Mitchell5 that the absence of a subjective memory complaint is a fair indicator of the absence of impending cognitive impairment. In our study, 78% of participants with no memory complaints on any examination remained cognitively intact.
Counseling for older adults with memory complaints should include information about cognitive changes associated with depression, anxiety, and health status. In addition, education may be needed about what memory or cognitive changes are age-appropriate.
Footnotes
Declaration of conflicting interests: The authors have reported no conflicts of interest.
Funding: The study was supported by grants from National Institute of Aging and National Institutes of Health (P30AG024978, P30AG008017, and R01AG024059).
References
- 1. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011; 7: 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reisberg B, Gauthier S. Current evidence for subjective cognitive impairment (SCI) as the pre-mild cognitive impairment (MCI) stage of subsequently manifest Alzheimer’s disease. Int Psychogeriatr 2008; 20: 1–16. [DOI] [PubMed] [Google Scholar]
- 3. Saykin AJ, Wishart HA, Rabin LA, et al. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology 2006; 67: 834–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jonker C, Geerlings MI, Schmand B. Are memory complaints predictive for dementia? A review of clinical and population-based studies. Int J Geriatr Psychiatry 2000; 15: 983–991. [DOI] [PubMed] [Google Scholar]
- 5. Mitchell AJ. The clinical significance of subjective memory complaints in the diagnosis of mild cognitive impairment and dementia: a meta-analysis. Int J Geriatr Psychiatry 2008; 23: 1191–1202. [DOI] [PubMed] [Google Scholar]
- 6. Reid LM, Maclullich AM. Subjective memory complaints and cognitive impairment in older people. Dement Geriatr Cogn Disord 2006; 22: 471–485. [DOI] [PubMed] [Google Scholar]
- 7. Slavin MJ, Brodaty H, Kochan NA, et al. Prevalence and predictors of “subjective cognitive complaints” in the Sydney Memory and Ageing Study. Am J Geriatr Psychiatry 2010; 18: 701–710. [DOI] [PubMed] [Google Scholar]
- 8. Mol ME, van Boxtel MP, Willems D, et al. Do subjective memory complaints predict cognitive dysfunction over time? A six-year follow-up of the Maastricht Aging Study. Int J Geriatr Psychiatry 2006; 21: 432–441. [DOI] [PubMed] [Google Scholar]
- 9. Reid M, Parkinson L, Gibson R, et al. Memory complaint questionnaire performed poorly as screening tool: validation against psychometric tests and affective measures. J Clin Epidemiol 2012; 65: 199–205. [DOI] [PubMed] [Google Scholar]
- 10. Kurt P, Yener G, Oguz M. Impaired digit span can predict further cognitive decline in older people with subjective memory complaint: a preliminary result. Aging Ment Health 2011; 15: 364–369. [DOI] [PubMed] [Google Scholar]
- 11. Minett TS, Da Silva RV, Ortiz KZ, et al. Subjective memory complaints in an elderly sample: a cross-sectional study. Int J Geriatr Psychiatry 2008; 23: 49–54. [DOI] [PubMed] [Google Scholar]
- 12. Vogel A, Stokholm J, Gade A, et al. Awareness of deficits in mild cognitive impairment and Alzheimer’s disease: do MCI patients have impaired insight? Dement Geriatr Cogn Disord 2004; 17: 181–187. [DOI] [PubMed] [Google Scholar]
- 13. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011; 7: 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Portet F, Ousset PJ, Visser PJ, et al. Mild cognitive impairment (MCI) in medical practice: a critical review of the concept and new diagnostic procedure. Report of the MCI Working Group of the European Consortium on Alzheimer’s Disease. J Neurol Neurosurg Psychiatry 2006; 77: 714–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment—beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med 2004; 256: 240–246. [DOI] [PubMed] [Google Scholar]
- 16. Kaye JA, Maxwell SA, Mattek N, et al. Intelligent systems for assessing aging changes: home-based, unobtrusive, and continuous assessment of aging. J Gerontol B: Psychol 2011; 66(Suppl. 1): i180–i190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kaye JA, Oken BS, Howieson DB, et al. Neurologic evaluation of the optimally healthy oldest old. Arch Neurol 1994; 51: 1205–1211. [DOI] [PubMed] [Google Scholar]
- 18. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–198. [DOI] [PubMed] [Google Scholar]
- 19. Hollingshead AB. Four factor index of social position. New Haven, CT: Yale University, 1975. [Google Scholar]
- 20. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1982; 17: 37–49. [DOI] [PubMed] [Google Scholar]
- 21. Pfeffer RI, Kurosaki TT, Harrah CH, Jr, et al. Measurement of functional activities in older adults in the community. J Gerontol 1982; 37: 323–329. [DOI] [PubMed] [Google Scholar]
- 22. Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc 1968; 16: 622–626. [DOI] [PubMed] [Google Scholar]
- 23. Parmelee PA, Thuras PD, Katz IR, et al. Validation of the cumulative illness rating scale in a geriatric residential population. J Am Geriatr Soc 1995; 43: 130–137. [DOI] [PubMed] [Google Scholar]
- 24. Jak AJ, Bondi MW, Delano-Wood L, et al. Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am J Geriatr Psychiatry 2009; 17: 368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jungwirth S, Fischer P, Weissgram S, et al. Subjective memory complaints and objective memory impairment in the Vienna-Transdanube aging community. J Am Geriatr Soc 2004; 52: 263–268. [DOI] [PubMed] [Google Scholar]
- 26. Clarnette RM, Almeida OP, Forstl H, et al. Clinical characteristics of individuals with subjective memory loss in Western Australia: results from a cross-sectional survey. Int J Geriatr Psychiatry 2001; 16: 168–174. [DOI] [PubMed] [Google Scholar]
- 27. Hänninen T, Reinikainen KJ, Helkala EL, et al. Subjective memory complaints and personality traits in normal elderly subjects. J Am Geriatr Soc 1994; 42: 1–4. [DOI] [PubMed] [Google Scholar]
- 28. Glodzik-Sobanska L, Reisberg B, De Santi S, et al. Subjective memory complaints: presence, severity and future outcome in normal older subjects. Dement Geriatr Cogn Disord 2007; 24: 177–184. [DOI] [PubMed] [Google Scholar]
- 29. Farias ST, Mungas D, Jagust W. Degree of discrepancy between self and other-reported everyday functioning by cognitive status: dementia, mild cognitive impairment, and healthy elders. Int J Geriatr Psychiatry 2005; 20: 827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Caselli RJ, Chen K, Locke DE, et al. Subjective cognitive decline: self and informant comparisons. Alzheimers Dement 2014; 10: 93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Iliffe S, Pealing L. Subjective memory problems. BMJ 2010; 340: c1425. [DOI] [PubMed] [Google Scholar]
- 32. Geerlings MI, Jonker C, Bouter LM, et al. Association between memory complaints and incident Alzheimer’s disease in elderly people with normal baseline cognition. Am J Psychiatry 1999; 156: 531–537. [DOI] [PubMed] [Google Scholar]
- 33. Ganguli M, Dodge HH, Shen C, et al. Mild cognitive impairment, amnestic type: an epidemiologic study. Neurology 2004; 63: 115–121. [DOI] [PubMed] [Google Scholar]
- 34. Han JW, Kim TH, Lee SB, et al. Predictive validity and diagnostic stability of mild cognitive impairment subtypes. Alzheimers Dement 2012; 8: 553–559. [DOI] [PubMed] [Google Scholar]
- 35. Mitchell J, Arnold R, Dawson K, et al. Outcome in subgroups of mild cognitive impairment (MCI) is highly predictable using a simple algorithm. J Neurol 2009; 256: 1500–1509. [DOI] [PubMed] [Google Scholar]
- 36. Binder LM, Iverson GL, Brooks BL. To err is human: “abnormal” neuropsychological scores and variability are common in healthy adults. Arch Clin Neuropsychol 2009; 24: 31–46. [DOI] [PubMed] [Google Scholar]
- 37. Dik MG, Jonker C, Comijs HC, et al. Memory complaints and APOE-epsilon4 accelerate cognitive decline in cognitively normal elderly. Neurology 2001; 57: 2217–2222. [DOI] [PubMed] [Google Scholar]
- 38. Clement F, Belleville S, Gauthier S. Cognitive complaint in mild cognitive impairment and Alzheimer’s disease. J Int Neuropsychol Soc 2008; 14: 222–232. [DOI] [PubMed] [Google Scholar]
- 39. Crumley JJ, Stetler CA, Horhota M. Examining the relationship between subjective and objective memory performance in older adults: a meta-analysis. Psychol Aging 2014; 29: 250–263. [DOI] [PubMed] [Google Scholar]
- 40. Wang L, van Belle G, Crane PK, et al. Subjective memory deterioration and future dementia in people aged 65 and older. J Am Geriatr Soc 2004; 52: 2045–2051. [DOI] [PubMed] [Google Scholar]
- 41. Lenehan ME, Klekociuk SZ, Summers MJ. Absence of a relationship between subjective memory complaint and objective memory impairment in mild cognitive impairment (MCI): is it time to abandon subjective memory complaint as an MCI diagnostic criterion? Int Psychogeriatr 2012; 24: 1505–1514. [DOI] [PubMed] [Google Scholar]


