Abstract
This 56-week, randomized, placebo-controlled trial examined the efficacy and safety of naltrexone plus bupropion as an adjunct to intensive behavior modification (BMOD). A total of 793 participants (BMI = 36.5 ± 4.2 kg/m2) was randomly assigned in a 1:3 ratio to: (i) placebo + BMOD (N = 202); or (ii) naltrexone sustained-release (SR, 32 mg/day), combined with bupropion SR (360 mg/day) plus BMOD (i.e., NB32 + BMOD; N = 591). Both groups were prescribed an energy-reduced diet and 28 group BMOD sessions. Co-primary end points were percentage change in weight and the proportion of participants who lost ≥5% weight at week 56. Efficacy analyses were performed on a modified intent-to-treat population (ITT; i.e., participants with ≥1 postbaseline weight while taking study drug (placebo + BMOD, N = 193; NB32 + BMOD, N = 482)). Missing data were replaced with the last observation obtained on study drug. At week 56, weight loss was 5.1 ± 0.6% with placebo + BMOD vs. 9.3 ± 0.4% with NB32 + BMOD (P < 0.001). A completers analysis revealed weight losses of 7.3 ± 0.9% (N = 106) vs. 11.5 ± 0.6% (N = 301), respectively (P < 0.001). A third analysis, which included all randomized participants, yielded losses of 4.9 ± 0.6 vs. 7.8 ± 0.4%, respectively (P < 0.001). Significantly more NB32 + BMOD- vs. placebo + BMOD-treated participants lost ≥5 and ≥10% of initial weight, and the former had significantly greater improvements in markers of cardiometabolic disease risk. NB32 + BMOD was generally well tolerated, although associated with more reports of nausea than placebo + BMOD. The present findings support the efficacy of combined naltrexone/bupropion therapy as an adjunct to intensive BMOD for obesity.
INTRODUCTION
Naltrexone is an opioid receptor antagonist that is approved for the treatment of alcohol and opioid dependence (1-3). Bupropion is a dopamine and norepinephrine reuptake inhibitor that was first approved for the treatment of depression (4-6) and later for smoking cessation (7,8). Two recent studies found that the combination of these medications was effective in producing weight loss in obese adults (9,10). The first, a 16-week randomized controlled trial, demonstrated that 50 mg/day of naltrexone immediate-release, combined with 300 mg/day of bupropion sustained-release (SR), produced a loss of 3.7% of baseline weight, compared with a significantly smaller 0.6% for placebo and 1.7% for naltrexone alone (9). Bupropion alone produced a loss of 3.2%, which did not differ significantly from the naltrexone/bupropion combination. A longer follow-up trial (of 24 weeks) examined the efficacy of 400 mg/day of bupropion SR in combination with three different doses of naltrexone immediate-release (10). Bupropion, combined with 16 or 32 mg of naltrexone, produced losses of 5.4%, which were significantly greater than losses resulting from placebo (0.8%), 48 mg of naltrexone alone (1.2%), or 400 mg of bupropion alone (2.7%). These latter data provide evidence that the naltrexone/bupropion combination produces weight loss that is superior to either medication alone.
The mechanisms by which the naltrexone/bupropion combination induces weight loss are not entirely understood. Pro-opiomelanocortin (POMC) producing neurons in the arcuate nucleus of the hypothalamus release α-melanocyte-stimulating hormone and β-endorphin (11-13). α-Melanocyte-stimulating hormone mediates the anorectic effect of POMC activation, whereas β-endorphin causes autoinhibitory feedback by activating opioid receptors on POMC neurons (12). Bupropion increases POMC firing (9) but is associated with modest weight loss as a monotherapy (14). The weight loss inducing effects of bupropion may be attenuated by the β-endorphin-mediated autoinhibitory feedback loop. The addition of naltrexone may prevent this negative feedback loop on POMC neurons (9) and, thus, facilitate continued weight reduction or improve the maintenance of lost weight.
The present 56-week study assessed the safety and efficacy of the naltrexone SR/bupropion SR combination (Contrave), as compared with placebo, each of which was combined with an intensive program of diet, exercise, and behavior therapy. Inclusion of a strong behavioral program has been recommended by an expert panel from the National Institutes of Health (15) and has been shown to significantly increase weight loss as compared with treatment by weight loss medication alone (16,17). This trial represents one of four phase 3 studies, which together comprise Orexigen Therapeutics’ Contrave Obesity Research (COR) program.
METHODS AND PROCEDURES
Study design
This was a 56-week, multicenter, randomized, double-blind, placebo-controlled trial. The protocol was approved by each site’s institutional review board, and all participants provided written informed consent. Implementation of the study was consistent with Good Clinical Practice standards and the Declaration of Helsinki.
Participants
Participants were recruited at nine academic medical centers in the United States. Participation was open to persons 18–65 years of age who had a BMI of 30–45 kg/m2, or a BMI of 27–45 kg/m2 in the presence of controlled hypertension and/or dyslipidemia. Women of child-bearing potential were required to use effective contraception throughout the study. Exclusion criteria included: type 1 or 2 diabetes mellitus; significant cerebrovascular, cardiovascular, hepatic, or renal disease; obesity of known endocrine origin; previous surgical (or device) intervention for obesity; loss or gain of >4 kg within the previous 3 months; use of medications known to affect body weight; a history of seizures; treatment with bupropion or naltrexone within the previous 12 months; and a history of drug or alcohol abuse within the previous 12 months. Current smokers and those who had used tobacco or other nicotine products within 6 months before screening were excluded, as were individuals with serious psychiatric illness (e.g., bipolar disorder, schizophrenia, bulimia, or conditions requiring psychotropic medications other than low doses of sedative hypnotics). A medical history and physical examination were completed for all participants during screening.
Procedures
Following screening, participants were randomized via a centralized automated voice response system in a 1:3 ratio (stratified by study center) to placebo or naltrexone SR 32 mg/day combined with bupropion SR 360 mg/day (NB32). Each treatment was added to intensive group behavior modification (BMOD). The two treatment groups are hereafter referred to as placebo + BMOD and NB32 + BMOD, respectively. NB32 (or placebo) was provided as a single tablet (with 8 mg naltrexone SR and 90 mg bupropion SR), and participants were instructed to take two tablets twice daily (i.e., morning and evening). Medication was initiated at one-quarter of the daily maintenance dose and increased weekly over the first 4 weeks (with the maintenance dose reached at the beginning of the fourth week). Participants who discontinued medication before the end of the study were encouraged to remain in the BMOD program and to return for scheduled study assessment visits at weeks 28 and 56 (for measurement of weight and waist circumference). Participants had study visits at baseline and every 4 weeks thereafter.
BMOD
All participants in both treatment groups received an intensive program of BMOD that was delivered to groups of 10–20 persons by registered dietitians, behavioral psychologists, or exercise specialists. Group meetings lasted 90 min (including the weigh-in) and were held weekly for the first 16 weeks, every other week for the next 12 weeks, and monthly thereafter (yielding a total of 28 sessions). All participants were instructed to consume a balanced deficit diet of conventional foods that provided ~15–20% of energy from protein, 30% or less energy from fat, and the remainder from carbohydrate. Individual goals for energy intake were based on initial body weight. Participants who weighed ≤249 lb were prescribed 1,200 kcal/day, whereas those 250–299 lb were prescribed 1,500 kcal/day, with higher allotments for heavier individuals (i.e., 300–349 lb, 1,800 kcal/day; ≥350 lb, 2,000 kcal/day). Participants were instructed in measuring portion sizes, counting calories (with a calorie counter provided), and keeping detailed daily records of their food intake. They also were encouraged, during the first 6 months, to gradually increase to 180 min/week of planned moderately vigorous physical activity (typically brisk walking). Participants were further instructed to keep daily records of their activity, to increase their lifestyle activity, and to engage in strength training, if desired. During months 7–12, they were encouraged to aim for up to 360 min of activity per week.
Group sessions typically began with a review of participants’ eating and activity records and other homework assignments. Group leaders then introduced a new topic in weight control which, during the first 16 weeks, included meal planning, stimulus control, slowing eating, problem solving, social support, and coping with high risk situations. Subsequent sessions covered skills required for maintaining lost weight. Treatment sessions were led following detailed treatment manuals that incorporated materials from the LEARN Program for Weight Management (18), the Diabetes Prevention Program (19), and other handouts used by the authors (G.D.F., P.M.O., C.L.R., and T.A.W.) in prior trials.
Outcome measures
Body weight
The study had two co-primary end points: (i) the percent change in baseline body weight at week 56; and (ii) the proportion of participants who lost ≥5% of baseline body weight at week 56. The proportion of participants who lost ≥10% of initial weight was included as a secondary end point, and the percentage achieving ≥15% was examined as a prespecified exploratory outcome. Weight was measured (at each visit) on a calibrated scale with participants dressed in light clothing, without shoes.
Markers of cardiometabolic risk
Waist circumference was measured at baseline and at weeks 28 and 56 following methods described previously (20). Fasting blood samples were collected on the same schedule and assayed for triglycerides, low-density lipoprotein and high-density lipoprotein cholesterol, glucose, insulin, and high-sensitivity C-reactive protein (hsCRP). All assays were conducted by a central laboratory (Quintiles, Atlanta, GA).
Psychosocial status and food cravings
Weight-related quality of life was assessed at baseline and weeks 8, 16, 28, and 56 using the Impact of Weight on Quality of Life-Lite questionnaire (IWQOL-Lite) (21). This measure provides a total score, as well as subscales for physical function, public distress, self-esteem, sexual life, and work. Cravings for specific foods were assessed using the Food Craving Inventory (22). Various dimensions of appetite, food craving, eating behavior, and mood were assessed (on the same schedule as quality of life) using the Control of Eating Questionnaire, a 21-item questionnaire consisting of a series of (100 mm) visual analogue scales (23).
Safety
Systolic blood pressure (SBP) and diastolic blood pressure (DBP), as well as pulse, were measured at each study visit (conducted every 4 weeks). On each occasion, three measurements (of each variable) were obtained after participants had been seated for ≥5 min before the first measurement, with a 2-min interval between the subsequent assessments. The average of these three measurements was used.
Mood was assessed at each study visit using the Inventory of Depressive Symptomatology-Self-Report (IDS-SR) (24). At screening, participants were required to have a total score <30 on the IDS-SR, as well as scores <2 on key items that assessed sadness (question #5), irritability (question #6), anxiety/tension (#7), and suicidality (#18). During the study, participants with scores ≥2 on questions 5, 6, 7, and 18, or with total scores ≥25 (or ≥30 if baseline score ≥25) were evaluated for treatment-emergent depressive or anxiety disorders and, if indicated, were referred to a mental health professional for further assessment.
Safety assessments also included the incidence and severity of treatment-emergent adverse events (AEs), as reported at study visits (or at BMOD sessions). Other parameters included evaluation of concomitant medications, physical examination findings, electrocardiograms, and clinical laboratory measures.
Statistical analyses
A total of 800 participants was determined to provide 99% power to detect a 5% point difference between groups in percent change in initial weight, assuming the percentage change in weight for placebo + BMOD was ~5% (e.g., 5 vs. 10%) on the first co-primary end point, and ~90% power to detect a 14% point difference for the second co-primary end point, assuming the proportion of subjects achieving >5% weight loss was 50% in the placebo + BMOD group (e.g., 50 vs. 64%). Unless otherwise specified, all efficacy analyses were performed on participants in the prespecified, modified intent-to-treat population (modified-ITT), which included all randomized participants with a baseline measurement of body weight and ≥1 postbaseline measurement of weight, obtained while on study drug (defined as the participant’s taking study drug within 24 h of the visit). Unless otherwise indicated, missing individual data thereafter were imputed using the last observation carried forward (LOCF) method, yielding a modified-ITT-LOCF analysis for all efficacy end points. (This analysis used the last observation obtained while participants were on study drug.)
A completers analysis (for weight loss) included all randomized participants who provided measurements of weight at baseline and at week 56 while on study drug; only observed values were included. Two sensitivity analyses were conducted to determine the effect of excluding (from the primary analysis of weight change) participants who did not provide a postbaseline measurement of weight on study drug. The first analysis included all randomized participants (i.e., randomized LOCF; N = 793), and missing data were imputed by carrying forward the last observed measurement of body weight, regardless of whether participants provided a postbaseline measurement of weight or whether they were on study drug. This analysis included the observed weights (at the time of last measurement) for all placebo + BMOD and NB32 + BMOD participants who discontinued study drug but were invited to continue to attend group treatment and subsequently provided a measurement of body weight at one or more scheduled assessment visits. The second analysis included only participants who provided a postbaseline assessment of weight, on or off study drug (N = 761). Results of these two sensitivity analyses were nearly identical and, thus, the latter findings are not reported.
Safety analyses were performed on the safety analysis set. It included all randomized participants who took ≥1 tablet of study drug and had ≥1 investigator contact at any time after beginning the study (N = 784). In post hoc analyses, changes in SBP and DBP, from baseline to week 56, were plotted against percent change in body weight (during the same time) for each treatment group. The corresponding Pearson correlation coefficients and associated P values also were obtained.
General linear models that included terms for treatment, study center, and baseline values (as covariates) were used to analyze the first co-primary end point and other continuous secondary end points. To minimize skewness, values for triglycerides, hsCRP, insulin, and homeostasis model assessment of insulin resistance were log10 transformed before running the general linear models. The percent change from baseline was calculated by back-transforming the least squares geometric mean minus one. The proportions of participants who achieved different weight loss categories (e.g., ≥5% loss) were analyzed using a logistic regression model that included treatment and study center as main effects and baseline body weight as a covariate.
Differences between the two groups on baseline characteristics, shown in Table 1, were examined using analysis of variance (with Fisher’s or χ2-tests used for noncontinuous variables). To control for multiple comparisons, secondary end points were analyzed in a predetermined sequence, beginning with the proportion of participants who lost ≥10% of initial weight and continuing with the change (or percent change for triglycerides, insulin, homeostasis model assessment of insulin resistance, and hsCRP) from baseline in each of the following variables: waist circumference, triglycerides, insulin, high-density lipoprotein-cholesterol, IWQOL-Lite total score, homeostasis model assessment of insulin resistance, hsCRP, glucose, and low-density lipoprotein-cholesterol. Formal testing was continued in a step-down manner until any end point failed at a two-sided significance level of 0.05, after which all statistical comparisons should be viewed as exploratory. Other statistical comparisons—including those for the IWQOL-Lite subscales, the Food Craving Inventory, and the Control of Eating Questionnaire—should be viewed as exploratory. All continuous data are presented as least squares mean ± s.e., unless otherwise indicated. All statistical analyses were performed using Windows SAS, version 9.1 (Cary, NC).
Table 1.
Baseline characteristics of study participants
| Placebo + BMOD |
NB32 + BMOD |
|
|---|---|---|
| Variable | N = 202 | N = 591 |
| Gender (% female) | 91.6 | 89.3 |
| Age (years) | 45.6 ± 11.4 | 45.9 ± 10.4 |
| Race/ethnicity (%) | ||
| White | 73.8 | 68.5 |
| African-American | 21.8 | 24.5 |
| Other | 4.5 | 6.9 |
| BMI (kg/m2) | 37.0 ± 4.2 | 36.3 ± 4.2 |
| Weight (kg) | 101.9 ± 15.0 | 100.2 ± 15.4 |
| Systolic blood pressure (mm Hg) |
116.7 ± 10.9 | 116.6 ± 10.1 |
| Diastolic blood pressure (mm Hg) |
77.1 ± 7.4 | 78.0 ± 7.3 |
| Pulse rate (b.p.m.) | 70.2 ± 8.9 | 70.7 ± 8.4 |
| IWQOL-Lite total score | 73.8 ± 15.6 | 71.9 ± 15.4 |
| Physical function subscale | 71.3 ± 18.3 | 69.2 ± 18.7 |
| Self-esteem subscale | 59.2 ± 23.4 | 56.2 ± 23.8 |
| Public distress subscale | 84.9 ± 18.5 | 85.2 ± 17.1 |
| Sexual life subscale | 80.2 ± 24.0 | 76.2 ± 23.9 |
| Work subscale | 86.6 ± 17.2 | 86.5 ± 17.4 |
| IDS-SR total score | 6.2 ± 5.3 | 5.8 ± 4.8 |
| Food Craving Inventory | ||
| Total score | 78.7 ± 19.4 | 78.8 ± 17.3 |
| Sweets subscale score | 21.0 ± 6.4 | 20.9 ± 6.1 |
| Carbohydrates subscale score |
19.2 ± 6.0 | 19.1 ± 5.7 |
| High fats subscale | 16.2 ± 5.6 | 16.2 ± 5.1 |
| Fast-food fats subscale | 10.6 ± 3.2 | 10.7 ± 2.9 |
Demographic data and those for weight, blood pressure, and IDS-SR total score are for the randomized population. All other data are for the modified-ITT population. Data shown are mean ± s.d.; percentages may not add up to 100 due to rounding.
BMOD, behavior modification; b.p.m., beats per minute; IDS-SR, Inventory of Depressive Symptomatology-Self-Report; IWQOL, Impact of Weight on Quality of Life.
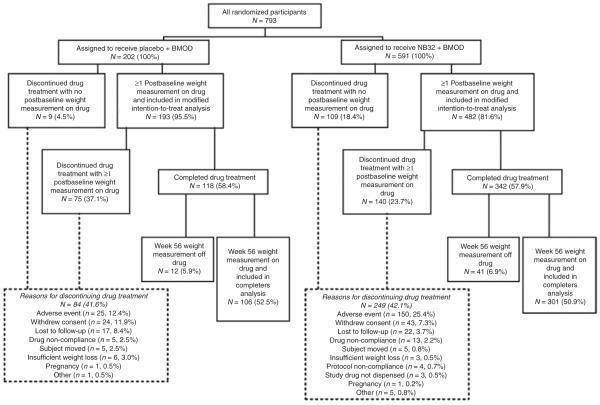
Retention
Figure 1 shows the flow of participants through the 56-week trial. Of the 202 participants randomized to placebo + BMOD, 193 (95.5%) had a baseline weight measurement and at least one post-baseline weight measurement while taking study drug, thus qualifying for inclusion in the modified-ITT analysis. Of the 591 NB32 + BMOD participants, 482 (81.6%) qualified for inclusion in the modified-ITT analysis. During the first 4 weeks of the study, 2.0% of participants in the placebo + BMOD group did not provide a postbaseline measurement of weight on study drug because of study drug discontinuation related to an AE, compared with 14.0% of participants in the NB32 + BMOD group (P = 0.038). Over the 56-week trial, 41.6% of participants in placebo + BMOD discontinued study drug, compared with 42.1% of NB32 + BMOD. A greater percentage of participants who received NB32 + BMOD vs. placebo + BMOD discontinued because of an AE (25.4 vs. 12.4%, P < 0.001). By contrast, a greater percentage of participants in the placebo + BMOD group than in NB32 + BMOD discontinued due to withdrawal of consent (11.9 vs. 7.3%, P = 0.042), lost to follow-up (8.4 vs. 3.7%, P = 0.008), or self-perceived insufficient weight loss (3.0 vs. 0.5%, P = 0.004).
Figure 1.
Flow of participants through the study. Participants were included in the modified-ITT analysis population if they had ≥1 postbaseline measurement of weight while on study drug. Participants were included in the completers analysis if they had a week 56 weight measurement on study drug. The discrepancy between the number of participants who completed the trial (N = 460) and those included in the completers analysis (N = 407) reflects participants who had not taken a dose of study drug within 24 h of their weight being measured at week 56. BMOD, behavior modification; ITT, intent-to-treat.
RESULTS
Co-primary end points: weight loss
Percent weight change
At week 56, participants treated with placebo + BMOD lost 5.1 ± 0.6% of initial weight, compared with a significantly (P < 0.001) greater 9.3 ± 0.4% for those who received NB32 + BMOD (as determined by the modified-ITT-LOCF analysis; see Figure 2). With the completers analysis, weight losses were 7.3 ± 0.9% with placebo + BMOD and 11.5 ± 0.6% with NB32 + BMOD (P < 0.001). The randomized-LOCF sensitivity analysis, which included all randomized participants, revealed weight losses of 4.9 ± 0.6 and 7.8 ± 0.4% for the two groups, respectively (P < 0.001).
Figure 2.
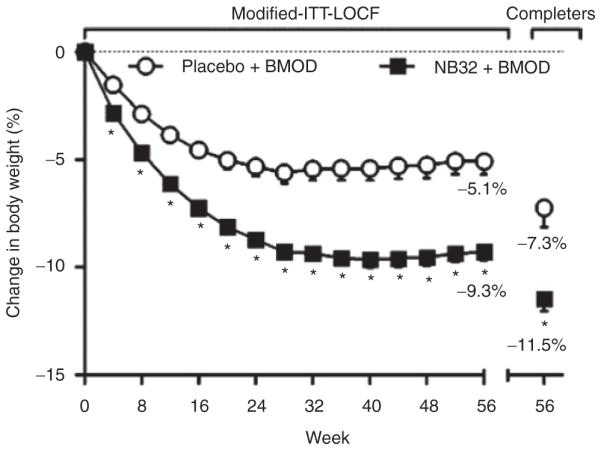
Percentage weight loss from baseline for the modified-ITT-LOCF population (placebo + BMOD, N = 193; NB32 + BMOD, N = 482) and the completer population (placebo + BMOD, N = 106; NB32 + BMOD, N = 301). *P < 0.001 for NB32 + BMOD vs. placebo + BMOD. BMOD, behavior modification; ITT, intent-to-treat; LOCF, last observation carried forward.
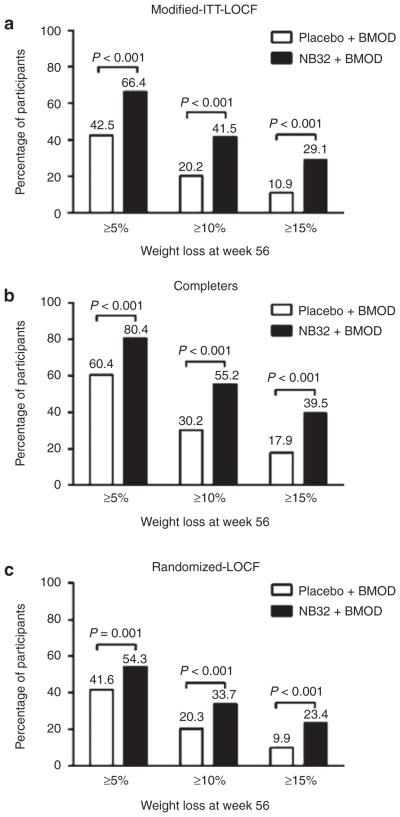
Categorical weight loss
The proportions of participants who achieved ≥5, ≥10, and ≥15% reductions in baseline weight were greater with NB32 + BMOD than with placebo + BMOD (P < 0.001 for all comparisons, using the modified-ITT-LOCF; see Figure 3a). More than 1.5 times as many participants who received NB32 + BMOD lost ≥5% of initial weight, and twice as many lost ≥10%, compared with participants treated with placebo + BMOD. Nearly three times as many participants in the NB32 + BMOD vs. placebo + BMOD group lost ≥15% of initial weight. Results of the completers analysis showed similar differences between treatment groups on all three categories of weight loss (P < 0.001 for all comparisons, Figure 3b), and a greater proportion of participants in both the NB32 + BMOD and placebo + BMOD groups reached the criterion weight losses. The randomized-LOCF sensitivity analysis, which included all randomized participants (N = 793), also revealed that more participants treated with NB32 + BMOD than with placebo + BMOD achieved all three categorical weight losses (P < 0.001 for all comparisons, Figure 3c). However, smaller percentages of participants in both treatment groups reached the categorical weight losses when the sensitivity analysis was employed, as compared with the modified-ITT-LOCF analysis. The smaller percentages of participants who achieved categorical weight losses in NB32 + BMOD, as determined by the sensitivity analysis, can be accounted for by the high attrition among these participants during the first 4 weeks of treatment.
Figure 3.
Percentage of study participants losing ≥5, ≥10, or ≥15% of their baseline body weight at week 56. (a) Modified-ITT-LOCF population (placebo + BMOD, N = 193; NB32 + BMOD, N = 482). (b) Completer population (placebo + BMOD, N = 106; NB32 + BMOD, N = 301). (c) Randomized-LOCF population (placebo + BMOD, N = 202; NB32 + BMOD, N = 591). BMOD, behavior modification; ITT, intent-to-treat; LOCF, last observation carried forward.
Session attendance and weight loss
Participants in the placebo + BMOD and NB32 + BMOD groups attended a similar number of group BMOD sessions (17.5 ± 7.3 and 18.7 ± 6.8, respectively; mean ± s.d.). A multiple linear regression analysis, which adjusted for baseline weight and treatment, revealed that the more sessions participants attended, the greater their percent reduction in initial weight (partial R2 = 0.28, P < 0.0001), as determined for the modified-ITT-LOCF population. Similar results were obtained with the completers population.
Key secondary end points
Markers of cardiometabolic risk
As shown in Table 2, waist circumference declined significantly (P < 0.001) more at week 56 with NB32 + BMOD than with placebo + BMOD, as did plasma triglycerides (P = 0.004), insulin (P = 0.003), and homeostasis model assessment of insulin resistance (P = 0.003). High-density lipoprotein-cholesterol increased significantly (P < 0.001) more with NB32 + BMOD than with placebo + BMOD. There were no statistically significant differences between groups in changes in hsCRP.
Table 2.
Change from baseline to week 56 in key secondary end points
| Variable | Placebo + BMOD | NB32 + BMOD | P value |
|---|---|---|---|
| Waist circumference (cm) | |||
| Baseline | 109.0 ± 11.8 | 109.3 ± 11.4 | |
| End point | 102.0 ± 13.1 | 99.1 ± 12.8 | |
| Change (95% CI) | −6.8 (−8.3, −5.3) | −10.0 (−10.9, −9.0) | <0.001b |
| Percent change (95% CI) | −6.1% (−7.5%, −4.7%) | −9.1 (−9.9%, −8.2%) | <0.001 |
| Triglycerides (mg/dl)a | |||
| Baseline | 104.6 ± 1.6 | 111.6 ± 1.6 | |
| End point | 95.6 ± 1.6 | 91.4 ± 1.6 | |
| Percent change (95% CI) | −8.5% (−13.7%, −3.0%) | −16.6% (−19.7%, −13.5%) | 0.004b |
| HDL cholesterol (mg/dl) | |||
| Baseline | 55.3 ± 12.9 | 53.6 ± 13.5 | |
| End point | 56.9 ± 13.4 | 58.5 ± 14.1 | |
| Change (95% CI) | +0.9 (−0.7, +2.4) | +4.1 (+3.1, +5.1) | <0.001b |
| Percent change (95% CI) | +2.8% (−0.3%, +6.0%) | +9.4% (+7.4%, +11.4%) | <0.001 |
| LDL cholesterol (mg/dl) | |||
| Baseline | 109.2 ± 27.3 | 109.5 ± 27.5 | |
| End point | 117.3 ± 33.2 | 115.0 ± 30.9 | |
| Change (95% CI) | +8.1 (+4.0, +12.3) | +5.4 (+2.8, +8.1) | 0.245 |
| Percent change (95% CI) | +10.0% (+5.7%, +14.3%) | +7.1% (+4.3%, +9.8%) | 0.219 |
| hsCRP (mg/l) a | |||
| Baseline | 4.2 ± 2.6 | 3.9 ± 2.7 | |
| End point | 3.1 ± 3.4 | 2.7 ± 3.1 | |
| Percent change (95% CI) | −16.9% (−28.3%, −3.7%) | −25.9 % (−32.6%, −18.5%) | 0.165 |
| Fasting blood glucose (mg/dl) | |||
| Baseline | 94.1 ± 20.1 | 92.4 ± 10.7 | |
| End point | 91.6 ± 14.0 | 90.0 ± 11.2 | |
| Change (95% CI) | −1.1 (−3.0, +0.8) | −2.4 (−3.6, −1.2) | 0.225 |
| Percent change (95% CI) | 0.0% (−2.1%, +2.1%) | −1.5% (−2.9%, −0.2%) | 0.185 |
| Insulin (μlU/ml) a | |||
| Baseline | 11.0 ± 1.7 | 11.3 ± 1.8 | |
| End point | 8.8 ± 1.8 | 7.8 ± 2.1 | |
| Percent change (95% CI) | −15.5% (−23.3%, −6.8%) | −28.0% (−32.4%, −23.3%) | 0.003b |
| HOMAIR a | |||
| Baseline | 2.5 ± 1.8 | 2.6 ± 1.9 | |
| End point | 2.0 ± 1.9 | 1.7 ± 2.2 | |
| Percent change (95% CI) | −16.6% (−25.0%, −7.1%) | −29.9% (−34.6%, −24.9%) | 0.003b |
| IWQOL-Lite total score | |||
| Baseline | 73.8 ± 15.6 | 71.9 ± 15.4 | |
| End point | 83.7 ± 14.8 | 85.6 ± 14.0 | |
| Change (95% CI) | +10.3 (+8.6, +12.0) | +13.4 (+12.3, +14.5) | <0.001b |
| Percent change (95% CI) | +17.7% (+14.7%, +20.7%) | +23.9% (+22.0%, +25.9%) | <0.001 |
Data are for the modified-ITT-LOCF population in which the last observation on study drug was carried forward. Ns for the placebo + BMOD group ranged from 141–178 across the different variables and from 386–448 for the NB32 + BMOD group. P values are for NB32 + BMOD vs. placebo + BMOD. Baseline and end point (week 56) data are mean ± s.d.; change and percent change data are LS mean (95% confidence interval).
BMOD, behavior modification; CI, confidence interval; HDL, high-density lipoprotein; HOMAIR, homeostasis model assessment of insulin resistance; ITT, intent-to-treat; IWQOL, Impact of Weight on Quality of Life; LDL, low-density lipoprotein; LS, least squares.
Based on log-transformed data. Baseline and end-point data are geometric mean ± s.d. Log-transformed data did not permit the calculation of a change score from baseline to week 56.
End points that were significant according to the prespecified sequential closed testing procedure conducted to correct for multiple comparisons.
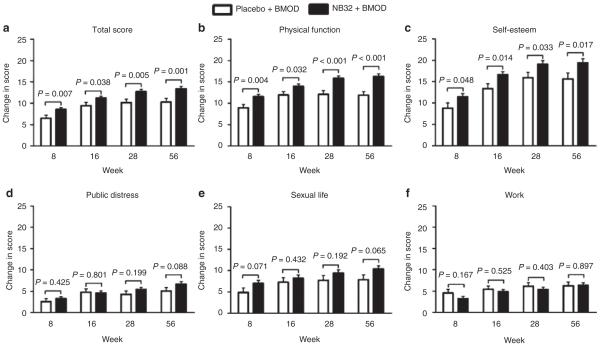
Quality of life
Overall weight-related quality of life, as measured by the IWQOL-Lite total score, improved significantly more at all assessment visits with NB32 + BMOD than with placebo + BMOD (P < 0.05 for all comparisons; see Figure 4 and Table 2). In exploratory analyses, shown in Figure 4, participants in the NB32 + BMOD group also reported greater improvements on the physical function and self-esteem subscales than did placebo + BMOD-treated participants.
Figure 4.
Change from baseline to week 56 in IWQOL-Lite total and subscale scores for the modified-ITT-LOCF population. ITT, intent-to-treat; IWQOL, Impact of Weight on Quality of Life; LOCF, last observation carried forward. Ns for the placebo + BMOD group ranged from 174–179 across the different variables and from 438–448 for the NB32 + BMOD group.
Food cravings and control of eating
In exploratory analyses, participants in both groups had reductions on the total score of the Food Craving Inventory at all assessment visits, including week 56, when values declined from baseline by 7.1 ± 0.7 with NB32 + BMOD and by 7.0 ± 1.0 with placebo + BMOD. However, there were no significant differences between groups at any time. A similar pattern of small reductions on all subscales of the Food Craving Inventory was observed. Similarly, with one exception, there were not consistent differences (at all assessment periods) between the two groups on the exploratory Control of Eating Questionnaire (23) that assessed aspects of appetite, food cravings, eating behavior, and mood. At all four assessments, however, participants in the NB32 + BMOD group reported greater improvements in their ability to control their eating than did participants treated by placebo + BMOD, with reductions at week 56 (as measured from baseline) of 13.8 ± 1.2 and 8.5 ± 1.8, respectively (P ≤ 0.007 for all comparisons).
Safety and tolerability
Vital signs and laboratory measures
Mean SBP and DBP in both groups were in the normal range at baseline (see Table 1). At week 56, SBP fell an average of 3.9 ± 0.7 mm Hg from baseline in the placebo + BMOD group, compared with a smaller reduction of 1.3 ± 0.5 mm Hg in participants treated with NB32 + BMOD (P = 0.002). DBP also declined more in placebo + BMOD-than NB32 + BMOD-treated participants (2.8 ± 0.5 and 1.4 ± 0.3 mm Hg, respectively; P = 0.017). A post hoc subset analysis of 50 participants with baseline SBP ≥130 mm Hg revealed that mean SBP in the NB32 + BMOD group declined from baseline at all visits, with mean reductions ranging from 3.4 to 11.4 mm Hg. In these same participants, the mean reduction in DBP ranged from 1.0 to 6.5 mm Hg over the duration of the study. Additional analyses revealed that, in both treatment groups, greater end-of-treatment weight losses were associated with greater reductions in blood pressure. In the NB32 + BMOD group, weight loss correlated with reductions in both SBP (r = 0.27, P = 0.001) and DBP (r = 0.29, P < 0.001). In the placebo + BMOD group, the correlation between the change in weight and SBP was r = 0.18 (P = 0.012) and that between change in weight and DBP was r = 0.19 (P = 0.007).
There were no differences (P = 0.139) between the two groups at week 56 in pulse, which was virtually unchanged in the placebo + BMOD group (+0.2 ± 0.5 beats per minute) and increased slightly in the NB32 + BMOD (+1.1 ± 0.4 beats per minute). Placebo-subtracted increases in pulse as great as 3.4 beats per minute were observed during the first 20 weeks in participants in NB32 + BMOD. However, differences between groups declined to <2.5 beats per minute all assessments thereafter. There were no consistent effects of NB32 + BMOD on clinical laboratory tests or electrocardiographic evaluations, and no evidence of treatment-related hepatotoxicity.
Mood
Mean baseline scores on the IDS-SR questionnaire were in the normal (nondepressed) range for the placebo + BMOD and NB32 + BMOD groups at baseline (6.2 ± 5.3 and 5.8 ± 4.8, respectively; mean ± s.d.) (24). (Normal range = 0–13.) Total scores in both groups remained low throughout the treatment. However, exploratory analyses showed that total scores increased (within normal limits) by 0.3–1.9 points in participants in the NB32 + BMOD group at all study assessments (conducted every 4 weeks) during the first 20 weeks, with placebo-subtracted difference of +0.9 to +1.3 points (P ≤ 0.009 for all comparisons during the first 20 weeks). Further analyses of the individual items on the IDS-SR questionnaire indicated that the difference between treatment groups, in the change in total score, was related to decreased weight and appetite in the NB32 + BMOD participants, as well as their increased report of constipation or diarrhea, changes in sleep patterns, and other nonspecific somatic symptoms. Table 3 summarizes the incidence over the 56-week trial of participants who had a total score of ≥25 (or ≥30 if baseline score was ≥25), as well as those who scored ≥2 on the key items that assessed sadness, irritability, anxiety/tension, and suicidality. As shown, there were no differences between the two groups in the percentages of participants who met any of these criterion values for evaluation of potential psychiatric treatment-emergent side effects (all P > 0.60).
Table 3.
Treatment-emergent changes in mood and depressive symptoms as determined by the Inventory of Depressive Symptomatology-Self-Report
| Placebo + BMOD |
NB32 + BMOD |
||
|---|---|---|---|
|
|
|||
| N = 193 | N = 483 | ||
|
|
|||
| Participants with: | n (%) | P value | |
| ≥1 Postbaseline score ≥2 on question #5 (sadness) |
7 (3.6) | 13 (2.7) | 0.615 |
| ≥1 Postbaseline score ≥2 on question #6 (irritability) |
6 (3.1) | 17 (3.5) | 1.000 |
| ≥1 Postbaseline score ≥2 on question #7 (anxiety/tension) |
10 (5.2) | 27 (5.6) | 1.000 |
| ≥1 Postbaseline score ≥2 on question #18 (suicidality) |
1 (0.5) | 2 (0.4) | 1.000 |
| ≥1 Postbaseline total score ≥25 | 6 (3.1) | 15 (3.1) | 1.000 |
| ≥1 Postbaseline total score ≥30 in participants with IDS-SR total score ≥25 at screening |
0 (0) | 0 (0) | — |
Data are observed values for the safety analysis set. P value is for NB32 + BMOD vs. placebo + BMOD. N and percentages are based on the number of participants who provided ≥1 postbaseline IDS-SR score.
BMOD, behavior modification; IDS-SR, Inventory of Depressive Symptomatology-Self-Report.
Adverse events
Table 4 presents AEs that occurred in ≥5% of participants in either treatment group and with greater incidence in NB32 + BMOD than in placebo + BMOD. Nausea was the most frequent AE, with 34.1% of participants treated by NB32 + BMOD reporting at least one event, compared to 10.5% for placebo + BMOD (P < 0.001). Nausea was mostly mild to moderate in intensity and occurred primarily during the first 4 weeks of the study (coinciding with drug titration), with a median duration of 10 days with NB32 + BMOD and 12 days with placebo + BMOD. Constipation, dizziness, dry mouth, tremor, upper abdominal pain, and tinnitus also occurred more often in the NB32 + BMOD group than in placebo + BMOD. With one exception, there were no differences between groups in the 10 most frequently observed psychiatric AEs. Depression, however, occurred more frequently in the placebo + BMOD group than in NB32 + BMOD (2.5 vs. 0.3%, P = 0.014). Two episodes of suicidal ideation were reported in the placebo + BMOD group, with none in participants treated by NB32 + BMOD.
Table 4.
Adverse events (AEs) with ≥5% incidence and greater incidence in the NB32 + BMOD vs. placebo + BMOD groups, the 10 most common psychiatric AEs, and AEs resulting in discontinuation with ≥0.5% incidence in the NB32 + BMOD group
| Placebo + BMOD |
NB32 + BMOD |
||
|---|---|---|---|
|
|
|||
| N = 200 | N = 584 | ||
|
|
|||
| Variabie | n (%) | P value | |
| All AEs | |||
| Nausea | 21 (10.5) | 199 (34.1) | <0.001 |
| Headache | 35 (17.5) | 139 (23.8) | 0.076 |
| Constipation | 28 (14.0) | 141 (24.1) | 0.003 |
| Dizziness | 9 (4.5) | 85 (14.6) | <0.001 |
| Vomiting | 13 (6.5) | 64 (11.0) | 0.074 |
| Insomnia | 12 (6.0) | 51 (8.7) | 0.291 |
| Dry mouth | 6 (3.0) | 47 (8.0) | 0.014 |
| Anxiety | 7 (3.5) | 30 (5.1) | 0.441 |
| Tremor | 2 (1.0) | 34 (5.8) | 0.003 |
| Abdominal pain, upper | 3 (1.5) | 32 (5.5) | 0.017 |
| Tinnitus | 1 (0.5) | 31 (5.3) | 0.001 |
| Psychiatric AEs | |||
| Insomnia | 12 (6.0) | 51 (8.7) | 0.291 |
| Anxiety | 7 (3.5) | 30 (5.1) | 0.441 |
| Sleep disorder | 6 (3.0) | 14 (2.4) | 0.610 |
| Depressed mood | 8 (4.0) | 11 (1.9) | 0.110 |
| Abnormal dreams | 4 (2.0) | 8 (1.4) | 0.514 |
| Middle insomnia | 2 (1.0) | 6 (1.0) | 1.000 |
| Tension | 1 (0.5) | 7 (1.2) | 0.687 |
| Depression | 5 (2.5) | 2 (0.3) | 0.014 |
| Stress | 4 (2.0) | 3 (0.5) | 0.074 |
| Dissociation | 0 (0) | 6 (1.0) | 0.347 |
| AEs resulting in discontinuation | |||
| Nausea | 0 (0) | 27 (4.6) | <0.001 |
| Urticaria | 1 (0.5) | 10 (1.7) | 0.306 |
| Anxiety | 3 (1.5) | 7 (1.2) | 0.721 |
| Disturbance in attention | 0 (0) | 6 (1.0) | 0.347 |
| Headache | 1 (0.5) | 5 (0.9) | 1.000 |
| Blood pressure increased | 0 (0) | 4 (0.7) | 0.577 |
| Dizziness | 0 (0) | 4 (0.7) | 0.577 |
| Vomiting | 0 (0) | 4 (0.7) | 0.577 |
| Depressed mood | 1 (0.5) | 3 (0.5) | 1.000 |
| Feeling abnormal | 1 (0.5) | 3 (0.5) | 1.000 |
| Abdominal pain | 0 (0) | 3 (0.5) | 0.574 |
| Abdominal pain upper | 0 (0) | 3 (0.5) | 0.574 |
| Disorientation | 0 (0) | 3 (0.5) | 0.574 |
| Dissociation | 0 (0) | 3 (0.5) | 0.574 |
| Feeling jittery | 0 (0) | 3 (0.5) | 0.574 |
| Insomnia | 0 (0) | 3 (0.5) | 0.574 |
| Rash | 0 (0) | 3 (0.5) | 0.574 |
P value is for NB32 + BMOD vs. placebo + BMOD. AEs are listed for all categories of AEs in the top portion of the table and for just the psychiatric category in the middle portion. The bottom portion of the table shows AEs associated into discontinuation. BMOD, behavior modification.
Two serious AEs occurred in the NB32 + BMOD group that were considered possibly related to study drug. Both involved cholecystitis in participants who had experienced marked weight loss (>15 kg). Both participants resumed blinded therapy after successful surgical treatment. No deaths occurred in the study.
Discontinuation due to AEs
As shown in the lower half of Table 4, nausea was the most frequent AE that resulted in study drug discontinuation (4.6% in NB32 + BMOD vs. 0% in placebo + BMOD; P < 0.001). Other frequent AEs that resulted in study drug discontinuation in >0.5% of NB32 + BMOD-treated participants included urticaria, anxiety, disturbance in attention, headache, increase in blood pressure, dizziness, and vomiting. However, in none of these cases did the incidence of discontinuation for a specific AE have a P value <0.05 for NB32 + BMOD vs. placebo + BMOD. In nearly 10% of the total 25.4% of NB32 + BMOD-treated participants who discontinued due to an AE, the AE’s contributing to discontinuation were of a wide variety that occurred at a very low frequency (i.e., ≤0.3%). Among the 15.2% of participants in the NB32 + BMOD group who discontinued study drug in the first month due to an AE, nausea was the most common event, accounting for 2.9% of discontinuations, compared with 0% in the placebo + BMOD group (P = 0.010). In general, the remaining study drug discontinuations due to an AE in the first month were attributable to the same AEs shown in Table 4.
DISCUSSION
Participants who received an intensive program of group BMOD (with placebo) lost an average of 5.1% of initial weight in 56 weeks of treatment. The addition of the NB32 combination to BMOD significantly increased weight loss to 9.3% (as determined by the modified-ITT analysis) and resulted in 66.4% of participants losing 5% or more of initial weight, as compared with 42.5% of those who received placebo + BMOD. Moreover, 41.5% of NB32 + BMOD participants lost ≥10% of initial weight and 29.1% lost ≥15%, as compared to values of 20.2 and 10.9%, respectively, for participants who received placebo + BMOD (as determined by the modified-ITT analysis).
The larger weight losses produced by the addition of NB32 also were associated with significantly greater reductions in markers of cardiometabolic risk, including waist circumference, triglyceride concentration, and fasting insulin concentration. In addition, NB32 + BMOD was associated with a significantly greater increase in high-density lipoprotein-cho-lesterol concentration than was placebo + BMOD.
SBP and DBP declined in both treatment groups but more in participants treated with placebo + BMOD than with NB32 + BMOD. This finding suggests that NB32 may attenuate the favorable effects of weight loss on blood pressure. The smaller reduction in blood pressure (as well as the small increase in pulse) in the NB32 + BMOD group is consistent with the pharmacological properties of bupropion (4) and has been reported previously (14,25). However, additional analyses revealed that the relationship of greater blood pressure reduction with greater weight loss was maintained in participants treated with NB32 + BMOD. Moreover, a post hoc analysis of participants with SBP ≥130 mm Hg revealed mean reductions in SBP and DBP at all assessments in participants who received NB32 + BMOD. Further study of the effects of NB32 on blood pressure, particularly in persons diagnosed with hypertension, is warranted to determine whether the medication attenuates improvements in blood pressure usually observed with weight loss.
Nausea is a well-known side effect of naltrexone (1-3) (and of bupropion to a lesser degree) and was the most common AE associated with NB32 + BMOD, reported by 34% of these participants, compared with 11% of those in the placebo + BMOD group. Most events in participants who received NB32 + BMOD were reported in the first 4 weeks of treatment, as the dosage was increased; few cases were reported after week 12. Nausea was generally transient, mostly mild to moderate in severity, and led to discontinuation in 4.6% of NB32 + BMOD-treated participants. Other side effects that were observed more frequently in the NB32 + BMOD than in the placebo + BMOD group included constipation, dizziness, dry mouth, tremor, upper abdominal pain, and tinnitus, all of which were consistent with prior AEs reported with either naltrexone or bupropion (1,4). None of these additional AEs was associated with significantly greater termination of study medication in the NB32 + BMOD than in the placebo + BMOD group. Urticaria (i.e., hives) was the second most common reason for medication termination in the NB32 + BMOD group, although this rate of occurrence did not differ significantly from that observed in the placebo + BMOD group (1.7 vs. 0.5%, respectively). Urticaria has been reported previously with both bupropion (4) and naltrexone (26), taken as monotherapy.
Two participants experienced serious AEs of cholecystitis that were judged to be potentially related to study drug. In both cases, study investigators believed that study drug may have contributed to the participants’ large weight loss (>15 kg), which was considered the proximal cause of cholecystitis (27).
NB32 + BMOD produced greater improvements in overall weight-related quality of life than did placebo + BMOD. Exploratory analyses revealed that participants in the former group also reported more favorable changes on the physical function and self-esteem subscales of the IWQOL-Lite questionnaire. The present results confirm prior findings of improvements in weight-related quality of life with a loss ≥5% of initial weight (28).
Weight loss with NB32 + BMOD did not appear to be associated with adverse effects on mood. Mean depression scores (as measured by the IDS-SR) in both groups were low at baseline and remained so throughout the study. Although mean total scores on the IDS-SR increased in NB32 + BMOD-treated participants during the first 20 weeks, increases were small and without clinical significance. There were no significant differences between groups on treatment-emergent increases on key items from the IDS-SR that assessed sadness, irritability, anxiety/tension, or suicidality. In addition, the incidence of depression AEs was low in both groups, and was lower in NB32 + BMOD than in placebo + BMOD. There were no reports of suicidal ideation in participants treated by NB32 + BMOD and two reports in those who received placebo + BMOD.
Strengths of this study included a large sample size and the provision of an intensive program of BMOD. We had expected the placebo + BMOD intervention to induce a larger weight loss—of about 7–8% of initial weight—as is typically observed with BMOD alone (16,29). It is possible that the provision of a placebo with BMOD diminished the efficacy of the latter therapy because of participants’ potential belief that, because they were taking medication, they could lose weight without working as hard on changing their eating and activity behaviors (16,30). Limitations of the study include the relative lack of male participants, as well as of participants with significant comorbidities, each of which is likely to have contributed to a greater-than-desired attrition rate (31). In addition, results of the prespecified modified-ITT analysis, which excluded the 18.4% of NB32 + BMOD-treated participants who did not have a postbaseline measurement of body weight on study drug, must be interpreted in light of the more conservative results obtained for the entire randomized population.
In conclusion, the combination of naltrexone SR/bupropion SR, combined with an intensive program of BMOD, produced significantly greater mean weight loss than BMOD alone and helped a significantly greater percentage of participants lose ≥5, ≥10, and ≥15% or more of initial weight. Naltrexone SR/bupropion SR was generally well tolerated, and the observed AEs are consistent with what has previously been reported for naltrexone and/or bupropion. The present findings provide additional support for the efficacy of naltrexone SR/bupropion SR combined therapy for weight management (9,10) and suggest the further benefit of combining medication with a comprehensive program of BMOD (16,32).
ACKNOWLEDGMENTS
This study was funded by Orexigen Therapeutics. The sponsor had primary responsibility for designing the study protocol, receiving and managing study data, planning statistical analyses (in consultation with the US Food and Drug administration), and contracting with an independent party to analyze the data. The authors from the academic sites collaborated with the sponsor on aspects of study design, developed the behavior modification intervention, and assessed and treated all study participants. T.A.W. and H.M. wrote the first draft of the manuscript which was revised with input from all of the authors. We thank inVentiv Clinical Solutions, Terry Rees (Orexigen), and Georgia Tsaroucha (Orexigen) for statistical and programming support, and Cynthia Buchner (Orexigen) for quality control support. We also thank Victoria Webb for editorial assistance and Sheri Volger for reviewing the manuscript.
Footnotes
DISCLOSURE
All academic authors received research grants from Orexigen Therapeutics (to their respective institutions) to conduct the study. T.A.W., J.O.H., S.K., P.M.O., and F.X.P.-S. have received honoraria for serving on Orexigen Therapeutic’s Advisory Board, and G.D.F. and P.M.O. have served as paid consultants to the company. H.M., D.D.K., and E.D. are employees of and hold stock in Orexigen Therapeutics, Inc. J.E. works for inVentiv Clinical Solutions, which is a consultant for Orexigen Therapeutics, Inc.
REFERENCES
- 1.Naltrexone hydrochloride (naltrexone hydrochloride) [package insert] Barr Laboratories; Pomona, NY: 2003. [Google Scholar]
- 2.Berg BJ, Pettinati HM, Volpicelli JR. A risk-benefit assessment of naltrexone in the treatment of alcohol dependence. Drug Saf. 1996;15:274–282. doi: 10.2165/00002018-199615040-00005. [DOI] [PubMed] [Google Scholar]
- 3.Lobmaier P, Kornør H, Kunøe N, Bjørndal A. Sustained-release naltrexone for opioid dependence. Cochrane Database Syst Rev. 2008;16:CD006140. doi: 10.1002/14651858.CD006140.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Wellbutrin SR. GlaxoSmithKline; Research Triangle Park, NC: 2009. (bupropion hydrochloride) [package insert] [Google Scholar]
- 5.Dhillon S, Yang LP, Curran MP. Bupropion: a review of its use in the management of major depressive disorder. Drugs. 2008;68:653–689. doi: 10.2165/00003495-200868050-00011. [DOI] [PubMed] [Google Scholar]
- 6.Stahl SM, Pradko JF, Haight BR, et al. A Review of the Neuropharmacology of Bupropion, a Dual Norepinephrine and Dopamine Reuptake Inhibitor. Prim Care Companion J Clin Psychiatry. 2004;6:159–166. doi: 10.4088/pcc.v06n0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zyban (bupropion hydrochloride) [package insert] GlaxoSmithKline; Research Triangle Park, NC: 2009. [Google Scholar]
- 8.Tong EK, Carmody TP, Simon JA. Bupropion for smoking cessation: a review. Compr Ther. 2006;32:26–33. doi: 10.1385/comp:32:1:26. [DOI] [PubMed] [Google Scholar]
- 9.Greenway FL, Whitehouse MJ, Guttadauria M, et al. Rational design of a combination medication for the treatment of obesity. Obesity (Silver Spring) 2009;17:30–39. doi: 10.1038/oby.2008.461. [DOI] [PubMed] [Google Scholar]
- 10.Greenway FL, Dunayevich E, Tollefson G, et al. Comparison of combined bupropion and naltrexone therapy for obesity with monotherapy and placebo. J Clin Endocrinol Metab. 2009;94:4898–4906. doi: 10.1210/jc.2009-1350. [DOI] [PubMed] [Google Scholar]
- 11.Cone RD, Cowley MA, Butler AA, et al. The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int J Obes Relat Metab Disord. 2001;25(Suppl 5):S63–S67. doi: 10.1038/sj.ijo.0801913. [DOI] [PubMed] [Google Scholar]
- 12.Cowley MA, Smart JL, Rubinstein M, et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 13.Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- 14.Jain AK, Kaplan RA, Gadde KM, et al. Bupropion SR vs. placebo for weight loss in obese patients with depressive symptoms. Obes Res. 2002;10:1049–1056. doi: 10.1038/oby.2002.142. [DOI] [PubMed] [Google Scholar]
- 15.National Institutes of Health/National Heart, Lung, and Blood Institute Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—the Evidence Report. Obes Res. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 16.Wadden TA, Berkowitz RI, Womble LG, et al. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med. 2005;353:2111–2120. doi: 10.1056/NEJMoa050156. [DOI] [PubMed] [Google Scholar]
- 17.Digenio AG, Mancuso JP, Gerber RA, Dvorak RV. Comparison of methods for delivering a lifestyle modification program for obese patients: a randomized trial. Ann Intern Med. 2009;150:255–262. doi: 10.7326/0003-4819-150-4-200902170-00006. [DOI] [PubMed] [Google Scholar]
- 18.Brownell KD. American Health Publishing; Dallas: 1998. The LEARN Program for Weight Control, 7th edn. [Google Scholar]
- 19.Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Heart, Lung, and Blood Institute & North American Association for the Study of Obesity . The Practical Guide: Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. National Institutes of Health; Bethesda, MD: 2000. [Google Scholar]
- 21.Kolotkin RL, Crosby RD, Kosloski KD, Williams GR. Development of a brief measure to assess quality of life in obesity. Obes Res. 2001;9:102–111. doi: 10.1038/oby.2001.13. [DOI] [PubMed] [Google Scholar]
- 22.White MA, Whisenhunt BL, Williamson DA, Greenway FL, Netemeyer RG. Development and validation of the food-craving inventory. Obes Res. 2002;10:107–114. doi: 10.1038/oby.2002.17. [DOI] [PubMed] [Google Scholar]
- 23.Wilcox CS, Oskooilar N, Erickson JS, et al. An open-label study of naltrexone and bupropion combination therapy for smoking cessation in overweight and obese subjects. Addict Behav. 2010;35:229–234. doi: 10.1016/j.addbeh.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 24.Rush AJ, Carmody T, Reimitz PE. The Inventory of Depressive Symptomatology (IDS): clinician (IDS-C) and self-report (IDS-SR) ratings of depressive symptoms. Int J Methods Psychiatr Res. 2000;9:45–59. [Google Scholar]
- 25.Anderson JW, Greenway FL, Fujioka K, et al. Bupropion SR enhances weight loss: a 48-week double-blind, placebo-controlled trial. Obes Res. 2002;10:633–641. doi: 10.1038/oby.2002.86. [DOI] [PubMed] [Google Scholar]
- 26.Vivtrol (naltrexone) [package insert] Alkermes; Cambridge, MA: 2009. [Google Scholar]
- 27.Weinsier RL, Ullmann DO. Gallstone formation and weight loss. Obes Res. 1993;1:51–56. doi: 10.1002/j.1550-8528.1993.tb00008.x. [DOI] [PubMed] [Google Scholar]
- 28.Kolotkin RL, Norquist JM, Crosby RD, et al. One-year health-related quality of life outcomes in weight loss trial participants: comparison of three measures. Health Qual Life Outcomes. 2009;7:53. doi: 10.1186/1477-7525-7-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foster GD, Makris AP, Bailer BA. Behavioral treatment of obesity. Am J Clin Nutr. 2005;82:230S–235S. doi: 10.1093/ajcn/82.1.230S. [DOI] [PubMed] [Google Scholar]
- 30.Hollon SD, DeRubeis RJ. Placebo-psychotherapy combinations: inappropriate representations of psychotherapy in drug-psychotherapy comparative trials. Psychol Bull. 1981;90:467–477. [PubMed] [Google Scholar]
- 31.Fabricatore AN, Wadden TA, Moore RH, et al. Attrition from randomized controlled trials of pharmacological weight loss agents: a systematic review and analysis. Obes Rev. 2009;10:333–341. doi: 10.1111/j.1467-789X.2009.00567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wadden TA, Berkowitz RI, Sarwer DB, Prus-Wisniewski R, Steinberg C. Benefits of lifestyle modification in the pharmacologic treatment of obesity: a randomized trial. Arch Intern Med. 2001;161:218–227. doi: 10.1001/archinte.161.2.218. [DOI] [PubMed] [Google Scholar]