Abstract
Background
Metastatic and recurrent, platinum resistant cervix cancer has an extremely poor prognosis. The Gynecologic Oncology Group has studied >20 cytotoxic drugs or drug combinations in the second-line, phase II setting of advanced, drug resistant cervix cancer.
Methods
Nanoparticle, albumin-bound paclitaxel (nab-paclitaxel) was administered at 125 mg/m2 IV over 30 minutes on days 1, 8 and 15 of each 28 day cycle to 37 women with metastatic or recurrent cervix cancer that had progressed or relapsed following first-line cytotoxic drug treatment. A flexible, 2-stage accrual design that allowed stopping early for lack of treatment activity was utilized. Because of slow patient accrual, the second stage was not completed.
Results
Of 37 patients enrolled, 2 were ineligible due to no prior cytotoxic chemotherapy, which left 35 eligible patients evaluable for response and tolerability. All of the eligible patients had 1 prior chemotherapy regimen and 27 of them had prior radiation therapy with concomitant cisplatin. The median number of nab-paclitaxel cycles were 4 (range 1–15). Ten (28.6%; CI 14.6%–46.3%) of the 35 patients had a partial response and another 15 patients (42.9%) had stable disease. The median progression-free and overall survival were 5.0 and 9.4 months, respectively. The only NCI CTCAE grade 4 event was neutropenia in 2 patients (5.7%) which resolved following dose reduction. Grade 3 neurotoxicity was reported in 1 (2.9%) patient and resolved to grade 2 following dose discontinuation.
Conclusions
Nab-paclitaxel has considerable activity and moderate toxicity in the treatment of drug resistant, metastatic and recurrent cervix cancer.
Keywords: Nab-paclitaxel, GOG, Cervix cancer, Drug resistant cervix cancer
Introduction
Cancer of the uterine cervix is the second most common cancer in women worldwide and represents 15% of all female cancers. In the United States, cervix cancer is the third most common gynecologic malignancy with approximately 12,700 new cases in 2011 [1]. For patients with advanced, persistent or recurrent squamous cell carcinoma of the cervix no longer amenable to surgical resection or radiation therapy, the prognosis is bleak. The need for effective therapy in this clinical setting is well recognized and optimal treatment has yet to be defined. The study of new agents for the treatment of advanced cervical cancer is clearly necessary.
Paclitaxel has a broad spectrum of activity against many gynecologic malignancies including ovarian, uterine and cervical cancer. McGuire et al. performed a phase II study of previously untreated patients with advanced squamous cell carcinoma of the cervix [2]. At a dose of 170 mg/m2 every three weeks, the primary and dose-limiting toxicity was neutropenia. A response rate of 17% was documented and the investigators concluded that paclitaxel had sufficient activity to merit further study. Rose et al. treated 47 patients with advanced squamous cell carcinoma of the cervix with a combination of paclitaxel and cisplatin with promising results; however, the majority of patients experienced grade 3 or 4 neutropenia and there were two toxic deaths [3].
Nab-paclitaxel is a nanoparticle formulation of albumin-bound paclitaxel [4]. No premedication is required as the risk of hypersensitivity is remote [5]. A phase I trial of nab-paclitaxel administered weekly for three weeks, followed by a one-week rest in patients with advanced solid tumors, was completed [6]. The MTDs for heavily and lightly pre-treated patients were 100 and 150 mg/m2 respectively. Dose-limiting toxicities included myelosuppression and peripheral neuropathy. Premedication was not required, and the observed adverse events were consistent with the known taxane safety profile. Phase II trials in the treatment of metastatic breast cancer have shown promising improvement in efficacy and improved safety of weekly nab-paclitaxel dosing which also takes advantage of its potential antiangiogenic properties similar to those of bevacizumab in squamous cell cancers of the cervix [7–10].
Given the lack of efficacious therapy for advanced cervical cancer, the demonstrable activity of paclitaxel in advanced cervical cancer, and the unique properties of nab-paclitaxel, it was considered appropriate to study this promising drug in patients with persistent or recurrent squamous cell carcinoma of the cervix who have progressed on one prior cytotoxic regimen not including drugs used concomitantly with radiation.
Methods
To be eligible for study, patients had persistent or recurrent squamous or non-squamous cell carcinoma of the cervix with documented disease progression. Histologic documentation of the original primary tumor was required via the pathology report. All patients had to have measurable disease, defined as at least one lesion that can be accurately measured in at least one dimension (longest dimension to be recorded). Each lesion had to be ≥20 mm when measured by conventional techniques, including palpation, plain X-ray, CT and MRI or ≥10 mm when measured by spiral CT. Patients had at least one “target-lesion” to be used to assess response on this protocol as defined by Response Evaluation Criteria in Solid Tumors (RECIST) guidelines v1.0 [11]. Patients had to have a GOG performance status of 0, 1 or 2 and must have recovered from effects of recent surgery, radiotherapy, or chemotherapy (i.e. discontinued at least 3 weeks prior to study registration).
Patients must have had one prior systemic chemotherapeutic regimen for management of advanced, metastatic, or recurrent squamous or non-squamous cell carcinoma of the cervix. Chemotherapy administered in conjunction with primary radiation as a radio-sensitizer was not counted as a systemic chemotherapy regimen. Patients must not have received prior treatment with a taxane or more than one previous cytotoxic chemotherapy regimen (either with single or combination cytotoxic drug therapy) but were allowed to receive one additional non-cytotoxic regimen for management of recurrent or persistent disease.
Patients must have had adequate: 1) bone marrow function (platelet count ≥100,000/mcl and ANC count ≥1500/mcl, equivalent to Common Terminology Criteria for Adverse Events (CTCAE) v3.0 Grade 1); 2) Renal function (creatine ≤1.5 × institutional upper limit normal, CTCAE v3.0 Grade 1); 3) Hepatic function (bilirubin ≤1.5 × upper limit of normal Common Toxicity Criteria (CTC) Grade 1, SGOT and alkaline phosphatase ≤2.5 × upper limit of normal, CTCAE v3.0 Grade 1); and 4) Neurologic function (neuropathy ≤ CTCAE v3.0 Grade 2). Finally, patients had to sign an approved informed consent and authorization permitting the release of personal health information, and those patients of childbearing potential must have had a negative serum pregnancy test prior to study entry.
Treatment plan
Eligible patients enrolled onto this phase II study received nab-paclitaxel 125 mg/m2, intravenously over 30 min on days 1, 8 and 15 of a 28 day cycle. No premedication for the prevention of hypersensitivity reaction (HSR), nausea or vomiting was required. The maximal body surface area used for dose calculations was 2.0 m2. Patients were allowed to continue on therapy indefinitely until (1) withdrawal of consent, (2) evidence of disease progression, (3) significant side effects precluding further administration, or (4) inability to tolerate the lowest doses due to toxicity.
Treatment modifications
Hematologic toxicity
Treatment modifications were based on the absolute neutrophil count (ANC). Subsequent cycles of therapy required the ANC to be ≥ 1500 cells/mcl (CTCAE v3.0 Grade 1) and the platelet count to be ≥ 100,000/mcl. Therapy could be delayed for a maximum of 2 weeks. Patients who failed to recover adequate counts after a 2 week delay were removed from study. Patients experiencing a first occurrence of febrile neutropenia, and/or documented grade 4 neutropenia persisting ≥ 7 days, underwent a one dose level reduction (dose level – 1:100 mg/m2, dose level – 2:80 mg/m2) in nab-paclitaxel dosage for subsequent cycles. For recurrent febrile neutropenia, and/or recurrent documented grade 4 neutropenia persisting ≥ 7 days (after initial dose reduction), prophylactic growth factors were administered. Patients with grade 4 thrombocytopenia underwent a 1 level dose reduction.
Non-hematologic toxicity
Grade 2 (or greater) peripheral neuropathy required a reduction of one dose level (to 100 mg/m2) and delay in subsequent therapy for a maximum of two weeks until recovered to grade 1. This modification was also required for a grade 2 (or greater) renal toxicity. Grade 3 (or greater) elevations in SGOT (AST), SGPT (ALT), alkaline phosphatase or bilirubin required a reduction of one dose level and delay in subsequent therapy for a maximum of two weeks until recovered to grade 1. Non-hematologic toxicities with an impact on organ function of grade 2 (or greater) required a reduction of one dose level and delay in subsequent therapy for a maximum of two weeks until recovered to grade 1, or pre-therapy baseline. Patients with grade 3 hypersensitivity reactions were allowed retreatment after standard paclitaxel premedication at the discretion of the investigator. Intolerance of level — 2 dosing (80 mg/m2) necessitated removal from study.
Study parameters and method of evaluation
Following registration but before first infusion, patients underwent a complete history and physical exam, including a bimanual pelvic evaluation, clinical tumor assessment, pre-existing toxicity assessment, routine hematological and blood chemistries, chest imaging (chest radiograph or computed tomography of the thorax), radiograph tumor measurement [for RECIST 1.0 “target lesion(s)”]. A CBC and differential were obtained weekly on therapy, with other laboratory blood testing (similar to baseline) obtained prior to each treatment cycle (28 days).
Standard GOG-RECIST (v1.0) criteria were utilized to evaluate response and progression on this trial. Confirmation of all documented responses by imaging and/or exam was required no sooner than four weeks following the initial documentation of response. PFS was calculated from study entry until documented disease progression, death or date of last contact. OS was defined from the date of study entry until death or date of last contact.
Statistical considerations
The primary endpoint of this study was frequency of objective tumor response. Secondary endpoints included frequency and severity of adverse effects, and duration of PFS and OS. The study used a flexible, two-stage accrual design that allowed stopping early for lack of treatment activity [11]. During the first stage of accrual, 22–29 patients were to be entered and evaluated; if more than 3 out of 22–24 or 4 out of 25–29 patients had a complete (CR) or partial response (PR), a second phase of accrual was to be initiated, with a total two-stage accrual of 53–60 patients. The regimen would be considered active if more than 10 out of 53, or 11 out of 54–57, or 12 out of 58–60 patients had a PR or CR. If the true response rate was 15%, the average probability of designating the treatment as active would be limited to 10%. On the other hand, if the true response rate was 30%, the probability of correctly classifying the treatments as active would be 90%.
Results
The objective response rate for the first stage accrual met the criteria to proceed to the second stage; however, because of slow patient registration to the second stage, patient accrual was halted at 37 patients. Two patients were ineligible, due to having had no prior chemotherapy. Table 1 presents the characteristics of the eligible and treated patients. All 35 eligible patients had at least one prior chemotherapy regimen and progressed and 27 of them had prior radiation therapy with concomitant cisplatin. The median number of nab-paclitaxel cycles administered was 4 (range 1–15); the most common reason for treatment discontinuation was disease progression, occurring in 25 (71.4%) patients.
Table 1.
Characteristics of evaluable patients (n=35).
| Characteristic | Number of cases | % |
|---|---|---|
| Age | ||
| <40 | 6 | 17.1 |
| 40–49 | 15 | 42.9 |
| 50–59 | 10 | 28.6 |
| 60–69 | 3 | 8.6 |
| >70 | 1 | 2.9 |
| Race | ||
| African American | 3 | 8.6 |
| Hispanic | 0 | 0.0 |
| American Indian | 1 | 2.9 |
| White | 30 | 85.7 |
| Unreported | 1 | 2.9 |
| Performance status | ||
| 0 | 19 | 54.3 |
| 1 | 10 | 28.6 |
| 2 | 6 | 17.1 |
| Cell type | ||
| Squamous | 22 | 62.8 |
| Adenocarcinoma | 9 | 25.7 |
| Small cell | 1 | 2.9 |
| Endometrioid adenocarcinoma | 1 | 2.9 |
| Undifferentiated | 1 | 2.9 |
| Adenosquamous | 1 | 2.9 |
| Grade | ||
| 1 | 3 | 8.6 |
| 2 | 17 | 48.6 |
| 3 | 14 | 40.0 |
| Unspecified | 1 | 2.9 |
| Prior chemotherapy | ||
| 1 regimen | 35 | 100.0 |
| Prior radiation | 27 | 77.1 |
| Cycles of treatment | ||
| 1 | 2 | 5.7 |
| 2 | 10 | 28.6 |
| 3 | 2 | 5.7 |
| 4 | 5 | 14.3 |
| 5 | 2 | 5.7 |
| >5 | 14 | 40.0 |
Table 2 presents the adverse events attributable to the study agent. The only NCI CTCAE grade 4 or higher event was grade 4 neutropenia in 2 patients (5.7%). Severe non-hematological toxicities were infrequent. Neurotoxicity was observed as grade 3 in one patient (2.9%) and grade 2 in eight patients (22.8%) (no grade 4 neurotoxicity). No grade 4 fatigue was reported in this cohort; however, there were five (14%) reported grade 3 symptoms. In seven patients, the dose was reduced to 100 mg/m2 and, for three of these patients, the dose was further reduced to 80 mg/m2.
Table 2.
All grade adverse events.
| Adverse event | Grade | ||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| Leukopenia | 11 | 10 | 12 | 3 | 0 |
| Thrombocytopenia | 30 | 4 | 0 | 1 | 0 |
| Neutropenia | 20 | 6 | 6 | 1 | 2 |
| Anemia | 3 | 6 | 22 | 4 | 0 |
| Nausea/vomiting | 15 | 11 | 9 | 0 | 0 |
| Other GI | 13 | 10 | 10 | 2 | 0 |
| GU | 34 | 1 | 0 | 0 | 0 |
| Neurotoxicity | 15 | 11 | 8 | 1 | 0 |
| Pain | 20 | 9 | 4 | 2 | 0 |
| Pulmonary | 30 | 1 | 2 | 2 | 0 |
| Cardiovascular | 34 | 0 | 1 | 0 | 0 |
| Constitutional (Fatigue) | 8 | 7 | 15 | 5 | 0 |
| Metabolic | 24 | 5 | 2 | 4 | 0 |
| Dermatologic | 30 | 1 | 3 | 1 | 0 |
| Alopecia | 17 | 3 | 15 | - | - |
| Musculoskeletal | 32 | 2 | 1 | 0 | 0 |
| Auditory | 31 | 0 | 1 | 0 | 0 |
| Infection | 27 | 0 | 4 | 4 | 0 |
| SGOT | 34 | 1 | 0 | 0 | 0 |
| Alkaline phosphatase | 34 | 1 | 0 | 0 | 0 |
| Lymphatics | 31 | 2 | 2 | 0 | 0 |
No treatment related deaths have been reported.
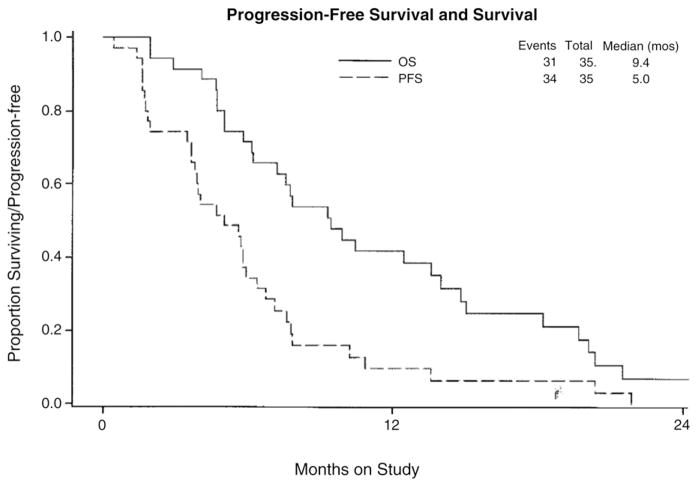
Of the 35 patients evaluable for efficacy, 10 (28.6%, 95% CI: 14.6%–46.3%) had partial responses (0 complete responses) lasting a median of 6.0 months (range 1.5 to 9.2); another 15 patients (42.9%) had stable disease lasting a median of 5.8 months (range 3.7 to 11.4 months), while eight (22.9%) patients had increasing disease. Response could not be evaluated in two (5.7%). Five of the partial responses were documented in viscera (i.e. liver in 2 patients, and 1 each in lung, pancreas and bladder), whereas the other 5 partial responses occurred in the retroperitoneal (i.e. 3 patients), mediastinal (i.e. 1 patient) and intraperitoneal (i.e. 1 patient) lymph nodes. Eight of the 35 patients had evidence at entry to this trial. Two (25%) of these patients responded to nab-paclitaxel in their in-field recurrences (i.e. left external iliac and left and right external iliac nodes were the primary target lesions). The median PFS and OS are 5 months and 9.4 months, respectively (Fig. 1). At this point, three patients were alive, one of which is without progression.
Fig. 1.
Kaplan–Meier progression free and overall survival curves for 35 eligible, relapsed cervix cancer patients treated with a weekly dosing schedule with nab-paclitaxel. Median progression-free survival was 5.0 months, while median overall survival was 9.4 months.
Discussion
Nab-paclitaxel is an FDA-approved drug for the treatment of meta-static breast cancer, using an every 3 week dosing schedule [9]. It also appears to have considerable activity in the management of metastatic non-small cell lung cancer, pancreatic and ovarian cancer and melanoma, using a weekly dosing schedule of 125 mg/m2 on days 1, 8 and 15 of a 28 day cycle [6,12,13]. It has proven to have an improved therapeutic and cost-effective index than docetaxel in direct comparisons [8,9].
Although nab-paclitaxel has been utilized mainly in the treatment of adenocarcinomas of the breast, lung and pancreas, there are few published data concerning its activity against squamous cell cancers of any type. In this multi-institutional, phase II trial, the GOG chose the weekly dosing schedule of 125 mg/m2 on days 1, 8 and 15 every 28 days to test the activity of nab-paclitaxel in patients, who had progressed or recurred after 1 prior cytotoxic regimen for meta-static cervix cancer of all histologic types. This nab-paclitaxel dosing schedule was selected to take advantage of its antiangiogenic properties, especially for squamous cell carcinomas of the cervix which express enhanced angiogenesis [10,14,15].
Because there are so few cytotoxic drugs with useful activity against metastatic cervix cancer, the GOG has set up a series of single agent studies for patients, who have progressed on, or shortly following, one prior cytotoxic drug treatment (i.e. for metastatic disease). Thus far, only cisplatin, carboplatin, topotecan and paclitaxel have proven to have useful activity against advanced squamous cell carcinoma of the cervix, but even these cytotoxic agents provide little extension of progression-free and/or overall survival [16].
This phase II single agent trial had a classical two-stage Chen design [12]. The first stage accrued its patients rapidly and met the statistical conditions required for a move into the second stage (with accrual of another 14 patients). In fact, the 28.9% objective response rate in the 35 eligible patients is the highest ever recorded in the GOG for a single-agent against drug refractory, platinum resistant disease [16–20,10]. It is of considerable interest that nab-paclitaxel therapy appeared equally active in patients, who had primarily lymph node metastases or visceral metastases. Because of slow patient registration to the second stage of the study, patient accrual was closed prematurely at 37 registered patients. Thus, while a high degree of activity was noted during the first study stage, no definitive conclusions are possible. As with other solid tumor studies with this agent, patients experienced less myelotoxicity and neurotoxicity than would be expected with weekly dosing schedules of paclitaxel in heavily pretreated populations. Thus, nab-paclitaxel may be considered a leading candidate for future studies of combinations of agents in both the adjuvant and advanced disease settings, especially evaluating weekly dosing schedules.
HIGHLIGHTS.
Nab-paclitaxel given on a weekly schedule was associated with a 28.6% objective response rate in drug resistant cervix cancer.
Nab-paclitaxel was also associated with an additional 42.9% stable disease rate in drug-resistant cervix cancer.
Severe adverse events were uncommon with grade 4 neutropenia in 5.4% and grade 3 neurotoxicity in 1 (2.9%) of patients.
Acknowledgments
This study was supported in part by the National Cancer Institute grants to the Gynecologic Oncology Group Administrative Office (CA 27469) and the Gynecologic Oncology Group Statistical and Data Center (CA 37517). We would also like to thank Abraxis Bioscience, LLC, (a wholly owned subsidiary of Celgene Corporation) for their generous support.
The following Gynecologic Oncology Group member institutions participated in this study: Roswell Park Cancer Institute; University of North Carolina; State University of New York at Brooklyn; Cleveland Clinic; State University of New York at Stony Brook; Cooper Hospital/University Medical Center; M.D. Anderson Cancer Center; Fox Chase Cancer Center; University of Oklahoma; Case Western Reserve University; University of Wisconsin Hospital; and the Community Clinical Oncology Program.
Footnotes
Conflict of interest statement
The authors report that there are no conflicts of interest to declare.
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics. CA Cancer J Clin. 2011;61:212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.McGuire WP, Blessing JA, Moore D, Lentz SS, Photopulos G. Paclitaxel has moderate activity in squamous cervix cancer: a Gynecologic Oncology Group study. J Clin Oncol. 1996;14:792–5. doi: 10.1200/JCO.1996.14.3.792. [DOI] [PubMed] [Google Scholar]
- 3.Rose PG, Blessing JA, Gershenson DM, McGehee R. Paclitaxel and cisplatin as a first-line therapy in recurrent or advanced squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. J Clin Oncol. 1999;17:2676–80. doi: 10.1200/JCO.1999.17.9.2676. [DOI] [PubMed] [Google Scholar]
- 4.Gradishar WJ. Albumin-bound paclitaxel: a next-generation taxane. Expert Opin Pharmacother. 2006:1041–53. doi: 10.1517/14656566.7.8.1041. [DOI] [PubMed] [Google Scholar]
- 5.Micha JP, Goldstein BH, Birk CL, Rettenmaier MA, Brown JV., III Abraxane in the treatment of ovarian cancer: the absence of hypersensitivity reactions. Gynecol Oncol. 2006;100:437–8. doi: 10.1016/j.ygyno.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Nyman DW, Campbell KJ, Hersh E, Long K, Richardson U, Trieu V, et al. Phase I and pharmacokinetics trial of ABI-007, a novel nanoparticle formulation of paclitaxel in patients with advanced nonhematologic malignancies. J Clin Oncol. 2005;23:7785–93. doi: 10.1200/JCO.2004.00.6148. [DOI] [PubMed] [Google Scholar]
- 7.Henderson IC, Bhatia V. Nab-paclitaxel for breast cancer: a new formulation with an improved safety profile and greater efficacy. Expert Rev Anticancer Ther. 2007;7:919–43. doi: 10.1586/14737140.7.7.919. [DOI] [PubMed] [Google Scholar]
- 8.Dranitsaris G, Coleman R, Gradishar W. Nab-paclitaxel weekly or every 3 weeks compared to standard docetaxel as first-line therapy in patients with metastatic breast cancer: an economic analysis of a prospective randomized trial. Cancer Res Treat. 2010;119:717–24. doi: 10.1007/s10549-009-0424-z. [DOI] [PubMed] [Google Scholar]
- 9.Gradishar WJ, Krasnojan D, Cheporov S, Makhson AW, Marikhas GM, Clawson A, et al. Significantly longer progression-free survival with nab-paclitaxel compared with docetaxel as first-line therapy for metastatic breast cancer. J Clin Oncol. 2009;27:3611–9. doi: 10.1200/JCO.2008.18.5397. [DOI] [PubMed] [Google Scholar]
- 10.Monk BJ, Sill MW, Burger RA, Gray HJ, Buekers TE, Roman LD. Phase II trial of Bevacizumab in the treatment of persistent or recurrent squamous cell carcinoma of the cervix: a Gynecologic Oncology Group Study. J Clin Oncol. 2009;27:1069–74. doi: 10.1200/JCO.2008.18.9043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumors. Revised RECIST guideline (version 1. 1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 12.Chen TT, Ng TH. Optimal flexible designs in phase II clinical trials. Stat Med. 1998;17:2301–12. doi: 10.1002/(sici)1097-0258(19981030)17:20<2301::aid-sim927>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 13.Paik PK, James LP, Riely GJ, Azzoli CG, Miller VA, Nq KK, et al. A phase 2 study of weekly albumin-bound paclitaxel (Abraxane) given as a two-hour infusion. Cancer Chemother Pharmacol. 2011;68:1331–7. doi: 10.1007/s00280-011-1621-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santin AD, Hermonat PL, Ravaggi A, Percorelli S, Cannon MJ, Parham GP. Secretion of vascular endothelial growth factor in adenocarcinoma and squamous cell carcinoma of the uterine cervix. Obstet Gynecol. 1999;94:78–82. doi: 10.1016/s0029-7844(99)00282-3. [DOI] [PubMed] [Google Scholar]
- 15.Lee IJ, Park KR, Lee KK, Song JS, Lee KG, Lee JY, et al. Prognostic value of vascular endothelial growth factor in Stage 1B carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys. 2002;54:768–79. doi: 10.1016/s0360-3016(02)02970-x. [DOI] [PubMed] [Google Scholar]
- 16.Tewari KS, Monk BJ. Gynecologic Oncology Group trials of chemotherapy for metastatic and recurrent cervical cancer. Curr Oncol Rep. 2005;7:419–34. doi: 10.1007/s11912-005-0007-z. [DOI] [PubMed] [Google Scholar]
- 17.Rose PG, Blessing JA, Arseneau J. Phase II evaluation of altretamine for advanced and recurrent squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecol Oncol. 1996;62:100–2. doi: 10.1006/gyno.1996.0196. [DOI] [PubMed] [Google Scholar]
- 18.Bookman MA, Blessing JA, Hanjani P, Herzog TJ, Anderson WA. Topotecan in squamous cell carcinoma of the cervix. A phase II Gynecologic Oncology Group study. Gynecol Oncol. 2000;77:446–9. doi: 10.1006/gyno.2000.5807. [DOI] [PubMed] [Google Scholar]
- 19.Rose PG, Blessing JA, Morgan M, Van Le L, Waggoner S. Prolonged oral etoposide in recurrent or advanced squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecol Oncol. 1998;70:263–6. doi: 10.1006/gyno.1998.5097. [DOI] [PubMed] [Google Scholar]
- 20.Schlider R, Blessing JA, Morgan M, Mangan CE, Rader JS. Evaluation of gemcitabine in patients with squamous cell carcinoma of the cervix: a phase II study of the Gynecologic Oncology Group study. Gynecol Oncol. 2000;76:204–7. doi: 10.1006/gyno.1999.5671. [DOI] [PubMed] [Google Scholar]