Abstract
Objectives
After surgical debulking and volume-directed irradiation of the pelvis/para-aortic lymph nodes, treatment was randomized to compare recurrence-free survival (RFS) and toxicity between two chemotherapy regimens for the treatment of women with advanced stage endometrial carcinoma.
Methods
Treatment was randomized between 6 cycles of cisplatin [C] (50 mg/m2) and doxorubicin [D] (45 mg/m2) with or without paclitaxel [P] (160 mg/m2). Initially in paclitaxel treated patients and, after May 2002, all patients received granulocyte growth factor with each cycle.
Results
Of 659 patients enrolled following surgery, 552 eligible patients were randomized to chemotherapy after irradiation. Accrual closed to Stage IV patients in June, 2003. Approximately 80% completed six cycles of chemotherapy. Three deaths resulted from bowel complications and one death was due to renal failure. Hematologic adverse events, sensory neuropathy and myalgia, were more frequent and severe in the paclitaxel arm (p< 0.01) which was confirmed by Quality of Life assessments. Percentage of patients alive and recurrence-free at 36 months was 62% for CD vs. 64% for CDP. The hazard of recurrence or death relative to the CD arm stratified by stage is 0.90 (95% CI is 0.69 to 1.17, p=0.21, one-tail). However, in subgroup analysis, CDP was associated with a 50% reduction in the risk of recurrence or death among patients with gross residual disease (95% CI: 0.26 to 0.92). Stage, residual disease, histology/grade, positive para-aortic node and cytology, pelvic metastases and age were significantly associated with RFS.
Conclusion
The addition of paclitaxel to cisplatin and doxorubicin following surgery and radiation was not associated with a significant improvement in RFS but was associated with increased toxicity.
Keywords: Advanced Endometrial Carcinoma, Surgery, Radiation and Chemotherapy
Introduction
The optimal adjuvant therapy following surgical staging and maximum cytoreduction in stage III and IV endometrial carcinoma is yet to be established. Postoperative abdomino-pelvic irradiation has been found to be efficacious for advanced endometrial cancer. [1–5]. Gynecologic Oncology Group (GOG) Protocol 94, a single arm phase II trial, established WAI to be tolerable with reasonable efficacy [6].
Chemotherapeutic options were explored in GOG Protocol 107, which compared single-agent doxorubicin with the combination of doxorubicin and cisplatin [7]. Although no differences in survival were seen, the response rate was significantly higher with the two-drug regimen and the data suggested an increase in progression-free survival.
The results of GOG Protocols 94 and 107 led to the design of GOG 122, which compared WAI to the combination of cisplatin and doxorubicin [8] in patients with advanced, optimally debulked (≤ 2 cm residual disease) endometrial cancer. In that study, WAI was prescribed as 30 Gy in 20 fractions to the abdomen followed by 15 Gy in 8 fractions to the pelvis. The chemotherapy regimen consisted of seven cycles of doxorubicin 60 mg/m2 and cisplatin 50 mg/m2 every 3 weeks followed by one additional cycle of cisplatin.
Paclitaxel appears to elicit good response in endometrial cancer based upon both phase II and phase III data. In GOG 129-C, as a second line single agent for persistent or advanced endometrial carcinoma, paclitaxel achieved a 28% response rate with a 6.5% compete response rate [9].
It was thought that further advantages may be realized by combining both adjuvant chemotherapy and radiation given prior results suggesting activity and/or feasibility of combining irradiation with chemotherapeutic in the treatment of advanced endometrial cancer. [10–12]. Considering the higher objective response rates reported in GOG 107 for the combination of doxorubicin and cisplatin in advanced endometrial carcinoma combined with the favorable irradiation results of GOG 94 and the positive findings of GOG 129-C with respect to paclitaxel, it seemed appropriate to combine tumor volume- directed pelvic irradiation with chemotherapy arms containing all active chemotherapeutic agents.
The general underlying hypothesis was that, following surgical cytoreduction and radiotherapy, combination chemotherapy may reduce both locoregional and systemic recurrence rates. Sequential rather than concurrent delivery of the two modalities was proposed in an attempt to limit the overall toxicity and allow a maximum therapeutic dosing of both modalities.
Therefore, the primary objective of this trial was to test the hypothesis of no increase in recurrence-free survival associated with the addition of paclitaxel to cisplatin and doxorubicin in patients with Stage III or IV endometrial carcinoma (≤ 2 cm residual disease) following initial surgery and tumor volume directed irradiation. Both acute and long term adverse events were to be assessed including patient-reported peripheral neuropathy.
Methods
Patients diagnosed with Stage III or IV endometrial carcinoma of any histology, including clear cell and serous papillary carcinomas, with disease limited to the pelvis and abdomen, were initially eligible. When the results of GOG 122 were reported demonstrating chemotherapy to be superior to radiation, patients with disease outside of the pelvis were no longer permitted to enroll in this study except patients with positive para-aortic nodes. As of June 2003, eligible patients had to have positive adnexa, tumor invading the uterine serosa, positive pelvic and/or para-aortic nodes, positive pelvic washings or vaginal involvement within the radiation port. Surgery must have included hysterectomy and bilateral salpingoophorectomy. Pelvic or para-aortic lymph node sampling was not required. Radiotherapy (RT) was to be initiated within 8 weeks after surgery, and chemotherapy was to be initiated within 8 weeks after radiation.
Tumor debulking must have resulted in a maximal residual diameter of 2 cm. All patients with positive para-aortic node were required to undergo scalene node biopsy and/or chest CT scan. Ineligibility would result if either test was positive for metastasis. Pre-entry chemistry requirements included: absolute neutrophil count (ANC) ≥ 1500/mcl, platelet count ≥ 100,000/mcl, SGOT, SGPT, and alkaline phosphatase ≤ 3 X normal, bilirubin ≤ 1.5 X normal, creatinine ≤ 1.6 mg/dl, and LVEF ≥ 50% measured within 6 months of entry. Patients must have had a GOG performance status of no more than two. IRB approval and informed consent were required.
Ineligible patients included those with recurrent disease, a history of pelvic or abdominal radiation therapy, a history of malignancy evident within the last 5 years or who had received prior chemotherapy or radiation therapy for that malignancy or a history of a serious comorbid illness that would preclude protocol therapy were ineligible and those with an expected survival of less than three months.
After obtaining informed consent, patients were registered with verification of eligibility, prior to initiation of radiation therapy. Tumor volume directed pelvic plus or minus para-aortic node irradiation with or without vaginal boost was then to be given to all patients.
All radiation treatments were delivered by megavoltage equipment ranging from 6 to 25 MV photons. The minimum source skin distance (SSD) was 80 cm. Cobalt 60 equipment was not acceptable. If an intravaginal boost was used, it was delivered with an intravaginal cylinder at high dose rate (HDR) or low dose rate (LDR). Acceptable isotopes included cobalt or iridium for HDR, radium or cesium for LDR. Localization films taken on the simulator were necessary in all cases. To participate in the trial, the institutions must have demonstrated ability to achieve an accuracy of ± 3% in measuring the output of their sources and ± 5% in delivering the prescribed dose.
Patients with pathologically negative para-aortic lymph nodes (at least 2 nodes sampled) were treated with only pelvic irradiation. If the para-aortic lymph nodes were positive, or if fewer than two para-aortic nodes were sampled, the patient received extended field pelvic/para-aortic radiotherapy. If there was tumor extension into the vagina, the external beam field was modified to include the disease volume with a 2 cm margin with the patient receiving intravaginal boost brachytherapy at the discretion of the radiation oncologist.
The pelvis was treated with a four-field box technique with a superior border at the L5/S1 interspace, the inferior border to the mid-portion of the obturator foramen, and the lateral borders 1.5 cm beyond the lateral margin of the true pelvis at its widest point.
The extended pelvic/para-aortic fields were treated with a four-field technique covering both areas, a four-field pelvic technique with an appropriately gapped AP/PA para-aortic (PAN) field, or a combined technique using AP/PA fields that covered both areas, and lateral fields that covered only the pelvis. The para-aortic field extended to the T11/T12 interspace, with lateral borders that had a margin of at least 1 cm on all lymph nodes. The pelvis was treated with a daily fraction size of 1.80 Gy to a total dose of 50.40 Gy. Para-aortic lymph nodes were treated with a daily fraction size of 1.50–1.80 Gy, to a total dose of 43.50 Gy.
The optional intravaginal boost was delivered with a vaginal cylinder. A dose of 7 Gy HDR in one fraction or 10 Gy LDR in one insertion, at .4–.65 Gy/hr, measured at a depth of 5 mm from the surface of the cylinder, was prescribed. Alternate fractionation schedules were accepted, so long as they delivered a biologically equivalent boost dose.
Following radiation therapy, the GOG Statistical and Data Center (SDC) randomly assigned the treatment regimen to patients agreeing to continue on study and who had no evidence of recurrent disease. The sequence of treatment assignments, allocated with equal probability within strata using balanced blocks, was concealed from institutions and patients until randomization. Stratum levels were defined by the use of extended field radiation.
The control regimen for this study consisted of doxorubicin (D) 45 mg/m2 IV followed immediately by cisplatin (C) 50 mg/m2 IV with optional filgrastim (G-CSF) 5mcg/kg/day on days 2–11. The maximum body surface area used for dose calculations was 2.0 m2. Beginning in May, 2002, filgrastim or pegfilgrastim was included in this regimen until the absolute neutrophil count (ANC) had reached 10,000/mm3 following the expected chemotherapy induced neutrophil nadir. The chemotherapy was to be administered every 21 days for a maximum of six cycles.
The experimental regimen for this trial was the same for doxorubicin (D) and cisplatin (C) on day 1 but on day 2 paclitaxel (P) 160 mg/m2 IV over 3 hours was added. Filgrastim 5 mcg/kg/day on days 3–12 or pegfilgrastim 6 mg on day 3 was to be given. The treatment interval was 21 days for a maximum of six cycles.
On day one, antiemetics included dexamethasone 10 mg IV and a 5HT3 antagonist. The paclitaxel premedication consisted of dexamethasone 20 mg 5–12 hours prior to paclitaxel.
For grade 4 hematologic toxicity, doxorubicin was reduced to 30 mg/m2, cisplatin to 30 mg/m2 and paclitaxel to 125 and 100 mg/m2. No cycle of study therapy was to be given until the ANC was ≥ 1000/mcl and the platelets ≥ 100,000/mcl. Treatment discontinuation was recommended in patients who experienced neutropenia lasting longer than 14 days, despiteG -CSF, and in patients on the lowest dose level with neutropenic fever or a treatment delay of more than seven days. All study treatment was discontinued in patients with progressive disease.
Data were collected and reviewed centrally at the SDC. The protocol study chairs (HDH and (SKG) reviewed patient data to assess protocol compliance and adverse events. The protocol Radiation Oncology Co-Chair (SKG) reviewed all reported grade 3–5 adverse events attributed to radiotherapy. Pathology materials were collected at the SDC and reviewed by members of the GOG Pathology Committee including slides documenting the primary and metastatic disease, histologic tumor cell type and grade. The GOG Gynecologic Oncology Committee centrally reviewed all patient eligibility data without knowledge of outcome. The GOG Radiation Oncology Committee centrally reviewed all radiation treatment materials including films, dosimetry and daily treatment records.
The primary endpoint used to compare the treatment regimens is recurrence-free survival (RFS). Recurrence-free survival is defined as the number of months a patient survives without reappearance or progression of disease starting from chemotherapy randomization. Patients alive without disease recurrence are censored at the date of last contact.
The accrual goal was originally 434 patients with follow-up until 218 RFS events were reported. This sample size was increased to 614 to offset the decrease in overall risk of recurrence when patients with stage IV disease were no longer enrolled. This increase was reviewed and approved without knowledge of treatment comparison results. Two hundred and eighteen events would provide statistical power of 80% to detect a proportional decrease of 29% in the hazard rate when testing at the level of 0.05 with a one-tail test [13]. A planned interim efficacy and futility analysis of RFS was performed when 137 disease recurrences or deaths were reported to the Data Monitoring Committee (DMC) in January 2005 [14]. The DMC voted to continue the study as planned.
The cumulative incidence of local-regional recurrences (LRR) counted recurrences when the initial site of recurrence was limited to the following areas: pelvis, vagina, and pelvic or para-aortic nodes. The cumulative incidence of distant recurrence (DR) counted any distant recurrence outside these areas of LRR. Deaths prior to recurrence were considered competing events for estimating cumulative incidence. Distant recurrences were also considered as competing events for calculating the cumulative incidence of local-regional recurrence. At the time of this report, OS data were not sufficiently mature for final analysis with only 181 deaths reported.
All acute adverse events, regardless of attribution, were graded using the Common Toxicity Criteria Version 2.0 for patients who received study chemotherapy. Adverse events occurring after completion of treatment were graded according to the RTOG/EORTC Late Radiation Morbidity Scoring Scheme. The cumulative incidence of treatment-related grade 3 or higher gastrointestinal (GI) effects during follow-up was estimated. Deaths prior to documentation of late GI toxicity were considered competing events.
A log rank test [15] stratified by FIGO Stage (III vs. IV) was used to test the independence of treatment with RFS in all eligible patients categorized by their random treatment assignment. The product-limit method [16] was used to obtain RFS life table estimates. The treatment effect on RFS, adjusting for FIGO Stage, was estimated using a Cox proportional hazards (PH) model [17]. Multiple variable PH regression models with baseline clinical, pathologic and host characteristic covariates were used to assess prognostic factors and subgroup effects for exploratory analyses.
Starting after August 27, 2001, the FACT/GOG-Ntx subscale was administered to patients to assess patient-reported peripheral neurotoxicity at baseline, end of treatment and 6 months after treatment [18]. Scores could range from 0 (severe neuropathy) to 44 (no neuropathy) and were prorated when more than 50% and less than 100% of items were answered.
Baseline Ntx scores were examined for their comparability between arms using a two-sample two-tailed t-test. The independence of the follow-up Ntx score and treatment was analyzed using a linear mixed model with unstructured covariance matrix accounting for correlations among the repeated measures and adjusted for the baseline score. Restricted maximum likelihood was used to estimate the covariance parameters. The denominator degrees of freedom were computed using a general Satterthwaite approximation. The interaction of treatment and assessment point was examined for constant treatment differences in the Ntx subscale scores across time. When the interaction effect was significant, treatment differences in the Ntx subscale score were tested at each time point using contrasts to obtain adjusted mean differences.
Results
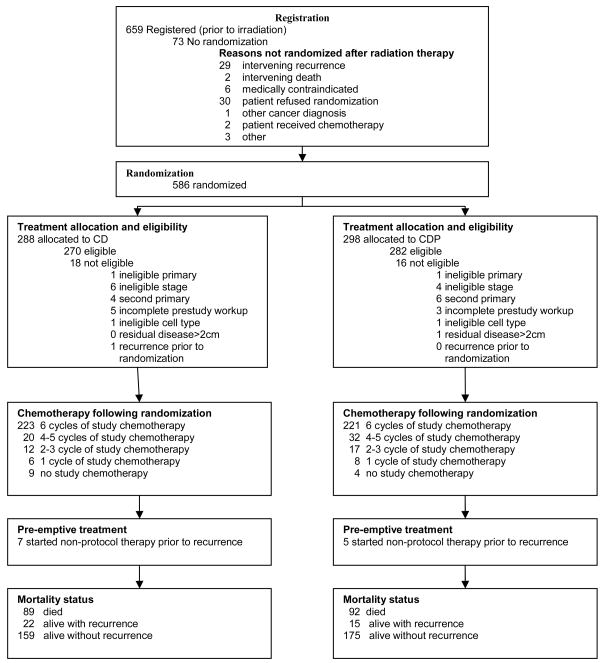
From July 3, 2000 to September 13, 2004, 659 patients were enrolled onto this trial following surgery and informed consent. Of the 659 registered patients, 552 were subsequently deemed eligible and, following irradiation, received a random treatment assignment. (Figure 1). Following irradiation, there were 30 patients who refused further treatment or randomization, 29 patients with intervening progression of cancer and 14 patients with various other reasons for not participating in the randomized component of this study (including two deaths prior to randomization, one attributable to radiation therapy) [18,19]. This report includes 218 RFS events with a median follow-up of 47 months among living patients without evidence of recurrence.
Figure 1.
CONSORT Diagram
As noted in Table 1 (Characteristics of Randomized Patients), the median age for both arms of the study was 58 and most (89%) of the patients were Caucasian. Of the 552 randomized patients, 18% had either clear cell or serous histology. Sixty-six Stage IV patients were randomized before the results of GOG 122 (June, 2003) were released and the eligibility criteria no longer permitted Stage IV patients to be enrolled. The median pretreatment CA 125 value was 32 mcg/ml.
Table 1.
Characteristics of Randomized Patients
| CD | CDP | ||||
|---|---|---|---|---|---|
| Characteristic | Category | n | % | n | % |
| Age Group | 30–39 | 16 | 5.9 | 4 | 1.4 |
| 40–49 | 42 | 15.6 | 46 | 16.3 | |
| 50–59 | 91 | 33.7 | 114 | 40.4 | |
| 60–69 | 78 | 28.9 | 83 | 29.4 | |
| 70–79 | 37 | 13.7 | 32 | 11.3 | |
| >=80 | 6 | 2.2 | 3 | 1.1 | |
| Age range | 26 to 84 | 36 to 86 | |||
| Median age | 58 | 58 | |||
| Ethnicity | Hispanic or Latino | 8 | 3.0 | 6 | 2.1 |
| Not Hispanic or Latino | 244 | 90.4 | 257 | 91.1 | |
| Unknown/Not specified | 18 | 6.7 | 19 | 6.7 | |
| Race | Race not specified | 5 | 1.9 | 6 | 2.1 |
| Asian | 10 | 3.7 | 3 | 1.1 | |
| Am Indian/Alaskan Native | 0 | 0.0 | 1 | 0.4 | |
| Native Hawaiian/PI | 3 | 1.1 | 1 | 0.4 | |
| White | 236 | 87.4 | 258 | 91.5 | |
| Black/African American | 16 | 5.9 | 13 | 4.6 | |
| Other | 18 | 6.7 | 11 | 4.0 | |
| Performance Status | 0 | 186 | 68.9 | 194 | 68.8 |
| 1 | 79 | 29.3 | 85 | 30.1 | |
| 2 | 5 | 1.9 | 3 | 1.1 | |
| Cell Type | Adenocarcinoma, NOS | 2 | 0.7 | 1 | 0.4 |
| Clear Cell | 12 | 4.4 | 13 | 4.6 | |
| Endometrioid | 185 | 68.5 | 197 | 69.9 | |
| Mucinous | 1 | 0.4 | 0 | 0.0 | |
| Mixed Epithelial | 24 | 8.9 | 30 | 10.6 | |
| Adenosquamous | 3 | 1.1 | 2 | 0.7 | |
| Undifferentiated | 7 | 2.6 | 2 | 0.7 | |
| Serous | 36 | 13.3 | 37 | 13.1 | |
| Histologic Grade | 1 | 46 | 17.0 | 45 | 16.0 |
| 2 | 98 | 36.3 | 100 | 35.5 | |
| 3 | 108 | 40.0 | 120 | 42.6 | |
| Not graded | 18 | 6.7 | 17 | 6.0 | |
| Stage | III | 238 | 88.1 | 248 | 87.9 |
| IV | 32 | 11.9 | 34 | 12.1 | |
| Residual disease size | Microscopic or no residual | 245 | 90.7 | 250 | 88.7 |
| Gross residual | 25 | 9.3 | 32 | 11.3 | |
CD = cisplatin and doxorubicin; CDP = cisplatin, doxorubicin and paclitaxel Am = American; PI = Pacific Islander; NOS = Not otherwise specified.
Among patients randomly assigned treatment, 97% received between 45 and 55.5 Gy to the pelvis, 49% were also treated to the para-aortic nodes, and 49% received an extended boost to the vagina (Table 2). Approximately 80% of eligible patients completed six cycles of chemotherapy (Table 3). Study treatment was discontinued early for recurrence in 3% of the patients and for toxicity in 10%, while 5% of the patients refused to complete six cycles. One patient died before completing all 6 cycles of CDP therapy. In each arm, approximately 80% of each drug’s cumulative planned total dose was given over a maximum total treatment time of 5 months.
Table 2.
Radiation Treatment
| CD | CDP | ||||
|---|---|---|---|---|---|
| Field | Dose (Gy) | n | % | n | % |
| Pelvis | 0 | 0 | 0.0 | 1 | 0.3 |
| <45.36 | 7 | 2.6 | 6 | 2.1 | |
| 45.36–55.44 | 262 | 97.0 | 274 | 97.2 | |
| >55.44 | 1 | 0.4 | 1 | 0.3 | |
| Para-aortic nodes | Not indicated and not given | 100 | 37.0 | 106 | 37.6 |
| Indicated but not given | 39 | 14.4 | 38 | 13.5 | |
| <39.15 | 2 | 0.7 | 0 | 0.0 | |
| 39.15–47.85 | 129 | 47.8 | 136 | 50.4 | |
| >47.85 | 0 | 0.0 | 2 | 0.7 | |
| Vaginal boost | Not given | 138 | 51.1 | 140 | 49.6 |
| Lower than protocol dose | 6 | 2.2 | 3 | 1.1 | |
| 90% – 150% protocol | 121 | 44.8 | 134 | 47.5 | |
| 150% – 225% protocol | 5 | 1.8 | 5 | 1.8 | |
CD = cisplatin and doxorubicin
CDP = cisplatin, doxorubicin and paclitaxel
Table 3.
Chemotherapy
| Number of cycles of study chemotherapy received | CD | CDP | |||
|---|---|---|---|---|---|
| Total | n | % | n | % | |
| 0 | 13 | 9 | 3.3 | 4 | 1.4 |
| 1 | 14 | 6 | 2.2 | 8 | 2.8 |
| 2 or 3 | 29 | 12 | 4.4 | 17 | 6.0 |
| 4 or 5 | 52 | 20 | 7.4 | 32 | 11.3 |
| 6 | 444 | 223 | 82.6 | 221 | 78.4 |
| Total | 552 | 270 | 100.0 | 282 | 100.0 |
CD = cisplatin and doxorubicin
CDP = cisplatin, doxorubicin and paclitaxel
In Table 4a is a summarization of the maximum grade of acute adverse events reported, regardless of attribution, over a maximum of 6 cycles of study chemotherapy for each treated patient within each adverse event category. Table 4b summarizes the maximum grade of any reported late adverse event occurring during the follow-up period. The cumulative probability of a late grade 3 or higher treatment-related gastrointestinal adverse event was 5%.
Table 4.
| Table 4a. Acute Adverse Events Among Treated Patients | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CD Grade Frequency (N = 261) | CDP Grade Frequency (N = 278) | |||||||||
| Adverse Event term or category | 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 |
| Leukopenia* | 28 | 43 | 58 | 100 | 32 | 7 | 21 | 37 | 95 | 118 |
| Neutropenia* | 71 | 32 | 36 | 63 | 59 | 21 | 21 | 46 | 58 | 132 |
| Thrombocytopenia* | 114 | 92 | 28 | 26 | 1 | 35 | 127 | 49 | 62 | 5 |
| Anemia* | 36 | 88 | 106 | 30 | 1 | 15 | 58 | 162 | 43 | 0 |
| Gastrointestinal | 109 | 79 | 57 | 13 | 3 | 108 | 82 | 67 | 18 | 3 |
| Nausea | 67 | 105 | 65 | 24 | 0 | 81 | 102 | 66 | 29 | 0 |
| Vomiting | 132 | 54 | 52 | 21 | 2 | 141 | 58 | 55 | 23 | 1 |
| Stomatitis | 228 | 22 | 10 | 0 | 1 | 237 | 26 | 13 | 2 | 0 |
| Genitourinary/Renal | 195 | 48 | 14 | 4 | 0 | 214 | 37 | 23 | 3 | 1 |
| Infection/Fever* | 247 | 3 | 7 | 3 | 1 | 239 | 4 | 13 | 19 | 3 |
| Febrile Neutropenia* | 259 | 1 | 1 | 0 | 0 | 261 | 0 | 3 | 13 | 1 |
| Sensory neuropathy* | 183 | 65 | 8 | 5 | 0 | 94 | 108 | 52 | 23 | 1 |
| Pain* | 126 | 72 | 46 | 16 | 1 | 108 | 69 | 74 | 27 | 0 |
| Myalgia* | 243 | 11 | 7 | 0 | 0 | 198 | 28 | 45 | 7 | 0 |
| Table 4b. Late Adverse Events Among Treated Patients | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD Grade Frequency (N = 261) | CDP Grade Frequency (N = 278) | |||||||||||
| Organ System | 0 | 1 | 2 | 3 | 4 | 5 | 0 | 1 | 2 | 3 | 4 | 5 |
| Bladder | 237 | 13 | 11 | 2 | 1 | 0 | 246 | 12 | 16 | 3 | 1 | 0 |
| Bone | 250 | 3 | 3 | 3 | 2 | 0 | 261 | 5 | 8 | 3 | 2 | 0 |
| Skin | 249 | 6 | 5 | 0 | 1 | 0 | 260 | 9 | 5 | 3 | 1 | 0 |
| Sm or Lg Intestine | 184 | 42 | 14 | 13 | 8 | 0 | 185 | 46 | 23 | 19 | 6 | 1 |
|
| ||||||||||||
| Grade 3–5 Sm or Lg Intestine AE re-review* | 5 | 13 | 3 | 0 | 16 | 7 | 2 | 1 | ||||
CD = cisplatin and doxorubicin
CDP = cisplatin, doxorubicin and paclitaxel
p<0.01
All patients were not at risk for the entire follow-up period due to competing risks. All grade 3–5 small or large intestine adverse effects reported were re-reviewed and graded for attribution to study treatment and not disease. Those adverse events thought to be related to study treatment during follow-up with obstruction or bleeding requiring surgery were classified as grade 3, with necrosis, perforation or fistula were classified as grade 4, or that resulted in death were classified as grade 5.
CD = cisplatin and doxorubicin
CDP = cisplatin, doxorubicin and paclitaxel
Sm = Small, Lg = Large
AE = Adverse Event
There were 422 eligible patients (206 in regimen I and 216 in regimen II) who received at least one cycle of chemotherapy and completed a baseline and at least one follow-up assessment. The mean scores of Patient-Reported Neurotoxicity are presented in Table 5. Baseline Ntx scores did not differ between the two regimens. After adjusting for baseline score, the fitted linear mixed model estimates indicated that the treatment effect on the Ntx scores were not constant within 4 weeks of last cycle and at 6 month post last cycle (p<0.001 for interaction between treatment and assessment times). Within 4 weeks of last cycle, the mean Ntx score was 32.9 points; 5.2 points worse (95% CI: 4.0~6.5; p<0.001) in the CDP arm than that in the CD arm (38.1 points). The difference in Ntx score between regimens is clinically and statistically significant. Six months after completing treatment, the difference was diminished but still remained statistically significant (difference=1.6; 95% CI: 0.3~2.8; p=0.014).
Table 5.
Patient-Reported Neurotoxicity Score Summary Statistics
| CD |
CDP |
||||||
|---|---|---|---|---|---|---|---|
| Assessment time | No. | Mean | SD | No. | Mean | SD | p-value |
| Pre-cycle 1 | 206 | 40.7 | 4.2 | 216 | 40.8 | 3.8 | 0.83 |
| Within 4 weeks of last cycle | 189 | 38.1 | 6.1 | 200 | 32.9 | 8.0 | <0.001 |
| 6 month post last cycle | 156 | 37.1 | 6.9 | 189 | 35.6 | 7.0 | 0.014 |
CD = cisplatin and doxorubicin
CDP = cisplatin, doxorubicin and paclitaxel
No.= number of patients; SD = standard deviation
test of difference in adjusted means from fitted model; p<0.001 for test of interaction between treatment and assessment time.
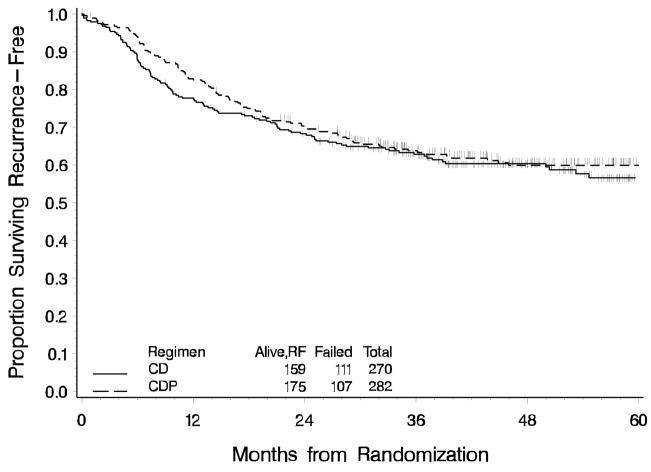
Sixty-two percent of patients on the cisplatin and doxorubicin (CD) arm were alive, recurrence free 36 months following randomization compared to 64% of patients on the cisplatin, doxorubicin and paclitaxel (CDP) arm. There is no statistically significant decrease in the risk of recurrence or death associated with the CDP regimen (p=0.21, one-tail) (Figure 2). The ratio of the hazard of recurrence or death relative to the CD arm stratified by stage is 0.90 (95% CI is 0.69 to 1.17). The median follow up time among those alive, recurrence-free is 46 months for CD and 47 months for CDP. Nearly 30% of patients had a distant recurrence and 10% had a local-regional recurrence (Figure 3). Survival data are not yet mature for final analysis.
Figure 2.
Recurrence-Free Survival for Chemotherapy Regimens
Figure 3.
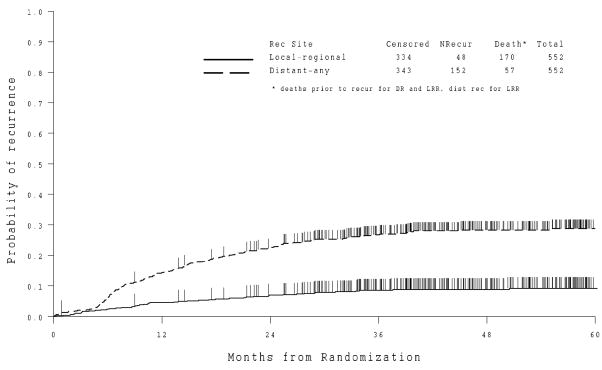
Cumulative Incidence of Recurrence by Site
The majority of deaths are due to disease, but 3 of the randomized patients died as a result of complications due to small bowel obstructions related to radiation effects. One patient who was never randomized had radiation interrupted by nausea and vomiting and died 3 weeks later. One patient died as a result of multiple events related to chemotherapy including acute renal failure.
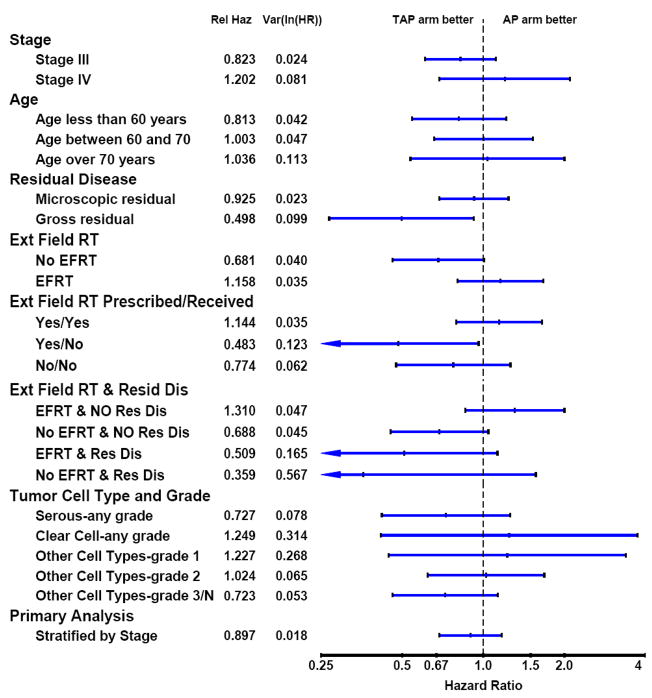
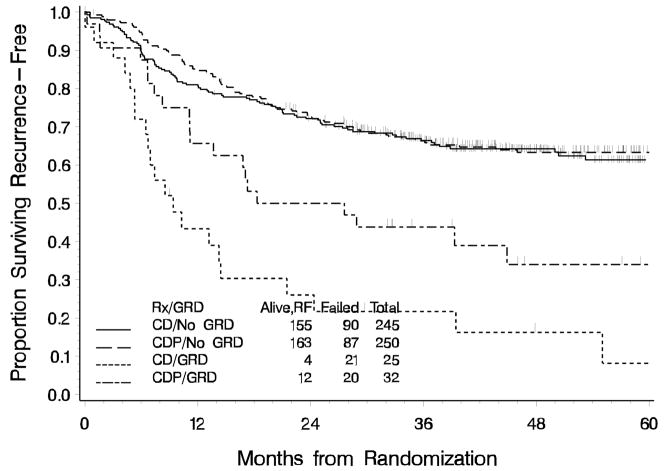
Exploratory analyses were performed to assess the consistency of treatment effect across subgroups and are summarized as forest plots in Figure 4. The p-value for the test for homogeneity of treatment effects within the subgroups defined by residual disease (none or microscopic vs. gross) at study entry was 0.076. There was a 50% (HR: 0.50; 95%CI: 0.27 to 0.92) reduction in the risk of recurrence or death in the CDP arm among those with gross residual disease (GRD) compared to the CD arm. (Figures 4,5). Among patients with no GRD who did not receive EFRT, the effect of treatment favors the CDP regimen. However, the effect of treatment among those with no GRD who did receive EFRT does not favor CDP; in fact, although not definitive, it slightly favors CD. However, CDP appears to be favored among those with GRD regardless of the radiation field used. Among the 77 patients who had one, negative, or no PA nodes sampled and did not receive EFRT, the treatment effect appears to favor CDP. Among those who were not prescribed EFRT, that is, only received pelvic RT, and had no gross residual disease there is little, if any, treatment difference. With the exception of GRD and extent of the radiation field, there is no other suggestion of a heterogeneous effect of treatment within subgroups.
Figure 4.
Recurrence-Free Survival Treatment Hazard Ratios by Subgroup
Figure 5.
Recurrence-Free Survival by Residual Tumor Status
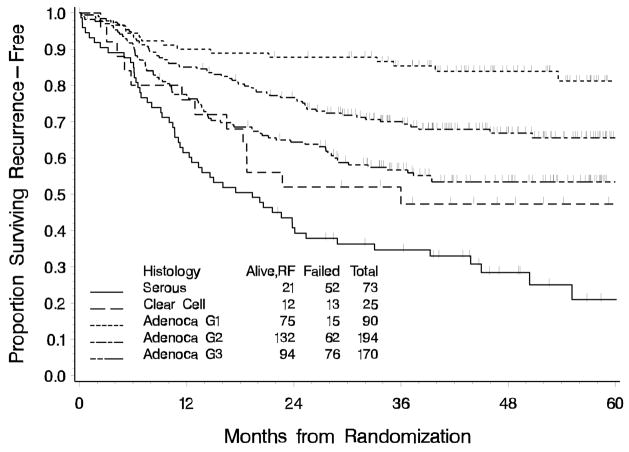
In a multiple variable proportional hazards regression model adjusting for stage (IV vs. III), residual disease (gross vs. none or microscopic), EFRT and interactions between treatment and both GRD and EFRT; age, histology and grade, positive para-aortic nodes, pelvic metastasis, and positive cytology were statistically significantly associated with RFS (Table 6). The unadjusted Kaplan-Meier estimates of RFS by histology and grade are displayed in Figure 6 showing lower RFS for patients with clear cell and papillary serous tumor histology. Once other factors are adjusted for, there was no statistically significant association of black racial designation and elevated CA 125 with RFS. Other factors not statistically significant at the p< 0.05 level in univariate models were positive pelvic nodes (any vs. none vs. not evaluated), GOG performance status, myometrial invasion (none vs. <50%, vs. >50%), and pathologically confirmed presence of lymphovascular space involvement, vaginal metastasis, bladder metastasis, and abdominal metastasis.
Table 6.
Multiple Variable Proportional Hazards Model Hazard Ratio Estimates adjusted for treatment, stage, gross residual disease (GRD), extended field radiation (EFRT) and interactions between GRD, EFRT and treatment
| Covariate Name | Covariate Value | RFS Hazard Ratio | 95% Confidence Interval |
|---|---|---|---|
| Age at study entry | Relative to a 1 year increase in age | 1.03 | (1.02, 1.05) |
| Tumor | Non-serous/non-clear cell grade 1 | 1.00 | Reference group |
| Histology and Grade | Non-serous/non-clear cell grade 2 | 2.18 | (1.23, 3.85) |
| Non-serous/non-clear cell grade 3 | 3.12 | (1.78, 5.47) | |
| Uterine clear cell carcinoma | 3.45 | (1.62, 7.36) | |
| Uterine papillary serous carcinoma | 4.43 | (2.45, 8.02) | |
| Pelvic Cytology | Positive vs. Negative | 1.58 | (1.17, 2.15) |
| Missing, suspicious, unknown vs. Negative | 1.23 | (0.80, 1.90) | |
| Para-aortic Nodes | Positive vs. Negative | 2.35 | (1.53, 3.62) |
| Not Evaluated vs. Negative | 1.53 | (1.01, 2.33) | |
| Pelvic metastasis | Tubes, Ovaries, or Serosa vs. All others without metastasis to the tubes, ovaries and serosa | 1.50 | (1.13, 1.99) |
GRD = gross residual disease
EFRT = extended field radiation therapy
RFS = Recurrence-free survival
Figure 6.
Recurrence-Free Survival by Tumor Histology and Grade
Discussion
In this trial, when added to cisplatin and doxorubicin following completion of surgery and radiation therapy, paclitaxel added toxicity including patient-reported peripheral neuropathy; however, it did not contribute to a significant improvement in recurrence-free survival. An exception may be noted in patients with macroscopic residual endometrial cancer.
Approximately 80% of patients completed six cycles of chemotherapy using either drug regimen, reflecting acceptable tolerance of chemotherapy after full radiation. Nearly all patients received growth factors beginning with the first course of chemotherapy. There was no significant gastrointestinal manifestation of toxicity from combining doxorubicin and radiation. The cumulative probability of a late grade 3 or higher treatment-related gastrointestinal adverse event was acceptable at 5%. On average, the patient-reported neurotoxicity scores at treatment completion and six months later were significantly worse for the paclitaxel arm. (Table 5)
After this trial was activated, paclitaxel combined with doxorubicin and cisplatin in a phase III (GOG 177) treatment trial for patients with measurable, advanced or recurrent endometrial cancer was reported to be superior (15 month median survival) to doxorubicin and cisplatin.[18]. Additionally, results of GOG 122 indicated that combination chemotherapy significantly improved progression-free and overall survival compared with WAI but was associated with worse acute toxicity. [7] There was an estimated 29% decrease in the risk of progression or death and a 32% decrease in the risk of death associated with the chemotherapy arm. At 60 months, 50% of the chemotherapy patients were predicted to be alive, recurrence-free compared with 38% of WAI patients. The deaths of eight patients (4%) on the chemotherapy arm and five patients (2%) on the WAI arm were attributable to study treatment.
The RFS estimates from GOG 122 appear worse for both arms than in the present trial. However there are some differences between the trials that may explain the lower percentage of patients alive, recurrence-free in GOG 122. In the present trial, RFS was calculated from time of randomization after completion of radiation therapy. Furthermore, of the 659 patients consented, 107 patients (16%) either did not meet eligibility or refused chemotherapy (4.4%) or had intervening recurrence (3.9%). This may have screened out patients with worse prognosis and did screen out patients with early recurrence. Additionally, enrollment of patients with Stage IV disease was stopped early in this trial, favoring a better overall prognosis. It is also possible that patient selection may have differed for these two sequential studies given the therapeutic options in each trial
The toxicity profiles of the two regimens (Table 4) revealed significantly more acute myelosuppression, including febrile neutropenia, infection, pain myalgia and sensory neuropathy, in patients on the CDP arm. In addition, the patient-reported neuropathy on the CDP arm was significantly worse than that on the CD arm. The magnitude of the difference assessed within 4 weeks of last cycle was substantial and clinically meaningful, when compared to other patient populations with significant neuropathy as measured by the same scale [18,20–21]. More details of the patient-reported neurotoxicity analysis will be reported elsewhere.
There were several significant prognostic factors found in exploratory analyses including: residual disease (gross vs none or microscopic), age, histology and grade, positive para-aortic nodes, pelvic metastasis, and positive cytology. Relative to grade 1 endometrioid adenocarcinoma, grade 3 endometrioid adenocarcinoma with a RFS hazard ratio of 3.12 was similar to 3.45 for clear cell histology. Serous histology had the largest effect on RFS relative to grade 1 endometrioid adenocarcinoma, with a hazard ratio of 4.43. This has been reported by others. [5,8,22,23]. The limited enrollment of patients with Stage IV disease may explain why abdominal metastasis was not considered a prognostic factor after adjusting for stage.
A RFS hazard ratio of 1.58 for positive cytology remains controversial in its significance [24,25]. Yet, on the relative scale, the RFS hazard ratio for positive cytology is in the range of positive adnexa (1.50), and less than that of positive para-aortic nodes (2.35) and serous histology (4.43).
From the acceptable toxicity noted in this trial, full dose chemotherapy with growth factor support can readily be given following pelvic radiation and extended field radiation with or without vaginal boost.
Given the caveats of subset analyses, the data from this study suggests that if there is any benefit due to paclitaxel, it is primarily observed among those patients with gross residual disease and possibly those with no gross residual disease who do not receive EFRT. It is possible that the treatment-by-gross residual disease (GRD) interaction result is spurious, however; the results of GOG 177 support the subgroup analysis results of improved RFS for gross residual disease in this study. The vast majority of patients in this study had no GRD. The results in this subgroup not treated with EFRT would need to be evaluated further in other studies.
EFRT was only prescribed for patients who had positive PA nodes or in whom 1 or fewer PA nodes were sampled. Thus, in this trial, it cannot be determined definitively if the lack of treatment difference among those with no gross residual tumor who received EFRT is purely due to the extended field radiation given. However, in an effort to address this, it was found that 77 patients who should have received EFRT did not. Among these patients, the treatment effect appears to favor CDP. However, the decision to use or not use EFRT in these patients is likely to be confounded with prognosis.
In conclusion, in patients with advanced local or regional stage III endometrial carcinoma with ≤ 2 cm maximum residual tumor following surgery and volume directed radiation, the addition of paclitaxel to cisplatin and doxorubicin was not associated with a significant improvement in RFS but was associated with increased morbidity. Because of the small number of patients with gross residual disease that may have benefited from the addition of paclitaxel, this should be used for hypothesis generating purposes. Three important high risk groups were identified: clear cell histology, papillary serous histology, grade 3 adenocarcinoma and patients with gross residual disease.
Acknowledgments
This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group (GOG) Administrative Office (CA 27469) and the GOG Statistical and Data Center (CA 37517).
The following GOG institutions participated in the related treatment study: Roswell Park Cancer Institute, Duke University Medical Center, Abington Memorial Hospital, Walter Reed Army Medical Center, Wayne State University, University of Minnesota Medical School, University of Mississippi Medical Center, Colorado Gynecologic Oncology Group PC, University of Washington/Puget Sound Oncology Consortium, University of Pennsylvania Cancer Center, Milton S. Hershey Medical Center, University of Cincinnati Medical Center, University of North Carolina School of Medicine, University of Iowa Hospitals and Clinics, University of Texas Southwestern Medical Center at Dallas, Indiana University School of Medicine, Wake Forest University School of Medicine, University of California-Irvine, Tufts-New England Medical Center, Rush-Presbyterian-St. Luke’s Medical Center, Magee Women’s Hospital, State University of New York-Downstate, University of Kentucky, University of New Mexico Health Sciences Center, Cleveland Clinic Foundation, State University of New York-Stony Brook, Washington University School of Medicine, Memorial Sloan-Kettering Cancer Center, Cooper Hospital/University Medical Center, Columbus Cancer Council, University of Massachusetts Medical School, Fox Chase Cancer Center, Women’s Cancer Center, University of Oklahoma, University of Virginia, University of Chicago, Tacoma General Hospital, ECOG Statistical Center, Mayo Clinic, Case Western Reserve University, Tampa Bay/H. Lee Moffitt Cancer Center, Gynecologic Oncology Network, Ellis Fischel Cancer Center, Fletcher Allen Health Care, Yale University, University of Texas – Galveston, and Ozarks Regional CCOP.
The authors would like to acknowledge Helen Huang, MS, Biostatistician, Gynecologic Oncology Group Statistical and Data Center, for the expert analysis and reporting of the patient-reported neurotoxicity data in this manuscript and Patty Brehm for her careful coordination of the clinical data.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest with the exception of Dr. Susan K. Gibbons who reports EntreMed Millennium Stock purchase 2003 <$1,500 and Dr. Harry J. Long III who reports stock ownership of <$10,000 per company: AstraZeneca, Amgen, Bristol Myers Squibb, Genentech, GlaxoSmithKline, Novartis, Sanofi-Aventis, Pfizer.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Greer B, Hamberger A. Treatment of intraperitoneal metastatic adenocarcinoma of the endometrium by the whole abdomen moving strip technique and pelvic boost irradiation. Gynecol Oncol. 1983;16:365–73. doi: 10.1016/0090-8258(83)90164-6. [DOI] [PubMed] [Google Scholar]
- 2.Leoffler J, Rosen E, Niloff J, Howes A, Knapp R. Whole abdominal irradiation for tumors of the uterine corpus. Cancer. 1988;61:1223–35. doi: 10.1002/1097-0142(19880401)61:7<1332::aid-cncr2820610710>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 3.Martinez A, Schray M, Podratz K, Stanhope R, Malkasian G. Postoperative whole abdomino-pelvic irradiation for patients with high risk endometrial cancer. Int J Radiat Oncol Biol Phys. 1989;17:371–77. doi: 10.1016/0360-3016(89)90453-7. [DOI] [PubMed] [Google Scholar]
- 4.Gibbons S, Martinez A, Schray M, et al. Adjuvant whole abdominopelvic irradiation for high risk endometrial carcinoma. Int J Radiat Oncol Biol Phys. 1991;21:1019–25. doi: 10.1016/0360-3016(91)90744-o. [DOI] [PubMed] [Google Scholar]
- 5.Sutton G, Axelrod JH, Bundy BN, Roy T, Homesley H, Lee RB, et al. Adjuvant whole abdominal irradiation in clinical stages I and II papillary serous or clear cell carcinoma of the endometrium: a phase II study of the Gynecologic Oncology Group. Gynecol Oncol. 2006;100:349–54. doi: 10.1016/j.ygyno.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 6.Sutton G, Axelrod JH, Bundy BN, Roy T, Homesley HD, Malfetano JH, et al. Whole abdominal radiotherapy in the adjuvant treatment of patients with stage III and IV endometrial cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 2005;97:755–63. doi: 10.1016/j.ygyno.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Thigpen JT, Brady MF, Homesley HD, Malfetano J, DuBeshter B, Burger RA, et al. Phase III trial of doxorubicin with or without cisplatin in advanced endometrial carcinoma: a gynecologic oncology group study. J Clin Oncol. 2004;22:3902–8. doi: 10.1200/JCO.2004.02.088. [DOI] [PubMed] [Google Scholar]
- 8.Randall ME, Filiaci VL, Muss H, Spirtos NM, Mannel RS, Fowler J, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2006;24:36–44. doi: 10.1200/JCO.2004.00.7617. [DOI] [PubMed] [Google Scholar]
- 9.Lincoln S, Blessing JA, Lee RB, Rocereto TF. Activity of paclitaxel as second-line chemotherapy in endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2003;88:277–81. doi: 10.1016/s0090-8258(02)00068-9. [DOI] [PubMed] [Google Scholar]
- 10.Pickel H, Lahousen M, Petru E, Stettner H, Hackl A, Kapp K, et al. Consolidation radiotherapy following carboplatin-based chemotherapy in radically operated advanced ovarian cancer. Gynecol Oncol. 1999;72:215–9. doi: 10.1006/gyno.1998.5184. [DOI] [PubMed] [Google Scholar]
- 11.Reisinger SA, Asbury R, Liao SY, Homesley HD. A phase I study of weekly cisplatin and whole abdominal radiation for the treatment of stage III and IV endometrial carcinoma: a Gynecologic Oncology Group pilot study. Gynecol Oncol. 1996;63:299–303. doi: 10.1006/gyno.1996.0326. [DOI] [PubMed] [Google Scholar]
- 12.Soper JT, Reisinger SA, Ashbury R, Jones E, Clarke-Pearson DL. Feasibility study of concurrent weekly cisplatin and whole abdominopelvic irradiation followed by doxorubicin/cisplatin chemotherapy for advanced stage endometrial carcinoma: a Gynecologic Oncology Group trial. Gynecol Oncol. 2004;95:95–100. doi: 10.1016/j.ygyno.2004.06.041. [DOI] [PubMed] [Google Scholar]
- 13.Schoenfeld DA. Sample size formula for the proportional hazards regression model. Biometrics. 1983;39:499–503. [PubMed] [Google Scholar]
- 14.DeMets DL, Lan G. The alpha spending function approach to interim data analyses. In: Thall PF, editor. Recent Advances in Clinical Trial Design and Analysis. Kluwer Academic Publishers; Boston: 1995. pp. 1–27. [DOI] [PubMed] [Google Scholar]
- 15.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemo Rep. 1966;50:163–70. [PubMed] [Google Scholar]
- 16.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 17.Cox DR. Regression model and life tables (with discussion) J R Stat Soc B. 1972;34:187–219. [Google Scholar]
- 18.Calhoun E, Welshman E, Chang CH, Lurain JR, Fishman DA, Hunt TL, et al. Psychometric evaluation of the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group –Neurotoxicity (FACT/GOG-Ntx) questionnaire for patients receiving systematic chemotherapy. Int J Gynecol Cancer. 2003;13:741–48. doi: 10.1111/j.1525-1438.2003.13603.x. [DOI] [PubMed] [Google Scholar]
- 19.Fleming GF, Brunetto VL, Cella D, Look KY, Reid GC, Munkarah AR, et al. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2004;22:2159–66. doi: 10.1200/JCO.2004.07.184. [DOI] [PubMed] [Google Scholar]
- 20.Kopec JA, Land SR, Cecchini RS, Ganz PA, Cella D, Costantino JP, et al. Validation of a self-reported neurotoxicity scale in patients with operable colon cancer receiving oxaliplatin. J Supportive Oncol. 2006;4:W1–W8. [Google Scholar]
- 21.Moore D, Donnelly J, McGuire W, Almadrones L, Cella DF, Herzog TJ, et al. Limited access trial using Amifostine for protection against Cisplatin and 3-Hour Paclitaxel-Included Neurotoxicity: A Phase II Study of the Gynecologic Oncology Group. J Clin Oncol. 2003;21:4207–13. doi: 10.1200/JCO.2003.02.086. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton CA, Liou WS, Osann K, Berman ML, Husain A, Teng NN, et al. Impact of adjuvant therapy on survival of patients with early-stage uterine papillary serous carcinoma. Intl J Radiat Oncol Biol Phys. 2005;63:839–44. doi: 10.1016/j.ijrobp.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 23.Martin JD, Gilks B, Lim P. Papillary serous carcinoma -- a less radio-sensitive subtype of endometrial cancer. Gynecol Oncol. 2005;98:299–303. doi: 10.1016/j.ygyno.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Dede M, Yenen MC, Goktolga U, Duru NK, Guden M, Dilek S, et al. Is adjuvant therapy necessary for peritoneal cytology-positive surgical-pathologic Stage I endometrial cancer? Preliminary results Eur J Gynaecol Oncol. 2004;25:591–3. [PubMed] [Google Scholar]
- 25.Tebeu PM, Popowski GY, Verkooijen HM, Casals J, Ludicke F, Zeciri G, et al. Impact of peritoneal cytology on survival of endometrial cancer patients treated with surgery and radiotherapy. Br J Cancer. 2003;89:2023–6. doi: 10.1038/sj.bjc.6601446. [DOI] [PMC free article] [PubMed] [Google Scholar]