One of the greatest challenges in geriatrics is the provision of optimal care for older adults with multiple chronic conditions, or “multimorbidity.”1–3 More than 50% of older adults have three or more chronic diseases, with distinctive cumulative effects for each individual.4
Multimorbidity is associated with higher rates of death, disability, adverse effects, institutionalization, use of healthcare resources, and poorer quality of life.1 Comprehensive strategies and interventions for common syndromes and organization of care in this population show promise, but what the best clinical management approaches are remains unclear.5
Most clinical practice guidelines (CPGs) focus on the management of a single disease, but CPG-based care may be cumulatively impractical, irrelevant, or even harmful for such individuals.3,6 CPG deficiencies are not based solely on shortcomings of guideline development and implementation.3,7 Older adults with multimorbidity are regularly excluded or underrepresented in trials and observational studies, which translates to less focus on older adults in meta-analyses, systematic reviews, and guidelines and affects appropriate interpretation of results.
Clinical management is defined as representing all types of care for chronic conditions, including pharmacological and nonpharmacological treatment and interventions (e.g., referral to specialists, physical and occupational therapy, use of pacemakers), screening, prevention, diagnostic tests, follow-up, and advanced illness care. The best strategies for prioritizing specific aspects of this management spectrum in a particular older adult with multimorbidity are unknown.
Clinicians need a management approach that will consider the challenges particular to each individual, including the often-limited available evidence; interactions among conditions or treatments; the patient’s preferences, goals, and prognosis; multifactorial geriatric problems and syndromes; and the feasibility of each management decision and its implementation.
The American Geriatrics Society (AGS) convened a panel with expertise in these topics. The goal was to develop an approach by which clinicians can care optimally for older adults with multimorbidity. This document is not a guideline. Therefore, it does not issue recommendations based on rigorous evaluation of the quality of evidence for specific clinical questions with assessments of harms and benefits. Rather it sets out guiding principles that, taken together, provide an approach to clinical management for older adults with multimorbidity.
Older adults with multimorbidity are heterogeneous in terms of illness severity, functional status, prognosis, personal priorities, and risk of adverse events even when diagnosed with the same pattern of conditions. Not only the individuals themselves, but also their treatment options will differ, necessitating more-flexible approaches to care in this population.
This executive summary presents important points from a full-length document, published in the online edition of this issue of the Journal of the American Geriatrics Society, and includes a minimal reference list. The full-length document, Guiding Principles for the Care of Older Adults with Multimorbidity: An Approach for Clinicians, can be found online at www.ags-online.org.
The aim is to encourage the development of an evidence base by which clinicians can make sound care decisions for this population. Not only must the healthcare community generate better evidence regarding intervention outcomes in older adults with multimorbidity, but it must also establish better methods for determining patient priorities and prognosis and for optimizing care plans. Such initiatives will maximize adherence and patient-important outcomes and will support changes to the healthcare system to allow these principles to be accommodated. This is a consensus document; it is hoped that evidence-based care approaches for this population will replace it in the future.
Many relevant clinical concerns are outside the scope of this document. Although it focuses on primary care management of older adults with multiple chronic conditions, many clinicians care for older adults with multimorbidity, who transition through multiple settings. Any clinician can use this approach, but a primary care clinician or medical home and an associated healthcare team are central to implementation. This document informs clinicians, researchers, public health professionals, payers, policy-makers, and others interested in the care of older adults.
METHODS
The document is organized around five domains relevant to the care of older adults with multimorbidity: Patient Preferences, Interpreting the Evidence, Prognosis, Clinical Feasibility, and Optimizing Therapies and Care Plans. An additional section on Barriers focuses on challenges to implementing this approach.
Literature Review Methods
This is not a systematic review. Two distinct literature review strategies were used: a structured PubMed literature search and a citation search of important articles. A detailed description of the search methods appears in the full-length document posted online.
External Review
A draft version was circulated for peer review to several organizations (see Acknowledgments) and was posted to the AGS website for public comment.
Approach to Older Adults with Multimorbidity
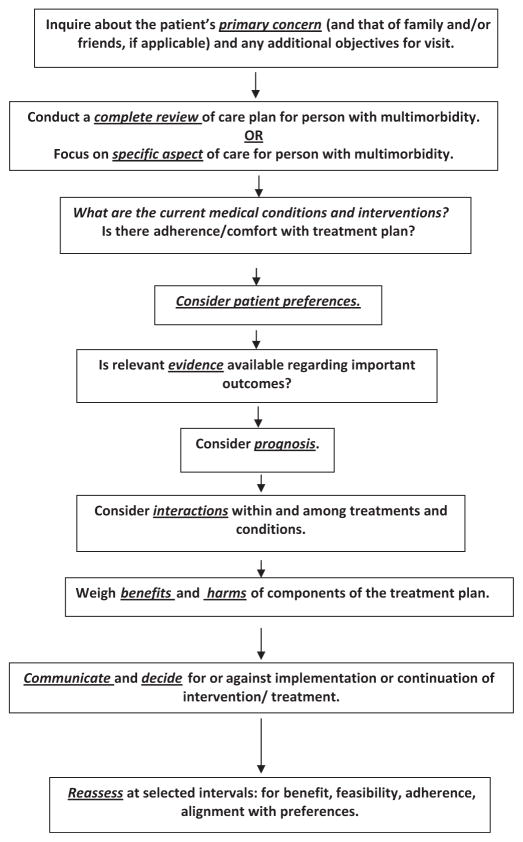
Clinicians treating older adults with multimorbidity face many challenges, including complex clinical management decisions, inadequate evidence, and time constraints and reimbursement structures that hinder the provision of efficient quality care.2 The flowchart in Figure 1 presents a useful approach to optimal management of these individuals. The five domains apply at various steps, which can be taken in other sequences with equal validity because approaches for addressing this population have not been compared. For example, patient preferences are often best elicited in the context of the individual’s prognosis.
Figure 1.
Approach to the evaluation and management of the older adult with multimorbidity.
GUIDING PRINCIPLES
I. PATIENT PREFERENCES DOMAIN
Guiding Principle: Elicit and incorporate patient preferences* into medical decision-making for older adults with multimorbidity.
Justification of Principle
Care provided in accordance to CPGs may not adequately address patient preferences, an important aspect of medical decision-making,8 but older people with multimorbidity are able to evaluate choices and prioritize their preferences for care within personal and cultural contexts.9 Some CPG recommendations are more preference sensitive than others, such as decisions involving multiple treatment options, lifelong implications of chronic disease management, and choices about treatments or interventions with important risks or uncertain benefits.10
How to Use in Clinical Practice
All clinical decisions require an assessment of patient preferences.11 Elicitation of preferences can be customized so that decision-making is abbreviated in less-complex situations and more expansive when many options need consideration.11 With multiple options, the process of eliciting patient preferences requires several steps.
Recognize when the older adult with multimorbidity is facing a “preference sensitive” decision
Older adults with multimorbidity are more likely to confront these kinds of decisions because of the burdens that many potential therapies impose, high risk of adverse events, and the possibility of limited benefits.3 Preference-sensitive decisions include therapy that may improve one condition but make another worse (e.g., corticosteroids for chronic obstructive pulmonary disease may exacerbate osteoporosis);12 therapy that may confer long-term benefits but cause short-term harm (e.g., preventive agents, such as statins, frequently have adverse effects);13 and multiple medications, each with benefits and harms that must be balanced.14
Ensure that older adults with multimorbidity are adequately informed about the expected benefits and harms of treatment options
Although clinicians may feel that some adverse effects are less important than expected benefits, patients often consider them to be highly significant.15
Numerical likelihoods should be provided to patients if available, because words such as “rarely” and “frequently” are variably interpreted and often misunderstood.16 Generally well-accepted recommendations include presenting the likelihood of the event occurring or not occurring, to avoid framing the outcome positively or negatively;17 offering absolute rather than relative risks; and providing visual aids.18 An important element of this step is to assess patient understanding of the information presented (e.g., using a “teach back” technique).
Elicit patient preferences only after the individual is sufficiently informed
Decision aids are available to help inform patients and elicit preferences,19 but these may fail to describe the likelihood of varying outcomes because of different comorbidity and risk factor pro-files.20 Decision analysis, for example, can involve the creation of a decision tree, which identifies all potential outcomes of each treatment option. Outcomes can then be compared using approaches such as the standard gamble and time trade-off21,22 or conjoint analysis.23 A simpler method may be to ask patients to prioritize a set of universal health outcomes that can be applied across individual diseases (e.g., living as long as possible, maintaining function, minimizing pain). Treatment options can then be considered in terms of their effects on these outcomes.24
Several important additional considerations should guide preference elicitation. First, clinicians need to distinguish between eliciting preferences and making a treatment decision.25 In the former, individuals voice their opinions about treatment options and potential outcomes based on personal values and priorities; in the latter, a specific option is chosen. Patients may wish to decide themselves, let the clinician decide, or share decision-making, but virtually all want their opinion to guide the process.26 Individuals may want family, friends, or caregivers to be included in decision-making or even to make the decision for them. For individuals with cognitive impairment who cannot understand the implications of different options, these significant others become surrogate decision-makers, working with clinicians to make decisions on behalf of the patient. Second, preferences may change over time and should be reexamined, particularly with a change in health status.27 Third, the principle of eliciting preferences and involving patients in the decision-making process does not mean that the patient has the right to demand any available treatment without a reasonable expectation of some benefit.28
II. INTERPRETING THE EVIDENCE DOMAIN
Guiding Principle: Recognizing the limitations of the evidence base, interpret and apply the medical literature specifically to older adults with multimorbidity.
Justification of Principle
CPGs evaluate the best current evidence from many types of studies, but most focus on only one to two clinical conditions and address comorbidities in limited ways, if at all.3 In addition, different conditions coexisting within the same patient may interact, changing the risks associated with each condition and its treatments. Consequently, determining whether the individual will benefit from a particular treatment is complicated.
Thoughtful standardized approaches for the interpretation of the medical literature29 (evidence-based medicine) provide tools for clinicians to evaluate the evidence base;30 one element of such methodologies that must not be neglected is whether the information applies to the individual under consideration.29 Significant evidence gaps exist concerning condition and treatment interactions, particularly in older adults with multimorbidity.
How to Use in Clinical Practice
To help evaluate whether specific information, from a guideline or other source of evidence, applies to an older person with multimorbidity, the evidence should be reviewed based on important clinical questions and concepts, described below31 (Table 1).
Table 1.
Questions to Ask Regarding the Medical Literature
| To what extent were older adults with multimorbidity included in the trials? Is there evidence of effect modification? |
| What is the quality of the evidence, using accepted evidence-based medicine methodologies? |
| What are the hoped- for outcomes of the treatment or intervention? Are these outcomes important to patients? |
| Is there meaningful variation in baseline risk for outcomes that the treatment or intervention is designed to affect? |
| Are the risks and side effects of the treatments and interventions in older patients with multimorbidity clearly known, so that a decision can be made whether the treatment for one condition will exacerbate another? |
| What are the comparator treatments or management strategies? |
| Is it known how long it takes to accrue the benefit or harms of the treatment or intervention? |
| Does the document give absolute risk reductions or merely relative risk reductions? Is it possible to estimate absolute risk reductions? |
| How precise are the findings? What are the confidence limits? |
Applicability and Quality of Evidence
To assess the “applicability” of the information, the clinician must try to ascertain whether people with multimorbidity, or even older people, were included in sufficient numbers to make the study findings relevant and, if so, whether specific comorbidities or multimorbidity modified intervention effects.32,33
Quality of evidence must also be considered. Even a strongly positive study may have design or analysis flaws.31,34,35 Existing approaches to evaluating quality of evidence are useful for clinicians seeking a balance between quality of evidence and applicability. For example, well-designed randomized clinical trials (RCTs) reduce problems of confounding seen in observational studies but often exclude older adults with multimorbidity. Observational studies, although considered of weaker quality than well-designed RCTs, often include older adults with multimorbidity and important data about adverse events.
Outcomes
Clinical trials often evaluate outcomes not of immediate importance to patients (e.g., intermediate or surrogate outcomes).31,34 Intermediate outcomes in themselves, such as high cholesterol, do not affect individuals as directly as patient-important outcomes such as stroke or myocardial infarction. Outcomes that may have higher priority for older adults with multimorbidity (e.g., quality of life or independent living) are often not addressed. An important question for the clinician is whether the outcomes reported are meaningful for the individual concerned.33
Harms and Burdens
When evaluating evidence regarding benefits versus potential harms and burdens in this population, clinicians must remember that short-term efficacy studies may not follow individuals long enough to fully determine adverse event rates, few clinical trials report treatment burden, and treating one disease may exacerbate another coexisting condition. Clinicians should ascertain whether adverse events were adequately reported, whether potential effects on other conditions were studied, and whether treatment interactions could occur. Financial costs and treatment complexity and burden must also be considered, because these often affect adherence.3
Absolute Risk Reduction
Study results are often conveyed in terms of relative risk reduction (RRR) rather than absolute risk reduction (ARR). RRR is uninterpretable if baseline risk (outcome without treatment) is not reported. ARR represents baseline risk minus the risk of the outcome with treatment, or the difference between two comparator treatments. A baseline risk of 2% without treatment minus a 1% risk with treatment produces a 50% RRR but only a 1% ARR. Baseline risk in older adults with multimorbidity may be higher or lower than that of the general population for many conditions. Even in trials without substantial numbers of older adults with multimorbidity, baseline risk may vary considerably and limit the application of the trial results even to the typical participant in the trial.36 When the patient has a baseline risk outside the typical trial, the limitations of application of the data are even greater. Baseline risk can be ascertained from RCT control groups, from observational studies or registries, or from prognostic indices. RRR is often assumed to be constant, regardless of baseline risk, suggesting that RRR, in combination with estimated baseline risks, can allow estimation of ARR even in people with different baseline risks.37 In considering the quality of evidence and its applicability to older adults with multimorbidity, RRR variability in this population has rarely been examined. Also, without knowing whether there are variations in baseline risk, results are difficult to interpret for older adults with multimorbidity.
Time Horizon to Benefit
Results are often discussed as number needed to treat (NNT) and number needed to harm (NNH), without consideration of time period to outcome. This can be misleading.38 NNT and NNH are most useful when the presentation includes a time factor. Also, there may be clinically meaningful benefits that occur more rapidly than the preestablished trial length. Clinicians should look for time horizon to benefit when making clinical management decisions for older adults with multimorbidity.39
Time horizon to benefit is the length of time needed to accrue an observable, clinically meaningful risk reduction for a specific outcome. Similarly, time horizon to harm is the length of time in which meaningful adverse events occur. For some chronic conditions, certain interventions are beneficial for certain outcomes only after lengthy treatment. For example, tight glycemic control is unlikely to help and more likely to harm older adults with multimorbidity who are at high risk of dying from other conditions.40 Clinicians and patients should therefore decide jointly whether anticipated benefits warrant potential harms of treatment.
III. PROGNOSIS DOMAIN
Guiding Principle: Frame clinical management decisions within the context of risks, burdens, benefits, and prognosis (e.g., remaining life expectancy, functional status, quality of life) for older adults with multimorbidity.
Justification of Principle
Clinical management decisions for this population necessitate the evaluation of prognosis to inform patient preferences and to adequately assess risks, burdens, and benefits,41 including remaining life expectancy, functional disability, and quality of life.42 Clinicians also need to evaluate risks of specific conditions (e.g., gastrointestinal hemorrhage with aspirin use for primary prevention of cardiovascular disease in men) as they consider prognosis.43
Each person’s prognosis informs, but does not dictate, clinical management decisions within the context of patient preferences.41 As stated above, the time horizon to benefit for a treatment may be longer than the individual’s projected life span, raising the risk of polypharmacy and drug–drug and drug–disease interactions, particularly in older adults with multimorbidity.44 Screening tests (e.g., colonoscopy) may also be nonbeneficial or even harmful if the time horizon to benefit exceeds remaining life expectancy, especially because associated harms and burdens increase with age and comorbidity.37
A discussion about prognosis can serve as a springboard for difficult conversations, facilitating decision-making and advance care planning, while addressing patient preferences, treatment rationales, and therapy prioritization.41 For example, the prognosis of an individual with cancer with a solid tumor and poor performance status usually worsens with chemotherapy.45 At the same time, progression through first- and second-line therapies has a low likelihood of treatment response, but a discussion about hospice offers patients and families options of greater home support, better quality of life, and possibly longer survival.
How to Use in Clinical Practice
Clinicians need to consider several issues when incorporating prognosis into decision-making, including framing a focused clinical question, determining the outcome (e.g., remaining life expectancy, quality of life, or condition-specific risk such as stroke), selecting a prognosis measure while recognizing its strengths and weaknesses, estimating prognosis, and integrating this information into the decision-making process.
Most older adults wish to discuss prognosis. Clinicians should use a culturally sensitive manner, because culture often influences priorities. (One tool, Doorway Thoughts,46 offers general considerations for particular ethnic groups to facilitate this conversation.) The dialogue needs to follow the ethical principles of autonomy (patient self-determination), beneficence (promotion of well-being), nonmaleficence (avoidance of harm), and justice (protection of vulnerable populations and fair allocation of resources).
Specific situations in which prognosis may inform clinical decision-making include disease prevention or treatment (e.g., whether to start or stop a medication or insert or replace a device), screening, a change in the patient’s clinical status, and health service utilization (e.g., hospitalization or intensive care unit treatment).37,41 The decision-making process includes which prognostic measure to use and what prognostic information to share with patients and families in the context of available evidence and patient preferences.47
Decisions can be prioritized based on life expectancy or other outcomes41 and categorized as short-term (within 1 year), midterm (within 5 years), and long-term (beyond 5 years).47 Individuals with limited life expectancy would focus on relevant short-term decisions such as intensity of glucose monitoring and control or whether to continue to live alone. Midterm or long-term decisions (e.g., lipid or colon cancer screening) would have lower priority. With such prioritization, the patient considers the treatments and interventions most likely to be beneficial, reducing the risk of harm without benefit.
Published prognostic tools are usually developed and tested in specific settings, potentially limiting their validity across settings (e.g., community vs nursing home).48 Also, clinicians need to consider which measure to use and how well it applies to older individuals with multimorbidity for outcomes that are disease-specific and non-disease specific. Tools for estimating remaining life expectancy have been the most widely studied and include measures for specific diseases as well as life tables broken down according to age, sex, and distribution of life expectancy for specified ages.37,41 Other approaches incorporate integrated measures or indices (e.g., Vulnerable Elders Survey [VES-13] and Palliative Prognostic Score [PaP]).49 Fewer measures exist to help predict functional disability or future quality of life.41,50
IV. CLINICAL FEASIBILITY DOMAIN
Guiding Principle: Consider treatment complexity and feasibility when making clinical management decisions for older adults with multimorbidity.
Justification of Principle
Treatment complexity and burden must be addressed in older adults with multimorbidity. Some guideline organizations, such as the Grading of Recommendations Assessment, Development and Evaluation (GRADE) working group, now encourage its routine consideration when making recommendations,51 although the definition of these concepts has been inconsistent.
A framework has been developed to break down treatment complexity and burden into individual components, such as steps in the task, number of choices, duration of execution, informed consent, and patterns of intervening distracting tasks, which may be useful when attempting to simplify individual components of care plans.52 The Medication Regimen Complexity Index (MRCI) also identifies factors that need to be considered when assessing medication regimen complexity.53
The more complex a treatment regimen, the higher the risk of nonadherence,54 adverse reactions,55 poorer quality of life, greater economic burden,3 and greater strain and depression in caregivers.56 Medication adherence also changes according to situational factors and perceptions of need, cost, or current symptoms.57
Education and assessments must be ongoing, multifaceted, individualized, and delivered using a variety of methods and settings because patients generally do not recall discussions with clinicians. Cognitive impairment frequently affects adherence.58
How to Use in Clinical Practice
An interdisciplinary team should assess adherence on an ongoing basis using tools such as the Medication Management Ability Assessment (MMAA), Drug Regimen Unassisted Grading Scale (DRUGS), Hopkins Medication Schedule (HMS), and Medication Management Instrument for Deficiencies in the Elderly (MedMaIDE).59 Patient-centered discussions must be held in collaboration with the support system (e.g., family, caregivers). Ongoing comprehensive medication reviews and medication management support result in fewer hospitalizations.60 Medication management interventions (e.g., reminder systems, education) have varying effects.61 Care transitions are important opportunities to reevaluate treatment complexity and adherence.
Clinical feasibility and patient preferences should inform treatment choices. Concordance between clinician and patient leads to greater motivation, persistence, and adherence62 and improves practitioners’ perspectives on prescribing,63 which helps prevent adverse reactions with problematic medications (e.g., anticoagulants), whose overuse or under use can lead to hospitalization.64 Education programs that teach self-management skills and improve self-efficacy for meeting realistic goals also improve adherence.65
V. OPTIMIZING THERAPIES AND CARE PLANS DOMAIN
Guiding Principle: Use strategies for choosing therapies that optimize benefit, minimize harm, and enhance quality of life for older adults with multimorbidity.
Justification of Principle
Older adults with multimorbidity are at risk of polypharmacy, suboptimal medication use, and potential harms from various interventions. Treatments and interventions must be prioritized to optimize adherence to the most essential pharmacological and nonpharmacological therapies, minimize risk exposure, and maximize benefit. Polypharmacy is associated with therapeutic omissions, less benefit from otherwise beneficial medications, and even harm in this population.66 Nonpharmacological interventions (e.g., implantable cardiac electronic devices), may prove more burdensome than beneficial if they are inconsistent with patient preferences.67
Persons with multimorbidity take more medications and have greater likelihood of adverse drug reactions exacerbated by age-related changes in pharmacokinetics and pharmacodynamics.68 Reducing the number of medications, particularly high-risk medications, can lower the risk of adverse drug reactions.
How to Use in Clinical Practice
In attempting to reduce the number of interventions, first identify treatments, procedures, and nonpharmacological therapies that may be inappropriate in older adults or in persons with multimorbidity. Several criteria exist to identify potentially inappropriate medications (see examples, Table 2). Algorithmic tools69 and sedative and anticholinergic indices are helpful in identifying medications associated with higher risk of adverse events.70 Similar strategies may be used in choosing testing, procedures, and nonpharmacological therapies. A clinician contemplating the use of an implantable cardiovascular electronic device can refer to an expert-derived consensus statement that considers benefits and risks, patient and family preferences, and quality of life.67
Table 2.
Commonly Cited Tools to Optimize Medication Therapy in Older Adults in the Outpatient Setting
| Criteria, Publication Year (Country) Target Group Basis of Criteria |
Definition of Content Validity of Criteria | Content of Criteria and Amount of Clinical Patient Information Included | Evidence of Association with Outcomes |
|---|---|---|---|
| Beers, 2012 (United States)87 Persons aged ≥65 Literature (published 2001–2011 in English) |
Modified Delphi consensus panel consisting of 11 interdisciplinary experts in geriatrics and pharmacotherapy, using a mail survey, conference calls, and in-person meeting followed by conference calls for final consensus | 53 medications or classes of medications in three categories: PIMs to avoid in older adults, PIMs to avoid in certain diseases and syndromes, and medications to be used with caution | Studies utilizing prior versions of the Beers criteria show conflicting evidence: studies link higher numbers of PIMs with drug-related problems, adverse drug events, hospitalization, and other adverse outcomes,88–90 but multiple studies have found no association between PIMs on the Beers list and adverse outcomes.91–95 Of hospitalizations for adverse drug reactions, Beers list medications accounted for only 6.6%, more than half of which was due to digoxin96 |
| STOPP and START, 2008 (Ireland)97 Persons aged ≥65 Evidence-based literature (not defined exactly in the article) Clinical experience of the investigators |
Two-round mail survey based on Delphi method with 18 experts (9 teaching hospital consultants in geriatric medicine, 3 clinical pharmacologists, 1 old-age psychiatric, 2 senior academic primary care physicians, 3 senior hospital pharmacists with interest in geriatric pharmacotherapy) | STOPP: 65 criteria focusing on prevalent problems associated with commonly prescribed medicines in older adults arranged according to physiological systems (42 criteria concerning avoidance of medications in certain disease states or conditions, 4 criteria concerning specific drug combinations to be avoided, 12 criteria concerning duration of drug therapies, 2 criteria concerning doses, 3 criteria concerning avoidance of prescribing without indication, 2 criteria concerning need for additional therapy) START: 22 evidence-based explicit prescribing indicators for common diseases in older adults |
Use of STOPP or START was associated with improvement in appropriate prescribing based on MAI and on the Assessment of Underutilization.98 No studies linking improvements in STOPP or START criteria prescribing and health outcomes98 |
| MAI, 1992, Summated 1994 (United States)68,99 Developed in persons in aged ≥65, but use not restricted to older adults Literature (Medline and manual search) published 1982–1990 Clinical experience of a clinical pharmacist and an internist geriatrician |
MAI: convenience sample of 10 academic clinicians judged MAI items to be definitely important or moderately important, providing an independent validation of their suitability Summated MAI: applying summated MAI to 105 medications prescribed to 10 elderly veterans. The results reflected the putative heterogeneity in prescribing appropriateness of 1,644 medications prescribed to 208 elderly veterans in the same clinic |
10 criteria (indication, effectiveness, dosage, correct directions, drug–drug interactions, drug–disease interactions, practical directions, costs, duplication, duration) worded as questions to assess the appropriateness of each prescribed drug, with instructions for use and operational definitions for each criterion | Most studies using this tool have used the MAI score as the outcome of interest. Decrease in MAI score during an in-patient intervention was linked with decrease in hospital readmission or emergency room visits 3 months after hospital discharge.100 Increase in MAI score linked with increasing risk of adverse drug events in primary care patients.101 MAI scores in Veterans Affairs outpatients were associated with higher numbers of subsequent hospital admissions and unscheduled visits102 |
| Good Palliative-Geriatric Practice algorithm, 2007 (Israel)103 Developed for use in disabled and frail older persons, no clear age recommendation provided Based on clinical practice as applied in nursing homes (not clearly defined in the article) |
Not validated | Flow diagram of items to evaluate (quality of evidence, valid indication, risk versus benefit ratio, possible symptoms or signs due to adverse reaction, alternative therapies, and feasibility of dose reduction) that aid in decisions to stop or switch a drug or reduce its dose | Study in nursing homes and geriatric centers in Israel showed a reduction in mortality and acute care in those randomized to receive an intervention using the algorithm.103 An outpatient study in 70 older persons demonstrated the ability to stop medications as recommended by the algorithm, with a low risk of adverse effects resulting from stopping medication69 |
Adapted with permission from Dimitrow MS, Airaksinen MSA, Kivela SL, Lyles A, Leikola SNS. Comparison of prescribing criteria to evaluate the appropriateness of drug treatment in individuals aged 65 and older: A systematic review. J Amer Geriatr Soc 2011;59:1521–1530. PIMs = potentially inappropriate medications; STOPP = Screening Tool of Older Person’s Prescriptions; START = Screening Tool to Alert doctors to Right Treatment; MAI = Medication Appropriateness Index.
Older adults with multimorbidity experience more healthcare transitions and utilization. A recent evaluation of potentially and actually inappropriate medications noted that 66% of hospitalized older adults used one of these medications and that 85% were still taking them at discharge.71 Medication appropriateness must be reevaluated at hospital admission and at intensive care and hospital discharge and repeated periodically in outpatients.
It can be complicated to identify interventions that should not be initiated or should be stopped in this population. Considerations include likelihood of reducing risk for a particular outcome, risk of harm, and time horizon to benefit or harm and the individual’s remaining life expectancy. For older adults with advanced disease or limited life expectancy, achievable benefits are unlikely to offset the risks and burdens of some aspects of clinical management.72 For example, secondary prevention interventions in diabetes mellitus to reduce risk of long-term complications are unlikely to provide meaningful benefit in this vulnerable subset of the population of older adults with diabetes mellitus and multimorbidity. Also, benefits may persist after discontinuation of some long-term therapies.
Adding medications for multiple conditions may produce less drug benefit and additional harms, burdens, and side effects.73 The so-called prescribing cascade may result when drug side effects are misidentified as a new medical condition, leading to additional prescriptions.74 To limit side effects and reduce costs to patients, nonpharmacological therapies (e.g., physical therapy) should be considered as alternatives to medication but may also add to treatment complexity and burden.75
Older adults with multimorbidity need good information about potential benefits and harms to help them make decisions, including clear explanations regarding uncertainty about potential benefits and harms. Often, individuals and their family and friends are poorly informed about possible adverse effects and benefits of medications.76 Although it may be easier to discuss stopping or not starting harmful interventions, decision-making about interventions with a high risk-to-benefit ratio, or a long time horizon to benefit may be more difficult. Decisions should be made after careful discussion with the individual, and the reasons for arriving at the decision should be documented.
Any decision to stop a medication needs a detailed plan for safe discontinuation. Although tapering is often unnecessary, stopping certain drug classes, especially those that act on the cardiovascular or central nervous system, requires caution.77 Generally, medications should be stopped one at a time.78 If there is uncertainty about discontinuation, a time-limited withdrawal can clarify whether the medication was needed in the first place.78 Partnering with pharmacists and other clinicians can optimize medication management.79
CONTROVERSIES AND CHALLENGES
Implementing this patient-centered approach in older patients with multimorbidity is challenging, especially with the dynamic health status of such individuals and use of multiple clinicians and settings. Even with appropriate decision tools and good communication, the need to make multiple simultaneous decisions makes it difficult to explain information and uncertainties about benefits and harms. This prevents individuals, families, and friends from fully participating in treatment decisions, communicating preferences, and prioritizing outcomes. Satisfactory evidence for clinical management of multimorbid individuals is scarce, as are reasonable prognostic measures; different prognostic tools often yield contrasting results for the same patient. Treatments meant to improve one outcome (e.g., survival) may worsen another (e.g., function). Many clinical management regimens are simply too complex to be feasible in this population, but as clinicians attempt to reduce polypharmacy and unnecessary interventions, they may fear liability regarding under use of therapies. Finally, such patient-centered approaches may simply be too time consuming for already overwhelmed clinicians within the current reimbursement structure and without an effective interdisciplinary team.
PROMISING APPROACHES TO OVERCOMING BARRIERS TO IMPLEMENTATION OF GUIDING PRINCIPLES IN THE CARE OF OLDER ADULTS WITH MULTIMORBIDITY
To implement these guiding principles, clinicians need an effective interdisciplinary healthcare team, as well as family, friends, and paid caregivers across sites of care, including the home; adequate training; reimbursement structures that reward patient-centered medical care; and an evidence base relevant to older adults with multimorbidity. These components are usually beyond an individual clinician’s immediate control. Few interventions have been developed that adequately address the restructuring of healthcare delivery, changes in clinicians’ behavior, and the support needed for patients and caregivers to improve care in this population.80
Coordination of Care and Patient-Centered Medical Homes
Because individuals with multimorbidity consult more clinicians, adequate systems of primary care medicine and central care coordination are needed to implement these principles. A “primary” clinician, or patient-centered medical home, may help older adults with multimorbidity make more informed decisions about their priorities, help coordinate medical and support services, and implement effective patient-centered care.
Collaboration with specialists (e.g., pharmacists, mental health professionals) may be challenging for some primary care clinicians because of inadequate communication systems, lack of established relationships, or accessibility problems. Also, specialists may not recognize serious problems facing older adults with multimorbidity, such as the importance of coordinating with a primary clinician and the complexity of managing multiple conditions. With appropriate education for all clinicians, patient-centered medical homes will help address such challenges.
Workforce Training: The Need for Curriculum Development and Training
Adequate evidence-based patient-centered care for older people with multimorbidity will require greater partnership among government agencies, professional organizations, and academic institutions, as well as resource investment in new curricula and training for all interdisciplinary team members to improve care for this population.81
Clinicians must learn how to move away from the single-disease approach to care and integrate family or friends into effective healthcare partnerships, because older adults with multimorbidity may need assistance with specific healthcare management tasks and healthcare decisions.82 Emerging evidence indicates the need for care facilitation and support for caregivers of older adults with complex health-related needs.80
Clinician training must address communication skills for discussions about prognosis and preferences, with awareness of ethnic and cultural concerns to improve treatment adherence and outcomes.83 Problematic “mismatches” may occur if clinician and patient styles differ. For example, a clinician with a paternalistic style may unintentionally antagonize someone who prefers shared decision-making.84 Healthcare literacy, numeracy, language barriers, and hearing and visual impairments may also affect outcomes. Printed educational materials in preferred languages may not be available for every chronic condition. Better communication will require improved patient educational materials to address these barriers.
Reimbursement Structure
The current reimbursement structure must change to care adequately for older people with multimorbidity. All necessary team members need appropriate compensation to allow enough time with patients and families. The current structure rewards acute, episodic, and specialist care for “quantity” of patients seen, rather than “quality” of care delivered,5 but care organized around single diseases may be inadequate because single-disease guidelines, rehabilitation, support, and education groups cannot meet the needs of complex, heterogeneous patients.3 Performance metrics based on single-disease guidelines to determine reimbursement in pay-for-performance formats may influence clinicians to provide unnecessary or potentially harmful care to older adults with multimorbidity.3,85 Thus, it is imperative to develop performance standards appropriate for this population and adequate for trial use in pay-for-performance demonstrations.86
Performance criteria are also needed to reward approaches known to improve patients’ health outcomes, functional status, and quality of life. Because Medicare and Medicaid are the main payment sources for health care in this population, they are the most appropriate agencies to implement innovative payment reform. The need to identify and support effective clinical management approaches will become more acute with time.
Building a Better Evidence Base
The lack of research focusing on the needs of older adults with multimorbidity has impeded optimal clinical management and educational advances (Table 3). A better evidence base regarding the outcomes of treatments and interventions is needed to guide the care of older adults with multimorbidity. Healthcare systems can improve the collection of relevant data with electronic medical records and other methods. Patient outcomes and system performance can thus be monitored, and quality improvement strategies, reimbursement options, and new performance measures can be developed and evaluated.
Table 3.
Proposed Topics for Research Agenda for Each Domain
| Topic | Research Agenda to Address Challenges |
|---|---|
| Patient preferences | Generate evidence regarding effects of treatment choices on outcomes other than survival, including functional status and quality of life. |
| Develop and test risk calculators and other tools to help clinicians inform patients appropriately by providing individualized outcome data according to each person’s multimorbidity profile. | |
| Compare methods to convey numerical information on benefits and harms and uncertainty to older adults with multimorbidity and their family or friends. | |
| Compare feasibility, acceptability, and results of using different methods of preference elicitation. | |
| Interpreting the evidence | Improve trial and study designs to include more older adults with multimorbidity, measure important outcomes, and evaluate time horizon to benefit. |
| Compare optimal methods for prioritizing the multiple possible treatment recommendations. | |
| Develop and test methods to help clinicians apply guidelines appropriately to older adults with multimorbidity. | |
| Design and test clinical decision support systems for this population. | |
| Prognosis | Develop, refine, externally validate, and test prognostic measures for feasibility and effect on clinical outcomes for this population. |
| Determine optimal approaches to communicating prognosis to inform clinical decision-making. | |
| Clinical feasibility | Develop sound and practical measures for describing treatment complexity and burden (pharmacological and nonpharmacological) in older adults with multimorbidity. |
| Evaluate use of such tools in clinical practice. | |
| Determine how overall treatment burden affects adherence and patient-important outcomes. | |
| Optimizing therapies and care plans | Develop evidence and tools to help clinicians recognize indications for discontinuing therapy and identify situations in which therapies should not be initiated at all. |
| Evaluate approaches for discontinuing medications, including communication with patients and family and friends. | |
| Incorporate a discontinuation arm or post discontinuation follow-up in trials. | |
| Overall | Investigate the best methods to implement principles in busy practices. |
CONCLUSION
Adoption of these guiding principles for management of older adults with multimorbidity may improve health care and outcomes in this population. Patients should be evaluated, and care plans should be designed and implemented according to the individual needs of each patient, but studies have not rigorously evaluated all approaches related to these guiding principles. Therefore, nonadoption of these principles should not imply medical liability or malpractice. These principles are intended to help guide clinicians. They highlight the urgent need for more research on the optimal management of this growing population.
Acknowledgments
Christine Weston, PhD, MSEd, provided research services. Katherine Addleman, PhD, provided editorial services. Joseph Douglas and Elvy Ickowicz, MPH provided additional research and administrative support. We are grateful to Milo Puhan, MD, PhD, and Bruce Leff, MD, for their thoughtful reviews of earlier versions of this manuscript.
The views expressed in this paper are those of the authors and do not necessarily represent the views of the Department of Health and Human Services, the Department of Veterans Affairs, the U.S. federal government, or other affiliated organizations.
The following organizations with special interest and expertise in the appropriate use of medications in older adults provided peer review of a preliminary draft of the long version document: American Academy of Family Physicians, American Academy of Home Care Physicians, American College of Cardiology, American College of Physicians, American Diabetes Association, American Medical Directors Association, American Nurses Association, Gerontological Society of America, Gerontological Advanced Practice Nurses Association, National Gerontological Nursing Association.
Dr. Boyd is a Paul Beeson Career Development Awardee funded by the NIA K23 AG032910, AFAR, The John A. Hartford Foundation, The Atlantic Philanthropies, The Starr Foundation and an anonymous donor. Dr. Boyd is an author for UpToDate, Inc. Dr. Brandt is on the Pharmacy and Therapeutics Committees at Omnicare and receives grants from Talyst (research grant), Econometrica (research grant), Health Resources and Services Administration (educational grant), and the State of Maryland Office of Health Care Quality (educational grant). Dr. Ritchie serves as a paid consultant to the University of Puerto Rico for a National Institute of Health research grant, receives grant funding from the Donald W. Reynolds Foundation (educational grant), and is an author for UpToDate, Inc.
Footnotes
By using the term “patient preferences” throughout this section, the aim is to keep the patient central to the decision-making process while recognizing that family and social supports play a vital role in management and decision-making whether or not cognitive impairment is present.
Author Contributions: All panel members contributed to the concept, design, and preparation of the manuscript.
Sponsor’s Role: AGS Staff participated in the final technical preparation and submission of the manuscript.
Conflict of Interest: The AGS and its Clinical Practice and Models of Care Committee convened and funded this work.
References
- 1.Boyd CM, Fortin M. Future of multimorbidity research: How should understanding of multimorbidity inform health system design? Public Health Rev. 2011;32:451–474. [Google Scholar]
- 2.Mercer SW, Smith SM, Wyke S, et al. Multimorbidity in primary care: Developing the research agenda. Fam Pract. 2009;26:79–80. doi: 10.1093/fampra/cmp020. [DOI] [PubMed] [Google Scholar]
- 3.Boyd CM, Darer J, Boult C, et al. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: Implications for pay for performance. JAMA. 2005;294:716–724. doi: 10.1001/jama.294.6.716. [DOI] [PubMed] [Google Scholar]
- 4.Anderson G. Chronic Care: Making the Case for Ongoing Care. Robert Wood Johnson Foundation; 2010. [Accessed June 19, 2012]. [on-line]. Available at http://www.rwjf.org/files/research/50968chronic.care.chartbook.pdf. [Google Scholar]
- 5.Boult C, Wieland GD. Comprehensive primary care for older patients with multiple chronic conditions: “Nobody rushes you through. JAMA. 2010;304:1936–1943. doi: 10.1001/jama.2010.1623. [DOI] [PubMed] [Google Scholar]
- 6.Tinetti ME, Bogardus ST, Jr, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med. 2004;351:2870–2874. doi: 10.1056/NEJMsb042458. [DOI] [PubMed] [Google Scholar]
- 7.Lugtenberg M, Burgers JS, Clancy C, et al. Current guidelines have limited applicability to patients with comorbid conditions: A systematic analysis of evidence-based guidelines. PLoS ONE. 2011;6:e25987. doi: 10.1371/journal.pone.0025987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chong CA, Chen IJ, Naglie G, et al. How well do guidelines incorporate evidence on patient preferences? J Gen Intern Med. 2009;24:977–982. doi: 10.1007/s11606-009-0987-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fried TR, Tinetti ME, Iannone L. Primary care clinicians’ experiences with treatment decision making for older persons with multiple conditions. Arch Intern Med. 2011;171:75–80. doi: 10.1001/archinternmed.2010.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durso S. The next frontier: Quantifying risks for interventions with no end in sight. Arch Intern Med. 2008;168:1230–1231. doi: 10.1001/archinte.168.11.1230-b. [DOI] [PubMed] [Google Scholar]
- 11.Reuben DB, Tinetti ME. Goal-oriented patient care—an alternative health outcomes paradigm. N Engl J Med. 2012;366:777–779. doi: 10.1056/NEJMp1113631. [DOI] [PubMed] [Google Scholar]
- 12.Loke YK, Cavallazzi R, Singh S. Risk of fractures with inhaled corticosteroids in COPD: Systematic review and meta-analysis of randomised controlled trials and observational studies. Thorax. 2011;66:699–708. doi: 10.1136/thx.2011.160028. [DOI] [PubMed] [Google Scholar]
- 13.Abd TT, Jacobson TA. Statin-induced myopathy: A review and update. Expert Opin Drug Saf. 2011;10:373–387. doi: 10.1517/14740338.2011.540568. [DOI] [PubMed] [Google Scholar]
- 14.Agostini JV, Han L, Tinetti ME. The relationship between number of medications and weight loss or impaired balance in older adults. J Am Geriatr Soc. 2004;52:1719–1723. doi: 10.1111/j.1532-5415.2004.52467.x. [DOI] [PubMed] [Google Scholar]
- 15.Fried TR, McGraw S, Agostini JV, et al. Views of older persons with multiple morbidities on competing outcomes and clinical decision-making. J Am Geriatr Soc. 2008;56:1839–1844. doi: 10.1111/j.1532-5415.2008.01923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakao MA, Axelrod S. Numbers are better than words. verbal specifications of frequency have no place in medicine. Am J Med. 1983;74:1061–1065. doi: 10.1016/0002-9343(83)90819-7. [DOI] [PubMed] [Google Scholar]
- 17.McNeil BJ, Pauker SG, Sox HC, Jr, et al. On the elicitation of preferences for alternative therapies. N Engl J Med. 1982;306:1259–1262. doi: 10.1056/NEJM198205273062103. [DOI] [PubMed] [Google Scholar]
- 18.Apter AJ, Paasche-Orlow MK, Remillard JT, et al. Numeracy and communication with patients: They are counting on us. J Gen Intern Med. 2008;23:2117–2124. doi: 10.1007/s11606-008-0803-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Connor AM, Bennett CL, Stacey D, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2009;3:CD001431. doi: 10.1002/14651858.CD001431.pub2. [DOI] [PubMed] [Google Scholar]
- 20.Fraenkel L, Fried TR. Individualized medical decision making: Necessary, achievable, but not yet attainable. Arch Intern Med. 2010;170:566–569. doi: 10.1001/archinternmed.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. [Accessed June 19, 2012];Assessing desirability of outcome states for medical decision making and cost-effectiveness analysis [on-line] Available at http://painconsortium.nih.gov/symptomresearch/chapter_24/index.htm.
- 22.Pauker SG, Kassirer JP. Decision analysis. N Engl J Med. 1987;316:250–258. doi: 10.1056/NEJM198701293160505. [DOI] [PubMed] [Google Scholar]
- 23.Farrar S, Ryan M, Ross D, et al. Using discrete choice modelling in priority setting: An application to clinical service developments. Soc Sci Med. 2000;50:63–75. doi: 10.1016/s0277-9536(99)00268-3. [DOI] [PubMed] [Google Scholar]
- 24.Fried TR, Tinetti M, Agostini J, et al. Health outcome prioritization to elicit preferences of older persons with multiple health conditions. Patient Educ Couns. 2011;83:278–282. doi: 10.1016/j.pec.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deber RB. Physicians in health care management: 8. The patient-physician partnership: Decision making, problem solving and the desire to participate. Can Med Assoc J. 1994;151:423–427. [PMC free article] [PubMed] [Google Scholar]
- 26.Levinson W, Kao A, Kuby A, et al. Not all patients want to participate in decision making. A national study of public preferences. J Gen Intern Med. 2005;20:531–535. doi: 10.1111/j.1525-1497.2005.04101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fried TR, Byers AL, Gallo WT, et al. Prospective study of health status preferences and changes in preferences over time in older adults. Arch Intern Med. 2006;166:890–895. doi: 10.1001/archinte.166.8.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luce JM. Physicians do not have a responsibility to provide futile or unreasonable care if a patient or family insists. Crit Care Med. 1995;23:760–766. doi: 10.1097/00003246-199504000-00027. [DOI] [PubMed] [Google Scholar]
- 29.Guyatt G, Rennie DE, editors. Users’ Guides to the Medical Literature: A Manual of Evidence-Based Clinical Practice. Chicago, IL: AMA Press; 2002. [Google Scholar]
- 30.Institute of Medicine. [Accessed June 19, 2012];Clinical practice guidelines we can trust [on-line] Available at http://www.iom.edu/~/media/Files/Report%20Files/2011/Clinical-Practice-Guidelines-We-Can-Trust/Clinical%20Practice%20Guidelines%202011%20Insert.pdf.
- 31.McClellan MB, McGinnis JM, Nabel EG, et al. IOM (Institute of Medicine) Evidence-Based Medicine and the Changing Nature of Health Care: 2007 IOM Annual Meeting Summary. Washington, DC: The National Academies Press; 2008. [PubMed] [Google Scholar]
- 32.Cox L, Kloseck M, Crilly R, et al. Underrepresentation of individuals 80 years of age and older in chronic disease clinical practice guidelines. Can Fam Physician. 2011;57:e263–2269. [PMC free article] [PubMed] [Google Scholar]
- 33.Fortin M, Contant E, Savard C, et al. Canadian guidelines for clinical practice: An analysis of their quality and relevance to the care of adults with comorbidity. BMC Fam Pract. 2011;12:74. doi: 10.1186/1471-2296-12-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. [Accessed June 19, 2012];GRADE Working Group [on-line] Available at http://www.gradeworking-group.org/index.htm.
- 35.AHRQ Effective Health Care Program Stakeholder Guide. Evidence-Based Medicine and the Changing Nature of Health Care: 2007 IOM Annual Meeting Summary. Rockville, MD: Agency for Healthcare Research and Quality; 2011. [Google Scholar]
- 36.Kent DM, Hayward RA. Limitations of applying summary results of clinical trials to individual patients: The need for risk stratification. JAMA. 2007;298:1209–1212. doi: 10.1001/jama.298.10.1209. [DOI] [PubMed] [Google Scholar]
- 37.Walter LC, Covinsky KE. Cancer screening in elderly patients: A framework for individualized decision making. JAMA. 2001;285:2750–2756. doi: 10.1001/jama.285.21.2750. [DOI] [PubMed] [Google Scholar]
- 38.Welch HG, Albertsen PC, Nease RF, et al. Estimating treatment benefits for the elderly: The effect of competing risks. Ann Intern Med. 1996;124:577–584. doi: 10.7326/0003-4819-124-6-199603150-00007. [DOI] [PubMed] [Google Scholar]
- 39.Koller MT, Raatz H, Steyerberg EW, et al. Competing risks and the clinical community: Irrelevance or ignorance? Stat Med. 2011;31:1089–1097. doi: 10.1002/sim.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blaum CS, Cigolle CT, Boyd C, et al. Clinical complexity in middle-aged and older adults with diabetes: The Health and Retirement Study. Med Care. 2010;48:327–334. doi: 10.1097/mlr.0b013e3181ca4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reuben DB. Medical care for the final years of life: “When you’re 83, it’s not going to be 20 years. JAMA. 2009;302:2686–2694. doi: 10.1001/jama.2009.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Covinsky KE, Hilton J, Lindquist K, et al. Development and validation of an index to predict activity of daily living dependence in community-dwelling elders. Med Care. 2006;44:149–157. doi: 10.1097/01.mlr.0000196955.99704.64. [DOI] [PubMed] [Google Scholar]
- 43.U S. Preventive Services Task Force. Aspirin for the prevention of cardiovascular disease: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;150:396–404. doi: 10.7326/0003-4819-150-6-200903170-00008. [DOI] [PubMed] [Google Scholar]
- 44.Brown AF, Mangione CM, Saliba D, et al. Guidelines for improving the care of the older person with diabetes mellitus. J Am Geriatr Soc. 2003;51:S265–280. doi: 10.1046/j.1532-5415.51.5s.1.x. [DOI] [PubMed] [Google Scholar]
- 45.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 46.Adler R, Kamel H. Doorway Thoughts: Cross-Cultural Health Care for Older Adults. III. Sudbury, MA: Jones and Bartlett Publishers; 2009. [Google Scholar]
- 47.Yourman LC, Lee SJ, Schonberg MA, et al. Prognostic indices for older adults: A systematic review. JAMA. 2012;307:182–192. doi: 10.1001/jama.2011.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Minne L, Ludikhuize J, de Rooij SE, et al. Characterizing predictive models of mortality for older adults and their validation for use in clinical practice. J Am Geriatr Soc. 2011;59:1110–1115. doi: 10.1111/j.1532-5415.2011.03411.x. [DOI] [PubMed] [Google Scholar]
- 49.Harrold J, Rickerson E, Carroll JT, et al. Is the palliative performance scale a useful predictor of mortality in a heterogeneous hospice population? J Palliat Med. 2005;8:503–509. doi: 10.1089/jpm.2005.8.503. [DOI] [PubMed] [Google Scholar]
- 50.Covinsky KE, Eng C, Lui LY, et al. The last 2 years of life: Functional trajectories of frail older people. J Am Geriatr Soc. 2003;51:492–498. doi: 10.1046/j.1532-5415.2003.51157.x. [DOI] [PubMed] [Google Scholar]
- 51.Kavanagh BP. The GRADE system for rating clinical guidelines. PLoS Med. 2009;6:e1000094. doi: 10.1371/journal.pmed.1000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nolan TW. System changes to improve patient safety. BMJ. 2000;320:771–773. doi: 10.1136/bmj.320.7237.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.George J, Phun Y, Bailey MJ, et al. Development validation of the medication regimen complexity index. Ann Pharmacother. 2004;38:1369–1376. doi: 10.1345/aph.1D479. [DOI] [PubMed] [Google Scholar]
- 54.George J, Vuong T, Bailey MJ, et al. Medication regimen complexity and adherence in patients at risk of medication misadventure. J Pharm Pract Res. 2006;36:99–102. [Google Scholar]
- 55.Cherubini A, Ruggiero C, Gasperini B, et al. The prevention of adverse drug reactions in older subjects. Curr Drug Metab. 2011;12:652–657. doi: 10.2174/138920011796504482. [DOI] [PubMed] [Google Scholar]
- 56.Giovannetti ER, Wolff JL, Xue QL, et al. Difficulty assisting with health care tasks among caregivers of multimorbid older adults. J Gen Intern Med. 2012;27:37–44. doi: 10.1007/s11606-011-1831-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morris RL, Sanders C, Kennedy AP, et al. Shifting priorities in multimorbidity: A longitudinal qualitative study of patient’s prioritization of multiple conditions. Chronic Illn. 2011;7:147–161. doi: 10.1177/1742395310393365. [DOI] [PubMed] [Google Scholar]
- 58.Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clin Proc. 2011;86:304–314. doi: 10.4065/mcp.2010.0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Farris KB, Phillips BB. Instruments assessing capacity to manage medications. Ann Pharmacother. 2008;42:1026–1036. doi: 10.1345/aph.1G502. [DOI] [PubMed] [Google Scholar]
- 60.Nobili A, Licata G, Salerno F, et al. Polypharmacy, length of hospital stay, and in-hospital mortality among elderly patients in internal medicine wards. The REPOSI study. Eur J Clin Pharmacol. 2011;67:507–519. doi: 10.1007/s00228-010-0977-0. [DOI] [PubMed] [Google Scholar]
- 61.Kripalani S, Yao X, Haynes RB. Interventions to enhance medication adherence in chronic medical conditions: A systematic review. Arch Intern Med. 2007;167:540–550. doi: 10.1001/archinte.167.6.540. [DOI] [PubMed] [Google Scholar]
- 62.Weiss M, Britten N. What is concordance? Pharm J. 2003;271:493. [Google Scholar]
- 63.Moen J, Norrgård S, Antonov K, et al. GPs’ perceptions of multiple-medicine use in older patients. J Eval Clin Pract. 2010;16:69–75. doi: 10.1111/j.1365-2753.2008.01116.x. [DOI] [PubMed] [Google Scholar]
- 64.Bell CM, Brener SS, Gunraj N, et al. Association of ICU or hospital admission with unintentional discontinuation of medications for chronic diseases. JAMA. 2011;306:840–847. doi: 10.1001/jama.2011.1206. [DOI] [PubMed] [Google Scholar]
- 65.Bodenheimer T, Lorig K, Holman H, et al. Patient self-management of chronic disease in primary care. JAMA. 2002;288:2469–2475. doi: 10.1001/jama.288.19.2469. [DOI] [PubMed] [Google Scholar]
- 66.Steinman MA, Landefeld CS, Rosenthal GE, et al. Does the number of coexisting chronic diseases affect the adverse association between polypharmacy and prescribing quality in older adults? Response J Am Geriatr Soc. 2007;55:803–804. doi: 10.1111/j.1532-5415.2007.01165.x. [DOI] [PubMed] [Google Scholar]
- 67.Lampert R, Hayes DL, Annas GJ, et al. HRS expert consensus statement on the management of cardiovascular implantable electronic devices (CI-EDs) in patients nearing end of life or requesting withdrawal of therapy. Heart Rhythm. 2010;7:1008–1026. doi: 10.1016/j.hrthm.2010.04.033. [DOI] [PubMed] [Google Scholar]
- 68.Hanlon JT, Wang X, Handler SM, et al. Potentially inappropriate prescribing of primarily renally cleared medications for older Veterans Affairs nursing home patients. J Am Med Dir Assoc. 2011;12:377–383. doi: 10.1016/j.jamda.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: Addressing polypharmacy. Arch Intern Med. 2010;170:1648–1654. doi: 10.1001/archinternmed.2010.355. [DOI] [PubMed] [Google Scholar]
- 70.Rudolph JL, Salow MJ, Angelini MC, et al. The Anticholinergic Risk Scale and anticholinergic adverse effects in older persons. Arch Intern Med. 2008;168:508–513. doi: 10.1001/archinternmed.2007.106. [DOI] [PubMed] [Google Scholar]
- 71.Morandi A, Vasilevskis EE, Pandharipande PP, et al. Inappropriate medications in elderly ICU survivors: Where to intervene? Arch Intern Med. 2011;171:1032–1034. doi: 10.1001/archinternmed.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Holmes HM, Hayley DC, Alexander GC, et al. Reconsidering medication appropriateness for patients late in life. Arch Intern Med. 2006;166:605–609. doi: 10.1001/archinte.166.6.605. [DOI] [PubMed] [Google Scholar]
- 73.Fitzgerald SP, Bean NG. An analysis of the interactions between individual comorbidities and their treatments-implications for guidelines and polypharmacy. J Am Med Dir Assoc. 2010;11:475–484. doi: 10.1016/j.jamda.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 74.Rochon PA, Gurwitz JH. Optimising drug treatment for elderly people: The prescribing cascade. BMJ. 1997;315:1096–1099. doi: 10.1136/bmj.315.7115.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schiff GD, Galanter WL, Duhig J, et al. Principles of conservative prescribing. Arch Intern Med. 2011;171:1433–1440. doi: 10.1001/archinternmed.2011.256. [DOI] [PubMed] [Google Scholar]
- 76.Modig S, Kristensson J, Ekwall AK, et al. Frail elderly patients in primary care—their medication knowledge and beliefs about prescribed medicines. Eur J Clin Pharmacol. 2009;65:151–155. doi: 10.1007/s00228-008-0581-8. [DOI] [PubMed] [Google Scholar]
- 77.Graves T, Hanlon JT, Schmader KE, et al. Adverse events after discontinuing medications in elderly outpatients. Arch Intern Med. 1997;157:2205–2210. [PubMed] [Google Scholar]
- 78.Bain KT, Holmes HM, Beers MH, et al. Discontinuing medications: A novel approach for revising the prescribing stage of the medication-use process. J Am Geriatr Soc. 2008;56:1946–1952. doi: 10.1111/j.1532-5415.2008.01916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Doucette WR, McDonough RP, Klepser D, et al. Comprehensive medication therapy management: Identifying and resolving drug-related issues in a community pharmacy. Clin Ther. 2005;27:1104–1111. doi: 10.1016/s0149-2918(05)00146-3. [DOI] [PubMed] [Google Scholar]
- 80.Wolff JL, Rand-Giovannetti E, Palmer S, et al. Caregiving and chronic care: The Guided Care Program for Family and Friends. J Gerontol A Biol Sci Med Sci. 2009;64A:785–791. doi: 10.1093/gerona/glp030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Turner J, Pugh J, Budiani D. “It’s always continuing”: First-year medical students’ perspectives on chronic illness and the care of chronically ill patients. Acad Med. 2005;80:183–188. doi: 10.1097/00001888-200502000-00017. [DOI] [PubMed] [Google Scholar]
- 82.Giovannetti ER, Xue Q, Reider L, et al. Factors Associated with Change in Health Care Task Difficulty Among Multimorbid Older Adults. Presented at the American Geriatrics Society Annual Meeting; May 2011; National Harbor, MD. [Google Scholar]
- 83.Mir G, Sheikh A. ‘Fasting and prayer don’t concern the doctors… they don’t even know what it is’: Communication, decision-making and perceived social relations of Pakistani Muslim patients with long-term illnesses. Ethn Health. 2010;15:327–342. doi: 10.1080/13557851003624273. [DOI] [PubMed] [Google Scholar]
- 84.Thorne SE, Ternulf Nyhlin K, Paterson BL. Attitudes toward patient expertise in chronic illness. Int J Nurs Stud. 2000;37:303–311. doi: 10.1016/s0020-7489(00)00007-9. [DOI] [PubMed] [Google Scholar]
- 85.Ostbye T, Yarnall KS, Krause KM, et al. Is there time for management of patients with chronic diseases in primary care? Ann Fam Med. 2005;3:209–214. doi: 10.1370/afm.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Multiple Chronic Conditions. A Strategic Framework: Optimum Health and Quality of Life for Individuals with Multiple Chronic Conditions. Washington, DC: U.S. Department of Health and Human Services; 2010. [Google Scholar]
- 87.The American Geriatrics Society; Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;2012 (60):616–631. doi: 10.1111/j.1532-5415.2012.03923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fick DM, Mion LC, Beers MH, et al. Health outcomes associated with potentially inappropriate medication use in older adults. Res Nurs Health. 2008;31:42–51. doi: 10.1002/nur.20232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dedhiya SD, Hancock E, Craig BA, et al. Incident use and outcomes associated with potentially inappropriate medication use in older adults. Am J Geriatr Pharmacother. 2010;8:562–570. doi: 10.1016/S1543-5946(10)80005-4. [DOI] [PubMed] [Google Scholar]
- 90.Akazawa M, Imai H, Igarashi A, et al. Potentially inappropriate medication use in elderly Japanese patients. Am J Geriatr Pharmacother. 2010;8:146–160. doi: 10.1016/j.amjopharm.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 91.Gnjidic D, Le Couteur DG, Abernethy DR, et al. Drug Burden Index and Beers criteria: Impact on functional outcomes in older people living in self-care retirement villages. J Clin Pharmacol. 2011;52:258–265. doi: 10.1177/0091270010395591. [DOI] [PubMed] [Google Scholar]
- 92.Sakuma M, Morimoto T, Matsui K, et al. Epidemiology of potentially inappropriate medication use in elderly patients in Japanese acute care hospitals. Pharmacoepidemiol Drug Saf. 2011;20:386–392. doi: 10.1002/pds.2110. [DOI] [PubMed] [Google Scholar]
- 93.Barnett K, McCowan C, Evans JM, et al. Prevalence and outcomes of use of potentially inappropriate medicines in older people: Cohort study stratified by residence in nursing home or in the community. BMJ Qual Saf. 2011;20:275–281. doi: 10.1136/bmjqs.2009.039818. [DOI] [PubMed] [Google Scholar]
- 94.Harugeri A, Joseph J, Parthasarathi G, et al. Potentially inappropriate medication use in elderly patients: A study of prevalence and predictors in two teaching hospitals. J Postgrad Med. 2010;56:186–191. doi: 10.4103/0022-3859.68642. [DOI] [PubMed] [Google Scholar]
- 95.Corsonello A, Pedone C, Lattanzio F, et al. Potentially inappropriate medications and functional decline in elderly hospitalized patients. J Am Geriatr Soc. 2009;57:1007–1014. doi: 10.1111/j.1532-5415.2009.02266.x. [DOI] [PubMed] [Google Scholar]
- 96.Budnitz DS, Lovegrove MC, Shehab N, et al. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365:2002–2012. doi: 10.1056/NEJMsa1103053. [DOI] [PubMed] [Google Scholar]
- 97.Gallagher P, Ryan C, Byrne S, et al. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert Doctors to Right Treatment) consensus validation. Int J Clin Pharmacol Ther. 2008;46:72–83. doi: 10.5414/cpp46072. [DOI] [PubMed] [Google Scholar]
- 98.Gallagher PF, O’Connor MN, O’Mahony D. Prevention of potentially inappropriate prescribing for elderly patients: A randomized controlled trial using STOPP/START criteria. Clin Pharmacol Ther. 2011;89:845–854. doi: 10.1038/clpt.2011.44. [DOI] [PubMed] [Google Scholar]
- 99.Samsa GP, Hanlon JT, Schmader KE, et al. A summated score for the Medication Appropriateness Index: Development and assessment of clinimetric properties including content validity. J Clin Epidemiol. 1994;47:891–896. doi: 10.1016/0895-4356(94)90192-9. [DOI] [PubMed] [Google Scholar]
- 100.Hellstrom LM, Bondesson A, Hoglund P, et al. Impact of the Lund Integrated Medicines Management (LIMM) model on medication appropriateness and drug-related hospital revisits. Eur J Clin Pharmacol. 2011;67:741–752. doi: 10.1007/s00228-010-0982-3. [DOI] [PubMed] [Google Scholar]
- 101.Lund BC, Carnahan RM, Egge JA, et al. Inappropriate prescribing predicts adverse drug events in older adults. Ann Pharmacother. 2010;44:957–963. doi: 10.1345/aph.1m657. [DOI] [PubMed] [Google Scholar]
- 102.Schmader KE, Hanlon JT, Landsman PB, et al. Inappropriate prescribing and health outcomes in elderly veteran outpatients. Ann Pharmacother. 1997;31:529–533. doi: 10.1177/106002809703100501. [DOI] [PubMed] [Google Scholar]
- 103.Garfinkel D, Zur-Gil S, Ben-Israel J. The war against polypharmacy: A new cost-effective geriatric-palliative approach for improving drug therapy in disabled elderly people. Isr Med Assoc J. 2007;9:430–434. [PubMed] [Google Scholar]