Summary
The temporal lobe is thought to be abnormal in autism, yet standard volumetric analyses are often unrevealing when age, sex, IQ and head size are controlled. Quantification of temporal lobe structures were obtained in male subjects with autism and controls, where subjects with head circumference (HC) defined macrocephaly were excluded, so that volume differences were not just related to the higher prevalence of macrocephaly in autism. Various statistical methods were applied to the analysis including a classification and regression tree (CART) method, a non-parametric technique that helps define patterns of relationships that may be meaningful in distinguishing temporal lobe differences between subjects with autism and age and IQ matched controls. Subjects with autism were also compared to a separate control group with reading disorder (RD), with the prediction that the temporal lobe morphometric analysis of the reading disorder controls would be more similar to that of the autism group. The CART method yielded a high specificity in classifying autism subjects from controls based on the relationship between the volume of the left fusiform gyrus (LFG) gray and white matter, the right temporal stem (RTS) and the right inferior temporal gyrus gray matter (RITG-GM). Reading disordered individuals were more similar to subjects with autism. Simple size differences did not distinguish the groups. These findings demonstrate different relationships within temporal lobe structures that distinguish subjects with autism from controls. Results are discussed in terms of pathological connectivity within the temporal lobe as it relates to autism.
Keywords: Autism, Temporal Lobe, CART Analysis, MRI
Introduction
Numerous theories along with morphometric neuroimaging studies and histological findings have implicated temporal lobe abnormalities in autism [1-4]. Given the core behavioral features of the disorder such as language impairment, deficits in social and emotional functioning and stereopathies, along with impairments in face processing, all have plausible relationships with temporolimbic abnormalities [5, 6]. However, no study to date has demonstrated a single or specific temporal lobe region or limbic structure abnormality that clearly differentiates subjects with autism from controls. Current thought on potential neuropathology of autism has focused on aberrant connectivity [7, 8], which would suggest that the relative association of temporal lobe regions, one with another, may be more important in differentiating those with autism from controls rather than size or morphology of a single structure or regional difference.
An attempt to identify temporolimbic structures critical in differentiating subjects with autism from controls poses several design and statistical challenges because of the complexity of temporolimbic structures and the heterogeneity of autism. For example, there are five main temporal lobe gyri comprised of both white and gray matter , two critical limbic structures (hippocampus and amygdala) and their connections and a major white matter region – the temporal stem – comprised of much of the afferent/efferent connections of the temporal lobe with the rest of the brain [9, 10]. At a minimum, taking just the aforementioned major regions equates to a total of 13 structures per hemisphere that can be isolated and studied, yielding a complex array of how these regions may differ or vary in comparison to one another and a statistical challenge as to how to control for family-wise error [11]. In order to overcome the family-wise error rate with so many simultaneous comparisons, standard parametric analyses requires a high alpha level before reaching any statistically significant difference. However, the classification and regression tree (CART) statistical method [10, 12, 13], provides a suitable technique to explore multifactorial brain relationships within autism and between comparison groups, especially when combined with logistic regression and discriminant analysis. Such analyses have been applied to complex neuroimaging data as a means of constructing the most efficient algorithm for predicating group differences [14, 15]. In the current study we applied the CART method in an attempt to identify which temporal structures most readily differentiated subjects with autism from controls.
Temporal lobe differences have been reported in subjects with a reading deficit (RD) when compared to typical developing, non-reading impaired controls [16, 17]. Furthermore, some have categorized features of the broader phenotype of language impairments in autism as having commonalities with verbal-based learning disabilities [18, 19]. Defining RD by reading ability greater than a standard deviation below IQ, we also applied the CART analysis between subjects with autism and RD controls without autism. If RD is associated with its own set of anomalous temporal lobe development, it would be predicted that in a CART analysis, RD would not be as readily discriminated from autism.
Since overall volume of the temporal lobe has been found to positively correlate with IQ [20, 21] it will be important to control for IQ differences that may relate to size differences. Another experimental design challenge in autism research has to do with head size differences [22], particularly since there is an increased prevalence of macrocephaly—defined as occipitofrontal circumference (OFC) > 97th percentile—in autism [see 23, 24]. Despite this increased rate of macrocephaly in autism, the majority of subjects with autism when assessed later in childhood, such as in this study, do not meet criteria for head circumference defined macrocephaly [see 25]. Therefore in the current study, both autism and control subjects that met OFC criteria for macrocephaly, were excluded.
With all of these design considerations in mind, we utilized the CART method to develop an algorithm based on characteristics of temporal lobe structures that had the highest likelihood of differentiating subjects with autism from typical developing controls and a separate RD control group comprised of individuals with reading impairment. All groups were matched on PIQ, OFC, age and all were male. Autism was rigorously defined and no subjects met criteria for mental retardation. We predicted that the CART method would distinguish quantitative temporal lobe differences most readily between subjects with autism and controls, but not necessarily between subjects with autism and RD.
Methods
Subjects
The majority of autistic and comparison subjects were ascertained from community sources, including social skills training groups, parent support groups, youth groups, and schools. Some of the autistic subjects had participated in other research at the University of Utah. Three subject groups were studied: an autism group, a control group of normal (typically developing) subjects and a reading deficit (RD) group. Subjects who met the qualification of macrocephaly (head circumference [HC] > 97th percentile for age and sex) were excluded from the analysis so that the HC in all three groups would be approximately comparable. Head circumference measurements were based on the methods outlined by Farkas et al. [26]. Autism associated with high vs. low IQ may differ in etiology and neuroanatomy. The same is true for autism in males vs. females and autism associated with causal medical conditions. Because we wanted our between group (autism vs. comparison) samples to be as homogeneous as possible in regard to IQ and gender, and our within group samples to be homogeneous in etiology, all subjects in the study were males with idiopathic autism who had PIQs of 62 or higher based on either the Wechsler Intelligence Scale for Children-III [WISC-III, 27], or the Wechsler Adult Intelligence Scale-III [WAIS-III, 28]. The comparison group consisted of normal (typically developing) subjects and subjects with RD, group-matched by age to the autism subjects. Demographic and descriptive variables including age at scan, HC, PIQ, handedness, total intracranial volume (TICV) and total brain volume (TBV) were similar across all groups (see Table 1).
TABLE 1.
Descriptive and demographic variable summary for each group
| Autism n = 33 | Normal Control n = 24 | Reading Disorder (RD) n = 24 | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Age at Scan | 13.94 (5.97) | 13.29 (5.15) | 14.21 (6.22) |
| PIQ | 97.14 (19.54) | 100.22 (12.70) | 101.33 (12.25) |
| Standardized HC | 0.49 (1.01) | 0.25 (1.09) | 0.11 (1.31) |
| Handedness | 62.10 (59.48) | 61.82 (66.95) | 79.05 (44.03) |
| TICV | 1472.60 (114.28) | 1477.06 (111.57) | 1443.08 (105.83) |
| TBV | 1354.14 (118.56) | 1367.15 (109.06) | 1341.91 (107.65) |
Legend: SD = standard deviation; PIQ = performance IQ; TICV = total intracranial volume; HC = head circumference; TBV = total brain volume.
Clinical Assessment
Autism
Autism was rigorously diagnosed. The subject's mother was interviewed using the Autism Diagnostic Interview-Revised (ADI-R), a semi-structured, investigator-based interview with good reliability and validity [29]. In addition, autistic subjects were directly assessed using the Autism Diagnostic Observation Schedule-Generic (ADOS-G), a semi-structured play and interview session designed to elicit social, communication, and stereotyped repetitive behaviors of autism [30]. All autistic subjects met ADI-R, ADOS-G, and DSM-IV criteria for autism.
Reading disorder (RD)
RD was operationally defined as reading ability significantly below the mean for their intellectual quotient (IQ). All subjects with RD had a documented history of a reading disorder and performed below their full scale IQ (FSIQ) scores on all of the following tests: Woodcock Johnson [31] word attack and letter word identification (tests of phonological processing); Wide Range Achievement Test [WRAT, 32] of spelling; and the Gray Oral Reading Test [33] passage score (reading speed and accuracy). All RD subjects scored 1.5 standard deviations or more below FSIQ on at least two of the tests or two standard deviations below FSIQ on one of the tests. RD subjects had no developmental or neurologic problems other than RD, and no severe psychiatric disorders, based on history, IQ, physical examination, and structured psychiatric assessment.
Typically developing subjects had no developmental, neurologic, or severe psychiatric disorders based on history, IQ, reading and language tests, physical examination, and structured psychiatric assessment. No RD or typically developing subject had an autism-spectrum disorder (ASD); those with ASD were excluded by history, direct observation, and an interview of the mother using the Family History Interview for Disorders of Social Development and Cognition [FHI, 34, 35]. The FHI was specifically designed to detect signs of autism-spectrum disorders and milder, isolated autism-like features.
IQ
Verbal skills are often diminished in autism [36]. In addition, there can be wide splits between verbal and performance IQ in autism, making full scale IQ difficult to interpret [37]. For these reasons, we defined level of intelligence on the basis of non-verbal abilities as measured by the performance intellectual quotient (PIQ) on either the WISC-III or the WAIS-III. All subjects had PIQs > 62 and the autism and comparison groups were matched on PIQ.
Handedness
Handedness was measured using the Edinburgh Handedness Inventory [38]. This inventory assesses what hand the subject uses most of the time for each of 10 skills, such as throwing a ball, writing, and eating with a spoon. Quantitative handedness = (# right-handed skills - # left-handed skills) / total number of skills performed. A score of 100 signifies complete right handedness and −100 indicates complete left handedness.
Neuroimaging
Structural magnetic resonance images (MRI) were acquired on a Philips 1.5 Tesla Scanner, details of which have been previously published [39]. Multiple protocols were run that were used for the clinical review and quantitative analyses. The 3D T1 and T2 weighted images, as will be explained below were used for the quantitative analyses. In some cases, sedation of autism subjects was necessary and followed a strict clinical protocol approved by the institutional review board of the university and performed by an onsite approved anesthesiologist in conjunction with the radiologist. The procedure was clearly explained, as best possible, to the subject and parent or guardian. In several situations rehearsal was used to ‘practice’ lying in the scanner. In all cases, informed consent was obtained prior to any imaging. No complications or untoward effects were encountered.
A neuroradiologist reviewed each scan clinically, with no clinically significant abnormalities identified. Quantitative analyses followed well-established protocols as previously published. Briefly, the co-registered T1 and T2 weighted images were segmented into white matter (WM) , gray matter (GM), and cerebral spinal fluid (CSF) pixels using the ANALYZE® [40] multispectral tool. Temporal lobe structures were identified according to the methods outlined by Bigler et al. [39] and included total, white and gray matter volumes of the five main gyri namely the superior temporal gyrus (STG), the middle temporal gyrus (MTG), the inferior temporal gyrus (ITG), the fusiform gyrus (FG), and the parahippocampal gyrus (PHG) and CSF volumes of the major temporal lobe sulci and the temporal horn along with the temporal stem. Total volume of the hippocampus and amygdala were also computed.
Data in Preparation for CART Analysis
Each of the 5 temporal gyri as well as the amygdala, hippocampus, and temporal stem volumes were included in the analysis. Left (L) and right (R) hemispheric measurements were obtained for each structure. Additionally, separate GM and WM measures were obtained for each gyrus. Thus, there were a total of 26 temporal lobe structures included in the analysis as summarized in Table 2. Table 2 also shows the brain volumes for the autism, control, and RD groups, as well as the significance of any differences. The cells with asterisks indicate those volumes that are significantly (or approaching significance) different than the autism group. The left amygdala, right parahippocampal gyrus gray matter (RPHG-GM), fusiform gyrus gray matter (FG-GM) both left and right, right middle temporal gyrus gray matter (RMTG-GM), and temporal stem white matter (TS-WM) are among the structures with asterisks in the autism vs. control comparison. None of these differences, however, remain significant after corrections for multiple testing. The RFG-GM, the MTG-GM, the right superior temporal gyrus gray matter (RSTG-GM), and the TS-WM are structures that differ between the autism and RD groups. Again, no significant differences remain after multiple testing corrections. Since this analysis is mainly exploratory in nature, the temporal lobe measures were kept as left and right measures initially, however since the correlations between right at left measures was high (between r = 0.6 and r = 0.8) the analysis was also done with the right and left values averaged together for one overall measure for each structure.
TABLE 2.
Descriptive information for the brain structures included in the analysis.
| Brain Structure | Autism (n = 33) Mean (SD) | Normal Control (n = 24) Mean (SD) | p value autism vs. control | RD (n = 24) Mean (SD) | p value autism vs. RD |
|---|---|---|---|---|---|
| L Amygdala | 3.23 (0.46) | 3.47 (0.45) | 0.053* | 3.26 (0.48) | 0.884 |
| R Amygdala | 3.17 (0.37) | 3.32 (0.42) | 0.172 | 3.17 (0.50) | 0.971 |
| L Hippocampus | 3.16 (0.44) | 2.96 (0.62) | 0.197 | 3.24 (0.65) | 0.580 |
| R Hippocampus | 3.11 (0.39) | 2.97 (0.69) | 0.390 | 3.20 (0.52) | 0.465 |
| L PHG-GM | 3.31 (0.49) | 3.15 (0.60) | 0.271 | 3.17 (0.52) | 0.321 |
| R PHG-GM | 3.21 (0.52) | 2.92 (0.56) | 0.051* | 3.11 (0.39) | 0.448 |
| L PHG-WM | 0.81 (0.41) | 0.87 (0.33) | 0.519 | 0.89 (0.35) | 0.467 |
| R PHG-WM | 0.85 (0.46) | 0.96 (0.28) | 0.273 | 0.91 (0.41) | 0.647 |
| L FG-GM | 4.28 (0.68) | 3.62 (0.71) | 0.001* | 4.01 (0.73) | 0.151 |
| R FG-GM | 4.25 (0.77) | 3.82 (0.71) | 0.039* | 3.80 (0.49) | 0.010* |
| L FG-WM | 0.87 (0.41) | 0.77 (0.24) | 0.262 | 0.89 (0.32) | 0.864 |
| R FG-WM | 0.91 (0.45) | 0.89 (0.27) | 0.837 | 0.90 (0.38) | 0.885 |
| L ITG-GM | 5.54 (0.99) | 5.10 (0.98) | 0.103 | 5.50 (0.82) | 0.871 |
| R ITG-GM | 5.50 (0.81) | 5.15 (1.12) | 0.170 | 5.44 (0.74) | 0.745 |
| L ITG-WM | 1.90 (0.67) | 2.05 (0.51) | 0.346 | 2.18 (0.66) | 0.118 |
| R ITG-WM | 2.01 (0.75) | 2.15 (0.62) | 0.442 | 2.12 (0.75) | 0.605 |
| L MTG-GM | 6.21 (1.08) | 5.71 (1.29) | 0.114 | 5.72 (0.53) | 0.029* |
| R MTG-GM | 6.90 (1.28) | 6.26 (1.35) | 0.074* | 6.30 (1.20) | 0.080* |
| L MTG-WM | 2.62 (0.83) | 2.79 (0.67) | 0.427 | 2.83 (0.74) | 0.346 |
| R MTG-WM | 3.06 (0.90) | 3.17 (0.80) | 0.652 | 3.06 (0.60) | 0.999 |
| L STG-GM | 9.26 (1.73) | 8.75 (1.74) | 0.277 | 8.65 (1.24) | 0.125 |
| R STG-GM | 9.84 (1.97) | 9.53 (2.34) | 0.593 | 8.75 (1.51) | 0.028* |
| L STG-WM | 3.05 (1.01) | 3.44 (1.11) | 0.182 | 3.30 (0.98) | 0.362 |
| R STG-WM | 3.83 (1.43) | 4.20 (1.09) | 0.278 | 3.77 (1.20) | 0.866 |
| L TS-WM | 1.06 (0.36) | 1.25 (0.30) | 0.038* | 1.24 (0.40) | 0.081* |
| R TS-WM | 0.97 (0.33) | 1.20 (0.32) | 0.013* | 1.17 (0.35) | 0.033* |
p value is significant or approaching significance
Legend: RD = reading disorder; GM = gray matter; WM = white matter; L = left; R = right; FG = fusiform gyrus; ITG = left inferior temporal gyrus; MTG = middle temporal gyrus; STG = superior temporal gyrus; TS = temporal stem.
Statistical Analysis
In this analysis, we use classification methods to compute a rule that will classify an individual as belonging to a certain group (i.e., either autism or normal control), based on brain scan measurements. Classification is a multivariate technique that helps in understanding the structure of the data by seeing how the collection of brain structures together differs in the autism and control groups, thus avoiding the problems of multiple testing. The computed classification rule is evaluated with the misclassification rate – how many of the subjects are wrongly classified based on the rule. In this analysis, we use cross-validated misclassification rate, in which each observation is excluded one at a time, from the classification rule computation. The excluded observation is then classified based on the constructed rule. Thus the same data are not used both to build and to validate the classification rule resulting in a better estimate of the true misclassification rate.
We mainly use Classification and Regression Tree (CART) analysis to identify structure in the data and logistic regression to compute the misclassification rate of the classification rule identified by CART. Stepwise discriminant analysis was used as a method of finding all structures with possible discriminatory power.
Classification and regression trees (CART)
CART is a nonparametric (does not require normality of observations) method of developing a classification rule [12, 13] and readily detects nonlinear relationships in the explanatory variables in order to help uncover the underlying structure of complex datasets.
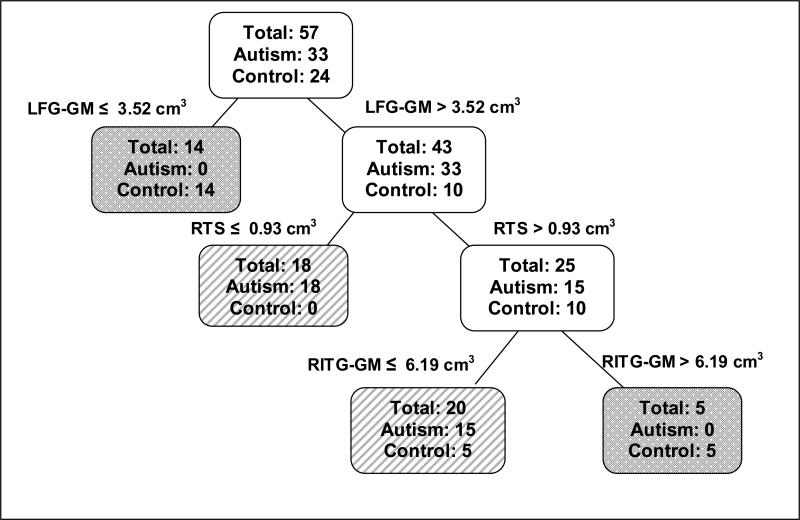
CART analysis creates a set of classification rules by building a binary decision tree that partitions the whole data set into disjoint subsets of homogenous groups. For example, Figure 1 illustrates the CART analysis for the autism and the control groups. In this analysis, there are 33 autism and 24 controls, and initially, all 57 observations in the dataset are together in the first set at the top of the tree. CART methods create a split of the data, based on a cutoff value of one of the explanatory variables, such that the subsets that are created have a higher proportion of observations from the same group. The goal is to create subsets that are composed of observations belonging to just one group.
Figure 1. Classification tree of autism vs. normal control groups.
Autism vs. Control Groups Classification Tree including left fusiform gyrus gray matter (LFG-GM), right inferior temporal gyrus gray matter (RITG-GM), and the right temporal stem (RTS).White boxes indicate locations on the tree that are still subject to splitting. Lined and checkered boxes are locations on the tree that have completed the splitting. Lined boxes have a higher proportion of autistic individuals and checkered boxes have a higher proportion of normal control individuals.
Partitioning or splitting of the tree will continue until a stopping criterion based on the overall purity of the tree is met. When a subset can no longer be split, at a location referred to as a terminal subset, a classification is made. While it is desirable to obtain a tree with only single-group terminal subsets, this method will often over fit the tree. Some of the tree splits usually need to be eliminated in order to find the simplest informative tree.
Logistic regression
Logistic regression is a regression procedure for a binary response variable that estimates the probability that an observation belongs to one of two response groups (i.e., autism vs. control) [41]. We use the variable identified in the CART classification rule as the explanatory variables in the logistic regression. The probability (pi) of observation i belonging to the autism group is estimated with the model; thus the probability of belonging to the control group is 1-pi. An observation is assigned to the autism group if pi is greater than some cutoff probability; otherwise the observation is assigned to the control group. The cutoff probability is chosen in such a way as to minimize the cross-validated misclassification error of both groups.
Discriminant analysis
Discriminant analysis, a classification tool first introduced in the 1930s by R. A. Fisher [42], computes a linear combination of the response variables that create the maximum distance between groups. Stepwise discriminant analysis finds the subset of the response variables that best reveals the differences among the groups. Discriminant analysis is one of the most common classification procedures and has been used in MRI studies [43]. One potential limitation with discriminant analysis is the assumption that the response variables are normally distributed with equal variances. In this study, stepwise discriminant analysis was used to determine which variables of all 26 variable structures, involved in the analysis, seemed to have discriminatory power between the groups.
Comparisons
The goal of this analysis was to uncover which subset of temporal lobe structures, by inclusion in a classification rule, suggested abnormalities that distinguished subjects with autism from controls. In order to directly compare the autism group with each comparison group, two separate classifications were performed. The first compared the autism and control (normal or typically developing) groups and the second compared the autism and the RD groups.
Results
Standard Morphometric Analyses
Mean volumetric values of the target temporal lobe structures are presented in Table 2. After applying a Bonferroni correction method, no temporal lobe structures differed significantly across groups, although the LFG approached significance compared to typical developing controls. Statistical trends in the parametric analyses did identify the main temporal lobe structures that yielded the highest classification algorithm, which will be discussed below.
Autism versus Control
The left fusiform gyrus gray matter (LFG-GM), the right temporal stem (RTS), the right inferior temporal gyrus gray matter (RITG-GM) and their interactions were temporal lobe structures that CART analysis showed to be important in distinguishing between the autism and control groups (see Figure 1). There were no autistic subjects with a LFG-GM volume below 3.52 cm3, but 14 control subjects were below this value. The rest of the ten control subjects that had LFG-GM volumes above this value also had RTS values above 0.93 cm3. Thus, the first two splits of the classification tree formed three groups: Subjects with LFG-GM < 3.52 cm3 (made up of 14 control and 0 autism), subjects with LFG-GM > 3.52 cm3 and RTS < 0.93 cm3 (made up of 0 control and 18 autism) and subjects with LFG-GM > 3.52 cm3and RTS > 0.93 cm3 (made up of 10 control and 15 autism). A further split of the last group on the RITG-GM shows that all 15 autism subjects have volumes < 6.19 cm3 while only half the controls have volumes below the same value.
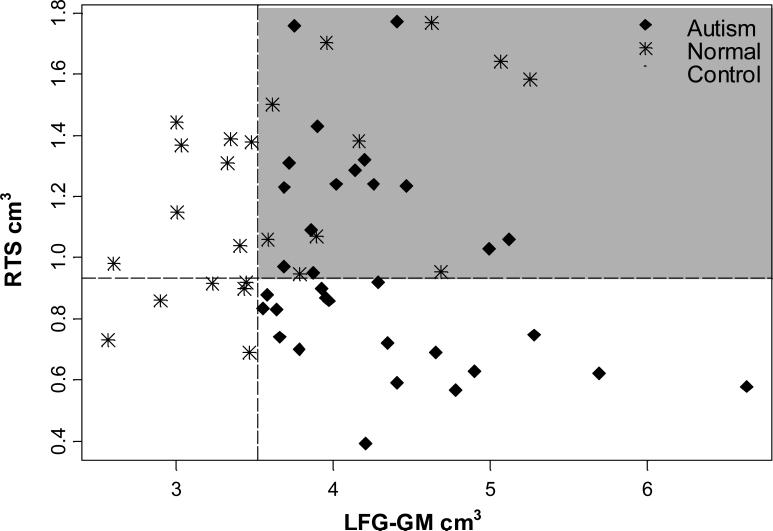
Figure 2 shows how the actual observations are separated based on the first two splits of the regression tree. The LFG-GM is on the x-axis. This represents the first split formed by the tree and the 14 stars on the left side of the vertical dotted line are the controls classified by the first split of the tree. The RTS is on the y-axis, representing the second split of the tree. There are 18 autism subjects classified by the first and second split shown to the right of the dotted vertical line and below the dotted horizontal line. The quadrant highlighted in gray indicates those individuals not classified by the first two splits. The graph shows a clear separation of points with only a smaller mixture of undetermined classification in the upper right gray quadrant. In other words, the majority of autism subjects had low RTS volume and larger LFG-GM volume.
Figure 2. Two dimensional classification tree of autism vs. normal control groups.
The two dimensional classification tree shows how the actual observations are separated based on the first two splits of the regression tree. The left fusiform gyrus gray matter (LFG-GM) is on the x-axis. This represents the first split formed by the tree and the 14 red stars on the left side of the orange line are the controls classified by the first split of the tree. The right temporal stem (RTS) is on the y-axis, representing the second split of the tree. There are 18 autism subjects classified by the first and second split shown to the right of the dotted vertical line and below the dotted horizontal line. The quadrant highlighted in gray indicates those individuals not classified by the first two splits. The graph shows a clear separation of points with only a smaller mixture of undetermined classification in the upper right quadrant. Although over half the subjects are classified here, a third variable split would still be informative.
A logistic regression analysis with only the LFG-GM, RTS, and RITG-GM and the two-way interactions validated the CART analysis. Twenty-eight of the 33 autistic subjects (85%) were correctly classified and 20 of the 24 control subjects (83%) were correctly classified for cross-validated misclassification error rates of 15% and 17% respectively.
The discriminant analysis identified the following 13 variables as having discriminatory power: L parahippocampal GM, L parahippocampal WM, R parahippocampal WM, LFG-GM, LITG-WM, RMTG-GM, LMTG-WM, RMTG-WM, LSTG-GM, RSTG-GM, LSTG-WM, RSTG-WM, then LTS.
Discrimination with the above thirteen variables had a misclassification rate of 24% in the autism group and 29% in the control group. These rates are worse than those used in the logistic regression analysis suggesting that some of the variables add noise to the analysis. However, the fact that they were chosen as discriminatory variable suggests the likelihood of these regions being related to the behavioral phenotype of autism.
Autism versus Reading Disorder
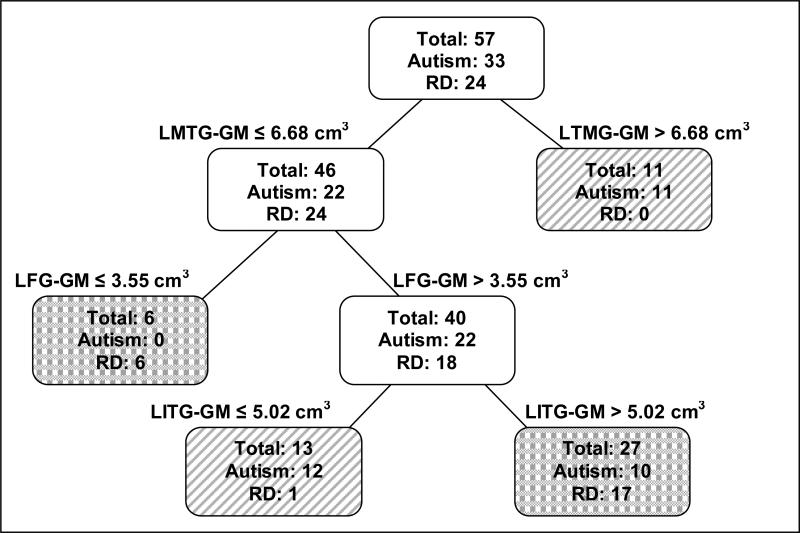
CART analysis included three temporal lobe structures in the analysis between the autism and RD groups (see Figure 3). These structures were the left middle temporal gyrus gray matter (LMTG-GM), the LFG-GM and left ITG gray matter (LITG-GM).
Figure 3. Classification tree of autism vs. RD control groups.
Classification tree for the Autism vs. reading disorder (RD) comparison including left middle temporal gyrus gray matter (LMTG-GM), left fusiform gyrus gray matter (LFG-GM), and left inferior temporal gyrus gray matter (LITG-GM). White boxes indicate locations on the tree that are still subject to splitting. Lined and dotted boxes are locations on the tree that have completed the splitting. Lined boxes have a higher proportion of autistic individuals and dotted boxes have a higher proportion of RD individuals.
There were no RD subjects and 11 autistic subjects with LMTG-GM volumes greater than 6.68 cm3. The remaining 22 autistic subjects all had LFG-GM volumes greater than 3.55 cm3; further, of those 22, thirteen had LITG-GM less than 5.02 cm3 and 12 had ITG-GM greater than this value. Thus the first two splits of the tree formed three groups: Subjects with LMTG-GM greater than 6.68 cm3 (autism = 11, RD = 0), subjects with LMTG-GM < 6.68 cm3 and LFG-GM < 3.55 cm3 (autism = 0, RD = 6) and subjects with LMTG-GM < 6.68 cm3, LFG-GM > 3.55 cm3, LITG-GM < 5.02 cm3 (autism = 12, RD = 1). A further split of the last group on LITG-GM shows that 17 of the 18 RD have volumes > 5.02 cm3 while only half of the autism group has volumes above the same value.
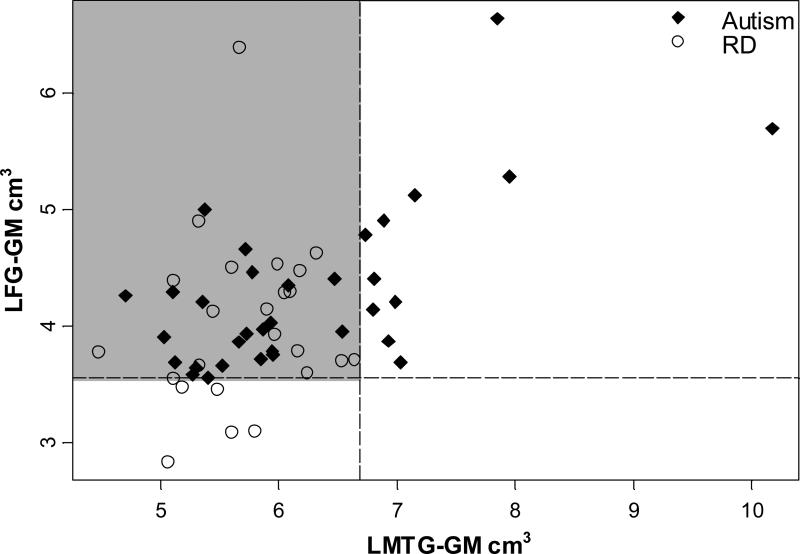
Figure 4 shows the separation of data points based on the first two splits of the tree. The LMTG-GM is on the x-axis and individuals with volumes greater than the cutoff (6.68 cm3) are to the right of the dotted vertical line. The LFG-GM is on the y-axis and the cutoff is shown with the dotted horizontal line. The gray highlighted quadrant in the upper left contains those individuals not classified with the first two splits. While separation of points exits, it is not as distinguishable as the autism vs. normal control comparison (contrast with Figure 2). This fact is especially exhibited by the mixture of points in the upper left quadrant. These points are left unclassified after the first two splits.
Figure 4. Two dimensional classification tree of autism vs. RD groups.
The two-dimensional classification tree shows the separation of data points based on the first two splits of the tree. The left middle temporal gyrus gray matter (LMTG-GM) is on the x-axis and individuals with volumes greater than the cutoff (6.68 cm3) are to the right of the dotted vertical line. The left fusiform gyrus gray matter (LFG-GM) is on the y-axis and the cutoff is shown with the dotted horizontal line. The upper left quadrant which is highlighted in gray are those individuals not classified with the first two splits. While separation of points exits, it is not as distinguishable as is the autism vs. normal control comparison. This fact is especially exhibited by the mixture of points in the upper left quadrant. These points are left unclassified after the first two splits. It seems as if at least a third variable split is necessary.
The logistic regression analysis with the LMTG-GM, LFG-GM, LITG-GM and their two-way interactions resulted in cross-validated misclassification rates close to or over 50%. When the right hippocampus was added to the model, the misclassification rates dropped to 33% for autism and 42% for RD. The high misclassification rates could be indicative of the fact the autism and RD group are more similar in the temporal lobe than the autism and control groups.
Discriminant analysis selected eight variables: R hippocampus, R parahippocampal GM, RFG-GM, LITG-WM, RMTG-GM, RSTG-GM, RSTG-WM, and RTS. Misclassification rates with these variables were 27% for the autism group and 25% for the control group. These variables suggest the existence of differences between the autism and RD.
The discriminant analysis, which produced a classification rule with more variables than the CART rule, resulted in smaller error rates that the CART/logistic regression. The more complicated model better explained group differences than the simpler model. This is the converse of the autism vs. normal control comparison.
Left and right averaged
With the left and right measures averaged together, the CART analysis again selected the FG-GM and the TS as the most significant areas of difference between the autism and normal control groups. The autism group tended to have larger gray matter and smaller temporal stem matter. However, the cross-validated error rates were higher than when the left and right were used separately with 3 of 33 autism subjects misclassified (9%) and 7 of 24 normal controls misclassified (29%). The amygdala was also indicated as a region of potential anomaly.
The autism vs. RD analysis also indicated the FG-GM, the ITG-GM and TS as regions of difference when the left and right measures were averaged together. Although separation was not as apparent, with error rates of 42% in the RD group and 3% in the autism group, the fact that the same temporal lobe structures were again seen in tree construction suggest regions of difference related to autism.
Discussion
Simple volumetric size differences did not reliably differentiate the groups. When compared to just the normal control group, the autism group had larger LFG-GM; however, once Bonferroni corrections were applied for the multiple comparisons (i.e., three groups times 26 brain measures) this difference only approached significance. Accordingly, when simply comparing size of a given temporal lobe structure between autism and control subjects, after controlling for age, head size, and PIQ, no significant differences were observed. Clearly, there is not a simple, straightforward temporal lobe structural anomaly that defines autism. The absence of such a finding emphasizes the importance of performing something like the CART analysis to examine relationships of a given temporal lobe structure to other temporal lobe regions in an attempt to identify a pattern that may distinguish autism.
Based upon the CART analysis, the combinations of differences between temporal lobe structures in the autism and typical developing controls distinguished the groups. As shown in Figure 1 larger LFG and RTS constituted the first two branches in the classification that differentiated the majority of controls from those with autism. The two-dimensional classification using just the first two branches of the CART shows this isolation of a large number of the controls who do not overlap with autism subjects. This suggests that size relationships between the temporal stem and fusiform may be very important in understanding the neurobiology of autism.
In contrast, the CART analysis yielded a less robust algorithm when it came to differentiating the RD group from those with autism. Since RD subjects would be expected to have a greater likelihood of temporal lobe anomalies [16, 17], the observation that the relationships between temporal lobe structures in RD are more similar to those with autism is not surprising.
Much has been written about the potential role of the FG in autism [44, 45]. The FG and ITG regions of the inferior temporal lobe are associated with face processing and face recognition abilities in individuals with autism are often impaired. Furthermore, the FG is important in social cognition and emotional regulation [44, 46]. That these temporal lobe structures were identified in the CART analysis suggests structural differences in the FG and ITG may be clinically meaningful for the behavioral phenotype of autism. In the comparison between autism and normal controls, the cross-validated misclassification rates were low, 15% in the autism group and 17% in the control group, verifying that that the LFG-GM, the TS-WM and RITG-GM volume distinguished autism subjects from controls fairly well in this sample.
However, the stepwise discriminant analysis certainly implicates a much broader network of potential structural differences that distinguish control subjects from those with autism. Three comments are in order concerning these structures. First, most are implicated in the broad aspect of both verbal and non-verbal communication as well as social cognition, clearly behavioral domains that are impaired in autism [44]. Secondly, much of the current thinking concerning the neuropathology of autism centers on problems of connectivity and integration between structures [47]. Anything that would interfere with how these structures develop, how they respond to programmed cell death and pruning during critical developmental stages and how they interconnect, clearly implicates an integration problem across temporal lobe structures [22]. Thirdly, the fact that the TS-WM is implicated is very important because this region of the temporal lobe houses the majority of afferent/efferent connections to the temporal lobe [9]. It is a relatively small structure given the overall size of the temporal lobe, but subtle, strategic pathology at this level could have widespread disruption with regards to functional associations of the temporal lobe [10].
In the autism vs. RD comparison, CART indicated the LMTG-GM, the LFG-GM and the LITG-GM as regions with group differences. The autism group in the current study tended to have larger LMTG-GM volume, larger LFG-GM volume and smaller LITG-GM volume; however these variables also seemed to work in an interactive way. Referring back to Figure 3, few RD subjects with LFG-GM volume >3.5 cm3 have LITG-GM < 5.02 cm3 while most all RD subjects with LFG-GM below the cutoff of 5.02 cm3 do have LITG-GM less than the cutoff value. Most all the autism subjects have LITG-GM <5.02 cm3, unless the MTG is > 6.68 cm3. The clinical implications of the relationship are not as clear as the autism vs. normal control comparison, as described above; however it is evident that there exist some temporal lobe differences between the autism and RD groups as well.
For autism versus normal control, the discriminant analysis cross-validated error rates, in which the classification rule included more variables, were much higher than the analysis using fewer variables. Conversely, when comparing autism and RD, the discriminant analysis cross-validated error rates were better than the classification rule which used few variables. This is further evidence that the autism and RD groups are more similar than the autism and normal controls. The more variables added to the classification in the former comparison adds noise to the analysis, while more variables added in the later comparison helps to better distinguish the groups. Thus, within the subjects examined in this study, it appears that autism does have its own set of temporal lobe anomalies as well as shared anomalies that may be common to temporal lobe based disorders, such as a reading disability.
There are several limitations of the current study. First, data are based on cross-sectional analyses in individuals seven years of age and older. Much of the developmental neuropathology of autism has been assumed to occur much earlier in life [48, 49] and therefore the differences observed at this age may not be the same in the more immature brain, which may be more critical in the initial expression of the disorder [22]. Second, we are inferring functional differences between the groups based on structural data, but have no direct measure of the identified regions that differentiate autism from controls as to their functional relationship. For example, a next step to truly assess whether some of the structural relationships identified in this study are functionally important, would be to design functional neuroimaging studies that could test involvement of these regions. Some studies have already demonstrated the role of several of the structures identified in this study [50], although current functional neuroimaging methods have limitations in inferring the simultaneity and integrative nature of behavioral function [51]. Accordingly, a third limitation of the current study has to do with not directly testing the ‘connectivity’ hypothesis of autism. For example, MRI-based diffusion tensor imaging (DTI) and functional neuroimaging methods that directly test connectivity will be important next steps in unraveling aberrant pathways specific to autism [52].
In summary, applying the CART method in the current study demonstrated that age and IQ matched autism subjects could be differentiated from controls via temporal lobe relationships between individual structures. Simple size differences did not distinguish the groups. These findings are supportive of current theories of autism that imply errors of connectivity as being central to the disorder.
Acknowledgments
This study was supported in part by the National Institutes of Child Health and Human Development 5 U19 HD035476-07 and the NICHD Collaborative Programs of Excellence in Autism (CPEA) and the Ira Fulton Foundation. The technical assistance of Tracy Abildskov is gratefully acknowledged, as is the editorial assistance of Jo Ann Petrie.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
E. Shannon NEELEY, Department of Statistics Brigham Young University.
Erin D. BIGLER, Departments of Psychology and Neuroscience Brigham Young University
Lori KRASNY, Department of Psychiatry The University of Utah.
Sally OZONOFF, The MIND Institute University of California at Davis.
William McMAHON, Department of Psychiatry The University of Utah.
Janet E. LAINHART, Department of Psychiatry The University of Utah
References
- 1.Gendry Meresse I, Zilbovicius M, Boddaert N, Robel L, Philippe A, Sfaello I, et al. Autism severity and temporal lobe functional abnormalities. Ann Neurol. 2005;58:466–469. doi: 10.1002/ana.20597. [DOI] [PubMed] [Google Scholar]
- 2.Salmond CH, Ashburner J, Connelly A, Friston KJ, Gadian DG, Vargha-Khadem F. The role of the medial temporal lobe in autistic spectrum disorders. Eur J Neurosci. 2005;22:764–772. doi: 10.1111/j.1460-9568.2005.04217.x. [DOI] [PubMed] [Google Scholar]
- 3.Schumann CM, Hamstra J, Goodlin-Jones BL, Lotspeich LJ, Kwon H, Buonocore MH, et al. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. J Neurosci. 2004;24:6392–6401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauman ML, Kemper TL, editors. The Neurobiology of Autism. Johns Hopkins University Press; Baltimore: 2004. [Google Scholar]
- 5.Schultz RT. Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. Int J Dev Neurosci. 2005;23:125–141. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Baron-Cohen S, Belmonte MK. Autism: a window onto the development of the social and the analytic brain. Annu Rev Neurosci. 2005;28:109–126. doi: 10.1146/annurev.neuro.27.070203.144137. [DOI] [PubMed] [Google Scholar]
- 7.Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127:1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- 8.Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. J Neurosci. 2004;24:9228–9231. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peuskens D, van Loon J, Van Calenbergh F, van den Bergh R, Goffin J, Plets C. Anatomy of the Anterior Temporal Lobe and the Frontotemporal Region Demonstrated by Fiber Dissection. Neurosurgery. 2004;55:1174–1184. doi: 10.1227/01.neu.0000140843.62311.24. [DOI] [PubMed] [Google Scholar]
- 10.Kier EL, Staib LH, Davis LM, Bronen RA. MR imaging of the temporal stem: anatomic dissection tractography of the uncinate fasciculus, inferior occipitofrontal fasciculus, and Meyer's loop of the optic radiation. AJNR Am J Neuroradiol. 2004;25:677–691. [PMC free article] [PubMed] [Google Scholar]
- 11.Brown BW, Russell K. Methods of correcting for multiple testing: operating characteristics. Stat Med. 1997;16:2511–2528. doi: 10.1002/(sici)1097-0258(19971130)16:22<2511::aid-sim693>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 12.Breiman L, Friedman JH, Olshen RA, Stone CJ. Classification and Regression Trees. Wadsworth International Group; Belmont, CA: 1984. [Google Scholar]
- 13.Zhang H, Crowley J, Sox HC, Olshen RA. Tree structured statistical methods. In: Armitage P, Colton T, editors. The Encyclopedia of Biostatistics. John Wiley & Sons; Chichester: 1998. pp. 4561–4573. [Google Scholar]
- 14.Godefroy O, Duhamel A, Leclerc X, Saint Michel T, Henon H, Leys D. Brain-behaviour relationships. Some models and related statistical procedures for the study of brain-damaged patients. Brain. 1998;121:1545–1556. doi: 10.1093/brain/121.8.1545. [DOI] [PubMed] [Google Scholar]
- 15.Li X, Jin H, Lu Y, Oh J, Chang S, Nelson SJ. Identification of MRI and 1H MRSI parameters that may predict survival for patients with malignant gliomas. NMR Biomed. 2004;17:10–20. doi: 10.1002/nbm.858. [DOI] [PubMed] [Google Scholar]
- 16.Brambati SM, Termine C, Ruffino M, Stella G, Fazio F, Cappa SF, et al. Regional reductions of gray matter volume in familial dyslexia. Neurology. 2004;63:742–745. doi: 10.1212/01.wnl.0000134673.95020.ee. [DOI] [PubMed] [Google Scholar]
- 17.Eckert MA, Leonard CM, Richards TL, Aylward EH, Thomson J, Berninger VW. Anatomical correlates of dyslexia: frontal and cerebellar findings. Brain. 2003;126:482–494. doi: 10.1093/brain/awg026. [DOI] [PubMed] [Google Scholar]
- 18.Tager-Flusberg H, Joseph RM. Identifying neurocognitive phenotypes in autism. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 2003;358(1430):303–314. doi: 10.1098/rstb.2002.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casanova MF, Araque J, Giedd J, Rumsey JM. Reduced brain size and gyrification in the brains of dyslexic patients. J Child Neurol. 2004;19:275–281. doi: 10.1177/088307380401900407. [DOI] [PubMed] [Google Scholar]
- 20.Peterson BS, Vohr B, Staib LH, Cannistraci CJ, Dolberg A, Schneider KC, et al. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA. 2000;284:1939–1947. doi: 10.1001/jama.284.15.1939. [DOI] [PubMed] [Google Scholar]
- 21.Bigler ED, Provencal S, Mortensen S, Fearing MA, McMahon W, Lainhart JE. Relationship between superior temporal gyrus and IQ in autism. Dev Neuropsychol. doi: 10.1080/87565640701190841. in press. [DOI] [PubMed] [Google Scholar]
- 22.Lainhart JE, Lazar M, Alexander AL, Bigler ED. The brain during life in autism: Advances in neuroimaging research. In: Casanova MF, editor. Recent developments in Autism research. Nova Biomedical Books; Hauppauge, NY: 2005. p. 215. [Google Scholar]
- 23.Courchesne E, Carper R, Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. JAMA. 2003;290:337–344. doi: 10.1001/jama.290.3.337. [DOI] [PubMed] [Google Scholar]
- 24.Lainhart JE. Increased rate of head growth during infancy in autism. JAMA. 2003;290:393–394. doi: 10.1001/jama.290.3.393. [DOI] [PubMed] [Google Scholar]
- 25.Aylward EH, Minshew NJ, Field K, Sparks BF, Singh N. Effects of age on brain volume and head circumference in autism. Neurology. 2002;59:175–183. doi: 10.1212/wnl.59.2.175. [DOI] [PubMed] [Google Scholar]
- 26.Farkas LG, Hreczko TA, Katie MJ. Craniofacial norms in North American Caucasians from birth to young adulthood. In: Farkas LG, editor. Anthropometry of the Head and Face. Raven Press; New York: 1994. [Google Scholar]
- 27.Wechsler D. Wechsler Intelligence Scale for Children-Third Edition (WISC-III) The Psychological Corporation; San Antonio: 1991. [Google Scholar]
- 28.Wechsler D. Wechsler Adult Intelligence Scale- Third Edition (WAIS-III) The Psychological Corporation; San Antonio: 1997. [Google Scholar]
- 29.Lord M, Rutter A, LeCouteur A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 30.Lord C, Rutter M, Goode S, Heemsbergen J, Jordan H, Mawhood L, et al. Autism diagnostic observation schedule: A standardized observation of communicative and social behavior. J Autism Dev Disord. 1989;19:185–212. doi: 10.1007/BF02211841. [DOI] [PubMed] [Google Scholar]
- 31.Woodcock RW, Johnson MB. Woodcock-Johnson Psycho-Educational Battery-Revised. Riverside Publishing; Itasca, IL: 1990. [Google Scholar]
- 32.Jastak S, Wilkinson G. The Wide Range Achievement Test-Revised. Jastak Associates; Wilmington, Del.: 1984. [Google Scholar]
- 33.Wiederholt JL, Bryant BR. Gray Oral Reading Test - 4th Edition (GORT-4) PRO ED; Austin: 1992. [Google Scholar]
- 34.Bolton P, Macdonald H, Pickles A, Rios P, Goode S, Crowson M, et al. A case-control family history study of autism. J Child Psychol Psychiatry. 1994;35:877–900. doi: 10.1111/j.1469-7610.1994.tb02300.x. [DOI] [PubMed] [Google Scholar]
- 35.Folstein SE, Santangelo SL, Gilman SE, Piven J, Landa R, Lainhart JE, et al. Predictors of cognitive test patterns in autism families. J Child Psychol Psychiatry. 1999;40:1117–1128. [PubMed] [Google Scholar]
- 36.Rapin I. Autism in search of a home in the brain. Neurology. 1999;52:902–904. doi: 10.1212/wnl.52.5.902. [DOI] [PubMed] [Google Scholar]
- 37.Deutsch CK, Joseph RM. Brief report: cognitive correlates of enlarged head circumference in children with autism. J Autism Dev Disord. 2003;33:209–215. doi: 10.1023/a:1022903913547. [DOI] [PubMed] [Google Scholar]
- 38.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 39.Bigler ED, Tate DF, Neeley ES, Wolfson LJ, Miller MJ, Rice SA, et al. Temporal lobe, autism, and macrocephaly. AJNR Am J Neuroradiol. 2003;24:2066–2076. [PMC free article] [PubMed] [Google Scholar]
- 40.Robb RA. ANALYZE: The biomedical imaging resource at Mayo Clinic. IEEE Trans Med Imaging. 2001;20:854–867. doi: 10.1109/42.952724. [DOI] [PubMed] [Google Scholar]
- 41.McCullagh P, Nelder JA. Generalized Linear Models. Chapman and Hall; London: 1989. [Google Scholar]
- 42.Fisher RA. The use of multiple measurements in taxonomic problems. Ann Eugenics. 1936;7:179–188. [Google Scholar]
- 43.DeCarli C, Murphy DG, McIntosh AR, Teichberg D, Schapiro MB, Horwitz B. Discriminant analysis of MRI measures as a method to determine the presence of dementia of the Alzheimer type. Psychiatry Res. 1995;57:119–130. doi: 10.1016/0165-1781(95)02651-c. [DOI] [PubMed] [Google Scholar]
- 44.Pelphrey K, Adolphs R, Morris JP. Neuroanatomical substrates of social cognition dysfunction in autism. Ment Retard Dev Disabil Res Rev. 2004;10:259–271. doi: 10.1002/mrdd.20040. [DOI] [PubMed] [Google Scholar]
- 45.Schultz RT, Gauthier I, Klin A, Fulbright RK, Anderson AW, Volkmar F, et al. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Arch Gen Psychiatry. 2000;57:331–340. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- 46.Adolphs R. Cognitive neuroscience of human social behaviour. Nature Reviews Neuroscience. 2003;4:165–178. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- 47.Casanova MF, Buxhoeveden D, Gomez J. Disruption in the inhibitory architecture of the cell minicolumn: implications for autism. Neuroscientist. 2003;9:496–507. doi: 10.1177/1073858403253552. [DOI] [PubMed] [Google Scholar]
- 48.Courchesne E, Pierce K. Brain overgrowth in autism during a critical time in development: implications for frontal pyramidal neuron and interneuron development and connectivity. Int J Dev Neurosci. 2005;23:153–170. doi: 10.1016/j.ijdevneu.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 49.Herbert MR. Large brains in autism: the challenge of pervasive abnormality. Neuroscientist. 2005;11:417–440. doi: 10.1177/0091270005278866. [DOI] [PubMed] [Google Scholar]
- 50.Herbert MR, Ziegler DA, Makris N, Filipek PA, Kemper TL, Normandin JJ, et al. Localization of white matter volume increase in autism and developmental language disorder. Ann Neurol. 2004;55:530–540. doi: 10.1002/ana.20032. [DOI] [PubMed] [Google Scholar]
- 51.Devlin JT, Russell RP, Davis MH, Price CJ, Moss HE, Fadili MJ, et al. Is there an anatomical basis for category-specificity? Semantic memory studies in PET and fMRI. Neuropsychologia. 2002;40:54–75. doi: 10.1016/s0028-3932(01)00066-5. [DOI] [PubMed] [Google Scholar]
- 52.Alexander AL, Lee JE, Lazar M, Boudos RM, Dubray MB, Oakes TR, et al. Diffusion tensor imaging of the corpus callosum in Autism. Neuroimage. doi: 10.1016/j.neuroimage.2006.08.032. in press. [DOI] [PubMed] [Google Scholar]