Abstract
Transplantable murine melanomas are well-established models for the study of experimental cancer therapies. The aim of this study was to explore the behaviour of four different B16 murine melanoma cell sublines after inoculation into C57BL/6J mice; and, more specifically to analyse skin changes, with respect to two specific parameters: clinical (tumour volume, melanin amount, erythema) and histological (H & E, S100, VEGF expression). Both non-invasive and invasive analysis showed that B164A5 is the most aggressive melanoma cell line for C57BL/6J's skin, followed by B16F10 and then by diminished aggressive growth pattern by the B16GMCSF and B16FLT3 cell lines.
Keywords: B164A5, B16F10, B16FLT3, B16GMCSF, C57BL/6J
Skin cancers include basal cell carcinoma, squamous cell carcinoma and malignant melanoma. The first two types of skin cancer are the most frequent malignant neoplasms among fair-skinned population (Andrade et al. 2012). Recent studies showed that the incidence of melanoma was also increasing especially in young adult women (Reed et al. 2012). Ultraviolet (UV) radiation can promote the limited proliferative capacity of melanocytes (Gupta et al. 2013). Although the incidence of melanoma among other types of skin cancer is a parameter with increased variability, the severity of this disease is undisputed. Due to its highly metastatic potential and resistance to chemotherapy, it is responsible for most deaths (Rigel 2005; Svobodova et al. 2006). Over the past years, Food and Drug Administration approved three agents for the treatment of melanoma, namely pegylated interferon alpha-2b, vemurafenib and ipilimumab (Lee et al. 2012). More research is still required to find a highly effective drug, with low side effects, against this challenging type of cancer. For this purpose, preclinical studies in animal models provide valuable clues for clinical trials.
Transplantable murine melanomas are well-established models for the study of experimental cancer therapies. Many immunotherapeutic protocols have been tested using the murine B16 melanoma cell line (and its sublines) that originates in the syngeneic C57BL/6 (H-2b) mouse strain. Although B164A5 is one of the most widely used cell line for the murine melanoma model, as evidenced by the latest papers in the field (Danciu et al. 2013a,b; Lee et al. 2013; Villareal et al. 2013; Ookubo et al. 2014), several subline derivates have been obtained to study different therapeutic strategies. The sublines B16F1 and B16F10 were derived from the mother B16 line by selection for their ability to form lung colonies in vivo after intravenous injection and subsequently established in vitro after one (B16F1) or 10 (B16F10) cycles of lung colony formation. B16F10 is a subline that possesses high lung metastatic ability, whereas B16F1 is a subline with low metastatic potential (Fidler 1973; Teicher 2010). B16GMCSF is a subline derived from B16F10 by transduction, employing an MFG retroviral vector-encoding murine Granulocyte - Macrophage Colony Stimulating Factor (GM-CSF) (Kumar et al. 1999). It has been shown that GM-CSF surface-modified B16F10 melanoma cell vaccine may induce protection against the wild-type tumour challenge (Gao et al. 2006; Danciu et al. 2013a,b). The fourth cell subline analysed in this study is B16FLT3. It was also obtained from the murine B16 cell line, which was transfected with the gene for the Fms-like tyrosine kinase 3 (Flt3)-L cytokine (Vargas et al. 2006; Danciu et al. 2013a,b). Although both GM-CSF and Flt3 ligand induce marked expansion of dendritic cells, it has been shown that GM-CSF-secreting tumour cells promoted higher levels of protective immunity than vaccination with FLT3-L-secreting tumour cells (Zarei et al. 2009).
The aim of this study was to analyse the behaviour of this four different B16 murine melanoma cell sublines in the C57BL/6J host, and more specifically to explore and analyse their skin targeted properties, with respect to two parameters: clinical (tumour volume, melanin amount, erythema) and histological features [Haematoxylin and Eosin (H&E), S100, Vascular Endothelial Growth Factor (VEGF) expression].
Materials and methods
Cells
Mouse adherent melanoma cell line B164A5 was purchased from ECACC (European Collection of Cell Cultures, Salisbury, UK). B16F10, B16GMCSF and B16FLT3 cells were provided by Prof. Radeke, Pharmazentrum Frankfurt/Center for Drug Research, Development, and Safety, Clinic of JW Goethe University, Frankfurt, Germany. Cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated foetal calf serum (FCS), 1% non-essential amino acids and 1% penicillin–streptomycin in a humidified atmosphere containing 5% CO2 at 37°C. All cell culture media and supplements were obtained from Life Technologies (Paisley, UK). To prepare the cells for inoculation, cells were trypsinized, counted using trypan blue, washed with PBS, resuspended at a concentration of 106 cell / 0.1 ml in saline solution and injected immediately as described below.
Animal studies
Animal studies were conducted on 7- to 8-week-old C57BL/6J female mice with an average weight of 20–25 g. Mice were purchased from Charles River (Sulzfeld, Germany). The work protocol followed all National Institute of Animal Health (NIAH) rules: animals were maintained during the experiment in standard conditions: 12 h light–dark cycle, food and water ad libitum, temperature 24°C and humidity above 55%. The experiment was conducted according to the rules of the Ethical Committee of the ‘Victor Babeş’ University of Medicine and Pharmacy of Timişoara, Romania. The number of mice included in the study was thirty, and the mice were divided equally in five groups as follows: group A – blank group, group B – mice inoculated with B164A5 cells, group C – mice inoculated with B16F10 cells, group D – mice inoculated with B16GMCSF cells and group E – mice inoculated with B16FLT3 cells. In day 0 of the experiment, mice in groups B, C, D and E were inoculated subcutaneously (s.c.) into the hair-depilated lateral abdomen with 0.1 ml of 106 cells/mouse. Group A, the blank group was injected with saline solution, the vehicle used for the injection of cells. Mice were inspected daily for the development of tumours or other changes. Tumour growth was measured in millimetres, daily, using callipers, and tumour volume was estimated by the formula: length × width2/2 (Giavazzi et al. 1986). On day 21 post-inoculation, after the last measurements had been taken, mice were sacrificed by cervical dislocation. Tumours were collected, measured, weighed, and afterwards histological analysis was performed.
Non-invasive skin measurements
All the measurements on mice skin were carried out with a Multiprobe Adapter System (MPA5) from Courage-Khazaka, Köln, Germany. For measurement of melanin and erythema Mexameter® MX 18 was used to obtain quantitative information regarding melanin and erythema (haemoglobin) amounts and to monitor modifications of these features during tumour evolution The device emits light over 3 wavelengths, namely 568, 660 and 880 nm, and measures remitted light over a 5 mm diameter. The erythema and melanin indices are determined as follows:
Mx = melanin index, Ex = erythema index and infrared/red/green = infrared/red/green remittance.
These indices are relative values, and the maximum ratio between each colour is 1:5. The range of values is 0–1000, a higher value representing more melanin or erythema, and a value of 500 representing a remittance ratio of 1:1. The measurement was carried out for four parts of the skin located near the tumour, and mean and standard deviations were calculated (Hoshino et al. 2010). Melanin and erythema values were measured at baseline (day 0) and every two days until day 17 of the experiment. The measurement area was 5 mm in diameter. Afterwards, the mice were sacrificed and the skin and main visceral organs were collected. Histological and immunohistochemical analyses were performed.
Histological and immunohistochemical analyses
For the histological analysis, skin samples were fixed in 10% formalin solution and were embedded in paraffin and cut at 4 μm. Finally, after dewaxing, the samples were stained with the conventional H & E method and examined.
Additional slides containing 5-μm thick sections were performed from each case and were stained with anti-VEGF antibody (monoclonal mouse anti-human antibody, Clone VG1, code no. M7273; Dako Co, Denmark) and with S100 protein antibody (polyclonal anti-human antibody, Dako Co, Denmark code 1573, ready-to-use). The dewaxing and rehydration of the sections was followed by heat-induced epitope retrieval in citrate buffer pH 9 for 20 min, respectively, pH 7.2 for 30 min (with PT link module; DakoCytomation, Denmark). The immunohistochemical technique continued with blocking of the endogenous peroxidases, using hydrogen peroxide 3%. Incubation with the VEGF primary antibody (dilution 1:30) and S100 primary antibody (dilution 1:100) was for 30 min. After incubation with the primary antibody, the slides were exposed to labelled streptavidin–biotin system and then 3,3-diamino-benzidine dihydrochloride was applied as chromogen. Nuclei were stained with Lillie's modified haematoxylin. The entire immunohistochemical procedure was performed with Dako Autostainer Plus (DakoCytomation). Image acquisition and analysis were performed using Nikon Eclipse E 600 microscope and Lucia G software for microscopic image analysis (NIKON, Germany).
For both antibodies, the intensity of reaction was assessed as 1 – low staining, 2 – moderate staining and 3 – intense staining. The immunostaining was considered 0 – negative when <10% of tumour cells showed positivity. Examination was performed with the microscope Eclipse E80i Nikon, and images were acquired with Lucia G soft for microscopic image analysis.
Statistical analysis
One-way anova followed by Bonferroni post-test was used to determine the statistical difference between various experimental and control groups.*, ** and *** indicate P < 0.05, P < 0.01 and P < 0.001 compared to control group.
Results
Clinical results
Our results (Figure1) show a linear development of the tumour, directly proportional with the number of days postinoculation in all four groups of mice. The results started to be statistically significant in all groups compared to the blank group from day 12 postinoculation (P = 0.021) until the end of the experiment (P = 0.009). In group B and C, tumours arose on day 6 postinoculation while in groups D and E, tumours arose on day 8 postinoculation. As a general observation, tumours were larger throughout the experiment in groups B and C, compared to groups D and E. At the end of the experiment, the average tumour volume was as follows: 1033.67 ± 400 mm3 in group B, 895.33 ± 445 mm3 in group C, 638.647 ± 426 mm3 in groups D and 732 ± 465 mm3 in group E. One-way anova followed by Bonferroni post-test showed no significant difference between the volume of the tumour in the four groups (B, C, D and E) at the end of the experiment (P = 0.702).
Figure 1.
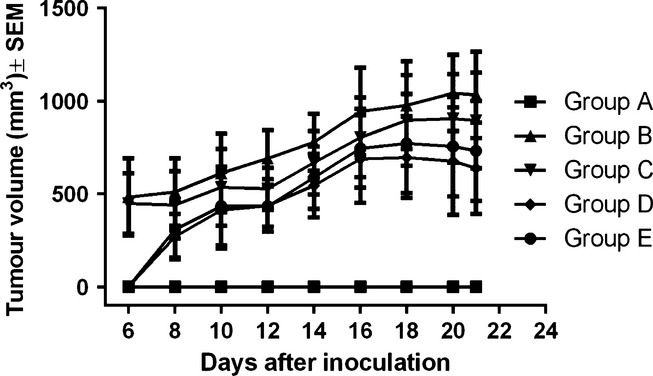
Tumour volume (mm3) evolution throughout the experiment.
Non-invasive measurements for melanin amount and degree of erythema were conducted every two days starting from day 0 until the end of the experiment. Normal values for melanin in the blank group (C57BL/6J host) varied between 650 and 665 arbitrary units (as explained in the Materials and Methods section). After inoculation the amount of melanin increases linearly in all four groups, as shown in Figure2. Until day 7 postinoculation, values were similar in all inoculated groups. Differences started to increase on day 9 postinoculation and continued until the end of the experiment. As a ‘general rule’ observed during days 9–21, mice in group B presented the highest values, corresponding to amounts of melanin. For groups C, D and E, the amount was quite similar during days 9–21. One-way anova followed by Bonferroni post-test showed no significant difference between the melanin amount in the four groups (B, C, D and E) at the end of the experiment (P = 0.570).
Figure 2.
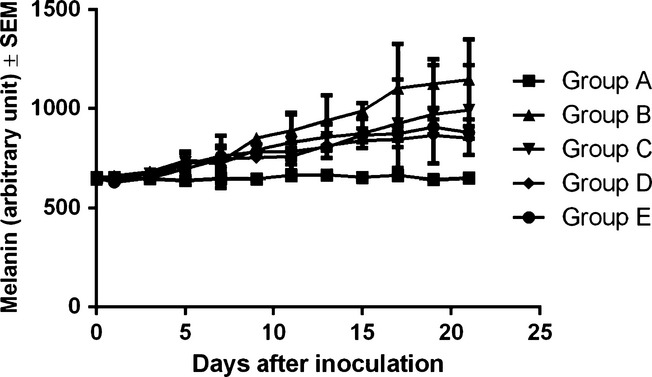
Melanin amount (arbitrary units) evolution in the different experimental groups among the 21 days of experiment.
The difference was not significant when compared to the blank group (P = 0.269). At the end of the experiment, the average amount of melanin was as follows: 650 ± 13 arbitrary units in group A, 1147 ± 287 arbitrary units in group B, 993 ± 322 arbitrary units in group C, 851 ± 128 arbitrary units in group D and 879 ± 108 arbitrary units in group E.
Together with the melanin amount determination, another non-invasive measurement that was conducted was the degree of erythema. Normal values for the blank group (C57BL/6J host) vary between 50 and 60 arbitrary units (as explained in the Materials and Methods section). After inoculation, the degree of erythema increased linearly in all four groups, as shown in Figure3. The values in all four inoculated groups (corresponding to a certain day of measurement) are quite similar. One-way anova followed by Bonferroni post-test showed no significant difference between the erythema degree in the four groups (B, C, D and E) at the end of the experiment (P = 0.704). The changes were significant when compared to the blank group (P = 0.008). At the end of the experiment, the average value corresponding to the degree of erythema was as follows: 59 ± 3 arbitrary units in group A, 191 ± 47 arbitrary units in group B, 193 ± 322 arbitrary units in group C, 163 ± 14 arbitrary units in group D and 185 ± 11 arbitrary units in group E.
Figure 3.
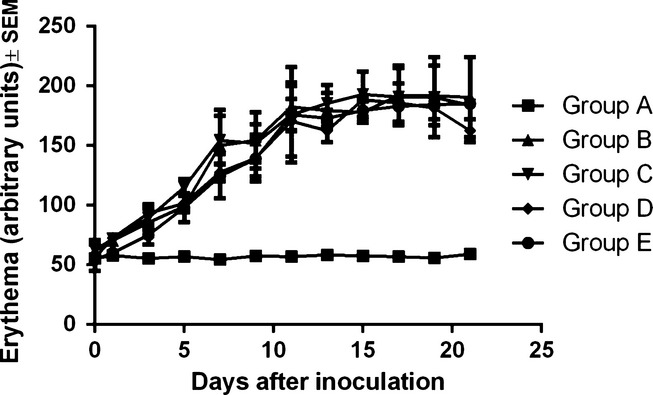
Erythema (arbitrary units) evolution in the different experimental groups among the 21 days of experiment.
Histological and immunohistochemical results
In group B, the conventional H&E analysis showed intense pigmentation in almost all tumour cells and with predominance at the periphery of the tumour (Figure4a). The tumour borders were infiltrative, and there were large areas of tumoral necrosis. There was no ulceration or lymph-vascular invasion. The mitotic index was 15 mitoses/1 mm2. In this group, S100 and VEGF were strongly positive in almost all tumour cells (Figure5a and 6a).
Figure 4.
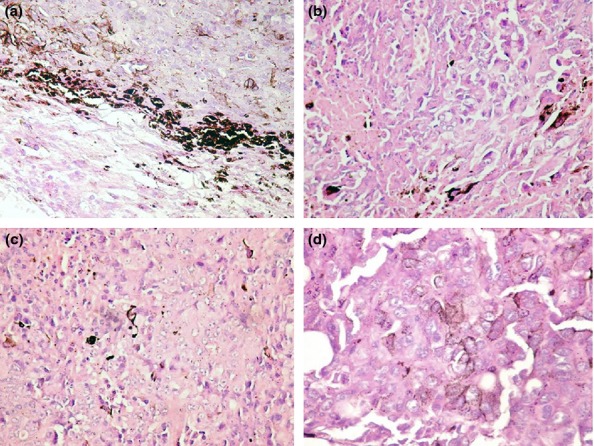
HE staining in the different groups with skin melanoma: (a) group B – intense pigmentation in almost all tumour cells with a predominance at the periphery of the tumour; (b) group C – moderate pigmentation with intense pigmentation in nests of tumour melanocytes; (c) group D – skin melanoma with local presence of melanin in isolated cells; (d) group E – presence of diffuse melanin with some loss of pigmentation.
Figure 5.
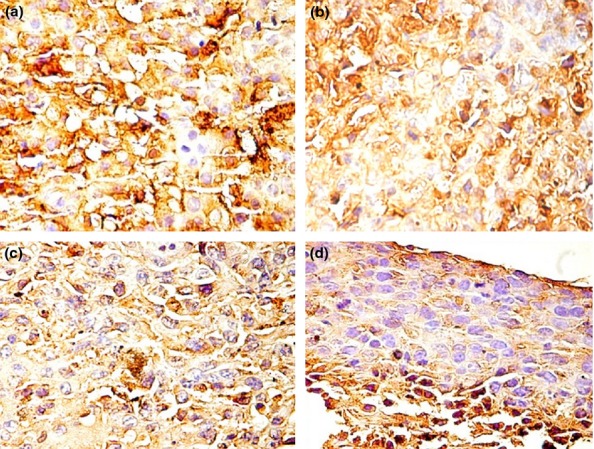
S100 staining in the different groups with skin melanoma: (a) group B – strongly positive, consistent, in almost all tumour cells; (b) group C – moderately and strongly positive, heterogeneous; (c) group D – moderate intensity with focal areas of intense positive; (d) group E – low positivity with areas of moderate expression of S100.
Figure 6.
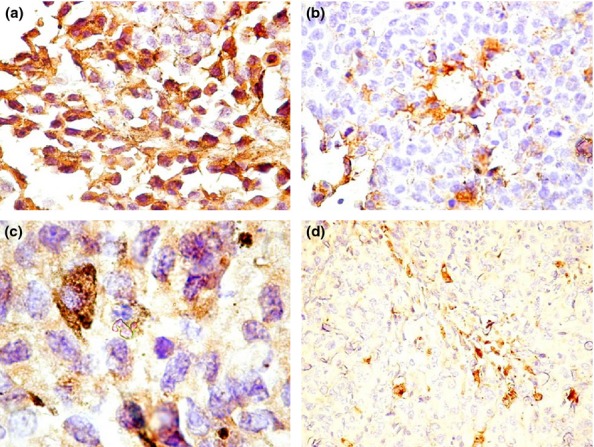
VEGF staining in the different groups with skin melanoma: (a) group B – intense VEGF expression in tumour cells in almost all cells; (b) group C – VEGF expression has a moderate intensity with a perivascular predominance; (c) group D – VEGF showing moderate intensity; the isolated pigmented cells with melanin have intense expression of VEGF and a granular appearance; (d) group E – moderate intensity of VEGF in isolated cells and nests.
The evaluation of H&E in group C revealed moderate pigmentation with intense pigmentation in nests of tumour melanocytes (Figure4b). There were large areas of tumoral necrosis with haemorrhage and infiltrative borders. There was no ulceration in this group and no lymph-vascular invasion. The mitotic index was 10 mitoses/1 mm2. S100 expression was heterogeneous with a moderately and strongly positivity in the tumour (Figure5b). The most important observation is the heterogeneity of VEGF expression in this group, with a low and medium intensity as predominant. The majority of the cells stained positively for VEGF and were perivascular in distribution (Figure6b). The cells with intense expression of VEGF were concentrated at the peripheri of the tumour.
In group D, melanin was present in isolated melanoma cells (Figure4c). There were few areas of necrosis and the tumour showed an infiltrative growth pattern. The ulceration was absent in this group, the lymph-vascular invasion was absent, and the mitotic index was 6 mitoses/1 mm2. The S100 assessment showed moderate positivity with focal areas of intense positive expression (Figure5c), while VEGF was variable from low to intense with a granular cytoplasmic pattern. The predominant positivity was moderate, and the isolate pigmented cells with melanin had intense expression of VEGF (Figure6c).
In group E, we detected the presence of melanin distributed diffusely and an overall loss of pigmentation (Figure4d). As in group D, there was no ulceration and no lymph–vascular invasion, the tumoral necrosis was isolated, the borders were infiltrative, and the mitotic index was 8 mitoses/1 mm2. In this group, S100 showed low positivity with areas of moderate expression (Figure5d). VEGF had a low to moderate intensity in melanoma cells. The cells were small with a spindle-like appearance (Figure6d).
Discussion
The present study shows that among the tested cell lines B164A5 is the most aggressive for C57BL/6J skin. Melanin is the most important pigment of skin, synthesized by melanocytes, as a normal defence to diverse stimuli (Pinon et al. 2011; Sass et al. 2013). The major cutaneous melanin role is protection against epidermal carcinogenesis and malignant melanomagenesis (Brozyna et al. 2008). Pinon et al. (2011). This is an index of the fact that melanocytes respond to UVB exposure and synthesise melanin and proliferate, instead of undergoing apoptosis. As melanoma is a malignant tumour of melanocytes, the role of these pigment-producing cells (Thomas et al. 2007), and moreover, the implication of melanin expression in melanoma, can give important clues about melanoma as a disease and its responses of melanomas to different therapeutic approaches. It is well known that melanin content is variable in different types of melanomas, melanomas being evaluated as ‘deeply’, ‘heavily’, ‘lightly’ pigmented or amelanotic, as Watts et al. (1981) reported in his work many years ago. Brozyna et al. (2008) demonstrated that amelanotic melanomas have a better response to radiotherapy than those with higher melanin content. Liu et al. (2006) showed that in human subjects, fast-growing melanoma (usually associated with a worse prognosis) have a higher percentage of amelanotic cells. Furthermore, recently the relationship between mitotic rate and the lack of pigment in melanoma has been described (Shen et al. 2014).
Our data show that in all B16 sublines, after inoculation, the level of melanin increased as compared to the control group. Among the tested groups, mice injected with B164A5 cells (group B) had the highest content of melanin. The values started to increase from day 9 after inoculation, as described in the Results section. The second high melanin level was registered in the group injected with B16F10 cells. Fedele et al.'s experiment with B16F10 melanoma cells implanted into C57BL/6J mouse strain, revealed that melanin was released in excess by the tumour cells, resulting in tumours with blackish colour extending into the subcutaneous tissue. Also, a large number of metastases in lung and liver parenchyma were noticed in their experiment (Fedele et al. 2013).
As reported previously, in most cases, melanin values can also be correlated with erythema values (Kaur & Saraf 2011; Danciu et al. 2013a,b; Hexse et al. 2013). Brenner et al. first described the erythematous rash that accompanies melanoma (Brenner & Wolf 1992; Mashiah et al. 2009). The erythematous eruption, termed ‘Brenner sign’, is correlated with angiogenesis (Russo et al. 2011). It is known that tumour growth in vivo is dependent on vascularization on the first day after inoculation. When tumours are not vascularized then tumour growth is slow and linear. After angiogenesis has occurred, the tumours begin to grow rapidly (Rose et al. 1999; Zhao et al. 2010; Şoica et al. 2012). The erythema and tumour growth measurements show that both parameters increased linearly after cell inoculation. Although the ‘behaviour’ of erythema values was similar for all four cell lines subtypes, as described in the Results section, tumour volume was higher in the group of B164A5, followed by B16F10. Ulceration is a very important prognostic factor in case of human melanoma (Balch et al. 2009). In this study, during the 21 days of observation, ulceration of the tumour was not detected in any of the experimental groups. Overwijk and Restifo (2001) reported that in the case of implanted B164A5 cells, tumours become necrotic in the centre and start to ulcerate or bleed when allowed to grow larger, for a period exceeding 21 days.
For all three non-invasive measurements, tumour volume, melanin index and erythema, inoculation with B16GMCSF and B16FLT3 cell lines leads to the lowest values. These results are consistent with other studies concerning vaccines expressing GM-CSF and Flt3 (Curran & Allison 2009). It is well known that GM-CSF is one of the most potent cytokine which are capable of inducing tumour-specific systemic immunity (Dranoff et al. 1993; Stagg et al. 2004; Curran & Allison 2009). Being associated with the growth and differentiation of hematopoietic progenitors, GM-CSF exerts its effects on antigen-presenting cells and recruitment of dendritic cells (Dranoff et al. 1993; Li et al. 2006). It also chemo-attracts macrophages, lymphocytes and granulocytes to the vaccine site (Curran & Allison 2009). Dranoff et al. reported that GM-CSF generates protection against a distant tumour, while other cytokines, such as IL-2, can induce locoregional tumour rejection (Dranoff et al. 1993; Stagg et al. 2004). The same protective role is played by the cytokine Flt3L When used prophylactically, it promotes tumour regression in experimental models (Curran & Allison 2009). Although it is known that B16-GMCSF can give better immune protection than B16-Flt3L, Curran and Allison (2009) reported that the Flt3L efficacy increases with higher doses, whereas GM-CSF activity decreases at higher expression levels.
Histological and immunohistochemical analyses support the above findings. As H&E staining shows, mice skin inoculated with B164A5 cell expressed the highest amount of pigment. Mouse skin inoculated with B16F10 and B16GMCSF cells showed moderate pigmentation with isolated areas of intense pigmentation. In mouse skin inoculated with the B16FLT3 cell line, the presence of diffuse melanin and loss of pigmentation is probably due to the dedifferentiation of malignant melanocytes.
S100 protein is widely accepted as a marker of choice in the immunohistochemical detection of malignant melanoma (Henze et al. 1997). S100 showed positivity in all groups with the most intense expression in mouse skin inoculated with the B164A5 cell line. In B16F10 and B16GMCSF inoculated groups, S100 was heterogeneous with moderate to intense positivity, while in B16FLT3 group, the expression was low to moderate. A strong expression of S100 protein is associated with tumour progression (Harpio & Einarsoon 2004). B164A5 is a cell line derived from the skin of a murine melanoma of a C57BL/6J mouse strain (Danciu et al. 2013a,b). S100 expression shows that this cell line is the most ‘well accepted’, in the C57BL/6J host, which is a pertinent behaviour, bearing in mind its origin.
VEGF is a signal protein produced by cells that stimulates the growth of new blood vessels, (Hoeben et al. 2004). An increased expression of VEGF in the tumour microenvironment is associated with progression of malignant melanomas (Brychtova et al. 2008). In this study, VEGF was positive in all groups. The most intense positivity was noticed in mouse skin inoculated with the B164A5A cell line while the other groups showed approximately equal moderate expression. In mouse skin inoculated with B16F10, perivascular predominance of VEGF expression was seen on many vessels.
Conclusions
Both clinical (tumour volume, melanin amount, erythema) and histological (H&E, S100, VEGF expression) approaches show that B164A5 is the most aggressive melanoma cell line for C57BL/6J's skin, followed by B16F10 and followed, in a similar, diminished manner of aggression, by the B16-GMCSF and B16FLT3 cell lines.
Acknowledgments
The work of the first author, Danciu Corina was supported by the UMFT grant Parteneriate in cercetarea fundamentala inovativa PIII-C2-PCFI-2015-2016.
Conflicts of interest
The authors declare no conflict of interest.
References
- Andrade P, Brites MM, Vieira R, et al. Epidemiology of basal cell carcinomas and squamous cell carcinomas in a Department of Dermatology: a 5 year review. An. Bras. Dermatol. 2012;87:212–219. doi: 10.1590/s0365-05962012000200004. [DOI] [PubMed] [Google Scholar]
- Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J. Clin. Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. Wolf R. The “red face” – a warning sign of malignant melanoma? Acta Derm. Venereol. 1992;72:464. [PubMed] [Google Scholar]
- Brozyna A, VanMiddlesworth L, Slominski AT. Inhibition of melanogenesis as a radiation sensitizer for melanoma therapy. Int. J. Cancer. 2008;123:448–456. doi: 10.1002/ijc.23664. [DOI] [PubMed] [Google Scholar]
- Brychtova S, Bezdekova M, Brychta T, Tichy M. The role of vascular endothelial growth factors and their receptors in malignant melanomas. Neoplasma. 2008;55:273–279. [PubMed] [Google Scholar]
- Curran MA. Allison JP. Tumor vaccines expressing Flt3 ligand synergize with CTLA-4 blockade to reject preimplanted tumors. Cancer Res. 2009;69:7747–7755. doi: 10.1158/0008-5472.CAN-08-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danciu C, Borcan F, Bojin F, Zupko I, Dehelean C. Effect of the isoflavone genistein on tumor size, metastasis potential and melanization in a B16 mouse model of murine melanoma. Nat. Prod. Commun. 2013a;8:343–346. [PubMed] [Google Scholar]
- Danciu C, Falamas A, Dehelean C, et al. A characterization of four B16 murine melanoma cell sublines molecular fingerprint and proliferation behavior. Cancer Cell Int. 2013b;13:75. doi: 10.1186/1475-2867-13-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dranoff G, Jaffee E, Lazenby A, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc. Natl Acad. Sci. USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedele TA, Galdos-Riveros AC, Jose de Farias e Melo H, Megalhaes A, Maria DA. Prognostic relationship of metabolic profile obtained of melanoma B16F10. Biomed. Pharmacother. 2013;67:146–156. doi: 10.1016/j.biopha.2012.10.013. [DOI] [PubMed] [Google Scholar]
- Fidler IJ. The relationship of embolic homogeneity, number, size and viability to the incidence of experimental metastasis. Eur. J. Cancer. 1973;9:223–227. doi: 10.1016/s0014-2964(73)80022-2. [DOI] [PubMed] [Google Scholar]
- Gao J, Huang S, Li M, Luo R, Wang X, Takashima A. GM-CSF-surface-modified B16. F10 melanoma cell vaccine. Vaccine. 2006;24:5265–5268. doi: 10.1016/j.vaccine.2006.04.031. [DOI] [PubMed] [Google Scholar]
- Giavazzi R, Campbell DE, Jessup JM, Cleary K, Fidler IJ. Metastatic behavior of tumor cells isolated from primary and metastatic human colorectal carcinomas implanted into different sites in nude mice. Cancer Res. 1986;46(4 Pt 2):1928–1933. [PubMed] [Google Scholar]
- Gupta A, Avci P, Dai T, Huang YY, Hamblin MR. Ultraviolet radiation in wound care: sterilization and stimulation. Adv. Wound Care (New Rochelle) 2013;2:422–437. doi: 10.1089/wound.2012.0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpio R. Einarsoon R. S100 proteins as cancer biomarkers with focus on S100B in malignant melanoma. Clin. Biochem. 2004;37:512–518. doi: 10.1016/j.clinbiochem.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Henze G, Dummer R, Joller-Jemelka HI, Böni R, Burg G. Serum S100-a marker for disease monitoring in metastatic melanoma. Dermatology. 1997;194:208–212. doi: 10.1159/000246103. [DOI] [PubMed] [Google Scholar]
- Hexse D, Caspary P, Dal Forno Dini T, Schilling-Souza J, Siega C. Variation of melanin levels in the skin areas exposed and not exposed to the sun following winter and summer. Surg. Cosmet. Dermatol. 2013;5:298–301. [Google Scholar]
- Hoeben A, Landuvt B, Highley MS, Wildiers H, Van Oosterom AT, De Brujin EA. Vascular endothelial growth factor and angiogenesis. Pharmacol. Rev. 2004;56:549–580. doi: 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- Hoshino T, Matsuda M, Yamashita Y, et al. Suppression of melanin production by expression of HSP70. J. Biol. Chem. 2010;285:13254–13263. doi: 10.1074/jbc.M110.103051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur CD. Saraf S. Skin care assessment on the basis of skin hydration, melanin, erythema and sebum at various body sites. Int. J. Pharm. Pharm. Sci. 2011;3:209–213. [Google Scholar]
- Kumar R, Yoneda J, Fidler IJ, Dong Z. GM-CSF-transduced B16 melanoma cells are highly susceptible to lysis by normal murine macrophages and poorly tumorigenic in immune-compromised mice. J. Leukoc. Biol. 1999;65:102–108. doi: 10.1002/jlb.65.1.102. [DOI] [PubMed] [Google Scholar]
- Lee B, Mukhi N, Liu D. Current management and novel agents for malignant melanoma. J. Hematol. Oncol. 2012;5:3. doi: 10.1186/1756-8722-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MH, Huang Z, Kim DJ, et al. Direct targeting of MEK1/2 and RSK2 by silybin induces cell-cycle arrest and inhibits melanoma cell growth. Cancer Prev. Res. 2013;6:455–465. doi: 10.1158/1940-6207.CAPR-12-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Lalani AS, Harding TC, et al. Vascular endothelial growth factor blockade reduces intratumoral regulatory T cells and enhances the efficacy of a GM-CSF-secreting cancer immunotherapy. Clin. Cancer Res. 2006;12:6808–6816. doi: 10.1158/1078-0432.CCR-06-1558. [DOI] [PubMed] [Google Scholar]
- Liu W, Dowling JP, Murray WK, McArthur WA, et al. Rate of growth in melanomas. Characteristics and associations of rapidly growing melanomas. Arch. Dermatol. 2006;142:1551–1558. doi: 10.1001/archderm.142.12.1551. [DOI] [PubMed] [Google Scholar]
- Mashiah J, Brenner S, Pessach Y, Barak V, Schachter J. Differences in cytokine levels in melanoma patients with and without redness (brenner sign) Anticancer Res. 2009;29:1793–1796. [PubMed] [Google Scholar]
- Ookubo N, Michiue H, Kitamatsu M, et al. The transdermal inhibition of melanogenesis by a cell-membrane-permeable peptide delivery system based on poly-arginine. Biomaterials. 2014;35:4508–4516. doi: 10.1016/j.biomaterials.2014.01.052. [DOI] [PubMed] [Google Scholar]
- Overwijk WW. Restifo NP. B16 as a mouse model for human melanoma. Curr. Protoc. Immunol. 2001 doi: 10.1002/0471142735.im2001s39. Chapter 20:Unit 20.1, doi: 10.1002/0471142735.im2001s39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinon A, Limami Y, Micallef L, et al. A novel form of melanoma apoptosis resistance: melanogenesis up-regulation in apoptotic B16-F0 cells delays ursolic acid-triggered cell death. Exp. Cell Res. 2011;317:1669–1676. doi: 10.1016/j.yexcr.2011.04.014. [DOI] [PubMed] [Google Scholar]
- Reed KB, Brewer JD, Lohse CM, Bringe KE, Pruitt CN, Gibson LE. Increasing incidence of melanoma among young adults: an epidemiological study in Olmsted County, Minnesota. Mayo Clin. Proc. 2012;87:328–334. doi: 10.1016/j.mayocp.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigel DS. Cancer of the Skin. China: Elsevier Saunders; 2005. [Google Scholar]
- Rose ML, Madren J, Bunzendahl H, Thurman RG. Dietary glycine inhibits the growth of B16 melanoma tumors in mice. Carcinogenesis. 1999;20:793–798. doi: 10.1093/carcin/20.5.793. [DOI] [PubMed] [Google Scholar]
- Russo J, Barr K, Scanlan L, Vincek V. Signet ring cell melanoma, Brenner sign, and elevated VEGF. J. Am. Acad. Dermatol. 2011;65:444–446. doi: 10.1016/j.jaad.2010.02.033. [DOI] [PubMed] [Google Scholar]
- Sass C, Bojin F, Dehelean C, Soica C, Păunescu V. Insights into melanoma. Histological aspects, progression and prognostic. Fiziologia. 2013;23:14–20. [Google Scholar]
- Shen S, Wolfe R, McLean CA, Haskett M, Kelly JW. Characteristics and associations of high-mitotic-rate melanoma. JAMA Dermatol. 2014;150((10)):1048–55. doi: 10.1001/jamadermatol.2014.635. ; doi: 10.1001/jamadermatol.2014.635. [DOI] [PubMed] [Google Scholar]
- Şoica C, Dehelean C, Danciu C, et al. Betulin complex in γ-cyclodextrin derivatives: properties and antineoplasic activities in in vitro and in vivo tumor models. Int. J. Mol. Sci. 2012;13:14992–15011. doi: 10.3390/ijms131114992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg J, Wu JH, Bouganim N, et al. Granulocyte-macrophage colony-stimulating factor and interleukin-2 fusion cDNA for cancer gene immunotherapy. Cancer Res. 2004;64:8795–8799. doi: 10.1158/0008-5472.CAN-04-1776. [DOI] [PubMed] [Google Scholar]
- Svobodova A, Walterova D, Vostalova J. Ultraviolet light induced alteration to the skin. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2006;150:25–38. doi: 10.5507/bp.2006.003. [DOI] [PubMed] [Google Scholar]
- Teicher BA. Tumor Models in Cancer Research. Boston, USA: Springer; 2010. p. 682. [Google Scholar]
- Thomas J, Liu T, Cotter MA, et al. Melanocyte expression of survivin promotes development and metastasis of UV-induced melanoma in HGF-transgenic mice. Cancer Res. 2007;67:5172–5178. doi: 10.1158/0008-5472.CAN-06-3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas P, Cortés C, Vargas L, Rosemblatt M, Bono MR. Immunization with antigen-pulsed dendritic cells significantly improves the immune response to weak self-antigens. Immunobiology. 2006;211:29–36. doi: 10.1016/j.imbio.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Villareal M, Han J, Matsuyama K, et al. Lupenone from erica multiflora leaf extract stimulates melanogenesis in B16 murine melanoma cells through the inhibition of ERK1/2 activation. Planta Med. 2013;79:236–243. doi: 10.1055/s-0032-1328189. [DOI] [PubMed] [Google Scholar]
- Watts KP, Fairchild RG, Slatkin DN, et al. Melanin content of hamster tissues, human tissues, and various melanomas. Cancer Res. 1981;41:467–472. [PubMed] [Google Scholar]
- Zarei S, Schwenter F, Luy P, et al. Role of GM-CSF signaling in cell-based tumor immunization. Blood. 2009;113:6658–6668. doi: 10.1182/blood-2008-06-161075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao QH, Zhang Y, Liu Y, et al. Anticancer effect of realgar nanoparticles on mouse melanoma skin cancer in vivo via transdermal drug delivery. Med. Oncol. 2010;27:203–212. doi: 10.1007/s12032-009-9192-1. [DOI] [PubMed] [Google Scholar]


