Abstract
Objective
To examine the temporal and dose-related effect of glucocorticoids (GCs) on body mass index (BMI) in children with rheumatic diseases.
Methods
Children initiating GCs for a rheumatic disease (n=130) were assessed every 3 months for 18 months. BMI, weight and height Z-score trajectories were described according to GC starting dosage in prednisone equivalents: high (≥1.0 mg/kg/day), low (<0.2 mg/kg/day to a maximum of 7.5 mg/d), and moderate (between high and low) dosage. The impact of GC dosing, underlying diagnosis, pubertal status, physical and disease activity on BMI Z-scores and on percent body fat was assessed with longitudinal mixed effects growth curve models.
Results
The GC starting dose was high in 59% and moderate in 39% of patients. The peak BMI Z score was +1.29 at 4 months with high-dose GCs and +0.69 at 4.2 months with moderate-dose GCs (p<0.001). Overall, 50% (95% confidence interval 41–59%) of children returned to within +0.25 standard deviations (SD) of their baseline BMI Z score. Oral GC dose over the preceding 3 months was the most significant determinant of BMI Z-score and percent body fat. The proportion of days in receipt of GCs, disease activity, and a diagnosis of systemic-onset juvenile idiopathic arthritis were also associated with BMI Z scores. The correlation between changes in BMI and changes in percent body fat was 0.09.
Conclusions
In children with rheumatic disease starting moderate and high doses of GCs, BMI Z score peaked at 4 months and only half returned to within +0.25 SD of their baseline BMI Z-score by 18 months.
Introduction
Glucocorticoids (GC) commonly used for the treatment of children with rheumatic disease can cause weight gain that is distressing to patients (1, 2) and their families. It is not known how many children restore their premorbid weight after discontinuing or reducing GC therapy. The risks associated with non-GC related obesity in pediatric patients (3, 4) include metabolic syndrome (5), increased arterial stiffness (6), advanced vascular age (7), increased left atrial size (8), increased risk of coronary artery disease (9, 10), and type 2 diabetes mellitus (11). Adolescent obesity frequently tracks into adulthood (12), and there are known obesity-associated health risks at all ages (13–16). Conversely, obese children who reduce weight can lower the associated health risks (17).
Childhood rheumatic diseases that may require GC treatment include juvenile idiopathic arthritis (JIA), particularly the systemic subtype of JIA; systemic lupus erythematosus (SLE); juvenile dermatomyositis (DM); chronic systemic vasculitis (CSV); and others. GCs are often prescribed in moderate to large doses for rapid control of inflammation, with subsequent tapering and ideally discontinuation if disease activity is controlled with treatment using other medications. There is a paucity of studies in the literature to guide practitioners in counseling children about anticipated weight changes following GC initiation. The only study examining body mass index (BMI) changes in pediatric rheumatic disease to date included 15 children on high-dose GCs. In this study, BMI percentiles increased 6 - 8 weeks after starting GCs and peaked at 6 - 9 months (18). Our reanalysis of data from the Tekano et al study (18) showed that 6 children had a BMI consistently above the 85th percentile at 24 months; these 6 patients had an initial BMI greater than the 75th percentile (re-analysis of data from reference (18).
Physical activity is an important aspect of healthy weight maintenance and potentially could impact the effect of GCs on BMI changes; however the aerobic and anaerobic exercise capacity in children and adolescents with JIA is significantly impaired compared to healthy controls, and those with more swollen joints tend to be less physically active (19). Reduced fitness may persist even when disease is in remission (20–22).
In the present study, we sought to describe the changes in BMI Z scores during the 18 months following initiation of GCs among children with rheumatic disease. We further aimed to determine the proportion of children who had a return of their BMI Z-score to the baseline level, and to assess the influence of the mean daily GC dose, days in receipt of GCs, disease type, as well as physical and disease activity on BMI Z scores.
Patients and Methods
We used data from the STeroid-associated Osteoporosis in the Pediatric Population (STOPP) study (members of the Canadian STOPP Consortium are shown in Appendix A). This prospective observational study involving 10 Canadian pediatric academic centers was principally designed to study bone health among children with GC-treated disorders. Details of the STOPP protocol for children initiating GC therapy for rheumatic diseases have been published previously (23, 24).
Patient Population
All children in the STOPP study receiving GCs for the treatment of a rheumatic disease with at least 1 follow-up visit were eligible for this study if they were ≤16 years of age at enrollment, and had a first-time requirement for intravenous or oral GC therapy as determined by their pediatric rheumatologist. The eligibility criteria for enrolment in the STOPP study have been described in detail elsewhere (24). Children were excluded from the study if they had previously taken GCs for the treatment of the underlying disease. Patients were also excluded if they had received intravenous or oral GC for > 14 consecutive days in the 12 months preceding study enrolment to treat any other medical condition (e.g. asthma), or if they had had complete immobilization for > 14 consecutive days prior to enrolment. The children were studied every 3 months for one year to allow accurate collection of the clinical data. The research ethics boards of each participating institution approved the study and informed consent/assent was obtained prior to enrolment.
Data Collection
The baseline visit was targeted within 30 days of GC initiation. Height and weight were measured using standardized procedures as previously described (25). BMI was calculated as weight in kilograms divided by the square of height in meters, and BMI Z scores were generated using Center for Disease Control and Prevention reference values (26) available in STATA software. Since these BMI Z scores are only available for children ≥2 years of age, data for patients <2 years old at enrollment were included in the graphical trajectories after their second birthday, but were excluded from the mixed-effects models and were not analyzed for return to baseline BMI Z scores. The Habitual Activity Estimation Scale (HAES) was used to measure physical activity (27, 28) and rheumatic disease activity was assessed with a 10-cm visual analog scale (VAS) by the treating rheumatologist (29). Puberty was assessed by physical examination according to the method of Marshall and Tanner (30, 31) at baseline and at 12 months, except for one study centre where it was recorded by self-report (32). Body composition was evaluated at baseline and every 6 months by dual energy x-ray absorptiometry (DXA). Patients with JIA who received their GC therapy for < 4 months underwent annual DXA scans (rather than DXA scans every 6 months) after the first year of the study.
GC Dosing
GC dosing was determined entirely at the discretion of the treating rheumatologist. Daily GC doses and changes were documented at follow-up by a chart review, patient or parent interview, and review of patient home GC diaries. When a patient missed a study visit, a research assistant called the family by telephone to record GC dose changes. GC doses were converted to mg/kg/day of prednisone equivalents.
Due to the paucity of available information on GC-related BMI changes, a focus group comprised of pediatric rheumatologists and allied health professionals convened to determine an a priori clinically meaningful framework to describe GC dosing. The focus group members recommended an analysis based on the initial GC dose, since this information coincides with what information is available at the time patients are counseled about the potential for GC-related weight gain; this is analogous to an intention-to-treat analysis. The starting dose was calculated as the mean daily combined intravenous and oral dose during the first 2 weeks of therapy. A high starting dosage was defined as ≥1.0 mg/kg/day of prednisone equivalents (33, 34), a low dosage as <0.2 mg/kg/day to a maximum of 7.5 mg/day (35), and moderate dose as any dose in between.
GC exposure between each 3 - month study visit was described in the analysis as follows: (1) the mean daily oral GC dosage (mg/kg/day); (2) the mean daily intravenous GC dosage (mg/kg/day), and (3) the proportion of days in receipt of GCs (the number of days in receipt of GCs between study visits divided by the number of days between study visits). The weight used to calculate the average daily oral and intravenous doses was estimated each day during the study period by simple linear interpolation of the weights from study visit data; this allowed the analyses to separately account for the route, dose, and number of days treated with GCs. Once- daily dosing and divided-daily dosing were not distinguished in the models because information on divided daily dosing was not available.
Physical Activity
The HAES is a validated measure of daily physical activity in children (27, 28) that has been used in multiple pediatric settings (36–38). Parents completed the HAES for children ages <13 years, and when possible, children ages ≥13 years completed the scale themselves. The HAES measures the amount of physical activity over the preceding week, and provides the duration of time spent in each of four levels of intensity (inactive, somewhat inactive, somewhat active and very active) for any awake day. Visits with >90% of awake time spent doing physical activity were removed from the analysis as incorrectly completed questionnaires.
Disease Activity
Disease activity was scored on a 10-cm VAS by a pediatric rheumatologist (29), where 0 cm = inactive disease and 10 cm = very active disease. The scale refers to the particular disease being scored. Differences across diseases in VAS scoring and effects on BMI (39), were accounted for in statistical modeling by adding variables for the following 5 groups of diseases: juvenile DM, SLE, systemic onset JIA, CSV, and other rheumatic diseases. CSV included Takayasu’s arteritis, granulomatosis with polyangiitis (Wegener’s), microscopic polyangiitis, perinuclear antineutrophil cytoplasmic antibody (pANCA) - positive renal limited vasculitis, and pANCA - positive vasculitis with Goodpasture’s syndrome. This statistical model allowed for the estimation of interactions between GC dose and disease groups.
Body Composition by DXA
Body composition was measured every 6 months with DXA using either QDR 4500 (Hologic) at 3 centers, Discovery (Hologic) at 2 centers, and Delphi (Hologic) at 1 center, or Lunar Prodigy (GE Medical Systems) at 4 centers. These instruments were cross-calibrated for bone mineral content in the spine but not for percent body fat data. The within-subject changes in percent fat could therefore be modeled, but not between-subject differences, as between subject differences would have been at least partially attributable to different machines. Percent fat was calculated as the number of grams of fat divided by grams of total body weight (the sum of fat mass, lean mass, and bone mineral content determined from the DXA scan).
Statistical Analysis
We described BMI, weight and height Z-score trajectories graphically by fitting splines in time from study entry using a longitudinal mixed-effects model. The degree of complexity for the spline representations was chosen based on Akaike’s Information Criterion (AIC) values, which indicated that 4 degrees of freedom were sufficient to accurately capture the underlying functional form of the relation. We defined return-to-baseline BMI as a return to within 0.25 standard deviations (SD) above baseline BMI Z-scores.
The models for growth were based on annualized, between-visit growth rates (change in growth parameter between visits divided by time in years between visits). To allow for variation in growth rates due to age and sex, we applied linear mixed effects models with a spline term for age and an interaction with sex. These terms were reduced to additive linear terms in age and sex based on estimated predictive accuracy of the models, as indicated by AIC values.
We used mixed effects multiple regression models to estimate the impact of a number of factors on BMI Z-scores. The between-subject variation in growth rate and sensitivity to GC dose were incorporated as random effects. Covariates representing baseline BMI Z score, pubertal status, physical activity, and underlying rheumatic disease were included. Time-dependent covariates in the preceding 3 months included: mean daily oral GC dosage between each study visit (mg/kg/day), mean daily intravenous GC dosage between each study visit (mg/kg/day), proportion of days between study visits in receipt of GCs, and disease activity. There were no interactions between GC dose and disease groups in this model.
The disease activity between study visits was calculated by averaging the VAS scores at each study visit with the VAS scores at the previous study visit to reflect the mean disease activity in the preceding 3-month interval. The reference category for disease type was juvenile DM. Tanner growth stages were categorized as early puberty (Tanner stages 1-2) or mid- to late puberty (Tanner stages 3 - 5) (40). Patients were then divided into 3 groups: early puberty at baseline and 12 months, mid- late puberty at both assessments, and early puberty at baseline but mid-late puberty at 12 months. The combined inactive and somewhat active hours in the week prior to each study visit as recorded by the HAES were included in the BMI Z score and DXA percent fat models, since these were deemed to be the most relevant measures for the models. Statistical significance was set at a p value less than 0.05.
Percent fat mass trajectories were modeled similarly to BMI Z score curves while controlling for height, age, and sex, which are known potential confounders. Independent variables included interval specific mean daily oral GC dose, interval specific average daily intravenous GC dose, interval specific proportion of days in receipt of GCs, physical activity, pubertal status, type of rheumatic disease, and disease activity. We did not control for baseline fat mass as we modeled changes from baseline and not absolute values. The correlation between percent fat mass and BMI changes was described using Pearson’s correlation coefficient. R was used for all analyses (41).
Results
The 130 eligible patients (96% of the total cohort [n=136]) had a mean age of 9.8 years and were predominantly female (66%). Six patients were excluded; 2 did not have a well-established rheumatic disease and 4 were excluded for lack of follow-up. Percent body fat mass was available for 127 of 130 patients. At baseline, 16% of the patients had a BMI greater than 85th percentile and 13% had a BMI greater than the 95th percentile, while at 18 months the these percentages were 24% and 16%, respectively. Additional baseline characteristics are shown in Table 1. GC dosing over the 18 months varied significantly among the children, in accordance with treatment decisions made by the attending rheumatologists based on disease manifestations. By 6 months, 22% had discontinued GCs and by 12 months, 31 % had discontinued GCs. In addition, 62% received GCs longer than 12 months, and 7% received GCs for ≥2 discrete periods separated by a minimum 6-week GC-free interval. The median starting GC dosage was 1.3 mg/kg/day of prednisone equivalents. Figure 1 shows the dosing pattern over time. Overall, 37% of the children were still receiving GCs within 2 weeks of the 18-month visit, at which time their median GC dosage was 0.37 mg/kg/day. Only 1 of the 4 patients in the low starting dose group and 5 of the 50 patients in the moderate starting dose group received intravenous pulse therapy at any point in the study.
Table 1.
Baseline patient characteristics
| Baseline characteristics (n=130) # | Result |
|---|---|
| Median age in years (IQR) | 9.8 (5.9, 13.8) |
| Female | 86 (66) |
| Some White ethnicity§, n (%) | 101 (78) |
| Median days after diagnosis at enrolment (IQR) | 24 (15, 24) |
| GC starting dosage, median (IQR) mg/kg/day | |
| Low | 0.09 (0.07, 0.10) |
| Moderate | 0.50 (0.35, 0.70) |
| High | 3.60 (1.60, 8.00) |
| Days in receipt of GC between study visits, median (IQR) % | |
| All patients | 74 (37, 100) |
| Low starting dose group | 5 (0.2, 20) |
| Moderate starting dose group | 66 (20, 96) |
| High starting dose group | 81 (46, 100) |
| Patients in each GC group⌘ | |
| Low | 4 (3) |
| Moderate | 50 (39) |
| High | 76 (59) |
| Diagnosis⌘ | |
| Juvenile idiopathic arthritis (all subtypes) | 49 (38) |
| Systemic arthritis | 24 (18) |
| Polyarthritis, rheumatoid factor negative | 8 (6) |
| Polyarthritis, rheumatoid factor positive | 5 (4) |
| Enthesitis related arthritis | 3 (2) |
| Oligoarticular arthritis | 3 (2) |
| Psoriatic arthritis | 2 (2) |
| Unclassified | 4 (3) |
| Juvenile dermatomyositis | 30 (23) |
| Systemic lupus erythematyosus | 20 (15) |
| Localized scleroderma | 10 (8) |
| Systemic scleroderma | 1 (1) |
| Overlap syndromes (including mixed connective tissue disease) | 5 (4) |
| Chronic systemic vasculitis* | 14 (11) |
| CNS vasculitis | 1 (1) |
Values are the number (percentage) unless otherwise indicated. IQR = interquartile range; GC = glucocorticoid; CNS = central nervous system.
Includes all patients with any amount of white ethnicity.
Total of 101% due to rounding.
Includes Takayasu’s arteritis, granulomatosis with polyangiitis (Wegener’s), microscopic polyangiitis, perinuclear antineutrophil cytoplasmic antibody (pANCA)-positive renal limited vasculitis, and pANCA-positive vasculitis with Goodpasture’s syndrome.
Figure 1.
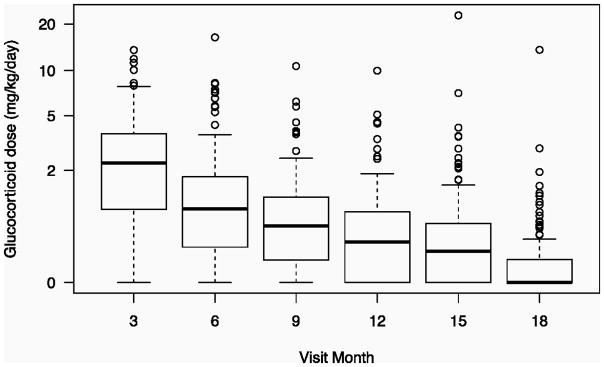
Boxplot of glucocorticoid doses at each study visit over the 18-month study period, during which the doses steadily decreased. The lines inside the boxes represents the median dose at each visit. The circles indicate outliers.
The GC starting dose was high in 59% of the patients, moderate in 39%, and low in 3% (the total is 101% because of rounding). BMI peaked at 4.0 months in the high-dose group and 4.2 months in the moderate-dose group; the curves of mean BMI Z scores over time differed significantly for the moderate- and high-dose GC groups (P< 0.001) (Figure 2). The mean peak BMI Z scores were 1.29 and 0.69 in the high- and moderate-dose groups, respectively. The low starting dose group was not analyzed graphically because it included only 4 children; however, this group was included in the longitudinal modeling. Despite the high level of significance, the R2 values of the graphical models shown in Figure 2 were low at 0.05 for the moderate starting dose group and 0.02 for the high starting dose group, indicating that these models have limited predictive ability for individual patients.
Figure 2.
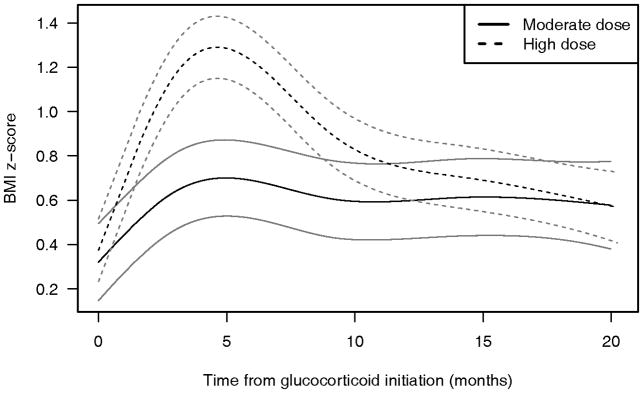
Body mass index (BMI) Z-score trajectories and 95% confidence intervals for children with rheumatic disease according to glucocorticoid (GC) starting dose (n=126). GC starting doses were defined as high (≥ 1.00 mg/kg/day) for 76 children and moderate for 50 children. The y-axis shows actual BMI Z-scores. BMI Z-scores start close to 0 because our population had a relatively normal BMI distribution at enrollment.
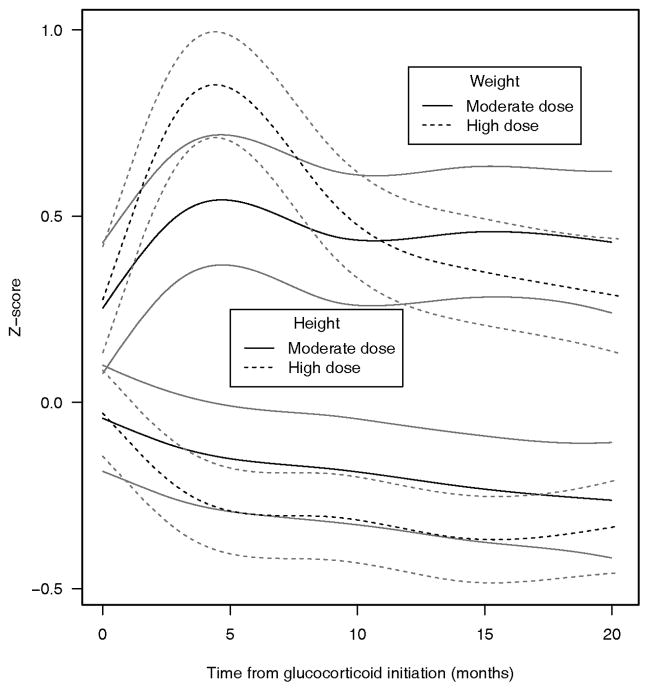
Data for assessment of return-to-baseline for BMI Z-scores were available for 112 (86%) of the 130 children. At 18 months, 50% (95% confidence interval 41–59%) of the subjects failed to return to their baseline BMI Z scores, and there was no difference between the moderate (48%) and high (52%) GC starting dose groups. Overall, 30% of the patients who returned to baseline BMI Z scores and 45% of patients who did not were still being treated with GCs within 2 weeks of their 18-month visit (P= 0.17), while 70% of the patients who returned to baseline BMI Z scores had stopped taking GCs. The estimated mean height Z scores declined with GC therapy and did not recover during the study period, while the mean weight Z score trajectories tended to parallel the trajectories of the BMI Z scores (Figure 3). The mean BMI and weight Z scores differed significantly between the moderate- and high-dose groups (P < 0.0001), whereas mean height Z scores did not.
Figure 3.
Weight and height Z-score trajectories and 95% confidence intervals for children with rheumatic disease according to glucocorticoid starting dose.
In mixed-effects modeling, after controlling for intravenous GC dose, baseline raw BMI, sex, pubertal status, physical activity, and rheumatic disease, the only significant predictors of BMI Z scores were oral GC dose, proportion of days in receipt of GCs, disease activity, and the presence of systemic-onset JIA (Table 2). The longitudinal model used the mean daily dosage of prednisone equivalents in mg/kg/day in the previous 3-month study interval to predict the BMI Z scores at each 3-month study visit. Age was significant when the model did not adjust for pubertal status (data not shown). All patients were included in the longitudinal model irrespective of their starting dose.
Table 2.
Regression coefficients for mixed effects modeling of BMI Z-scores for 18 months after GC therapy was started (n=112)¥
| Variable | Coefficient (SE) | p-value |
|---|---|---|
| Oral GC$ (mg/kg/day)$ | 4.29 (0.45) | <0.00 |
| Intravenous GC$ (mg/kg/day)$ | 0.38 (0.25) | 0.12 |
| Proportion of days in receipt of GC+ | -0.98 (0.22) | <0.00 |
| Male# | -0.35 (0.19) | 0.06 |
| Age, years | 0.05 (0.04) | 0.19 |
| Inactive HAES scoreπ | 0.01 (0.01) | 0.68 |
| Entered pubertyβ | 0.22 (0.62) | 0.72 |
| Post-pubertal at baseline¶ | 0.06 (0.29) | 0.83 |
| SLEф | 0.17 (0.31) | 0.59 |
| Systemic-onset JIAф | 0.57 (0.28) | 0.04 |
| CSVф§ | -0.22 (0.31) | 0.48 |
| Other rheumatic diseaseф | 0.44 (0.02) | 0.08 |
| Disease activity* | 0.15 (0.05) | <0.00 |
| Baseline raw BMI | 0.04 (0.02) | 0.05 |
Coefficients show the effect over 1 year, and can be divided to show the effect over shorter time intervals. For example, dividing coefficients in half would show the effect over a 6-month period.
BMI=body mass index; GC=glucocorticoid; HAES=Habitual Activity Estimation Scale; SLE=systemic lupus erythematosus; JIA= juvenile idiopathic arthritis; CSV=chronic systemic vasculitis
Mean daily dosage during the preceding 3-month interval
Proportion of days in receipt of GC during the preceding 3-month interval
Compared to female
The inactive component of the HAES (sum of somewhat inactive and inactive hours)
Entered puberty sometime during the18-month study period
Compared to remaining pre-pubertal throughout the 18-month study period
Compared to remaining pre-pubertal throughout the 18-month study period
Juvenile dermatomyositis was the reference category for all disease groups
CSV includes Takayasu’s arteritis, granulomatosis with polyangiitis (Wegener’s), microscopic polyangiitis, perinuclear antineutrophil cytoplasmic antibody (pANCA) - positive renal limited vasculitis, and pANCA-positive vasculitis with Goodpasture’s syndrome
Disease activity reflects the average activity during the preceding 3-month interval
The correlation between raw BMI results (not BMI Z scores) and body fat at baseline was 0.75, and at 6 months the correlation was 0.78; however, the correlation between the change in raw BMI results and the change in percent body fat from baseline to 6 months after initiation of GCs was quite low at 0.09. Similar to the BMI Z score model, the mean daily oral GC dose (25.75, SE 3.10, P < 0.00) and proportion of days in receipt of GCs (-5.21, SE 1.79, p<0.00) had significant effects on changes in percent body fat, although in this model the disease group termed other was also significant (4.64, SE 1.69, P = 0.01) when controlling for age, height, sex, mean daily intravenous GC dose, pubertal status, disease type, disease activity, and physical activity. Although lean body mass generally increased following GC initiation, there was an abundance of variability in this parameter. In the raw data, fat mass appeared to contribute more to increases in mass than lean body mass, resulting in a relative decrease in the percent lean body mass (data not shown).
Discussion
The prospective longitudinal tracking of changes in BMI Z scores over the 18-month period after starting GCs for GC-naive children with rheumatic diseases showed that BMI Z scores peaked at 4 months, with a mean peak Z score of 1.3 in those starting high-dose GCs and 0.7 in those starting moderate-dose GCs. Half of these children did not return to their baseline BMI Z score by 18 months, and this failure to return to baseline values was equally likely in patients in the moderate and high GC starting dose groups. Oral GC dose, proportion of days in receipt of GCs, and disease activity significantly predicted BMI Z scores. Although observations were limited to 3-month intervals, peak BMI Z scores occurred sooner than we had predicted from pilot data (18), while physical activity, disease type, and disease activity had minimal impact on BMI Z score trajectories, in keeping with our a priori hypothesis. The graphical mean trajectories of BMI Z scores significantly differed between children initiating moderate-and high-dose GCs, as expected, but the degree of variability in the data was quite large. Therefore these trajectories provided a clear picture of population-level data, but were of limited value for individual patient predictions.
Larger BMI Z scores were associated with a combination of height deficits and increased weight. Although decreased height Z scores may have partially explained the initial increases in BMI Z scores, after BMI Z score trajectories peaked, they returned toward their baseline values and diverged from height Z scores trajectories, while the height Z score trajectories showed continued height deficits. The weight Z score curves paralleled the BMI Z score trajectories more closely, indicating that increased weight, and not just height deficits, was responsible for the shape of the BMI trajectories.
A widely held held belief based on long-term clinical experience is that alternate day steroids are less lipogenic than daily steroids. Surprisingly, once accounting for all covariates, including GC dose and route of administration, an increased proportion of days in receipt of GCs was associated with a smaller increase in BMI. In our model (while maintaining all other variables constant), for the same average daily GC dose regardless of whether the doses were administered orally or intravenously, each 10% increase in the proportion of days in receipt of GCs reduced the BMI Z score by 0.1 unit over the course of a year. One explanation for this could be that large, intermittent GC pulses may have different and longer-lasting effects than alternate day lower-dose oral GCs; however, our model could not test this possibility. Our findings may also be a result of reverse causation, whereby the physicians decreased the GC dose or changed the route of administration when there was a large increase in BMI.
The relationships between obesity and GC therapy in the treatment of children with other chronic pediatric diseases have been described, but because of the disease-specific variation in GC dosing strategies, it is difficult to extrapolate these findings to children with chronic rheumatic diseases. However, GC-induced obesity has been reported to occur in 35–41% of children with nephrotic syndrome, although the extent to which their obesity resolved was not clearly described (42–44).
To our knowledge, there are no published studies examining the degree to which physical activity impacts GC-associated weight changes. In our study, physical activity did not attenuate the GC-associated increase in BMI. Additionally, disease activity assessed by the treating physician did not significantly impact the BMI trajectories in our models.
Although BMI is easily obtained, it does not always reflect percent body fat (45), whereas DXA is a direct measure of adiposity. In this study, the within-subject changes in raw BMI did not correlate with changes in percent body fat, even when accounting for height, age, and sex as potential confounders. Changes in percent body fat are small relative to absolute values; therefore, the relative error in these changes is much larger than the relative error for the absolute values, which may partially explain the poor correlation between the changes in BMI and percent body fat. Previous findings from small studies have suggested that there is a discrepancy between BMI measurements and percent body fat (46) and a higher percent body fat (47) in patients with a history of a pediatric rheumatic disease compared to controls.
A number of limitations to this study could threaten the generalizability of our findings. The requirement of study visits every 3 months potentially precluded the participation of patients who were not able to meet the STOPP study demands. Furthermore, participants were enrolled from tertiary care pediatric centers, where the majority of Canadian pediatric rheumatologists practice; conversely, the number of children with rheumatic diseases undergoing care solely outside of these centers is likely very small. Our study findings may not be generalizable to non-Canadian settings where health care access is not universal or where physicians other than pediatric rheumatologists provide care for these patients, since these physicians may have a different approach to GC prescription. Additionally, although we considered modeling the World Health Organization Organization BMI Z scores, when we examined the baseline distribution of these BMI Z scores in our population, the large number of outliers present indicated that these BMI curves did not reflect our population as well as the Centers for Disease Control and Prevention BMI curves. Furthermore, indirect measures of physical activity, such as the one we used, tend to overestimate physical activity when compared to direct measures (48). Finally, data on changes in caloric intake were not collected in this study.
GC-induced weight gain is a distressing side effect of therapy for children and youth with rheumatic diseases (1). This study provides data from a relatively large cohort of children and adolescents with rheumatic diseases initiating GC therapy for the first time for their rheumatic disease. It describes how much and how soon BMI Z scores increase, to what extent BMI Z scores are restored to the previous status over 18 months, and how these changes relate to the GC starting dose. The observed BMI Z score trajectories will help health care providers to counsel children with rheumatic diseases and their families about the expected timing and degree of GC-induced BMI changes at a population level, although the BMI trajectories cannot be used for predictions at an individual level. Further work is necessary to clarify BMI changes beyond 18 months after GC initiation. Our surprising finding, that increased days in receipt of GCs was associated with a smaller increase in BMI Z scores, requires additional evaluation.
Innovation
This is the first study to prospectively describe how body mass index (BMI), height and weight Z scores change over time after glucocorticoid (GC) initiation for pediatric rheumatic disease.
Significance
After peaking at 4 months, only half of children returned to their baseline BMI Z scores by 18 months.
Oral GC dose was the most significant determinant of BMI gain after adjusting for physical activity, diagnosis, disease activity, baseline BMI Z scores, pubertal status, age, sex, intravenous GCs, and days in receipt of GCs.
Changes in BMI correlated poorly with changes in percent body fat among GC-treated children with rheumatic disease.
Acknowledgments
This study was funded primarily by the Canadian Institutes for Health Research (FRN 64285), with additional funding from the Children’s Hospital of Eastern Ontario, and Women and Children’s Health Research Institute, University of Alberta. Dr. Natalie Jane Shiff received support from the Clinician Investigator Program, University of British Columbia and a CIHR Frederick Banting and Charles Best Canada Graduate Scholarship Master Award for a component of this project. Dr. Leanne Ward has been the recipient of a Canadian Institutes for Health Research New Investigator Award, a Canadian Child Health Clinician Scientist Career Enhancement Award, and Research Chair Award from the University of Ottawa.
The Canadian STOPP Consortium would like to thank the following individuals: The children and their families who participated in the study and without whom the STOPP study would not have been possible.
Research Associates who managed the study at the co-ordinating center (the Children’s Hospital of Eastern Ontario Ottawa, Ontario): Elizabeth Sykes (STOPP Project Manager), Maya Scharke (STOPP Data Analyst and Database Manager), Monica Tomiak (Statistical Analyses), Victor Konji (STOPP Publications and Presentations Committee Liaison and hand morphometry measurements), Steve Anderson (Children’s Hospital of Eastern Ontario Pediatric Bone Health Program Research Manager), Catherine Riddell (STOPP National Study Monitor); Research Associates who took care of the patients from the following institutions: Alberta Children’s Hospital, Calgary, Alberta: Eileen Pyra; British Columbia Children’s Hospital, Vancouver British Columbia: Terry Viczko, Angelyne Sarmiento, Jacqueline Page; Children’s Hospital of Eastern Ontario, Ottawa, Ontario: Heather Cosgrove, Josie MacLennan, Catherine Riddell; Children’s Hospital, London Health Sciences Centre, London, Ontario: Vinolia ArthurHayward, Leila MacBean, Mala Ramu; McMaster Children’s Hospital, Hamilton, Ontario: Susan Docherty-Skippen; IWK Health Center, Halifax, Nova Scotia: Aleasha Warner; Montréal Children’s Hospital, Montréal, Québec: Valérie Gagné, Diane Laforte, Maritza Laprise; Ste. Justine Hospital, Montréal, Québec: Claude Belleville, Natacha Gaulin Marion; Stollery Children’s Hospital, Edmonton, Alberta: Ronda Blasco, Germaine, McInnes, Amanda Mullins, Toronto Hospital for Sick Children, Toronto, Ontario: Michele Petrovic; Winnipeg Children’s Hospital, Winnipeg, Manitoba: Dan Catte, Erika Bloomfield. The Research Nurses, Support Staff and all the STOPP collaborators from the various Divisions of Nephrology, Oncology, Rheumatology and Radiology who have contributed to the care of the children enrolled in the study.
Abbreviations
- AIC
Akaike information criterion
- BMI
Body mass index
- CI
Confidence interval
- CSV
Chronic systemic vasculitis
- DXA
Dual energy x-ray absorptiometry
- GC
Glucocorticoid(s)
- HAES
Habitual Activity Estimation Scale
- DM
Juvenile dermatomyositis
- JIA
Juvenile idiopathic arthritis
- SD
Standard deviation
- SE
Standard error
- SLE
Systemic lupus erythematosus
- JIA
Systemic onset juvenile idiopathic arthritis
- VAS
Visual analog scale
Appendix A. Members of the Canadian STeroid-associated Osteoporosis in the Pediatric Population (STOPP) Consortium (a pan-Canadian, pediatric bone health working group)
Co-ordinating Center
Children’s Hospital of Eastern Ontario, Ottawa, Ontario: Leanne M. Ward#,*,§ (Study Principal Investigator), Ciaran Duffy (Rheumatology), Janusz Feber*,§ (Nephrology), Jacqueline Halton*,§ (Oncology), Roman Jurencak (Rheumatology), MaryAnn Matzinger (Radiology, Central Radiograph Analyses), Johannes Roth (Rheumatology), Nazih Shenouda§ (Radiology, Central Radiograph Analyses); Ottawa Hospital Research Institute, Ottawa Methods Centre Ottawa, Ontario: David Moher*,§ (Research Methods), Tim Ramsay (Statistics).
Participating Centers
Alberta Children’s Hospital, Calgary, Alberta: David Stephure (Site Principal Investigator), Reinhard Kloiber (Radiology), Victor Lewis (Oncology), Julian Midgley (Nephrology), Paivi Miettunen (Rheumatology); British Columbia Children’s Hospital, Vancouver, British Columbia: David Cabral* (Site Principal Investigator), David B. Dix (Oncology), Kristin Houghton (Rheumatology), Helen R. Nadel (Radiology).
British Columbia Women’s Hospital and Health Sciences Center, Vancouver, British Columbia: Brian C. Lentle§ (Radiology); Brock University, Faculty of Applied Health Sciences, St. Catharines, Ontario: John Hay§ (Physical Activity Measurements); Children’s Hospital, London Health Sciences Centre, University of Western Ontario, London, Ontario: Robert Stein (Site Principal Investigator), Elizabeth Cairney (Oncology), Cheril Clarson (Bone Health), Guido Filler (Nephrology) §, Joanne Grimmer (Nephrology), Keith Sparrow (Radiology), Scott McKillop (Radiology); IWK Health Center, Halifax, Nova Scotia: Elizabeth Cummings (Site Principal Investigator), Conrad Fernandez (Oncology), Adam M. Huber§ (Rheumatology), Bianca Lang*,§ (Rheumatology), Kathy O’Brien (Radiology); McMaster Children’s Hospital, Hamilton, Ontario: Stephanie Atkinson*,§ (Site Principal Investigator), Steve Arora (Nephrology), Ronald Barr§ (Oncology), Craig Coblentz (Radiology), Peter B. Dent (Rheumatology), Maggie Larché (Rheumatology), Colin Webber* (DXA Methodology); Montreal Children’s Hospital, Montréal, Québec: Celia Rodd§ (Site Principal Investigator), Sharon Abish (Oncology), Lorraine Bell (Nephrology), Claire LeBlanc (Rheumatology), Rosie Scuccimarri (Rheumatology); Shriners Hospital for Children, Montréal, Québec: Frank Rauch*,§ (Co-Chair, Publications and Presentations Committee and Ancillary Studies Committee), Francis Glorieux*; Ste. Justine Hospital, Montréal, Québec: Nathalie Alos* (Site Principal Investigator), Josée Dubois (Radiology), Caroline Laverdière (Oncology), Véronique Phan (Nephrology), Claire Saint-Cyr (Rheumatology); Stollery Children’s Hospital, Edmonton, Alberta: Robert Couch* (Site Principal Investigator), Janet Ellsworth (Rheumatology), Maury Pinsk (Nephrology), Kerry Siminoski§ (Radiology), Beverly Wilson (Oncology); Toronto Hospital for Sick Children, Toronto, Ontario: Ronald Grant* (Site Principal Investigator), Martin Charron (Radiology), Diane Hebert (Nephrology) Université de Sherbrooke, Department of family medicine, Sherbrooke, Québec: Isabelle Gaboury*,§ (Biostatistics); Winnipeg Children’s Hospital, Winnipeg, Manitoba: Shayne Taback§ (Site Principal Investigator), Tom Blydt-Hansen (Nephrology), Sara Israels (Oncology), Kiem Oen (Rheumatology), Martin Reed (Radiology).
Footnotes
Principal Investigator;
Executive Committee Member;
Publications and Presentations Committee Member
Financial Disclosure:
Dr. Celia Rodd has received less than $10,000 honorarium and speaking fees from the Association of Endocrinologists of Quebec and BC Children’s Hospital. None of the other authors have any disclosures.
References
- 1.Nashel DJ, Ulmer CC. Systemic lupus erythematosus: Important considerations in the adolescent. J Adolesc Health Care. 1982 Jun;2(4):273–8. doi: 10.1016/s0197-0070(82)80062-4. [DOI] [PubMed] [Google Scholar]
- 2.Rogers CC, Alloway RR, Buell JF, Boardman R, Alexander JW, Cardi M, et al. Body weight alterations under early corticosteroid withdrawal and chronic corticosteroid therapy with modern immunosuppression. Transplantation. 2005 Jul 15;80(1):26–33. doi: 10.1097/01.tp.0000164290.17030.bc. [DOI] [PubMed] [Google Scholar]
- 3.Dietz WH. Health consequences of obesity in youth: Childhood predictors of adult disease. Pediatrics. 1998 Mar;101(3):518–25. [PubMed] [Google Scholar]
- 4.Dietz WH, Robinson TN. Clinical Practice: Overweight children and adolescents.[see comment] N Engl J Med. 2005 May 19;352(20):2100–9. doi: 10.1056/NEJMcp043052. [DOI] [PubMed] [Google Scholar]
- 5.Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004 Jun 3;350(23):2362–74. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 6.Sakuragi S, Abhayaratna K, O’Reilly C, Srikusalanukul W, Gravenmaker K, Budge M, et al. Influence of adiposity on arterial stiffness in healthy children. Circulation. 2008;118:S1115. doi: 10.1161/HYPERTENSIONAHA.108.123364. [DOI] [PubMed] [Google Scholar]
- 7.Le J, Spencer M, McCrary D, Zhang D, Jie C, Raghuveer G. Advanced “vascular age” in children with dyslipidemia. Circulation. 2008;118:S1056. [Google Scholar]
- 8.Ayer JG, Sholler G, Celermajer DS. Left atrial size is independently associated with body mass index in children. Circulation. 2008;(118):S1154. [Google Scholar]
- 9.Baker JL, Olsen LW, Sorensen TI. Childhood body-mass index and the risk of coronary heart disease in adulthood. N Engl J Med. 2007 Dec 6;357(23):2329–37. doi: 10.1056/NEJMoa072515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ludwig DS, Ebbeling CB. Overweight children and adolescents. N Engl J Med. 2005 Sep 8;353(10):1070–1. doi: 10.1056/NEJMc051637. author reply 1070–1. [DOI] [PubMed] [Google Scholar]
- 11.Daniels S, Jacobson M, McCrindle B, Eckel R, McHugh SB. American Heart Association Childhood Obesity Research Summit Report. Circulation. 2009;119:e489–e517. doi: 10.1161/CIRCULATIONAHA.109.192216. [DOI] [PubMed] [Google Scholar]
- 12.Guo SS, Chumlea WC. Tracking of body mass index in children in relation to overweight in adulthood. Am J Clin Nutr. 1999 Jul;70(1):145S–8. doi: 10.1093/ajcn/70.1.145s. [DOI] [PubMed] [Google Scholar]
- 13.Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. 1997 Sep 25;337(13):869–73. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- 14.Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA. 2003 Jan 8;289(2):187–93. doi: 10.1001/jama.289.2.187. [DOI] [PubMed] [Google Scholar]
- 15.Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006 Aug 24;355(8):763–78. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 16.Hu FB, Willett WC, Li T, Stampfer MJ, Colditz GA, Manson JE. Adiposity as compared with physical activity in predicting mortality among women. N Engl J Med. 2004 Dec 23;351(26):2694–703. doi: 10.1056/NEJMoa042135. [DOI] [PubMed] [Google Scholar]
- 17.Ford AL, Hunt LP, Cooper A, Shield JP. What reduction in BMI SDS is required in obese adolescents to improve body composition and cardiometabolic health? Arch Dis Child. 2010 Apr;95(4):256–61. doi: 10.1136/adc.2009.165340. [DOI] [PubMed] [Google Scholar]
- 18.Tekano JL, Tucker L, Khattra P. Limiting obesity in children with rheumatic diseases requiring corticosteroid therapy. Arthritis Rheum. 2006;54(9):S529. [Google Scholar]
- 19.Takken T, van der Net J, Helders PJ. Relationship between functional ability and physical fitness in juvenile idiopathic arthritis patients. Scand J Rheumatol. 2003;32(3):174–8. doi: 10.1080/03009740310002524. [DOI] [PubMed] [Google Scholar]
- 20.van Brussel M, Lelieveld OT, van der Net J, Engelbert RH, Helders PJ, Takken T. Aerobic and anaerobic exercise capacity in children with juvenile idiopathic arthritis. Arthritis Rheum. 2007 Aug 15;57(6):891–7. doi: 10.1002/art.22893. [DOI] [PubMed] [Google Scholar]
- 21.Klepper S. Making the case for exercise in children with juvenile idiopathic arthritis: What we know and where we go from here. Arthritis Rheum. 2007 Aug 15;57(6):887–90. doi: 10.1002/art.22902. [DOI] [PubMed] [Google Scholar]
- 22.Lelieveld OT, van Brussel M, Takken T, van Weert E, van Leeuwen MA, Armbrust W. Aerobic and anaerobic exercise capacity in adolescents with juvenile idiopathic arthritis. Arthritis Rheum. 2007 Aug 15;57(6):898–904. doi: 10.1002/art.22897. [DOI] [PubMed] [Google Scholar]
- 23.Huber AM, Gaboury I, Cabral DA, Lang B, Ni A, Stephure D, Taback S, Dent P, Ellsworth J, LeBlanc C, Saint-Cyr C, Scuccimarri R, Hay J, Lentle B, Matzinger M, Shenouda N, Moher D, Rauch F, Siminoski K, Ward LM. Canadian Steroid-Associated Osteoporosis in the Pediatric Population (STOPP) Consortium. Prevalent vertebral fractures among children initiating glucocorticoid therapy for the treatment of rheumatic disorders. Arthritis Care Res (Hoboken) 2010 Apr;62(4):516–2. doi: 10.1002/acr.20171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodd C, Lang B, Ramsay T, Alos N, Huber AM, Cabral DA, Scuccimarri R, Miettunen PM, Roth J, Atkinson SA, Couch R, Cummings EA, Dent PB, Ellsworth J, Hay J, Houghton K, Jurencak R, Larche M, LeBlanc C, Oen K, Saint-Cyr C, Stein R, Stephure D, Taback S, Lentle B, Matzinger M, Shenouda N, Moher D, Rauch F, Siminoski K, Ward LM Canadian Steroid-Associated Osteoporosis in the Pediatric Population (STOPP) Consortium. Incident vertebral fractures among children with rheumatic disorders 12 months after glucocorticoid initiation: A national observational study. Arthritis Care Res (Hoboken) 2012 Jan;64(1):122–31. doi: 10.1002/acr.20589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halton J, Gaboury I, Grant R, Alos N, Cummings EA, Matzinger M, et al. Advanced vertebral fracture among newly diagnosed children with acute lymphoblastic leukemia: Results of the Canadian Steroid-Associated Osteoporosis in the Pediatric Population (STOPP) research program. J Bone Miner Res. 2009 Jul;24(7):1326–34. doi: 10.1359/jbmr.090202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: Improvements to the 1977 national center for health statistics version. Pediatrics. 2002 Jan;109(1):45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 27.Hay JA. Development and testing of the Habitual Activity Estimation Scale. Eur J Phys Ed. 1997;2:110. [Google Scholar]
- 28.Hay JA, Cairney J. Development of the Habitual Activity Estimation Scale for clinical research: A systematic approach. Pediatric Exercise Science. 2006;18(2):193. [Google Scholar]
- 29.Rider LG, Feldman BM, Perez MD, Rennebohm RM, Lindsley CB, Zemel LS, et al. Development of validated disease activity and damage indices for the juvenile idiopathic inflammatory myopathies: I. Physician, parent, and patient global assessments. Juvenile Dermatomyositis Disease Activity Collaborative Study Group. Arthritis Rheum. 1997 Nov;40(11):1976–83. doi: 10.1002/art.1780401109. [DOI] [PubMed] [Google Scholar]
- 30.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969 Jun;44(235):291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970 Feb;45(239):13–2. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morris NM, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. J Youth and Adolescence. 1980;9(3):271–80. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- 33.Cassidy J, Petty R, Laxer R, Lindsley C, editors. The Textbook of Pediatric Rheumatology. 6. Philadelphia, PA, USA: Elsevier Saunders; 2011. [Google Scholar]
- 34.DeWitt E, Kimura Y, Beukelman T, Nigrovic P, One K, Prahalad S, et al. Consensus treatment plans for new-onset systemic juvenile idiopathic arthritis. Arthritis Care & Research. doi: 10.1002/acr.21625. (accepted, non-copy edited electronic version) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buttgereit F, da Silva JA, Boers M, Burmester GR, Cutolo M, Jacobs J, et al. Standardised nomenclature for glucocorticoid dosages and glucocorticoid treatment regimens: Current questions and tentative answers in rheumatology. Ann Rheum Dis. 2002 Aug;61(8):718–22. doi: 10.1136/ard.61.8.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wells GD, Wilkes DL, Schneiderman-Walker J, Elmi M, Tullis E, Lands LC, et al. Reliability and validity of the Habitual Activity Estimation Scale (HAES) in patients with cystic fibrosis. Pediatr Pulmonol. 2008 Apr;43(4):345–53. doi: 10.1002/ppul.20737. [DOI] [PubMed] [Google Scholar]
- 37.Schneiderman-Walker J, Wilkes DL, Strug L, Lands LC, Pollock SL, Selvadurai HC, et al. Sex differences in habitual physical activity and lung function decline in children with cystic fibrosis. J Pediatr. 2005 Sep;147(3):321–6. doi: 10.1016/j.jpeds.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 38.van Gent R, van der Ent CK, van Essen-Zandvliet LE, Rovers MM, Kimpen JL, de Meer G, et al. No differences in physical activity in (un)diagnosed asthma and healthy controls. Pediatr Pulmonol. 2007 Nov;42(11):1018–23. doi: 10.1002/ppul.20672. [DOI] [PubMed] [Google Scholar]
- 39.Souza RB, Borges CT, Takayama L, Aldrighi JM, Pereira RM. Systemic sclerosis and bone loss: The role of the disease and body composition. Scand J Rheumatol. 2006 Sep-Oct;35(5):384–7. doi: 10.1080/03009740600704296. [DOI] [PubMed] [Google Scholar]
- 40.Thayu M, Denson LA, Shults J, Zemel BS, Burnham JM, Baldassano RN, et al. Determinants of changes in linear growth and body composition in incident pediatric Crohn’s disease. Gastroenterology. 2010 Aug;139(2):430–8. doi: 10.1053/j.gastro.2010.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.R Development Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2011. URL: http://www.R-project.org/ [Google Scholar]
- 42.Foster BJ, Shults J, Zemel BS, Leonard MB. Risk factors for glucocorticoid-induced obesity in children with steroid-sensitive nephrotic syndrome. Pediatr Nephrol. 2006 Jul;21(7):973–80. doi: 10.1007/s00467-006-0100-z. [DOI] [PubMed] [Google Scholar]
- 43.Elzouki AY, Jaiswal OP. Long-term, small dose prednisone therapy in frequently relapsing nephrotic syndrome of childhood: Effect on remission, statural growth, obesity, and infection rate. Clin Pediatr (Phila) 1988 Aug;27(8):387–92. doi: 10.1177/000992288802700807. [DOI] [PubMed] [Google Scholar]
- 44.Merritt RJ, Hack SL, Kalsch M, Olson D. Corticosteroid therapy-induced obesity in children. Clin Pediatr (Phila) 1986 Mar;25(3):149–52. doi: 10.1177/000992288602500304. [DOI] [PubMed] [Google Scholar]
- 45.Neovius M, Linne Y, Barkeling B, Rossner S. Discrepancies between classification systems of childhood obesity. Obes Rev. 2004 May;5(2):105–14. doi: 10.1111/j.1467-789X.2004.00136.x. [DOI] [PubMed] [Google Scholar]
- 46.Lilleby V, Haugen M, Morkrid L, Frey Froslie K, Holven KB, Forre O. Body composition, lipid and lipoprotein levels in childhood-onset systemic lupus erythematosus. Scand J Rheumatol. 2007 Jan-Feb;36(1):40–7. doi: 10.1080/03009740600907881. [DOI] [PubMed] [Google Scholar]
- 47.Mul D, van Suijlekom-Smit LW, ten Cate R, Bekkering WP, de Muinck Keizer-Schrama SM. Bone mineral density and body composition and influencing factors in children with rheumatic diseases treated with corticosteroids. J Pediatr Endocrinol. 2002 Feb;15(2):187–92. doi: 10.1515/jpem.2002.15.2.187. [DOI] [PubMed] [Google Scholar]
- 48.Adamo K, Prince S, Tricco A, Connor-Gorber S, Tremblay M. A comparison of indirect versus direct measures for assessing physical activity in the pediatric population: A systematic review. International Journal of Pediatric Obesity. 2009;4:2–27. doi: 10.1080/17477160802315010. [DOI] [PubMed] [Google Scholar]