Abstract
Retinal ganglion cells transmit the visual signal from the retina to the brain. We have previously shown that the AP-2δ (TFAP2D) transcription factor is expressed in one-third of ganglion cells in developing retina suggesting a specialized role for these AP-2δ-expressing cells. Here, we address the role of AP-2δ in retina by in ovo electroporation of RCAS/AP-2δ retroviral constructs into the eyes of chick embryos at day 2 of gestation. Ectopic expression of AP-2δ does not affect lineage differentiation in the developing retina. However, immunostaining of retinal tissue with markers associated with axonal growth such as GAP43 and PSA-NCAM demonstrates axonal misrouting and abnormal axonal bundling. Treatment of AP-2δ-misexpressing retinal cell cultures with Endo-N, an enzyme that removes PSA from NCAM, decreases AP-2δ-induced axonal bundling. Our data suggest a role for AP-2δ in polysialylation of NCAM, with ectopic expression of AP-2δ resulting in premature bundling of emerging axons and misrouting of axons. We propose that expression of AP-2δ in a subset of ganglion cells contributes to the fine-tuning of axonal growth in the developing retina.
Keywords: AP-2delta, retinal ganglion cells, axon bundling, GAP43, PSA-NCAM
INTRODUCTION
Activator Protein 2 (AP-2) is a family of five transcription factors (AP-2α, β, γ, δ and ε) which play critical roles during development. AP-2α knock-out mice die around birth with severe defects in cranial and body wall closure and skeletal structures (Schorle et al. 1996, Zhang et al. 1996). AP-2β knock-out mice die perinatally due to patent ductus arteriosus, noradrenaline deficiency and/or massive apoptosis of renal tubular epithelia (Moser et al. 1997, Zhao et al. 2011, Hong et al. 2008). AP-2γ−/− mice die after gastrulation due to defective placenta development (Auman et al. 2002, Werling & Schorle 2002), whereas AP-2ε−/− mice show disorganization of the olfactory bulb (Feng et al. 2009).
AP-2δ (TFAP2D, AP2D) is the most divergent member of the AP-2 family. Of the eight residues in the transactivation domain deemed critical for AP-2 function, only three are conserved in AP-2δ (Wankhade et al. 2000, Li et al. 2008). The binding affinity of AP-2δ for consensus AP-2 regulatory elements is lower than that of other AP-2 proteins (Zhao et al. 2001). Furthermore, AP-2δ is the only member of the AP-2 family that does not retain neural crest inducing function in an AP-2-depleted background in zebrafish (Van Otterloo et al. 2012). In adult mouse brain, AP-2δ is expressed in the posterior midbrain, as well as in the cortex, dorsal thalamus and superior colliculus. The latter structure receives input from the eye and other sensory systems (Hesse et al. 2011). AP-2δ−/− mice are viable but lack part of the posterior midbrain due to increased apoptosis in this part of the brain starting at the end of embryogenesis (Hesse et al. 2011). Despite this loss, AP-2δ−/− mice appear to retain at least some higher auditory function, suggesting an alternate auditory route that allows response to individual tones (Hesse et al. 2011).
The vertebrate retina is derived from neuroectodermal progenitor cells that differentiate into six major classes of neurons (ganglion, amacrine, bipolar, horizontal, cone photoreceptors and rod photoreceptors) and one class of glial cells (Müller glia). These cells are distributed into three nuclear layers, with ganglion cells located in the innermost ganglion cell layer (GCL), photoreceptors in the outer nuclear layer (ONL), and the remaining cell types distributed in specific regions of the inner nuclear layer (INL). Visual information is conveyed to the brain via the only output neuron of the retina, the ganglion cells. These cells produce long axons that travel along the innermost retina (nerve fiber layer) towards the optic disc. Ganglion cell fibers exit the eye through the optic disc and form the optic nerve which projects to the brain via the optic chiasm. AP-2 transcription factors have specific distribution profiles in the retina. For example, AP-2α and AP-2β are expressed in the amacrine and horizontal cells of the developing chick and mammalian retina (Bisgrove & Godbout 1999, Bassett et al. 2007, Li et al. 2010), whereas AP-2δ protein is restricted to a subset of retinal ganglion cells (Li et al. 2008). At embryonic day 7 (E7) in chick retina, approximately one-third of retinal ganglion cells express AP-2δ. AP-2δ-positive ganglion cells are still present in the differentiated E15 retina, albeit in lower numbers (Li et al. 2008).
Here, we express AP-2δ in the chick retina by in ovo electroporation of a RCAS/GFP-AP-2δ retroviral expression construct. We show that misexpression of AP-2δ in the developing retina results in the production of ectopic bundles of fibers characterized by the expression of GAP43 and PSA-NCAM. As both GAP43 and PSA-NCAM have previously been associated with the growth and regrowth of axons, these results suggest a role for AP-2δ in the regulation of factors involved in axonal growth.
MATERIALS AND METHODS
Retrovirus constructs and chick embryo injection
The 5′ end of chicken AP-2δ cDNA (1 - 834 bp) was PCR-amplified and inserted into the pSlax12Nco-GFP shuttle vector (Morgan & Fekete 1996) at the EcoRI and PstI sites (pSlax12Nco-GFP-AP-2δN). The 3′ end of AP-2δ was PCR-amplified and inserted into pSlax12Nco-GFP-AP-2δN at the PstI site. A ClaI fragment containing either GFP or GFP-AP-2δ was then inserted into the ClaI site of the avian replication-competent retrovirus vector (RCASBP(B)) (Morgan & Fekete 1996). In ovo electroporation of RCASBP(B)-GFP and RCASBP(B)-GFP-AP-2δ DNA was carried out as previously described (Gao et al. 2010).
Fertilized eggs from White Leghorn chickens were obtained from the University of Alberta Farm poultry unit and incubated for ~40 h prior to in ovo electroporation. At Hamburger Hamilton (HH) stages 10 to 12 (E1.5 to E2), eggs were windowed and 1.5 ml albumin was removed. Approximately 1 μl of RCASBP(B) DNA (3 μg/μl) mixed with 0.1% of Fast Green (1 μl dye/15 μl DNA) was injected into the right optic vesicle using a PV820 pneumatic picopump (World Precision Instruments, Inc). Straight-tipped electrodes were spaced 2 mm apart at a 70°C angle to the longitudinal axis of the embryo such that the current path would pass through the posterior half of the right eye. Five square pulses of 15 V at 25 ms were applied at 950 ms intervals using a BTX Square Wave Pulse generator (ECM 830 electroporator). Embryos were harvested at E7, E8, E10 or E13, and screened for GFP expression by epifluorescence. Chick embryo research was carried out with institutional approval following the Canadian Council on Animal Care guidelines.
Western blot analysis
Retinal tissue from E9 embryos in ovo electroporated with GFP or GFP-AP-2δ was homogenized in RIPA buffer. Proteins were separated in 10% SDS-PAGE gel, transferred to a nitrocellulose membrane and AP-2δ was detected using rabbit anti-AP-2δ (1:1000 dilution) antibody. Immunoblotting with mouse anti-actin (1:100,000 dilution; Sigma) antibody served as the loading control. For analysis of GAP43 expression, retinal tissue from control and GFP-AP-2δ in ovo electroporated E9 embryos was homogenized in RIPA buffer. Proteins were separated in 10% SDS-PAGE gel, transferred to a nitrocellulose membrane and GAP43 was detected using mouse anti-GAP43 (1:1000 dilution; Sigma) antibody.
Immunofluorescence analysis of retinal tissue sections and dissociated retinal cells
Retinal tissue was fixed in 4% paraformaldehyde, cryoprotected in sucrose and embedded in OCT along the dorsal-ventral axis. Tissue sections (6 – 7 μm) were prepared at E7, E8, E9, E10 and E13. For dissociation experiments, retinal tissue was trypsinized and cultured for 24 h or 48 h on poly-D-lysine-coated coverslips. For cleavage of PSA from NCAM, dissociated retinal cultures were prepared from E7 chick embryos and cultured in the presence of 27 units/ml Endo-N for 48 h (Canger & Rutishauser 2004). Retinal tissue and dissociated cells were double-stained with goat anti-GFP antibody (1:1000 dilution; Abcam) and one of the following: mouse anti-GAP43 (1:2000 dilution) (7B10; Sigma), mouse anti-Brn3a (1:200 dilution; Chemicon), rabbit anti-AP-2β (1:400 dilution) (H87; Santa Cruz), mouse anti-glutamine synthetase (1:500 dilution; Transduction laboratories), sheep anti-CHX10 (1:3000 dilution) (obtained from Dr. Rod Bremner), mouse anti-CRX (1:2000; Abnova), mouse anti-neurofilament (1:5000 dilution) (RT-97; DSHB), mouse anti-PSA-NCAM (1:20 dilution) (5A5; DSHB), followed by secondary antibodies conjugated with Alexa 488 or Alexa 555. F-actin was labeled with phalloidin and DNA was labeled with DAPI. Images were captured using a Zeiss LSM710 confocal microscope. For three dimensional reconstructions, E7 and E13 chick retinas overexpressing either GFP or GFP-AP-2δ were dissected, flat-mounted and fixed in 4% paraformaldehyde followed by permeabilization in 0.2% Triton-X-100. Tissues were immunostained with anti-GFP and anti-GAP43 antibody followed by secondary antibody linked to Alexa 488 or Alexa 555. Coverslips were mounted with FluorSave reagent (Calbiochem). Image acquisition was performed at room temperature using Zeiss LSM710 confocal miscroscope with NA 1.3 Plan-Apochromat oil immersion lens. The signal was detected by photomultiplier tube. Images were acquired with ZEN 2011 software.
TUNEL assay
Apoptotic cells were stained using the In Situ Cell Death Detection kit, TMR red, following the manufacturer’s directions (Roche). Images were captured using a Zeiss LSM710 confocal microscope.
Statistical analysis
Statistical analysis was performed using the Student’s t-test with cell counts from three GFP and three GFP-AP-2δ in ovo electroporated eyes obtained from different embryos. A p-value of < 0.05 is considered significant.
RESULTS
Ectopic expression of AP-2δ disrupts the nuclear structure of the retina
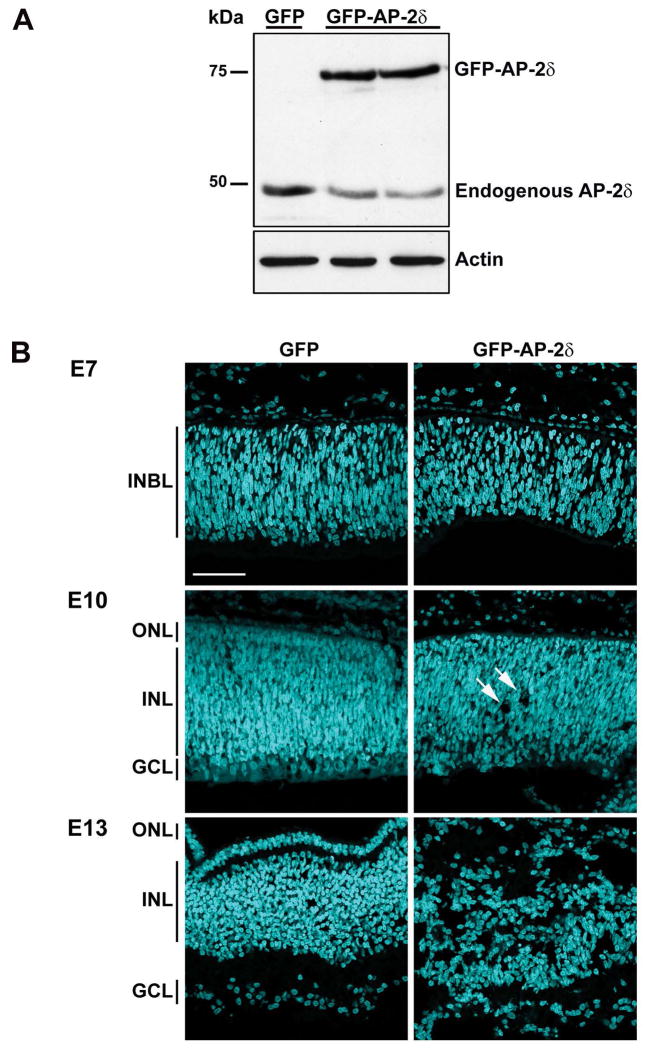
To investigate the role of AP-2δ in retinal ganglion cells, we inserted either GFP or GFP-AP-2δ cDNA into the RCAS retrovirus vector and in ovo electroporated RCAS/GFP or RCAS/GFP-AP-2δ into the eyes of E2 chick embryos. Western blot analysis shows that the GFP-AP-2δ fusion protein is expressed in in ovo electroporated retinal tissue (Fig. 1A). We then examined the effect of AP-2δ overexpression on the retinal structure by harvesting in ovo electroporated retinas at E7, E10 or E13 and staining the tissue with DAPI to label the nuclei. Because the retina differentiates along the central to peripheral, and dorsal to ventral gradients, we examined comparable GFP-positive regions in each retina (Fig. 1B). By E10, holes or gaps were observed in the GFP-AP-2δ-positive regions of the retina (see arrows). At E13, there was massive disruption of the nuclear structure of the retina in GFP-AP-2δ-positive regions.
Figure 1. Disruption of the layers of the retina upon overexpression of AP-2δ.
(A) Protein lysates from E9 embryos in ovo electroporated with either GFP or GFP-AP-2δ RCAS expression constructs (two different eyes for GFP-AP-2δ) were separated in a 10% SDS-PAGE gel and transferred to a nitrocellulose membrane. AP-2δ was detected with rabbit anti-AP-2δ antibody. (B) Retinal tissue sections from E7, E10 and E13 embryos in ovo electroporated with GFP or GFP-AP-2δ RCAS expression constructs were stained with DAPI to label the nuclei. INBL, inner neuroblastic layer; ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer. Photographs were taken with a 40X lens using a Zeiss LSM710 confocal microscope. Arrows indicate holes. Scale bar = 50 μm.
Ectopic expression of AP-2δ in retinal cells disrupts the structure of the retina. To determine whether AP-2δ-overexpressing cells are undergoing programmed cell death, we carried out the TUNEL assay on E7 and E10 retinas positive for either GFP or GFP-AP-2δ. Although increased programmed cell death was observed in GFP-AP-2δ-positive cells compared to GFP control, the majority of GFP-AP-2δ-positive cells were non-apoptotic (Fig. S1).
Ectopic expression of AP-2δ does not affect retinal cell differentiation
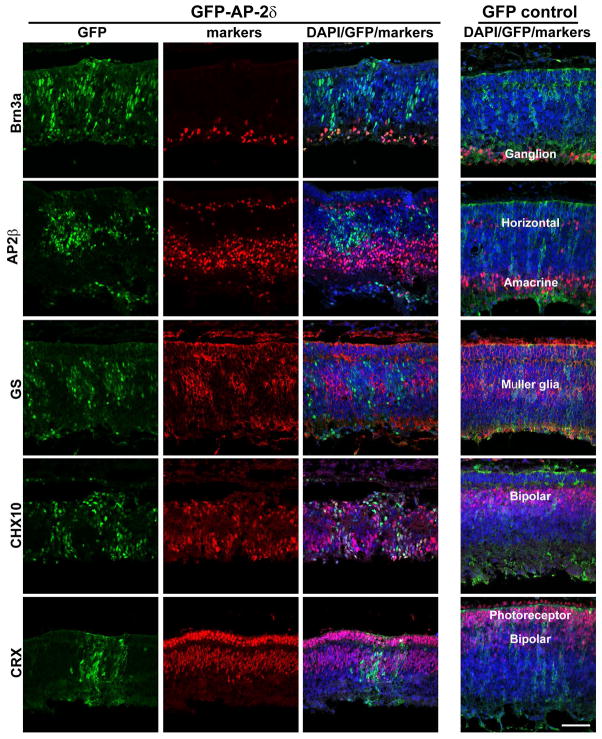
AP-2δ is normally restricted to a subset of ganglion cells found in the innermost layer of the retina. Retinal cells expressing GFP-AP-2δ are observed throughout the retinal layers. To determine the lineage of GFP-AP-2δ expressing cells, we immunostained E10 retinal sections with Brn3a, a well-characterized early marker of ganglion cells. Brn3a was primarily found in the retinal ganglion cell (RGC) layer of both RCAS/GFP and RCAS/GFP-AP-2δ-infected retinas (Fig. 2 – top panels). Thus, GFP-AP-2δ-positive cells outside the RGC layer do not appear to be misplaced ganglion cells.
Figure 2. Co-expression of lineage-specific markers and AP-2δ in E10 embryos.
Retinal tissue expressing either GFP or GFP-AP-2δ was double-stained with anti-GFP and anti-Brn3a, anti-AP-2β, anti-GS, anti-CHX10 or anti-CRX antibodies, followed by secondary antibodies linked to Alexa 488 or 555. Sections were counterstained with DAPI to label the nuclei. The location of specific retinal cell types is indicated in GFP control retinal tissue. Photographs were taken with a Zeiss LSM710 confocal microscope equipped with a 40X lens. Scale bar = 50 μm.
To further address the differentiation state of GFP-AP-2δ-positive cells residing outside the ganglion cell layer, we immunostained GFP- and GFP-AP-2δ-positive retinal tissue sections with markers specific to the different cell lineages of the retina: amacrine and horizontal cells (AP-2β), Müller glial cells (glutamine synthetase, GS), bipolar cells (CHX10) and photoreceptor cells [CRX; this marker is also weakly expressed in bipolar cells (Glubrecht et al. 2009)]. While expression of CHX10 and CRX was compatible with AP-2δ expression as indicated by co-labeling of GFP/CHX10 and GFP/CRX (Figs. 2 and S2), AP-2δ was excluded from GS-positive cells and rarely observed in AP-2β-positive cells (Fig. 2). Based on these results, AP-2δ expression does not interfere with neurogenesis and cell differentiation, at least in the case of bipolar and photoreceptor cells.
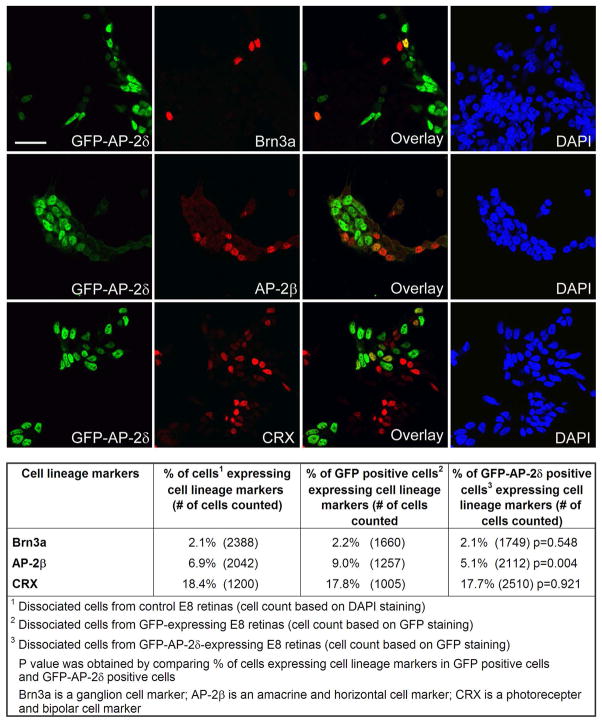
The percentage of GFP-AP-2δ-positive cells expressing specific retinal cell lineage markers was quantitated by dissociating retinal tissue from RCAS-GFP or RCAS-GFP-AP-2δ in ovo electroporated E8 embryos, as well as from non-electroporated E8 embryos, and culturing the cells for 24 h prior to immunostaining with Brn3a, AP-2β and CRX antibodies. Nuclei were stained with DAPI and GFP was detected by immunostaining with anti-GFP antibody. The percentages of Brn3a-positive (ganglion) and CRX-positive (photoreceptor and bipolar) cells were similar in non-electroporated control, GFP control and GFP-AP-2δ-positive cultures (Fig. 3). In contrast, fewer AP-2β-positive (amacrine and horizontal) cells were observed in GFP-AP-2δ-positive cultures compared to GFP and non-electroporated control cultures. These data are consistent with our in ovo observations and indicate that: (i) ectopic expression of GFP-AP-2δ does not promote differentiation along the ganglion cell lineage, and (ii) the majority of GFP-AP-2δ-positive cells are not ganglion cells.
Figure 3. Co-expression of lineage-specific markers and GFP-AP-2δ in dissociated cells from E8 retina.
E2 embryos were in ovo electroporated with a GFP-AP-2δ RCAS expression construct. Retinal cells were dissociated and immunostained with anti-GFP and anti-Brn3a, anti-AP-2β, or anti-CRX antibodies, followed by secondary antibodies linked to Alexa 488 or Alexa 555. Nuclei were labeled with DAPI. Scale bar = 25 μm. The table shows the percentage of DAPI-stained control cells, GFP-positive cells and GFP-AP-2δ-positive cells expressing the indicated cell lineage markers.
GFP-AP-2δ-positive cells form extended neurites in culture
As AP-2δ is normally expressed in ganglion cells, the output neurons of the retina, we examined whether AP-2δ-misexpressing cells can form the extended axonal fibers that are characteristic of ganglion cells. Dissociated retinal cells from GFP-AP-2δ-in ovo electroporated embryos were cultured for 24 h and immunostained with neuron-specific β-tubulin TUJ1 antibody. GFP-AP-2δ-positive cells formed extended TUJ1-positive fibers in culture, indicating that GFP-AP-2δ expression is compatible with formation of long axon-like fibers (Fig. S3A).
The lineage of the fiber-producing GFP-AP2δ-positive cells was identified by co-staining dissociated retinal cells with phalloidin (which detects filamentous actin found in axons) or TUJ1, and either ganglion cell markers (Brn3a and Islet1) or non-ganglion cell markers (CRX and AP-2β). As expected, GFP-AP-2δ-expressing cells that were positive for ganglion cell-specific markers formed long axonal-like fibers in culture (Fig. S3B). In contrast, GFP-AP-2δ-expressing cells that were positive for CRX and AP-2β did not form extended neurites in culture (data not shown).
Expression of GAP43 in GFP-AP-2δ-positive cells
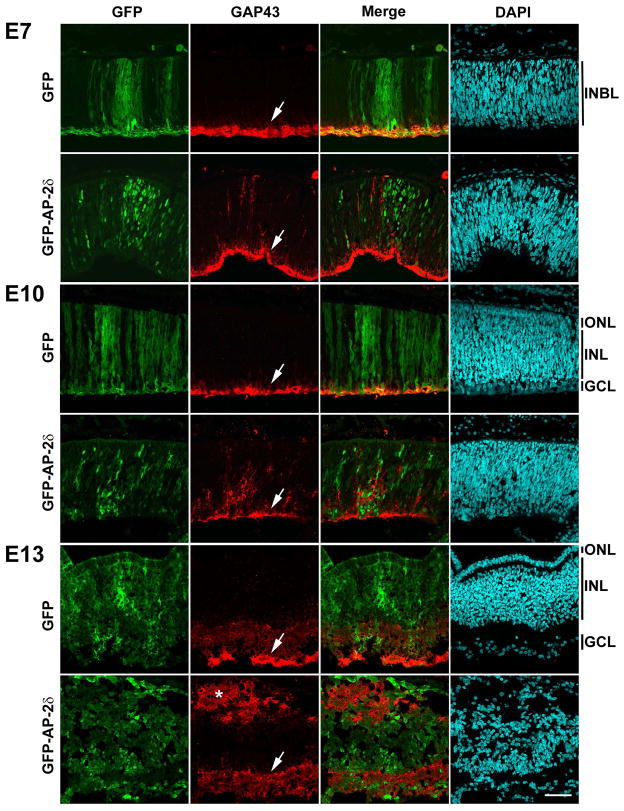
Next, we examined whether growth-associated protein 43 (GAP43), a marker of retinal ganglion cell axons, was induced upon AP-2δ misexpression. Western blot analysis showed similar levels of GAP43 in comparable regions of control and GFP-AP-2δ-positive E9 retinas (data not shown). To investigate GAP43 distribution in GFP-AP-2δ electroporated retinas, we carried out immunofluorescence analysis. A strong GAP43 signal was observed in the nerve fiber layer of GFP (control) retinas at E7, E10 and E13 (Fig. 4). Intriguingly, GAP43 immunoreactivity was also observed outside of the nerve fiber layer in retinas electroporated with GFP-AP-2δ RCAS retroviral expression constructs. At E7 and E10, these GAP43-positive fibers vertically spanned the GFP-positive regions of GFP-AP-2δ-electroporated retinas (Fig. 4). By E13, when there is significant structural disruption of the retinal layers, two parallel GAP43-positive layers were observed, one corresponding to the normal nerve fiber layer (indicated by arrow), the other located at the outer edge of the retina (indicated by asterisk) (Fig. 4). This second parallel fiber layer was not only found at sites that were proximal to GFP-AP-2δ positive cells, but at sites distal to GFP-AP-2δ-positive regions, indicating that ectopic axonal growth can span long distances.
Figure 4. Overexpression of AP-2δ induces axonal growth in developing chick retina.
Retinal tissue sections from E7, E10 and E13 embryos in ovo electroporated with GFP and GFP-AP-2δ RCAS expression constructs were immunostained with anti-GFP and anti-GAP43 antibodies followed by secondary antibody conjugated to Alexa 488 or Alexa 555. Nuclei were labeled with DAPI. Photographs were taken with a Zeiss LSM710 confocal microscope equipped with a 40X lens. The arrows point to the nerve fiber layer and the asterisk indicates the parallel fiber layer. Scale bar = 50 μm. Abbreviations: INBL, inner neuroblastic layer; ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer.
When dissecting RCAS-GFP-AP-2δ-injected eyes, we observed what appeared to be holes or blistering at the sites of GFP-AP-2δ positivity, indicating that the integrity of the retinal pigmented epithelium was compromised (Fig. 5A). Blistering was never observed in RCAS-GFP-control eyes. As others have reported ocular phenotypes caused by gene misexpression outside the eye (Bassett et al. 2010), we examined four GFP-AP-2δ-injected embryos for the presence of a GFP signal outside of the retina. The absence of a GFP signal in the non-retinal tissue of GFP-AP-2δ injected eyes (two eyes are shown in Fig. S4) suggests that the ocular phenotype observed in GFP-AP-2δ-injected embryos is caused by AP-2δ misexpression within the retina.
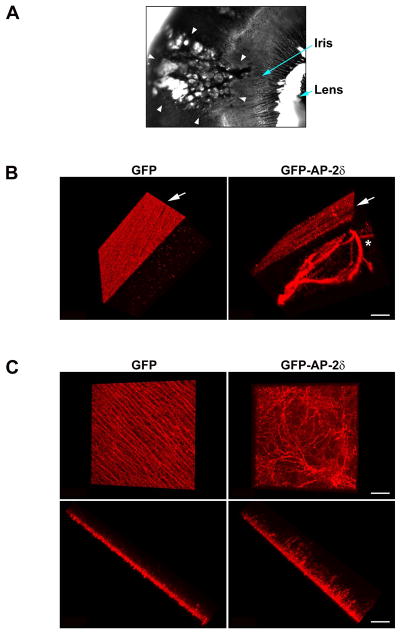
Figure 5. AP-2δ-overexpressing retinal cells form axonal bundles.
(A) An eye taken from an E13 GFP-AP-2δ-electroporated embryo was photographed under visible light using a 2.5X lens. The pock-marked GFP-positive region of the eye is indicated by the arrowheads. This pock-marked appearance was never observed in eyes electroporated with the GFP RCAS expression construct. (B) Retinal tissue from E13 embryos expressing GFP or GFP-AP-2δ were immunostained with anti-GAP43 antibody followed by secondary antibody linked to Alexa 555. Retina tissue was flat-mounted onto glass slides. Z-stack sections were generated at 0.98 μm intervals using a Zeiss LSM710 confocal microscope with 40X lens. Three dimensional structures were reconstructed using Imaris x64 software. One angle of the fibers (vertical view through the width of the flat-mount retina) is shown. Arrows point to the optic fibers generated from the ganglion cells. The asterisk indicates fibers generated from AP-2δ-overexpressing retinal cells. Scale bar = 40 μm. (C) Retinal tissue from E7 embryos expressing GFP or GFP-AP-2δ were immunostained as described in (B). Three dimensional structures were reconstructed as described in (B). Two angles are shown: horizontal view from the top of the flat-mount retina (top), and vertical view through the width of the flat-mount retina (bottom). Scale bar = 40 μm.
As AP-2δ overexpression leads to abnormal vertical fiber formation in the retina, it is possible that these clear patches represent sites of axonal outgrowth through the retina and retinal pigmented epithelium. In order to three-dimensionally visualize the location of GAP43-positive fibers in GFP-AP-2δ-positive retinas, we flat-mounted E13 retinal tissue from both RCAS-GFP (control) and RCAS-GFP-AP-2δ-infected eyes onto glass slides. A series of Z-stack sections at 0.98 μm intervals spanning all the layers of the retina were generated. One angle of the three-dimensionally reconstructed retina is shown in Fig. 5B. The well-ordered ganglion nerve fiber layer (red, indicated by arrow) which is characteristic of the normal differentiated retina can easily be visualized in control (GFP) eyes. In contrast, GFP-AP-2δ-positive eyes have both a nerve fiber layer (indicated by arrow), and a poorly-organized parallel layer characterized by the presence of thick coils of GAP43-positive fibers (indicated by asterisk). Of note, the density of fibers in the nerve fiber layer of GFP-AP-2δ-positive regions of the retina was often reduced compared to control retina, in keeping with results shown in Fig. 4.
To identify the origin of the ectopic fibers produced as the result of GFP-AP-2δ-expression, we carried out three dimensional reconstruction of the GAP43 signal using E7 retina, an early stage of development at which fibers can be easily detected. Significant disruption of the emerging fiber layer is already apparent at E7 in GFP-AP-2δ-positive regions of the retina (Fig. 5C). Furthermore, the majority, if not all, the fibers extending into the inner nuclear layer appear to originate in the ganglion cell layer (Fig. 5C).
Ectopic expression of AP-2δ induces polysialylation of NCAM
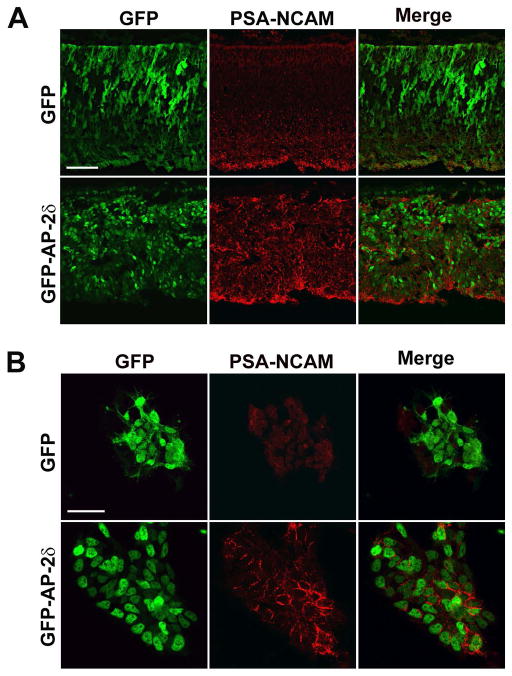
NCAM is a member of the immunoglobulin superfamily of adhesion molecules. Like GAP43, NCAM expression is closely associated with axonal outgrowth and regrowth (Anderson et al. 2005, Bates et al. 1999). Polysialic acid (PSA) is a large carbohydrate homopolymer that is almost exclusively found on NCAM. Polysialylation of NCAM increases cell motility and promotes axon growth, guidance and fasciculation by interfering with NCAM-protein interactions and reducing contact-dependent interactions between cells (Rutishauser & Landmesser 1996, Durbec & Cremer 2001, Rutishauser 2008). To investigate whether PSA-NCAM levels are induced as a consequence of GFP-AP-2δ expression, we immunostained E10 retina sections with an antibody that specifically recognizes the polysialylated form of NCAM. PSA-NCAM was primarily found in the ganglion cell layer of GFP (control) retina (Fig. 6A – top panels) as well as non-electroporated retina (data not shown). Induction of PSA-NCAM was observed in GFP-AP-2δ-positive regions of the retina (Fig. 6A – bottom panels). Dissociated cultures of GFP- and GFP-AP-2δ-positive retina clearly demonstrate induction of PSA-NCAM in GFP-AP-2δ-positive cells (Fig. 6B – bottom panels) with PSA reactivity primarily found at sites of cell-cell contacts as previously reported for dissociated subventricular zone neuroblasts in culture (Rockle et al. 2008).
Figure 6. Induction of PSA-NCAM as a consequence of ectopic expression of AP-2δ.
(A) Retinal tissue sections from E10 embryos in ovo electroporated with GFP and GFP-AP-2δ RCAS expression constructs were immunostained with anti-GFP and anti-PSA-NCAM antibodies. Scale bar = 50 μm. (B) Dissociated retinal cells from E8 embryos in ovo electroporated with GFP and GFP-AP-2δ were immunostained with anti-GFP and anti-PSA-NCAM antibodies. Scale bar = 25 μm.
We then carried out co-immunostaining of PSA-NCAM and GAP43 to investigate the extent to which these two axonal markers co-localize in GFP-AP-2δ-positive regions of the retina. Although PSA-NCAM and GAP43 were both ectopically expressed in GFP-AP-2δ-positive regions of the retina, PSA-NCAM distribution was more widespread than that of GAP43 (Fig. S5). These results suggest that GFP-AP-2δ-positive cells other than ganglion cells may be producing PSA-NCAM.
Cleavage of PSA by Endo-N reverses axonal bundling
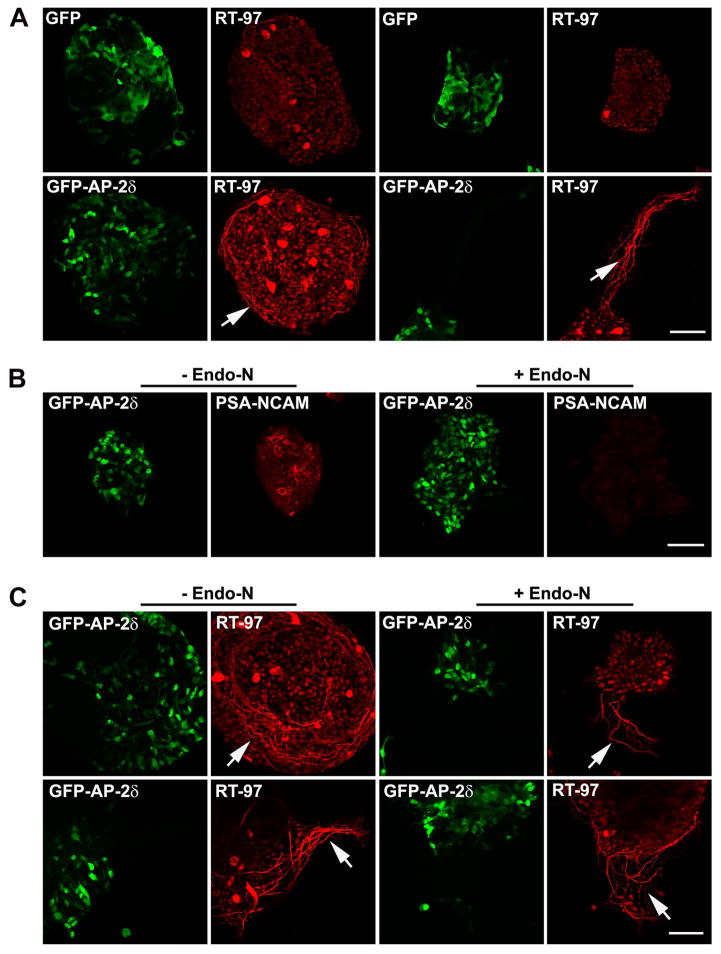
To further characterize the fibers in GFP-AP-2δ-positive regions of the retina, dissociated E7 GFP control and GFP-AP-2δ retinas were cultured for 48 h, and immunostained with RT-97, an antibody that recognizes a phosphoepitope in the C-terminal tail of neurofilament heavy chain. This phosphoepitope is associated with mature axons (Veeranna et al. 2008, Sanchez et al. 2000). A striking difference was observed between GFP (control) and GFP-AP-2δ dissociated retinal cultures upon immunostaining with RT-97, with the latter revealing either coils of fibers circling individual clumps of GFP-AP-2δ-positive cells (Fig. 7A – bottom left) or offshoots of bundled fibers emerging from clumps of GFP-AP-2δ-positive cells (Fig. 7A – bottom right). In contrast, few RT-97-positive fibers were observed in GFP control cultures (Fig. 7A – top).
Figure 7. Endo-N treatment relaxes the coils of fibers produced by GFP-AP-2δ-positive cells in vitro.
(A) E2 embryos were in ovo electroporated with GFP or GFP-AP-2δ expression constructs. E7 retinas were dissociated with trypsin and cultured for 48 h. Cells were immunostained with anti-GFP (green) and RT-97 (red) antibodies. Cultures from two separate experiments are shown. Scale bar = 50 μm. (B, C) E2 embryos were in ovo electroporated with GFP-AP-2δ expression construct. E7 retinas were dissociated with trypsin and cultured for 48 h in the presence of Endo-N. Cells were immunostained with anti-GFP (green) and anti-PSA-NCAM (red) (B) or RT-97 (red) (C) antibodies. Cultures from two separate experiments are shown in (C). Scale bar = 50 μm. The arrows point to the coils of RT-97-positive fibers.
The importance of PSA-NCAM in the production of the RT-97-positive fibers observed in GFP-AP-2δ cultures was investigated by treating retinal cultures with endoneuraminidase (Endo-N), an enzyme that cleaves PSA from PSA-NCAM (Canger & Rutishauser 2004). As shown in Fig. 7B, PSA was no longer detectable in GFP-AP-2δ-positive cultures treated with Endo-N. When Endo-N-treated cells were immunostained with RT-97, a general loosening-up of the bundles of axon-like fibers was noted (Fig. 7C arrows). These results suggest a role for PSA-NCAM in the formation of the ectopic axonal bundles observed in GFP-AP-2δ-positive retina.
DISCUSSION
AP-2δ is primarily expressed in midbrain, although it has also been detected at lower levels in other parts of the central nervous system (Zhao et al. 2003, Li et al. 2008). As predicted based on AP-2δ distribution patterns, the main structural defect found in AP-2δ−/− mice is absence of the colliculus inferior auditory center (Hesse et al. 2011). We have previously shown that AP-2δ is expressed in a small subset of retinal cells in the chick eye, with approximately one-third of retinal ganglion cells expressing AP-2δ from E7 to E10, peak stages of axonal growth (Li et al. 2008, Mey & Thanos 1991). Expression of AP-2δ in a subset of ganglion cells suggests specialized tasks for these cells that may not easily be identified using a gene knock-out or knock-down approach. For this reason, we carried out ectopic expression analyses in the easily accessible chick eye to gain insight into the role of AP-2δ in the developing retina. GFP (control) and GFP-AP-2δ retroviral expression constructs were in ovo electroporated into the optic vesicle (precursor to the eye) of chick embryos at embryonic day 2 and the retina examined at different stages of differentiation. Ectopic expression of AP-2δ in the retina revealed an important link between AP-2δ and molecules involved in axonal routing and bundling. Specifically, our data indicate that fibers characterized by the expression of GAP43 and PSA-NCAM are misrouted in the developing GFP-AP-2δ-positive retina. This misrouting is accompanied by the formation of thick bundles of axons that initially run perpendicular then parallel to the normal fiber layer.
A fundamental aspect of the retinal maturation process is the routing of ganglion cell axons along the innermost layer of the retina to the optic nerve. The first ganglion cells to differentiate in the retina establish the correct path to the optic nerve by interacting with molecules that inhibit and/or promote axon growth such as chondroitin sulfate proteoglycans and members of the Slit family (Snow & Letourneau 1992, Yu & Bargmann 2001, Oster et al. 2004). The importance of these molecules is demonstrated by the fact that their disruption leads to disorganization of axons in the retina (Jin et al. 2003, Brittis et al. 1992). Once the axon path has been established in the retina, newly emerging axons can fasciculate with previously established axons, thus facilitating basic pathfinding processes within the retina. Our results indicate that axon growth abnormalities and vertical growth of axons are readily apparent by E7, an early stage of retinal differentiation (Dutting et al. 1983), in GFP-AP-2δ-positive regions of the retina, suggesting that misexpression of AP-2δ affects the growth of pioneer axons which initially establish the correct path to the optic nerve.
NCAM and its polysialylated derivatives are steadily gaining stature as key molecules in central nervous system circuitry, including axon growth/guidance/fasciculation, synapse formation and synaptic plasticity (Hildebrandt et al. 2007, Rutishauser 2008, Durbec & Cremer 2001). PSA, a large negatively-charged carbohydrate homopolymer of α-2,8-sialic acid residues is almost exclusively found on NCAM. Because of its negative charge, PSA is believed to have repulsive properties. However, both PSA and PSA-NCAM have been shown to form filamentous bundles in vitro suggesting that PSA may have associative as well as repulsive properties (Toikka et al. 1998). In vivo studies indicate that PSA may increase or decrease fasciculation depending on the system being studied. For example, experiments using the chick hindlimb indicate that the increased number of sensory projections from dorsal root ganglia observed upon PSA removal may be caused by increased fasciculation resulting in sensory axons tracking closer to neighboring axons (Honig & Rutishauser 1996). Subsequent experiments using dorsal root explants have demonstrated that removal of PSA results in increased fasciculation of growth cones (Honig et al. 1998). In contrast, PSA removal has been associated with defasciculation in chick optic tract and tectum (Yin et al. 1995). Furthermore, removal of PSA caused defasciculation of neuronal processes in chick cochleo-vestibular ganglion-otocyst cultures as well as in rat primary cortical cultures (Hrynkow et al. 1998, Wakade et al. 2013). In retina, PSA-NCAM has been associated with promoting and directing retinal ganglion cell axonal growth (Monnier et al. 2001, Murphy et al. 2009).
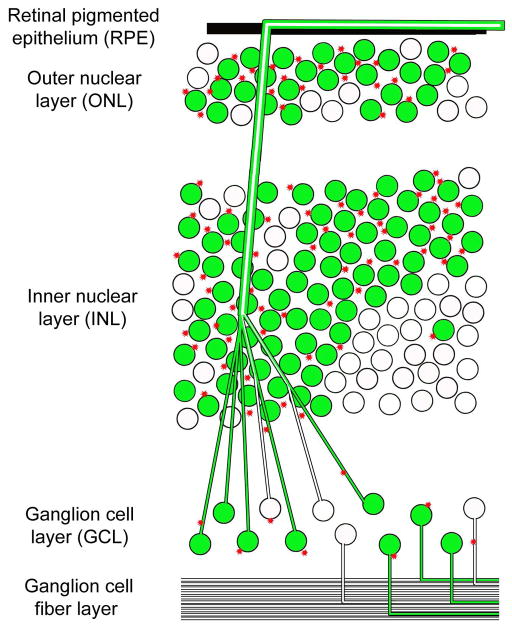
Knockout of NCAM in mice results in an increased number of retinal ganglion cells; however, NCAM−/− retinal ganglion cells can still project to their correct targets in brain (Murphy et al. 2007). The importance of NCAM polysialylation on axon growth in the mouse was revealed by germ-line disruption of two polysialyltransferases, ST8Sia2 and ST8Sia4, involved in polysialylation of NCAM (Weinhold et al. 2005). ST8Sia2−/−ST8Sia4−/− (polySia-ve) double knock-out mice die shortly after birth. These mice have undetectable PSA in brain, and show massive brain axon tract defects, including complete absence of the anterior commissure connecting the olfactory nuclei and temporal parts of the cortex (Weinhold et al. 2005, Galuska et al. 2006). Analysis of polySia-ve/NCAM+ve versus polySia-ve/NCAM-ve mouse brain axons reveals both hyperfasciculation and defasciculation defects (Schiff et al. 2011). As PSA is found on axons as well as surrounding structures, it has been postulated that absence of PSA may lead to competing axon-axon and axon-environment interactions (Schiff et al. 2011, Hrynkow et al. 1998). On the basis of these observations, we propose that excessive production of PSA by GFP-AP-2δ-positive non-ganglion cells alters axon-environment interactions, resulting in the increased axonal fasciculation and routing defects observed in our chick retina model (Fig. 8).
Figure 8. Model depicting axonal misrouting and bundling upon overexpression of GFP-AP-2δ in developing retina.
GFP-AP-2δ-positive (green) non-ganglion cells may produce substrate or signaling molecules (e.g. PSA-NCAM; indicated by asterisks) that cause misrouting and increased bundling of ganglion cell axons.
A consequence of misexpressing AP-2δ outside the ganglion cell layer during retinal development may be ectopic regulation of genes involved in the polysialylation of NCAM and/or axonal growth. A number of putative target genes have been identified for AP-2δ, including FGFR3 which is associated with neurite outgrowth and retinal ganglion cell survival in vitro (Kinkl et al. 2003, Tan et al. 2009) and Pou4f3 (Brn3c) which plays an essential role in the inner ear (Hesse et al. 2011). Known Pou4f3 target genes such as Bdnf and Calbindin2 are down-regulated in the midbrain of AP-2δ-deficient mice (Hesse et al. 2011). As Pou4f3 promotes ganglion cell differentiation in chick (Liu et al. 2000), and AP-2δ overexpression does not appear to affect cell lineage differentiation in the retina, it seems unlikely that Pou4f3 is an AP-2δ target gene in chick retina. In agreement with this, semi-quantitative RT-PCR analysis indicates that there is no difference in the levels of Pou4f3 RNA in GFP control versus GFP-AP-2δ-positive chick retina (Persad, A., Li, X and Godbout, R, unpublished data). Similarly, no significant differences were noted in FGFR3 RNA levels at E9 between GFP-electroporated and GFP-AP-2δ-electroporated chick retinas. These data suggest that neither FGFR3 nor Pou4f3 are targets of AP-2δ in developing chick retina. The identification of AP-2δ target genes in developing retina will be the subject of future studies.
Unlike fish, adult ganglion cells from mammalian and chicken retina do not regrow their axons after injury to the optic nerve. However, in the absence of their normal environment and/or under the correct permissive cues, axonal regeneration is possible (Weise et al. 2000, Koeberle & Bahr 2004). While the mechanisms underlying axonal regrowth remain poorly understood, regrowth is believed to be prevented by inhibitory molecules at the site of injury and/or lack of positive cues at the cut site (Charalambous et al. 2008). Of note, it has been postulated that the presence of GAP43 is required to overcome inhibitory signals during axonal outgrowth (Kusik et al. 2010, Erskine & Herrera 2007, Biffo et al. 1990). In support of this hypothesis, GAP43 is induced in the regenerating axons of fish and ectopic expression of GAP43 in mice can stimulate axonal regrowth (Korshunova & Mosevitsky 2010, Kaneda et al. 2008, Kaneda et al. 2010). Similarly, PSA-NCAM is induced in regenerating adult mouse ganglion cell axons in vitro (Bates et al. 1999), PSA-NCAM increases ganglion cell survival in mouse (Murphy et al 2009) and removal of PSA results in loss of ganglion cells and reduced axonal elongation in an in vitro chicken model system (Zhang et al. 1992). Induction of PSA-NCAM as a consequence of GFP-AP-2δ expression in chick retina, combined with the observed misrouting and bundling of axons, suggest a key role for AP-2δ in axonal growth and bundling.
In conclusion, our data indicate that ectopic expression of AP-2δ is sufficient to cause axon misrouting/bundling in the developing retina. We favor a model whereby ectopic fibers originating from either normal ganglion cells or GFP-AP-2δ-positive ganglion cells undergo premature fasciculation and misrouting as a consequence of increased PSA-NCAM levels in non-ganglionic layers of the developing retina. This model is based on the following observations: (i) the overall number of ganglion cells does not increase with ectopic expression of AP-2δ, with most ganglion cells remaining in the ganglion cell layer, (ii) the ectopic axons originate from the ganglion cell layer, and (iii) PSA-NCAM induction in GFP-AP-2δ-positive regions of the retina is more widespread than GAP43 expression, suggesting a possible role for PSA-NCAM is non-ganglion cells. Regardless of exact mechanism of action, AP-2δ may serve as a useful tool to re-direct axonal growth and/or promote fasciculation in diseased or injured retina.
Supplementary Material
Acknowledgments
We thank Drs. Urs Rutihauser and Rod Bremner for generously supplying Endo-N and anti-Chx10 antibody, respectively. We are grateful to Dr. Cairine Logan for teaching us the in ovo electroporation technique, and to Drs. Stephen Hughes, Bruce Morgan and Donna Fekete for sharing the pSLAX12 and RCAS constructs. We also thank Amit Persad for his contributions to the work, Dr. Xuejun Sun and Gerry Barron for their assistance with the 3-dimensional reconstruction of the retina, and Devon Germain and Dr. Lei Li for their comments regarding the manuscript. This work was supported by the Canadian Institutes of Health Research – Funding Reference Number 114993.
Abbreviations
- AP-2
Activator Protein 2
- E7
embryonic day 7
- Endo-N
endoneuraminidase
- GAP43
growth-associated protein 43
- GCL
ganglion cell layer
- GFP
green fluorescent protein
- GS
glutamine synthetase
- INBL
inner neuroblastic layer
- INL
inner nuclear layer
- NCAM
neural cell adhesion molecule
- ONL
outer nuclear layer
- PSA
polysialic acid
- RGC
retinal ganglion cells
Footnotes
The authors declare no conflicts of interest.
References
- Anderson AA, Kendal CE, Garcia-Maya M, Kenny AV, Morris-Triggs SA, Wu T, Reynolds R, Hohenester E, Saffell JL. A peptide from the first fibronectin domain of NCAM acts as an inverse agonist and stimulates FGF receptor activation, neurite outgrowth and survival. J Neurochem. 2005;95:570–583. doi: 10.1111/j.1471-4159.2005.03417.x. [DOI] [PubMed] [Google Scholar]
- Auman HJ, Nottoli T, Lakiza O, Winger Q, Donaldson S, Williams T. Transcription factor AP-2gamma is essential in the extra-embryonic lineages for early postimplantation development. Development. 2002;129:2733–2747. doi: 10.1242/dev.129.11.2733. [DOI] [PubMed] [Google Scholar]
- Bassett EA, Pontoriero GF, Feng W, Marquardt T, Fini ME, Williams T, West-Mays JA. Conditional deletion of activating protein 2alpha (AP-2alpha) in the developing retina demonstrates non-cell-autonomous roles for AP-2alpha in optic cup development. Mol Cell Biol. 2007;27:7497–7510. doi: 10.1128/MCB.00687-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett EA, Williams T, Zacharias AL, Gage PJ, Fuhrmann S, West-Mays JA. AP-2alpha knockout mice exhibit optic cup patterning defects and failure of optic stalk morphogenesis. Hum Mol Genet. 2010;19:1791–1804. doi: 10.1093/hmg/ddq060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates CA, Becker CG, Miotke JA, Meyer RL. Expression of polysialylated NCAM but not L1 or N-cadherin by regenerating adult mouse optic fibers in vitro. Exp Neurol. 1999;155:128–139. doi: 10.1006/exnr.1998.6972. [DOI] [PubMed] [Google Scholar]
- Biffo S, Verhaagen J, Schrama LH, Schotman P, Danho W, Margolis FL. B-50/GAP43 Expression Correlates with Process Outgrowth in the Embryonic Mouse Nervous System. Eur J Neurosci. 1990;2:487–499. doi: 10.1111/j.1460-9568.1990.tb00440.x. [DOI] [PubMed] [Google Scholar]
- Bisgrove DA, Godbout R. Differential expression of AP-2alpha and AP-2beta in the developing chick retina: repression of R-FABP promoter activity by AP-2. Dev Dyn. 1999;214:195–206. doi: 10.1002/(SICI)1097-0177(199903)214:3<195::AID-AJA3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Brittis PA, Canning DR, Silver J. Chondroitin sulfate as a regulator of neuronal patterning in the retina. Science. 1992;255:733–736. doi: 10.1126/science.1738848. [DOI] [PubMed] [Google Scholar]
- Canger AK, Rutishauser U. Alteration of neural tissue structure by expression of polysialic acid induced by viral delivery of PST polysialyltransferase. Glycobiology. 2004;14:83–93. doi: 10.1093/glycob/cwh014. [DOI] [PubMed] [Google Scholar]
- Charalambous P, Hurst LA, Thanos S. Engrafted chicken neural tube-derived stem cells support the innate propensity for axonal regeneration within the rat optic nerve. Invest Ophthalmol Vis Sci. 2008;49:3513–3524. doi: 10.1167/iovs.07-1473. [DOI] [PubMed] [Google Scholar]
- Durbec P, Cremer H. Revisiting the function of PSA-NCAM in the nervous system. Mol Neurobiol. 2001;24:53–64. doi: 10.1385/MN:24:1-3:053. [DOI] [PubMed] [Google Scholar]
- Dutting D, Gierer A, Hansmann G. Self-renewal of stem cells and differentiation of nerve cells in the developing chick retina. Brain Res. 1983;312:21–32. doi: 10.1016/0165-3806(83)90117-7. [DOI] [PubMed] [Google Scholar]
- Erskine L, Herrera E. The retinal ganglion cell axon’s journey: insights into molecular mechanisms of axon guidance. Dev Biol. 2007;308:1–14. doi: 10.1016/j.ydbio.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Feng W, Simoes-de-Souza F, Finger TE, Restrepo D, Williams T. Disorganized olfactory bulb lamination in mice deficient for transcription factor AP-2epsilon. Mol Cell Neurosci. 2009;42:161–171. doi: 10.1016/j.mcn.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galuska SP, Oltmann-Norden I, Geyer H, Weinhold B, Kuchelmeister K, Hildebrandt H, Gerardy-Schahn R, Geyer R, Muhlenhoff M. Polysialic acid profiles of mice expressing variant allelic combinations of the polysialyltransferases ST8SiaII and ST8SiaIV. J Biol Chem. 2006;281:31605–31615. doi: 10.1074/jbc.M606516200. [DOI] [PubMed] [Google Scholar]
- Gao Z, Monckton EA, Glubrecht DD, Logan C, Godbout R. The early isoform of disabled-1 functions independently of Reelin-mediated tyrosine phosphorylation in chick retina. Mol Cell Biol. 2010;30:4339–4353. doi: 10.1128/MCB.00545-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glubrecht DD, Kim JH, Russell L, Bamforth JS, Godbout R. Differential CRX and OTX2 expression in human retina and retinoblastoma. J Neurochem. 2009;111:250–263. doi: 10.1111/j.1471-4159.2009.06322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse K, Vaupel K, Kurt S, Buettner R, Kirfel J, Moser M. AP-2delta is a crucial transcriptional regulator of the posterior midbrain. PLoS One. 2011;6:e23483. doi: 10.1371/journal.pone.0023483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt H, Muhlenhoff M, Weinhold B, Gerardy-Schahn R. Dissecting polysialic acid and NCAM functions in brain development. J Neurochem. 2007;103(Suppl 1):56–64. doi: 10.1111/j.1471-4159.2007.04716.x. [DOI] [PubMed] [Google Scholar]
- Hong SJ, Lardaro T, Oh MS, Huh Y, Ding Y, Kang UJ, Kirfel J, Buettner R, Kim KS. Regulation of the noradrenaline neurotransmitter phenotype by the transcription factor AP-2beta. J Biol Chem. 2008;283:16860–16867. doi: 10.1074/jbc.M709106200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig MG, Petersen GG, Rutishauser US, Camilli SJ. In vitro studies of growth cone behavior support a role for fasciculation mediated by cell adhesion molecules in sensory axon guidance during development. Dev Biol. 1998;204:317–326. doi: 10.1006/dbio.1998.9093. [DOI] [PubMed] [Google Scholar]
- Honig MG, Rutishauser US. Changes in the segmental pattern of sensory neuron projections in the chick hindlimb under conditions of altered cell adhesion molecule function. Dev Biol. 1996;175:325–337. doi: 10.1006/dbio.1996.0118. [DOI] [PubMed] [Google Scholar]
- Hrynkow SH, Morest DK, Bilak M, Rutishauser U. Multiple roles of neural cell adhesion molecule, neural cell adhesion molecule-polysialic acid, and L1 adhesion molecules during sensory innervation of the otic epithelium in vitro. Neuroscience. 1998;87:423–437. doi: 10.1016/s0306-4522(98)00156-0. [DOI] [PubMed] [Google Scholar]
- Jin Z, Zhang J, Klar A, Chedotal A, Rao Y, Cepko CL, Bao ZZ. Irx4-mediated regulation of Slit1 expression contributes to the definition of early axonal paths inside the retina. Development. 2003;130:1037–1048. doi: 10.1242/dev.00326. [DOI] [PubMed] [Google Scholar]
- Kaneda M, Nagashima M, Mawatari K, Nunome T, Muramoto K, Sugitani K, Kato S. Growth-Associated Protein43 (GAP43) Is a Biochemical Marker for the Whole Period of Fish Optic Nerve Regeneration. Adv Exp Med Biol. 2010;664:97–104. doi: 10.1007/978-1-4419-1399-9_12. [DOI] [PubMed] [Google Scholar]
- Kaneda M, Nagashima M, Nunome T, et al. Changes of phospho-growth-associated protein 43 (phospho-GAP43) in the zebrafish retina after optic nerve injury: a long-term observation. Neurosci Res. 2008;61:281–288. doi: 10.1016/j.neures.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Kinkl N, Ruiz J, Vecino E, Frasson M, Sahel J, Hicks D. Possible involvement of a fibroblast growth factor 9 (FGF9)-FGF receptor-3-mediated pathway in adult pig retinal ganglion cell survival in vitro. Mol Cell Neurosci. 2003;23:39–53. doi: 10.1016/s1044-7431(03)00070-8. [DOI] [PubMed] [Google Scholar]
- Koeberle PD, Bahr M. Growth and guidance cues for regenerating axons: where have they gone? J Neurobiol. 2004;59:162–180. doi: 10.1002/neu.10345. [DOI] [PubMed] [Google Scholar]
- Korshunova I, Mosevitsky M. Role of the growth-associated protein GAP-43 in NCAM-mediated neurite outgrowth. Adv Exp Med Biol. 2010;663:169–182. doi: 10.1007/978-1-4419-1170-4_11. [DOI] [PubMed] [Google Scholar]
- Kusik BW, Hammond DR, Udvadia AJ. Transcriptional regulatory regions of gap43 needed in developing and regenerating retinal ganglion cells. Dev Dyn. 2010;239:482–495. doi: 10.1002/dvdy.22190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Glubrecht DD, Godbout R. AP2 transcription factor induces apoptosis in retinoblastoma cells. Genes Chromosomes Cancer. 2010;49:819–830. doi: 10.1002/gcc.20790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Glubrecht DD, Mita R, Godbout R. Expression of AP-2delta in the developing chick retina. Dev Dyn. 2008;237:3210–3221. doi: 10.1002/dvdy.21744. [DOI] [PubMed] [Google Scholar]
- Liu W, Khare SL, Liang X, Peters MA, Liu X, Cepko CL, Xiang M. All Brn3 genes can promote retinal ganglion cell differentiation in the chick. Development. 2000;127:3237–3247. doi: 10.1242/dev.127.15.3237. [DOI] [PubMed] [Google Scholar]
- Mey J, Thanos S. Ontogenetic changes in the regenerative ability of chick retinal ganglion cells as revealed by organ explants. Cell Tissue Res. 1991;264:347–355. doi: 10.1007/BF00313973. [DOI] [PubMed] [Google Scholar]
- Monnier PP, Beck SG, Bolz J, Henke-Fahle S. The polysialic acid moiety of the neural cell adhesion molecule is involved in intraretinal guidance of retinal ganglion cell axons. Dev Biol. 2001;229:1–14. doi: 10.1006/dbio.2000.9970. [DOI] [PubMed] [Google Scholar]
- Morgan BA, Fekete DM. Manipulating gene expression with replication-competent retroviruses. Methods Cell Biol. 1996;51:185–218. doi: 10.1016/s0091-679x(08)60629-9. [DOI] [PubMed] [Google Scholar]
- Moser M, Pscherer A, Roth C, et al. Enhanced apoptotic cell death of renal epithelial cells in mice lacking transcription factor AP-2beta. Genes Dev. 1997;11:1938–1948. doi: 10.1101/gad.11.15.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JA, Franklin TB, Rafuse VF, Clarke DB. The neural cell adhesion molecule is necessary for normal adult retinal ganglion cell number and survival. Mol Cell Neurosci. 2007;36:280–292. doi: 10.1016/j.mcn.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Oster SF, Deiner M, Birgbauer E, Sretavan DW. Ganglion cell axon pathfinding in the retina and optic nerve. Semin Cell Dev Biol. 2004;15:125–136. doi: 10.1016/j.semcdb.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Rockle I, Seidenfaden R, Weinhold B, Muhlenhoff M, Gerardy-Schahn R, Hildebrandt H. Polysialic acid controls NCAM-induced differentiation of neuronal precursors into calretinin-positive olfactory bulb interneurons. Dev Neurobiol. 2008;68:1170–1184. doi: 10.1002/dneu.20649. [DOI] [PubMed] [Google Scholar]
- Rutishauser U. Polysialic acid in the plasticity of the developing and adult vertebrate nervous system. Nat Rev Neurosci. 2008;9:26–35. doi: 10.1038/nrn2285. [DOI] [PubMed] [Google Scholar]
- Rutishauser U, Landmesser L. Polysialic acid in the vertebrate nervous system: a promoter of plasticity in cell-cell interactions. Trends Neurosci. 1996;19:422–427. doi: 10.1016/0166-2236(96)10041-2. [DOI] [PubMed] [Google Scholar]
- Sanchez I, Hassinger L, Sihag RK, Cleveland DW, Mohan P, Nixon RA. Local control of neurofilament accumulation during radial growth of myelinating axons in vivo. Selective role of site-specific phosphorylation. J Cell Biol. 2000;151:1013–1024. doi: 10.1083/jcb.151.5.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff M, Rockle I, Burkhardt H, Weinhold B, Hildebrandt H. Thalamocortical pathfinding defects precede degeneration of the reticular thalamic nucleus in polysialic acid-deficient mice. J Neurosci. 2011;31:1302–1312. doi: 10.1523/JNEUROSCI.5609-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorle H, Meier P, Buchert M, Jaenisch R, Mitchell PJ. Transcription factor AP-2 essential for cranial closure and craniofacial development. Nature. 1996;381:235–238. doi: 10.1038/381235a0. [DOI] [PubMed] [Google Scholar]
- Snow DM, Letourneau PC. Neurite outgrowth on a step gradient of chondroitin sulfate proteoglycan (CS-PG) J Neurobiol. 1992;23:322–336. doi: 10.1002/neu.480230311. [DOI] [PubMed] [Google Scholar]
- Tan CC, Walsh MJ, Gelb BD. Fgfr3 is a transcriptional target of Ap2delta and Ash2l-containing histone methyltransferase complexes. PLoS One. 2009;4:e8535. doi: 10.1371/journal.pone.0008535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toikka J, Aalto J, Hayrinen J, Pelliniemi LJ, Finne J. The polysialic acid units of the neural cell adhesion molecule N-CAM form filament bundle networks. J Biol Chem. 1998;273:28557–28559. doi: 10.1074/jbc.273.44.28557. [DOI] [PubMed] [Google Scholar]
- Van Otterloo E, Li W, Garnett A, Cattell M, Medeiros DM, Cornell RA. Novel Tfap2-mediated control of soxE expression facilitated the evolutionary emergence of the neural crest. Development. 2012;139:720–730. doi: 10.1242/dev.071308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeranna, Lee JH, Pareek TK, et al. Neurofilament tail phosphorylation: identity of the RT-97 phosphoepitope and regulation in neurons by cross-talk among proline-directed kinases. J Neurochem. 2008;107:35–49. doi: 10.1111/j.1471-4159.2008.05547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakade CG, Mehta SH, Maeda M, Webb RC, Chiu FC. Axonal fasciculation and the role of polysialic acid-neural cell adhesion molecule in rat cortical neurons. J Neurosci Res. 2013;91:1408–1418. doi: 10.1002/jnr.23268. [DOI] [PubMed] [Google Scholar]
- Wankhade S, Yu Y, Weinberg J, Tainsky MA, Kannan P. Characterization of the activation domains of AP-2 family transcription factors. J Biol Chem. 2000;275:29701–29708. doi: 10.1074/jbc.M000931200. [DOI] [PubMed] [Google Scholar]
- Weinhold B, Seidenfaden R, Rockle I, Muhlenhoff M, Schertzinger F, Conzelmann S, Marth JD, Gerardy-Schahn R, Hildebrandt H. Genetic ablation of polysialic acid causes severe neurodevelopmental defects rescued by deletion of the neural cell adhesion molecule. J Biol Chem. 2005;280:42971–42977. doi: 10.1074/jbc.M511097200. [DOI] [PubMed] [Google Scholar]
- Weise J, Ankerhold R, Bahr M. Degeneration and regeneration of ganglion cell axons. Microsc Res Tech. 2000;48:55–62. doi: 10.1002/(SICI)1097-0029(20000115)48:2<55::AID-JEMT1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Werling U, Schorle H. Transcription factor gene AP-2 gamma essential for early murine development. Mol Cell Biol. 2002;22:3149–3156. doi: 10.1128/MCB.22.9.3149-3156.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, Watanabe M, Rutishauser U. Effect of polysialic acid on the behavior of retinal ganglion cell axons during growth into the optic tract and tectum. Development. 1995;121:3439–3446. doi: 10.1242/dev.121.10.3439. [DOI] [PubMed] [Google Scholar]
- Yu TW, Bargmann CI. Dynamic regulation of axon guidance. Nat Neurosci. 2001;4(Suppl):1169–1176. doi: 10.1038/nn748. [DOI] [PubMed] [Google Scholar]
- Zhang H, Miller RH, Rutishauser U. Polysialic acid is required for optimal growth of axons on a neuronal substrate. J Neurosci. 1992;12:3107–3114. doi: 10.1523/JNEUROSCI.12-08-03107.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Hagopian-Donaldson S, Serbedzija G, Elsemore J, Plehn-Dujowich D, McMahon AP, Flavell RA, Williams T. Neural tube, skeletal and body wall defects in mice lacking transcription factor AP-2. Nature. 1996;381:238–241. doi: 10.1038/381238a0. [DOI] [PubMed] [Google Scholar]
- Zhao F, Bosserhoff AK, Buettner R, Moser M. A heart-hand syndrome gene: Tfap2b plays a critical role in the development and remodeling of mouse ductus arteriosus and limb patterning. PLoS One. 2011;6:e22908. doi: 10.1371/journal.pone.0022908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Lufkin T, Gelb BD. Expression of Tfap2d, the gene encoding the transcription factor Ap-2 delta, during mouse embryogenesis. Gene Expr Patterns. 2003;3:213–217. doi: 10.1016/s1567-133x(02)00067-4. [DOI] [PubMed] [Google Scholar]
- Zhao F, Satoda M, Licht JD, Hayashizaki Y, Gelb BD. Cloning and characterization of a novel mouse AP-2 transcription factor, AP-2delta, with unique DNA binding and transactivation properties. J Biol Chem. 2001;276:40755–40760. doi: 10.1074/jbc.M106284200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.