Supplemental digital content is available in the text.
Key words/Abbreviations: reaction time, anxiety, depression, allostatic load, GHQ = General Health Questionnaire, HADS = Hospital Anxiety and Depression Scale, HbA1c = glycosolated hemoglobin, HDL = high-density lipoprotein, SD = standard deviation
ABSTRACT
Objective
To examine the relation between reaction time in adolescence and subsequent symptoms of anxiety and depression and investigate the mediating role of sociodemographic measures, health behaviors, and allostatic load.
Methods
Participants were 705 members of the West of Scotland Twenty-07 Study. Choice reaction time was measured at age 16. At age 36 years, anxiety and depression were assessed with the 12-item General Health Questionnaire (GHQ) and the Hospital Anxiety and Depression Scale (HADS), and measurements were made of blood pressure, pulse rate, waist-to-hip ratio, and total and high-density lipoprotein cholesterol, C-reactive protein, albumin, and glycosolated hemoglobin from which allostatic load was calculated.
Results
In unadjusted models, longer choice reaction time at age 16 years was positively associated with symptoms of anxiety and depression at age 36 years: for a standard deviation increment in choice reaction time, regression coefficients (95% confidence intervals) for logged GHQ score, and square-root–transformed HADS anxiety and depression scores were 0.048 (0.016–0.080), 0.064 (0.009–0.118), and 0.097 (0.032–0.163) respectively. Adjustment for sex, parental social class, GHQ score at age 16 years, health behaviors at age 36 years and allostatic load had little attenuating effect on the association between reaction time and GHQ score, but weakened those between reaction time and the HADS subscales. Part of the effect of reaction time on depression was mediated through allostatic load; this mediating role was of borderline significance after adjustment.
Conclusions
Adolescents with slower processing speed may be at increased risk for anxiety and depression. Cumulative allostatic load may partially mediate the relation between processing speed and depression.
INTRODUCTION
There is evidence from several cross-sectional studies of adults that depression is often accompanied by less efficient cognitive function, as indicated by slower speed of information processing. People diagnosed as having major depression and those who report symptoms of depression have been shown to perform less well on a range of measures of processing speed, such as the Processing Speed Index of the Wechsler Adult Intelligence Scale-III (1), the Trailmaking Test (2), the Stroop Color-Word Test (3), inspection time (4), and reaction time (5). Slowed processing speed has also been found in depressed children (6). Whether the presence of anxiety is accompanied by slower processing speed has been much less studied, but the few investigations of people with various types of anxiety disorder—all based on small samples—have found no evidence of this (7,8).
It is unclear from these studies whether the slower processing speed observed in people with depression was an effect of the disorder or whether it preceded the onset of illness and was in fact a risk factor for it. Findings that people who have recovered from an episode of depression tend to have slower processing speed than healthy controls suggests the deficit might be trait dependent rather than state dependent (2,9), but evidence from longitudinal studies is needed to establish whether slower processing speed is a risk factor for onset of depression. To our knowledge, there have been no such studies to date. There is, however, a body of longitudinal evidence that lower scores on tests of intelligence or general cognitive ability in youth are a risk factor for later diagnosis with depression or anxiety and for reporting symptoms of these disorders (10–15).
Scores on tests of intelligence are moderately highly correlated with reaction time and scores on other measures of processing speed, such that people with higher intelligence tend to process information faster (16–19). It is therefore plausible that scores on a measure of processing speed in youth may be predictive of depression and anxiety later in life.
The concept of allostatic load may provide a potential biological mechanism for understanding any links between processing speed in youth and later mental health. Allostasis refers to the long-term functional changes that take place in physiological systems to maintain stability in the face of stressors (20). Such adaptations, which may affect the operating range of biological systems, are protective in the short-term but can come at a cost in terms of increased risk of morbidity or death (21). This cost, termed allostatic load by McEwen and Stellar (22), may result in maladaptive stress responding (23).
Slower processing speed and its links to lower intelligence may lead to increased stress and difficulties responding to adversity earlier in life—increasing the burden of allostatic load. This can lead to a vicious circle in that prolonged elevated allostatic load can adversely affect neurological processes, particularly in the prefrontal cortex and hippocampus (23,24). These brain regions are important for cognitive functioning, including processing speed, and neurobiological impairments in these regions have also been implicated in mental disorders. Evidence in 17-year-olds has shown that increased allostatic load is associated with cognitive deficits in the form of poorer working memory (25). Conversely, higher ability may increase the likelihood of entry into a healthy and stimulating environment that, in turn, offsets increases in allostatic load. This also increases the likelihood of making healthy life-style choices, which again reduce allostatic load. Animal models suggest that the psychological sequelae of high allostatic load may include depression and anxiety (26), but evidence for this in humans is still relatively limited (27). It is possible that any link in humans between processing speed and subsequent symptoms of depression and anxiety might be mediated through allostatic load.
We used data from the population-based West of Scotland Twenty-07 Study to investigate the prospective relationship between processing speed, as measured by reaction time, at around age 16 years and symptoms of depression and anxiety 20 years later, and to explore the potential mediating role of cumulative allostatic load.
METHODS
Participants
The Twenty-07 Study was established in the West of Scotland in 1986 to investigate longitudinally the processes producing or maintaining inequalities in health (28,29). It consists of three age cohorts, born around 1932, 1952, and 1972, members of which were randomly selected from the Central Clydeside Conurbation. Comparison of these cohorts with an equivalent sample from the United Kingdom's 1991 Census Samples of Anonymised Records revealed no significant differences in terms of sex, social class, car ownership, or household tenure (30). Baseline interviews (Wave 1) were carried out in 1987/1988, and the most recent wave of data collection (Wave 5) took place in 2007/2008. In the current study, we use data collected at Wave 1 and Wave 5 on the 1972-born cohort. We restricted our study to this youngest cohort as levels of anxiety and depression at Wave 1 when the cohort was aged approximately 16 years were low; therefore, there was less likelihood that any association we found between reaction time at that age and subsequent symptoms of anxiety or depression would be due to reverse causation. The age of this cohort also made it unlikely that somatic illness at Wave 1 would have affected both reaction time and propensity to anxiety or depression.
Ethical approval for Wave 1 was granted in 1986 by the ethics subcommittee of the West of Scotland Area Medical Committees and the GP Sub-Committee of Greater Glasgow Health Board. Wave 5 was approved by Tayside Committee on Medical Research Ethics A. Written consent to participate was obtained from parents at Wave 1 (when participants were aged 16 years) and from the participants at Wave 5.
Measures
Reaction Time
Four-choice reaction time was measured at Wave 1 with a portable device designed for the UK Health and Lifestyle Survey (31), as has been described previously (16). The participant rested the second and third fingers of the left and right hands on keys marked 1, 2, 3, and 4, respectively. When a number (between 1 and 4) appeared on the liquid crystal display screen, the participant attempted to press the correct key as quickly as possible. There were eight practice trials and 40 test trials, and an interstimulus interval that varied between 1 and 3 seconds. The mean and standard deviation (SD) of correct and incorrect trials were calculated separately. The current analyses are based on the mean of the correct trials.
Symptoms of Anxiety and Depression
Symptoms of anxiety and depression were assessed at Wave 5 using the 12-item General Health Questionnaire (GHQ) (32) and the Hospital Anxiety and Depression Scale (HADS) (33). The latter has two subscales each of seven items. Both these self-completion questionnaires measure the common mental health problems of anxiety and depression. The GHQ items include four response options (0–3), giving a total score that ranges from 0 to 36. Higher scores indicate more severe distress.(34) The HADS items include four response options (0–3), giving a total score for each subscale ranging from 0 to 21, with higher scores indicating more severe symptoms (35).
Allostatic Load
We used data from Wave 5 on nine biomarkers representing different contributing factors to allostatic load, namely, C-reactive protein, glycosolated hemoglobin, albumin, total and high-density lipoprotein (HDL) cholesterol, systolic and diastolic blood pressure, pulse rate, and waist-to-hip ratio. Nonfasting venous blood samples were collected and assayed for C-reactive protein (using latex-enhanced turbidimetry), glycosylated hemoglobin (HBA1c; using Menarini method), albumin (using cholesterol oxidase), and total and HDL cholesterol (using the accelerator selective detergent method) at the Glasgow Royal Infirmary. The coefficient of variation for each of the assays was as follows: C-reactive protein, ≤6%; glycosolated hemoglobin, ≤1%; albumin, ≤3.8%; and total cholesterol, ≤3%. Systolic and diastolic blood pressure and pulse rate were measured using an Omron HEM-705CP automated oscillometric device. Waist and hip circumferences were measured by a trained nurse using a standard protocol, and their ratio subsequently calculated. Participants provided information about current medication. For the small number of participants who were currently using antihypertensive medication (of our analytical sample, n = 12), diabetes medication (n = 5), statins (n = 11), diuretics (n = 2), or β-blockers (n = 5), values of systolic and diastolic blood pressure, total and HDL cholesterol, and C-reactive protein were adjusted to take account of the effect of the medication as follows. Systolic and diastolic blood pressures were increased by 10 and 5 mm Hg, respectively, in those on antihypertensive medication; total cholesterol was increased by 1.8 mM in those on statins and reduced by 4% in those on diuretics (36,37); HDL cholesterol was increased by 10% in those on β-blockers (37); HbA1c was increased by 1% in those on medication for diabetes (38); and C-reactive protein by increased by 0.02 mg/dl in those on statins (39). Participants who reported having an operation or accident within the last month were excluded from analyses.
There remains much debate about the best way to operationalize allostatic load (27). Here, we calculated SD scores (zero mean, unit SD) for each component of allostatic load (systolic and diastolic blood pressures, pulse rate, total and HDL cholesterol, albumin, HbA1c, C-reactive protein, and waist-to-hip ratio) for men and women separately, and the sum of these SD scores was taken as a measure of allostatic load. Table S1 (Supplemental Digital Content 1, http://links.lww.com/PSYMED/A211) shows mean (SD) or median (interquartile range) values of allostatic load and each of its components and the correlations between these variables in the sample on which our analysis is based.
Other Covariates
Symptoms of anxiety and depression at Wave 1 were assessed using the 12-item GHQ. Parental occupational social class was based on the father's current or previous job or, if no father was present, the mother's current or previous job at Wave 1, classified in six categories (professional, managerial/technical, skilled nonmanual, skilled manual, partly skilled, unskilled) (40). We regarded GHQ score at Wave 1 and parental social class as potential confounding factors. Educational attainment by Wave 5 was based on years of full-time education. Participants also provided information at Wave 5 on their smoking status (never, ex-smoker, current smoker), their current alcohol consumption (units of alcohol per week), and their physical activity (number of brisk walks undertaken in an average week). We regarded educational attainment and health behaviors as potential mediating factors. Health-related behaviors such as smoking, alcohol intake, and physical activity are likely to contribute to allostatic load by altering its biomarkers.
Statistical Methods
We used Pearson correlation coefficients to examine the relation between choice reaction time at age 16 years, the measures of mental health at age 36 years, and the covariates. To allow us to adjust for the covariates, we then used linear regression to examine the relation between choice reaction time and the mental health outcomes. The distribution of scores for all three mental health outcomes was positively skewed. To convert scores to a nearer-normal distribution, we used a logarithmic transformation for the GHQ-12 scores and a square-root transformation for the HADS anxiety and depression scores. We used these transformed scores as the dependent variables in the linear regression analyses. Because some studies show a nonlinear relation between alcohol intake and anxiety and depression, we created a categorical variable for the regression analyses, defining participants as abstainers (no alcohol), or drinkers within or above sex-specific recommended weekly limits (≤21 versus 22+ units for men, ≤14 versus 15+ units for women) (41). We used the Sobel-Goodman mediation tests in STATA version 12 to examine the extent to which mediating variables carried the influence of choice reaction time at age 16 years to mental health outcomes at age 36 years.
There were 1515 participants at Wave 1, of whom 942 (62%) took part in Wave 5. Nonrespondents at Wave 5 had a slightly longer mean choice reaction time (575 versus 563) at Wave 1 than respondents (p = .020), but there was no difference between these groups in Wave 1 GHQ-12 score (p = .35). The analyses that follow are based on 705 participants with complete data on choice reaction time, mental health outcomes, and the covariates.
RESULTS
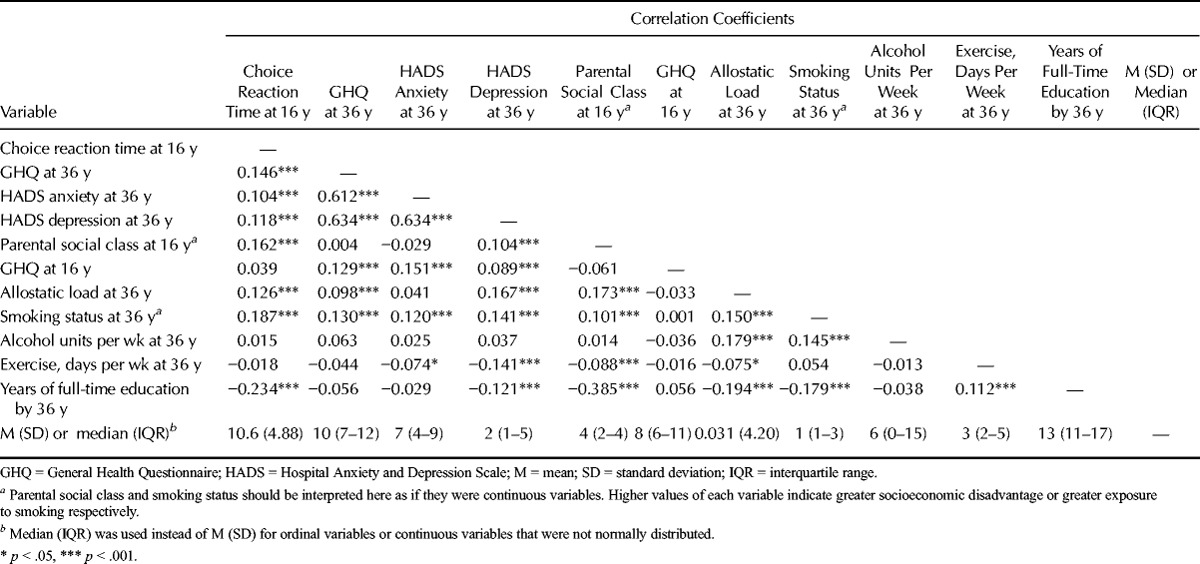
At the time of the Wave 5 follow-up, the study participants (54% female) were aged 36.6 (SD = 0.42) years. Table 1 shows the correlations among choice reaction time at age 16 years, scores on the GHQ, HADS anxiety and HADS depression scales at follow-up, allostatic load at follow-up, and the covariates. Longer choice reaction time—in other words, slower processing speed—at age 16 years was associated with greater parental socioeconomic disadvantage (r = 0.162), poorer mental health as indicated by higher scores on the GHQ and HADS anxiety and depression scales at age 36 years (r = 0.146, 0.104, and 0.118), higher allostatic load at age 36 (r = 0.128), fewer years of full-time education (r = −0.234), and greater likelihood of ever having smoked (r = 0.187). Greater allostatic load was associated with greater parental socioeconomic disadvantage, fewer years of full-time education, higher scores on the GHQ and HADS depression scales, and poorer health behavior in terms of smoking, alcohol consumption, and exercise. Allostatic load was also positively correlated with scores on the HADS anxiety subscale, but this association was not statistically significant. Table S2 (Supplemental Digital Content 2, http://links.lww.com/PSYMED/A212) shows correlations of allostatic load and its components with choice reaction time at age 16 years, GHQ score at age 16 years, GHQ score at age 36 years, and HADS anxiety and depression scores at age 36 years. The size of the correlations between each of the allostatic load components and reaction time, GHQ, or HADS scores varied.)
TABLE 1.
Pearson Correlations Among Choice Reaction Time at Age 16 Years, GHQ and HADS Scores at Age 36 Years, and the Covariates
The associations between choice reaction time at 16 years and logged GHQ score, and square-root-transformed HADS anxiety score and HADS depression score at 36 years did not differ significantly by sex. In the case of logged GHQ score, the coefficient for the interaction term was −0.028 (p = .38). In the case of square-root–transformed HADS anxiety or HAD depression scores, the coefficients for the interaction terms were −0.022 (p = .70) and −0.099 (p = .14), respectively. Hence, in each case, the strength of the association between reaction time and the mental health measure was slightly stronger in women than in men, but none of these differences were statistically significant. In the linear regression analyses, we therefore pooled data for men and women.
Table 2 shows the results of linear regression analyses of the relation between choice reaction time at age 16 years, expressed as SD scores, and logged GHQ scores at age 36 years. In the unadjusted Model 1, 1-SD longer choice reaction time was associated with a 5% (95% confidence interval [CI] = 2%∓8%) higher GHQ score. This model explained 1% of the variance in GHQ score. Adjustment for sex, GHQ score at age 16 years, parental socioeconomic status, years of full-time education (Model 2), then in addition for health behaviors at age 36 years (Model 3), and then allostatic load at age 36 years (Model 4) had negligible effects on this association. Adding the covariates did little to increase the predictive power of the initial model: the model containing all the covariates explained the most variance in GHQ score at 4.1%. The results of Sobel-Goodman mediation tests confirmed that education, allostatic load, and the health behaviors had no significant mediating effects.
TABLE 2.
The Association Between Choice Reaction Time at Age 16 Years With GHQ Scores at Age 36 Years (Unadjusted and Adjusted Models)
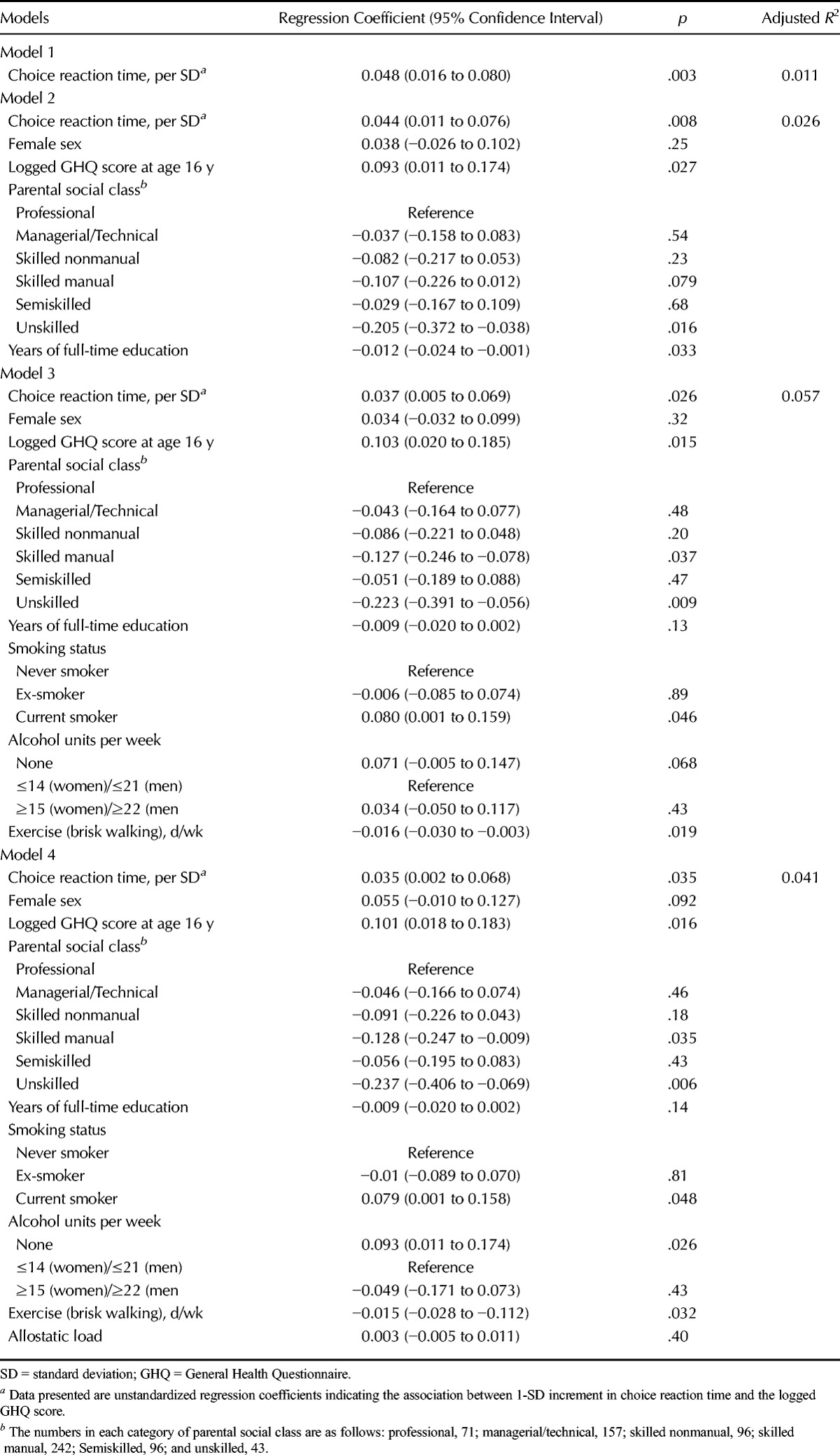
Table 3 shows the results of linear regression analyses of the relation between choice reaction time at age 16 years, expressed as SD scores, and HADS anxiety scores at age 36 years. In the unadjusted Model 1, 1-SD longer choice reaction time at age 16 years was associated with an increase in the square-root–transformed HADS anxiety score at age 36 years of 0.06 (95% CI = 0.01−0.12). This model explained 0.6% of the variance in the HADS anxiety score. Adjustment for sex, GHQ score at age 16 years, parental social class, and years of full-time education (Model 2) had little attenuating effect on this association. Further adjustment for health behaviors (Model 3) weakened the association, and it became of borderline significance. Additional adjustment for allostatic load (Model 4) had only slight attenuating effects. Model 3 explained the largest proportion of the variance, at 5.9%. Results of Sobel-Goodman mediation tests showed that of the health behaviors, only current smoking status had a significant mediating effect (p = .038). Of the total effect of reaction time at age 16 years on HADS anxiety score at age 36 years, 17% was mediated through smoking status.
TABLE 3.
The Association Between Choice Reaction Time at Age 16 Years With the Hospital Anxiety and Depression Scale Anxiety Subscale Score At Age 36 Years (Unadjusted and Adjusted Models)
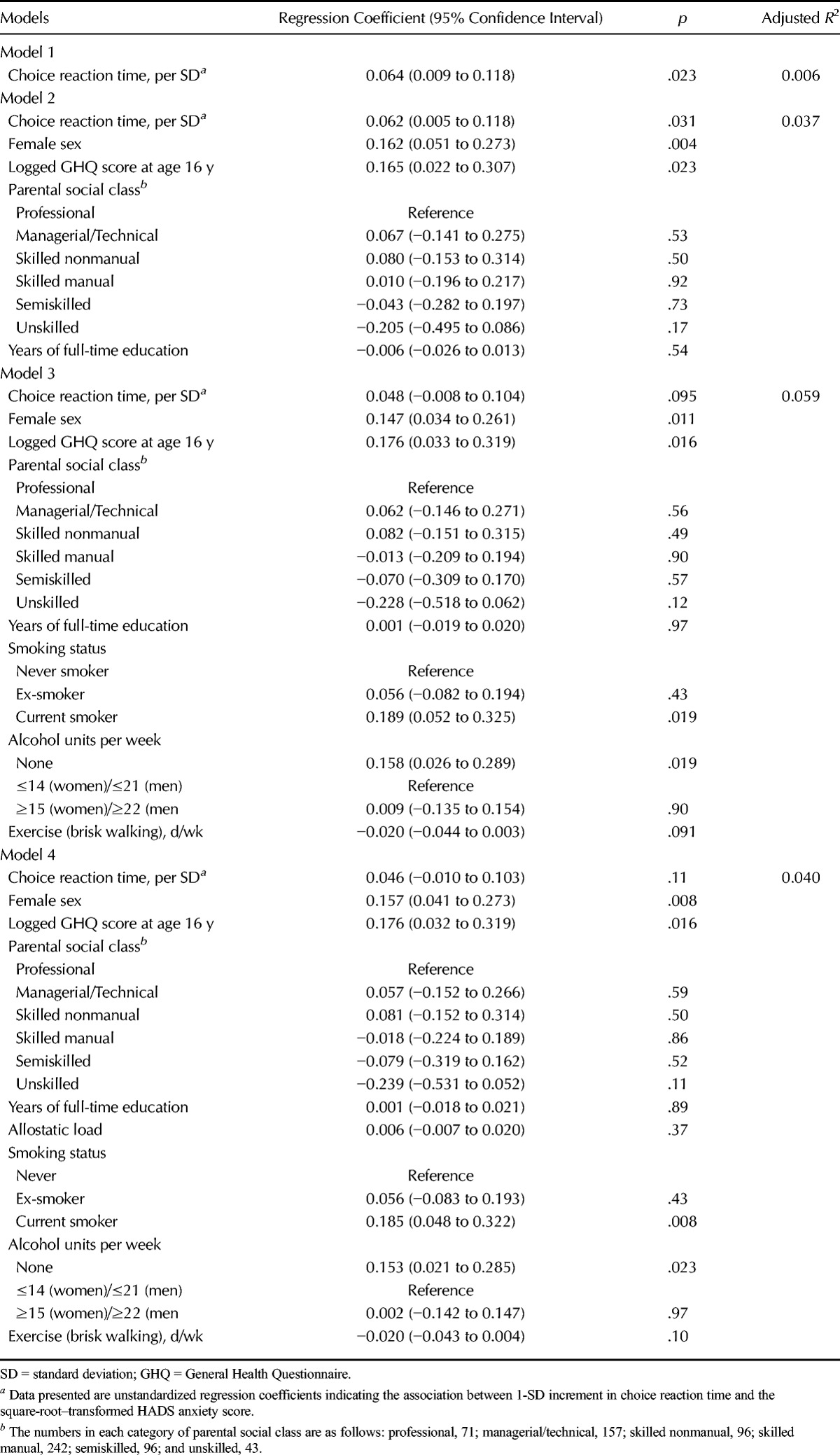
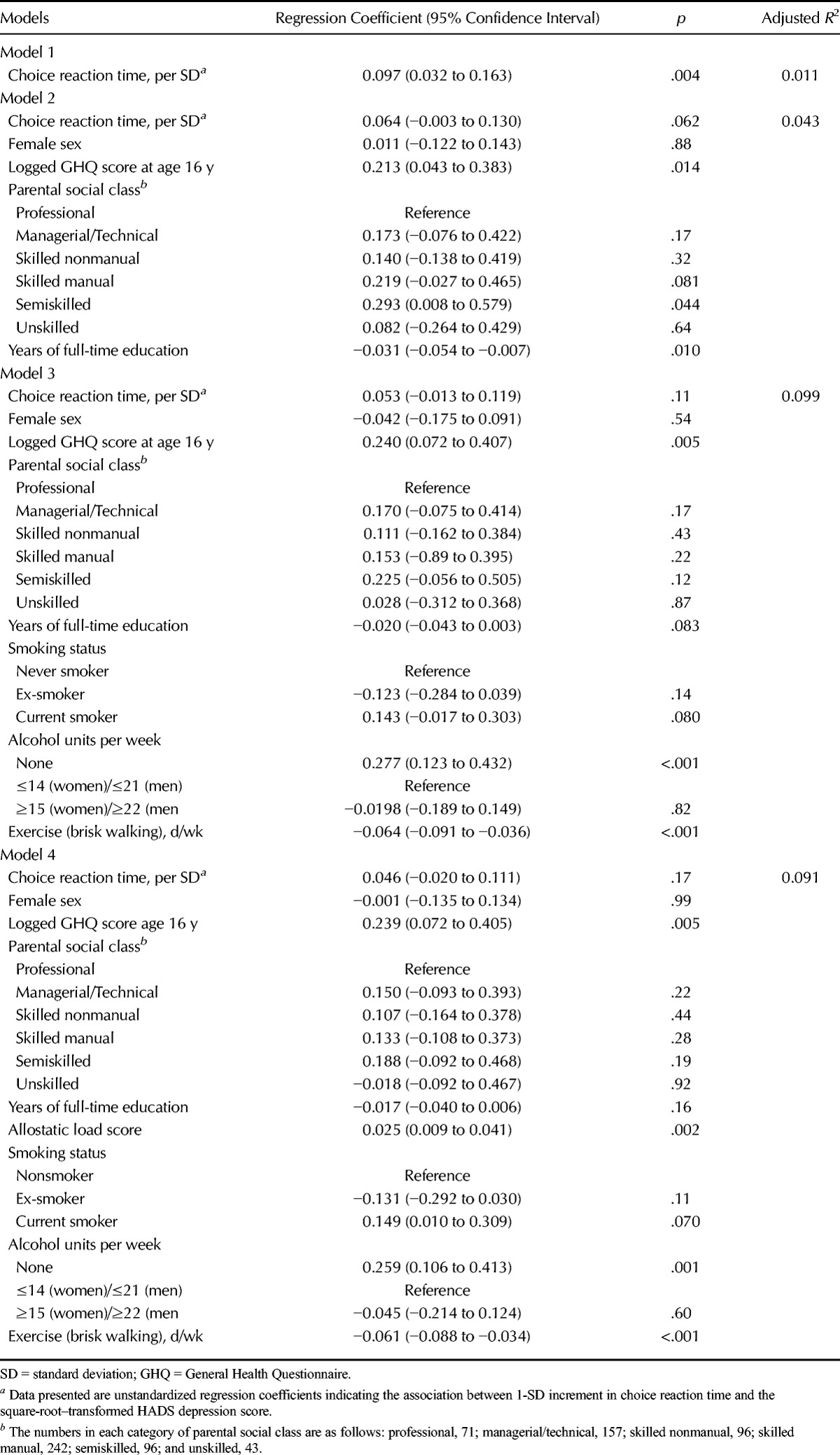
Table 4 shows the results of linear regression analyses of the relation between choice reaction time at age 16 years, expressed as SD scores, and HADS depression scores at age 36 years. In the unadjusted Model 1, 1-SD longer choice reaction time was associated with an increase in the square root–transformed HADS depression score of 0.10 (95% CI = 0.03−0.16). This amounts to 1.1% of the variance. After adjustment for sex, GHQ score at age 16 years, parental socioeconomic status, and years of full-time education (Model 2), this association became of borderline statistical significance. It was further attenuated and no longer significant after additional adjustment for health behaviors (Model 3) and allostatic load (Model 4). Model 3 explained 9.9% of the variance in HADS depression score. Results of Sobel-Goodman mediating tests showed that before adjusting for any of the covariates, allostatic load and years of full-time education each had a significant mediating effect on this association: 17% was mediated through allostatic load (p = .010) and 26% through years of full-time education (p = .005). After adjustment for the other covariates, the percentage mediated by allostatic load fell slightly to 16% (p = .074) and that mediated through education fell to 20% (p = .059), and in neither case were these statistically significant at conventional levels.
TABLE 4.
The Association Between Choice Reaction Time at Age 16 Years With the Hospital Anxiety and Depression Scale Depression Subscale Score at Age 36 Years (Unadjusted and Adjusted Models)
In total, 18% of the participants who had data on reaction time at age 16 years and mental health at age 36 years had missing data on one or more of the covariates, primarily allostatic load, so they were excluded from our analytical sample. To investigate whether this exclusion might have biased the associations, we reran the initial models that were adjusted for sex only in the 861 people with data on reaction time and mental health. Associations were almost identical to those found in the 705 people in our analytical sample.
DISCUSSION
In this 20-year longitudinal study of 705 adolescents, boys and girls who had longer choice reaction time at age 16 years had more symptoms of anxiety and depression at age 36 years, as measured by their scores on the GHQ and the HADS anxiety and depression subscales. All these effect sizes were small. The strength of the association between reaction time and score on the GHQ persisted and was only slightly changed, after adjustment for the potential confounding or mediating variables, sex, GHQ score at age 16 years, parental social class, educational attainment, allostatic load, and health behaviors that contribute to allostatic load. The associations between reaction time and scores on the HADS anxiety and depression subscales were attenuated after these adjustments and no longer statistically significant. Formal tests of mediation showed that part of the effect of reaction time on HADS anxiety score was significantly mediated through smoking status at age 36 years. Part of the effect of reaction time on HADS depression score was mediated through cumulative allostatic load and educational attainment, but formal tests of these mediating effects did not reach conventional levels of significance after adjustment.
So far as we are aware, there have been no previous longitudinal studies of the relation between reaction time and subsequent symptoms of anxiety and depression. Cross-sectional studies, mostly based on clinical samples, have found that people with more severe depressive symptoms tend to have slower reaction times and other cognitive deficits (9,42,43). These findings have generally been interpreted as indicating that the presence of depression can cause psychomotor slowing and have an adverse effect on task performance. However, that a few studies have found persisting cognitive deficits in people who have recovered from depression suggests that they may not be simply epiphenomena of depression, but may reflect enduring cognitive traits (9). The few small studies that have examined the cross-sectional relation between reaction time and anxiety disorders have found no association (7). In the present larger study, we found small but significant associations between longer reaction time in adolescence and higher scores on the GHQ and on the HADS anxiety and depression subscales 20 years later. These associations was only slightly changed by adjustment for the confounding factors, parental social class, and GHQ score at age 16 years, but were attenuated to varying degrees by adjustment for mediating factors. In the present study, there was little variance in mood among our sample: most of the participants had low scores on the GHQ and HADS. Further longitudinal studies are needed—preferably in samples with greater variation in levels of psychological distress—to confirm our observations that longer reaction time might be a risk factor for symptoms of anxiety and depression.
We found only partial support for the hypothesis that cumulative allostatic load might mediate links between reaction time in adolescence and later symptoms of anxiety and depression. Allostatic load at age 36 years was not associated with scores on the HADS anxiety subscale or on the GHQ scale, but it was significantly positively correlated with HADS depression score. Of the total effect of reaction time in adolescence on depression scores at age 36 years, 17% was mediated through allostatic load before adjustment for covariates, although this mediating effect did not reach statistical significance after adjustment. It is possible that the potential explanatory effect of allostatic load might have been more pronounced if there had been greater variance in depression scores among our participants. A few studies have reported cross-sectional (44,45) and longitudinal associations (46) between cumulative allostatic load and severity of depressive symptoms in people 60 years and older. Our findings suggest that this link may be present at a much younger age.
In our study, people who smoked at age 36 years had higher levels of anxiety and depression. This might, at least in part, reflect the use of cigarettes as a method of relieving or coping with psychological symptoms. However, there is a growing body of evidence that smoking in childhood or adolescence leads to increased levels of anxiety and heightened risk of developing anxiety disorders later in life (47,48). Various biological pathways may potentially underlie this link, including neurotransmitter systems, inflammation, oxidative and nitrosative stress, mitochondrial dysfunction, neurotrophins, or neurogenesis (49). Here, we found that slower reaction time at age 16 years was a significant predictor of still being a current smoker at age 36 years. Although it is impossible to be certain of the direction of the effect between smoking and poorer mental health in our study, the finding that 17% of the effect of reaction time at aged 16 years on HADS anxiety scores was mediated through current smoking could plausibly reflect the anxiogenic impact of long-term nicotine intake. Smoking is highly likely to contribute to allostatic load, but in this study, we found no evidence that allostatic load mediated the association between reaction time and HADS anxiety score. Smoking did not have a mediating role in the association between reaction time and HADS depression score.
Our study has some limitations. First, a recent review of more than 50 studies found that the factor structure of the HADS varied across studies and populations, with the number of factors ranging from one to four (50). The authors subsequently suggested that it might be more appropriate to view the HADS as an assessment of emotional distress given that it does not provide good separation between symptoms of anxiety and depression (51). In view of this, our findings using the anxiety and depression subscales need to be viewed with caution. Second, our measure of physical activity—number of brisk walks per week—may not adequately capture the extent or intensity of physical activity among our participants at age 36 years, although it is worth noting that it was significantly inversely related to HADS subscale scores. Third, we were unable to adjust for substance abuse in adolescence, which might potentially confound associations between reaction time and later symptoms of anxiety and depression. Finally, we had no data on intelligence in adolescence so we were not able to use our study sample to investigate the suggestion that faster processing speed might help explain links between higher intelligence and reduced risk of anxiety and depression (10). Reaction time is moderately correlated with intelligence (16), and there is some evidence that it accounts, to a very large extent, for the association between higher intelligence and lower mortality (52). The extent to which it explains associations between intelligence and mental health needs to be investigated in future studies.
In this prospective study of adolescents, we found that those with slower processing speed, as indexed by longer choice reaction time, reported higher levels of anxiety and depression 20 years later. Effect sizes were modest. Part of the effects of reaction time on later levels of anxiety or depression was mediated through smoking or allostatic load, respectively, although the latter's role ceased to be statistically significant after adjustment for covariates. Further prospective studies of the relation between reaction time and mental health outcomes in other samples are needed to gauge whether reaction time is a true risk factor for mental disorders and to confirm the mediating roles played by smoking and allostatic load.
Supplementary Material
Acknowledgments
Source of Funding and Conflicts of Interest: The West of Scotland Twenty-07 Study is funded by the UK Medical Research Council (MRC; MC_US_A540_53462). G. Der (MC_US_A540_5TK30) and C. Gale (MC-A620-5TF00) are funded by the MRC. C. Gale, I. Deary, G. Der, and G.D. Batty are members of The University of Edinburgh Centre for Cognitive Ageing and Cognitive Epidemiology, part of the cross council Lifelong Health and Wellbeing Initiative (MR/K026992/1). Funding from the Biotechnology and Biological Sciences Research Council and MRC is gratefully acknowledged. The authors have no conflicts of interest to declare.
Footnotes
Supplemental Content
REFERENCES
- 1. Gorlyn M, Keilp JG, Oquendo MA, Burke AK, Sackeim HA, John Mann J. The WAIS-III and major depression: absence of VIQ/PIQ differences. J Clin Exper Neuropsychol 2006; 28: 1145– 57. [DOI] [PubMed] [Google Scholar]
- 2. Smith DJ, Muir WJ, Blackwood DH. Neurocognitive impairment in euthymic young adults with bipolar spectrum disorder and recurrent major depressive disorder. Bipolar Disord 2006; 8: 40– 6. [DOI] [PubMed] [Google Scholar]
- 3. Stordal KI, Lundervold AJ, Egeland J, Mykletun A, Asbjornsen A, Landro NI, Roness A, Rund BR, Sundet K, Oedegaard KJ, Lund A. Impairment across executive functions in recurrent major depression. Nord J Psychiatry 2004; 58: 41– 7. [DOI] [PubMed] [Google Scholar]
- 4. Tsourtos G, Thompson JC, Stough C. Evidence of an early information processing speed deficit in unipolar major depression. Psychol Med 2002; 32: 259– 65. [DOI] [PubMed] [Google Scholar]
- 5. Iverson GL. Sensitivity of computerized neuropsychological screening in depressed university students. Clin Neuropsychol 2006; 20: 695– 701. [DOI] [PubMed] [Google Scholar]
- 6. Cataldo MG, Nobile M, Lorusso ML, Battaglia M, Molteni M. Impulsivity in depressed children and adolescents: a comparison between behavioral and neuropsychological data. Psychiatry Res 2005; 136: 123– 33. [DOI] [PubMed] [Google Scholar]
- 7. Airaksinen E, Larsson M, Forsell Y. Neuropsychological functions in anxiety disorders in population-based samples: evidence of episodic memory dysfunction. J Psychiatr Res 2005; 39: 207– 14. [DOI] [PubMed] [Google Scholar]
- 8. Purcell R, Maruff P, Kyrios M, Pantelis C. Neuropsychological deficits in obsessive-compulsive disorder: a comparison with unipolar depression, panic disorder, and normal controls. Arch Gen Psychiatry 1998; 55: 415– 23. [DOI] [PubMed] [Google Scholar]
- 9. Austin MP, Mitchell P, Goodwin GM. Cognitive deficits in depression—possible implications for functional neuropathology. Br J Psychiatry 2001; 178: 200– 6. [DOI] [PubMed] [Google Scholar]
- 10. Gale CR, Batty GD, Tynelius P, Deary IJ, Rasmussen F. Intelligence in early adulthood and subsequent hospitalisation and admission rates for the whole range of mental disorders: longitudinal study of 1,049,663 men. Epidemiology 2010; 21: 70– 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gale CR, Deary IJ, Boyle SH, Barefoot J, Mortensen LH, Batty GD. Cognitive ability in early adulthood and risk of five specific psychiatric disorders in mid life: the Vietnam Experience Study. Arch Gen Psychiatry 2008; 65: 1410– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gale CR, Hatch SL, Batty GD, Deary IJ. Intelligence in childhood and risk of psychological distress in adulthood: the 1958 National Child Development Survey and the 1970 British Cohort Study. Intelligence 2009; 37: 592– 9. [Google Scholar]
- 13. Austin EJ, Hofer SM, Deary IJ, Eber HW. Interactions between intelligence and personality: results from two large samples. Pers Individ Dif 2000; 29: 405– 27. [Google Scholar]
- 14. Koenen KC, Moffitt TE, Roberts AL, Martin LT, Kubzansky L, Harrington H, Poulton R, Caspi A. Childhood IQ and adult mental disorders: a test of the cognitive reserve hypothesis. Am J Psychiatry 2009; 166: 50– 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hatch SL, Jones PB, Kuh D, Hardy R, Wadsworth ME, Richards M. Childhood cognitive ability and adult mental health in the British 1946 birth cohort. Soc Sci Med 2007; 64: 2285– 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deary IJ, Der G, Ford G. Reaction times and intelligence differences—a population-based cohort study. Intelligence 2001; 29: 389– 99. [Google Scholar]
- 17. Hunt E. Information processing and intelligence: where we are and where we are going. In: Sternberg RJ, Pretz JE, editors. Cognition and Intelligence: Identifying Mechanisms of Mind. Cambridge: Cambridge University Press; 2005: 1– 25. [Google Scholar]
- 18. Jensen AR, Cohn SJ, Cohn CMG. Speed of information-processing in academically gifted youths and their siblings. Pers Individ Dif 1989; 10: 29– 33. [Google Scholar]
- 19. Tucker-Drob EM, Briley DA, Starr JM, Deary IJ. Structure and correlates of cognitive aging in a narrow age cohort. Psychol Aging 2014; 29: 236– 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sterling P, Eyer J. Biological bases of stress-related mortality. In: Fisher S, Reason J, editors. Handbook of Life Stress, Cognition and Health. New York: Wiley; 1981: 629– 49. [Google Scholar]
- 21. Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc Natl Acad Sci U S A 2001; 98: 4770– 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Arch Intern Med 1993; 153: 2093– 101. [PubMed] [Google Scholar]
- 23. Lupien SJ, Ouellet-Morin I, Hupback A, Tu MT, Buss C, Walker D, Pruessner J, McEwen BS. Beyond the stress concept: allostatic load—a developmental, biological and cognitive perspective. In: Cicchetti D, Cohen D, editors. Developmental Psychopathology. Hoboken, NJ: Wiley; 2006. [Google Scholar]
- 24. Sapolsky RM. Why stress is bad for your brain. Science 1996; 273: 749– 50. [DOI] [PubMed] [Google Scholar]
- 25. Evans GW, Schamberg MA. Childhood poverty, chronic stress, and adult working memory. Proc Natl Acad Sci U S A 2009; 106: 6545– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Beauchaine TP, Neuhaus E, Zalewski M, Crowell SE, Potapova N. The effects of allostatic load on neural systems subserving motivation, mood regulation, and social affiliation. Dev Psychopathol 2011; 23: 975– 99. [DOI] [PubMed] [Google Scholar]
- 27. Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev 2010; 35: 2– 16. [DOI] [PubMed] [Google Scholar]
- 28. Benzeval M, Der G, Ellaway A, Hunt K, Sweeting H, West P, Macintyre S. Cohort profile: West of Scotland Twenty-07 Study: health in the community. Int J Epidemiol 2009; 38: 1215– 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. MacIntyre S, Annandale R, Ecob G, Ford JB, Maciver S, West P, Wyke S. The West of Scotland Twenty-07 Study: health in the community. In: Martin C, MacQueen D, editors. Readings for a New Public Health, Edinburgh: Edinburgh University Press; 1989. [Google Scholar]
- 30. Der G. A Comparison of the West of Scotland Twenty-07 Study Sample and the 1991 Census SARs. Glasgow: MRC Medical Sociology Unit; 1998. [Google Scholar]
- 31. Cox BJ, Huppert F, Whichelow M. The Health and Lifestyle Survey: Seven Years on. Aldershot: Dartmouth; 1993. [Google Scholar]
- 32. Goldberg DP, Williams P. A User's Guide to the General Health Questionnaire. Windsor, UK: Nfer-Nelson; 1988. [Google Scholar]
- 33. Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983; 67: 361– 70. [DOI] [PubMed] [Google Scholar]
- 34. Goldberg DP, Gater R, Sartorius N, Ustun TB, Piccinelli M, Gureje O, Rutter C. The validity of two versions of the GHQ in the WHO study of mental illness in general health care. Psychol Med 1997; 27: 191– 7. [DOI] [PubMed] [Google Scholar]
- 35. Austin EJ, Deary IJ, Whiteman MC, Fowkes FGR, Pedersen NL, Rabbitt P, Bent N, McInnes L. Relationships between ability and personality: does intelligence contribute positively to personal and social adjustment? Pers Individ Dif 2002; 32: 1391– 411. [Google Scholar]
- 36. Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ 2003; 326: 1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Weir MR, Moser M. Diuretics and beta-blockers: is there a risk for dyslipidemia? Am Heart J 2000; 139: 174– 83. [DOI] [PubMed] [Google Scholar]
- 38. Sherifali D, Nerenberg K, Pullenayegum E, Cheng JE, Gerstein HC. The effect of oral antidiabetic agents on A1C levels. Diab Care 2010; 38: 1859– 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Albert MA, Danielson E, Rifai N, Ridker PM, Investigators P. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA 2001; 286: 64– 70. [DOI] [PubMed] [Google Scholar]
- 40. Office of Population Censuses and Surveys Classification of Occupations 1980. London: HMSO; 1980. [Google Scholar]
- 41. Scottish Government Changing Scotland's Relationship With Alcohol: A Framework for Action. Edinburgh: Scottish Government; 2009. [Google Scholar]
- 42. McDermott LM, Ebmeier KP. A meta-analysis of depression severity and cognitive function. J Affect Disord 2009; 119: 1– 8. [DOI] [PubMed] [Google Scholar]
- 43. Gualtieri CT, Morgan DW. The frequency of cognitive impairment in patients with anxiety, depression, and bipolar disorder: an unaccounted source of variance in clinical trials. J Clin Psychiatry 2008; 69: 1122– 30. [DOI] [PubMed] [Google Scholar]
- 44. Kobrosly RW, Seplaki CL, Cory-Slechta DA, Moynihan J, van Wijngaarden E. Multisystem physiological dysfunction is associated with depressive symptoms in a population-based sample of older adults. Int J Geriatr Psychiatry 2013; 28: 718– 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Seplaki CL, Goldman N, Weinstein M, Lin YH. Measurement of cumulative physiological dysregulation in an older population. Demography 2006; 43: 165– 83. [DOI] [PubMed] [Google Scholar]
- 46. Goldman N, Turra CM, Glei DA, Lin YH, Weinstein M. Physiological dysregulation and changes in health in an older population. Exp Gerontol 2006; 41: 862– 70. [DOI] [PubMed] [Google Scholar]
- 47. Johnson JG, Cohen P, Pine DS, Klein DF, Kasen S, Brook JS. Association between cigarette smoking and anxiety disorders during adolescence and early adulthood. JAMA 2000; 284: 2348– 51. [DOI] [PubMed] [Google Scholar]
- 48. Moylan S, Gustavson K, Karevold E, Overland S, Jacka FN, Pasco JA, Berk M. The impact of smoking in adolescence on early adult anxiety symptoms and the relationship between infant vulnerability factors for anxiety and early adult anxiety symptoms: the TOPP Study. PLoS One 2013; 8: e63252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Moylan S, Jacka FN, Pasco JA, Berk M. How cigarette smoking may increase the risk of anxiety symptoms and anxiety disorders: a critical review of biological pathways. Brain Behav 2013; 3: 302– 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cosco TD, Doyle F, Ward M, McGee H. Latent structure of the Hospital Anxiety and Depression Scale: a 10-year systematic review. J Psychosom Res 2012; 72: 180– 4. [DOI] [PubMed] [Google Scholar]
- 51. Norton S, Cosco T, Doyle F, Done J, Sacker A. The Hospital Anxiety and Depression Scale: a meta confirmatory factor analysis. J Psychosom Res 2013; 74: 74– 81. [DOI] [PubMed] [Google Scholar]
- 52. Deary IJ, Der G. Reaction time explains IQ's association with death. Psychol Sci 2005; 16: 64– 9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


