Abstract
Objective
Using disease-modifying antirheumatic drugs (DMARDs) improves outcomes in rheumatoid arthritis (RA) and is a nationally endorsed quality measure. We investigated the prevalence and predictors of receiving glucocorticoids alone for the treatment of RA in a nationwide sample of Medicare beneficiaries.
Methods
Among individuals ages ≥65 years with RA enrolled in the Part D prescription drug benefit in 2009, we compared those with ≥1 DMARD claim to those receiving glucocorticoid monotherapy, defined as no DMARD claim and an annual glucocorticoid supply of ≥180 days or an annual dose of ≥900 mg of prednisone or equivalent. We fit multivariable models to determine the sociodemographic and clinical factors associated with glucocorticoid monotherapy.
Results
Of 8,125 beneficiaries treated for RA, 10.2% (n = 825) received glucocorticoids alone. Beneficiaries with low incomes were more likely to receive glucocorticoids alone (12.3%; 95% confidence interval [95% CI] 10.9–13.8% versus 9.4%; 95% CI 8.6–10.1%), as were those living in certain US regions. More physician office visits and hospitalizations were associated with glucocorticoid monotherapy. Individuals who had no contact with a rheumatologist were significantly more likely to receive glucocorticoids alone (17.5%; 95% CI 16.0–19.0% versus 8.5%; 95% CI 7.4–9.5% for those with no rheumatology visits versus 1–4 visits).
Conclusion
Approximately 1 in 10 Medicare beneficiaries treated for RA received glucocorticoids without DMARDs in 2009. Compared to DMARD users, glucocorticoid users were older, had lower incomes, were more likely to live in certain US regions, and were less likely to have seen a rheumatologist, suggesting persistent gaps in quality of care despite expanded drug coverage under Part D.
INTRODUCTION
Significant advances in therapy for rheumatoid arthritis (RA) have occurred in the last decade with the development of an increasing number of effective nonbiologic and biologic disease-modifying antirheumatic drugs (DMARDs). Given the substantial personal and societal costs associated with RA and the proven role of DMARDs in decreasing pain, disability, and mortality, DMARD use has become the standard of care for patients with RA and is recommended by professional society guidelines and national quality measures (1–4).
Despite the recommendation to use DMARDs in patients with RA, previous studies have found lower than expected rates of DMARD use in the US, with significant variation between sociodemographic groups and geographic regions (5–8). We reported that only 63% of Medicare managed care beneficiaries with RA received DMARDs between 2005 and 2008 (6). In this and other studies, lower-income, African American, and older patients were less likely to receive DMARDs, suggesting potential disparities in DMARD use. However, one aspect that has not been fully addressed in prior studies is whether there are systematic differences in the treatment of RA with non-DMARDs, specifically strategies that use glucocorticoids alone.
To address this issue, we created a cohort of Medicare fee-for-service beneficiaries with RA enrolled in Medicare’s Part D prescription drug program. Implemented in 2006, Part D provides subsidized drug coverage for Medicare beneficiaries. Detailed prescription drug events are generated each time a medication is dispensed, allowing us to examine drug use for RA at the population level. The primary aim of our analysis was to understand the prevalence and predictors of the use of DMARDs compared to the use of glucocorticoids alone for the treatment of RA. We sought to control for factors that might reasonably limit the use of DMARDs, including medical comorbidities, while investigating patient sociodemographic characteristics and health care system factors associated with the use of glucocorticoid monotherapy for the treatment of RA.
MATERIALS AND METHODS
This study was approved by the Centers for Medicare and Medicaid Services and was submitted and determined exempt from review by the Institutional Review Board of the University of California, San Francisco.
Data sources
We used nationwide data from the Medicare program, a national insurance program administered by the US Federal Government that provides health insurance for those ages ≥65 years as well as younger people with disabilities. Medicare offers all recipients a defined benefit. Hospital care is covered under Part A and outpatient services are covered under Part B. In addition, since 2006, a prescription drug benefit, Part D, went into effect as part of the Medicare Modernization Act of 2003. This study used data from Medicare Parts B and D to examine drug utilization.
We used 2009 Carrier Claims Files for a 5% random sample of Medicare beneficiaries, including all Part B claims. We linked these files to several additional data sources. We used the Medicare Beneficiary Summary File to determine patient sociodemographic characteristics. The Beneficiary Annual Summary File was used both to determine inpatient hospitalizations and to exclude those with long-term nursing stays during the study period, since we could not observe drug use during such stays. We used Part D Drug Event Files as well as Part B claims for infusible or injectable RA drugs to assess medication use, and the Part D Provider Characteristics Files to determine the medical specialty of prescribing physicians. Finally, we used the Area Resource File to determine community and local health care system characteristics (9).
Study population
Our 5% sample included 22,017 continuously enrolled Medicare beneficiaries ages ≥65 years residing in the 50 states, who survived through the measurement year and had ≥2 face-to-face encounters with different dates of service in an ambulatory or non-acute patient setting with a diagnosis of RA (International Classification of Diseases, Ninth Revision [ICD-9] codes 714.0, 714.1, 714.2, 714.4, 714.81, or 714.89) (6) within the year. Consistent with the Healthcare Effectiveness Data and Information Set measure regarding DMARD use, we excluded patients with a diagnosis of human immunodeficiency virus during the measurement year (6). From this larger cohort, we limited our analysis to those individuals being treated with RA, since our primary goal was to understand variation in drug use among patients receiving medications for active RA. Therefore, individuals who received no DMARDs or did not meet the criteria for glucocorticoid monotherapy were excluded (n = 4,509). In addition, we excluded individuals who were not enrolled in Part D, since we could not observe their drug utilization (n = 9,355). Figure 1 outlines the study inclusion and exclusion criteria and summarizes how we arrived at our final sample of 8,125 individuals.
Figure 1.
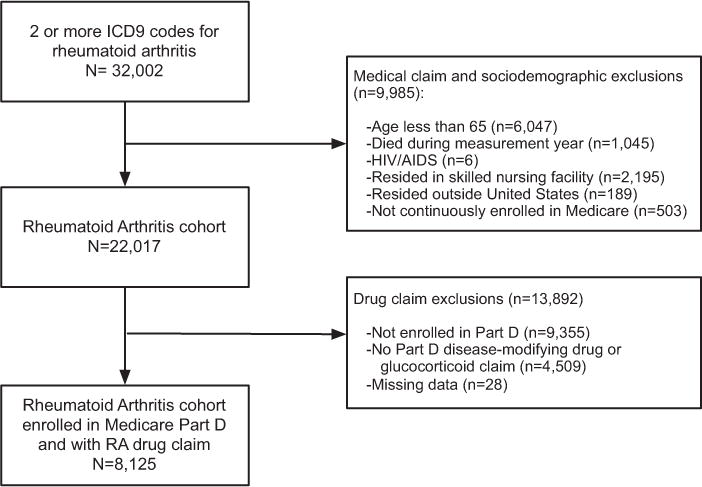
Diagram depicting inclusion and exclusion criteria for treatment study of Medicare beneficiaries with rheumatoid arthritis (RA) enrolled in the Part D prescription drug program during 2009. ICD9 = International Classification of Diseases, Ninth Revision; HIV = human immunodeficiency virus; AIDS = acquired immunodeficiency syndrome.
Outcome measure
We compared 2 mutually exclusive groups of patients who received ≥1 prescription drugs for RA during the measurement year. The first group, “DMARD users,” consisted of individuals who had ≥1 DMARD dispensed or administered. All nonbiologic DMARDs (hydroxychloroquine, methotrexate, leflunomide, minocycline, penicillamine, sulfasalazine, gold, cyclophosphamide, cyclosporine, and azathioprine) and biologic DMARDs (etanercept, adalimumab, infliximab, rituximab, abatacept, and anakinra) available in 2009 were included.
The second group, “glucocorticoid monotherapy users,” consisted of individuals who had no DMARD dispensed through either Part D or Part B during the measurement year but did receive glucocorticoids. We a priori defined and used a conservative and clinically relevant definition of glucocorticoid monotherapy: those receiving an annual dispensed glucocorticoid supply of ≥180 days corresponding to 6 months, which is the definition of established RA (2), or an annual dispensed dose of ≥900 mg of oral prednisone or equivalent (representing >5 mg/day for 180 days), included to capture those with substantial total glucocorticoid exposure. We converted steroid doses for oral forms of cortisone, dexamethasone, hydrocortisone, and methylprednisolone to prednisone equivalents. Use of glucocorticoids without DMARDs was the primary outcome in this study.
Covariates
Covariates included age, sex, race/ethnicity, and medical comorbidities (Charlson comorbidity score) (10). We used 2 measures of socioeconomic status. The first was personal income, defined by Medicare eligibility for a low-income subsidy under Part D. Low-income beneficiaries have to meet both income and asset tests to receive subsidies, unless they are enrolled in Medicaid, in which case they automatically receive a subsidy. We also examined area-level income, defined by the percentage of individuals in the residential county at or below 125% of the federal poverty limit, obtained from the Area Resource File (9).
We included several measures of health care utilization, including the number of inpatient and outpatient clinical encounters and the involvement of rheumatologists in care. For this latter variable, we examined the number of Part D prescription drug events originating from a rheumatologist by linking the Part D Drug Event File to the Part D Providers Characteristics File. We also calculated the annual number of Part B outpatient claims from a rheumatologist. Finally, we included county health professional shortage areas and census geographic regions using the Area Resource File.
Statistical analysis
We compared the demographic, socioeconomic, clinical, and health care utilization characteristics of Medicare beneficiaries receiving glucocorticoid monotherapy versus those of beneficiaries receiving DMARDs using chi-square tests.
We fit multivariable logistic regression models in which use of glucocorticoid monotherapy was the outcome. All covariates were tested to ensure noncollinearity. To gauge model fit, we applied the Hosmer-Lemeshow goodness-of-fit test and calculated a C statistic; the C statistic can be interpreted as the probability that one randomly selected person receiving a DMARD and one randomly selected person receiving glucocorticoid monotherapy are correctly classified by the regression model. We calculated the adjusted group percentages and 95% confidence intervals (95% CIs) from the predicted marginals derived from our model. We tested for interactions among variables of interest, including between income and contact with a rheumatologist, either through Part B or through Part D events, and between age and comorbidities.
In sensitivity analyses, we varied our original, clinically-based definition of glucocorticoid monotherapy. We decreased the requirement for an annual dispensed glucocorticoid supply to ≥120 days (~4 months) or an annual dispensed dose of ≥600 mg of oral prednisone. We also repeated the analysis including only those who received prolonged courses of glucocorticoid monotherapy at a moderate or high dose (≥180 days of glucocorticoids and ≥900 mg).
As additional checks, we performed analyses excluding individuals with ≥2 codes for polymyalgia rheumatica (ICD-9 diagnosis code 725) or giant cell arteritis (ICD-9 diagnosis code 446.5) (11), conditions that can initially present with symptoms suggestive of RA and for which glucocorticoid monotherapy is the standard of care. We also considered whether individuals in our sample might have inactive RA but be taking long-term glucocorticoids for an unrelated condition, such as asthma or chronic obstructive pulmonary disease. Among those with RA receiving glucocorticoid monotherapy, we searched for those meeting administrative definitions of asthma, defined as 2 physician claims with ICD-9 codes of 493.0–493.2, 493.8, and 493.9 during the year (12). No patients met this definition. We also evaluated those with ≥2 physician claims for chronic obstructive pulmonary disease (ICD-9 codes 491–493 or 496) (13); 35 individuals or 4% of the 824 glucocorticoid monotherapy users met this definition. We repeated our analyses excluding these individuals to test the robustness of our findings. All analyses were performed using SAS statistical software, version 9.2.
RESULTS
Characteristics of the Medicare beneficiaries with RA tabulated by whether they received a DMARD versus glucocorticoid monotherapy are listed in Table 1. Among 8,125 treated individuals, 10.2% (95% CI 9.5–10.8%) received glucocorticoid monotherapy. For glucocorticoid monotherapy users, the median annual prednisone equivalent dose was 800 mg (interquartile range [IQR] 1,350–2,700). For DMARD users, the median annual prednisone equivalent dose was 105 mg (IQR 0–1,200). DMARDs used by Medicare beneficiaries in 2009 are summarized in Supplementary Tables 1–3 (available in the online version of this article at http://onlinelibrary.wiley.com/doi/10.1002/acr.22312/abstract).
Table 1.
Characteristics of Medicare beneficiaries who received glucocorticoids alone versus disease-modifying antirheumatic drugs (DMARDs) for rheumatoid arthritis in 2009
| Total sample (n = 8,125), no. (%) |
Glucocorticoid monotherapy users (n = 825), no. (%) |
DMARD users (n = 7,300), no. (%) |
P* | |
|---|---|---|---|---|
| Sociodemographic characteristics | ||||
| Age, years | < 0.0001 | |||
| 65–69 | 1,877 (23) | 120 (15) | 1,757 (24) | |
| 70–74 | 2,191 (27) | 157 (19) | 2,034 (28) | |
| 75–79 | 1,813 (22) | 187 (23) | 1,626 (22) | |
| 80–84 | 1,387 (17) | 187 (23) | 1,200 (16) | |
| ≥85 | 857 (11) | 174 (21) | 683 (9) | |
| Women | 6,610 (81) | 670 (81) | 5,940 (81) | 0.9122 |
| Race/ethnicity | 0.0040 | |||
| White | 6,929 (85) | 682 (83) | 6,247 (86) | |
| African American | 696 (9) | 96 (12) | 600 (8) | |
| Other | 500 (6) | 47 (6) | 453 (6) | |
| Comorbid illness | ||||
| Charlson comorbidity score | < 0.0001 | |||
| 1 | 5,500 (68) | 479 (58) | 5,021 (69) | |
| 2 | 1,683 (21) | 200 (24) | 1,483 (20) | |
| ≥3 | 942 (12) | 146 (18) | 796 (11) | |
| Health care utilization | ||||
| ≥1 prescriptions originating from rheumatologist (versus none) | 5,547 (68) | 359 (44) | 5,188 (71) | < 0.0001 |
| Claims from rheumatologist | < 0.0001 | |||
| None | 2,389 (30) | 498 (60) | 1,891 (26) | |
| 1–4 | 3,185 (39) | 243 (29) | 2,942 (40) | |
| ≥5 | 2,551 (31) | 84 (10) | 2,467 (34) | |
| Annual physician office visits | 0.0026 | |||
| 0–8 | 2,796 (34) | 294 (36) | 2,502 (34) | |
| 9–15 | 2,761 (34) | 238 (29) | 2,523 (35) | |
| ≥16 | 2,568 (32) | 293 (35) | 2,275 (31) | |
| Annual hospitalizations | < 0.0001 | |||
| None | 6,287 (77) | 543 (66) | 5,744 (79) | |
| 1 | 1,208 (15) | 159 (19) | 1,049 (14) | |
| ≥2 | 630 (8) | 123 (15) | 507 (7) | |
| Socioeconomic status | ||||
| Area-level socioeconomic status† | 0.4701 | |||
| Quintile 1 (lowest-income counties) | 1,762 (22) | 172 (21) | 1,590 (22) | |
| Quintile 2 | 1,847 (23) | 178 (21) | 1,669 (23) | |
| Quintile 3 | 1,828 (22) | 181 (22) | 1,647 (23) | |
| Quintile 4 | 1,639 (20) | 186 (23) | 1,453 (20) | |
| Quintile 5 (highest-income counties) | 1,049 (13) | 108 (13) | 941 (13) | |
| Personal income: enrolled in low-income subsidy program | 1,873 (23) | 264 (32) | 1,609 (22) | < 0.0001 |
| Area-level physician supply | ||||
| Health professional shortage area | 6,905 (85) | 691 (84) | 6,214 (85) | 0.2982 |
| Census geographic divisions | < 0.0001 | |||
| New England | 422 (5) | 46 (6) | 376 (5) | |
| Mid-Atlantic | 993 (12) | 149 (18) | 844 (12) | |
| East North Central Midwest | 1,183 (15) | 112 (14) | 1,071 (15) | |
| West North Central Midwest | 763 (9) | 62 (8) | 701 (10) | |
| South Atlantic | 1,668 (21) | 182 (22) | 1,486 (20) | |
| East South Central | 610 (8) | 57 (7) | 553 (8) | |
| West South Central | 1,063 (13) | 97 (12) | 966 (13) | |
| Mountain | 482 (6) | 37 (4) | 445 (6) | |
| Pacific | 941 (12) | 83 (10) | 858 (12) |
Computed from chi-square tests.
Quintiles calculated based on percentage of individuals in residential county at or below 125% of the federal poverty limit.
Table 2 lists the unadjusted and adjusted percentages of beneficiaries receiving glucocorticoid monotherapy, calculated from the predicted marginals derived from the regression model. The column of percentages adjusts for each of the variables in the multivariable model, shown as the rows. The factors most strongly associated with glucocorticoid monotherapy in the unadjusted analysis were age, with older patients being significantly more likely to receive glucocorticoid monotherapy (20.3%; 95% CI 17.6–23.0% among age ≥85 years versus 6.4%; 95% CI 5.3–7.5% among age 65–69 years), and lack of Part B claims from a rheumatologist (20.9%; 95% CI 19.2–22.5% among those with no rheumatology claims versus 3.3%; 95% CI 2.6–4.0% among those with ≥5 rheumatology claims).
Table 2.
Factors associated with glucocorticoid monotherapy use among Medicare beneficiaries with rheumatoid arthritis
| Unadjusted
|
Adjusted
|
|||
|---|---|---|---|---|
| Glucocorticoid monotherapy users, % (95% CI) | P* | Glucocorticoid monotherapy users, % (95% CI) | P† | |
| Overall glucocorticoid monotherapy use Sociodemographic characteristics |
10.2 (9.5–10.8) | 10.2 (9.5–10.8) | ||
| Age, years | < 0.0001 | < 0.0001 | ||
| 65–69 | 6.4 (5.3–7.5) | 6.7 (5.6–7.8) | ||
| 70–74 | 7.2 (6.1–8.2) | 7.9 (6.8–9.1) | ||
| 75–79 | 10.3 (8.9–11.7) | 10.6 (9.2–12.0) | ||
| 80–84 | 13.5 (11.7–15.3) | 12.5 (10.9–12.0) | ||
| ≥85 | 20.3 (17.6–23.0) | 16.3 (14.1–18.5) | ||
| Sex | 0.9122 | 0.2689 | ||
| Female | 10.2 (8.7–11.8) | 10.9 (9.4–12.5) | ||
| Male | 10.1 (9.4–10.5) | 10.0 (9.3–10.7) | ||
| Race/ethnicity | 0.0040 | 0.0925 | ||
| African American | 13.8 (11.2–16.4) | 10.9 (8.8–13.0) | ||
| Other | 9.4 (6.8–12.0) | 7.6 (5.5–9.8) | ||
| White | 9.8 (9.1–10.5) | 10.3 (9.6–11.0) | ||
| Comorbid illness | ||||
| Charlson comorbidity score | < 0.0001 | 0.0877 | ||
| 1 | 8.7 (8.0–9.5) | 9.8 (9.0–10.6) | ||
| 2 | 11.9 (10.3–13.4) | 10.1 (8.8–11.4) | ||
| ≥3 | 19.5 (16.4–22.6) | 11.9 (10.1–13.8) | ||
| Health care utilization | ||||
| Prescriptions originating from rheumatologist | < 0.0001 | < 0.0001 | ||
| None | 18.1 (16.6–19.6) | 13.2 (12.0–14.4) | ||
| ≥1 | 6.5 (5.8–7.1) | 8.0 (7.2–8.7) | ||
| Claims from rheumatologist | < 0.0001 | < 0.0001 | ||
| None | 20.9 (19.2–22.5) | 17.5 (16.0–19.0) | ||
| 1–4 | 7.6 (6.7–8.6) | 8.5 (7.4–9.5) | ||
| ≥5 | 3.3 (2.6–4.0) | 3.7 (2.9–4.4) | ||
| Annual physician office visits | 0.0026 | < 0.0050 | ||
| 0–8 | 10.5 (9.4–11.7) | 9.3 (8.3–10.4) | ||
| 9–15 | 8.6 (7.6–9.7) | 9.5 (8.4–10.6) | ||
| ≥16 | 11.4 (10.2–12.6) | 11.9 (10.6–13.2) | ||
| Annual hospitalizations | < 0.0001 | < 0.0001 | ||
| None | 8.6 (7.9–9.3) | 9.0 (8.3–9.7) | ||
| 1 | 13.2 (11.3–15.1) | 12.6 (10.9–14.4) | ||
| ≥2 | 19.5 (16.4–22.6) | 15.4 (12.8–19.0) | ||
| Socioeconomic status | ||||
| Area-level socioeconomic status‡ | 0.4710 | 0.4761 | ||
| Quintile 1 (lowest-income counties) | 9.8 (8.4–11.1) | 9.4 (8.0–10.8) | ||
| Quintile 2 | 9.6 (8.3–11.0) | 10.3 (8.9–11.6) | ||
| Quintile 3 | 9.9 (8.5–11.3) | 10.4 (9.0–11.7) | ||
| Quintile 4 | 11.4 (9.8–12.9) | 11.1 (9.6–12.6) | ||
| Quintile 5 (highest-income counties) | 10.3 (8.5–12.1) | 9.4 (7.7–11.2) | ||
| Personal income | < 0.0001 | 0.0002 | ||
| Not low | 9.0 (8.3–9.7) | 9.4 (8.6–10.1) | ||
| Enrolled in low-income subsidy program | 14.1 (12.5–15.7) | 12.3 (10.9–13.8) | ||
| Area-level physician supply | ||||
| Health professional shortage area | 0.2982 | 0.4264 | ||
| No shortage area | 11.0 (9.2–12.7) | 10.8 (9.0–12.6) | ||
| Shortage area | 10.0 (9.3–10.7) | 10.0 (9.3–10.8) | ||
| Census geographic divisions | < 0.0001 | < 0.0001 | ||
| New England | 10.9 (7.9–13.9) | 11.0 (8.1–14.0) | ||
| Mid-Atlantic | 15.0 (12.8–17.2) | 14.4 (12.3–16.5) | ||
| East North Central Midwest | 9.5 (12.8–17.2) | 10.4 (8.6–12.2) | ||
| West North Central Midwest | 8.1 (6.2–10.1) | 8.1 (6.2–9.9) | ||
| South Atlantic | 10.9 (9.4–12.4) | 11.2 (9.7–12.7) | ||
| East South Central | 9.3 (7.0–11.7) | 8.8 (6.7–11.0) | ||
| West South Central | 9.1 (7.4–10.9) | 9.2 (7.5–10.9) | ||
| Mountain | 7.7 (5.3–10.1) | 7.2 (5.1–9.3) | ||
| Pacific | 8.8 (7.0–10.6) | 8.5 (6.7–10.2) | ||
Computed from chi-square tests.
Computed from a logistic regression model adjusting for all variables listed.
Quintiles calculated based on percentage of individuals in residential county at or below 125% of the federal poverty limit.
In the unadjusted analysis, we found that African Americans were more likely than whites to receive glucocorticoid monotherapy (13.8%; 95% CI 11.2–16.4% versus 9.8%; 95% CI 9.1–10.5%). This difference did not remain statistically significant in our adjusted analysis.
However, in our adjusted analysis, differences by personal income persisted. Individuals with low personal incomes were more likely to receive glucocorticoid monotherapy (12.3%; 95% CI 10.9–13.8% versus 9.4%; 95% CI 8.6–10.1% in higher-income groups). Higher rates of glucocorticoid monotherapy were found among beneficiaries with more frequent use of health services. Among individuals who had ≥2 annual hospitalizations, 15.4% (95% CI 12.8–19.0%) received glucocorticoid monotherapy compared to 9.0% (95% CI 8.3–9.7%) of individuals who had no hospitalizations. Higher rates of glucocorticoid monotherapy were also found among beneficiaries with ≥16 annual physical visits (11.9%; 95% CI 10.6–13.2%) compared to those who had 0–8 annual visits (9.3%; 95% CI 8.3–10.4%).
Finally, we found geographic differences in glucocorticoid monotherapy use after adjusting for other factors. For example, glucocorticoid monotherapy use was more prevalent in the Mid-Atlantic region (14.4%; 95% CI 12.3–16.5%) compared to the Pacific region (8.5%; 95% CI 6.7–10.2%). The lowest rates of glucocorticoid monotherapy use were observed in the Mountain region (7.2%; 95% CI 5.1–9.3%) and the West North Central Midwest region (8.1%; 95% CI 6.2–9.9%). The C statistic for the adjusted model was 0.78, indicating good explanation by the association model.
There were no statistically significant interactions between socioeconomic variables and rheumatology contact. In Figure 2, the prevalence of glucocorticoid monotherapy by income and involvement of a rheumatologist in care is shown. Those with low personal incomes and no rheumatology contact were the most likely to receive glucocorticoid monotherapy, with 31.1% (95% CI 26.6–35.5%) of patients not receiving a DMARD compared to 22.2% (95% CI 19.6–24.8%) in other income groups with no rheumatology contact. For those with rheumatology contact, low-income individuals were only slightly more likely to receive glucocorticoid monotherapy (8.3%; 95% CI 6.8–9.8% versus 6.4%; 95% CI 5.7–7.1% in other income groups). We found a statistically significant interaction between age and medical comorbidity. Among those ages 65–74 years, those with greater comorbid disease were significantly more likely to receive glucocorticoid monotherapy when compared to those with fewer comorbidities (10.5%; 95% CI 7.9–13.1% versus 6.8%; 95% CI 6.0–7.7%). However, among those ages ≥85 years, those with greater comorbid disease were less likely to receive glucocorticoid monotherapy when compared to those with fewer comorbidities (15.4%; 95% CI 9.7–21.1% versus 16.5%; CI 14.2–18.9%).
Figure 2.
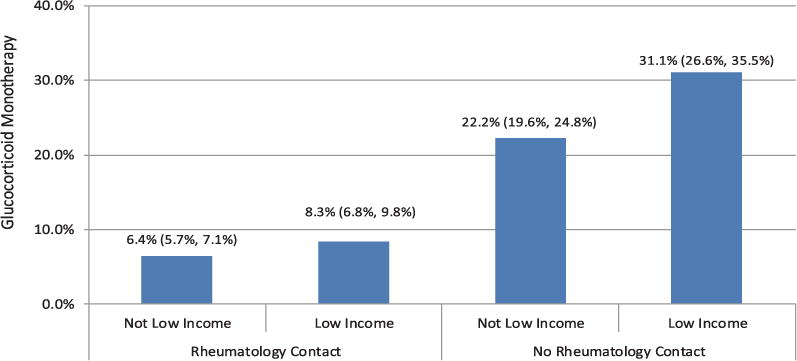
Adjusted percentages and 95% confidence intervals of glucocorticoid monotherapy users with rheumatoid arthritis by contact with rheumatologist and low-income status. Glucocorticoid monotherapy users were defined as those who had no disease-modifying antirheumatic drug dispensed during the measurement year and had ≥180 days or ≥900 mg of prednisone (or steroid equivalent) dispensed. Rheumatology contact was defined as ≥1 claims originating from a rheumatologist during 2009.
We performed sensitivity analyses to check the robustness of our findings. First, when we decreased the requirement for an annual dispensed glucocorticoid supply from ≥180 days to ≥120 days (~4 months) or an annual dispensed dose of ≥600 mg of oral prednisone or equivalent, our findings remained unchanged. There was a small increase in the percentage of patients classified as receiving glucocorticoid monotherapy (additional n = 87 [11.3% of total sample on glucocorticoid monotherapy]; 95% CI 10.6–12.0%). Older individuals and those with low personal income, less rheumatology contact, more physician office visits, and a greater number of hospitalizations were still statistically significantly more likely to receive glucocorticoid monotherapy. Second, when we repeated the analysis including only those who received prolonged courses of moderate-dose glucocorticoid monotherapy (≥180 days and ≥900 mg of prednisone equivalent), our findings remained unchanged except for a decreased likelihood of glucocorticoid monotherapy use among non-white and non-Hispanic races/ethnicities (see Supplementary Tables 1–3, available in the online version of this article at http://onlinelibrary.wiley.com/doi/10.1002/acr.22312/abstract). Finally, excluding the 20 individuals classified as having polymyalgia rheumatica or giant cell arteritis or the 35 individuals classified as having comorbid chronic obstructive pulmonary disease did not impact our findings.
DISCUSSION
In this nationwide study of Medicare beneficiaries enrolled in the Part D prescription drug program, we found that 10.2% of individuals treated for RA received glucocorticoids without DMARDs. Compared to DMARD users, glucocorticoid monotherapy users were more likely to be older, have a low income, and reside in certain US regions, and were less likely to have a rheumatologist prescribing their RA medication. Although older age and accompanying medical comorbidities may in some cases limit DMARD use, differences by income or geographic region may suggest persistent gaps in quality of care for RA despite expanded prescription drug coverage under Medicare Part D.
These results add to our understanding of DMARD use among individuals with RA in the US. Individuals treated with daily, moderate-dose glucocorticoids can reasonably be assumed to have active RA requiring therapy. Therefore, unexplained differences in treatment by sociodemographic characteristics such as income, geography, or contact with a rheumatologist likely represent differences in access to or quality of care for RA. We investigated several potential mechanisms that may underlie these findings. For example, we found that despite having minimal or no cost sharing for prescription drugs in Part D, the subset of individuals with low personal incomes who receive a low-income subsidy had a lower rate of DMARD use. Although the magnitude of this association was small, it is consistent with prior studies showing income-based disparities in DMARD use (6,14). Moreover, our findings imply that expanding drug coverage for low-income individuals has not been sufficient to eliminate these income-based disparities. However, as shown in Figure 2, we found that contact with a rheumatologist significantly attenuated income-based differences in DMARD use, suggesting that access to or use of specialty care is likely a key mechanism to reduce socioeconomic disparities in health care quality for RA.
We found that even minimal contact with a rheumatologist (i.e., one encounter per year) was associated with significantly higher DMARD use. As care for RA becomes increasingly complex, raising awareness regarding recommended treatment regimens among generalists and enhancing access to rheumatologists will become more important. Recent survey data confirm that although a majority of primary care physicians feel comfortable diagnosing RA, more than half who prescribed DMARDs felt either “somewhat” or “very” uncomfortable doing so. In addition, 37% stated that they do not initiate DMARDs (15). The study also found that 44% of physicians reported that their patients had significant difficulty accessing rheumatology care. It is possible that reluctance to prescribe DMARDs explains the higher use of glucocorticoid monotherapy among patients seeing generalists for RA in our study. Our findings also corroborate those of previous studies regarding the impact of rheumatology specialty care on DMARD use (5,7,16). For patients with geographic barriers to care, innovations in care delivery, including preconsultation exchange, telemedicine, and other technologies, are currently being pursued and appear promising in improving access to specialty care (17,18).
Our finding that glucocorticoid monotherapy was more prevalent with increasing age warrants discussion. There are several plausible explanations for this finding. Comorbid illness, including severe liver or renal disease and cancer, may appropriately limit DMARD use. Interestingly, controlling for comorbidities attenuated, but did not eliminate, the relationship between age and use of glucocorticoid monotherapy. It is possible that our models did not fully account for relevant comorbidities, or that additional factors, such as patient preferences or adherence, may explain the findings. However, it is also possible that DMARDs are underutilized in older patients, representing a gap in quality of care. This would be consistent with previous findings that some elderly patients are under-treated for RA because concerns regarding frailty or toxicity may prevent physicians from using DMARDs (19). Studies to date suggest that these fears may not always be justified, since side effects and efficacy of DMARDs may be similar in older patients (20–22). In addition, the moderate doses of glucocorticoids found in our study can be associated with significant toxicity in the elderly, where there is added concern regarding accelerated bone loss, skeletal fractures, cataracts, and diabetes mellitus, among other side effects. While we cannot definitively determine the factors driving higher glucocorticoid monotherapy use in older adults, our data suggest that further studies examining treatment strategies and outcomes in this group of patients are needed. In addition, although previous US guidelines have not directly addressed the role of glucocorticoids in RA treatment, achieving professional consensus on this topic might reduce practice variation in the treatment of this group of patients (2).
Finally, glucocorticoid monotherapy was associated with greater health care utilization, including more frequent hospitalizations, even after adjusting for factors such as age and comorbidities. Our data do not allow us to determine whether this association is causal. It is possible that patients who are not receiving DMARDs fare more poorly and are therefore hospitalized frequently and use more physician services. This would be consistent with a large body of literature suggesting that DMARDs protect against a variety of poor outcomes (2,23), and may represent missed opportunities to provide appropriate subspecialty care in both the inpatient setting as well as during care transitions to the outpatient setting. Alternatively, those with higher health care utilization may have comorbidities that preclude the use of DMARDs. The former hypothesis regarding a protective effect of DMARDs is supported by the fact that adjusting for comorbidities attenuated but did not eliminate the association between utilization and DMARD use, but further research, using both administrative and other data sources such as registries, is needed to definitively understand this relationship.
Our study has limitations that are important to consider. Data on prescriptions filled outside of Medicare Part D or Part B were unavailable. However, we expect that few individuals are likely to obtain outside medications, since discount suppliers such as Walmart do not carry DMARDs or oral glucocorticoids. Second, because our study used administrative data, information on variables of clinical interest, including disease activity scores, patient preferences, and other factors, were not available. Third, in our clinical experience, some patients are not candidates for DMARDs, and it is likely that glucocorticoid monotherapy was appropriate for some patients in our study. However, insofar as we found significant unexplained differences in the use of glucocorticoid monotherapy by factors such as socioeconomic status or geographic location, our results provide evidence for underutilization of DMARDs in specific groups of patients. Fourth, we used an administrative definition of RA that has only a moderate positive predictive value for RA (24). Although there has been some debate in the literature about the predictive value of RA diagnoses in administrative data (25,26), most studies suggest moderate sensitivity and specificity for RA codes in administrative data (24,27). We expect that the degree of misclassification in our study is substantially lower than in prior studies using administrative data, since all patients were receiving either a DMARD or glucocorticoids. Finally, racial/ethnic comparisons in our study were limited to examining African Americans and whites because other ethnicities (e.g., Hispanic/Latino) are not reliably coded in Medicare administrative data (28).
Our study has important implications for efforts to improve the quality of care for RA in the US. First, we found that individuals from traditionally at-risk populations, including the elderly and those with low socioeconomic status, were more likely to receive glucocorticoids without a DMARD for RA. These sociodemographic differences as well as the geographic variability in our study suggest disparities in the use of DMARD-containing regimens for the treatment of RA. Second, we observed these differences despite the fact that our study population had drug coverage under Medicare Part D, implying that expanding drug coverage alone has been insufficient to eliminate disparities in DMARD use. Instead, our findings provide evidence that access to rheumatology specialty care may play a key role in attenuating socioeconomic disparities in DMARD use for individuals with RA. These results may inform the efforts of payers and health systems seeking to improve quality of care for RA, particularly as health reform accelerates.
Supplementary Material
Significance & Innovations.
This is the first study to comprehensively evaluate the use of disease-modifying antirheumatic drugs (DMARDs) compared to glucocorticoids alone for the treatment of rheumatoid arthritis (RA) using newly available data from the Medicare Part D prescription drug program.
Older individuals and those with lower socioeconomic status were more likely to receive glucocorticoids without DMARDs for RA. Individuals residing in certain US regions and those who did not see a rheumatologist were also more likely to receive glucocorticoids alone.
Since DMARD use is recommended for individuals with RA, unwarranted variations in treatment may suggest persistent gaps in quality of care for RA despite expanded drug coverage under Part D.
Acknowledgments
Supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (awards K23-AR-060259 and P60-AR-053308), the University of California, San Francisco Resource Evaluation and Allocation Committee Grant Program, and the Rosalind Russell Medical Research Center for Arthritis.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Dr. Lin has received consulting fees, speaking fees, and/or honoraria (less than $10,000) from Informed Medical Decisions Foundation.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Yazdany had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Yazdany, Tonner, Schmajuk.
Acquisition of data. Yazdany, Tonner.
Analysis and interpretation of data. Yazdany, Tonner, Schmajuk, Lin, Trivedi.
References
- 1.Birnbaum H, Pike C, Kaufman R, Marynchenko M, Kidolezi Y, Cifaldi M. Societal cost of rheumatoid arthritis patients in the US. Curr Med Res Opin. 2010;26:77–90. doi: 10.1185/03007990903422307. [DOI] [PubMed] [Google Scholar]
- 2.Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, Kremer JM, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64:625–39. doi: 10.1002/acr.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Committee for Quality Assurance. HEDIS and quality measurement. http://ncqa.org/tabid/59/default.aspx.
- 4.Smolen JS, Landewe R, Breedveld FC, Buch M, Burmester G, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73:492–509. doi: 10.1136/annrheumdis-2013-204573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacLean CH, Louie R, Leake B, McCaffrey DF, Paulus HE, Brook RH, et al. Quality of care for patients with rheumatoid arthritis. JAMA. 2000;284:984–92. doi: 10.1001/jama.284.8.984. [DOI] [PubMed] [Google Scholar]
- 6.Schmajuk G, Trivedi AN, Solomon DH, Yelin E, Trupin L, Chakravarty EF, et al. Receipt of disease-modifying antirheumatic drugs among patients with rheumatoid arthritis in Medicare managed care plans. JAMA. 2011;305:480–6. doi: 10.1001/jama.2011.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmajuk G, Schneeweiss S, Katz JN, Weinblatt ME, Setoguchi S, Avorn J, et al. Treatment of older adult patients diagnosed with rheumatoid arthritis: improved but not optimal. Arthritis Rheum. 2007;57:928–34. doi: 10.1002/art.22890. [DOI] [PubMed] [Google Scholar]
- 8.Lee SJ, Kremer J, Kavanaugh A, for the Consortium of Rheumatology Researchers of North America (CORRONA) Treatment disparity related to race/ethnicity and education in rheumatoid arthritis patients: comment on the article by Constantinescu et al [letter] Arthritis Rheum. 2009;61:1141–2. doi: 10.1002/art.24727. [DOI] [PubMed] [Google Scholar]
- 9.US Department of Health and Human services, Health Resources and Services Administration, Bureau of Health Professions. Area Resource File (ARF) :2009–2012. [Google Scholar]
- 10.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–9. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 11.Bernatsky S, Lix L, Hanly JG, Hudson M, Badley E, Peschken C, et al. Surveillance of systemic autoimmune rheumatic diseases using administrative data. Rheumatol Int. 2011;31:549–54. doi: 10.1007/s00296-010-1591-2. [DOI] [PubMed] [Google Scholar]
- 12.Gershon AS, Wang C, Guan J, Vasilevska-Ristovska J, Cicutto L, To T. Identifying patients with physician-diagnosed asthma in health administrative databases. Can Respir J. 2009;16:183–8. doi: 10.1155/2009/963098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gershon AS, Wang C, Guan J, Vasilevska-Ristovska J, Cicutto L, To T. Identifying individuals with physician diagnosed COPD in health administrative databases. COPD. 2009;6:388–94. doi: 10.1080/15412550903140865. [DOI] [PubMed] [Google Scholar]
- 14.Solomon DH, Yelin E, Katz JN, Lu B, Shaykevich T, Ayanian JZ. Treatment of rheumatoid arthritis in the Medicare Current Beneficiary Survey. Arthritis Res Ther. 2013;15:R43. doi: 10.1186/ar4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garneau KL, Iversen MD, Tsao H, Solomon DH. Primary care physicians’ perspectives towards managing rheumatoid arthritis: room for improvement. Arthritis Res Ther. 2011;13:R189. doi: 10.1186/ar3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solomon DH, Ayanian JZ, Yelin E, Shaykevich T, Brookhart MA, Katz JN. Use of disease-modifying medications for rheumatoid arthritis by race and ethnicity in the National Ambulatory Medical Care Survey. Arthritis Care Res (Hoboken) 2012;64:184–9. doi: 10.1002/acr.20674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jong M, Kraishi M. A comparative study on the utility of telehealth in the provision of rheumatology services to rural and northern communities. Int J Circumpolar Health. 2004;63:415–21. doi: 10.3402/ijch.v63i4.17758. [DOI] [PubMed] [Google Scholar]
- 18.Harrington JT, Walsh MB. Pre-appointment management of new patient referrals in rheumatology: a key strategy for improving health care delivery. Arthritis Rheum. 2001;45:295–300. doi: 10.1002/1529-0131(200106)45:3<295::AID-ART263>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 19.Tutuncu Z, Reed G, Kremer J, Kavanaugh A. Do patients with older-onset rheumatoid arthritis receive less aggressive treatment? Ann Rheum Dis. 2006;65:1226–9. doi: 10.1136/ard.2005.051144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bathon JM, Fleischmann RM, van der Heijde D, Tesser JR, Peloso PM, Chon Y, et al. Safety and efficacy of etanercept treatment in elderly subjects with rheumatoid arthritis. J Rheumatol. 2006;33:234–43. [PubMed] [Google Scholar]
- 21.Genevay S, Finckh A, Ciurea A, Chamot AM, Kyburz D, Gabay C, for the Physicians of the Swiss Clinical Quality Management Program for Rheumatoid Arthritis Tolerance and effectiveness of anti–tumor necrosis factor α therapies in elderly patients with rheumatoid arthritis: a population-based cohort study. Arthritis Rheum. 2007;57:679–85. doi: 10.1002/art.22688. [DOI] [PubMed] [Google Scholar]
- 22.Hirshberg B, Muszkat M, Schlesinger O, Rubinow A. Safety of low dose methotrexate in elderly patients with rheumatoid arthritis. Postgrad Med J. 2000;76:787–9. doi: 10.1136/pmj.76.902.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schoels M, Wong J, Scott DL, Zink A, Richards P, Landewe R, et al. Economic aspects of treatment options in rheumatoid arthritis: a systematic literature review informing the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis. 2010;69:995–1003. doi: 10.1136/ard.2009.126714. [DOI] [PubMed] [Google Scholar]
- 24.Kim SY, Servi A, Polinski JM, Mogun H, Weinblatt ME, Katz JN, et al. Validation of rheumatoid arthritis diagnoses in health care utilization data. Arthritis Res Ther. 2011;13:R32. doi: 10.1186/ar3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ng B, Aslam F, Petersen NJ, Yu HJ, Suarez-Almazor ME. Identification of rheumatoid arthritis patients using an administrative database: a Veterans Affairs study. Arthritis Care Res (Hoboken) 2012;64:1490–6. doi: 10.1002/acr.21736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmajuk G, Yazdany J. Underestimation of the reliability of codes for rheumatoid arthritis within administrative data: comment on the article by Ng et al [letter] Arthritis Care Res (Hoboken) 2013;65:835–6. doi: 10.1002/acr.21911. [DOI] [PubMed] [Google Scholar]
- 27.Gabriel SE. The sensitivity and specificity of computerized databases for the diagnosis of rheumatoid arthritis. Arthritis Rheum. 1994;37:821–3. doi: 10.1002/art.1780370607. [DOI] [PubMed] [Google Scholar]
- 28.Arday SL, Arday DR, Monroe S, Zhang J. HCFA’s racial and ethnic data: current accuracy and recent improvements. Health Care Financ Rev. 2000;21:107–16. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


