Abstract
Background
Stereotactic body radiation therapy (SBRT) is a promising option for patients with pancreatic cancer (PCA); however, limited data support its efficacy. This study reviews our institutional experience of SBRT in the treatment of locally advanced (LAPC) and borderline resectable (BRPC) PCA.
Methods
Charts of all PCA patients receiving SBRT at our institution from 2010 to 2014 were reviewed. Most patients received pre-SBRT chemotherapy. Primary endpoints included overall survival (OS) and local progression-free survival (LPFS). Patients received a total dose of 25–33 Gy in five fractions.
Results
A total of 88 patients were included in the analysis, 74 with LAPC and 14 with BRPC. The median age at diagnosis was 67.2 years, and median follow-up from date of diagnosis for LAPC and BRPC patients was 14.5 and 10.3 months, respectively. Median OS from date of diagnosis was 18.4 months (LAPC, 18.4 mo; BRPC, 14.4 mo) and median PFS was 9.8 months (95 % CI 8.0–12.3). Acute toxicity was minimal with only three patients (3.4 %) experiencing acute grade ≥3 toxicity. Late grade ≥2 gastrointestinal toxicity was seen in five patients (5.7 %). Of the 19 patients (21.6 %) who underwent surgery, 79 % were LAPC patients and 84 % had margin-negative resections.
Conclusions
Chemotherapy followed by SBRT in patients with LAPC and BRPC resulted in minimal acute and late toxicity. A large proportion of patients underwent surgical resection despite limited radiographic response to therapy. Further refinements in the integration of chemotherapy, SBRT, and surgery might offer additional advancements toward optimizing patient outcomes.
Pancreatic cancer (PCA) remains one of the most deadly cancers in the United States (US), contributing to more than 37,500 deaths in 2013.1 Despite aggressive combined modality treatment, 5-year survival remains dismal at <5 %.1,2
Of the current treatment modalities, surgical resection appears to be the only potentially curable option.3 Unfortunately, most patients are unresectable at initial diagnosis with <20 % being deemed surgical candidates.4 Furthermore, even resected patients have a poor prognosis (5-year survival rate of 7–25 %) due to high rates of margin-positivity and development of local and/or distant disease.
Currently, the standard of care in the US for unresectable, locally advanced (LAPC) and borderline resectable pancreatic cancer (BRPC) patients includes a combination of chemotherapy and radiation therapy (RT); however, optimal treatment sequence, radiation technique, and total dose are controversial.5 Combined chemotherapy and chemoradiation (CRT) appears to be particularly effective in BRPC due to its ability to improve local control (LC) and increase the likelihood of a margin-negative resection.
Conventional external beam radiation therapy (EBRT) with concurrent chemotherapy may require up to 7 weeks to complete and can result in acute and late toxicity.4 Recent advancements in RT techniques have resulted in an increased use of stereotactic body radiation therapy (SBRT). Reduced fractionation, increased feasibility, and established efficacy in other disease sites have further substantiated this modality.6,7 Earlier studies evaluating SBRT in patients with LAPC have reported excellent LC rates but have also resulted in significant late grade 2–4 gastrointestinal toxicity.8–11 Notably, these studies used larger fraction sizes (15 Gy × 3, 25 Gy × 1) and lacked standardized dose constraints for adjacent normal structures, such as the small bowel and stomach.
We report our institutional experience utilizing definitive five-fraction SBRT for LAPC and BRPC patients.
METHODS AND MATERIALS
All patients with histologically confirmed borderline resectable or locally advanced pancreatic adenocarcinoma who underwent definitive SBRT treatment at our institution from January 2010 to 2014 were retrospectively reviewed. Definitive SBRT is defined as SBRT given to patients as the primary treatment modality with or without chemotherapy. Patients were excluded if they had: (1) radiographic evidence of metastatic disease at the time of SBRT, (2) received adjuvant SBRT following surgery, or (3) received SBRT as salvage therapy following previous chemoradiation.
All patients provided informed consent before treatment and, when applicable, study approval was granted by the internal institutional review board (IRB). The population included 40 LAPC patients treated on two institutional prospective studies (NCT01146054, NCT01781728) and 48 who were treated off protocol. Staging of BRPC or LAPC was based on review of imaging at our institutional multidisciplinary pancreatic clinic or tumor board, following criteria defined by the Americas Hepato-Pancreato-Biliary Association/Society of Surgical Oncology/Society for Surgery of the Alimentary Tract.12,13
Treatment Intervention
All patients received SBRT at our institution, whereas chemotherapy administration was allowed at outside institutions. Thirty-two patients (36.3 %) were included as part of a larger multi-institutional trial in which patients received up to 3 weeks of gemcitabine before delivery of SBRT followed by gemcitabine until progression, toxicity, or surgical exploration. Another eight patients were treated on a single-institution trial that allowed chemotherapy before SBRT delivered using identical technique and dose as the multicenter trial. Nonprotocol patients also received chemotherapy before SBRT. Pre-SBRT chemotherapy regimens consisted of: (1) gemcitabine alone; (2) gemcitabine-based regimens involving cisplatin, 5-fluorouracil, or nab-paclitaxel; or (3) 5-fluorouracil, leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX)-based regimens. Patients often resumed chemotherapy 1–4 weeks following SBRT.
Before CT simulation (1–4 weeks before SBRT), the majority of patients had gold fiducials implanted into or near the pancreatic tumor using endoscopic ultrasound guidance.14 CT simulation, diagnostic pancreas-protocol CT, and positron emission/computed tomography were used for treatment planning.
Dose Constraints
The gross tumor volume (GTV) was identified from a combination of CT, PET/CT, and magnetic resonance imaging (MRI) images when available. The planning target volume (PTV) was defined as the GTV plus a 2- to 3-mm margin to account for microscopic extension and set-up error. A modified PTV was created by editing back the PTV when there was overlap with duodenum, small bowel, and/or stomach.
The PTV received a total dose of 25–33 Gy in five fractions (5–6.6 Gy/fraction), allowing for up to 30 % heterogeneity within the PTV. Greater than 90 % of the PTV received 100 % of the prescribed dose and no more than 1 cc of the PTV received >130 % of the prescription dose. If organs at risk, such as the duodenum, small bowel, and/or stomach, were within 1 cm of the PTV, they were labeled as “proximal structures” with specific radiation dose constraints. In some cases, the fraction and total dose was limited to <33 Gy to meet these normal tissue dose constraints.
Statistical Analysis
For comparison, overall survival (OS), progression-free survival (PFS), and local progression-free survival (LPFS) were calculated from both date of pathological diagnosis and start of SBRT treatment. For PFS and LPFS analysis, patients were censored at the date of last scan. Local progression was defined based on blinded centralized review using the revised Response Evaluation Criteria in Solid Tumors (RECIST) guidelines, version 1.1.15 Acute toxicity was defined as toxicity occurring ≤90 days of SBRT, whereas late toxicity comprises those occurring >90 days after SBRT. The toxicities reported were those definitively or possibly attributed to the intervention.
Survival was analyzed using the Kaplan–Meier method, and differences between patient groups defined at baseline were estimated with Cox proportional hazards models that included terms for age, sex, race and a time-dependent covariate representing the time from diagnosis to treatment. Differences in patient groups defined post-SBRT were estimated using the same approach but did not include the time-dependent covariate for treatment. P < 0.05 was considered significant in this unadjusted exploratory analysis. Statistical analysis was performed using R version 3.03 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Patient Characteristics
Of the 105 patients who were treated between January 2010 and 2014, 88 were included in the final analysis. Patient demographics and disease characteristics are summarized in Table 1. The median age at diagnosis was 67.2 years [interquartile range (IQR) 59.9–72.3 years]. A total of 74 patients (84 %) had LAPC, whereas 14 (16 %) were diagnosed with BRPC. The median SBRT dose delivered was 6.6 Gy in 5 fractions.
TABLE 1.
Patient demographics and baseline disease characteristics
| Characteristic | Protocol patients, n (%) |
Nonprotocol patients, n (%) |
Total, n (%) | p value |
|---|---|---|---|---|
| Total | 40 | 48 | 88 | |
| Age (year), median (range) | 66.8 (35.6–85.7) | 67.2 (45–87.5) | 67.1 (35.6–87.5) | 0.887 |
| Locally advanced | 40 (100) | 34 (71) | 74 (84) | <0.001* |
| Borderline resectable | 0 (0) | 14 (29) | 14 (16) | |
| Male gender | 23 (57) | 24 (50) | 47 (53) | 0.525 |
| Caucasian | 36 (90) | 41 (85) | 77 (88) | 0.748 |
| Baseline ECOG performance status | 0.667 | |||
| 0 | 16 (40) | 22 (46) | 38 (43) | |
| ≥1 | 24 (60) | 26 (54) | 50 (57) | |
| Location of tumor | 0.547 | |||
| Head/Uncinate/Neck | 31 (77) | 38 (79) | 69 (72) | |
| Body/tail | 9 (22) | 10 (21) | 19 (28) | |
| Pre-SBRT chemotherapy | 77 (88) | >0.99 | ||
| Gemcitabine-based | 29 (72) | 30 (62) | 59 (76) | |
| FOLFIRINOX-based | 6 (15) | 12 (25) | 18 (24) | |
| Baseline CA19-9, median (range) | 186 (0–3,790.2) | 111.2 (0–5,951.1) | 168.6 (0–5,951.1) | 0.638 |
| Post-SBRT CA19-9, median (range) | 60.6 (0–4,087.9) | 78 (0–7,065.1) | 62.7(0–7,065.1) | 0.966 |
| Fraction size | <0.001* | |||
| 6.6 Gy | 37 (92) | 17 (35) | 54 (61) | |
| Other | 3 (8) | 31 (64) | 34 (39) | |
| Pre-SBRT stent placement | 10 (25) | 18 (37) | 28 (32) | 0.254 |
Statistically significant (p < 0.05)
The median time from date of diagnosis to first day of SBRT treatment was 2.9 months (IQR 1.4–6.0). Thirty-two patients received SBRT < 2 months following initial diagnosis and 56 patients received SBRT ≥ 2 months following diagnosis. For the entire cohort, the median time of follow-up from the first day of SBRT was 13.1 months (IQR 8.9–19.1): 10.3 months for BRPC patients and 14.6 months for LAPC patients. At last follow-up, 38 patients were alive.
Treatment Outcomes
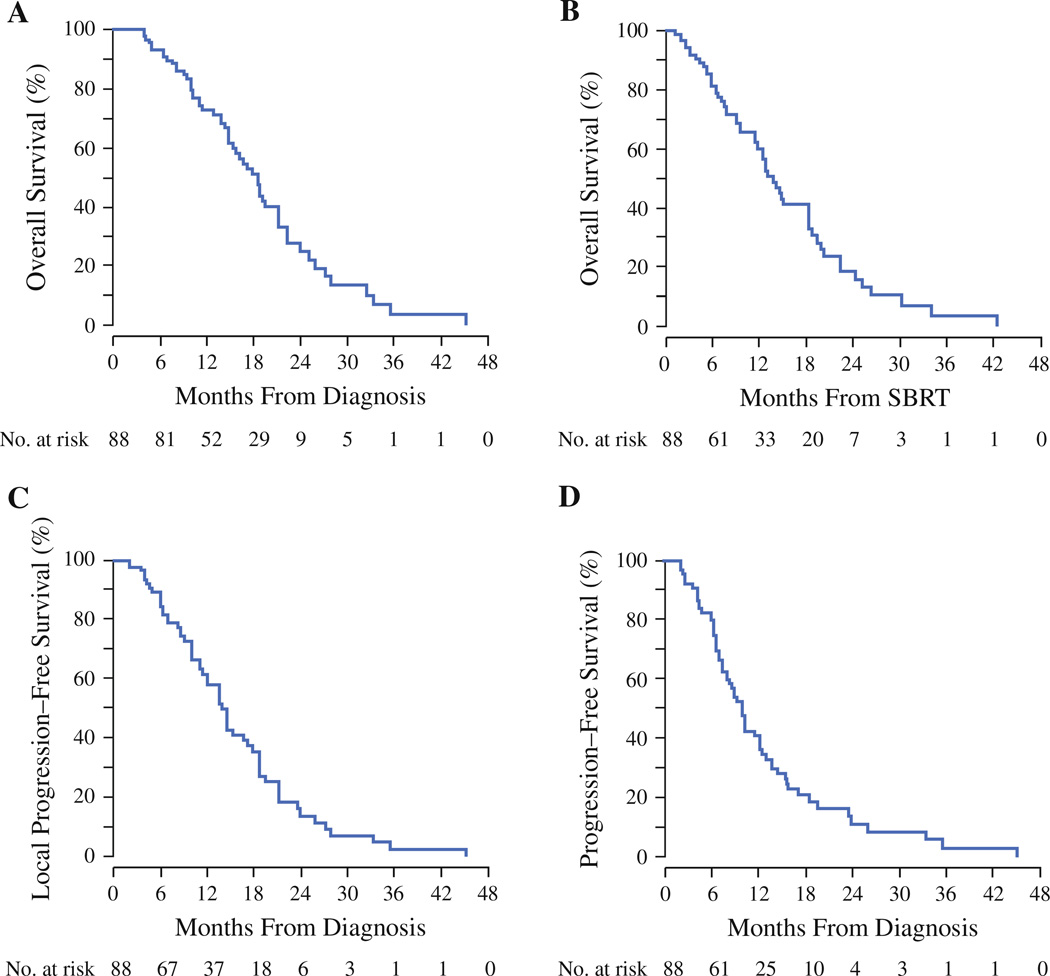
Median OS from date of diagnosis for the entire cohort was 18.4 months (95 % CI 15.3–21.3; Fig. 1a). One- and 2-year OS rates were 73 and 24 %, respectively. Median OS from start of SBRT treatment for the entire cohort was 13.7 months (95 % CI 11.4–16.0), with 1- and 2-year OS rates of 60 and 15 %, respectively (Fig. 1b). Patients with LAPC had a median OS of 18.4 months, whereas patients with BRPC had a median OS of 14.4 months (p = 0.87).
FIG. 1.
Kaplan–Meier curves depicting a overall survival from the date of diagnosis, b overall survival from the start of SBRT, c local progression-free survival from the date of diagnosis, and d progression-free survival from date of diagnosis
Median LPFS and PFS are illustrated in Fig. 1c, d, respectively. Median LPFS from date of diagnosis was 13.9 months (95 % CI 12.02–17.87). One- and 2-year LPFS rates, from date of diagnosis, were 61 and 14 %, respectively (Fig. 1c). Median PFS from date of diagnosis for the entire cohort was 9.9 months (95 % CI 8.0–12.3), with 1- and 2-year PFS of 41 and 11 %, respectively (Fig. 1d).
The 77 patients who received pre-SBRT chemotherapy survived a median of 18.8 months (95 % CI 16.4–21.3) from the date of diagnosis compared with 9.0 months (95 % CI 8.0–45.2) for those patients who did not receive chemotherapy (14 %; p = 0.1). Forty-five patients received gemcitabine only regimens, 14 received gemcitabine-based regimens, and 18 received FOLFIRINOX. Eleven (79 %) BRPC patients received chemotherapy before SBRT compared with 66 (89 %) of LAPC patients. There was no significant difference in OS in patients who received more versus less aggressive chemotherapy regimens (i.e., FOLFIRINOX vs. Gemcitabine; p > 0.05).
The median carbohydrate antigen 19-9 (CA19-9) level prior to SBRT was 168 U/µL. Patients who had a baseline CA19-9 ≥ 168 U/µL had a lower median OS compared with patients with a CA19-9 < 168 U/µL (p = 0.1). Additional survival outcomes are described in Table 2.
TABLE 2.
Overall survival (OS) outcomes from the date of pathological diagnosis or from start of SBRT treatment
| N | Median overall survival (mo) |
HRa | (95 % CI) | p value | |
|---|---|---|---|---|---|
| Total | 88 | 18.4 (15.3–21.3) | |||
| OS from date of diagnosis | |||||
| Protocol patients | 40 | 18.83 (14.8–23.8) | 1.00 | – | |
| Nonprotocol patients | 48 | 17.08 (14.6–27.2) | 1.13 | (0.66–1.96) | 0.65 |
| Tumor in body or tail | 19 | NA | 1.00 | – | |
| Tumor in head, neck, or uncinate | 69 | 17.08 (14.6–21.2) | 2.00 | (0.76–4.9) | 0.17 |
| Baseline ECOG 0 | 38 | 18.86 (16.7–27.2) | 1.00 | – | |
| Baseline ECOG ≥ 1 | 50 | 15.34 (11.3–22.2) | 1.47 | (0.85–2.53) | 0.16 |
| No baseline pain | 47 | 19.52 (16.1–27.9) | 1.00 | – | |
| Baseline pain | 41 | 15.34 (14.4–21.2) | 1.58 | (0.87–2.62) | 0.14 |
| Baseline CA19-9 < 168 U/µL | 41 | 19.12 (17.1–45.2) | 1.00 | – | |
| Baseline CA19-9 ≥ 168 U/µL | 42 | 16.07 (11.0–21.3) | 1.59 | (0.91–2.77) | 0.1 |
| Pre-SBRT chemotherapy | 77 | 18.83 (16.1–22.2) | 0.54 | (0.23–1.09) | 0.1 |
| No pre-SBRT chemotherapy | 11 | 9.00 (8.0–45.2) | 1.00 | ||
| Borderline resectable | 14 | 14.39 (11.0–45.2) | 0.88 | (0.31–2.49) | 0.81 |
| Locally advanced | 74 | 18.41 (15.3–21.3) | 1.00 | – | |
| Pre-SBRT stent placed | 28 | 17.87 (11.3–45.2) | 1.05 | (0.58–1.89) | 0.88 |
| Pre-SBRT no stent placed | 60 | 18.83 (14.8–22.2) | 1.00 | – | |
| OS from start of SBRT | |||||
| Protocol patients | 40 | 18.2 (11.7–22.3) | 1.00 | – | |
| Nonprotocol patients | 48 | 12.7 (9.4–18.2) | 1.61 | (0.92–2.82) | 0.09 |
| No surgical resection | 69 | 12.75 (9.7–18.2) | 1.00 | ||
| Surgical resection | 19 | 20.24 (12.5–42.4) | 0.34 | (0.10–1.08) | 0.07 |
| Post SBRT ECOG 0 | 44 | 18.27 (14.6–42.4) | 1.00 | – | |
| Post SBRT ECOG ≥ 1 | 44 | 11.73 (7.8–18.2) | 1.56 | (0.89–2.71) | 0.12 |
| Post SBRT CA19-9 < 62 U/µL | 36 | 15.01 (12.8–24.3) | 1.00 | – | |
| Post SBRT CA19-9 ≥ 62 U/µL | 39 | 12.42 (9.2–19.9) | 1.34 | (0.75–2.37) | 0.32 |
| Fraction size 6.6 Gy/fx | 54 | 13.7 (9.7–19.9) | 1.00 | – | |
| Fraction size < 6.6 Gy/fx | 34 | 13.0 (12.4–42.4) | 1.12 | (0.63–1.99) | 0.69 |
Statistically significant (p < 0.05)
Hazard ratios from diagnosis are adjusted for age, sex, race and a time-dependent covariate for the time between diagnosis and treatment. Hazard ratios from treatment are adjusted for age, sex, and race only
Surgical Response
A total of 19 patients, 15 LAPC and 4 BRPC, underwent surgery following chemotherapy and SBRT (Table 3). All but one patient had surgery at our institution. Surgical resection occurred at a median of 2.1 months (range 0.1–10.5) from the end of SBRT. Of these patients, 16 (84 %) had margin-negative resections and 14 patients (74 %) had lymph node-negative resections. Based on surgical pathology review, 16 % of patients had a pathologic complete response (pCR), 10 % had a near pCR, and 74 % had a partial response. There were no significant postoperative complications seen in these patients. Resected patients had a median OS of 20.2 months (95 % CI 12.5–42.4) from SBRT compared with 12.3 months for unresected patients (p = 0.07).
TABLE 3.
Surgical characteristics
| Characteristics | Value n (%) |
|---|---|
| Total | 19 (22) |
| Locally advanced | 15 (79) |
| Borderline resectable | 4 (21) |
| Pathology | |
| Pathologic complete response | 3 (16) |
| Pathologic near complete responsea | 2 (10) |
| T stage following SBRT | |
| T1 | 7 (37) |
| T2 | 3 (33) |
| T3 | 5 (26) |
| SMV-PV involvement | |
| >180° | 8 (42) |
| SMA involvement | |
| >180° | 4 (21) |
| CHA involvement | |
| >180° | 6 (31) |
| Celiac artery involvement | |
| >180° | 5 (26) |
| Tumor diameter (cm) | |
| ≤3 | 14 (74) |
| >3 | 5 (26) |
| Margin-negative | 16 (85) |
| Pre-SBRT chemotherapy | 19 (100) |
| Gemcitabine-based | 11 (58) |
| FOLFIRINOX-based | 8 (42) |
LAPC locally advanced pancreatic cancer; BRPC borderline resectable pancreatic cancer; SMA superior mesenteric artery; SMV superior mesenteric vein; PV portal vein; CHA common hepatic artery
One case of microscopic foci of glandular carcinoma in situ and one case of scattered foci of a residual viable tumor of undetermined size
Toxicity
Gastrointestinal toxicity in this cohort of patients was minimal. Acute grade ≥3 gastrointestinal toxicity (3.4 %) included one duodenal ulcer (grade 3), one case of gastritis (grade 3), and one grade 4 gastrointestinal bleed (Table 4). Five patients (5.7 %) experienced significant late grade ≥2 gastrointestinal toxicity, including three duodenal ulcers (grade 3), one enteric fistula (grade 4), and one gastrointestinal bleed (grade 5).
TABLE 4.
Acute gastrointestinal toxicities within 90 days of radiation therapy
| Category | Grade 2 (%) |
Grade 3 (%) |
Grade 4 (%) |
Grade 5 (%) |
|---|---|---|---|---|
| Acute toxicity | ||||
| Nonhematologic | ||||
| Enteritis | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Fistula | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Gastritis | 0 (0) | 1 (1.1) | 0 (0) | 0 (0) |
| Ulcer | 0 (0) | 1 (1.1) | 0 (0) | 0 (0) |
| Other GI toxicities | 0 (0) | |||
| Abdominal pain | 4 (4.5) | 0 (0) | 0 (0) | 0 (0) |
| Anorexia | 5 (5.7) | 0 (0) | 0 (0) | 0 (0) |
| Constipation | 1 (1.1) | 0 (0) | 0 (0) | 0 (0) |
| Dehydration | 0 (0) | 0 (0) | 0 (0) | 1 (1.1)a |
| Diarrhea | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Dyspepsia/heartburn | 1 (1.1) | 0 (0) | 0 (0) | 0 (0) |
| Fatigue | 7 (8.0) | 0 (0) | 0 (0) | 0 (0) |
| Other | 0 (0) | 2 (2.3)b | 1 (1.1)c | 0 (0) |
| Hematologic | ||||
| Anemia | 6 (6.8) | 0 (0) | 0 (0) | 0 (0) |
| Lymphopenia | 13 (14.7) | 5 (5.7) | 0 (0) | 0 (0) |
| Neutropenia | 2 (2.3) | 1 (1.1) | 0 (0) | 0 (0) |
| Thrombocytopenia | 4 (4.5) | 1 (1.1) | 0 (0) | 0 (0) |
Death secondary to Clostridium difficile dehydration
Two patients were hospitalized due to sepsis
GI bleed requiring blood transfusion and stabilization
DISCUSSION
To date, there is no clear standard treatment paradigm for LAPC or BRPC patients in the US and in cases where RT is used, the proper sequence and delivery of chemotherapy and RT is uncertain. Due to inadequate LC rates observed with standard dose CRT in the management of PCA (~50 %), more emphasis has shifted to dose escalation to the primary pancreatic tumor with intensity-modulated radiation therapy (IMRT) or SBRT.8–10,16 The majority of the literature on SBRT in PCA is in the locally advanced setting. The role of SBRT in patients with BRPC is less understood.4,7–11,17,18
An initial phase I trial at Stanford University evaluated single-fraction SBRT (25 Gy) in six LAPC patients.8 Results demonstrated excellent LC and no acute grade ≥3 toxicity, thereby establishing the feasibility of single-fraction 25 Gy SBRT. Follow-up phase II studies from the Stanford group reported good rates of LC and median OS, although the rate of late grade ≥2 toxicity was high (47 %).9,10 Similarly, Hoyer et al. demonstrated an increased rate of significant late gastrointestinal toxicity associated with 30 Gy SBRT in 3 fractions.11
The high rates of toxicity observed in these studies led to increased interest in fractionated SBRT regimens. Subsequent retrospective analyses investigating 3–5 fraction SBRT regimens in patients with LAPC have reported comparable rates of LC (>70 %) with much lower rates of grade ≥3 toxicity.4,5,19 This study also reports minimal gastrointestinal toxicities with the use of 5-fraction SBRT (<6 % late-grade ≥2 gastrointestinal toxicity). The use of prophylactic proton-pump inhibitors in all patients and exclusion of patients who had direct extension of the tumor into the bowel or stomach on endoscopy starting in 2012 likely contributed to the favorable toxicity profile of this regimen.
The LC rate observed in our study (1-year LPFS, 61 %) is slightly lower than those reported in previous studies.8–10,20 It is difficult to directly compare our LPFS values with other studies given the various definitions of LPFS and limited follow-up time. Also, several of our patients progressed at the first post-SBRT scan. It is possible that this initial enlargement was due to inflammation from SBRT as opposed to true progression.
Nevertheless, the median OS of 13.7 months from the start of SBRT in this study is comparable to previous reports of fractionated SBRT.8–10,21 It is important to note, however, that this study reports on both BRPC and LAPC patients, whereas the majority of previous studies reported outcomes on patients with LAPC only. Interestingly, our study demonstrates no significant difference in survival between LAPC and BRPC patients, which could be due to the heterogeneity of chemotherapy regimens or limited median follow-up time of patients with BRPC (10.3 months) when compared to LAPC (14.6 months).
Recently, Chuong et al. also reported promising results using induction gemcitabine, Taxotere, and capecitabine chemotherapy followed by SBRT (median dose delivered to the tumor was 25 Gy in 5 fractions and 35 Gy to the portion of tumor surrounding vasculature) in BRPC and LAPC.5 Median OS was 16.4 and 15.0 months in BRPC and LAPC patients, respectively, and 1-year LC was 81 % with minimal grade ≥3 toxicity.5 In our study, we did not use this “dose painting” approach that conserves the dose of radiation to the tumor and adds a boost to the surrounding vasculature. Instead, we gave a uniform dose to the entire tumor, allowing for up to 30 % heterogeneity within the tumor. Dose heterogeneity allowed for personalized treatment planning in which the radiation dose to both the tumor and the vasculature was optimized, likely leading to improved rates of resectability and pCR. Using this method, we found similar rates of surgical resection and median OS after chemotherapy and SBRT. Interestingly, a higher percentage of patients who were initially deemed unresectable were able to undergo successful surgical resection in our study (20.3 vs. 12.5 %), in addition to having higher rates of pCR.
A subset of our cohort (21.6 %) underwent successful surgical resection, with an 84 % margin-negative rate, following chemotherapy and SBRT. Although the number of resected patients is small, these results are higher than other reported studies, especially for patients with LAPC.5,22 For example, Rajagopalan et al. recently reported their experience with neoadjuvant SBRT (24 Gy in 1 fraction, 36 Gy in 3 fractions) in five BRPC and seven LAPC patients. Similar to our results, they reported a high margin-negative resection rate of 92 %.22 Notably, 79 % of our resected patients had LAPC. This is a particularly high rate of conversion from unresectable to resectable disease in comparison to previous reports.5,22
Although the role of SBRT in patients with LAPC and BRPC is yet to be fully defined, our single-institution experience suggests that SBRT results in minimal toxicity and comparable survival to historical reports of patients treated with conventional CRT. We report that a high proportion of patients with LAPC are able to undergo margin-negative resections and can demonstrate pCR; while encouraging, longer follow-up on these resected patients is needed to see how pCR correlates with survival. Currently, in a multi-center study with Stanford we are evaluating FOLFIRINOX with or without SBRT (8 Gy × 5) in patients with LAPC. In addition, SBRT is being considered in several prospective and cooperative group studies in BRPC and resected PCA. However, additional prospective studies are necessary to determine how to optimally integrate SBRT with more aggressive systemic regimens in an attempt to improve resection rates and survival in patients with LAPC and BRPC.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1245/s10434-014-4274-5) contains supplementary material, which is available to authorized users.
DISCLOSURE None.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362(17):1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 3.Wagner M, Redaelli C, Lietz M, Seiler CA, Friess H, Buchler MW. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg. 2004;91(5):586–594. doi: 10.1002/bjs.4484. [DOI] [PubMed] [Google Scholar]
- 4.Mahadevan A, Jain S, Goldstein M, et al. Stereotactic body radiotherapy and gemcitabine for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2010;78(3):735–742. doi: 10.1016/j.ijrobp.2009.08.046. [DOI] [PubMed] [Google Scholar]
- 5.Chuong MD, Springett GM, Freilich JM, et al. Stereotactic body radiation therapy for locally advanced and borderline resectable pancreatic cancer is effective and well tolerated. Int J Radiat Oncol Biol Phys. 2013;86(3):516–522. doi: 10.1016/j.ijrobp.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 6.Iyengar P, Westover K, Timmerman RD. Stereotactic ablative radiotherapy (SABR) for non-small cell lung cancer. Semin Respir Crit Care Med. 2013;34(6):845–854. doi: 10.1055/s-0033-1358554. [DOI] [PubMed] [Google Scholar]
- 7.Timmerman RD, Kavanagh BD, Cho LC, Papiez L, Xing L. Stereotactic body radiation therapy in multiple organ sites. J Clin Oncol. 2007;25(8):947–952. doi: 10.1200/JCO.2006.09.7469. [DOI] [PubMed] [Google Scholar]
- 8.Koong AC, Le QT, Ho A, et al. Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2004;58(4):1017–1021. doi: 10.1016/j.ijrobp.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Koong AC, Christofferson E, Le QT, et al. Phase II study to assess the efficacy of conventionally fractionated radiotherapy followed by a stereotactic radiosurgery boost in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2005;63(2):320–323. doi: 10.1016/j.ijrobp.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Schellenberg D, Goodman KA, Lee F, et al. Gemcitabine chemotherapy and single-fraction stereotactic body radiotherapy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2008;72(3):678–686. doi: 10.1016/j.ijrobp.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 11.Hoyer M, Roed H, Sengelov L, et al. Phase-II study on stereotactic radiotherapy of locally advanced pancreatic carcinoma. Radiother Oncol. 2005;76(1):48–53. doi: 10.1016/j.radonc.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 12.Callery MP, Chang KJ, Fishman EK, Talamonti MS, Traverso WL, Linehan DC. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: Expert consensus statement. Ann Surg Oncol. 2009;16(7):1727–1733. doi: 10.1245/s10434-009-0408-6. [DOI] [PubMed] [Google Scholar]
- 13.Pawlik TM, Laheru D, Hruban RH, et al. Evaluating the impact of a single-day multidisciplinary clinic on the management of pancreatic cancer. Ann Surg Oncol. 2008;15(8):2081–2088. doi: 10.1245/s10434-008-9929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khashab MA, Kim KJ, Tryggestad EJ, et al. Comparative analysis of traditional and coiled fiducials implanted during EUS for pancreatic cancer patients receiving stereotactic body radiation therapy. Gastrointest Endosc. 2012;76(5):962–971. doi: 10.1016/j.gie.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 16.Patel M, Hoffe S, Malafa M, et al. Neoadjuvant GTX chemotherapy and IMRT-based chemoradiation for borderline resectable pancreatic cancer. J Surg Oncol. 2011;104(2):155–161. doi: 10.1002/jso.21954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. http://www.sciencedirect.com/science/article/pii/S009377541400284X. [Google Scholar]
- 18.Goyal K, Einstein D, Ibarra RA, et al. Stereotactic body radiation therapy for nonresectable tumors of the pancreas. J Surg Res. 2012;174(2):319–325. doi: 10.1016/j.jss.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polistina F, Costantin G, Casamassima F, et al. Unresectable locally advanced pancreatic cancer: A multimodal treatment using neoadjuvant chemoradiotherapy (gemcitabine plus stereotactic radiosurgery) and subsequent surgical exploration. Ann Surg Oncol. 2010;17(8):2092–2101. doi: 10.1245/s10434-010-1019-y. [DOI] [PubMed] [Google Scholar]
- 20.Rwigema JC, Parikh SD, Heron DE, et al. Stereotactic body radiotherapy in the treatment of advanced adenocarcinoma of the pancreas. Am J Clin Oncol. 2011;34(1):63–69. doi: 10.1097/COC.0b013e3181d270b4. [DOI] [PubMed] [Google Scholar]
- 21.Chang DT, Schellenberg D, Shen J, et al. Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer. 2009;115(3):665–672. doi: 10.1002/cncr.24059. [DOI] [PubMed] [Google Scholar]
- 22.Rajagopalan MS, Heron DE, Wegner RE, et al. Pathologic response with neoadjuvant chemotherapy and stereotactic body radiotherapy for borderline resectable and locally-advanced pancreatic cancer. Radiat Oncol. 2013;8 doi: 10.1186/1748-717X-8-254. 254-717X-8-254. [DOI] [PMC free article] [PubMed] [Google Scholar]