Abstract
Body mass index (BMI) has a strong genetic basis, with a heritability around 0.75, but is also influenced by numerous behavioral and environmental factors. Aspects of the built environment (e.g., environmental walkability) are hypothesized to influence obesity by directly affecting BMI, by facilitating or inhibiting behaviors such as physical activity that are related to BMI, or by suppressing genetic tendencies toward higher BMI. The present study investigated relative influences of physical activity and walkability on variance in BMI using 5,079 same-sex adult twin pairs (70% monozygotic, 65% female). High activity and walkability levels independently suppressed genetic variance in BMI. Estimating their effects simultaneously, however, suggested that the walkability effect was mediated by activity. The suppressive effect of activity on variance in BMI was present even with a tendency for low-BMI individuals to select into environments that require higher activity levels. Overall, our results point to community- or macro-level interventions that facilitate individual-level behaviors as a plausible approach to addressing the obesity epidemic among U.S. adults.
Keywords: body mass index, built environment, physical activity, gene-by-environment interaction
The prevalence of obesity, defined as a body mass index (BMI) exceeding 30 kg/m2, among U.S. adults aged 20 years or older was 35% in 2011-2012 based on national surveillance data, compared to 31% one decade ago (1999-2000 national data) and 23% two decades ago (1988-1994 national data) (Flegal et al., 2002; Flegal et al., 2010; Hedley et al., 2004; Ogden et al., 2006; Ogden et al., 2014). Obesity is considered a major public health threat because of its high prevalence and because it is associated with adverse and costly conditions including type 2 diabetes, cardiovascular disease, and certain types of cancer (ADA, 2008; Anderson et al., 2005; Flegal et al., 2005; NIH 1998; Pronk et al., 1999; Wang et al., 2011). As the obesity epidemic in the U. S. continues to worsen, researchers have increased their efforts to unravel the complex causes of obesity, identify its consequences, and explore possible proactive or preventative measures that individuals and communities can take to combat this chronic condition. The present study evaluates the roles of physical activity, and aspects of the built environment that promote physical activity, on BMI and genetic factors that influence BMI.
Genetic Etiology of BMI and Obesity
Body mass appears to have a strong genetic basis. The fact that lean individuals exist in obesogenic environments—and obese individuals in food-limited environments—is support for a biological propensity toward low (or high) BMI (O’Rahilly & Farooqi, 2008). Genetically informed studies estimate the heritability of BMI to be between 24–90%, averaging around 75% (Elks et al., 2012) and typically decreasing with age following adolescence (Dubois et al., 2012; Elks et al., 2012; McCaffery et al., 2009; Min et al., 2013). Studies of monozygotic twin pairs have found between-family variance to be greater than within-family variance across a wide range of indicators and correlates of weight gain and adiposity following periods of overfeeding (Bouchard et al., 1990; Poehlman et al., 1986a; Poehlman et al., 1986b), further support for a genetic basis of body mass and weight gain. Genetic studies of humans and animals have identified over 500 candidate genes related to obesity (Vimaleswaran et al., 2012), with FTO generating the greatest attention (O’Rahilly & Farooqi, 2008; Fawcett & Barroso, 2010).
Behavioral and Environmental Influences on Genetic Variation in BMI
Research suggests that behavioral and environmental factors which protect against obesity and weight gain also interact with an individual’s genetic makeup to predict obesity. For example, physical activity mitigates the genetic propensity toward high BMI (McCaffery et al., 2009; Mustelin et al., 2009) and large waist circumference (Karnehed et al., 2006; Mustelin et al., 2009). Further, weight gain at 6-year follow-up was smaller and more heritable in active males, and was greater and strongly environmental in inactive males (Heitmann et al., 1997), suggesting that physical activity promotes expression of genes that maintain weight levels.
Studies investigating the interaction of physical activity with candidate genes converges with evidence from twin studies. Numerous studies have detected an interaction between FTO risk alleles and physical inactivity to predict greater risk for obesity (Ahmad et al., 2013; Cauchi et al., 2009; Corella et al., 2012; Kilpeläinen et al., 2011), higher BMI (Andreason et al., 2008; Cauchi et al., 2009; Corella et al., 2012; Jacobsson et al., 2009; Lee et al., 2010; Rampersaud et al., 2008; Scott et al., 2010; Sonestedt et al., 2009; Vimaleswaran et al., 2009; Xi et al., 2010), higher body fat percentage (Ruiz et al., 2010), and larger waist circumference (Corella et al., 2012; Ruiz et al., 2010; Vimaleswaran et al., 2009). Other obesity-related genes similarly interact with physical inactivity to predict lower BMI, including ACE (Moran et al., 2005), ADRB2 (Corbalán et al., 2002; Lagou et al., 2012; Meirhaeghe et al., 1999), ADRB3 (Marti del Moral et al., 2002), PPARGC1A (Ridderstråle et al., 2006), SEC16B (Ahmad et al., 2013), and UPC3 (Otabe et al., 2000). Although several other studies have failed to observe such interactive effects (Berentzen et al., 2005; Cauchi et al., 2009; Edwards et al., 2013; Jonsson et al., 2009; Jóźków et al., 2011; Kaakinen et al., 2010; Lappaläinen et al., 2009; Liem et al., 2010; Liu et al., 2010; Nakashima et al., 2013; Tan et al., 2008) or have found interaction effects in the opposite direction (Alonso et al., 2005), several experimental studies of interventions for weight loss that compare wild type individuals and persons with a risk allele have provided compelling evidence for physical activity as a moderator of genetic influences on high adiposity or obesity (de Luis et al., 2006; Shiwaku et al., 2003; but see Müller et al., 2008).
Other non-behavioral factors, such as low socioeconomic status, are related to increased risk for being overweight or obese, presumably because they limit access to exercise resources (Gordon-Larsen et al., 2006). Recently, there has been an upsurge in interest in the built environment and its effects on obesity. Neighborhood greenness (i.e., amount of healthy green vegetation) is negatively associated with BMI and changes in BMI over a two-year period in children and adolescents (Bell et al., 2008). Neighborhood design features such as environmental “walkability,” are also related to lower BMI (Black & Macinko, 2008; Papas et al., 2007). Such features are thought to facilitate or inhibit other behaviors, such as physical activity, that are also related to BMI. For example, individuals in highly walkable environments who increase their physical activity show more weight loss and waist circumference reduction compared with similarly active individuals in less walkable environments (Li et al., 2009). Despite evidence that such neighborhood design characteristics may protect against high BMI, no study to date has investigated whether this effect exists within families as well as between them. If aspects of the built environment (such as walkability) do not protect against high BMI within families, then this effect cannot be causal and instead represents nonrandom exposure (via genes or family characteristics) to environments that promote healthier body weight.
Resources which promote or inhibit health behavior have also been shown to affect genetic variance in BMI. High socioeconomic status, for example, decreases genetic variance in BMI (Johnson & Krueger, 2005; Johnson et al., 2011). As noted above, walkability—like socioeconomic status—is hypothesized to facilitate or inhibit behaviors such as physical activity that are related to variability in BMI, yet previous studies also have not examined the interactive effects between neighborhood design characteristics and variability in BMI. If such an effect exists, it would provide a cornerstone for community-level interventions that successfully combat the obesity epidemic faced by modern-day Americans.
Nonrandom Exposure to Obesogenic Environments
Although environments (or behaviors) may enhance or suppress the expression of a genetic propensity for high BMI, it is an unrealistic assumption that individuals encounter such environments (or engage in such behaviors) at random. Similarly, the assumption that the same BMI-influencing genotypes are present across all levels of environments (or behaviors) is far too simplistic. Lower-BMI individuals may be both leaner and more active because of genetic reasons, but the activity phenotype, which itself is a culmination of both genetic and environmental factors (Carlsson et al., 2006; den Hoed et al., 2013; Duncan et al., 2008; Joosen et al., 2005; Perusse et al., 1989; Simonen et al., 2002; Stubbe et al., 2006), may simultaneously be acting to suppress the expression of obesogenic genes or enhance the expression of genes related to leanness. Lifting the assumptions that genes and environments are independent of one another and static over time, and instead examining systematically their plasticity with respect to one another, is important for understanding the biological or environmental pathways through which BMI is determined (c.f. Johnson, 2007).
Some researchers have found evidence for selection effects in the BMI–physical activity association. Two studies using unrelated individuals demonstrated (phenotypically) that indices of obesity predict later sedentary time, but sedentary time did not predict subsequent obesity or weight gain (Ekelund et al., 2008; Metcalf et al., 2009). Other studies using genetically informative samples have provided evidence that genetic-based selection factors may also play a role in the BMI–physical activity association. Variants of the MC4R (Loos et al., 2005) and FTO (Cecil et al., 2008; Jonnson et al., 2009) genes are related to physical inactivity. Interestingly, these FTO variants also show positive gene-environment correlation with BMI. Quantitative genetic research examining environmental influences on BMI has demonstrated that the protective effect of high socioeconomic status is entirely attributable to negative gene-environment correlation (Johnson & Kruger, 2005; Johnson et al., 2011; Osler et al., 2007; Silventoinen et al., 2004; Webbink et al., 2010). Previous quantitative genetic approaches to environments (or behaviors) as modifiers of the heritability of BMI, although sophisticated and replicated (Heitmann et al., 1997; Karnehed et al., 2006; McCaffery et al., 2009; Mustelin et al., 2009), have not controlled for genetic selection where it likely exists. Results from these studies, although convergent with candidate gene research (which are vulnerable to the same shortcomings (VanderWeele et al., 2013)), must be interpreted cautiously, as they are subject to inflated type I error rates or biased estimates of environmental effects on variance in BMI (van der Sluis et al., 2012).
Present Study
The present study investigates the association between both physical activity and environmental walkability and variation in BMI. We use a sample of adult twins to reinvestigate the suppressive effect of physical activity on genetic variance in BMI, and we seek to extend existing G×Activity findings to include aspects of the built environment. We have three primary research questions:
Do aspects of (a) the built environment (walkability) and (b) physical activity protect against high BMI within families as well as between families? We predict that twins living in more walkable neighborhoods will also have lower BMIs than their co-twins living in less walkable neighborhoods. We expect similar findings for physical activity.
Is there an effect of (a) environmental walkability and (b) physical activity on genetic variance in BMI controlling for potential selection factors (e.g., gene-environment correlation)? We predict that genetic variance will be reduced in individuals living in more walkable neighborhoods and in more active individuals regardless of nonrandom exposure to environments that predict differences in BMI.
Do physical activity and environmental walkability have independent effects on variance in BMI, or does physical activity mediate the influence of environmental walkability? We predict that the modifying effect of environmental walkability on variance in BMI will not be fully mediated by physical activity level.
Methods
Participants
The University of Washington Twin Registry (UWTR) is a community-based sample of adult twins reared together; construction methods are described in detail elsewhere (Afari et al., 2006; Strachan et al., 2013). Twins completed a survey with items on sociodemographics, health, and lifestyle behaviors. Standard questions about childhood similarity that determine zygosity with greater than 90% accuracy when compared with DNA-based methods were used to classify twins as identical (monozygotic; MZ) or fraternal (dizygotic; DZ) (Eisen et al., 1989; Spitz et al., 1996; Torgersen, 1979). Written informed consent was provided as approved by the university’s institutional review board. Information for physical activity was available for 3,316 female (2,303 MZ; 1,013 DZ) and 1,763 male (1,239 MZ; 524 DZ) pairs. Prior to 2008, twins’ residential street addresses (used to calculate walkability) were not available; therefore, we have walkability information for a large subsample of the total sample described above (1,331 MZ females; 611 DZ females; 755 MZ males; 301 DZ males). Overall, the sample was young (39.4 ± 17.6 years; range = 18–96; 25th percentile = 24.2; 75th percentile = 53.2), well-educated (93% with a high school degree and 40% with a Bachelor’s degree or higher), and predominantly white (85% Caucasian, 2% African-American, 3% Asian, 1% Pacific Islander, 1% Hispanic, 1% Native American, 7% multi-ethnic). The subsample of twins with walkability information was representative of the total sample and did not differ in terms of demographics, BMI, or physical activity levels.
Measures
Body Mass Index
The primary outcome was BMI (kg/m2) from self-reported height and weight. Approximately 2.8% of the sample was underweight (BMI < 18.5), 50.5% normal weight (BMI ranging from 18.5–24.9), 28.5% overweight (BMI ranging from 25–29.9), and 18.2% obese (BMI ≥ 30). Among 200 twin pairs from the UWTR, we found that self-reported BMI was highly correlated (r = 0.98, p < 0.01) with directly measured BMI, indicating a high degree of construct validity in our sample, although we did observe a tendency for higher BMI individuals to under-report to a greater extent than lower BMI individuals (r = −0.27, p < 0.01; each kg/m2 increase in measured BMI was associated with under-reporting BMI by 0.05 kg/m2). However, this discrepancy was not correlated with activity level (r = −0.01, p = 0.80) or walkability (r = −0.03, p = 0.59).
Moderate-to-Vigorous Physical Activity (MVPA)
Twins reported the number of days per week they engaged in vigorous physical activity for at least 20 minutes and moderate physical activity for at least 30 minutes. A single, continuous activity measure was constructed by summing moderate and vigorous physical activity days weighted by their respective durations. This measure provides an estimate that corresponds to activity levels recommended for health (i.e., U.S. adults should engage in moderate-intensity activity for ≥ 30 minutes per day on ≥ 5 days per week for a total of ≥ 150 minutes per week, vigorous-intensity activity for ≥ 20 minutes per day on ≥ 3 days per week for a total of ≥ 75 minutes per week, or a combination of moderate- and vigorous-intensity activity to achieve a total energy expenditure of ≥ 500-1,000 metabolic equivalent (MET) minutes per week) (Garber et al., 2011; U.S. Department of Health and Human Services, 1996). In a sample of 104 twins who wore accelerometers and GPS devices over a two-week period to collect objective measures of physical activity, this subjective measure of MVPA correlated moderately (r = .46, p < .01) with objectively measured MVPA. This correlation is consistent with the literature in which accelerometers are used to objectively measure physical activity (Craig et al., 2003; Prince et al., 2008; Statistics Canada, 2014). There was a tendency for more active individuals to under-report MVPA to a greater extent than less active individuals (r = −.71, p < .01; each minute increase in accelerometer-measured MVPA was associated with underreporting of MVPA by .64 minutes). This discrepancy was not associated with BMI, however (r = .13, p = .18).
Although MVPA was our primary physical activity indicator, we also examined walking within the neighborhood as an indicator of physical activity. Twins reported the number of days per week and the average duration that they walked in their neighborhood, as described in the Walk and Bike Community Project (Lee & Moudon, 2006; Moudon et al., 2006). Responses of <15 minutes were coded as 10 minutes, whereas responses of ≥90 minutes were top coded as 90 minutes.
Environmental Walkability
Walkability, a measure of the “built” environment around each twin’s residence, was estimated using Walkability (Walk Score, 2012). Addresses were entered into the Walk Score® website, which uses data from business listings, road networks, schools, and public transit to map walking distance to amenities in nine different categories (e.g., schools, parks, restaurants, etc.), with each category weighted by importance (Walk Score, 2011). The algorithm then uses distances, counts, and weights to create a continuous score normalized on a scale of 0-100, with 0 representing the least and 100 the most “walkable” neighborhoods (Walk Score, 2011). This index is a valid measure of walkability (Carr et al., 2010, 2011), and correlated strongly (r = 0.78, p = 0.01) with another commonly cited walkability index (Frank et al., 2005) in a subsample of 3,162 UWTR twins.
Statistical Analyses
Primary analyses used the maximum likelihood estimation option in the structural equation modeling program Mplus (v. 7.0, Los Angeles, CA) (Muthen and Muthen, 2012). Analyses were conducted for like-sex pairs only and controlled for linear effects of age, gender, ethnicity (White/non-White), income, and education. In addition, we controlled for the linear effects of age (which has been shown to modify biometric variance components in BMI; McCaffery et al., 2009) and gender in the prediction of residual variance in BMI.
Univariate biometric decomposition
Although it was not the primary goal of our analysis, we used the classical twin model to decompose variation in BMI, MVPA, and walkability into three components: additive genetic (A), shared environmental (C), and nonshared environmental (E) variance. The A variance components, which represent the additive effect of an individual’s genes, correlate r = 1.0 between MZ twins (who share 100% of their genetic sequence) and r = 0.5 between DZ twins (who share on average 50% of their segregating genes). The C variance components correlate at 1.0 regardless of degree of genetic relatedness, because it represents environmental experiences that make members of the same family more alike. The E variance components, which represent environmental experiences unique to the individual, do not correlate between twins.
Causal Pathways vs. Gene-Environment Correlation (rGE).
The association between environmental walkability (or physical activity) and BMI within pairs of MZ and DZ twins raised in the same family provides the closest approximation of the causal effect of walkability (or physical activity) on BMI short of random assignment to neighborhood or activity levels. That is, using twin pairs raised together allows us to decompose the main effect of walkability (or physical activity) on BMI association into a genetic regression (or gene-environment correlation, rGE) and a nonshared environmental regression.1 A causal relationship between walkability (or physical activity) and BMI is supported when the association is observed both between twin pairs (pairs who live in more walkable environments or who exercise more on average have lower BMIs) and within twin pairs (the pair member who lives in the more walkable neighborhood or who exercises more has a lower BMI than his or her co-twin living in a less walkable neighborhood or engaging in less physical activity). On the other hand, if the walkability–BMI association is not observed within families, a non-causal process that operates through genetic pathways can be inferred.
The twin design does not control for all possible confounds of a causal relationship, but it does control for all those that are shared by pairs of twins who were raised together, measured or unmeasured. Because of the quasi-experimental nature of the co-twin control design, we choose to use the term quasi-causal (used interchangeably with nonshared environmental effect or nonshared pathway in the Results section of this report) to refer to causal pathways (Turkheimer & Harden, 2014).
Gene-By-Environment (G×E) Interaction in the Presence of rGE
Classical twin models use correlations of MZ and DZ twins to partition variance in outcome into additive components attributable to genetic and environmental variance. These models can be extended by dropping the assumption of additivity and testing for G×E interaction by permitting the magnitudes of genetic and environmental variances of BMI to vary as a function of a moderating variable such as MVPA or walkability:
| (1) |
In this equation, the ACE variance components of BMI are expressed as linear functions of a moderating variable, Mod (MVPA or walkability in the current analysis); the regression coefficients have the subscripts Au, Cu, or Eu, which indicate to which ACE variance component the regression coefficients correspond; the squares of the b0 terms yield the values of the ACE variances where Mod = 0; and the b1 terms represent the rate of increase or decrease in a respective variance component as a function of Mod. Other moderating variables can be included in Equation 1 as well.
Models of G×E interaction must also account for rGE. As we noted above, it is possible, for example, that individuals genetically predisposed to lower BMI select into environments (such as neighborhoods that support more walking) or choose environmental experiences (such as regularly attending a gym) that require more physical activity. Indeed, the tendency of highly active individuals to demonstrate a reduced genetic influence on BMI may be due to (a) suppression of genetic effects on high BMI by physical activity (i.e., G×E interaction), (b) lower genetic susceptibility to high BMI in more active individuals (i.e., rGE), or (c) a combination of the two. Further, rGE may not be constant across all levels of physical activity. Equation 1 can be extended to account for any effects the environmental moderator variable may have on variance in BMI (Purcell, 2002; Johnson, 2007; van der Sluis et al., 2012):
| (2) |
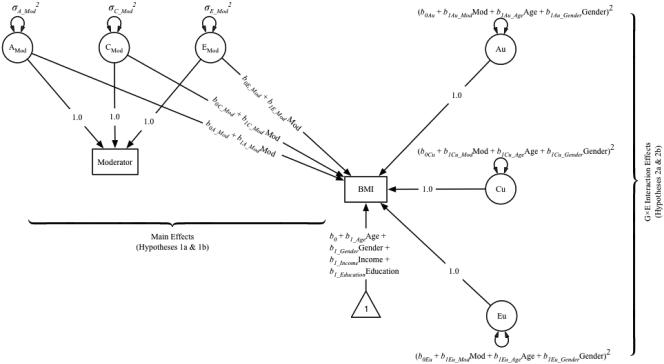
where the subscripts A, C, and E correspond to the regressions of BMI on the ACE components of Mod. It should be noted that these regression of BMI on the ACE components of walkability (or physical activity) correspond to the genetic and quasi-causal pathways described in the previous section, and comprise the test of Hypotheses 1a and 1b. The regression equations that represent the residual ACE components of BMI comprise the test of Hypotheses 2a and 2b. Figure 1 illustrates a path diagram of the G×E in the presence of rGE model fit to the data.
Figure 1.
Path diagram of gene-by-environment interaction in the presence of a gene-environment correlation, with moderation of variance in body mass index (BMI). The paths representing the ACE covariances between the moderator (walkability or MVPA) and BMI vary as a function of the moderator; these regression paths test Hypotheses 1a and 1b. Au, Cu, and Eu are the residual variance components of BMI (i.e., the variance in BMI that remains after accounting for variance shared with the moderating variable), and are functions of the moderator and covariates age, and gender; these paths test Hypotheses 2a and 2b. Only one twin is shown for clarity; the A and C components of the moderator and BMI correlate across twins (rA = 1.0 for monozygotic twins, 0.5 for dizygotic twins; rC = 1.0 for all pair types); the E components are not correlated across twins. Individual-level covariates income and education were allowed to correlate across twins.
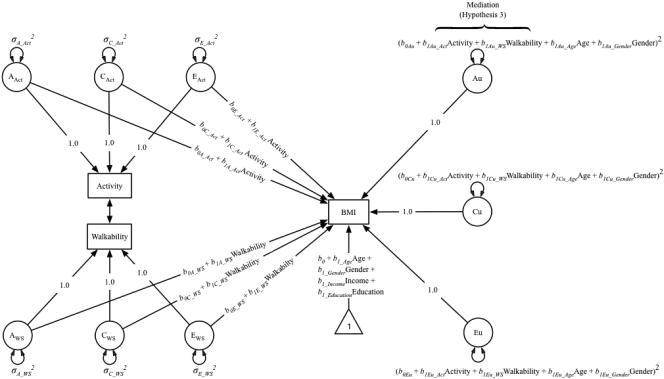
In the present analyses, we examined G×E in the presence of rGE for each moderator separately. After demonstrating that both walkability and physical activity modified variance in BMI, we included each moderator in a combined model to test for mediation of the effect of walkability by physical activity (test of Hypothesis 3). Figure 2 illustrates a path diagram of the fully moderated, combined model that we fit in our primary analyses.
Figure 2.
Path diagram of G×E interaction in the presence of rGE, with moderation of variance in BMI by both environmental walkability and moderate-to-vigorous physical activity (MVPA). This model is an extension of the model illustrated in Figure 1. The residual variances Au, Cu, and Eu vary as a function of walkability, MVPA, and covariates age, and gender; these paths test Hypothesis 3. Only one twin is shown for clarity; the A and C components of the phenotypes correlate across twins (rA = 1.0 for monozygotic twins, 0.5 for dizygotic twins; rC = 1.0 for all pair types); the E components are not correlated across twins. Individual-level covariates income and education correlated across twins.
Results
Univariate Biometric Decomposition of Phenoytpes
Descriptive information, intraclass correlations, and variance components are shown in Table 1. The variance in BMI was largely accounted for by additive genetic variation (75%), with no evidence of contribution from the shared environmental variance and a modest contribution from the nonshared environment (25%). MVPA was influenced little by shared environmental factors (2%), moderately by additive genetic factors (31%), and substantially by nonshared environmental factors (67%). Similarly, neighborhood walking showed no evidence of contribution from the shared environment, modest influence from additive genetic factors (28%), and large contributions from nonshared environmental factors (72%). Walkability was influenced by additive genetic (19%), shared environmental (21%), and nonshared environmental (60%) factors. Because the shared environmental variance in BMI was estimated to be 0%, we did not model shared environmental variance common to both the environmental exposures (i.e., physical activity and walkability) and BMI or residual shared environmental variance in BMI.
Table 1.
Descriptive statistics, twin correlations, and variance components for body mass index, moderate to vigorous physical activity, neighborhood walking, and environmental walkability.
| Walkability | Moderate-to- Vigorous Physical Activity (min/week) |
Walking (min/week) |
Body Mass Index (kg/m2) |
|
|---|---|---|---|---|
| Descriptive | ||||
| Statistics | ||||
| M | 40.591 | 119.592 | 87.806 | 25.751 |
| SD | 28.073 | 91.820 | 100.571 | 5.540 |
| Median | 38 | 100 | 60 | 24.442 |
| Range | 0–100 | 0–350 | 0–630 | 13.7–64.2 |
| 1st Quartile | 15 | 50 | 15 | 21.853 |
| 3rd Quartile | 63 | 180 | 120 | 28.319 |
| Sibling | ||||
| Correlations | ||||
| MZ | .401 (.018) | .327 (.015) | .287 (.015) | .752 (.006) |
| DZ | .316 (.028) | .164 (.023) | .132 (.024) | .370 (.018) |
| ACE Estimates | ||||
| h2 | .187 (.066) | .310 (.054) | .281 (.017) | .752 (.006) |
| c2 | .214 (.059) | .015 (.048) | .000 (.008) | .000 (.000) |
| e2 | .599 (.018) | .675 (.015) | .719 (.015) | .248 (.006) |
| Phenotypic | ||||
| Correlations | ||||
| Walkability | 1 | |||
| MVPA | .027 (.012) | 1 | ||
| Walking | .081 (.012) | .355 (.009) | 1 | |
| BMI | −.029 (.013) | −.156 (.010) | −.115 (.010) | 1 |
Note: Standard errors in parentheses.
Causal Pathways, rGE, and G×E
We examined whether the biometric decomposition of BMI varied as a function of environmental walkability and physical activity in the presence of rGE. We present estimates for these two models in Table 2, and discuss our findings in terms of our stated hypotheses.
Table 2.
Parameter estimates, body mass index moderated by environmental walkability (left column) and physical activity (middle and right columns).
| Parameter | Walkability (N = 2086 MZ pairs, 912 DZ pairs) |
MVPA (N = 3542 MZ pairs, 1537 DZ pairs) |
Walking (N = 3241 MZ pairs, 1411 DZ pairs) |
|---|---|---|---|
| Main Effects of Moderator on BMI | |||
| A Regression | |||
| b 0A_Mod | .146 (.123) | −.023 (.207) | −1.588 (211) |
| b 1A_Mod | −.065 (.023) | −.413 (064) | .338 (.065) |
| E Regression | |||
| b 0E_Mod | .016 (.066) | −.720 (077) | −.043 (.060) |
| b 1E_Mod | −.003 (.014) | .206 (.026) | −.072 (.020) |
|
Interactive Effects of Moderator on
Residual ACE Components of BMI |
|||
| A Path | |||
| b 0Au | 5.290 (.221) | 6.407 (.167) | 5.339 (.160) |
| b 1Au_Mod | − .049 (.023) | −.399 (031) | −.100 (028) |
| E Path | |||
| b 0Eu | 1.793 (142) | 2.283 (109) | 1.952 (100) |
| b 1Eu_Mod | −.015 (.018) | −.160 (021) | −.043 (.026) |
Note: Statistically significant (p < .05) parameter estimates bolded; estimates that are significant at the α = .01 level italicized. Standard errors in parentheses.
Hypothesis 1a: Main Effects of Environmental Walkability on BMI
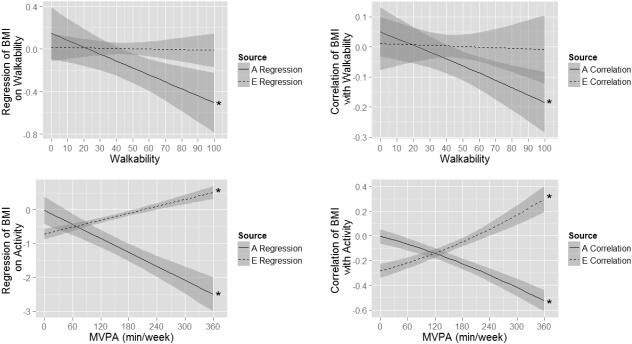
The main effects of walkability on BMI are presented in the Walkability column of Table 2 under the heading Main Effects of Moderator on BMI. Walkability protected against high BMI, but was non-causal and acted through the genetic pathway from walkability to BMI (i.e., gene-environment correlation). However, as illustrated in the top left panel of Figure 3, which shows the magnitude of the genetic and nonshared environmental regressions of BMI on walkability as a function of level of walkability, this effect only emerged when WalkScore® exceeded approximately 45 (or approached a level of walkability in which individuals have reduced dependence on cars; b1A_Walkability = −.065, p < .001). Our first hypothesis that walkability would protect against high BMI through nonshared environmental pathways was not supported; as evident in the top left panel of Figure 3, the nonshared environmental correlation between walkability and BMI was not statistically distinguishable from zero at any level of walkability. That is, high-BMI genes are more likely to be present in individuals who live in less walkable neighborhoods. These regression lines are re-represented as genetic and nonshared environmental correlations between walkability and BMI as a function of level of walkability in the top right panel of Figure 3.
Figure 3.
Regression of BMI on environmental walkability (top left panel) and physical activity (bottom left panel) and 95% confidence intervals (shaded areas). Right panels show the same relations, but represented as genetic and nonshared environmental correlations. The asterisks (*) indicate that the magnitude of the regression (or correlation) of BMI on MVPA (or walkability)
Illustrative analyses further highlight this gene-environment correlation. In Figure 4a, we show pair differences in BMI as a function of pair differences in walkability within randomly paired individuals (phenotypic difference; dotted line) and within MZ twin pairs (solid line). Comparison of these lines suggests that differences in walkability do not predict differences in BMI within families, only between them. If the protective effect of walkability on BMI was causal, the slopes of these lines would closely approximate one another, but they do not.
Figure 4.
Illustrative analysis of the main effects of walkability (a) and MVPA (b) on BMI. Figure 4a shows pair differences in BMI as a function of pair differences in walkability in the population (referred to as the “phenotypic difference”) and within pairs of MZ twins (referred to as the “MZ difference”). Figure 4b shows BMI at lower (0-2 hours per week; left panel) and higher (4-6 hours per week; right panel) levels of MVPA; within each panel, the twin engaging in less physical activity (dark gray) is compared with the twin engaging in more physical activity (light gray) in MZ and DZ twin pairs.
Hypothesis 1b: Main Effects of Physical Activity on BMI
Overall, results using neighborhood walking were consistent with those using MVPA. Although in Table 2 we provide estimates for both MVPA and neighborhood walking as predictors of BMI and variance in BMI, for simplicity, we only discuss results for MVPA as an indicator of physical activity.
The main effects of MVPA on BMI are presented in the MVPA column of Table 2 under the heading Main Effects of Moderator on BMI. MVPA protected against high BMI, and acted through both genetic pathways (rGE) and nonshared environmental (i.e., quasi-causal) pathways from MVPA to BMI. As shown in the bottom left panel of Figure 3, the magnitude of the genetic regression of BMI on MVPA grew stronger with greater levels of MVPA (b1A_MVPA = − .413, p < .001) and became statistically distinguishable from zero after approximately 90 minutes per week of MVPA, whereas the magnitude of the protective quasi-causal effect grew weaker (b1A_MVPA = .206, p < .001), becoming indistinguishable from zero at approximately 180 minutes per week and positive at higher levels of MVPA (>270 minutes per week). Therefore, our first hypothesis that physical activity has a nonshared environmental effect on BMI was only partially supported. As we observed with walkability, high-BMI genes are more likely to be present in individuals engaging in less MVPA, but there does appear to be a causal influence of MVPA on lowering BMI as well. As with walkability, these regression coefficients are re-illustrated as correlations in the bottom right panel of Figure 3.
To demonstrate what these effects look like within and between twins, we conducted an illustrative analysis of the main effect of MVPA on BMI and how it varies according to the level of MVPA, which we present in Figure 4b. We identified twin pairs concordant for lower MVPA levels (0 to 2 hours) and pairs concordant for higher MVPA levels (4 to 6 hours). Within these groups, we then compared the mean BMI of the less active twins (dark gray) with that of the more active twins (light gray). Overall, there is a main effect of MVPA on BMI such that greater activity levels are associated with lower BMIs on average (this is evident comparing the height of the bars in the left panel to those in the right panel). Examining the relation at the within-pair level, however, shows that at lower levels of MVPA (left panel) there is little difference between MZ or DZ co-twins regardless of who exercises more within the pair, consistent with genetic selection (or rGE). At higher activity levels (right panel), MZ twins still demonstrate no differences, but the DZ difference is much larger, demonstrating that the genetic selection process is greater at higher levels of physical activity. Also evident is that the small nonshared effect appears to disappear at higher MVPA.
Hypothesis 2a: Moderating Effect of Walkability on Genetic Variance in BMI
The moderating effects of walkability on variance BMI are presented in the Walkability column of Table 2 under the heading Interactive Effects of Moderator on Residual ACE Components of BMI. We observed a significant effect of environmental walkability on genetic variance in BMI even while controlling for the nonrandom exposure to environments that predict differences in BMI, supporting our second hypothesis that walkability predicted variance in BMI. Holding constant the effects of age and gender, walkability suppressed the genetic risk for high BMI (b1Au_Walkability = −.049, p = .032) but did not moderate residual nonshared environmental variance (b1Eu_Walkability = −.015, p = .404). When considered within the context of the main effect of walkability on BMI, this finding can be interpreted substantively as walkability having a suppressive effect on the genetic risk for high BMI.
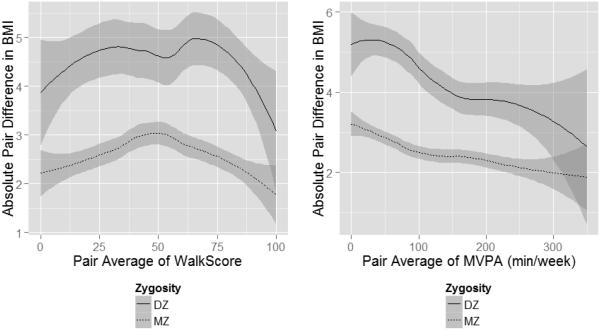
These model results are illustrated in the left panel of Figure 5. The stacked variance plot shows how the genetic variance is decreasing as a function of walkability holding constant the interactive effects of age and gender. This can be interpreted substantively as walkability modifying the influence that high-BMI genes have on the body mass phenotype. We also conducted additional illustrative analyses. We plotted absolute pair differences in BMI for MZ and DZ twins against pair average walkability (see Figure 6, left panel). In this plot, the gap between the MZ and DZ loess curves (i.e., the tendency for MZ pairs to be more similar than DZ pairs) represents the additive genetic variance in BMI. Evident in this plot is that the distance between these lines decreases, although only at very high levels of walkability.
Figure 5.
Gene by environment interaction between body mass index and walkability (left panel) and moderate-to-vigorous physical activity (right panel). The stacked variances illustrate how the total variance in BMI decreases as a function of walkability and MVPA. White dotted lines represent the 95% confidence intervals of each variance component of BMI as a function of walkability and MVPA.
Figure 6.
Absolute pair difference in body mass index as a function of the pair average environmental walkability (left panel) and pair average moderate to vigorous physical activity (right panel). Shaded region represents the 95% confidence interval around the smoothed loess curves fit to the data. The area between the MZ and DZ loess curves represents the additive genetic variance in BMI.
Hypothesis 2b: Moderating Effect of Physical Activity on Genetic Variance in BMI
The moderating effects of walkability on variance in BMI are presented in the MVPA column of Table 2 under the heading Interactive Effects of Moderator on Residual ACE Components of BMI. We also detected a significant interaction effect of MVPA on variance in BMI, supporting our second hypothesis that physical activity predicts genetic variance in BMI. Holding constant the effects of age and gender, both genetic variance and nonshared environmental variance decreased with respect to MVPA (b1Au_MVPA = −.399, p < .001; b1Eu_MVPA = −.160, p < .001). Like walkability, MVPA suppresses the genetic risk for high BMI, but it also reduces the influence of individual-level environmental exposures on the risk for high BMI.
These model results are illustrated in the right panel of Figure 5, which shows how both the genetic, nonshared environmental, and total variances in BMI decrease as a function of increasing MVPA. A plot of absolute pair differences in BMI as a function of pair-level MVPA in MZ and DZ twins (right panel of Figure 6) also clearly shows decreased total variance and decreased genetic variance in BMI as a function of MVPA. A simple regression of absolute pair differences in BMI on the interaction between zygosity and pair average MVPA (controlling for age and gender) shows that this absolute pair difference decreases faster in DZ twins relative to MZ twins (p = .005).2
Mediating Effects of Physical Activity on the Effect of Walkablity on Variance in BMI
Our hypotheses that environmental (a) walkability and (b) physical activity predicted genetic variance in BMI were supported. High environmental walkability is hypothesized to promote exercise-related behaviors (Ewing et al., 2003; Frank et al., 2004; Frank et al., 2007; Frank et al., 2006; Frank et al., 2005; Moudon et al., 2006; Moudon et al., 2007; Saelens et al., 2003) which in turn influence BMI (Papas et al., 2007). Consistent with past research, we observed small but positive correlations between environmental walkability and MVPA (r = .064, p < .001) and neighborhood walking (r = .092, p < .001). This led us to test whether walkability and physical activity have independent effects on variance in BMI when included in the same model. Because a reverse causation argument (i.e., that greater exercise-related behaviors cause increased environmental walkability) has questionable plausibility, this follow-up analysis can be considered a type of mediation analysis (cf. Baron & Kenny, 1986; James & Brett, 1984; Judd & Kenny, 1981). We examined changes in the magnitude and statistical significance of the effect of environmental walkability on variance in BMI after controlling for analogous effects exerted by the mediator, physical activity.
Hypothesis 3: Independent Effects of Walkability and Physical Activity on Genetic Variance in BMI
Parameter estimates for this model are presented in Table 3. Estimates were largely unchanged compared with models using MVPA or walkability alone. The suppressive effect of walkability on genetic variance in BMI was no longer statistically distinguishable from zero (b1Au_Walkability = −.036, p = .117) in the context of MVPA (b1Au_MVPA = −.418, p < .001), however, suggesting that MVPA mediates this relation. Compared with models using MVPA, we observed a similar, although attenuated, effect of walking on genetic variance in BMI (b1Au_Walking = −.116, p = .001). Unlike MVPA, however, walking did not mediate the effect of walkability on A variance in BMI (b1Au_Walkability = −.048, p = .043). That is, in contrast with MVPA, in the context of neighborhood walking walkability still has a significant influence on genetic variance in BMI.
Table 3.
Parameter estimates, body mass index moderated by environmental walkability and physical activity (MVPA is presented in the left column, neighborhood walking in the right column).
| Parameter |
MVPA
(N = 2069 MZ pairs, 897 DZ pairs) |
Walking
(N = 1898 MZ pairs, 833 DZ pairs) |
|---|---|---|
| Main Effects of Walkability on BMI | ||
| A Regression | ||
| b 0A_Walkability | .192 (.209) | .045 (.227) |
| b 1A_Walkability | −.104 (.039) | −.070 (.039) |
| E Regression | ||
| b 0E_Walkability | −.014 (.062) | −.006 (.066) |
| b 1E_Walkability | .002 (.013) | .001 (.013) |
| Main Effects of Activity on BMI | ||
| A Regression | ||
| b 0A_Activity | .416 (.273) | −1.621 (273) |
| b 1A_Activity | −.569 (.082) | .394 (.082) |
| E Regression | ||
| b 0E_Activity | −.863 (096) | −.047 (.076) |
| b 1E_Activity | .259 (031) | − .076 (.024) |
|
Interactive Effects of Moderator on
Residual ACE Components of BMI |
||
| A Path | ||
| b 0Au | 6.086 (242) | 5.280 (233) |
| b 1Au_Walkability | −.036 (.023) | − .048 (.024) |
| b 1Au_Activity | −.418 (044) | −.116 (036) |
| E Path | ||
| b 0Eu | 2.310 (.174) | 1.976 (.161) |
| b 1Eu_Walkability | −.015 (.018) | −.025 (.018) |
| b 1Eu_Activity | −.165 (.028) | −.043 (.034) |
Note: Statistically significant (p < .05) parameter estimates bolded; estimates that are significant at the α = .01 level italicized. Standard errors in parentheses.
Discussion
Nearly two decades of research documents a variety of non-additive relations between environments or behaviors and genetic variation in BMI. Physical activity mitigates an individual’s genetic propensity toward weight gain or obesity (e.g., Heitmann et al., 1997; McCaffery et al., 2009; Mustelin et al., 2009), whereas physical inactivity tends to exacerbate such a predisposition (Ahmad et al., 2013; Heitmann et al., 1997; Karnehed et al., 2006). Surprisingly, no research exists on the effects of the built environment—and aspects of it which promote physical activity in particular—on the heritability of BMI or obesity. The present study is the first examining the modification of genetic variance in BMI by environmental walkability and exploring the mediating effects that physical activity has on this association.
We tested three hypotheses in the current study. Our first hypothesis that walkability and physical activity had protective effects on BMI that operated through nonshared environmental pathways was not supported. The association between walkability and physical activity was driven primarily by common genetic factors. That is, the genes contributing to low BMI were also the same genes contributing to selection of more walkable neighborhoods and greater physical activity levels, or active gene-environment correlation.
Our second hypothesis that walkability and physical activity predicted genetic variance in BMI, controlling for selection factors (i.e., the gene-environment correlation noted above), was supported. We observed that both walkability and physical activity performed at a moderate or vigorous intensity suppressed the genetic risk for high BMI. We also found that this suppressive influence remained after accounting for the tendency for individuals with low BMI to be more active and select into environmental experiences that require more activity. Our physical activity results were consistent with past family- and population-based findings, but we expand on past research by demonstrating that these effects exist in the context of genetic-based selection factors that induce a correlation between these phenotypes. We also show the first evidence that aspects of the built environment interact with an individual’s genetic makeup to predict BMI.
Our last hypothesis approached the nonadditive effect of walkability on BMI from a more mechanisms of action perspective, predicting that physical activity would not fully mediate the association between walkability and genetic variance in BMI—that is, that walkability would operate directly on genetic variance in BMI rather than through a behavioral mediator. We observed that although walkability decreased genetic variance in BMI, it had no independent effect on variability in BMI when simultaneously adjusting for the effect of MVPA. That is, it appears that walkability operates indirectly to reduce an individual’s genetic predisposition toward adiposity. Although these data were cross-sectional and quasi-experimental and we therefore cannot conclude with certainty that walkability causes physical activity or BMI, our results are consistent with prior research on the main effects of walkability, which suggest that more walkable neighborhoods have stronger influences on individuals’ BMI or predict greater weight loss compared with less walkable neighborhoods, but only if the individuals within those neighborhoods utilize walkability as an exercise-promoting resource (Li et al., 2009). Of course, the present study is the only report of the interactive effects of environmental walkability on genetic variance in BMI and is by no means conclusive evidence that walkability has no effects on BMI independent of physical activity.3 We operationalized environmental walkability using Walk Score®, an algorithm which is primarily an index of utilitarian destinations within the neighborhood accessible by walking—and to a lesser extent neighborhood density—and does not give due consideration to other measures of urban form that correlate with physical activity and BMI, such as smaller street blocks and greater street connectivity (Frank et al., 2006; Moudon et al., 2006; Moudon et al., 2007). It is possible that other measures of environmental walkability could demonstrate different effects from those presented here. For example, recent studies using different indicators of walkability suggest that physical activity only partially mediates the association between walkability and BMI (Brown et al., 2013; Van Dyck et al., 2010).
Limitations and Strengths
We note several limitations of this study. First, we note that our data were cross-sectional and quasi-experimental, limiting our ability to conclude with certainty that any of our results are causal. It is plausible, for example, that BMI (and variance in BMI) instead predicts environmental walkability and physical activity. Our sample, although representative of Washington State, was largely white and well-educated, limiting generalizability. Additionally, MVPA was based on self-report and thus subject to measurement bias. It also did not capture activity type (e.g., running and swimming) or domain (e.g., household-, occupational-, and transportation-related activity), and was not sensitive to durations lasting less than or more than 20 minutes of vigorous or 30 minutes of moderate activity. Our measure of MVPA, however, actually under-rather than overestimates activity level, based on our own data of this measure compared against levels of MVPA determined by accelerometry and a survey instrument common in the published activity literature (Craig et al., 2003). Thus, when considered in the context of existing gene-activity findings, our estimate of MVPA was unlikely to have created spurious results. Third, the correlation between physical activity and environmental walkability is lower than might be expected (r = 0.027 for MVPA and r = 0.081 for neighborhood walking). The likely explanation for the small correlation between MVPA and environmental walkability is that MVPA may or may not occur within the home neighborhood (e.g., you can do MVPA at a gym, MVPA could be a long bike ride that takes you well outside of the bounds of your neighborhood, you could play tennis at a tennis court in a park that is outside of your neighborhood, etc.) and environmental walkability features do not necessarily support all forms of MVPA (e.g., having utilitarian destinations within proximity to your home, a measure of environmental walkability, does not necessarily support running, biking, or playing tennis). The explanation for the low correlation between neighborhood walking and environmental walkability (although higher than the correlation noted between MVPA and walkability) is slightly more nuanced. The primary reason for the low correlation is that there are relatively few “walkable” environments in the U.S. Indeed, the mean score of 40.5 in this sample would be representative of a “car dependent” environment in which almost all (walkability = 0–24) or most errands (walkability 25–49) require a car, based on the walkability index used in this paper. On the other hand, a “walker’s paradise,” in which daily errands to not require a car, is in the 90– 100 range. Given the descriptive information about walkability in Table 1, there are few subjects in “very walkable” (70–89) and “walker’s paradise” (90–100) categories. In a separate study that is currently under peer review in which we quantify neighborhood walking bouts objectively with accelerometry, we found that the average number of bouts increased systematically over the four walkability levels in a dose-response manner. Similarly, scatterplots of the data used in the current study suggest that the relation between environmental walkability and MVPA or neighborhood walking is stronger at higher walkability levels—that is, that the relation between walkability and physical activity in this report may be misrepresented as weaker than it actually us (at least at very high walkability levels). It is possible that a replication study conducted in a sample characterized by higher overall walkability, or in a country with such characteristics, may demonstrate a stronger relation between walkability and physical activity than that presented in this report (and indeed may show stronger mediating effects of physical activity on the walkability–genetic variance in BMI association). Fourth, our use of self-reported zygosity may have resulted in an underestimation of total genetic variance in BMI due to possible misreporting of some DZ twins as MZ twins; such a misclassification would make within-pair variances in MZ twins more similar to DZ twins (Elks et al., 2012). Although we used a highly valid nonserological measure of zygosity, this possibility still exists and cannot be controlled for. Nevertheless, it is unlikely that misreporting of DZ twins as MZ twins varies systematically across levels of activity or walkability; therefore, bias in our moderation parameters due to zygosity misclassification is unlikely. Finally, the twin model itself, both in its classical and more sophisticated forms, makes several important assumptions that should be considered when interpreting the results, namely that the environments of MZ pairs are no more similar than those of DZ pairs and that the twins do not have reciprocal causal effects on each other.
Our study also has several key strengths. First, we performed the first genetically informed study of the effect of environmental walkability on BMI and variance in BMI. Although our G × walkability finding became nonsignificant when controlling for the effects of physical activity, it represents an important first step toward identifying factors that may serve to maximize the obesity-suppressing effects of physical activity. Second, we also demonstrated that the suppressive effect of physical activity on genetic variance in BMI cannot be attributed to genetic selection or the differential expression of genes based on level of physical activity, a significant contribution to the current genetically informed literature documenting this association. This finding also provides robust evidence for physical activity as a behavioral intervention with potential to counteract genetic risk of obesity. Third, although our multiple regression approach to moderating variance in BMI has been proposed or used in previous reports (e.g., McCaffery et al., 2009; Purcell, 2002), here we demonstrate how this framework may be used to examine the mediating effects of one moderator by another. This in itself is a contribution to the existing quantitative behavior genetics literature on gene-by-environment interaction, because it opens up a world of research that uses a mechanistic approach to G×E analysis.
Implications and Future Directions
The results we present in this report have important implications for individuals who have a genetic predisposition for being overweight because their heritable risk for high BMI may be counteracted, at least to some extent, by increasing their level of physical activity, and more specifically that performed at a moderate or higher level. Living in neighborhoods that are more conducive to activity has benefit, although this appears to be because such neighborhoods promote physical activity rather than directly suppressing variance in BMI. Nevertheless, the results from our study suggest that macro-level obesity interventions warrant further investigation.
Our finding that physical activity suppresses the genetic risk for high BMI despite nonrandom selection into more active environments warrants further investigation. Specifically, examining the main and interactive effects of physical activity on BMI over time will help elucidate the process by which activity influences the body mass phenotype. Most past G×Activity research (including the present report) has used cross-sectional data to demonstrate this effect (McCaffery et al., 2009; Mustelin et al., 2009; but see Heitmann et al., 1997; Karnehed et al., 2006). Past longitudinal research on the main effects of physical activity or inactivity on BMI shows that BMI is more predictive of activity than vice versa (Ekelund et al., 2008; Metcalf et al., 2009). A genetically-informed longitudinal study could help explain some of the processes involved in this observation. Based on the evidence we provide here, we predict that lower BMI would predict subsequent higher physical activity through genetic pathways. If the suppression of genetic risk for high BMI by physical activity is a true phenomenon, we would also expect to observe decay or amplification of earlier genetic influences as a function of activity level during any given time lag.
Conclusions
Epidemiologic study of interactions between genes and the environment aids in our understanding of disease etiology. Using a twin modeling approach, we demonstrated that high levels of moderate intensity physical activity suppress genetic risk for high BMI, even when activity and BMI share an underlying genetic etiology. Walkability alone also had moderating effects on genetic variance in BMI, yet these effects were mediated by physical activity. Our results support interventions that focus on increasing physical activity, including those that improve aspects of the built environment which in turn may promote more physical activity, to reduce obesity.
Acknowledgments
This research was supported by grants from the National Institutes of Health (R01 AG042176 and RC2 HL103416). The NIH played no role in the study design; data collection, analysis, or interpretation; manuscript preparation; or the decision to submit it for publication.
The authors thank Ally Avery, Scientific Operations Manager, and the entire Registry staff for their diligent work in data collection, and the twins for taking part in the Registry.
Footnotes
Shared environmental factors (e.g., poor diet during childhood) may also be inducing this correlation. We chose to use rGE in our example, however, because in our sample BMI contains no variance attributable to shared environmental influences.
In contrast, it should be noted that no such interaction effect was evident when predicting absolute pair differences in BMI from walkability (p = .992) except at higher (>70) levels of walkability (p = .087).
Indeed, we observed that walking within the neighborhood did not mediate the interactive effect of walkability on variance in BMI.
Contributor Information
Erin E. Horn, Department of Psychology, University of Virginia
Eric Turkheimer, Department of Psychology, University of Virginia.
Eric Strachan, Department of Psychiatry and Behavioral Sciences, University of Washington School of Medicine.
Glen E. Duncan, Department of Epidemiology, Nutritional Sciences Program, University of Washington
References
- Afari N, Noonan C, Goldberg J, Edwards K, Gadepalli K, Osterman B, Evanoff C, Buchwald D. University of Washington Twin Registry: construction and characteristics of a community-based twin registry. Twin Res Hum Genet. 2006;9:1023–1029. doi: 10.1375/183242706779462543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad S, Rukh G, Varga TV, Ali A, Kurbasic A, Shungin D, et al. Gene × physical activity interactions in obesity: Combined analysis of 111,421 individuals of European ancestry. PLOS Genet. 2013;9:e1003607. doi: 10.1371/journal.pgen.1003607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso A, Martí A, Corbalán MS, Martínez-González MA, Forga L, Martínez JA. Association of UCP3 gene −55C>T polymorphism and obesity in a Spanish population. Ann Nutr Metab. 2005;49:183–188. doi: 10.1159/000086883. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association Economic costs of diabetes in the U.S. in 2007. Diabetes Care. 2008;31:596–615. doi: 10.2337/dc08-9017. [DOI] [PubMed] [Google Scholar]
- Anderson LH, Martinson BC, Crain AL, Pronk NP, Whitebird RR, O'Connor PJ, Fine LJ. Health care charges associated with physical inactivity, overweight, and obesity. Prev Chronic Dis. 2005;2:A09. [PMC free article] [PubMed] [Google Scholar]
- Andreason CH, Stender-Petersen KL, Mogensen MS, Torekov SS, Wegner L, Andersen G, et al. Low physical activity accentuates the effect of the FTO rs9939609 polymorhism on body fat accumulation. Diabetes. 2008;57:95–101. doi: 10.2337/db07-0910. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Pers Soc Psych. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bell JF, Wilson JS, Liu GC. Neighborhood greenness and 2-year changes in body mass index of childre and youth. Am J Prev Med. 2008;35:547–553. doi: 10.1016/j.amepre.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berentzen T, Dalgaard LT, Petersen L, Pedersen O, Sørensen TIA. Interactions between physical activity and variants of the genes encoding uncoupling proteins –2 and –3 in relation to body weight changes during a 10-y follow-up. Int J Obes. 2005;29:93–99. doi: 10.1038/sj.ijo.0802841. [DOI] [PubMed] [Google Scholar]
- Black JL, Macinko J. Neighborhoods and obesity. Nutr Rev. 2008;66:2–20. doi: 10.1111/j.1753-4887.2007.00001.x. [DOI] [PubMed] [Google Scholar]
- Bouchard C, Tremblay A, Després J-P, Nadeau A, Lupien PJ, Thériault G, et al. The response to long-term overfeeding in identical twins. N Engl J Med. 1990;24:1477–1482. doi: 10.1056/NEJM199005243222101. [DOI] [PubMed] [Google Scholar]
- Brown BB, Smith KR, Hanson H, Fan JX, Kowaleski-Jones L, Zick CD. Neighborhood design for walking and biking: physical activity and body mass index. Am J Prev Med. 2013;44:231–238. doi: 10.1016/j.amepre.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson S, Andersson T, Lichtenstein P, Michaelsson K, Ahlbom A. Genetic effects on physical activity: results from the Swedish Twin Registry. Med Sci Sports Exerc. 2006;38:1396–1401. doi: 10.1249/01.mss.0000228941.17034.c1. [DOI] [PubMed] [Google Scholar]
- Carr LJ, Dunsiger SI, Marcus BH. Walk Score (TM) as a global estimate of neighborhood walkability. Am J Prev Med. 2010;39:460–463. doi: 10.1016/j.amepre.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr LJ, Dunsiger SI, Marcus BH. Validation of Walk Score for estimating access to walkable amenities. Brit J Sports Med. 2011;45:1144–1148. doi: 10.1136/bjsm.2009.069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauchi S, Stutzmann F, Cavalcanti-Prença C, Durand E, Pouta A, Hartikainen A-L, et al. Combined effects of MC4R and FTO common genetic variants on obesity in European general populations. J Mol Med. 2009;87:537–546. doi: 10.1007/s00109-009-0451-6. [DOI] [PubMed] [Google Scholar]
- Cecil JE, Tavendale R, Watt P, Hetherington MM, Palmer CAN. An obesity-associated FTO gene variant and increased energy intake in children. N Engl J Med. 2008;359:2258–2266. doi: 10.1056/NEJMoa0803839. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention About Healthy Places. 2007 http://www.cdc.gov/healthyplaces/about.htm. Accessed on August 27, 2013.
- Corbalán MS, Marti A, Forga L, Martínez-González MA, Martínez JA. The 27Glu polymorphism of the β2-adrenergic receptor gene interacts with physical activity influencing obesity risk among female subjects. Clin Genet. 2002;61:305–307. doi: 10.1034/j.1399-0004.2002.610411.x. [DOI] [PubMed] [Google Scholar]
- Corella D, Ortega-Azorin C, Sorli JV, Covas MI, Carrasco P, Salas-Salvadó J, et al. Statistical and biological gene-lifestyle interactions of MC4R and FTO with diet and physical activity on obesity: New effects of alcohol consumption. PLoS One. 2012;7:e52344. doi: 10.1371/journal.pone.0052344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- de Luis DA, Aller R, Izaola O, Sagrado MG, Conde R. Influence of ALA54THR polymorphism of fatty acid binding protein 2 on lifestyle modification response in obese subjects. Ann Nutr Metab. 2006;50:354–360. doi: 10.1159/000094299. [DOI] [PubMed] [Google Scholar]
- den Hoed M, Brage S, Zhao JH, Westgate K, Nessa A, Ekelund U, et al. Heritability of objectively assessed daily physical activity and sedentary behavior. Am J Clin Nutr. 2013;98:1317–1325. doi: 10.3945/ajcn.113.069849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois L, Kyvik KO, Girard M, Tatone-Tokuda F, Pérusse D, Hjelmborg J, et al. Genetic and environmental contributions to weight, height, and BMI from birth to 19 years of age: An international study of over 12,000 twin pairs. PLoS One. 2012;7:e30153. doi: 10.1371/journal.pone.0030153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan GE, Goldberg J, Noonan C, Moudon AV, Hurvitz P, Buchwald D. Unique environmental effects on physical activity participation: a twin study. PLoS One. 2008;3:e2019. doi: 10.1371/journal.pone.0002019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DRV, Naj AC, Monda K, North KE, Neuhouser M, Magvanjav O, et al. Gene-environment interactions and obesity traits among postmenopausal African-American and Hispanic women in the Women’s Health Initiative SHARe Study. Hum Genet. 2013;132:323–336. doi: 10.1007/s00439-012-1246-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen S, Neuman R, Goldberg J, Rice J, True W. Determining zygosity in the Vietnam Era Twin Registry: an approach using questionnaires. Clin Genet. 1989;35:423–432. doi: 10.1111/j.1399-0004.1989.tb02967.x. [DOI] [PubMed] [Google Scholar]
- Ekelund U, Brage S, Besson H, Sharp S, Wareham NJ. Time spent being sedentary and weight gain in healthy adults: reverse or bidirectional causality? Am J Clin Nutr. 2008;88:612–617. doi: 10.1093/ajcn/88.3.612. [DOI] [PubMed] [Google Scholar]
- Elks CE, den Hoed M, Zhao JH, Sharp SJ, Wareham NJ, Loos RJ, Ong KK. Variability in the heritability of body mass index: a systematic review and meta-regression. Front Endocrinol. 2012;3:29. doi: 10.3389/fendo.2012.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing R, Schmid T, Killingsworth R, Zlot A, Raudenbush S. Relationship between urban sprawl and physical activity, obesity, and morbidity. Am J Health Promot. 2003;18:47–57. doi: 10.4278/0890-1171-18.1.47. [DOI] [PubMed] [Google Scholar]
- Fawcett KA, Barroso I. The genetics of obesity: FTO leads the way. Trends Genet. 2010;26:266–274. doi: 10.1016/j.tig.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999-2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293:1861–1867. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- Frank LD, Andresen MA, Schmid TL. Obesity relationships with community design, physical activity, and time spent in cars. Am J Prevent Med. 2004;27:87–96. doi: 10.1016/j.amepre.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Frank LD, Saelens BE, Powell KE, Chapman JE. Stepping towards causation: do built environments or neighborhood and travel preferences explain physical activity, driving, and obesity? Soc Sci Med. 2007;65:1898–914. doi: 10.1016/j.socscimed.2007.05.053. [DOI] [PubMed] [Google Scholar]
- Frank LD, Sallis JF, Conway TL, Chapman JE, Saelens BE, Bachman W. Many pathways from land use to health: Associations between neighborhood walkability and active transportation, body mass index, and air quality. J Am Plann Assoc. 2006;72:75–87. [Google Scholar]
- Frank LD, Schmid TL, Sallis JF, Chapman J, Saelens BE. Linking objectively measured physical activity with objectively measured urban form: findings from SMARTRAQ. Am J Prev Med. 2005;28:117–125. doi: 10.1016/j.amepre.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, Nieman DC, Swain DP. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011;43:1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- Garriguet D, Colley RC. A comparison of self-reported leisure-time physical activity and measured moderate-to-vigorous physical activity in adolescents and adults. 2014 Jul 16; Retrieved January 12, 2015, from http://www.statcan.gc.ca/pub/82-003-x/2014007/article/14038-eng.htm. [PubMed]
- Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999-2002. JAMA. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- Heitmann BL, Kaprio J, Harris JR, Rissanen A, Korkeila M, Koskenvuo M. Are genetic determinants of weight gain modified by leisure-time physical activity? A prospective study of Finnish twins. Amer. J. Clin. Nutr. 1997;66:672–678. doi: 10.1093/ajcn/66.3.672. [DOI] [PubMed] [Google Scholar]
- Jacobsson JA, Risérus U, Axelsson T, Lannfelt L, Schiöth HB, Fredriksson R. The common FTO variant rs9939609 is not associated with BMI in a longitudinal study on a cohort of Swedish men borh 1920-1924. BMC Med Genet. 2009;10:131. doi: 10.1186/1471-2350-10-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James LR, Brett JM. Mediators, moderators and tests for mediation. J Appl Psychol. 1984;69:307–321. [Google Scholar]
- Johnson W. Genetic and environmental influences on behavior: Capturing all the interplay. Psychol Rev. 2007;114:423–440. doi: 10.1037/0033-295X.114.2.423. [DOI] [PubMed] [Google Scholar]
- Johnson W, Krueger RF. Genetic effects on physical health: Lower at higer income levels. Behavior Genetics. 2005;35:579–590. doi: 10.1007/s10519-005-3598-0. [DOI] [PubMed] [Google Scholar]
- Johnson W, Kyvik KO, Skytthe A, Deary IJ, Sørensen TIA. Education modifies genetic and environmental influences on BMI. PLoS One. 2011;6:e16290. doi: 10.1371/journal.pone.0016290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joosen AM, Gielen M, Vlietinck R, Westerterp KR. Genetic analysis of physical activity in twins. Am J Clin Nutr. 2005;82:1253–1259. doi: 10.1093/ajcn/82.6.1253. [DOI] [PubMed] [Google Scholar]
- Jonsson A, Franks PW. Obesity, FTO gene variant, and energetic intake in children. New Engl J Med. 2009;360:1571–1572. doi: 10.1056/NEJMc090017. [DOI] [PubMed] [Google Scholar]
- Jonsson A, Renström F, Lyssenko V, Brito EC, Isomaa B, Berglund G, et al. Assessing the effect of interaction between an FTO variant (rs9939609) and physical activity on obesity in 15,925 Swedish and 2,511 Finnish adults. Diabetologia. 2009;52:1334–1338. doi: 10.1007/s00125-009-1355-2. [DOI] [PubMed] [Google Scholar]
- Jóźków P, Slowińska-Lisowska M, Łaczmański Ł , Jakubiec D, Mędraś M. Melanocortin-4 receptor gene polymorphism and the level of physical activity in men (HALS Study) Endocr. 2011;39:62–68. doi: 10.1007/s12020-010-9412-7. [DOI] [PubMed] [Google Scholar]
- Judd CM, Kenny DA. Estimating the effects of social interventions. Cambridge University Press; New York: 1981. [Google Scholar]
- Kaakinen M, Läära E, Pouta A, Hartikainen A-L, Laitinen J, Tammelin TH, et al. Life-course analysis of a fat mass and obesity-associated (FTO) gene variant and body mass index in the Northern Finland Birth Cohort 1966 using structural equation modeling. Am J Epidemiol. 2010;172:653–665. doi: 10.1093/aje/kwq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnehed N, Tynelius P, Heitmann BL, Rasmussen F. Physical activity, diet and gene-environment interactions in relation to body mass index and waist circumference: The Swedish Young Male Twins Study. Public Health Nutr. 2006;9:851–858. doi: 10.1017/phn2005926. [DOI] [PubMed] [Google Scholar]
- Kilpeläinen TO, Qi L, Brage S, Sharp SJ, Sonestedt E, Demerath E, et al. Physical activity attenuates the influence of FTO variants on obesity risk: A meta-analysis of 218,166 adults and 19,268 children. PLoS Med. 2011;8:e1001116. doi: 10.1371/journal.pmed.1001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagou V, Liu G, Zhu H, Stallman-Jorgensen IS, Gutin B, Dong Y, Snieder H. Lifestyle and socioeconomic-status modify the effects of ADRB2 and NOS3 on adiposity in European-American and African-American adolescents. Obesity. 2012;19:595–603. doi: 10.1038/oby.2010.224. [DOI] [PubMed] [Google Scholar]
- Lappalainen TJ, Tolppanen A-M, Kolehmainen M, Schwab U, Lindström J, Tuomilehto J, et al. The common variant in the FTO gene did not modify the effect of lifestyle changes on body weight: The Finnish Diabetes Prevention Study. Obesity. 2009;17:832–836. doi: 10.1038/oby.2008.618. [DOI] [PubMed] [Google Scholar]
- Lee H-J, Jim IK, Kang JH, Ahn Y, Han B-G, Lee J-Y, Song J. Effects of common FTO gene variants associated with BMI on dietary intake and physical activity in Koreans. Clin Chim Acta. 2010;411:1716–1722. doi: 10.1016/j.cca.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Lee C, Moudon AV. Correlates of Walking for Transportation or Recreation Purposes. J Phys Act Health. 2006;3:S77–S98. doi: 10.1123/jpah.3.s1.s77. [DOI] [PubMed] [Google Scholar]
- Li F, Harmer P, Cardinal BJ, Bosworth M, Johnson-Shelton D, Moore M, et al. Built environment and 1-year change in weight and waist circumference in middle-aged and older adults: Portland neighborhood environment and health study. Am J Epidem. 2009;169:401–408. doi: 10.1093/aje/kwn398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Zhao JH, Luan J, Ekelund U, Luben RN, Khaw K-T, et al. Physical activity attenuates the genetic predisposition to obesity in 20,000 men and women from EPIC-Norfolk Prospective Population Study. PLoS Med. 2010;7:e1000332. doi: 10.1371/journal.pmed.1000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem ET, Vonk JM, Sauer PJJ, van der Steege G, Oosterom E, Stolk RP, Snieder H. Influence of common variants near INSIG2, in FTO, and near MC4R genes on overweight and the metabolic profile in adolescence: The TRAILS (Tracking Adolescents’ Individual Lives Survey) Study. Am J Clin Nutr. 2010;91:321–328. doi: 10.3945/ajcn.2009.28186. [DOI] [PubMed] [Google Scholar]
- Liu G, Zhu H, Lagou V, Gutin B, Stallmann-Jorgensen IS, Treiber FA, et al. FTO variant rs9939609 is associated with body mass index and waist circumference, but now with energy intake of physical activity in European- and African-American youth. BMC Med Genet. 2010;11:57. doi: 10.1186/1471-2350-11-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loos RJF, Rankinen T, Tremblay A, Pérusse L, Chagnon Y, Bouchard C. Melanocortin-4 receptor gene and physical activity in the Québec Family Study. Int J Obes. 2005;29:420–428. doi: 10.1038/sj.ijo.0802869. [DOI] [PubMed] [Google Scholar]
- Marti del Moral A, Corbalán MS, Martínez-Gonzalez MA, Martinez JA. TRP64ARG polymorphism on the β3-adrenergic receptor gene and obesity risk: Effect modification by a sedentary lifestyle. Diabetes Obes Metab. 2002;4:428–430. doi: 10.1046/j.1463-1326.2002.00227.x. [DOI] [PubMed] [Google Scholar]
- McCaffery JM, Papandonatos GD, Bond DS, Lyons MJ, Wing RR. Gene X environment interaction of vigorous exercise and body mass index among male Vietnam-era twins. Amer. J. Clin. Nutr. 2009;89:1011–1018. doi: 10.3945/ajcn.2008.27170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meirhaeghe A, Helbecque N, Cottel D, Amouyel P. β2-adrenoreceptor gene polymorphism, body weight, and physical activity. Lancet. 1999;353:896. doi: 10.1016/S0140-6736(99)00251-2. [DOI] [PubMed] [Google Scholar]
- Metcalf BS, Hosking J, Jeffery AN, Voss LD, Henley W, Wilkin TJ. Fatness leads to inactivity, but inactivity does not lead to fatness: A longitudinal study in children (EarlyBird 45) Arch Dis Child. 2011;96:942–947. doi: 10.1136/adc.2009.175927. [DOI] [PubMed] [Google Scholar]
- Min J, Chiu DT, Wang Y. Variation in the heritability of body mass index based on diverse twin studies: A systematic review. Obes Rev. 2013;14:871–882. doi: 10.1111/obr.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran CN, Vassilopoulos C, Tsiokanos A, Jamurtas AZ, Bailey MES, Wilson RH, Pitsiladis YP. Effects of interaction between angiotensin I-converting enzyme polymorphism and lifestyle on adiposity in adolescent Greeks. Obes Res. 2005;13:1499–1504. doi: 10.1038/oby.2005.181. [DOI] [PubMed] [Google Scholar]
- Moudon AV, Lee C, Cheadle AD, Garvin C, Johnson D, Schmid TL, et al. Operational definitions of walkable neighborhood: theoretical and empirical insights. J Physical Activity and Health. 2006;3:S99–S117. doi: 10.1123/jpah.3.s1.s99. [DOI] [PubMed] [Google Scholar]
- Moudon AV, Lee C, Cheadle AD, Garvin C, Rd DB, Schmid TL, et al. Attributes of environments supporting walking. Am J Health Promot. 2007;21:448–59. doi: 10.4278/0890-1171-21.5.448. [DOI] [PubMed] [Google Scholar]
- Müller TD, Hinney A, Scherag A, Nguyen TT, Schreiner F, Schäfer H, et al. ‘Fat mass and obesity associated’ gene (FTO): No significant association of variant rs9939609 with weight loss in a lifestyle intervention and lipid metabolism markers in German obese children and adolescents. BMC Med Genet. 2008;9:85. doi: 10.1186/1471-2350-9-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustelin L, Silventoinen K, Pietilainen K, Rissanen A, Kaprio J. Physical activity reduces the influence of genetic effects on BMI and waist circumference: a study in young adult twins. Int J Obes. 2009;33:29–36. doi: 10.1038/ijo.2008.258. [DOI] [PubMed] [Google Scholar]
- Muthen LK, Muthen BO. User's Guide. 7th Muthen & Muthen; Los Angeles, CA: 2012. Mplus. Statistical analysis with latent variables. [Google Scholar]
- Nakashima H, Omae K, Nomiyama T, Tamano Y, Takebayashi T, Sakurai Y. Beta-3-adrenergic rceptor Trp64Arg polymorphism: Does it modulate the relationship between exercise and percentage body fat in youn adult Japanese males? Environ Health Prev Med. 2013;18:323–329. doi: 10.1007/s12199-012-0325-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Heart Lung and Blood Institute Predictors of obesity, weight gain, diet, and physical activity workshop. 2004 http://www.nhlbi.nih.gov/meetings/workshops/predictors/summary.htm. Accessed August 27, 2013.
- National Institutes of Health Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health. Obesity Res. 1998;6:51S–209S. [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and aduly obesity in the United States, 2011-2012. JAMA. 2006;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rahilly S, Farooqi IS. Human obesity: A heritable neurobehavioral disorder that is highly sensitive to environmental conditions. Diabetes. 2008;57:2905–2910. doi: 10.2337/db08-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osler M, McGue M, Christensen K. Socioeconomic position and twins’ health: A life-course analysis of 1266 pairs of middle-aged Danish twins. Int J Epidemiol. 2007;36:77–83. doi: 10.1093/ije/dyl266. [DOI] [PubMed] [Google Scholar]
- Otabe S, Clement K, Pelloux V, Guy-Grand B, Froguel P, Vasseur F. A genetic variation in the 5’ flanking region of the UCP3 gene is associated with body mass index in humans in interaction with physical activity. Diabetologia. 2000;43:245–249. doi: 10.1007/s001250050037. [DOI] [PubMed] [Google Scholar]
- Papas MA, Alberg AJ, Ewing R, Helzlsouer KJ, Gary TL, Klassen AC. The built environment and obesity. Epidemiol Rev. 2007;29:129–143. doi: 10.1093/epirev/mxm009. [DOI] [PubMed] [Google Scholar]
- Perusse L, Tremblay A, Leblanc C, Bouchard C. Genetic and environmental influences on level of habitual physical activity and exercise participation. Am J Epidemiol. 1989;129:1012–1022. doi: 10.1093/oxfordjournals.aje.a115205. [DOI] [PubMed] [Google Scholar]
- Poehlman ET, Tremblay A, Després J-P, Fontaine E, Pérusse L, Thériault G, Bouchard C. Genotype-controlled changes in body composition and fat morphology following overfeeding in twins. Am J Clin Nutr. 1986a;43:723–731. doi: 10.1093/ajcn/43.5.723. [DOI] [PubMed] [Google Scholar]
- Poehlman ET, Tremblay A, Fontaine E, Després JP, Nadeau A, Dussault J, Bouchard C. Genotype dependency of the thermic effect of a meal and associated hormonal changes following short-term overfeeding. Metabolism. 1986b;35:30–36. doi: 10.1016/0026-0495(86)90092-2. [DOI] [PubMed] [Google Scholar]
- Prince SA, Adamo KB, Hamel ME, Hardt J, Gorbor SC, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: A systematic review. Int J Behav Nutr Phys Act. 2008;5:56. doi: 10.1186/1479-5868-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronk NP, Goodman MJ, O'Connor PJ, Martinson BC. Relationship between modifiable health risks and short-term health care charges. JAMA. 1999;282:2235–2239. doi: 10.1001/jama.282.23.2235. [DOI] [PubMed] [Google Scholar]
- Purcell S. Variance components models for gene-environment interaction in twin analysis. Twin Res Hum Genet. 2002;5:554–571. doi: 10.1375/136905202762342026. [DOI] [PubMed] [Google Scholar]
- Rampersaud E, Mitchell BD, Pollin TI, Fu M, Shen H, O’Connell JR, et al. Physical activity and the association of common FTO gene variants with body mass index and obesity. Arch Intern Med. 2008;168:1791–1797. doi: 10.1001/archinte.168.16.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderstråle M, Johansson LE, Rastam L, Lindblad U. Increased risk of obesity associated with the variant allele of the PPARGC1A Gly482Ser polymorphism in physically inactive elderly men. Diabetologia. 2006;49:496–500. doi: 10.1007/s00125-005-0129-8. [DOI] [PubMed] [Google Scholar]
- Ruiz JR, Labayen I, Ortega FB, Legry V, Moreno LA, Dallongeville J, et al. Attenuation of the effect of the FTO rs9939609 polymorphism on total and central body fat by physical activity in adolescents: The HELENA Study. Arch Pediatr Adolesc Med. 2010;164:328–333. doi: 10.1001/archpediatrics.2010.29. [DOI] [PubMed] [Google Scholar]
- Saelens BE, Sallis JF, Black JB, Chen D. Neighborhood-based differences in physical activity: an environment scale evaluation. Am J Pub Health. 2003;93:1552–8. doi: 10.2105/ajph.93.9.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schousboe K, Willemsen G, Kyvik KO, Mortensen J, Boomsma DI, Cornes BK, et al. Sex differences in heritability of BMI: a comparative study of results from twin studies in eight countries. Twin Res Hum Genet. 2003;6:409–421. doi: 10.1375/136905203770326411. [DOI] [PubMed] [Google Scholar]
- Scott RA, Bailey MES, Moran CN, Wilson RH, Fuku N, Tanaka M, et al. FTO genotype and adiposity in children: Physical activity levels influence the effect of the risk genotype in adolescent males. Eur J Hum Genet. 2010;18:1339–1343. doi: 10.1038/ejhg.2010.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiwaku K, Nogi A, Anuurad E, Kitajima K, Enkhmaa B, Shimono K, Yamane Y. Difficulty in losing weight by behavioral intervention for women with Trp64Arg polymorphism of the β3-adrenergic receptor gene. Int J Obes. 2003;27:1028–1036. doi: 10.1038/sj.ijo.0802375. [DOI] [PubMed] [Google Scholar]
- Silventoinen K, Sarlio-Lähteenkorva S, Koskenvuo M, Lahelma E, Kaprio J. Effect of environmental and genetic factors on education-associated disparities in weight and weight gain: A study of Finnish adult twins. Am J Clin Nutr. 2004;80:815–822. doi: 10.1093/ajcn/80.4.815. [DOI] [PubMed] [Google Scholar]
- Simonen RL, Perusse L, Rankinen T, Rice T, Rao DC, Bouchard C. Familial aggregation of physical activity levels in the Quebec Family Study. Med Sci Sports Exerc. 2002;34:1137–1142. doi: 10.1097/00005768-200207000-00014. [DOI] [PubMed] [Google Scholar]
- Sonestedt E, Roos C, Gullberg B, Ericson U, Wirfält E, Orho-Melander M. Fat and carbohydrate intake modify the association between gentic variation in the FTO genotype and obesity. Am J Clin Nutr. 2009;90:1418–1425. doi: 10.3945/ajcn.2009.27958. [DOI] [PubMed] [Google Scholar]
- Spitz E, Moutier R, Reed T, Busnel MC, Marchaland C, Roubertoux PL, Carlier M. Comparative diagnoses of twin zygosity by SSLP variant analysis, questionnaire, and dermatoglyphic analysis. Behav Genet. 1996;26:55–63. doi: 10.1007/BF02361159. [DOI] [PubMed] [Google Scholar]
- Strachan E, Hunt C, Afari N, Duncan G, Noonan C, Schur E, et al. University of washington twin registry: poised for the next generation of twin research. Twin Res Hum Genet. 2013;16:455–462. doi: 10.1017/thg.2012.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbe JH, Boomsma DI, Vink JM, Cornes BK, Martin NG, Skytthe A, et al. Genetic influences on exercise participation in 37,051 twin pairs from seven countries. PLoS One. 2006;1:e22. doi: 10.1371/journal.pone.0000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JT, Dorajoo R, Seielstad M, Sim XL, Ong RT-H, Chia KS, et al. FTO variants are associated with obesity in the Chinese and Malay populations in Singapore. Diabetes. 2008;57:2851–2857. doi: 10.2337/db08-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgersen S. The determination of twin zygosity by means of a mailed questionnaire. Acta Genet Med Gemellol. 1979;28:225–236. doi: 10.1017/s0001566000009077. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services . Physical activity and health: a report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; Atlanta, GA: 1996. [Google Scholar]
- van der Sluis S, Posthuma D, Dolan CV. A note on false positives and power in G x E modelling of twin data. Behav Genet. 2012;42:170–186. doi: 10.1007/s10519-011-9480-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyck D, Cerin E, Cardon G, Deforche B, Sallis JF, Owen N, de Bourdeaudhuij I. Physical activity as a mediator of the associations between neighborhood walkability and adiposity in Belgian adults. Health Place. 2010;16:952–960. doi: 10.1016/j.healthplace.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Vinaleswaran KS, Li S, Zhao JH, Luan J, Bingham SA, Khaw K-T, et al. Physical activity attenuates the body mass index-increasing influence of genetic variation in the FTO gene. Am J Clin Nutr. 2009;90:425–428. doi: 10.3945/ajcn.2009.27652. [DOI] [PubMed] [Google Scholar]
- Vimaleswaran KS, Tachmazidou I, Zhao JH, Hirschhorn JN, Dudbridge F, Loos RJF. Candidate genes for obesity-susceptibility show enriched assocaition within a large genome-wide association study for BMI. Hum Mol Genet. 2012;21:4537–4542. doi: 10.1093/hmg/dds283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walk Score® Walk Score methodology. 2011 http://www.walkscore.com/methodology.shtml. Accessed August 27, 2013.
- Walk Score® Get your Walk Score - A walkability score for any address. 2012 http://www.walkscore.com. Accessed August 27, 2013.
- Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378:815–825. doi: 10.1016/S0140-6736(11)60814-3. [DOI] [PubMed] [Google Scholar]
- Webbink D, Martin NG, Visscher PM. Does education reduce the probability of being overweight? J Health Econ. 2010;29:29–38. doi: 10.1016/j.jhealeco.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Xi B, Shen Y, Zhang M, Liu X, Zhao X, Wu L, et al. The common rs9939609 variant of the fat mass and obesity-associated gene with obesity risk in children and adolescents of Beijing, China. BMC Med Genet. 2010;11:107. doi: 10.1186/1471-2350-11-107. [DOI] [PMC free article] [PubMed] [Google Scholar]