Abstract
Purpose/Objectives
Optimizing androgen suppression may provide better control of localized prostate cancer (PCa). Numerous trials have supported the benefit of combining androgen deprivation with definitive radiotherapy in men with locally advanced or high-grade disease. Addition of abiraterone to LHRH agonist (LHRHa) with radiation has not been reported. We examined the safety of this combination as well as its impact on androgen suppression.
Materials/Methods
A prospective, phase II study was conducted in men with localized PCa treated with 6 months of neoadjuvant and concurrent abiraterone with LHRHa and radiation. Duration of adjuvant LHRHa was at discretion of treating clinician. Prostate biopsies were obtained prior to start of therapy and prior to radiation. Serum and tissue androgen levels were measured by liquid chromatography-tandem mass spectrometry.
Results
22 men with intermediate (3) and high-risk PCa (19) received study therapy. 16 men completed the intended course of abiraterone, and 19 men completed planned radiation to 77.4–81 Gy. Radiation to pelvic nodes was administered in 20 men. The following grade 3 toxicities were reported: lymphopenia (14),, fatigue (1), transaminitis (2), hypertension (2), and hypokalemia (1). There were no grade 4 toxicities. All 21 men who complied with at least 3 months of abiraterone had pre-radiation PSA nadir of <0.3. Median levels of tissue androgens downstream of CYP17A were significantly suppressed after treatment with abiraterone, and upstream steroids were increased. At median follow-up of 21 months (range 3–37), only one patient (who had discontinued abiraterone at 3 months) had biochemical relapse.
Conclusions
Addition of abiraterone to LHRHa with radiation is safe and achieves effective prostatic androgen suppression. Preliminary analysis of the clinical data is also promising with excellent PSA nadir and no relapse to date in this high-risk population.
Introduction
Numerous studies have demonstrated the benefit of combining androgen deprivation (ADT) with radiation therapy in preclinical models as well as for men with locally advanced or high-grade prostate cancer [1–8]. While studies have varied with respect to entry criteria, radiation dose and field, as well as timing and duration of ADT, all have shown significant improvements in long-term local, distant, and biochemical control. Recent preclinical work in prostate cancer cell line models have demonstrated the role of the androgen receptor in regulating DNA repair genes, which provides a potential mechanism for ADT synergizing with ionizing radiation[9,10] The traditional backbone of ADT is a luteinizing-hormone-releasing-hormone agonist (LHRHa) with or without a nonsteroidal antiandrogen such as bicalutamide or flutamide. The clinical safety and therapeutic effect of further suppressing the androgen axis by newer agents such as abiraterone in combination with radiation is still unknown.
Abiraterone acetate is a prodrug of abiraterone, which is an inhibitor of cytochrome P450 17alpha-hydroxylase/17,20 lyase (CYP17), a rate limiting enzyme of steroidogenesis. It can inhibit all sources of androgens including testicular, adrenal, prostate, and intratumoral androgens [11]. The addition of abiraterone to LHRHa in men with castration resistant prostate cancer can result in undetectable serum testosterone levels (< 1 ng/dL) within 8 days of starting therapy [12]. Abiraterone acetate in combination with prednisone is currently approved for use in men with metastatic castration resistant prostate cancer both in the pre- and post-chemotherapy settings. The use of abiraterone in high-risk localized prostate cancer in combination with radiation has never been reported.
We hypothesized that the addition of abiraterone to LHRHa would result in lower serum and intraprostatic androgens than treatment with LHRHa and bicalutamide. We designed a trial to investigate the safety and feasibility of adding abiraterone to LHRHa as neoadjuvant and concurrent ADT with external beam radiation, and measured tissue and serum abiraterone and hormone levels prior to and after 3 months of therapy. We propose that enhanced androgen suppression achieved with abiraterone can minimize activation of the androgen receptor and improve outcomes for patients following radiation without increasing toxicity.
Patients and Methods
Patient Population
This open label, single-arm, phase 2 study was approved by institutional review boards and all subjects signed written informed consent. Eligible men had clinically localized prostate cancer and were candidates for short- or long-term ADT in combination with external beam radiation therapy based on the following criteria: intermediate-risk disease (Gleason 7, or PSA 10–20) or high-risk disease (T3/4, Gleason 8–10 or PSA > 20). Prior pharmacologic therapy or radiation for prostate cancer, including drugs affecting the androgen axis and metabolism, history of pituitary or adrenal dysfunction, uncontrolled hypertension, congestive heart failure, active or chronic hepatitis or inflammatory bowel disease were exclusionary. Men were required to have a serum testosterone ≥ 100 ng/dL and normal blood counts, creatinine, and transaminases.
Study Procedures
All patients were assigned to receive 24 weeks of pharmacologic therapy with LHRHa of goserelin or leuprolide, abiraterone acetate (1000 mg daily), and prednisone (5 mg daily). After the initial 12 weeks (neoadjuvant phase), patients underwent placement of fiducials and radiation planning followed by external beam radiation therapy (concurrent phase).
Radiotherapy planning and delivery methods were per each center’s standard practice. Treatment was 3D planned and delivered by intensity-modulated radiation therapy (IMRT) techniques employing 6–15MV photons. The location of the prostatic apex was determined by either retrograde urethrography or by soft tissue matching of a treatment planning MRI fused to the treatment planning CT. Radiation targets were the pelvic lymph nodes and the prostatic primary. Dose to the initial fields was 5,040 cGy at 180 cGy per fraction. After completion of the pelvic irradiation, there was an additional coned-down boost field encompassing the prostate and at least 1.5 cm of proximal seminal vesicles (full seminal vesicle if grossly involved) to an additional 3,060 cGy, for a total dose of 8100 cGy (normal tissue dose constraints permitting).
Intermediate-risk patients received no further ADT following completion of radiation unless the treating physician felt that further ADT was warranted. High-risk patients continued to receive adjuvant ADT in the form of LHRHa monotherapy, with total treatment duration at the discretion of the treating physician.
The primary endpoint was to determine the safety of concurrent abiraterone with radiation therapy. Toxicity assessments using CTCAE criteria were performed every 4 weeks during the neoadjuvant treatment phase, every 4 weeks during radiotherapy, and every 12 weeks through 24 months following completion of radiotherapy. The exploratory endpoint was to assess the impact of LHRHa plus abiraterone on androgen suppression compared to LHRHa plus bicalutamide.
Serum and Tissue Acquisition
Prior to initiation of pharmacologic therapy, patients underwent a mandatory ultrasound-guided prostate biopsy, which was separate from their initial diagnostic biopsy. After 12 weeks of neoadjuvant therapy, patients underwent another ultrasound-guided prostate biopsy at the time of fiducial placement, prior to radiation. At both stages, 8 cores were obtained and snap frozen. Non-fasting blood samples were obtained before treatment initiation and after 12 weeks of therapy and kept at −80°C until assayed.
Clinical assays
Serum PSA was analyzed by CLIA-certified clinical pathology laboratories. Laboratory results were extracted from the medical record and entered into study-specific records with minimal identifiers. These data were linked with results of the experimental laboratory assays by IRB-approved personnel.
Androgen and Abiraterone Measurements
Serum and tissue androgen levels were determined by a liquid chromatography-tandem mass spectrometry assay in a blinded manner, using a modification of methods we have previously described [13,14]. In brief, frozen needle biopsy tissue cores were weighed, added to 60°C water containing deuterated internal standards, heated at 60°C for 10 minutes, homogenized using a Precellys tissue homogenizer (Bertin, Rockville, MD), supernatant extracted twice with hexane: ethyl acetate (80:20 v/v), organic layer dried in a SpeedVac (Thermo Scientific, Waltham, MA), derivatized with 0.025-M hydroxylamine hydrochloride for 24 hours at room temperature to form oximes, and quantified using liquid chromatography electrospray-ionization tandem mass spectrometry.
Statistical Analyses
A sample size of 25 was planned to detect appreciable toxicities and to compare androgen blockade with the bicalutamide study. The safety of combined androgen blockade with IMRT has been examined in prior studies. The incidence of grade 3 or greater acute (≤ 90 days from completion of radiation) is 0–7% [15,16]. The incidence of grade 3 or greater late toxicity (> 90 days from completion of radiation) is 3–4% [17,18]. Taking into account the potential for disproportionate effect of events in a small study, an unacceptable rate of toxicity for the abiraterone-treated patients was set to that seen in heavily chemotherapy-treated patients, or 25%. Per protocol, accrual to the study was to be suspended and analysis of toxicity reported to the relevant IRB for reasonable evidence that the true rate for serious toxicity (≥ grade 3) exceeded 0.25. Reasonable evidence was defined by a Clopper-Pearson exact one-sided 80% confidence interval for the true rate of events excluding values of 0.25 or lower [19]. This stopping rule was to be triggered if 3 or more of 5 patients, 5 or more of 10 patients, 6 or more of 15 patients, or 8 or more of 20 patients experienced grade 3 or greater toxicity. The study protocol was amended prior to first stopping threshold to exclude transient grade 3 lymphopenia as a serious adverse event, based on the high lymphopenia rates and subsequent recovery of patients undergoing radiation therapy to the pelvis for gynecologic cancers [20]. The stopping threshold was not crossed prior to study completion. Mann-Whitney U test was used to compare tissue and serum androgen levels.
Results
Patient Characteristics
Twenty-four patients with intermediate to high-risk prostate adenocarcinoma were enrolled; two patients are excluded from analyses as they withdrew from the study prior to receiving any study treatments. The study was stopped prior to reaching accrual goal of 25 due to interruption of funding. Three patients were intermediate-risk and 19 patients were high-risk with median age of 61. Median PSA prior to therapy was 19 ng/mL (range 2.87–155.36). Median dose of radiation delivered was 81 Gy (range: 48.60–81). Patient characteristics are summarized in Table 1.
Table 1.
Patient Characteristics
| Characteristic | N (%) |
|---|---|
| PSA (ng/mL) | |
| <10 | 8 (36) |
| 10–20 | 4 (18) |
| >20 | 10 (45) |
| Gleason sum | |
| 3+4 | 8 (36) |
| 4+3 | 2 (9) |
| 4+4 | 1 (5) |
| 4+5 | 10 (45) |
| 5+4 | 1 (5) |
| Clinical T stage | |
| T1 | 3 (14) |
| T2 | 13 (59) |
| T3 | 6 (27) |
Toxicities
Adverse events during the study were recorded and graded according to NCI-CTCAE v.4.0. Table 2 outlines grade 1 and 2 toxicities > 10% and grade 3 toxicities that were either possibly or probably related to radiation, abiraterone/prednisone or LHRHa. There were no grade 4 toxicities, and the stopping rules were not triggered due to the exclusion of grade 3 lymphopenia or other toxicities as significant adverse events. Abiraterone was discontinued early in 6 patients for the following reasons: grade 2 fatigue (1), grade 3 fatigue (1), grade 2 tinea pedis (1), grade 2 atrial fibrillation and grade 3 hypertension (1), and grade 3 elevated liver function tests (2). Radiation therapy was stopped early in 3 patients for the following reasons: cost (1), inconvenience (1), and grade 3 fatigue (1). No increased toxicity was seen during the radiation portion that could be attributed to abiraterone, and there were no delays or holds in administration of radiation related to abiraterone. No acute or late grade ≥ 2 genitourinary or gastrointestinal toxicities were seen with radiation (median follow up 21 months). Late toxicities were defined as any toxicities occurring ≥ 90 days after the start of radiation. Grade 3 lymphopenia developed in 14 patients (64%) during radiation, subsequently resolved in all patients and was not associated with any serious infections.. The overall rate of grade 3 toxicities was 27%, which was at the acceptable limit of anticipated grade 3 toxicity of 25%. All of the grade 3 toxicities were known adverse events related to abiraterone or androgen deprivation such as chemical hepatitis, hypertension, hypokalemia and fatigue.
Table 2.
Adverse Events
| Toxicity | All Grades N (%) |
Grade 3 N (%) |
|---|---|---|
| Lymphopenia | 19 (86) | 14 (64) |
| Hypertension | 8 (36) | 2 (9) |
| Elevated liver function tests | 7 (32) | 2 (9) |
| Fatigue | 15 (68) | 1 (5) |
| Hypokalemia | 12 (55) | 1 (5) |
| Hot flashes | 15 (68) | 0 (0) |
| Hyperbilirubinemia | 5 (23) | 0 (0) |
| Insomnia | 3 (14) | 0 (0) |
| Decreased libido | 7 (32) | 0 (0) |
| Mood swings | 4 (18) | 0 (0) |
| Sexual dysfunction | 3 (14) | 0 (0) |
| Diarrhea | 9 (41) | 0 (0) |
| Dysuria | 5 (23) | 0 (0) |
| Nocturia | 5 (23) | 0 (0) |
| Urinary frequency | 9 (41) | 0 (0) |
PSA nadir
Median pre-radiation PSA at 12 weeks was 0.05 ng/mL (range: undetectable – 0.81 ng/mL). All patients had nadirs ≤ 0.3 ng/mL except one patient who was not compliant with abiraterone (PSA = 0.81 ng/mL). Four men had an undetectable PSA at this time point. Post radiation PSA was undetectable in 19/22 patients and in all patients who completed the neoadjuvant and concurrent portions of the study (N=16).
Recurrence
At median follow-up of 21 months (range 3–37), 21/22 patients had not experienced biochemical relapse. Nine patients remain on adjuvant ADT with LHRHa. The median duration of LHRHa use in high-risk patients was 20 months. The one patient with biochemical relapse did not complete the initial three months of neoadjuvant abiraterone and received only 9 total months of LHRHa therapy for high-risk disease (due to noncompliance).
Serum hormone effects of LHRHa plus abiraterone at 12 weeks
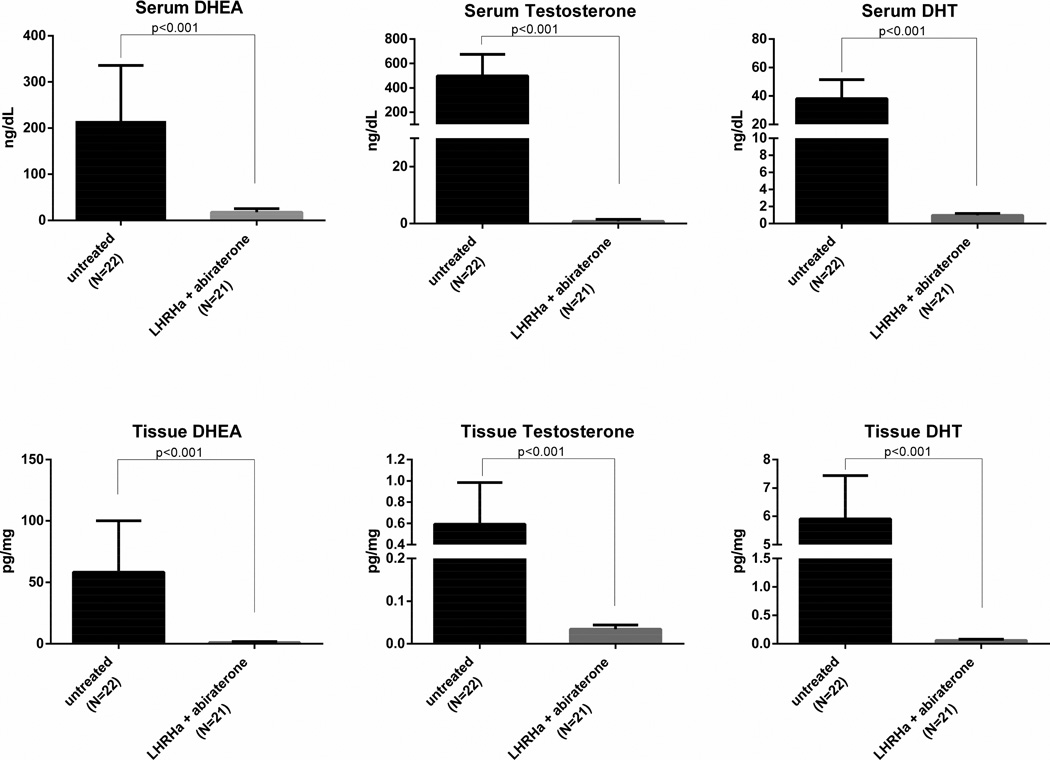
Serum pregnenolone and progesterone, hormones that are upstream of CYP17, had a 486% increase and 2772% increase after treatment, respectively. Serum androgens downstream of CYP17A (DHEA, testosterone, DHT, and androstenedione) are significantly decreased with changes of −91%, −99.9%, −97%, and −99%, respectively. Median serum hormone levels prior to therapy and following 12 weeks of treatment with LHRHa and abiraterone are shown in Table 3. Serum hormone levels from a prior trial of LHRHa and bicalutamide (given for 12 weeks prior to prostatectomy in men with localized prostate cancer) are included for comparison [21]. In that study, the percent difference in mean serum testosterone, DHT, DHEA, and androstenedione levels in patients who received 12 weeks of LHRHa and bicalutamide compared to those who received no treatment were −92% (p<0.001), −68% (p<0.001), +16% (p not significant), and +16% (p not significant), respectively. The top row of Figure 1 shows the differences in DHEA, testosterone, and DHT between untreated patients, those receiving 12 weeks of LHRHa and bicalutamide (from the previously published study), or 12 weeks of LHRHa and abiraterone (current study).
Table 3.
Median serum hormone levels prior to treatment and following 12 weeks of LHRHa plus abiraterone.
| Median serum androgen |
Pre- treatment [90% CI] |
LHRHa + bicalutamide |
LHRHa + abiraterone [90% CI] |
% change (pre- treatment vs LHRHa + abiraterone) |
p-value |
|---|---|---|---|---|---|
| DHT (ng/dL) | 37.3 [31.3–74.4] |
6.6 | 0.98* [0.98-0.98] |
−97% | <0.001 |
| Testosterone (ng/dL) | 490.6 [397.4–586.9] |
19.4 | 0.49* [0.49-0.49] |
−99.9% | <0.001 |
| Androstenedione (ng/dL) | 71.5 [56.2–77.9] |
69.6 | 0.90 [0.68–2.37] |
−99% | <0.001 |
| DHEA (ng/dL) | 179.7 [128.1–292.2] |
386 | 16.9 [13.0–20.3] |
−91% | <0.001 |
| DHEA-S (µg/dL) | 130.2 [84.8–305.8] |
71.0 | 2.35 [0.44–3.04] |
−98% | <0.001 |
| Progesterone (ng/mL) | 0.05 [0.04–0.06] |
-- | 1.35 [0.74–1.84] |
+2600% | <0.001 |
| Pregnenolone (ng/mL) | 1.86 [1.60–2.97] |
-- | 10.9 [9.27–15.2] |
+486% | <0.001 |
indicates levels at the lower limit of detection; --indicates data not available
Fig. 1.
Changes in serum and tissue DHEA, testosterone, and DHT following 12 weeks of LHRHa plus abiraterone.
Tissue hormone effects of LHRHa plus abiraterone at 12 weeks
Similar to the changes seen in serum levels, pregnenolone and progesterone are significantly increased in intraprostatic tissue after abiraterone treatment, with 670% and 1265% increases, respectively. Tissue androgens downstream of CYP17A (DHEA, testosterone, DHT, and androstenedione) are significantly decreased following therapy, with changes of −98%, −94%, −99%, and −97%, respectively. Median tissue hormone levels prior to and following 12 weeks of treatment are shown in Table 4. Again, for comparison, tissue hormone data from a prior trial following 12 weeks of LHRHa and bicalutamide are included in the table [21]. In that study, the percent difference in mean tissue testosterone, DHT, DHEA, and androstenedione levels in patients who received 12 weeks of LHRHa and bicalutamide compared to those who received no treatment were −73% (p<0.001), −78% (p<0.001), +18% (p not significant), and +169% (p not significant) respectively. The bottom row of Figure 1 shows differences in DHEA, testosterone, and DHT between untreated patients, patients receiving 12 weeks of LHRHa and bicalutamide (from the previously published study), and patients receiving 12 weeks of LHRHa and abiraterone (current study).
Table 4.
Median intraprostatic hormone levels prior to treatment and following 12 weeks of LHRHa plus abiraterone.
| Median tissue androgen (pg/mg) |
Pre- treatment [90% CI] |
LHRHa + bicalutamide |
LHRHa + abiraterone [90% CI] |
% change (pre- treatment vs LHRHa + abiraterone |
p-value |
|---|---|---|---|---|---|
| DHT | 5.92 [5.15–6.80] |
0.798 | 0.050* [0.050-0.050] |
−99% | <0.001 |
| Testosterone | 0.487 [0.372–0.686] |
0.047 | 0.030* [0.030-0.030] |
−94% | <0.001 |
| Androstenedione | 0.305 [0.218–0.449] |
0.648 | 0.010* [0.010-0.010] |
−97% | <0.001 |
| DHEA | 43.7 [28.6–62.7] |
26.3 | 0.660* [0.500–1.23] |
−98% | <0.001 |
| DHEA-S | 0.320 [0.209–0.542] |
-- | 0.0083 [0.0019–0.0182] |
−97% | <0.001 |
| Progesterone | 0.159 [0.112–0.218] |
-- | 1.17 [0.944–1.74] |
636% | <0.001 |
| Pregnenolone | 31.7 [23.4–43.6] |
-- | 244 [183–510] |
670% | <0.001 |
indicates levels at the lower limit of detection; --indicates data not available.
Abiraterone levels
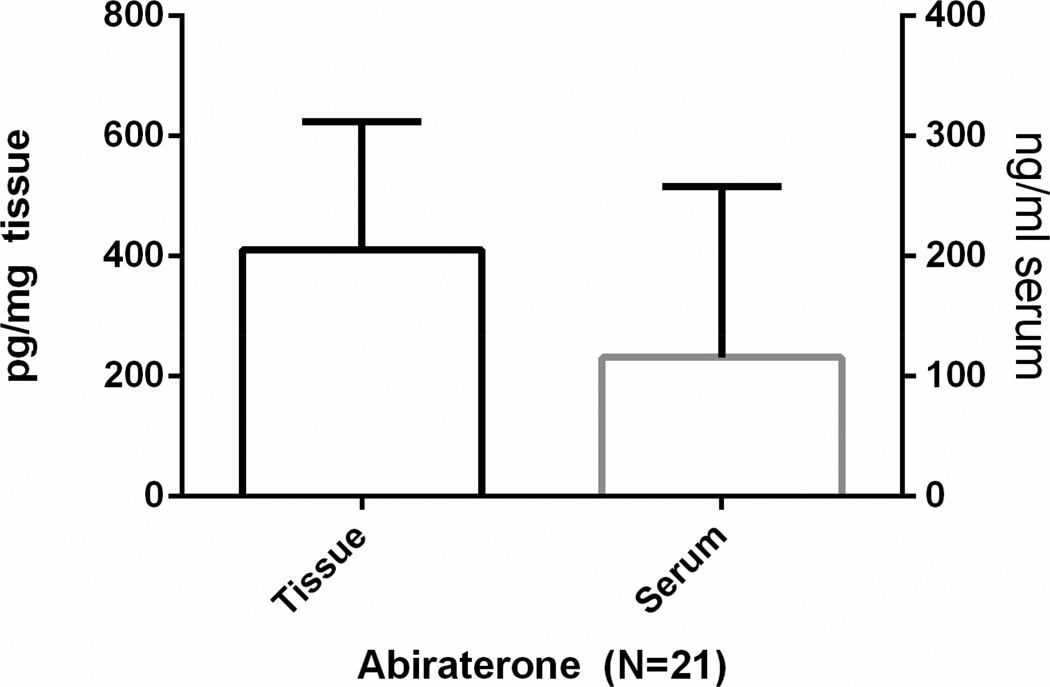
Abiraterone was detectable in serum and prostate tissue for all men compliant with therapy at 12 weeks, as shown in Figure 2 (N=21; 95%). Serum and tissue abiraterone levels were strongly correlated (Spearman r=0.838, p<0.001). Serum abiraterone levels also showed a positive correlation with serum progesterone levels at the 12-week time point (Spearman r=0.631, p=0.002) although significant correlations at 12 weeks between abiraterone and other hormone levels in the tissue and serum, or with serum PSA were not observed.
Fig. 2.
Serum and tissue abiraterone levels following 12 weeks of LHRHa plus abiraterone.
Discussion
This study is the first to report abiraterone acetate with LHRHa as neoadjuvant and concurrent ADT in patients undergoing radiation for localized prostate cancer. Overall, the study treatment was well tolerated, with the toxicities observed generally reflecting those expected with ADT and radiation. Safety data from two randomized controlled trials of abiraterone in metastatic castration resistant patients reported grade ≥ 3 hypertension in 2%, grade ≥ 3 hypokalemia in 4%, grade ≥ 3 edema in 1%, grade ≥ 3 transaminase elevations in 4%, and grade ≥ 3 fatigue in 2% [22,23]. Our numbers are similar when accounting for small sample size. With regards to radiation-related toxicities, no patients had CTCAE grade ≥ 2 genitourinary or gastrointestinal events during or after radiation. Although hematologic toxicities are not routinely assessed during standard radiation for localized prostate cancer, complete blood count assessments were performed every 4 weeks as part of our protocol. We found grade 3 lymphopenia in 64% of patients, which is similar to a previously reported rate of 73% for patients undergoing pelvic irradiation to 50.4 Gy for gynecologic neoplasms [20].
Twelve weeks of treatment with abiraterone plus LHRHa led to significant increases in both serum and tissue hormones that are upstream of CYP17A (progesterone and pregnenolone) and significant decreases in both serum and tissue hormones that are downstream of CYP17A (DHEA-S, DHEA, androstenedione, testosterone, and DHT). Compared to 12 weeks of treatment with LHRHa plus bicalutamide [21], treatment with LHRHa plus abiraterone resulted in significantly greater suppression of all downstream steroids. Our results in serum and tissue are similar in range and magnitude to those recently reported in a neoadjuvant study of LHRHa plus abiraterone for 12 or 24 weeks prior to prostatectomy [21], but our study is the first to report measurements of intraprostatic DHEA-S and tissue abiraterone.
The suppression of tissue DHEA-S, DHEA and androstenedione (which are not affected by LHRHa and bicalutamide) is significant as these androgens can be converted to testosterone and DHT by prostate basal epithelial cells and may provide a potential source of continued androgen receptor activation under castrate conditions [24]. While the suppression of serum DHEA-S to 10–15% of baseline levels is consistent with findings in the recent study of abiraterone prior to prostatectomy [25], it was previously unknown whether there remained a similar reservoir of DHEA-S in prostate tissue after abiraterone. Our data now show that although serum DHEA-S levels are three orders of magnitude higher than DHEA (consistent with DHEA-S serving as a reservoir in serum), levels of intraprostatic DHEA-S in abiraterone-treated tissues are much lower than levels of intraprostatic DHEA. This finding suggests that DHEA-S is not a major reservoir of androgens in tissue, likely due to sulfatases in prostate tissue which rapidly convert DHEA-S to DHEA [26]. However, serum DHEA-S may still serve as a reservoir for prostate androgens in abiraterone-treated tissue as correlation between serum DHEA-S and tissue DHEA-S/DHEA levels are strong after abiraterone treatment (Spearman r=0.79 and 0.63 respectively, p<0.01 for both).
Abiraterone was detectable in serum and prostate tissue for all men compliant with therapy at the time of their 12-week biopsy. Strong correlation between serum and tissue abiraterone levels is consistent with data from a recent study using neoadjuvant abiraterone prior to prostatectomy [25]. The positive correlation between serum abiraterone and serum progesterone, which is a hormone that is upstream of CYP17A, is also consistent with data from the neoadjuvant prostatectomy study (presented in abstract form – Mostaghel, et al. ASCO 2014 abstract 5015), although significant positive correlations with pregnenolone and negative correlations with DHEA noted in that report were not seen in our patients. Differences in the results may be related to small sample size or differences in sampling technique (hormone levels were measured from core biopsy samples for our patients whereas hormone levels were measured from prostatectomy specimens in the other study).
The impact of abiraterone with LHRHa in the neoadjuvant setting was reflected in significant PSA declines. All men who were compliant with therapy (n=21; 95%) had a nadir ≤ 0.3 ng/mL, which is in contrast to published data for men on LHRHa without abiraterone where pre-radiation PSA nadir ≤ 0.3 ng/mL was achieved in only 60% of patients [27]. Analysis of men in that study showed that pre-radiation PSA nadir of ≤ 0.3 ng/mL was associated with improved long-term biochemical tumor control (hazard ratio = 0.546, p < 0.001), reduction in distant metastases (hazard ratio = 0.62, p=0.003), and prostate cancer-related death (hazard ratio =0.595, p=0.01). [28] Post-radiation PSA-nadir in men treated with LHRHa and radiation has also been demonstrated to be a strong prognostic indicator of post-treatment outcomes including prostate cancer-specific mortality [29,30]All compliant men in our study had a post-radiation nadir of 0.2 or less, and 19 men had undetectable nadirs. Although it is too early to determine whether post-radiation PSA-nadir can serve as a valid surrogate endpoint in patients receiving neoadjuvant/concurrent abiraterone, it is promising that among our group of intermediate and high-risk patients, only one patient (5%) has had biochemical relapse at a median follow-up of 21 months, and no patient who completed full intended course of therapy has had a biochemical relapse. Continued follow-up will allow for better assessment of the long-term impact of abiraterone in our study population.
The results of our study demonstrate that combining LHRHa and abiraterone with radiation is a feasible and well-tolerated approach to treating intermediate and high-risk localized prostate cancer. We have also established a successful model for performing tissue-based analyses in the context of a radiation therapy study. The addition of abiraterone to LHRHa improves suppression of intraprostatic testosterone and DHT to levels considerably lower than that which can be achieved with LHRHa and bicalutamide. Although preliminary, our limited clinical outcomes are quite promising. Whether more potent suppression of testosterone and DHT with abiraterone results in enhanced clinical efficacy when with combined with radiation will be addressed by Radiation Therapy Oncology Group Protocol 1115 (Phase III trial of dose escalated radiation therapy and standard androgen deprivation therapy (ADT) with a GnRH agonist vs. enhanced ADT with GnRH agonist and TAK-700 for high risk prostate cancer). Tissue determinants of sensitivity to abiraterone remain unknown, and analysis of pretreatment tissue from this study and the Stand Up to Cancer (SU2C) dream team efforts will provide further insights. These investigations may help identify the patients who will benefit most from enhanced androgen suppression in localized and advanced prostate cancer.
A prospective phase 2 study of abiraterone in combination with LHRH agonist as neoadjuvant and concurrent therapy with external-beam radiation was conducted in men with intermediate and high-risk localized prostate cancer. The treatment was well-tolerated and achieved significantly greater serum and intraprostatic androgen suppression compared to changes seen with LHRH agonist with bicalutamide. Preliminary analysis of the clinical data is also promising with excellent PSA nadir and no relapse to date.
Acknowledgements
Pacific Northwest Prostate Cancer Spore P50CA097186
Prostate Cancer Foundation
Wayne D. Kuni and Joan E. Kuni Foundation
Johnson and Johnson, Inc
NCI T32 CA009515
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: none
REFERENCES
- 1.Zietman AL, Prince EA, Nakfoor BM, Park JJ. Androgen deprivation and radiation therapy: Sequencing studies using the shionogi in vivo tumor system. International journal of radiation oncology, biology, physics. 1997;38:1067–1070. doi: 10.1016/s0360-3016(97)00309-x. [DOI] [PubMed] [Google Scholar]
- 2.Kaminski JM, Hanlon AL, Joon DL, Meistrich M, Hachem P, Pollack A. Effect of sequencing of androgen deprivation and radiotherapy on prostate cancer growth. International journal of radiation oncology, biology, physics. 2003;57:24–28. doi: 10.1016/s0360-3016(03)00539-x. [DOI] [PubMed] [Google Scholar]
- 3.Hanks GE, Pajak TF, Porter A, Grignon D, Brereton H, Venkatesan V, Horwitz EM, Lawton C, Rosenthal SA, Sandler HM, Shipley WU Radiation Therapy Oncology G. Phase iii trial of long-term adjuvant androgen deprivation after neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate: The radiation therapy oncology group protocol 92-02. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21:3972–3978. doi: 10.1200/JCO.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 4.Horwitz EM, Bae K, Hanks GE, Porter A, Grignon DJ, Brereton HD, Venkatesan V, Lawton CA, Rosenthal SA, Sandler HM, Shipley WU. Ten-year follow-up of radiation therapy oncology group protocol 92-02: A phase iii trial of the duration of elective androgen deprivation in locally advanced prostate cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:2497–2504. doi: 10.1200/JCO.2007.14.9021. [DOI] [PubMed] [Google Scholar]
- 5.Bolla M, Gonzalez D, Warde P, Dubois JB, Mirimanoff RO, Storme G, Bernier J, Kuten A, Sternberg C, Gil T, Collette L, Pierart M. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. The New England journal of medicine. 1997;337:295–300. doi: 10.1056/NEJM199707313370502. [DOI] [PubMed] [Google Scholar]
- 6.Roach M, 3rd, Bae K, Speight J, Wolkov HB, Rubin P, Lee RJ, Lawton C, Valicenti R, Grignon D, Pilepich MV. Short-term neoadjuvant androgen deprivation therapy and external-beam radiotherapy for locally advanced prostate cancer: Long-term results of rtog 8610. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:585–591. doi: 10.1200/JCO.2007.13.9881. [DOI] [PubMed] [Google Scholar]
- 7.Pilepich MV, Winter K, Lawton CA, Krisch RE, Wolkov HB, Movsas B, Hug EB, Asbell SO, Grignon D. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma--long-term results of phase iii rtog 85-31. International journal of radiation oncology, biology, physics. 2005;61:1285–1290. doi: 10.1016/j.ijrobp.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 8.D'Amico AV, Manola J, Loffredo M, Renshaw AA, DellaCroce A, Kantoff PW. 6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: A randomized controlled trial. JAMA : the journal of the American Medical Association. 2004;292:821–827. doi: 10.1001/jama.292.7.821. [DOI] [PubMed] [Google Scholar]
- 9.Goodwin JF, Schiewer MJ, Dean JL, Schrecengost RS, de Leeuw R, Han S, Ma T, Den RB, Dicker AP, Feng FY, Knudsen KE. A hormone-DNA repair circuit governs the response to genotoxic insult. Cancer discovery. 2013;3:1254–1271. doi: 10.1158/2159-8290.CD-13-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polkinghorn WR, Parker JS, Lee MX, Kass EM, Spratt DE, Iaquinta PJ, Arora VK, Yen WF, Cai L, Zheng D, Carver BS, Chen Y, Watson PA, Shah NP, Fujisawa S, Goglia AG, Gopalan A, Hieronymus H, Wongvipat J, Scardino PT, Zelefsky MJ, Jasin M, Chaudhuri J, Powell SN, Sawyers CL. Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer discovery. 2013;3:1245–1253. doi: 10.1158/2159-8290.CD-13-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Donnell A, Judson I, Dowsett M, Raynaud F, Dearnaley D, Mason M, Harland S, Robbins A, Halbert G, Nutley B, Jarman M. Hormonal impact of the 17alpha-hydroxylase/c(17,20)-lyase inhibitor abiraterone acetate (cb7630) in patients with prostate cancer. British journal of cancer. 2004;90:2317–2325. doi: 10.1038/sj.bjc.6601879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Attard G, Reid AH, Yap TA, Raynaud F, Dowsett M, Settatree S, Barrett M, Parker C, Martins V, Folkerd E, Clark J, Cooper CS, Kaye SB, Dearnaley D, Lee G, de Bono JS. Phase i clinical trial of a selective inhibitor of cyp17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:4563–4571. doi: 10.1200/JCO.2007.15.9749. [DOI] [PubMed] [Google Scholar]
- 13.Kalhorn TF, Page ST, Howald WN, Mostaghel EA, Nelson PS. Analysis of testosterone and dihydrotestosterone from biological fluids as the oxime derivatives using high-performance liquid chromatography/tandem mass spectrometry. Rapid communications in mass spectrometry : RCM. 2007;21:3200–3206. doi: 10.1002/rcm.3205. [DOI] [PubMed] [Google Scholar]
- 14.Tamae D, Byrns M, Marck B, Mostaghel EA, Nelson PS, Lange P, Lin D, Taplin ME, Balk S, Ellis W, True L, Vessella R, Montgomery B, Blair IA, Penning TM. Development, validation and application of a stable isotope dilution liquid chromatography electrospray ionization/selected reaction monitoring/mass spectrometry (sid-lc/esi/srm/ms) method for quantification of keto-androgens in human serum. The Journal of steroid biochemistry and molecular biology. 2013;138:281–289. doi: 10.1016/j.jsbmb.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryu JK, Winter K, Michalski JM, Purdy JA, Markoe AM, Earle JD, Perez CA, Roach M, 3rd, Sandler HM, Pollack A, Cox JD. Interim report of toxicity from 3d conformal radiation therapy (3d-crt) for prostate cancer on 3dog/rtog 9406, level iii (79.2 gy) International journal of radiation oncology, biology, physics. 2002;54:1036–1046. doi: 10.1016/s0360-3016(02)03006-7. [DOI] [PubMed] [Google Scholar]
- 16.De Meerleer G, Vakaet L, Meersschout S, Villeirs G, Verbaeys A, Oosterlinck W, De Neve W. Intensity-modulated radiotherapy as primary treatment for prostate cancer: Acute toxicity in 114 patients. International journal of radiation oncology, biology, physics. 2004;60:777–787. doi: 10.1016/j.ijrobp.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 17.Lawton CA, Bae K, Pilepich M, Hanks G, Shipley W. Long-term treatment sequelae after external beam irradiation with or without hormonal manipulation for adenocarcinoma of the prostate: Analysis of radiation therapy oncology group studies 85-31, 86-10, and 92-02. International journal of radiation oncology, biology, physics. 2008;70:437–441. doi: 10.1016/j.ijrobp.2007.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taussky D, Bae K, Bahary JP, Roach M, 3rd, Lawton CA, Shipley WU, Sandler HM. Does timing of androgen deprivation influence radiation-induced toxicity? A secondary analysis of radiation therapy oncology group protocol 9413. Urology. 2008;72:1125–1129. doi: 10.1016/j.urology.2007.11.067. [DOI] [PubMed] [Google Scholar]
- 19.Clopper CaP ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–413. [Google Scholar]
- 20.Lissoni P, Meregalli S, Bonetto E, Mancuso M, Brivio F, Colciago M, Gardani G. Radiotherapy-induced lymphocytopenia: Changes in total lymphocyte count and in lymphocyte subpopulations under pelvic irradiation in gynecologic neoplasms. Journal of biological regulators and homeostatic agents. 2005;19:153–158. [PubMed] [Google Scholar]
- 21.Mostaghel EA, Nelson PS, Lange P, Lin DW, Taplin ME, Balk S, Ellis W, Kantoff P, Marck B, Tamae D, Matsumoto AM, True LD, Vessella R, Penning T, Hunter Merrill R, Gulati R, Montgomery B. Targeted androgen pathway suppression in localized prostate cancer: A pilot study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32:229–237. doi: 10.1200/JCO.2012.48.6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB, Jr, Saad F, Staffurth JN, Mainwaring P, Harland S, Flaig TW, Hutson TE, Cheng T, Patterson H, Hainsworth JD, Ryan CJ, Sternberg CN, Ellard SL, Flechon A, Saleh M, Scholz M, Efstathiou E, Zivi A, Bianchini D, Loriot Y, Chieffo N, Kheoh T, Haqq CM, Scher HI Investigators C-A- Abiraterone and increased survival in metastatic prostate cancer. The New England journal of medicine. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, Fizazi K, Mainwaring P, Piulats JM, Ng S, Carles J, Mulders PF, Basch E, Small EJ, Saad F, Schrijvers D, Van Poppel H, Mukherjee SD, Suttmann H, Gerritsen WR, Flaig TW, George DJ, Yu EY, Efstathiou E, Pantuck A, Winquist E, Higano CS, Taplin ME, Park Y, Kheoh T, Griffin T, Scher HI, Rathkopf DE Investigators C-A- Abiraterone in metastatic prostate cancer without previous chemotherapy. The New England journal of medicine. 2013;368:138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luu-The V, Belanger A, Labrie F. Androgen biosynthetic pathways in the human prostate. Best practice & research Clinical endocrinology & metabolism. 2008;22:207–221. doi: 10.1016/j.beem.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Taplin ME, Montgomery B, Logothetis CJ, Bubley GJ, Richie JP, Dalkin BL, Sanda MG, Davis JW, Loda M, True LD, Troncoso P, Ye H, Lis RT, Marck BT, Matsumoto AM, Balk SP, Mostaghel EA, Penning TM, Nelson PS, Xie W, Jiang Z, Haqq CM, Tamae D, Tran N, Peng W, Kheoh T, Molina A, Kantoff PW. Intense androgen-deprivation therapy with abiraterone acetate plus leuprolide acetate in patients with localized high-risk prostate cancer: Results of a randomized phase ii neoadjuvant study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014 doi: 10.1200/JCO.2013.53.4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein H, Molwitz T, Bartsch W. Steroid sulfate sulfatase in human benign prostatic hyperplasia: Characterization and quantification of the enzyme in epithelium and stroma. Journal of steroid biochemistry. 1989;33:195–200. doi: 10.1016/0022-4731(89)90294-x. [DOI] [PubMed] [Google Scholar]
- 27.Zelefsky MJ, Gomez DR, Polkinghorn WR, Pei X, Kollmeier M. Biochemical response to androgen deprivation therapy before external beam radiation therapy predicts long-term prostate cancer survival outcomes. International journal of radiation oncology, biology, physics. 2013;86:529–533. doi: 10.1016/j.ijrobp.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 28.McGuire SE, Lee AK, Cerne JZ, Munsell MF, Levy LB, Kudchadker RJ, Choi SL, Nguyen QN, Hoffman KE, Pugh TJ, Frank SJ, Corn PG, Logothetis CJ, Kuban DA. Psa response to neoadjuvant androgen deprivation therapy is a strong independent predictor of survival in high-risk prostate cancer in the dose-escalated radiation therapy era. International journal of radiation oncology, biology, physics. 2013;85:e39–e46. doi: 10.1016/j.ijrobp.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lamb DS, Denham JW, Joseph D, Matthews J, Atkinson C, Spry NA, Duchesne G, Ebert M, Steigler A, Delahunt B, D'Este C. A comparison of the prognostic value of early psa test-based variables following external beam radiotherapy, with or without preceding androgen deprivation: Analysis of data from the trog 96.01 randomized trial. International journal of radiation oncology, biology, physics. 2011;79:385–391. doi: 10.1016/j.ijrobp.2009.10.071. [DOI] [PubMed] [Google Scholar]
- 30.D'Amico AV, Chen MH, de Castro M, Loffredo M, Lamb DS, Steigler A, Kantoff PW, Denham JW. Surrogate endpoints for prostate cancer-specific mortality after radiotherapy and androgen suppression therapy in men with localised or locally advanced prostate cancer: An analysis of two randomised trials. The Lancet Oncology. 2012;13:189–195. doi: 10.1016/S1470-2045(11)70295-9. [DOI] [PubMed] [Google Scholar]