Abstract
Certain membrane-active cationic steroids are known to also possess both anti-inflammatory and antimicrobial properties. This combined functionality is particularly relevant for potential therapies of infections associated with elevated tissue damage, e.g. cystic fibrosis airway disease, a condition characterized by chronic bacterial infections and ongoing inflammation. In this study, six novel cationic glucocorticoids were synthesized using beclomethasone, budesonide, and flumethasone. Products were either monosubstituted or disubstituted, containing one or two steroidal groups, respectively. In vitro evaluation of biological activities demonstrated dual anti-inflammatory and antimicrobial properties with limited cytotoxicity for all synthesized compounds. Budesonide-derived compounds showed the highest degree of both glucocorticoid and antimicrobial properties within their respective mono- and disubstituted categories. Structure-activity analyses revealed that activity was generally related to the potency of the parent glucocorticoid. Taken together, these data indicate that these types of dual acting cationic lipids can be synthesized with the appropriate starting steroid to tailor activities as desired.
Keywords: Cationic Lipids, Antimicrobial, Anti-inflammatory, Lipofection, Cystic Fibrosis
Graphical abstract
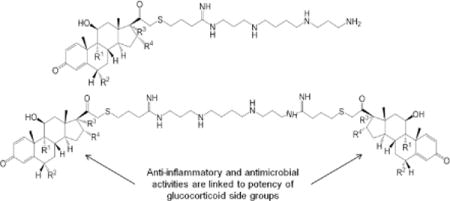
Cystic fibrosis (CF) is a monogenic disorder characterized by persistent bacterial infections of the airway. Although gene therapy could be an ideal treatment for this single-gene disease, development of an effective gene delivery system is complicated by the chronically infected and inflamed airway environment typical of many CF patients. Infections inhibit proper gene transfer, regardless of whether they are on-going or recently resolved at the time of gene administration.1 The mechanism behind this observed effect was linked to the immune response occurring at the time of gene delivery. Compounds with dual antimicrobial and anti-inflammatory properties may prove to be useful in preventing this effect from occurring, particularly if co-administered with the gene carrier during delivery. Additionally, such compounds may also be useful as locally applied antibiotics for prevention and symptomatic treatment of chronic bacterial lung infection, a dominant cause of death in CF subjects.
By the age of 17 years, nearly 70% of CF patients are infected with an opportunistic pathogen known as Pseudomonas aeruginosa. Once acquired, the organism is difficult to completely eradicate from the CF airway, leading to persisting infection and inflammation that eventually causes permanent pulmonary damage.2 Pathophysiological symptoms of this condition are cyclic in intensity, with the more intense periods termed “exacerbations.” Such exacerbations are associated with high bacterial (usually, P. aeruginosa) concentrations in the airway sputum, and aggressive antibiotic treatment is typically required to improve lung function.3,4 However, while early P. aeruginosa infections are susceptible to common anti-pseudomonal antibiotics (e.g. β-lactam antibiotics, aminoglycosides, and fluoroquinolones), later infections are more difficult to treat as antibiotic resistance emerges with the patient’s age.2 Resistance develops under the pressure of continued heavy use of antibiotics, which facilitates the selection of resistant strains, and is additionally linked to increased occurrence of hypermutable P. aeruginosa isolates.5 The emergence of these isolates is associated with the ongoing oxidative stress caused by the chronic polymorphonuclear leukocyte inflammation seen in many CF patients.6 Thus, a novel antibiotic with a dual anti-inflammatory function may also prove useful for treating and preventing additional exacerbations of CF airway disease.
Previous cationic sterol-based cationic lipids synthesized by our laboratory, dexamethasone spermine (DS)7 and disubstituted dexamethasone spermine (D2S)8, exhibited dual antimicrobial and anti-inflammatory functions. This study presents the synthesis and characterization of six new cationic steroids of similar structure to further understanding of this family of compounds. Instead of dexamethasone (dex), the six novel lipids presented (Figure 1) here contain other glucocorticoids (GCs) as the steroidal side groups: flumethasone in Compounds 1 and 2 (FS and F2S), budesonide in Compounds 3 and 4 (BuS and Bu2S), and beclomethasone in Compounds 5 and 6 (BeS and Be2S). These cationic lipids were the result of linking a polyamine, i.e. spermine, to the 21-OH position of each steroid. Products from this linkage reaction were either monosubstituted or disubstituted, with linkages occurring at one or both terminal amino groups of spermine, respectively. Final products were evaluated for glucocorticoid, antimicrobial, and DNA lipofection activities, as well as for any potential cytotoxic effects on mammalian cells.
Figure 1.
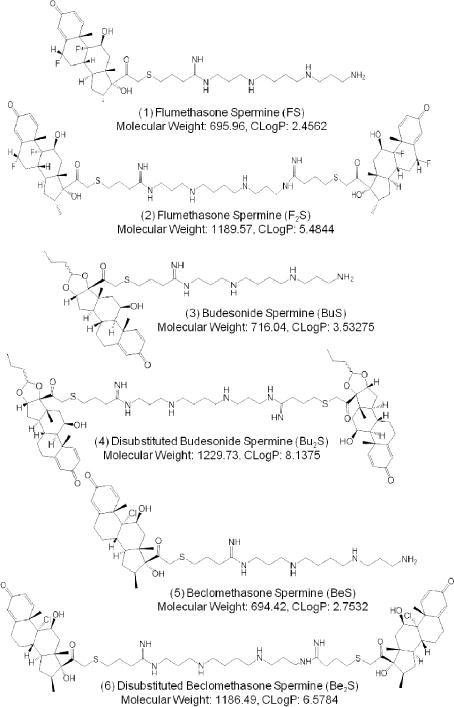
Structures, molecular weights, and CLogP values of FS (Compound 1), F2S (Compound 2), BuS (Compound 3), Bu2S (Compound 4), BeS (Compound 5), and Be2S (Compound 6).
A one-pot reaction with the appropriate GC mesylate, Traut’s reagent (TR), and spermine (shown in Figure 2) yielded the monosubstituted steroid (Compounds 1, 3, or 5) as the major product and the disubstituted steroid (Compounds 2, 4, or 6) as the minor product. The primary amines on either end of spermine reacted with TR to cause a selective ring-opening, resulting in an exposed sulfhydryl (-SH) end group. This end group then interacted with the α-keto mesylate of the modified GCs to form an α-keto thioether linkage between the steroid and polycation tail (i.e. the spermine-TR conjugate), yielding the monosubstituted cationic steroid. To form the disubstituted product, the primary amine of the monosubstituted lipid reacted with another TR molecule to eventually result in another thioether linkage with a separate GC molecule.
Figure 2. Synthesis of Compounds 1–6.
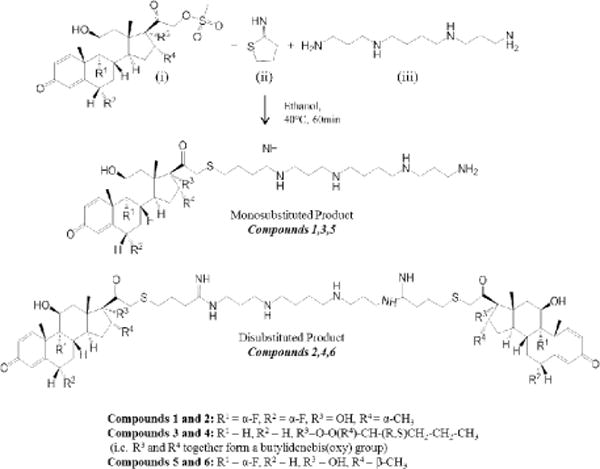
Reagents: (i) flumethasone-21-mesylate (Compounds 1 and 2), budesonide-21-mesylate (Compounds 3 and 4), beclomethasone-21-mesylate (Compounds 5 and 6); (ii) 2-iminothiolane-HCl (Traut’s Reagent); (iii) spermine.
Desired compounds were purified from excess starting reactants and unwanted reaction intermediates with semi-preparative HPLC, and then their molecular weights and chemical structures were verified with mass spectroscopy (MS) and 1H and 13C NMR. MS results are shown in Supplementary Figures 1–3. 1H and 13C NMR spectra and chemical shifts are shown in Supplementary Figures 4–15. Overall yield for the entire process (synthesis to purification) was ~60–70%, i.e. ~60–70% conversion of the parent steroid mesylate to the monosubstituted cationic product and a subsequent ~6–10% conversion of the monosubstituted product to the disubstituted product in a one-pot reaction.
Our ultimate goal for these compounds is to apply them in conjunction with potential gene therapies of CF airway disease. To identify the products with the highest therapeutic potential for this application, we evaluated the following in vitro properties of each compound: anti-inflammatory activity, antimicrobial activity, ability to deliver DNA (transfection activity), and cytotoxicity.
Anti-inflammatory activity was determined by measuring the glucocorticoid activity of each compound. This involved evaluating their abilities to trigger nuclear localization of the glucocorticoid receptor (GR) and to induce the glucocorticoid response element (GRE) in bovine aortic endothelial cells (BAECs). Results are shown in Figures 3 and 4, respectively. Ability to trigger some degree of nuclear localization of the GR was observed for all cationic lipids, though some exhibited more potency than others (Figure 3). Only budesonide-derived compounds (Compounds 3 and 4) showed activity equivalent to that seen with the positive control dex. Flumethasone and beclomethasone compounds (Compounds 1–2 and Compounds 5–6, respectively) showed less potency than equivalent concentrations of dex at all values tested (p<0.01). For induction of the GRE, the budesonide-derived products are, again, the only compounds to achieve expression equivalent to that seen with dex (Figure 4). Interestingly, beclomethasone products showed a comparatively limited ability to trigger GRE induction; no cellular response was observed at 1 μM BeS or Be2S (data not shown). At 5 μM, Be2S trigged only a modest response, while BeS triggered no effect at all. Flumethasone-derived products were able to achieve equivalently limited responses at 1 μM for both FS and F2S.
Figure 3. Steroid-induced nuclear localization of GFP-GR in BAECs.
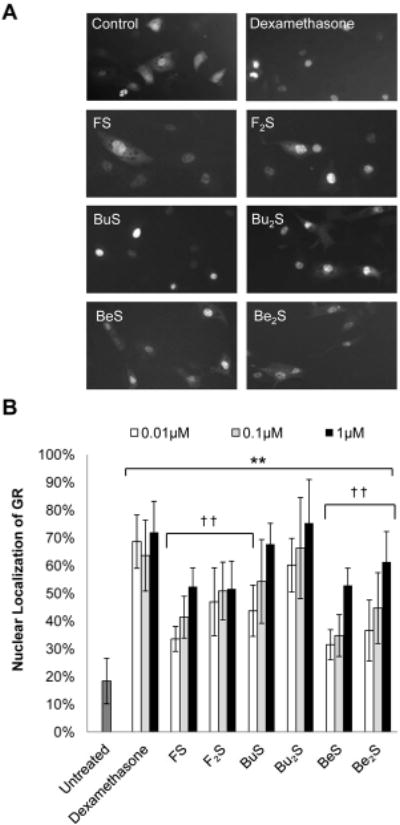
(A) GFP-GR translocation from cytosol to nucleoplasm. BAECs were transfected with pGFP-GR using Lipofectamine 2000 and then treated with compounds to stimulate GFP-GR nuclear localization. (B) Quantitative evaluation of cellular fluorescence localized within the nucleoplasm after treatment. Cells were treated with dexamethasone (positive control), FS, F2S, BuS, Bu2S, BeS, and Be2S at various concentrations. After 4 hours of treatment, cells were imaged using fluorescence microscopy, and percentage of cellular fluorescence localized with in the nucleus was assessed using ImageJ. At least 10 cells per condition were evaluated. Results are presented as means, and error bars represent the standard deviation. All experimental compounds showed some degree of GC activity greater than the untreated control at all concentrations tested (** p<0.01, Student’s t -test). Only BuS (0.1–1 μM) and Bu2S (0.01–1 μM), however, showed activity equivalent to that seen at equivalent concentrations of the positive control dexamethasone. † † indicates statistical significance (p<0.01, Student’s t-test) when
Figure 4. Steroid-induced activation of GRE promoter in BAECs.
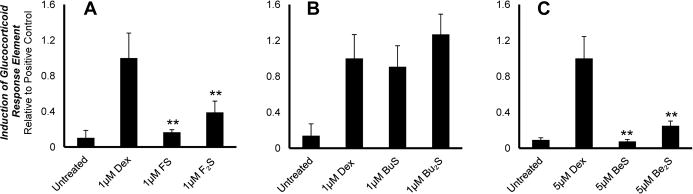
Cells were transfected with pGRE-SEAP using Lipofectamine 2000 and then treated with (A) 1 μM FS and F2S, (B) 1 μM BuS and Bu2S, and (C) 5 μM BeS and Be2S to induce activation of the GRE-SEAP gene construct. After 24 hours of treatment, media was harvested and evaluated for SEAP levels. Results were normalized relative to the positive control, dexamethasone (dex), and are presented as means. Error bars represent the standard deviation. Only BuS and Bu2S showed GRE activation comparable to that seen with dex. Flumethasone and beclomethasone products, on the other hand, showed significantly less GRE activation (** p<0.01, Student’s t- test) than that seen with eqivalent concentration of dex
To evaluate antimicrobial activity, Compounds 1–6 were screened for in vitro bactericidal activity against various strains of bacteria, including several clinical isolates of P. aeruginosa. The specific strains and corresponding minimal inhibitory concentrations (MICs) and minimal bactericidal concentrations (MBCs) are listed in Table 1. For clarity and easy interpretation, results are also depicted in the form of a clustergram in Figure 5. While generally more effective against the gram-positive bacterium tested (MRSA Xen30), many of the monosubstituted products were unable to achieve inhibitory effects at concentrations less than 128 μg/ml with the other bacteria tested. BuS (Compound 3) was the exception in this general trend, showing activity nearly equivalent to that seen with the more potent disubstituted products. Notably, BuS and all disubstituted products (Compounds 2, 4, and 6) displayed bactericidal activity greater than that observed with the positive controls, tobramycin and cathelicidin LL-37. Bu2S (Compound 4) showed the greatest potency of all compounds tested. Additional bacterial killing data, i.e. MBC/MIC ratios, can be found in Supplementary Table 1.
Table 1. Minimal inhi bitory concentration (MIC) and minimal bactericidal concentration (MBC) of selected steroids against various pathogens.
Specified pathogens were treated with FS, F2S, BuS, Bu2S, BeS, and Be2S as well as known antibiotic compounds, i.e. tobramycin (TOB), cathelicidin LL-37, DS, and D2S, to provide reference MIC and MBC values. Concentrations are in μg/ml. Clinical isolates of P. aeruginosa (PA) are indicated by a single asterisk (*).
| Bacteria Strain | MIC (μg/mL)/MBC (μg/mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TOB | LL-37 | DS | D2S | FS | F2S | BuS | Bu2S | BeS | Be2S | |
| MRSA Xen30 | ND | 8/16 | 64/64 | 4/8 | 64/128 | 4/4 | 8/8 | 4/8 | 64/64 | 2/4 |
| E. coli | ND | 16/32 | 256/256 | 8/8 | 256/256 | 16/64 | 8/64 | 1/1 | 256/>256 | 8/8 |
| PA 14 | ND | 128/256 | 256/256 | 8/16 | 256/>256 | 8/8 | 64/64 | 8/8 | 256/>256 | 16/32 |
| PA Xen5 | 64/128 | 64/128 | 256/256 | 16/32 | 128/128 | 8/16 | 8/32 | 1/1 | 256/>256 | 8/8 |
| PAM | 16/64 | 32/64 | 128/256 | 32/64 | 256/>256 | 32/32 | 8/16 | 4/4 | 256/>256 | 8/8 |
| PA*-2 | ND | ND | 256/256 | 32/64 | 256/256 | 16/16 | 32/64 | 8/16 | 64/128 | 16/32 |
| PA*-3 | 64/128 | >256/>256 | 256/>256 | 32/64 | 256/>256 | 16/16 | 64/128 | 32/32 | 256/>256 | 64/64 |
| PAM | 32/64 | >256/>256 | 256/256 | 16/32 | 256/256 | 32/64 | 32/32 | 8/16 | 256/>256 | 32/64 |
| PA*-5 | >256/>256 | >256/>256 | 256/256 | 16/32 | >256/>256 | 16/32 | 128/128 | 8/8 | 256/256 | 32/32 |
| PA*-20 | 32/64 | 64/256 | 128/256 | 32/64 | 256/>256 | 16/32 | 16/32 | 4/8 | 256/>256 | 32/64 |
| PA*-21 | 32/64 | 64/256 | 256/256 | 64/64 | 128/256 | 32/32 | 32/64 | 16/16 | 256/>256 | 32/64 |
| PA*-22 | >256/>256 | >256/>256 | 128/128 | 16/16 | 128/256 | 16/16 | 64/256 | 8/16 | 128/256 | 16/32 |
| PA*-63 | 64/256 | 128/256 | 128/128 | 32/32 | 128/128 | 8/8 | 16/64 | 8/16 | 128/256 | 16/16 |
| PA*-67 | >256/>256 | >256/>256 | >256/>256 | 32/32 | >256/>256 | 16/64 | 128/128 | 8/8 | >256/>256 | 16/32 |
Figure 5. Cluster heat map of mini mal bacteri cidal concent rations (MBCs,μg/ml) of cat ionic steroids against various pathogens.
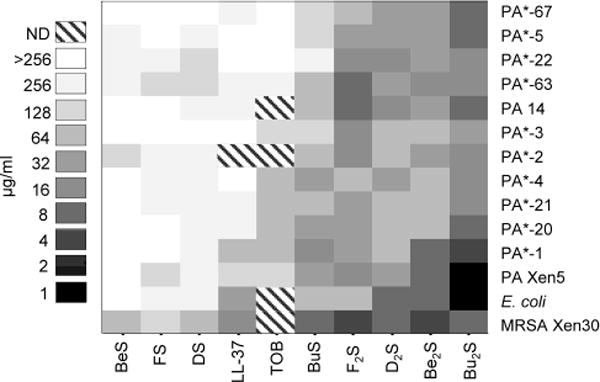
Specified pathogens were treated with FS, F2S, BuS, Bu2S, BeS, and Be2S as well as known antibiotic compounds, i.e. tobramycin (TOB), cathelicidin LL-37, DS, and D2S, to provide reference MBC values. Results are presented in a cluster heat map to visually associate compounds with similar activities. Patterned boxes indicate compound/pathogen combinations that were not determined (ND). Clinical isolates of P. aeruginosa (PA) are indicated by a single asterisk (*).
Transfection activities of cationic lipids were assessed using mixtures of varying monosubstituted to disubstituted lipid ratios on BAECs or A549 cells. Results are shown in Figure 6. With either cell type, transfection mixtures containing only monosubstituted lipids showed zero to very little transfection activity. Mixtures containing only disubstituted products achieved transfection activity generally comparable to that seen with the positive control, Lipofectamine 2000. Interestingly, 60/40 and 40/60 mol% FS/F2S mixtures showed activity equivalent to that seen with 100% F2S with either cell type. For budesonide and beclomethasone compounds, trends in activity appeared to be cell specific. 40/60 mol% mono/disubstituted mixtures appeared more effective with BAECs, while 60/40 mol% mixtures were more effective with A549 cells.
Figure 6. Transfection of BAECs and A549 cells with Compounds 1–6 using GFP transgene.
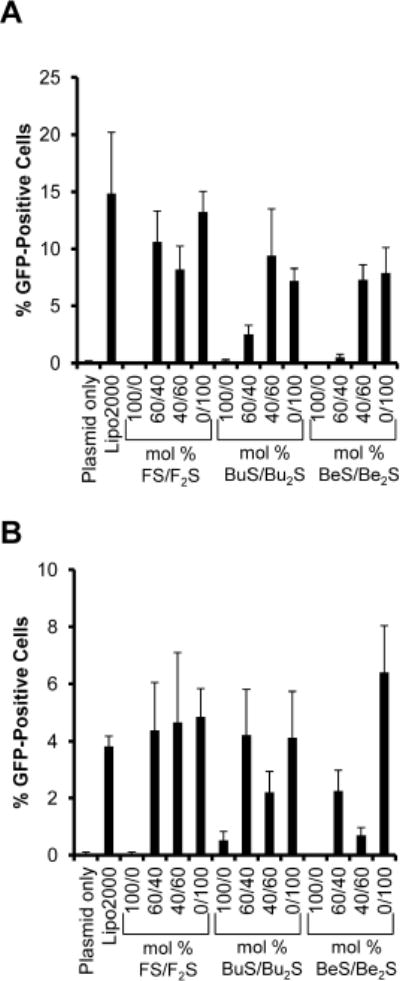
(A) BAECs and (B) A549 were transfected with cationic lipids at a charge ratio of 6:1 or Lipofectamine 2000 (i.e. Lipo2000, positive control) at a ratio of 2:1 (w/w) ratio with DNA. Cells were harvested 24 hours later and sorted for GFP-positivity using flow cytometry. Results are represented as means, and error bars show the standard deviation from four replicates of 500–1000 events per condition.
Compounds showed limited toxicity on BAECs and A549 cells at concentrations less than 50 μM (~30 μg/ml for monosubstituted compounds, ~60 μg/ml for disubstituted compounds), as shown in Figure 7. At these lower concentrations, loss in cell viability was never greater than ~10% for BAECs and ~20% for A549 cells. Furthermore, toxic effects on viability never exceeded that which was seen with dex at equivalent concentrations. At 50 μM, however, much more dramatic effects were observed. Treatment with any disubstituted compound resulted in a dramatic loss in cell viability (85–100%) with either cell type. This degree of toxicity was not observed at 50 μM dex, although this concentration proved to be more toxic than lesser concentrations of the compound. Interestingly, monosubstituted products also did not exhibit this amount of cytotoxicity at 50 μM concentration. BuS treatment showed loss in viability comparable to that seen with dex at this concentration, while FeS and BeS barely caused a ~5–10% loss in viability.
Figure 7. Cell vi ability post -t reatment with Compounds 1–6.
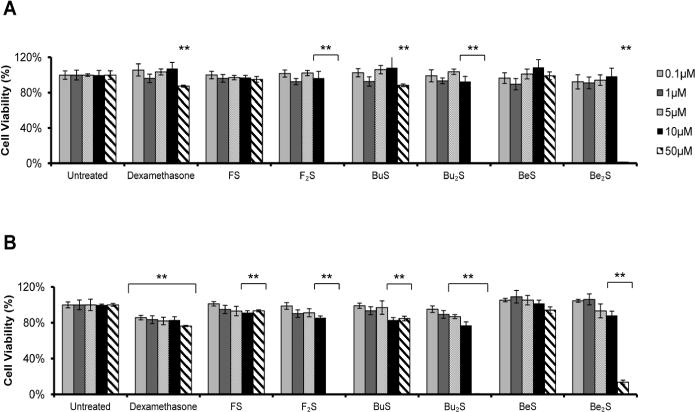
(A) BAECs and (B) A549 cells were treated with cationic lipids at the specified concentrations for 24 hours before cell viability was assessed. Results represent means, and error bars indicated standard deviation from four replicates at each condition. At concentrations below 50 μM, compounds showed limited toxicity, i.e. no more than 10% or 25% loss in viability with BAECs or A549 cells, respectively. Disubstituted products showed significant toxicity at 50 μM only, resulting in complete or near complete loss in viability with both cell types. In all other cases, toxicity was never greater than that observed with the dexamethasone control. ** indicates statistical significance (p < 0.01, Student’s t-test) when compared to untreated control.
Toxicity toward eukaryotic cell membranes was assessed by measurement of hemoglobin (Hb) release from human red blood cells (RBCs), and results are shown in Figure 8. Significant Hb release was observed only with the higher concentrations (≥50 μM) of the disubstituted compounds. Bu2S caused the most Hb release at these concentrations, i.e. ~17% Hb release at 50 μM and ~36% release at 100 μM, while Be2S resulted in the least, i.e. 0% release at 50 μM and ~8% release a 100 μM. All monosubstituted products failed to cause >1% Hb release at all concentrations tested.
Figure 8. Red blood cell lysis in response to treatment with Compounds 1–6.
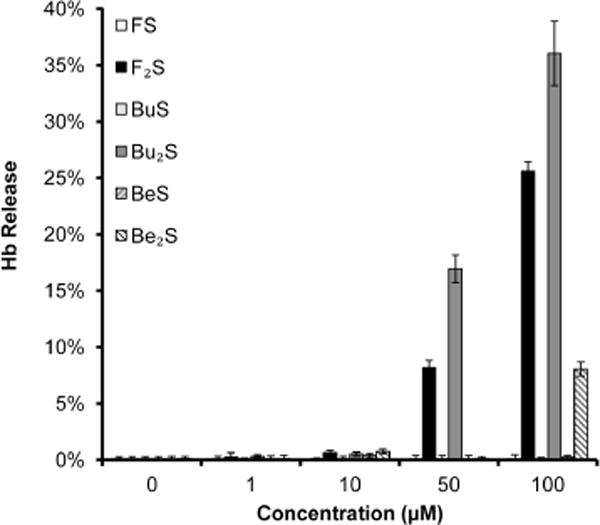
Freshly isolated human RBCs were treated with specified amounts of cationic lipids, and hemoglobin (Hb) release was measured after one hour of treatment at 37°C. Results are represented as means, and error bars show the standard deviation from four replicates at each condition. Monosubstituted products show very little to no RBC hemolysis at all concentrations tested. Disubstituted products show hemolysis at concentrations greater than 50 μM.
In conclusion, the compounds presented here exhibited a range of anti-inflammatory and antimicrobial activities that generally correlated with potencies of the parental steroidal groups used to synthesize them. Results additionally demonstrated that these lipids can be used without causing dramatic toxicities at the concentrations necessary to achieve desired pharmacological activities.
GC activity is known to correlate with the size and polarity of the substituent at the 16-C position.9 Hydrophobic residues at this position, like the cyclic acetal group of budesonide, result in higher GC potency. In fact, unmodified budesonide demonstrates GC activity that is ~10 times more potent than that of dex9 and a GR affinity for that is ~8 times the GR affinity of dex10. Meanwhile, unmodified flumethasone and beclomethasone have GC activities that are only 2–3 times higher than that of dex.9 Consequently, the comparatively high degree of GC activity of BuS and Bu2S is not particularly surprising. However, the lack of GC activity from flumethasone- and beclomethasone-derived compounds is. This finding suggests that addition of the cationic tails to these steroids causes decreased membrane permeability and/or decreased GR affinity to ultimately result in significantly lower GC activity.
In general, disubstituted products demonstrated more potency than their monosubstituted analogues in GC activity and many of the other categories tested. This is an expected observation since the disubstituted products, as demonstrated by their CLogP values (Figure 1), are more lipophilic than their monosubstituted analogues. With higher hydrophobic character, these compounds are able to penetrate the hydrophobic core of a cell membrane more easily and trigger the appropriate response once inside the cell. This enhanced membrane activity is also reflected in their ability to trigger Hb release from RBCs at concentrations that triggered no such response with the monosubstituted products (Figure 5). Additionally, for anti-inflammatory activity in particular, the enhanced activities of disubstituted products can also be attributed to the presence of two steroidal side groups, rather than one. With an additional ligand to bind appropriate receptors, these compounds are more likely to bind and activate the cytosolic GR once they have bypassed the cell membrane.
Beyond specific protein targets like the glucocorticoid receptor, steroid-spermine conjugates can also interact with micron-scale targets such as biological membranes. For the cationic lipids reported, one important metric of pharmaceutic activity is their differential interaction with mammalian cell membranes and bacterial cell membranes. We not only demonstrate that these molecules are nontoxic to mammalian cell plasmalemma membranes (Figure 8), they also have endosomolytic activity (Figure 6), which is an important feature in nucleic acid therapies. For the disubstituted spermine conjugates, the endosomolytic activity for DNA delivery was quite high as was their bactericidal activity, compared to the monosubstituted spermine conjugates. Given the relevance of new therapies involving nucleic acid (even against Ebola12), this functional membrane activity is a relevant metric of the pharmacological function of these molecules and we address this issue with quantitative measurements (Figures 5–8).
Interestingly, at concentrations above 0.01 μM, one monosubstituted compound, BuS (Compound 3), was able to achieve levels of GC activity equivalent to that observed with dex and its disubstituted counterpart (Bu2S, Compound 4) without demonstrating significant membrane disruption activity with RBCs. At the concentrations tested (0–100 μM), treatment of RBCs with BuS did not cause any Hb release, indicating that the plasma membrane had not been compromised. Together, these phenomena suggest that BuS is membrane permeable without being membrane disruptive, as it must bypass the cell membrane in order to bind the cytosolic GR for GC activity. Likely, the smaller size of BuS, relative to that of the disubstituted compounds, allows it to pass through the membrane without disrupting the structural integrity.
Trends observed in bacteria killing ability are generally analogous to those seen with GC activity. The disubstituted compounds are more effective than their monosubstituted counterparts, and the explanations are likely the same, despite the differences in lipid membrane constituents in prokaryotic and eukaryotic cells. Disubstituted compounds are larger and more hydrophobic, enabling them to more easily associate with a lipid bilayer membrane and cause disruptions in the structural integrity when they do so. Notably, however, BuS once again shows unexpectedly high activity. Despite being less effective than its disubstituted counterpart, it was much more effective than any of the other monosubstituted compounds tested. In fact, the antimicrobial activity of BuS was more comparable to that of the disubstituted compounds than that of the monosubstituted ones.
Results from the bacteria killing assay also demonstrated two other key findings. First, BuS and all disubstituted compounds tested were generally more effective at killing the tested pathogens than antimicrobial compounds cathelicidin LL-37 and tobramycin were, and the latter is frequently used to treat bacterial infections during CF airway exacerbations. Second, all compounds of this study, both mono- and disubstituted, showed particularly high efficacies against the Gram-positive bacterium MRSA Xen30. Gram-positive bacteria are generally more susceptible to cationic lipids,11 but they may show especial susceptibility with cationic antibiotics because of their peptidoglycan outer layer. Anionic polymers, i.e. teichoic acids, are exposed on the surface of Gram-positive bacteria and thread through their peptidoglycan outer layer. They impart to it a negative charge that facilitates easy association with cationic antibiotics. Gram-negative bacteria, on the other hand, have an outer membrane layer that contains LPS molecules, which reduce the fluidity, and thus the permeability, of the membrane. LPS typically allows resistance against hydrophobic antibiotics for this reason, but the antibiotics presented here are also cationic in nature. This characteristic likely enables them to weaken the LPS structure by displacing the divalent cations (Ca2+ and Mg2+) that normally stabilize it, thereby allowing permeabilization of the outer membrane that render the bacteria unviable.
In using these compounds for potential gene therapies of cystic fibrosis airway disease, we evaluated their ability to be used as DNA delivery agents. Essentially all CF patients have chronic airway infection and inflammation, thus motivating the molecular design of the synthesized molecules. While no compound was more efficacious than the commercially available Lipofectamine 2000, many of the formulations tested showed comparable activity. Those formulations could therefore be used to facilitate equivalent gene transfer while also effecting anti-inflammatory and antimicrobial changes as well. The value of these compounds is not in enhancing any single particular effect but in achieving simultaneous actions with a single formulation.
The compounds presented here offer a unique advantage in treating infections associated with elevated tissue inflammation. As novel antibiotics with dual anti-inflammatory and bactericidal activities, they are able to treat the infection itself as well as the inflammatory environment that promotes the development of hypermutable bacterial strains and antibiotic resistance. In particular, BuS (Compound 3) is a particularly promising candidate for future therapeutic applications, as it exhibits high potencies in GC and antimicrobial activities but little in cytotoxic and hemolytic activities. The abilities of BuS and the other compounds presented here to facilitate gene transfer provide additional value with treatments that target the relevant diseases at their genetic source.
Ultimately, the results presented in this study additionally demonstrate that these compounds can be synthesized with different parent glucocorticoids to produce cationic steroids that retain their dual functionalities but achieve potencies that correlate with that of the starting steroid. Synthesis of these lipids can thus be modified accordingly to tailor potential treatments for a variety of applications and potencies.
Supplementary Material
Acknowledgments
We gratefully acknowledge Dr. George Furst (University of Pennsylvania, Philadelphia, PA) for performing the NMR analysis.
The work was supported in part by NIH HL-66565 (S.L.D), National Science Foundation Graduate Research Fellowship under Grant No. DGE-1321851 (M.M.), and the National Science Centre (Poland) under Grant No. UMO-2012/07/B/NZ6/03504 (R.B.). Any opinions, findings, and conclusions or recommendations expressed in this article are those of the authors and do not necessarily reflect the views of the funding agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Data
Supplementary data (synthetic procedures, analytical data, and in vitro biological experimental details) associated with this article can be found in the online version.
References and Notes
- 1.Myint M, Limberis MP, Bell P, et al. In vivo evaluation of adeno-associated virus gene transfer in airways of mice with acute or chronic respiratory infection. Hum Gene Ther. 2014;25(11):966–76. doi: 10.1089/hum.2014.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goss CH, Burns JL. Exacerbations in cystic fibrosis. 1: Epidemiology and pathogenesis. Thorax. 2007;62(4):360–7. doi: 10.1136/thx.2006.060889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Regelmann WE, Elliott GR, Warwick WJ, Clawson CC. Reduction of sputum Pseudomonas aeruginosa density by antibiotics improves lung function in cystic fibrosis more than do bronchodilators and chest physiotherapy alone. Am Rev Respir Dis. 1990;141(4 Pt 1):914–21. doi: 10.1164/ajrccm/141.4_Pt_1.914. [DOI] [PubMed] [Google Scholar]
- 4.Ramsey BW, Pepe MS, Quan JM, et al. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. Cystic Fibrosis Inhaled Tobramycin Study Group. N Engl J Med. 1999;340(1):23–30. doi: 10.1056/NEJM199901073400104. [DOI] [PubMed] [Google Scholar]
- 5.Oliver A, Cantón R, Campo P, Baquero F, Blázquez J. High frequency of hypermutable Pseudomonas aeruginosa in cystic fibrosis lung infection. Science. 2000;288(5469):1251–4. doi: 10.1126/science.288.5469.1251. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10818002. Accessed December 19, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Ciofu O, Riis B, Pressler T, Poulsen HE, Høiby N. Occurrence of hypermutable Pseudomonas aeruginosa in cystic fibrosis patients is associated with the oxidative stress caused by chronic lung inflammation. Antimicrob Agents Chemother. 2005;49(6):2276–82. doi: 10.1128/AAC.49.6.2276-2282.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gruneich J, Price A, Zhu J, Diamond SL. Cationic corticosteroid for nonviral gene delivery. Gene Ther. 2004;11(8):668–74. doi: 10.1038/sj.gt.3302214. [DOI] [PubMed] [Google Scholar]
- 8.Fein DE, Bucki R, Byfield F, Leszczynska K, Janmey PA, Diamond SL. Novel cationic lipids with enhanced gene delivery and antimicrobial activity. Mol Pharmacol. 2010;78(3):402–10. doi: 10.1124/mol.110.066670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grossmann C, Scholz T, Rochel M, et al. Transactivation via the human glucocorticoid and mineralocorticoid receptor by therapeutically used steroids in CV-1 cells: a comparison of their glucocorticoid and mineralocorticoid properties. Eur J Endocrinol. 2004;151(3):397–406. doi: 10.1530/eje.0.1510397. Available at: http://www.ncbi.nlm.nih.gov/pubmed/15362971. Accessed January 9, 2015. [DOI] [PubMed] [Google Scholar]
- 10.Esmailpour N, Högger P, Rohdewald P. Binding kinetics of budesonide to the human glucocorticoid receptor. Eur J Pharm Sci. 1998;6(3):219–23. doi: 10.1016/s0928-0987(97)00082-1. Available at: http://www.ncbi.nlm.nih.gov/pubmed/9795066. Accessed January 10, 2015. [DOI] [PubMed] [Google Scholar]
- 11.Silhavy TJ, Kahne D, Walker S. The bacterial cell envelope. Cold Spring Harb Perspect Biol. 2010;2(5):a000414. doi: 10.1101/cshperspect.a000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thi EP, Mire CE, Lee ACH, et al. Lipid nanoparticle siRNA treatment of Ebola-virus-Makona-infected nonhuman primates. Nature. 2015 doi: 10.1038/nature14442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


